Abstract
Salbutamol was included in the prohibited list of the World Anti‐Doping Agency (WADA) in 2004. Although systemic intake is banned, inhalation for asthma is permitted but with dosage restrictions. The WADA established a urinary concentration threshold to distinguish accordingly prohibited systemic self‐administration from therapeutic prescription by inhalation. This study aimed at evaluating the ability of the WADA threshold to differentiate salbutamol therapeutic use from violation of antidoping rules. Concentration‐time profile of salbutamol in plasma and its excretion in urine was characterized through a model‐based meta‐analysis of individual and aggregate data collected after administration of a large range of doses following different modes of administration and under a variety of conditions. The developed model adequately fitted salbutamol plasma and urine concentration‐time profiles of the 13 selected studies. Model‐based simulations confirmed that a wide range of salbutamol urine concentrations might be measured after drug intake. Although violation of the WADA Code can be strongly suspected in individuals showing very high salbutamol urine concentrations, uncertainty remains for values close to the WADA threshold as they can be compatible with both permitted therapeutic use and violation. Although not entirely discriminant, the current WADA rule is globally supported by our appraisal. It could be further improved by a slight and reasonable adjustment of inhaled daily dosages allowed for therapeutic use. Our model might help antidoping experts in the evaluation of suspected doping cases through confronting the athlete's urine measurements with their allegations about salbutamol treatment.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Salbutamol is included in the list of prohibited substances and methods of the World Anti‐Doping Agency (WADA) banning systemic intake and permitting inhalation but with dosage restrictions. WADA also established a urinary concentration threshold to distinguish therapeutic from prohibited use.
WHAT QUESTION DID THIS STUDY ADDRESS?
Is the WADA threshold adequate to distinguish therapeutic use from violation of antidoping rules?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
Although violation of the antidoping rules can be strongly suspected in individuals showing very high salbutamol urine concentrations, uncertainty remains for values close to the WADA threshold as they can be compatible with both permitted therapeutic use and violation. These results support the decrease in maximum daily dose per administration established in the WADA 2022 rules. Complete discrimination between allowed and prohibited administration would require reducing further the maximum permitted daily dose.
HOW MIGHT THIS CHANGE DRUG DISCOVERY, DEVELOPMENT, AND/OR THERAPEUTICS?
This model will help antidoping experts in the evaluation of suspected doping cases through confronting the athlete's urine measurements with their allegations about salbutamol treatment.
INTRODUCTION
Salbutamol is a fast‐ and short‐acting β2‐adrenergic receptor agonist prescribed for the treatment of asthma attacks and the prevention of exercise‐induced bronchospasm. It is one of the medications most frequently used by athletes, generally for its bronchodilating effect, but it can also be abused for its ergogenic properties. Some evidence indicates a positive effect of systemic salbutamol on physical performance, whereas no significant effect is demonstrated for salbutamol inhaled at therapeutic doses probably due to the insufficient systemic exposure. 1 , 2 , 3 After oral administration, salbutamol undergoes an important first‐pass metabolism. It is predominantly metabolized into an inactive sulfoconjugated metabolite. 4 After oral intake, roughly one third of the dose is excreted in the urine unchanged (free) and one half as sulfoconjugate. 4 Another small fraction (<3%) is found as a glucuroconjugated metabolite. 5 Conversely, salbutamol is not extensively metabolized in the lungs, 6 and after inhalation, the fraction of the dose actually absorbed in the circulation through this route is mainly eliminated in the urine as the free form. 5 However, an important fraction of the dose administered by inhalation settles along the oral cavity and the throat or is carried back from the tracheobronchial tree by mucociliary clearance (CL), thus resulting into ingestion, gastrointestinal absorption, liver first pass, and transformation into metabolites.
Salbutamol was included in the List of Prohibited Substances and Methods of the World Anti‐Doping Agency (WADA) in 2004 after a complicated history of abuse, prohibition, and therapeutic exemption. 7 Although systemic salbutamol is banned, inhaled salbutamol is permitted but with dosage regimen restrictions. According to the WADA 2017 rules, the dose should not exceed “1600 µg over 24 hours in divided doses not to exceed 800 µg over 12 hours starting from any dose.” 8 This represents twice the maximum daily dose recommended in the summary of product characteristics, 9 which amounts to 200 µg four times a day (q.i.d.). The revised WADA 2022 rules modified the dosing time intervals without affecting the maximum authorized daily dose, allowing a “maximum 1600 µg over 24 hours in divided doses not to exceed 600 µg over 8 hours starting from any dose.” 10 The WADA established a urinary concentration threshold (T) of 1000 ng/ml based on the measurement of free and glucuroconjugated salbutamol to distinguish between prohibited and therapeutic use. The choice of this T was based on routine doping control analytical data, excretion studies, and cases reviewed by the International Olympic Committee. To ensure harmonization across all accredited laboratories, the WADA has defined a decision limit (DL) of 1200 ng/ml, taking into account the measurement uncertainty. 11 Nevertheless, urinary concentrations of some athletes inhaling salbutamol within the dosages allowed by the WADA might theoretically exceed the DL, whereas others taking prohibited systemic salbutamol might excrete salbutamol in urine at concentrations below the DL because of variable absorption and disposition kinetics among individuals. This variability challenges the discriminative power of urinary salbutamol controls. Moreover, some uncertainty remains about the optimality of the cutoff to discriminate prohibited systemic administration from acceptable inhalation therapy.
This study thus aimed at characterizing the concentration‐time typical profile and variability of salbutamol in plasma and its excretion in urine after either oral administration or inhalation under different dosages. We developed a model‐based meta‐analysis (MBMA) of salbutamol plasma and urine disposition relying on data already available. The population pharmacokinetic (PK) model developed by MBMA allowed evaluating the ability of the WADA T and DL to differentiate salbutamol therapeutic use from potential violation of antidoping rules.
METHODS
Data
Studies were selected by the WADA based on both a literature search using PubMed and the projects supported by the WADA itself.
Quantification methods and results reporting varied between studies: although some authors quantified only the free form (unchanged parent compound), others measured the nonsulfated salbutamol content of urine samples (including the salbutamol released from the glucuroconjugated metabolite).
The systematic correction of salbutamol urine concentrations when urine specific gravity (USG) exceeds 1.020 is required by the WADA to determine an adverse analytical finding (AAF). This allows taking into account the dehydration status of individuals at the time of urine sample collection. 11 Either unadjusted or USG‐corrected or both concentrations were reported in the available studies.
