Abstract
The thymus is a major target organ in human immunodeficiency virus type 1 (HIV-1)-infected children and feline immunodeficiency virus (FIV)-infected young cats (G. A. Dean and N. C. Pedersen, J. Virol. 72:9436–9440, 1998; J. L. Heeney, Immunol. Today 16:515–520, 1995; S. M. Schnittman et al., Proc. Natl. Acad. Sci. USA 87:7727–7731, 1990; T. A. Seemayer et al., Hum. Pathol. 15:469–474, 1984; H.-J. Shuurn et al., Am. J. Pathol. 134:1329–1338, 1989; J. C. Woo et al., J. Virol. 71:8632–8641, 1997; J. C. Woo et al., AIDS Res. Hum. Retrovir. 15:1377–1388, 1999). It is likely that the accelerated disease process in children and cats is due to infection of the thymus during the time when generation of naive T lymphocytes is needed for development of the mature immune system. Zidovudine (ZDV) monotherapy, which is used to prevent and treat perinatal HIV-1 infection (R. Sperling, Infect. Dis. Obstet. Gynecol. 6:197–203, 1998), previously had been shown to reduce viral burden in FIV-infected young cats (K. A. Hayes et al., J. Acquir. Immune Defic. Syndr. 6:127–134, 1993). The purpose of this study was to evaluate the effect of drug-induced reduction of viral burden in the thymus on virus-mediated thymic involution and peripheral blood CD4 decline using FIV-infected cats as a model for pediatric HIV-1 infection. Eight-week-old cats were randomly assigned to uninfected, saline-treated; uninfected, ZDV-treated; FIV-infected, saline-treated; and FIV-infected, ZDV-treated groups. Parameters measured included blood lymphocyte numbers, viral load in blood and thymic tissue, and thymic histopathology. While the viral burden was significantly reduced by ZDV monotherapy in peripheral blood lymphocytes, plasma, and thymus, thymic lesions were similar for the treated and untreated FIV-infected cats. Further, markedly lowering the viral burden did not increase blood CD4 lymphocyte numbers or prevent their decline. The data suggest that an inflammatory process continued in spite of reduced virus replication. These observations imply that reducing virus load and limiting thymic inflammation are separate factors that must be addressed when considering therapeutic strategies aimed at preserving thymic function.
In humans and most mammals, the thymus is active in producing T lymphocytes from before birth until sexual maturity at which time it undergoes age-related involution. In human immunodeficiency virus type 1 (HIV-1) infection, the thymus carries a heavy viral burden and is decimated by the cytopathic effects of the virus on thymocytes as well as supporting stromal elements (4, 6). The effects of thymic infection are especially devastating in HIV-1-infected children, where destruction of the thymus occurs while thymic function is still needed for the generation of antigenically diverse T lymphocytes (23, 24). HIV-related accelerated thymic involution is a common histological finding in pediatric AIDS and is marked by severe depletion of both lymphoid and epithelial cells (5, 6). Thymitis and dysinvolution also are common features which may precede the accelerated involution associated with pediatric AIDS (5, 14, 25). It is likely that HIV-1 infection of the thymus of children figures prominently in their heightened rate of disease progression relative to that of HIV-1-infected adults (14).
Zidovudine (ZDV) was the first antiviral approved for use against HIV-1 infection in humans, and its clinical and pharmacological effects have been extensively evaluated (2, 16, 17, 22, 27). ZDV monotherapy has become the standard in newborns and infants for prevention and treatment of HIV infection (27). Because of the importance of the thymus to the developing immune system, it is essential to determine if antiviral therapies are effective in reducing the viral burden in the thymus and what impact this has on CD4 lymphocyte numbers in an immature immune system.
Feline immunodeficiency virus (FIV) infection of cats is a unique model system in which to evaluate the effects of antiviral therapy on disease pathogenesis. FIV infection leads to progressive impairment of immune function (in vitro mitogen responsiveness, loss of delayed-type hypersensitivity, opportunistic infections, and neoplasia) and depletion in the peripheral pool of CD4 T lymphocytes which occurs over a period of months to years (9, 13, 20, 28, 32). Further, the thymus of FIV-infected cats carries a heavy viral burden (3, 7, 11, 30, 31). Thymic function is compromised in the face of extensive pathological change in the tissue. Similar to changes seen in pediatric HIV-1 infection, thymuses of young FIV-infected cats show follicular (B-cell) hyperplasia, premature cortical involution and/or cortical atrophy, thymitis, and resulting architectural distortion (3, 19, 30, 31).
Previous work in the FIV model system showed that prophylactic ZDV monotherapy was effective in reducing acute CD4 lymphocyte loss in young cats inoculated with FIV during the drug treatment period (4 weeks) and yet did not prevent infection and later CD4 lymphocyte decline (10, 11). More directly, another study of chronically infected adult cats (>6 months of age) placed on ZDV therapy for 12 weeks revealed that the thymuses of treated cats increased in overall cellularity as well as in numbers of CD1-, CD4-, and CD8-positive lymphocytes (K. A. Hayes and L. E. Mathes, unpublished data). Taken together, the data suggested that antiviral therapy afforded some level of protection to the thymic compartment.
In this study, we used FIV infection of young cats, where seeding of the lymphocyte pool still depends on thymic output, as a model of pediatric HIV-1 infection. Using this model, we studied the impact of antiviral therapy on lentiviral infection of the thymic compartment.
MATERIALS AND METHODS
Cats.
Eight-week-old specific-pathogen-free cats (Liberty Laboratories, Waverly, N.Y.) were randomly assigned as follows: saline treated and uninfected (n = 6), saline treated and FIV infected (n = 6), ZDV treated and uninfected (n = 6), and ZDV treated and FIV infected (n = 6). All work was performed in accordance with the guidelines of the University Laboratory Animal Care and Use Committee and the “Guide for the Care and Use of Laboratory Animals” (17a). The experimental period was 12 weeks.
