Abstract
Sodium stibogluconate (Sbb), a leishmanicidal drug, was studied for its in vivo effect on the formation of reactive oxygen species (ROS), assessed by chemiluminescence (CL) in the whole blood of mice infected with Leishmania infantum. Stimulation of ROS formation induced ex vivo by zymosan particles or the protein kinase C activator phorbol myristate acetate (PMA) was reduced by approximately 25% (P < 0.05) after infection of mice. Treatment of infected mice with Sbb (50 to 400 mg/kg of body weight) enhanced the blood CL induced by zymosan and PMA (47 to 96%, P < 0.01). The drug potentiation effect also occurred in uninfected mice. In vitro treatment of normal human blood with Sbb (1, 10, or 100 μg/ml) for 1 h primed the CL response to PMA (29 to 54%). The priming effect of Sbb was also observed on the production of superoxide by isolated polymorphonuclear leukocytes stimulated either by PMA and zymosan or by the chemoattractants N-formyl-Met-Leu-Phe and platelet-activating factor. These data provide the first evidence of priming of the phagocyte respiratory burst by Sbb. This novel property of Sbb may contribute to the drug's leishmanicidal effect.
Stimulation of phagocytes (polymorphonuclear cells, monocytes, and macrophages) by particle agents or soluble mediators such as chemoattractants triggers the generation of large amounts of superoxide anion (O2·) and related reactive oxygen species (ROS), such as hydrogen peroxide (H2O2) and hydroxyl radicals (OH·) and singlet oxygen (1O2). This generation, termed respiratory burst, plays a major role in the destruction of invading pathogens by phagocytes (4). In certain circumstances, the phagocyte respiratory burst is enhanced after cell treatment with low concentrations of certain agonists or drugs. This phenomenon, termed “priming” (14), may have beneficial or detrimental biological effects. Leishmania is a parasite of macrophages that causes a broad range of diseases, including cutaneous, mucocutaneous, and acute visceral leishmaniasis (27). It has been proposed that activated macrophages kill intracellular parasites by generating ROS and nitric oxide (20, 22, 27, 30). Visceral leishmaniasis is associated with depression of immune defenses, and Leishmania spp. appear to be resistant to oxygen-dependent toxicity (8, 17). al-Mofleh et al. have shown that the phagocyte respiratory burst was down-regulated in animals receiving antigens of Leishmania major (2). These antigens were also found to inhibit the respiratory burst of isolated polymorphonuclear leukocytes (PMN) in vitro (3). The importance of PMN in the early control of parasite load and spreading of parasitism in cutaneous leishmaniasis was recently reported (21).
Pentavalent antimonials, available as sodium stibogluconate (Pentostam) (Sbb) or meglumine antimoniate (Glucantime), are the first-choice therapy for visceral leishmaniases of immunocompetent patients but appear to be inefficacious in immunocompromised patients. Moreover, some authors have suggested that nonspecific cell defense was required for a completely successful cure (17). These drugs were shown to interact with Leishmania and inhibit its viability in vitro (6). However, the effects of these drugs on host cell reactivity and their mode of action are not fully understood. To gain insight into the mechanism of Sbb's leishmanicidal effect, we studied the in vivo drug effect on the generation of ROS, as measured by luminol-dependent chemiluminescence (CL) assay in the whole blood of Leishmania infantum-infected and uninfected mice. We further analyzed the effects of Sbb in vitro on the CL response of whole blood of healthy volunteers and on the production of superoxide anion by purified PMN stimulated by various agents.
MATERIALS AND METHODS
Reagents.
Dextran T500 was from Pharmacia (Uppsala, Sweden), and other reagents were from the Sigma Chemical Co. (St. Louis, Mo.). Stock solutions of N-formyl-Met-Leu-Phe (fMLP), platelet-activating factor (PAF), and phorbol myristate acetate (PMA) were prepared in dimethyl sulfoxide. Zymosan particles were opsonized by incubating 10 mg of zymosan in 1 ml of human serum for 30 min at 37°C. The zymosan suspension was washed twice in phosphate-buffered saline and used at a final concentration of 0.5 mg/ml. A stock solution of Sbb (Wellcome, London, United Kingdom) was used at a concentration of 100 mg/ml in phosphate-buffered saline.