We converted PK profiles of aggregate data (i.e., data compiling observations from several individuals into summary statistics) summarized in different formats (i.e., median or mean) into arithmetic means according to classic methods. 12
PK modeling
We conducted a population PK analysis using the nonlinear mixed effect modeling software NONMEM version 7.4.3 assisted by Pirana version 2.9.7 and PsN version 4.9.0. We used the R software version 3.6.1 (run with Rstudio version 1.2.1335) for statistical and graphical analyses.
Initially, we developed the model for plasma concentrations without distinction between individual‐ and study‐level values. We subsequently included urine data. We added statistical considerations for the MBMA once a satisfactory description of all data was achieved. Usual model building, selection, and validation strategies were followed (Supplementary Material S1).
Base structural model
Figure 1 illustrates the structural model retained for the description of salbutamol PK.
FIGURE 1.
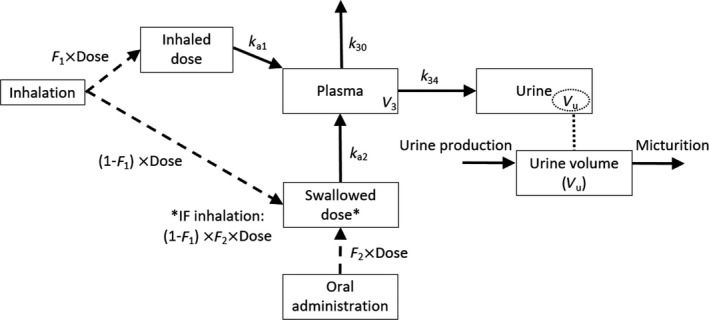
Compartmental model used to describe salbutamol plasma and urine concentration‐time profiles. F 1, fraction of the dose directly transferred into the lungs after inhalation; F 2, bioavailability after oral administration; k a1, absorption rate constant after inhalation; k a2, absorption rate constant after oral administration; k 34, urinary excretion rate constant from plasma to urine; k 30, elimination rate constant for free plasma salbutamol; V 3, salbutamol central volume of distribution; V u, urine volume
After inhalation, the major fraction (80%–90%) of the inhaled dose is deposited in the oral cavity and throat and then swallowed and ingested. 13 This was taken into account by defining two separate compartments for the lungs and the gut after inhalation, that is, the inhaled and the swallowed dose compartments shown in Figure 1, respectively. 14 Because of the paucity of plasma concentrations, the fraction of the dose transferred into the lungs (i.e., F 1) was fixed to 20%, assuming the remaining 80% reaches the circulation through the gastrointestinal tract. This allowed the estimation of the absorption rate constants after inhalation and oral administration (i.e., k a1 and k a2, respectively) and of the bioavailability after oral administration (i.e., F 2; under the assumption of full absorption of the inhaled fraction of the dose F 1). The absorption process of the fraction swallowed after inhalation was characterized by the same oral bioavailability F 2. Because the pulmonary absorption of salbutamol is considered a fast process, 15 a zero‐order absorption process following inhalation was tested. This absorption model was kept after the inclusion of urine data by fixing the estimated parameters (i.e., F 1, F 2, k a1, and k a2) to allow a precise and plausible estimation of all the other parameters. One‐ and two‐compartment disposition models were compared considering CL and the central volume of distribution independent of the route of administration.
First, we modeled nonsulfated urine and free‐salbutamol concentrations in a unique compartment with the same urinary excretion rate constant from plasma to urine (k 34; Figure 1). Second, we tested a compartment per compound with two distinct excretion constant rates. Because urine volumes were not available, we added a separate urine compartment assuming a constant urine production (UR_PROD). This compartment approximates physiological micturition as it describes the filling and voiding of the bladder. We fitted urine concentration data dividing the predicted amount of salbutamol in the urine compartment by the volume of urine produced over the corresponding period. The bladder was assumed to be voided before each salbutamol administration.
We performed multiple tests to evaluate the influence of the correction for USG on urine concentrations (Supplementary material S1).
Statistical model
All PK parameters were assumed to be log‐normally distributed except F 1 and F 2. A logit transformation of F 2 was implemented to constrain individual estimates to range between 0 and 1. Interindividual variability (IIV) was tested sequentially on all parameters. We compared proportional, additive, and combined residual error models.
During the analysis of the combined data set (USG‐corrected data for studies reporting both data and uncorrected concentrations when USG‐corrected data were not available; Supplementary Material S1), we investigated the estimation of two separate IIVs on urine production for uncorrected (UR_PRODuncorrected) and USG‐corrected urine concentrations (UR_PRODcorrected). Indeed, USG correction is expected to decrease the variability in urine production as it depends less on the individual hydration status. We also tested distinct residual errors for uncorrected and USG‐corrected data.
In our data set, correlations exist between observations within a study because individuals originate from a common population. According to MBMA, we first handled these correlations by testing multiple levels of random effects in the model. 16 We investigated separate individual‐ and study‐level IIVs and residual errors. Compared with the approach generally used in traditional meta‐analyses with inverse variance weighting, weighting by sample size is commonly employed in MBMA because it is more often reported in the original studies. 16 , 17 Therefore, for aggregate data, we weighted IIVs and residual effects by the inverse square root of the study sample size according to the following equation to increase confidence in studies conducted in larger populations:
with CL being the individual salbutamol clearance, TVCL the typical CL in the population, ETA1 the IIV on CL, and Nobs the number of individuals who contributed to aggregate plasma or urine PK profiles.
For aggregate plasma and urine PK profiles, we also tested a random effect in the residual error model. 18
Covariate exploration and model
Available covariates were either categorical (i.e., presence or absence of physical exercise during the PK session) or continuous (i.e., age, body weight). We did not evaluate other relevant covariates such as sex or hydration status because all studies either were conducted in males or reported no sex information. Hydration status was most often missing in the studies. We analyzed the correlation between post hoc individual estimates of the PK parameters and individuals’ or studies’ characteristics initially by graphic exploration and eventually by testing their inclusion into the model. We limited the evaluation of the influence of continuous covariates on salbutamol PK to an exploration so as to avoid the risk of ecological bias. 19
Parameter estimation and model selection
The model was implemented as a system of ordinary differential equations (ADVAN 13 subroutine), and salbutamol concentrations were fitted using the first‐order conditional method with interaction.