FIV inoculation.
One thousand 50% tissue culture infective doses of the Maryland strain of FIV (FIV-MD) was administered by intravenous injection to anesthetized cats.
Antiviral drug treatment.
ZDV was obtained as a powder kindly supplied by the AIDS Reference and Reagents Program. ZDV was prepared for injection by dissolving the powder at a concentration of 10 mg/ml in infusion-grade saline. ZDV was administered at 15 mg/kg of body weight/day divided into two subcutaneous injections at 12-h intervals. Saline-treated cats were given equivalent volumes per kilogram in the same manner. Treatment began 48 h prior to FIV inoculation and continued for 12 weeks until sacrifice and necropsy of the animals.
Sample collection.
Weekly blood samples were collected for complete blood counts, lymphocyte phenotyping, leukocyte DNA isolation, serology, and viremia assays. The cats also were evaluated weekly for weight gain and clinical signs of illness.
Processing of thymic tissue samples for nucleic acid extraction.
Thymus samples were collected at necropsy (12 weeks postinfection [p.i.]), sectioned, embedded in OCT compound (Finetek Sakura USA, Torrance, Calif.), and quickly frozen in liquid nitrogen-cooled isopentane (Sigma Chemical Co., St. Louis, Mo.). Tissue blocks were stored at −70°C pending further processing. Thick sections (20 μm) were obtained by cryostat sectioning, and five thick sections per tissue block were immersed in 600 μl of cold cell lysis buffer (for RNA, PureScript RNA kit; Gentra Systems, Minneapolis, Minn.; for DNA, PureGene DNA kit; Gentra Systems) and homogenized with a microcentrifuge tube pestle.
Nucleic acid extraction from thymic tissue and peripheral blood leukocytes.
RNA was obtained from thymic tissue using the PureScript RNA kit (Gentra Systems) according to the manufacturer's instructions with the addition of two DNase-free RNase (Gibco BRL, Gaithersburg, Md.; 20 U/sample, 15 min at 37°C) treatments followed by phenol-chloroform extraction and ethanol precipitation. DNA from thymic tissue and DNA from blood were obtained using the PureGene DNA kit (Gentra Systems) according to the manufacturer's instructions. Nucleic acid samples were quantitated spectrophotometrically (GeneQuant; Pharmacia, Piscataway, N.J.) and standardized to contain 5 ng/μl in nuclease-free water containing 20 μg of glycogen per ml as carrier (Boehringer Mannheim, Indianapolis, Ind.).
Plasma FIV antigenemia determinations.
FIV CA p24 levels were determined by commercial enzyme-linked immunosorbent assay (ELISA) (IDEXX, Westbrook, Maine). Picograms per milliliter were obtained by extrapolation against a standard curve. The level of detection of the FIV antigen ELISA was ≥64 pg/ml.
Quantitative-competitive DNA PCR for blood lymphocytes and thymus.
A quantitative-competitive PCR assay (QC-PCR), based on that described by Diehl et al. (8), was adapted for detection of FIV-MD. The PCR mix consisted of 1× PCR buffer (Gibco BRL), 3 mM MgCl2, 200 μM deoxynucleoside triphosphates (dNTPs), 0.125 μM (each) primer (KH3, 5′-GATCCAAAAATGGTGTCC-3′, and KH4, 5′-CCTATTCCCATAATCTCTGC-3′), and 0.125 U of Platinum Taq DNA polymerase (Gibco BRL). To 46 μl of PCR mix was added serial 1:4 dilutions of pFIV-2 competitor (kindly provided by E. Hoover, Colorado State University) and 10 ng of sample DNA (corresponding to approximately 1,600 lymphocytes). Each sample was run against five 1:4 dilutions of competitor (total competitor copy number ranged from 30,000 to 2). The competitor range was chosen individually for each sample based on an initial screening by semiquantitative PCR. Each sample was tested at least two times. Reaction mixtures underwent 40 cycles of 94°C for 20 s, 55°C for 30 s, and 72°C for 15 s. Amplified products were separated on 4% Metaphor gels (FMC, Rockland, Maine). The gels were stained with ethidium bromide and imaged with a video camera-based system (Alpha Innotech, San Leandro, Calif.). Data were analyzed with the AlphaEase data analysis package (Alpha Innotech). Integrated density values were adjusted for size and ethidium bromide incorporation and converted to log values (21). The ratio of the log intensities of the wild-type and competitor bands was plotted against the log of the competitor copy number to obtain the equivalence point for that sample (21). The limit of detection for this assay was 30 copies/10 ng of DNA (corresponding to 1,600 cells).
Extraction of FIV RNA from plasma.
Plasma samples which had been stored at −70°C were quickly thawed, and 200 μl was added to siliconized 1.5-ml microcentrifuge tubes. Each sample was subsequently diluted to 1,600 μl with the addition of ice-cold TNE (0.01 M Tris, 0.1 M NaCl, 0.001 M EDTA, pH 7.2). The samples were centrifuged at 4°C and 17,000 × g for 90 min. The supernatants were decanted off, and 250 μl of TNE was added to each pellet. The samples were then extracted with TriReagent LS (MRC, Cincinnati, Ohio) as per the manufacturer's instructions with the following modification: prior to ethanol precipitation, 20 μg of molecular biology-grade glycogen (Boehringer Mannheim) was added to each sample. After ethanol precipitation, each sample pellet was resuspended in 20 μl of diethyl pyrocarbonate-treated water and stored at −70°C until use.
Preparation of thymic tissue for QC-RT-PCR.