Model evaluation
We computed secondary PK parameters such as time of maximum concentration (Tmax) and half‐life (t 1/2) to assess the plausibility of estimates by comparison with reference values.
We performed nonparametric bootstrap using 500 replicates of the initial data set. 20 We also conducted a prediction‐corrected visual predictive check (pcVPC) on the final model. 21
Model‐based simulations
We performed simulations to evaluate the ability of the current WADA approach to differentiate permitted salbutamol use from forbidden abuse at either supratherapeutic inhaled doses or administration through the oral route. We computed simulations accounting for both IIV in salbutamol PK and residual error.
Additional information concerning the methods used in this analysis and the simulation scenarios is provided in Supplementary Material S1.
RESULTS
Literature data
Literature research identified 22 candidate studies to inclusion in our MBMA. 5 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 After screening, 13 full‐text studies met the inclusion criteria 5 , 23 , 26 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 39 , 40 as described in the flow diagram in Figure S2. Table S3 describes the characteristics of the studies included in the analysis.
Figure 2 presents salbutamol plasma and urine concentration‐time profiles used for the final model. Overall, the analysis of the combined data set (Supplementary Material S1) included 121 concentrations collected in plasma (43 individual data and 78 mean profile points) and 796 in urine (747 individual data and 49 mean profile points). Median salbutamol doses per administration after inhaled and oral administration were 800 µg (from 200 to 1600 µg) and 8000 µg (from 4000 to 12,000 µg), respectively. Daily doses ranged from 200 to 1600 µg (median 1600 µg) for inhalation and from 4000 to 12000 µg (median 8000 µg) for oral administration.
FIGURE 2.
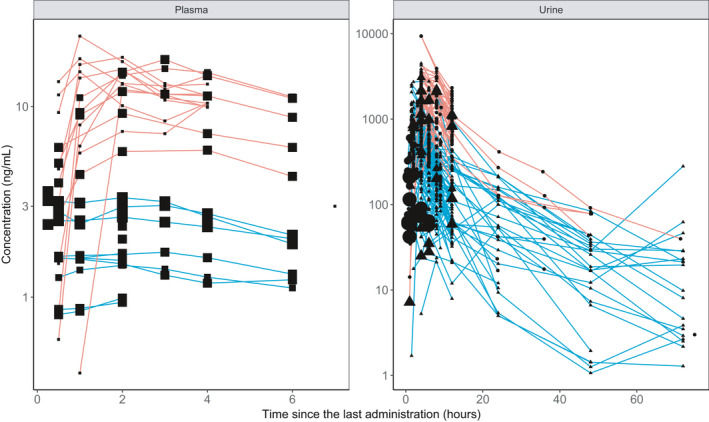
Observed salbutamol concentrations versus time after dose used for the final model. Concentrations of the same individual (individual data) or of individuals participating in the same study (aggregate data) are joined by lines. Red and blue lines represent urine concentrations obtained after oral or inhaled administration, respectively. The size of the points indicates the number of individuals who contributed to pharmacokinetic profiles (smallest symbols, individual data; biggest symbols, aggregate data from 30 individuals). For urine data, circles represent a measure of nonsulfated salbutamol, whereas triangles show free salbutamol measurements
Base structural and statistical models
Plasma concentrations
Salbutamol plasma concentrations were adequately fitted by a one‐compartment model with first‐order absorption following both inhalation and oral administrations. The addition of IIV on any absorption or disposition parameter was not supported by the model because of the paucity of plasma data. Parameter estimates are shown in Table S4.
Urine concentrations
Urine concentrations were first analyzed regardless of the measured salbutamol fraction in a data set combining uncorrected and USG‐corrected concentrations. In addition, IIV common for individual‐ and study‐level data were assigned onto CL and UR_PROD. A proportional residual error model was estimated for plasma data, whereas a combined error model improved urine data (difference in objective function value ∆OFV = −49.1 compared with the proportional error model; p < 0.05).
Graphical explorations of urine data did not indicate different concentrations for non‐sulfated compared to free salbutamol (Figure 2). The differentiation of the two entities in the model did not improve the fit (difference in Akaikeʼs criteria ∆AIC = +15). The proportionality factor between the excretion rates k 35 and k 34 when differentiating free and nonsulfated urinary salbutamol (Supplementary Material S1) was estimated at 0.001 with bad precision (relative standard error = 34495%), emphasizing the inability of the model to discriminate between both fractions. This result confirms the findings of previous studies about negligible urinary concentrations of salbutamol glucuronide both after inhalation and oral administrations. 5 Model development was thus pursued without distinguishing urinary fractions.
Visual inspection of the data did not indicate any systematic difference between uncorrected and USG‐corrected salbutamol concentrations (Supplementary Material S5). Parameter estimates were similar, although the variability on UR_PRODcorrected was lower compared with that on UR_PRODuncorrected (Supplementary Material S5). Model development was pursued with the combined data set. Parameter estimates of the final base model are described in Supplementary Material S5.
Statistical model
The final base model was further refined by considering the correlation within studies. All of the random effects were weighted by . Residual errors were estimated separately for individual and aggregate data. The estimation of IIV on the residual error for plasma and urine aggregate data did not improve the fit (∆OFV = +4.2 and +13.0 for plasma and urine, respectively; p > 0.05).
Unique IIVs were estimated for CL, UR_PRODuncorrected, and UR_PRODcorrected because the estimation of separate individual‐ and study‐level IIVs did not improve the fit (∆OFV > −2.4; p > 0.05; Table S6).
Covariate analysis
Physical exercise showed a significant impact on salbutamol PK profile, with urine production decreasing by 15% compared with individuals at rest or with missing data (∆OFV = −8.8; p < 0.05). The inclusion of this covariate explained 17% of the IIV on UR_PRODuncorrected and 7% of the IIV on UR_PRODcorrected.
The graphical exploration of the influence of body weight on CL revealed a positive trend; however, this is not included in the model for the reasons mentioned in the Covariate Exploration and Model section.
Model evaluation
Table 1 shows the final PK parameter estimates with bootstrap results. Residual errors for aggregate plasma concentrations ranged between 46% and 40% when Nobs = 8 or 13, respectively. For aggregate urine data, residual error varied between 78% and 56% when Nobs = 8 or n = 30, respectively. Figure S7 presents goodness‐of‐fit plots.
TABLE 1.
Population parameter estimates of salbutamol plasma and urine concentrations obtained with the MBMA including covariates
| Final MBMA | Bootstrap | |||
|---|---|---|---|---|
| Estimate | RSE a (%) | Median | 95% CI | |
| F 1 | 0.2 FIX | |||
| logitF 2 | −0.328 FIX | |||
| k a1 (h−1) | 31.6 FIX | |||
| k a2 (h−1) | 1.47 FIX | |||
| V 3 (L) | 205 | 2 | 203 | 188–230 |
| k 34 (h−1) | 0.0468 | 14 | 0.0432 | 0.0295–0.0601 |
| CL (L h−1) | 28 | 8 | 28 | 25–32 |
| ω CL (CV%) b | 39 | 12 | 37 | 27–47 |
| UR_PROD (L h−1) | 0.0467 | 4.8 × 10−6 | 0.0426 | 0.0290–0.0562 |
| θ physical,UR_PROD | −0.153 | 43 | −0.153 | −0.254 to −0.020 |
| ω UR_PRODuncorrected (CV%) b | 74 | 49 | 89 | 47–141 |
| ω UR_PRODcorrected (CV%) b | 38 | 16 | 37 | 27–49 |
| Proportional error plasma, individual (CV%) | 40 | 7 | 40 | 34–47 |
| Proportional error plasma, aggregate (CV%) | 77 | 12 | 76 | 53–101 |
| Proportional error urine, individual (CV%) | 46 | 2 | 46 | 41–51 |
| Additive error urine, individual (ng/ml) | 18 | 29 | 18 | 11–24 |
| Proportional error urine, aggregate (CV%) | 131 | 12 | 128 | 57–154 |
CL, salbutamol clearance; CV%, coefficient of variation expressed as percentage; F 1, fraction of the dose directly transferred into the lungs after inhalation; F 2, bioavailability after oral administration computed as ; k a1, absorption rate constant after inhalation; k 34, urinary excretion rate constant; k a2, absorption rate constant after oral administration; MBMA, model‐based meta‐analysis; RSE, relative standard error; UR_PROD, urine production per hour; θ physical,UR_PROD, influence of physical exercise on UR_PROD expressed as ; V 3, salbutamol central volume of distribution, ω inter‐individual variability expressed as CV%.
All random effects for aggregate data are weighted by : and where is the unit‐level random variance for each parameter, the residual error (CV%), is the unweighted unit‐level variance for each parameter, the unweighted residual error, and Nobs is the number of individuals who contributed to aggregate plasma or urine pharmacokinetic profiles.
RSE of the estimate defined as SEestimate/estimate, expressed as percentage, with SEestimate the standard error directly retrieved from the NONMEM output file.
Interindividual variability, expressed as CV%.
Parameter estimates obtained with the final MBMA translated into Tmax values of 0.17 h following inhalation (without considering the ingested fraction) and 1.8 h following oral administration (Supplementary Material S1). These results are in fair accordance with reported values of about 0.20 and 1.8 h after inhalation with mouth rinsing and oral administration, respectively. 43 , 44 An intermediate Tmax of 1.45 h was calculated when taking into account both inhaled and ingested fractions after inhalation (Supplementary Material S1). In addition, a t 1/2 of 5 h was calculated, comparable with values reported by the manufacturer. Average hourly urine production was estimated at 0.0467 L h−1 and 0.0396 L h−1 in individuals at rest and during physical exercise, respectively, in fair accordance with the typical daily urine volume of 1–2 L produced at rest and with the decrease in urine output during exercise.
The pcVPC revealed an adequate agreement between observed and simulated plasma and urine concentrations (Figure 3). Differences between bootstrap median values and population estimates did not exceed 11% for all the parameters but UR_PRODuncorrected for which it was 25%.
FIGURE 3.
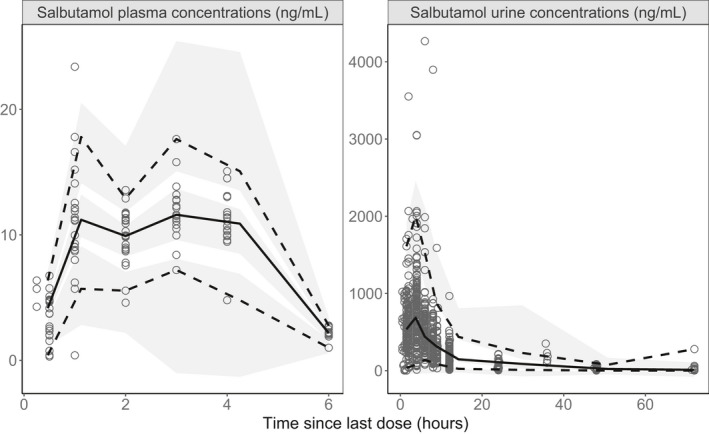
Prediction‐corrected visual predictive check of the final model‐based meta‐analysis with covariates. Open circles represent salbutamol plasma concentrations (left) and urine concentrations (right). The continuous line represents the median observed concentration, and the dashed lines represent the observed 2.5% and 97.5% percentiles. Shaded areas represent the model‐based 95% confidence interval for the median and 2.5% and 97.5% percentiles
Model‐based simulations
An example of a simulated salbutamol plasma PK profile is presented in Figure S8 part 1, with the associated urine concentrations. The results outlined next focus on urine concentrations.
Figure 4 compares simulated steady‐state urinary PK profiles as a function of the tested dosage regimens. None of the concentrations measured under the maximum recommended dosage for therapeutic use of 200 µg q.i.d. would exceed the T and the DL. Conversely, 2.4% (T) and 1.2% (DL) of the concentrations measured under a dosage of 800 µg b.i.d. (twice daily; WADA 2017 rules) would be above these limits during the entire dosing interval. After the inhalation of a dose of 400 µg at time 0, followed by 600 µg at times 8 and 16 h (WADA rules 2022), 1.9% of the overall concentrations would exceed the T and 0.9% the DL. Finally, when salbutamol is administered orally at the dose of 8 mg once daily (qd), 66% and 58% of measured concentrations would overcome the T and the DL, respectively. In the worst‐case scenario (i.e., detection of urine concentrations at Tmax), the average simulated maximal concentration (Cmax) obtained under a 200 µg q.i.d. regimen by inhalation would be 174 ng/ml, still well below the WADA limits. Following inhalation of 800 µg b.i.d., 4.9% (T) and 2.4% (DL) of Cmax values would be above the WADA limits. These proportions increase up to 83% and 76% after oral administration of 8 mg q.d. (Figure S8, part 2).