Total RNA was isolated from frozen thymic tissue (Purescript RNA isolation kit; Gentra Systems), and the samples were twice treated with RNase-free DNase I to remove any contaminating genomic DNA. Following acid phenol-chloroform extraction and ethanol precipitation, the RNA samples were quantitated spectrophotometrically (GeneQuant) and standardized to contain 5 ng of total RNA/μl by dilution in diethyl pyrocarbonate-treated water (Sigma) containing 20 μg of glycogen (Boehringer Mannheim) per ml as carrier. The samples were screened by semiquantitative reverse transcription-PCR (RT-PCR) with and without reverse transcriptase in order to define the competitor range before performing QC-RT-PCR.
QC-RT-PCR for thymus tissue and plasma.
Competitor RNA was generated as previously described (8), and panels of fourfold dilutions (ranging from 30 to 5 × 105 copies) were prepared and frozen at −70°C. cDNA was prepared by combining 2 μl of sample RNA with 2 μl of competitor and 0.075 pg of random hexamers (Gibco BRL). The samples were incubated at 68°C for 5 min followed by 25°C for 10 min. After this denaturation step, the RT reaction mix (4 μl) was added such that each reaction mixture contained 1× RT buffer (Boehringer Mannheim), 1.5 mM dNTPs (Gibco BRL), 0.3 U of RNase inhibitor (5 Prime→3 Prime, Boulder, Colo.), and 0.125 U of avian myeloblastosis virus reverse transcriptase (Boehringer Mannheim). The RT reaction was carried out for 16 h at 42°C followed by heat inactivation at 95°C for 5 min. After preparation of the cDNA, 90 μl of PCR mix was added to each sample such that each reaction mixture contained 1× PCR buffer (Gibco BRL), 3 mM MgCl2, 0.2 mM dNTPs, 0.125 μM (each) primer (KH3 and KH4), and 0.125 U of Platinum Taq DNA polymerase (Gibco BRL). Reaction mixtures underwent 55 cycles of 94°C for 20 s, 55°C for 30 s, and 72°C for 15 s. Gel electrophoresis and analysis were as described above. The limits of detection of these assays were 200 copies/ng of total RNA for tissue and 6,000 copies/ml of plasma.
Lymphocyte phenotyping.
Lymphocyte subpopulations in peripheral blood were enumerated by immunostaining and flow cytometry as previously described (10, 11).
Immunohistochemistry.
Cryostat sections of thymus were stained for CD1, CD4, CD8, cytokeratin, and CD21 expression by standard immunohistological methods (29).
Histopathology.
Standard histopathology evaluations were performed on all cats.
Statistics.
Differences between groups were determined by Student's t test or the Mann-Whitney nonparametric test, where appropriate.
RESULTS
ZDV treatment reduced FIV burden in the peripheral blood lymphocytes and plasma.
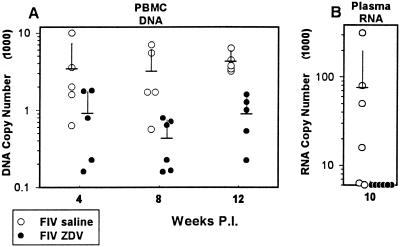
The effect of ZDV treatment on the virus load in the peripheral blood compartment was determined by measurement of viral DNA copy number in peripheral blood lymphocytes (QC-PCR), plasma antigenemia levels (ELISA), and plasma viral RNA levels (QC-RT-PCR). Measurement of peripheral blood lymphocyte viral DNA load was made at 4, 8, and 12 weeks p.i. and is expressed as copies per 1,600 lymphocytes (corresponding to 10 ng of DNA) (Fig. 1A). The mean copy numbers for the saline-treated FIV-infected group were 3,550 (standard deviation [SD], 3,750) (week 4), 3,280 (SD, 2,780) (week 8), and 4,420 (SD, 1,370) (week 12) versus 940 (SD, 790), 450 (SD, 300), and 920 (SD, 550) for the ZDV-treated FIV-infected group (Fig. 1A, P = 0.015 at 8 weeks and P = 0.001 at 12 weeks). Plasma antigenemia peaked at 2 weeks p.i. for both the saline-treated FIV-infected and ZDV-treated FIV-infected groups with mean peak levels of 1,940 (SD, 1,130) pg/ml for the saline-treated FIV-infected group versus 90 (SD, 50) pg/ml in the ZDV-treated FIV-infected group (P = 0.001) (data not shown). By week 3 p.i., plasma antigenemia levels for both groups were below the level of assay detection (64 pg/ml) (data not shown). In order to confirm the efficacy of ZDV treatment in suppression of plasma viremia at a later time point in the experiment, QC-RT-PCR was performed on viral RNA extracted from plasma. Plasma viral RNA at the 10-week point was below the level of detection (6,000 copies/ml of plasma) for all of the ZDV-treated FIV-infected cats and averaged 78,000 copies/ml of plasma for the saline-treated FIV-infected cats (P = 0.0087) (Fig. 1B). Thus, the viral DNA load in peripheral blood mononuclear cells was reduced by 75 to 85% with respect to the saline-treated FIV-infected group during the course of the experiment. Further, the level of cell-free viral antigen in the plasma was reduced by 96% during peak expression (week 2 p.i.), and the level of viral RNA in plasma was <6,000 copies/ml at 10 weeks p.i.
FIG. 1.