FIGURE 4.
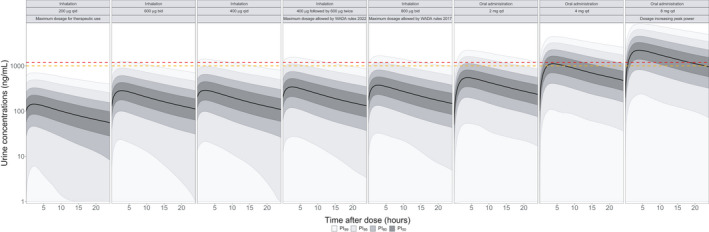
Comparison of simulated steady‐state salbutamol urine concentrations under several dosage regimens. Bladder is voided before last administration. Urine concentrations represent urine specific gravity–corrected values. Concentrations are simulated in individuals at rest. Continuous black lines represent the smoothed population median prediction based on 10,000 simulated individuals. Shaded areas represent the smoothed prediction intervals. The dashed red and orange lines represent the WADA salbutamol threshold (1000 ng/ml) and decision limit (1200 ng/ml), respectively. b.i.d., twice daily; PI, prediction interval; q.d., once daily; q.i.d., 4 times a day; WADA, World Anti‐Doping Agency
Figure 4 shows that essentially none of the concentrations measured after inhalation of 400 µg b.i.d. of salbutamol would be above 1000 or 1200 ng/ml. The number of measured concentrations above the DL reaches 0.2% and 0.6% after three (i.e., 400 µg t.i.d.) or four (i.e., 400 µg q.i.d.) administrations per day, respectively. When inhaling 600 µg b.i.d., 0.7% and 0.3% of the measured concentrations exceed the WADA T and DL, respectively. These results suggest that reducing the permitted dose to medically more appropriate dosages would allow better differentiating therapeutic use from violation based on the current WADA cutoffs.
The large overlap between urine PK profiles of 400 µg q.i.d., 800 µg b.i.d., or 2 mg q.d. (Figure 4) demonstrates the difficulty to differentiate between urine concentrations resulting from therapeutic use or violation of the antidoping rules. Salbutamol urine concentrations above 2000 ng/ml are mostly reached with oral doses of at least 4 mg q.d.
The discrimination between therapeutic use and violation is further complicated by the variations of urine concentrations between different conditions, as exemplified in Figure 5. Unique administration of the same dose, micturition, and doubled urine production decrease salbutamol urine concentrations, whereas physical exercise has the opposite effect. As expected, the absence of correction by USG markedly increases predicted IIV.
FIGURE 5.
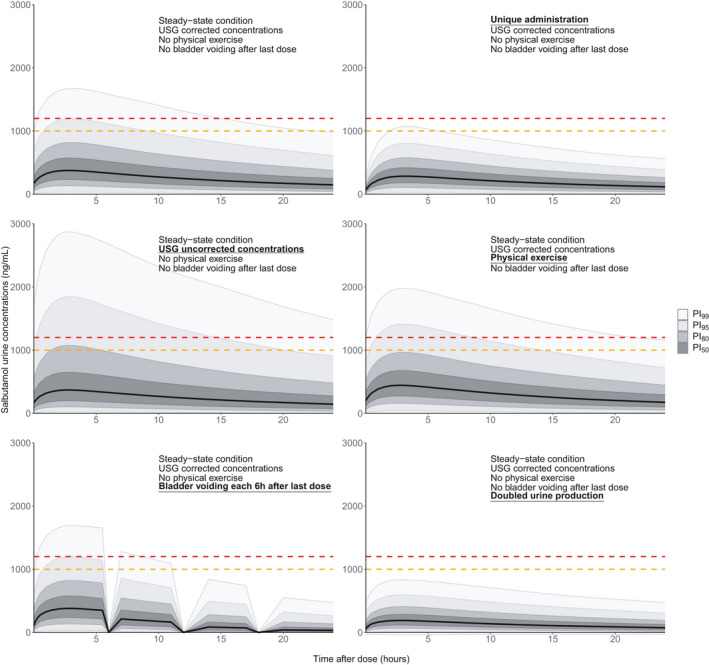
Simulated salbutamol urine concentrations after inhalation of a 800 µg b.i.d. regimen, under different conditions. Continuous black lines represent the smoothed population median predictions based on 10,000 simulated individuals. Shaded areas represent the smoothed prediction intervals. The dashed red and orange lines represent the World Anti‐Doping Agency salbutamol threshold (1000 ng/ml) and decision limit (1200 ng/ml), respectively. PI, prediction interval; USG, urine specific gravity
Finally, Figure 6 shows that following an arbitrary dosage regimen respecting the WADA 2017 recommendations (Supplementary Material S1), 5.2% and 2.5% of the measured urine concentrations would be above 1000 and 1200 ng/ml 2 h after drug administration, respectively. These results support the decrease in maximum daily dose per administration established in the WADA 2022 rules.
FIGURE 6.