(A) Results from QC-PCR analysis for viral DNA load in peripheral blood lymphocytes. Data are expressed as copies per 10 ng of DNA (approximately 1,600 lymphocytes). Each point designates an individual animal with samples measured at 4, 8, and 12 weeks p.i. Open circles denote FIV-infected, saline-treated cats, and closed circles denote FIV-infected, ZDV-treated cats. Means and SDs for each group of data points are represented by horizontal and vertical bars, respectively. Differences between the ZDV-treated FIV-infected and saline-treated FIV-infected groups were statistically significant at weeks 8 (P = 0.015) and 12 (P = 0.001). PBMC, peripheral blood mononuclear cells. (B) Results from QC-RT-PCR analysis for viral RNA in plasma from the 10-week-p.i. point. Data are expressed as copies per milliliter of plasma. Each point designates an individual animal. Open circles denote FIV-infected, saline-treated cats, and closed circles denote FIV-infected, ZDV-treated cats. Means and SDs for each group of data points are represented by horizontal and vertical bars, respectively. The difference between the ZDV-treated FIV-infected and saline-treated FIV-infected groups was statistically significant (P = 0.0087).
ZDV treatment reduced the FIV burden in the thymic tissue compartment.
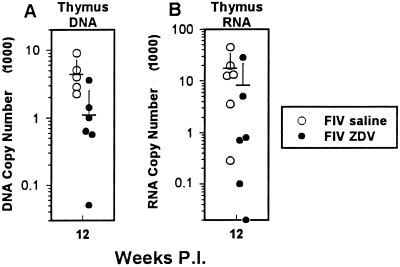
QC-PCR and QC-RT-PCR were used to determine the effect of ZDV treatment on thymic tissue virus load (Fig. 2). (i) The viral DNA measurement for thymic tissue was made at the 12-week point and expressed as copies per 1,600 lymphocytes. The mean viral DNA copy number for the saline-treated FIV-infected group was 4,600 (SD, 2,600) copies versus 1,200 (SD, 1,400; P = 0.02) copies for the ZDV-treated FIV-infected group (Fig. 2A). (ii) The mean viral RNA measurement for thymic tissue was made at the 12-week point and expressed as copies per nanogram of total RNA. Thymic tissue viral RNA copy number was 18,600 (SD, 15,700) copies for the saline-treated FIV-infected group versus 7,000 (SD, 12,000; P = 0.075) copies for the ZDV-treated FIV-infected group (Fig. 2B). Therefore, the mean value for viral DNA load in the thymus was reduced by 74% and that for viral RNA load was reduced by 62% with respect to the saline-treated FIV-infected group.
FIG. 2.
(A) Results from QC-PCR analysis for viral DNA in thymic tissue. Data are expressed as copies per 10 ng of DNA (approximately 1,600 lymphocytes). Each point designates an individual animal. Open circles denote FIV-infected, saline-treated cats, and closed circles denote FIV-infected, ZDV-treated cats. Samples were collected at the 12-week point. Means and SDs for each group of data points are represented by horizontal and vertical bars, respectively. The difference between the ZDV-treated FIV-infected and saline-treated FIV-infected groups was statistically significant (P = 0.02). (B) Results from QC-RT-PCR analysis for viral RNA in thymic tissue. Data are expressed as copies per 10 ng of RNA. Each point designates an individual animal, with the sample collected at the 12-week point only. Open circles denote FIV-infected, saline-treated cats, and closed circles denote FIV-infected, ZDV-treated cats. Means and SDs for each group of data points are represented by horizontal and vertical bars, respectively. The difference between the ZDV-treated FIV-infected and saline-treated FIV-infected groups was not significant (P = 0.075).
Histopathologic and immunohistologic examination of thymic tissues.
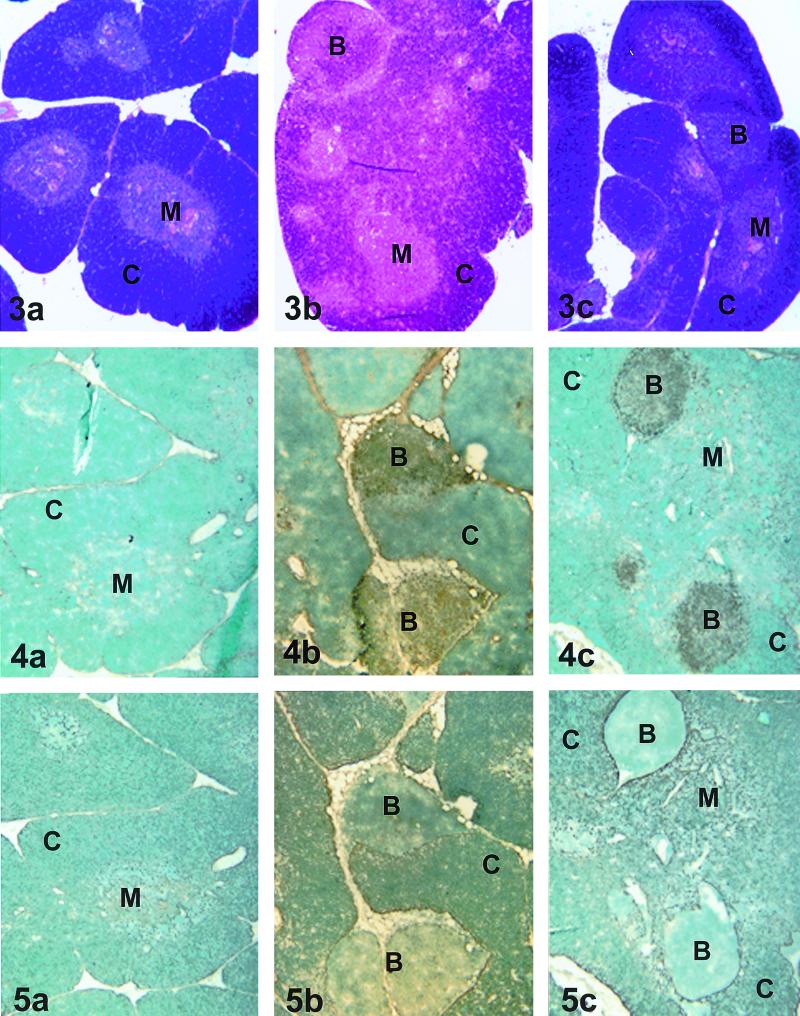
Histopathologic examination of thymic tissue from cats in the saline-treated uninfected and ZDV-treated uninfected groups showed normal tissue architecture with well-demarcated corticomedullary junctions (Fig. 3a). It is important to note that there was no histologic evidence of age-related thymic involution in the cats in the present study. As there were no differences histologically between the saline-treated uninfected and the ZDV-treated uninfected cats, we show one sample representative of both groups. In contrast, thymic tissue from the saline-treated FIV-infected and ZDV-treated FIV-infected groups was abnormal with architectural distortion, loss of distinct corticomedullary junctions, follicular hyperplasia, interlobular lymphocytic infiltration, and mild to moderate cortical atrophy (Fig. 3b and c). Immunohistochemical analysis showed that the distortion was due to the presence of prominent B-cell follicles which were present in all FIV-infected cats without regard to drug treatment (Fig. 4b and c) and which were absent in uninfected cats (Fig. 4a). In addition, there was a subjectively moderate reduction in CD4-positive lymphocytes in both the cortical and medullary regions of the thymus of both FIV-infected groups with respect to the uninfected groups (data not shown). The numbers of CD8-positive lymphocytes were only slightly reduced in the FIV-infected groups (data not shown). Cytokeratin staining supported the observation of abnormal architecture of the thymuses from FIV-infected cats irrespective of drug treatment (Fig. 5). There were no apparent effects on thymic architecture or numbers of CD4-, CD8-, or CD21-positive lymphocytes due to drug treatment in the absence of FIV infection (data not shown).