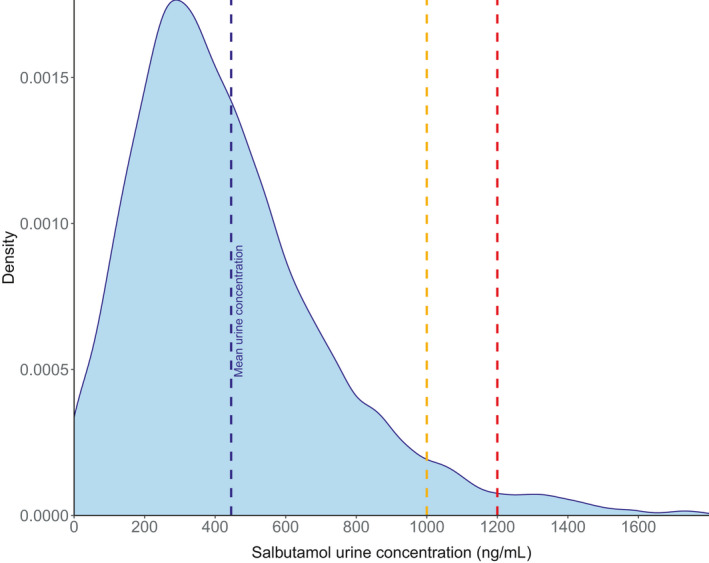
Density plots predicted 2 h after the last dose of an arbitrary dosage regimen (400 µg 4 times a day from time 0–36 h, then 800 µg twice daily from time 48–96 h, and bladder voided each 6 h, as described in Supplementary Material S1). Urine concentrations represent urine specific gravity–corrected concentrations. Concentrations are simulated in individuals at rest. The dashed blue line represents the mean simulated urine concentration. The dashed red and orange lines represent the World Anti‐Doping Agency salbutamol threshold (1000 ng/ml) and decision limit (1200 ng/ml), respectively
DISCUSSION
A one‐compartment model adequately fitted the salbutamol plasma concentration–time profile. The fact that all plasma samples were drawn <6 h after the last administration prevented the characterization of a peripheral compartment. 14 , 45 This is probably of limited relevance considering that athletes take salbutamol shortly before a physical effort, which usually lasts less than 6 h, prior to the doping control collection. The parameters estimated in this study were close to overall reported values. 4 , 14 The estimation of separate IIV for USG‐corrected and uncorrected concentrations definitely improved the fit, indicating that USG correction increases the informativeness of urinary concentrations. Part of the IIV was explained by integrating physical exercise as a binary covariate on the rate of urine production. However, the intensity of physical exercise, which might better correlate with urine production, was not specified in the model. Other factors such as individual demographic characteristics, genetic polymorphism, 46 drug interactions, 47 or inhalation technique may also contribute to the high variability of salbutamol disposition.
Considering the high IIVs and residual errors, an isolated determination of salbutamol concentration in urine sampled at an unknown time after dose intake is of limited relevance for determining an AAF. However, even in the worst‐case scenario of individuals taking 400 µg followed by 600 µg twice (maximum dosage allowed by the WADA 2022 rules), the false positive detection rate does not exceed 0.9%, which is more than acceptable. The highest urine concentrations are achieved rather specifically with the oral administration of high doses of salbutamol. Thus, violation of the WADA Code can be strongly suspected in individuals showing very high salbutamol urine concentrations (>2000 ng/ml). However, uncertainty remains for urine concentrations close to 1000 or 1200 ng/ml, which can be compatible with both permitted administration for therapeutic use and violation. Improving the discrimination between allowed and prohibited administrations would require reducing further the maximum daily dose of 1600 µg. This would limit the permitted dosage regimen to 600 µg b.i.d. or 400 µg b.i.d. or t.i.d., for instance. Following inhalation of such regimens, salbutamol urine concentrations are less likely to exceed the WADA T or DL. In addition, such doses are closer to doses recommended by the latest medical practice 9 compared with the current maximum permitted dosage regimen. It has been reported that maximum bronchodilatation is obtained at a cumulative dose of 110 µg in healthy individuals. 48 Although higher doses can be required in individuals who are asthmatic, largely supratherapeutic exposure has been shown possibly deleterious and should not be recommended even in athletes.
Assuming that sufficient information about salbutamol administration (dosage and time schedule) and frequency of bladder voiding is available, our model can be exploited to predict the probability of reaching a urine concentration above the WADA T and DL. Such an approach based on density probability could help WADA experts in identifying suspicions of violation or in exonerating legitimate salbutamol exposures through the confrontation of an athlete's allegation about drug intake and his/her urinary findings. However, in the setting of doping control, the frequent absence of such essential information largely contributes to the difficulty of interpreting the result of a doping analysis. Since 2009, athletes with a salbutamol urine concentration exceeding 1000 ng/ml can prove that this abnormal value results from a dosage regimen within the WADA limits via a controlled excretion study. The results of such a PK study could be confronted to a priori predictions derived from our model to calculate maximum likelihood values of the individual's PK parameters through a Bayesian approach. Moreover, the repetition of measurements in an athlete under controlled conditions could reduce the prediction uncertainty around his individual curve, thus contributing to better define the likelihood of therapeutic use versus risk of abuse of salbutamol.
Some limitations of our study should be acknowledged. First, the relative paucity of plasma concentrations prevented us to estimate IIV on the absorption parameters, while several factors such as inhalation technique or medical conditions are known to influence salbutamol absorption. 49 Second, the variability on urinary production rate is probably underestimated by the model. A constant urine production is assumed, whereas a variable production rate would certainly reflect more accurately the physiological micturition. This could be even more variable with dehydration and subsequent rehydration during prolonged exposure to hard physical exercise in professional competition. This limitation could be overcome with the exact measure of the urine volume for each urine concentration measurement during the study.
Several strengths of this study should be emphasized nevertheless. The reliance on modern MBMA approaches enables the largest possible base of available evidence to be taken into account to support our answers to the study questions. The modeling of aggregate data supplemented with individual data allowed a remarkably precise estimation of PK parameters despite the relative data paucity. The model was developed using data obtained following administration of a wide range of doses, with different modes of administration, and under several conditions, providing confidence in the estimation of PK parameters. Handling such a diversity of data types requires a thorough comprehension and an extensive visual exploration of the data to find the best manner to combine them in a unique MBMA. The effect of each data characteristic must be carefully evaluated on both fixed and random effects to select the most appropriate strategy reflecting the data heterogeneity in a suitable and relevant way.
In conclusion, although not entirely satisfactory, the current WADA rules regarding the definition of AAFs related to salbutamol appear globally supported by our model. Their application could possibly be improved by a slight and reasonable modification of inhalation dosages allowed in therapeutic exemption.
CONFLICT OF INTEREST
The authors declared no competing interests for this work.
AUTHOR CONTRIBUTIONS
P.C. wrote the manuscript. I.M., O.R., T.B., and J.B. designed the research. P.C., M.G., and T.B. performed the research. P.C. analyzed the data.
Supporting information
Supplementary Material S1
Supplementary Material S2
Supplementary Material S3
Supplementary Material S4
Supplementary Material S5
Supplementary Material S6
Supplementary Material S7
Supplementary Material S8
Courlet P, Buclin T, Biollaz J, Mazzoni I, Rabin O, Guidi M. Model‐based meta‐analysis of salbutamol pharmacokinetics and practical implications for doping control. CPT Pharmacometrics Syst Pharmacol. 2022;11:469‐481. doi: 10.1002/psp4.12773
Funding information
This study was supported by funds from the World Anti‐Doping Agency.