Phenotype analysis of peripheral blood lymphocyte numbers.
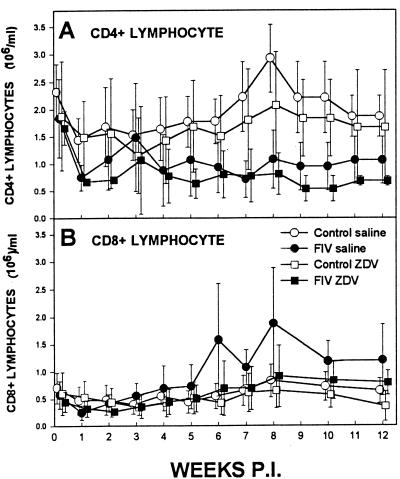
Even with the significant decrease in virus load in the thymus and peripheral blood due to ZDV treatment, lymphocyte numbers did not expand normally compared to those in age-matched controls. CD4 lymphocyte numbers for both the saline-treated FIV-infected and ZDV-treated FIV-infected groups declined over the course of the 12-week study to approximately half the number of the saline-treated uninfected and ZDV-treated uninfected groups (Fig. 6A). Meanwhile, numbers of CD8-positive lymphocytes were similar among all four groups with little change over the course of the study (Fig. 6B). Note that the mean CD8-positive lymphocyte numbers for the saline-treated FIV-infected group were elevated during the last 6 weeks due to one outlier cat.
FIG. 6.
(A) Numbers of peripheral blood CD4 lymphocytes obtained by immunostaining and flow cytometry analysis. Data are presented as mean numbers from each of the four groups at weekly time points (vertical bars represent SDs). Open circles denote saline-treated uninfected cats (n = 6), closed circles denote saline-treated FIV-infected cats (n = 6), open squares denote ZDV-treated uninfected cats (n = 6), and closed squares denote ZDV-treated FIV-infected cats (n = 6). The data were not significantly different at any time point. (B) Numbers of peripheral blood CD8 lymphocytes obtained by immunostaining and flow cytometry analysis. Data are presented as mean numbers from each of the four groups at weekly time points (vertical bars represent SDs). Open circles denote saline-treated uninfected cats (n = 6), closed circles denote saline-treated FIV-infected cats (n = 6), open squares denote ZDV-treated uninfected cats (n = 6), and closed squares denote ZDV-treated FIV-infected cats (n = 6). The data were not significantly different at any time point.
DISCUSSION
The thymuses of FIV-infected cats show significant pathological damage presumably due to the virus infection. If viral load in thymic tissue is directly correlated with thymic damage, it would be expected that any reduction in viral load would result in concomitant amelioration of the lesions in the thymus. We observed that even with reductions of 74% in proviral (DNA) load and 62% viral RNA load in the thymus, there were no differences histologically between FIV-infected cats with or without ZDV treatment. These observations led us to hypothesize that the viral infection may initiate a chronic inflammatory response which itself is damaging to the tissue.
On the other hand, it is possible that the level of virus suppression due to ZDV treatment may not have been significant enough to slow or prevent thymic damage. The moderate reductions seen in thymic viral load (less than 2 orders of magnitude) were much less than that seen in the plasma compartment where ZDV reduced plasma virus load below the level of assay detection (6,000 copies/ml of plasma) compared to an average of 78,000 copies of viral RNA/ml of plasma in the saline-treated FIV-infected cats. The degree of antiviral efficacy is thought to be correlated with the extent of reduction of plasma virus load. Importantly, with the demonstration of significant suppression of plasma viremia with ZDV treatment, we did not see as dramatic a reduction of virus load in the thymic compartment.
Treatment of AIDS patients with highly active antiretroviral therapy frequently results in a bimodal rebound of peripheral blood CD4 lymphocytes with initial increases in memory cells followed by a second phase of rebound in naive cells, provided that there was residual thymic tissue (15). In the scid-hu mouse system, human thymic implants infected with HIV-1 and allowed to become depleted of CD4-CD8-double-positive thymocytes demonstrated a transient renewal of thymopoiesis in mice treated with ZDV, didanosine, and indinavir (1, 29). The transient nature of the response resulted from a resurgence of the virus infection of the CD4-CD8-double-positive thymocyte population, however (1). In contrast to these studies, the cats in the present study were treated during the acute phase of infection before significant loss of peripheral CD4 lymphocytes occurs.
We observed significant disruption of normal thymic architecture in both the ZDV-treated and saline-treated FIV-infected cats at the termination of the study (12 weeks p.i.). Similarly, in another study, the thymus in cats infected with the FIV Petaluma strain showed, in addition to age-related thymic involution, significant structural damage with disruption of lobular architecture, loss of corticomedullary junctions, and thymitis beginning approximately 6 weeks p.i. (3, 30). Woo et al. determined that all subtypes of thymocytes as well as infiltrating B lymphocytes contain virus (30). Interestingly, single-positive (mature) CD8 lymphocyte numbers in the thymus and peripheral blood appeared unaffected by FIV infection (30). We also observed this although it is impossible to know if the CD8 lymphocytes survived maturation in an infected thymus or had trafficked to the thymus from the periphery. Although cortical CD4-CD8-double-positive lymphocytes are infected and killed by FIV, it was proposed previously that preferential differentiation or restricted cytopathicity might account for the selective preservation of mature CD8 lymphocytes (30). Dean and Pedersen reported that the thymus carries the heaviest viral burden in the acute phase of infection and that there was an increase in type 2 cytokines which may contribute to the observed inflammation (7). While viral DNA was disseminated throughout most lymphoid tissues at 70 days p.i., viral RNA was produced almost exclusively in the thymus during the acute phase of infection (18). We have demonstrated heavy viral burden in the thymic tissues of FIV-infected young cats monitored for over 1 year (11), suggesting a strong correlation between virus infection of the thymus and subsequent pathologic change. However, in this study, thymic pathology was not proportional to virus load in the ZDV-treated FIV-infected cats.
Previous work in our FIV model system showed that ZDV monotherapy initiated prior to infection was effective in preventing acute CD4 lymphocyte loss and acute plasma antigenemia in young cats inoculated with FIV and yet did not prevent infection (10, 11). In that study, drug treatment at 30 mg/kg/day was initiated 48 h prior to FIV inoculation and continued for 4 weeks. The cats were then monitored for an additional 48 weeks before sacrifice. We postulated that the early beneficial effect of ZDV on CD4 lymphocyte numbers might have been due to protection of the thymic compartment during the drug treatment period (4 weeks) and that this protection was manifested by production of normal numbers of peripheral blood CD4 lymphocytes (10). However, upon withdrawal of ZDV, CD4 lymphocytes declined to the level observed for the untreated FIV-infected cats (11). The present study used a 12-week course of drug treatment at 15 mg/kg/day, with the idea that protection was directly related to maintaining a low virus load. Even with a significant reduction in virus load in the blood and thymus, we observed that peripheral blood CD4 lymphocyte numbers dropped acutely p.i. and continued to be suppressed during the course of the study with or without ZDV treatment while CD8 lymphocyte numbers were unaffected. Immunohistochemical evaluation of CD1, CD4, CD8, and CD21 staining of thymic tissues confirmed previously reported pathologic changes due to FIV infection including disruption of normal architecture by infiltration of B-cell follicles and cortical atrophy due to a reduction in CD4-CD8- and CD4-positive lymphocytes (30, 31). Further, the histopathologic changes in the thymus (follicular lymphoid hyperplasia, cortical atrophy, and thymitis) were similar in the FIV-infected cats in the present study, irrespective of drug treatment.
It is apparent from the data from our work and others that a simple cause-and-effect relationship between virus load and thymus damage is not tenable. Based on the histologic evidence of inflammation and increased prevalence of cytokines gamma interferon, interleukin-4 (IL-4), IL-10, and IL-12 in the thymus during the latter part of the acute phase of FIV infection, it must be considered that multiple processes in the thymus may separately or together contribute to thymic damage (7, 30, 31). Further, the level of virus suppression in the plasma afforded by antiviral therapy is not necessarily attained in lymphoid compartments. It will be important to evaluate the impact of therapy which affords more complete suppression of virus replication in the thymic compartment. The implication from this work, building on that of others, is that antiviral therapy may need to be combined with other strategies to allow the host to maintain or reconstitute thymic function.
FIG. 3-5.
Fig. 3. Histopathologic evaluation of thymic tissue. (a) Thymic tissue representative of saline-treated uninfected and ZDV-treated uninfected groups. (b and c) Thymic tissue representative of the saline-treated FIV-infected (b) and ZDV-treated uninfected (c) groups. C, cortex; M, medulla; B, B-cell follicle. Magnification, ×33. Fig. 4. Immunohistochemical analysis of thymic tissue stained for B-cell CD21 expression. (a) Thymic tissue representative of the saline-treated uninfected and ZDV-treated uninfected groups. (b and c) Thymic tissue representative of the saline-treated FIV-infected (b) and ZDV-treated FIV-infected (c) groups. C, cortex; M, medulla; B, B-cell follicle. Magnification, ×33. Fig. 5. Immunohistochemical analysis of thymic tissue stained for cytokeratin expression. (a) Thymic tissue representative of the saline-treated uninfected and ZDV-treated uninfected groups. (b and c) Thymic tissue representative of the saline-treated FIV-infected and ZDV-treated FIV-infected groups. C, cortex; M, medulla; B, B-cell follicle. Magnification, ×33.
ACKNOWLEDGMENTS
We acknowledge the support of the Center for Retrovirus Research and the OSU Comprehensive Cancer Center and Arthur G. James Cancer Hospital and Solove Research Institute, The Ohio State University. This project was funded in part by grants R01 AI 40855 from the National Institutes of Health and P30 CA16058 from the National Cancer Institute.