REFERENCES
- 1. Pluim BM, de Hon O, Staal JB, et al. beta(2)‐Agonists and physical performance: a systematic review and meta‐analysis of randomized controlled trials. Sports Med. 2011;41:39‐57. [DOI] [PubMed] [Google Scholar]
- 2. Dickinson J, Molphy J, Chester N, Loosemore M, Whyte G. The ergogenic effect of long‐term use of high dose salbutamol. Clin J Sport Med. 2014;24:474‐481. [DOI] [PubMed] [Google Scholar]
- 3. Goubault C, Perault M‐C, Leleu E, et al. Effects of inhaled salbutamol in exercising non‐asthmatic athletes. Thorax. 2001;56:675‐679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Morgan DJ, Paull JD, Richmond BH, Wilson‐Evered E, Ziccone SP. Pharmacokinetics of intravenous and oral salbutamol and its sulphate conjugate. Br J Clin Pharmacol. 1986;22:587‐593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mareck U, Guddat S, Schwenke A, et al. Determination of salbutamol and salbutamol glucuronide in human urine by means of liquid chromatography‐tandem mass spectrometry. Drug Test Anal. 2011;3:820‐827. [DOI] [PubMed] [Google Scholar]
- 6. Hindle M, Chrystyn H. Determination of the relative bioavailability of salbutamol to the lung following inhalation. Br J Clin Pharmacol. 1992;34:311‐315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Allen H, Backhouse SH, Hull JH, Price OJ. Anti‐doping policy, therapeutic use exemption and medication use in athletes with asthma: a narrative review and critical appraisal of current regulations. Sports Med. 2019;49:659‐668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. The World Anti‐Doping Code The World Anti‐Doping Agency. International standard. Prohibited list. Accessed September 27, 2021. https://www.wada‐ama.org/sites/default/files/resources/files/2016‐09‐29_‐_wada_prohibited_list_2017_eng_final.pdf. Published 2017.
- 9.GlaxoSmithKline Inc. Ventolin HFA, product monograph. Accessed September 1, 2021. https://ca.gsk.com/media/592944/ventolin‐hfa.pdf. Published 2021.
- 10. The World Anti‐Doping Code . The World Anti‐Doping Agency. International standard. Prohibited list. Accessed October 1, 2021. https://www.wada‐ama.org/sites/default/files/resources/files/2022list_final_en.pdf. Published 2022.
- 11.The World Anti‐Doping Agency. WADA statement on the salbutamol threshold/decision limit. Accessed May 4, 2020. https://www.wada‐ama.org/sites/default/files/note_15may.pdf. Published 2018.
- 12. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chrystyn H. Methods to identify drug deposition in the lungs following inhalation. Br J Clin Pharmacol. 2001;51:289‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Heuberger J, van Dijkman SC, Cohen AF. Futility of current urine salbutamol doping control. Br J Clin Pharmacol. 2018;84:1830‐1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Borghardt JM, Weber B, Staab A, et al. Investigating pulmonary and systemic pharmacokinetics of inhaled olodaterol in healthy volunteers using a population pharmacokinetic approach. Br J Clin Pharmacol. 2016;81:538‐552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boucher M, Bennetts M. Many flavors of model‐based meta‐analysis: part II – modeling summary level longitudinal responses. CPT: Pharm Syst Pharmacol. 2018;7:288‐297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ahn JE, French JL. Longitudinal aggregate data model‐based meta‐analysis with NONMEM: approaches to handling within treatment arm correlation. J Pharmacokinet Pharmacodyn. 2010;37:179‐201. [DOI] [PubMed] [Google Scholar]
- 18. Van Wart SA, Shoaf SE, Mallikaarjun S, Mager DE. Population‐based meta‐analysis of hydrochlorothiazide pharmacokinetics. Biopharm Drug Dispos. 2013;34:527‐539. [DOI] [PubMed] [Google Scholar]
- 19. Blakely TA, Woodward AJ. Ecological effects in multi‐level studies. J Epidemiol Community Health. 2000;54:367‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lindbom L, Pihlgren P, Jonsson EN. PsN‐Toolkit–a collection of computer intensive statistical methods for non‐linear mixed effect modeling using NONMEM. Comput Methods Programs Biomed. 2005;79:241‐257. [DOI] [PubMed] [Google Scholar]
- 21. Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction‐corrected visual predictive checks for diagnosing nonlinear mixed‐effects models. AAPS J. 2011;13:143‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Backer V, Hemmersbach P, Schanzer W. Beta2 adrenoreceptor agonist and elite athletes: pharmacokinetics, physiological and pharmacogenetic studies. Accessed May 4, 2020. https://www.wada‐ama.org/sites/default/files/resources/files/08e02vb_backer_1.pdf. Published 2018.
- 23. Backer V, Hostrup M. Pharmacological properties of inhaled beta2‐agonists in athletes with special emphasis on salmeterol and terbutaline (13D12VB). Accessed May 4, 2020. https://www.wada‐ama.org/sites/default/files/resources/files/13d12vb_dr._backer_summary.pdf. Published 2015.
- 24. Backer V, Pedersen LE, Elers J, Dalhof K, Hemmersbach P. The pharmacokinetic of repetitive doses of inhaled salbutamol, terbutaline and formoterol and cardio‐respiratory side effects of high doses beta‐agonists. Accessed May 4, 2020. https://www.wada‐ama.org/sites/default/files/resources/files/backer_0.pdf. Published 2015.
- 25. Bergés R, Segura J, Ventura R, et al. Discrimination of prohibited oral use of salbutamol from authorized inhaled asthma treatment. Clin Chem. 2000;46:1365‐1375. [PubMed] [Google Scholar]
- 26. Delbeke F, Van Thuyne W. Development of analtycal methods for the quantitative determination of beta‐agonists and determination of detecting times after therapeutic use. Accessed May 4, 2020. https://www.wada‐ama.org/en/resources/science‐medicine/development‐of‐analytical‐methods‐for‐the‐quantitative‐determination‐of. Published 2009.