We thank Philip Johnson and Robert Olmsted for providing the Mount Airy, Maryland isolate of FIV and Edward Hoover for the generous gift of the pFIV competitor plasmid. ZDV used in this study was obtained from the AIDS Reference and Reagents Program. Tissue histopathology evaluation by Steven E. Weisbrode is gratefully acknowledged. We thank Katherine Beachy and Julia Grossman for excellent technical assistance.
REFERENCES
- 1.Amado R G, Jamieson B D, Cortado R, Cole S W, Zack J A. Reconstitution of human thymic implants is limited by human immunodeficiency virus breakthrough during antiretroviral therapy. J Virol. 1999;73:6361–6369. doi: 10.1128/jvi.73.8.6361-6369.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balis F M, Pizzo P A, Eddy J, Wilfert C, McKinney R, Scott G, Murphy R F, Jarosinski P F, Falloon J, Poplack D G. Pharmacokinetics of zidovudine administered intravenously and orally in children with human immunodeficiency virus infection. J Pediatr. 1989;114:880–884. doi: 10.1016/s0022-3476(89)80158-1. [DOI] [PubMed] [Google Scholar]
- 3.Beebe A M, Dua N, Faith T G, Moore P F, Pedersen N C, Dandekar S. Primary stage of feline immunodeficiency virus infection: viral dissemination and cellular targets. J Virol. 1994;68:3080–3091. doi: 10.1128/jvi.68.5.3080-3091.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calabro M L, Zanotto C, Calderazzo F, Crivellaro C, Del Mistro A, DeRossi A, Chieco-Bianchi L. HIV-1 infection of the thymus: evidence for a cytopathic and thymotropic variant in vivo. AIDS Res Hum Retrovir. 1995;11:11–19. doi: 10.1089/aid.1995.11.11. [DOI] [PubMed] [Google Scholar]
- 5.Cunningham-Rundles S, Chen C, Bussel J B, Blankenship C, Veber M B, Sanders-Laufer D, Hinds T, Cervia J S, Edelson P. Human immune development: implications for congenital HIV infection. Ann N Y Acad Sci. 1993;693:20–34. doi: 10.1111/j.1749-6632.1993.tb26254.x. [DOI] [PubMed] [Google Scholar]
- 6.Davis A E., Jr The histopathologic changes in the thymus gland in acquired immune deficiency syndrome. Ann N Y Acad Sci. 1984;437:493–502. doi: 10.1111/j.1749-6632.1984.tb37173.x. [DOI] [PubMed] [Google Scholar]
- 7.Dean G A, Pedersen N C. Cytokine response in multiple lymphoid tissues during the primary phase of feline immunodeficiency virus infection. J Virol. 1998;72:9436–9440. doi: 10.1128/jvi.72.12.9436-9440.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diehl L J, Mathiason-DuBard C K, O'Neill L L, Hoover E A. Longitudinal assessment of feline immunodeficiency virus kinetics in plasma by use of a quantitative-competitive reverse transcriptase PCR. J Virol. 1995;69:2328–2332. doi: 10.1128/jvi.69.4.2328-2332.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.English R V, Nelson P, Johnson C M, Nasisse M, Tompkins W A, Tompkins M B. Development of clinical disease in cats experimentally infected with feline immunodeficiency virus. J Infect Dis. 1994;170:543–552. doi: 10.1093/infdis/170.3.543. [DOI] [PubMed] [Google Scholar]
- 10.Hayes K A, Lafrado L J, Ericson J G, Marr J M, Mathes L E. Prophylactic ZDV therapy prevents early viremia and lymphocyte decline but not primary infection in feline immunodeficiency virus-inoculated cats. J Acquir Immune Defic Syndr. 1993;6:127–134. [PubMed] [Google Scholar]
- 11.Hayes K A, Wilkinson J G, Frick R, Francke S, Mathes L E. Early suppression of viremia by ZDV does not alter the spread of feline immunodeficiency virus infection in cats. J Acquir Immune Defic Syndr. 1995;9:114–122. [PubMed] [Google Scholar]
- 12.Heeney J L. AIDS: a disease of impaired T-cell renewal? Immunol Today. 1995;16:515–520. doi: 10.1016/0167-5699(95)80043-3. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman-Fezer G, Thum J, Ackley C, Herbold M, Mysliwietz J, Thefeld S, Hartmann K, Kraft W. Decline in CD4+ cell numbers in cats with naturally acquired feline immunodeficiency virus infection. J Virol. 1992;66:1484–1488. doi: 10.1128/jvi.66.3.1484-1488.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kourtis A P, Ibegbu C, Nahmias A J, Lee F K, Clark W S, Sawyer M K, Nesheim S. Early progression of disease in HIV-infected infants with thymus dysfunction. N Engl J Med. 1996;335:1431–1436. doi: 10.1056/NEJM199611073351904. [DOI] [PubMed] [Google Scholar]
- 15.Lederman M, Connick E, Landay A, Kuritzkes D R, Spritzler J, St. Clair M, Kotzin B L, Fox L, Chiozzi M H, Leonard J M, Rousseau F, Wade M, Roe J D, Martinez A, Kessler H. Immunologic responses associated with 12 weeks of combination antiretroviral therapy consisting of zidovudine, lamivudine, and ritonavir: results of AIDS Clinical Trials Group Protocol 315. J Infect Dis. 1998;178:70–79. doi: 10.1086/515591. [DOI] [PubMed] [Google Scholar]
- 16.McKinney R E, Jr, Pizzo P A, Scott G B, Parks W P, Maha M A, Lehrman S N, Riggs M, Eddy J, Lane B A, Eppes S C. Safety and tolerance of intermittent intravenous and oral zidovudine therapy in human immunodeficiency virus-infected pediatric patients. Pediatric Zidovudine Phase I Study Group. J Pediatr. 1990;116:640–647. doi: 10.1016/s0022-3476(05)81619-1. [DOI] [PubMed] [Google Scholar]
- 17.McKinney R E, Jr, Maha M A, Connor E M, Feinberg J, Scott G B, Wulfsohn M, McIntosh K, Borkowsky W, Modlin J F, Weintrub P. Multicenter trial of oral zidovudine in children with advanced human immunodeficiency virus disease. The Protocol 043 Study Group. N Engl J Med. 1991;324:1018–1025. doi: 10.1056/NEJM199104113241503. [DOI] [PubMed] [Google Scholar]
- 17a.National Institutes of Health. Guide for the care and use of laboratory animals. Publication no. NIH 74-23. Bethesda, Md: National Institutes of Health, U.S. Department of Health and Human Services; 1985. [Google Scholar]
- 18.Ohkura T, Shin Y S, Wakamiya N, Iwa N, Kurimura T. Detection of proviruses and viral RNA in the early stages of feline immunodeficiency virus infection in cats: a possible model of the early stage of HIV infection. Exp Anim. 1997;46:31–39. doi: 10.1538/expanim.46.31. [DOI] [PubMed] [Google Scholar]
- 19.Orandle M S, Papadi G P, Bubenik L J, Dailey C I, Johnson C M. Selective thymocyte depletion and immunoglobulin coating in the thymus of cats infected with feline immunodeficiency virus. AIDS Res Hum Retrovir. 1997;13:611–620. doi: 10.1089/aid.1997.13.611. [DOI] [PubMed] [Google Scholar]
- 20.Pedersen N C, Ho E W, Brown M L, Yamamoto J K. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science. 1987;235:790–793. doi: 10.1126/science.3643650. [DOI] [PubMed] [Google Scholar]
- 21.Piatak M, Jr, Luk K-C, Williams B, Lifson J D. Quantitative competitive polymerase chain reaction for accurate quantitation of HIV DNA and RNA species. BioTechniques. 1993;14:70–79. [PubMed] [Google Scholar]
- 22.Pizzo P A, Eddy J, Falloon J, Balis F M, Murphy R F, Moss H, Wolters P, Brouwers P, Jarosinski P, Rubin M. Effect of continuous intravenous infusion of zidovudine (AZT) in children with symptomatic HIV infection. N Engl J Med. 1988;319:889–896. doi: 10.1056/NEJM198810063191401. [DOI] [PubMed] [Google Scholar]
- 23.Rosenzweig M, Clark D P, Gaulton G N. Selective thymocyte depletion in neonatal HIV-1 thymic infection—short communication. AIDS. 1993;7:1601–1605. doi: 10.1097/00002030-199312000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Schnittman S M, Denning S M, Greenhouse J J, Justement J S, Baseler M, Kurtzburg J, Haynes B F, Fauci A S. Evidence for susceptibility of intrathymic T-cell precursors and their progeny carrying T-cell antigen receptor phenotypes TCRαβ+ and TCRγδ+ to human immunodeficiency virus infection: a mechanism for CD4+(T4) lymphocyte depletion. Proc Natl Acad Sci USA. 1990;87:7727–7731. doi: 10.1073/pnas.87.19.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seemayer T A, Laroche A C, Rosso R, Lebranche R, Arnoux E, Guerin J-M, Pierre G, Dupuy J-M, Gartner J G, Lapp W S, Spira T J, Elie R. Precocious thymic involution manifest by epithelial injury in the acquired immune deficiency syndrome. Hum Pathol. 1984;15:469–474. doi: 10.1016/s0046-8177(84)80082-9. [DOI] [PubMed] [Google Scholar]
- 26.Shuurn H-J, Krone W A J, Broekhuizen R, van Baarlen J, van Veen P, Goldstein A L, Huber J, Goudsmit J. The thymus in acquired immune deficiency syndrome: comparison with other types of immunodeficiency diseases, and presence of components of human immunodeficiency virus type 1. Am J Pathol. 1989;134:1329–1338. [PMC free article] [PubMed] [Google Scholar]
- 27.Sperling R. Zidovudine. Infect Dis Obstet Gynecol. 1998;6:197–203. doi: 10.1002/(SICI)1098-0997(1998)6:5<197::AID-IDOG2>3.0.CO;2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Torten M, Franchini M, Barlough J E, George J W, Mozes E, Lutz H, Pedersen N C. Progressive immune dysfunction in cats experimentally infected with feline immunodeficiency virus. J Virol. 1991;65:2225–2230. doi: 10.1128/jvi.65.5.2225-2230.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Withers-Ward E S, Amado R G, Koka P S, Jamieson B D, Kaplan A H, Chen I S Y, Zack J A. Transient renewal of thymopoiesis in HIV infected thymic implants following antiviral therapy. Nat Med. 1997;3:1102–1109. doi: 10.1038/nm1097-1102. [DOI] [PubMed] [Google Scholar]
- 30.Woo J C, Dean G A, Pedersen N C, Moore P F. Immunopathologic changes in the thymus during the acute stage of experimentally induced feline immunodeficiency virus infection in juvenile cats. J Virol. 1997;71:8632–8641. doi: 10.1128/jvi.71.11.8632-8641.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woo J C, Dean G A, Lavoy A, Clark R, Moore P F. Investigation of recombinant human insulin-like growth factor type I in thymus regeneration in the acute stage of feline immunodeficiency virus infection. AIDS Res Hum Retrovir. 1999;15:1377–1388. doi: 10.1089/088922299310089. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto J K, Sparger E E, Ho E W, Anderson P R, O'Connor T P, Mandell C, Lowenstine L, Munn R, Pedersen N C. Pathogenesis of experimentally induced feline immunodeficiency virus infection in cats. Am J Vet Res. 1988;49:1246–1262. [PubMed] [Google Scholar]