- 27. Dickinson J, Hu J, Chester N, Loosemore M, Whyte G. Acute impact of inhaled short acting b2‐agonists on 5 km running performance. J Sports Sci Med. 2014;13:271‐279. [PMC free article] [PubMed] [Google Scholar]
- 28. Dickinson J, Hu J, Chester N, Loosemore M, Whyte G. Impact of ethnicity, gender, and dehydration on the urinary excretion of inhaled salbutamol with respect to doping control. Clin J Sport Med. 2014;24:482‐489. [DOI] [PubMed] [Google Scholar]
- 29. Elers J, Morkeberg J, Jansen T, Belhage B, Backer V. High‐dose inhaled salbutamol has no acute effects on aerobic capacity or oxygen uptake kinetics in healthy trained men. Scand J Med Sci Sports. 2012;22:232‐239. [DOI] [PubMed] [Google Scholar]
- 30. Elers J, Pedersen L, Henninge J, et al. Urine concentrations of repetitive doses of inhaled salbutamol. Int J Sports Med. 2011;32:574‐579. [DOI] [PubMed] [Google Scholar]
- 31. Elers J, Pedersen L, Henninge J, et al. The pharmacokinetic profile of inhaled and oral salbutamol in elite athletes with asthma and nonasthmatic subjects. Clin J Sport Med. 2012;22:140‐145. [DOI] [PubMed] [Google Scholar]
- 32. Elers J, Pedersen L, Henninge J, et al. Blood and urinary concentrations of salbutamol in asthmatic subjects. Med Sci Sports Exerc. 2010;42:244‐249. [DOI] [PubMed] [Google Scholar]
- 33. Haase CB, Backer V, Kalsen A, et al. The influence of exercise and dehydration on the urine concentrations of salbutamol after inhaled administration of 1600 microg salbutamol as a single dose in relation to doping analysis. Drug Test Anal. 2016;8:613‐620. [DOI] [PubMed] [Google Scholar]
- 34. Hostrup M, Kalsen A, Auchenberg M, et al. Urine concentrations of oral salbutamol in samples collected after intense exercise in endurance athletes. Drug Test Anal. 2014;6:528‐532. [DOI] [PubMed] [Google Scholar]
- 35. Jacobson GA, Walters EH. Enantioselective pharmacokinetics of salbutamol and application to doping control. Accessed May 4, 2020. https://www.wada‐ama.org/sites/default/files/resources/files/research_13d24gj.pdf. Published 2019.
- 36. Pichon A, Venisse N, Krupka E, Perault‐Pochat MC, Denjean A. Urinary and blood concentrations of beta2‐agonists in trained subjects: comparison between routes of use. Int J Sports Med. 2006;27:187‐192. [DOI] [PubMed] [Google Scholar]
- 37. Pillard F, Lavit M, Cances VL, et al. Medical and pharmacological approach to adjust the salbutamol anti‐doping policy in athletes. Respir Res. 2015;16:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schweizer C, Saugy M, Kamber M. Doping test reveals high concentrations of salbutamol in a Swiss track and field athlete. Clin J Sport Med. 2004;14:312‐315. [DOI] [PubMed] [Google Scholar]
- 39. Sporer BC, Sheel AW, McKenzie DC. Dose response of inhaled salbutamol on exercise performance and urine concentrations. Med Sci Sports Exerc. 2008;40:149‐157. [DOI] [PubMed] [Google Scholar]
- 40. Sporer BC, Sheel AW, Taunton J, Rupert JL, McKenzie DC. Inhaled salbutamol and doping control: effects of dose on urine concentrations. Clin J Sport Med. 2008;18:282‐285. [DOI] [PubMed] [Google Scholar]
- 41. Ventura R, Segura J, Bergés R, et al. Distinction of inhaled and oral salbutamol by urine analysis using conventional screening procedures for doping control. Ther Drug Monit. 2000;22:277‐282. [DOI] [PubMed] [Google Scholar]
- 42. Whyte G, Dickinson J, Hu J, et al. The Ergogenic and Pharmacokinetic Impact of Short Acting β2‐Agonists on Association Football Performance in Male and Females . 2009.
- 43. Clark DJ, Gordon‐Smith J, McPhate G, Clark G, Lipworth BJ. Lung bioavailability of generic and innovator salbutamol metered dose inhalers. Thorax. 1996;51:325‐326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Du XL, Zhu Z, Fu Q, Li DK, Xu WB. Pharmacokinetics and relative bioavailability of salbutamol metered‐dose inhaler in healthy volunteers. Acta Pharmacol Sin. 2002;23:663‐666. [PubMed] [Google Scholar]
- 45. Vet NJ, de Winter BCM, Koninckx M, et al. Population pharmacokinetics of intravenous salbutamol in children with refractory status asthmaticus. Clin Pharmacokinet. 2020;59:257‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Boulton DW, Fawcett JP. The pharmacokinetics of levosalbutamol: what are the clinical implications? Clin Pharmacokinet. 2001;40:23‐40. [DOI] [PubMed] [Google Scholar]
- 47. Bian HS, Ngo SYY, Tan W, et al. Induction of human sulfotransferase 1A3 (SULT1A3) by glucocorticoids. Life Sci. 2007;81:1659‐1667. [DOI] [PubMed] [Google Scholar]
- 48. Barnes PJ, Pride NB. Dose‐response curves to inhaled beta‐adrenoceptor agonists in normal and asthmatic subjects. Br J Clin Pharmacol. 1983;15:677‐682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Melchor R, Biddiscombe MF, Mak VH, Short MD, Spiro SG. Lung deposition patterns of directly labelled salbutamol in normal subjects and in patients with reversible airflow obstruction. Thorax. 1993;48:506‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Miller RC, Brindle E, Holman DJ, et al. Comparison of specific gravity and creatinine for normalizing urinary reproductive hormone concentrations. Clin Chem. 2004;50:924‐932. [DOI] [PubMed] [Google Scholar]
- 51. WADA Laboratory Expert Group . WADA technical document–TD2019DL v. 2.0. Accessed July 17, 2020. https://www.wada.ama.org/sites/default/files/resources/files/td2019dl_v2_finalb.pdf. Published 2019.
- 52. Rohatagi A. WebPlotDigitizer. Accessed January 6, 2021. https://automeris.io/WebPlotDigitizer. Published 2020.
- 53. Mould DR, Upton RN. Basic concepts in population modeling, simulation, and model‐based drug development‐part 2: introduction to pharmacokinetic modeling methods. CPT: Pharmacometrics Syst Pharmacol. 2013;2:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hostrup M, Kalsen A, Auchenberg M, Bangsbo J, Backer V. Effects of acute and 2‐week administration of oral salbutamol on exercise performance and muscle strength in athletes. Scand J Med Sci Sports. 2016;26:8‐16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material S1
Supplementary Material S2
Supplementary Material S3
Supplementary Material S4
Supplementary Material S5
Supplementary Material S6
Supplementary Material S7
Supplementary Material S8