Animals and parasites.
A murine model of visceral leishmaniasis was used as previously described by Paul et al. (23). Thirty BALB/c mice were infected intravenously on day 0 with 107 stationary-phase promastigotes of L. infantum MON 1 (MHOM/PT/93/CRE69), identified by the Reference Center of the World Health Organization (Montpellier, France). The infected mice were randomly assigned to four groups of five mice, which were treated subcutaneously with 50, 100, 200, or 400 mg of Sbb/kg of body weight on days 14, 16, and 18 postinfection. A control group of 10 infected mice received normal saline at the same time. Four groups of 5 uninfected mice received the same Sbb treatment, while 10 uninfected mice received normal saline.
On day 21 after infection, the mice's blood was collected and heparinized (10 IU/ml) and the animals were killed by cervical dislocation. Drug efficacy was assessed by evaluating the parasite burden and calculating the 50% effective dose (ED50) and ED90 according to the Stauber count (28). The Guiding Principles for Biomedical Research Involving Animals, published by the Council for International Organizations of Medical Sciences (Geneva, Switzerland) in 1985, were followed during all procedures.
PMN preparation.
Human venous blood, heparinized at 10 U/ml, was obtained from healthy volunteers. PMN were isolated by a first-step sedimentation of whole blood on 2% dextran T500 followed by centrifugation of the granulocyte-rich supernatant on a cushion of a mixture of Ficoll and Hypaque (Eurobio, Les Ulis, France) as previously described (26). The purified PMN (97%) were subjected to hypotonic lysis, washed, and resuspended in Hanks balanced salt solution (HBSS) (pH 7.4) containing 10 mM HEPES.
CL assay.
CL, i.e., light that is generated from singlet oxygen and other molecules, has been used largely to study the respiratory burst of phagocytes (1, 18, 29). The in vitro CL response of whole blood (18, 29) was measured at 37°C using a Bio-orbit or Picolite luminometer, both from Packard. Briefly, the reaction mixture consisted of 200 μl of heparinized blood diluted at 1:10 in 100 μl of HBSS containing 10 μM luminol (18). Light emission was induced by two phagocyte activators, i.e., opsonized zymosan (0.5 mg/ml) and a direct activator of protein kinase C (PKC), PMA (1 μg/ml). The CL response was recorded at 26-s intervals for 15 min, and the peak value expressed in millivolts was used to assess differences between the groups. The CL response by purified PMN was studied with suspensions of 106 cells under conditions described in the figure legends. Results represent the peak PMN CL and are expressed in either millivolts or counts per minute.
Superoxide production.
Production of superoxide anion by purified PMN was studied by continuous monitoring of cytochrome c reduction (25) using a Perkin-Elmer Lambda 40 spectrophotometer equipped with thermostated (37°C) cuvette holder and magnetic stirrer. Suspensions of 2 × 106 PMN in 2 ml of HBSS were treated in the absence (control) or presence of Sbb for 1 h at 37°C before stimulation with various agents known to trigger respiratory burst through different signaling pathways, including PMA, zymosan particles, and two chemoattractants, fMLP and PAF (7). Results are presented as nanomoles of superoxide generated by 106 PMN.
Statistics.
Differences in the effects of Sbb in vivo were assessed using the Mann-Whitney U test. Differences of the in vitro drug effects were assessed using Student's t test. P values of <0.05 were considered statistically significant.
RESULTS
ROS production in a murine model of leishmaniasis and in vivo effect of Sbb.
The influence of leishmaniasis on ROS formation was determined in vitro by comparing the CL responses of the whole blood of infected and uninfected mice. The peak CL response (mean ± standard deviation) induced by PMA or zymosan in the blood of uninfected animals was, respectively, 0.69 ± 0.04 or 1.44 ± 0.8 mV, whereas that induced in the blood of infected mice was significantly depressed (P <0.05), by 22 or 27%, respectively (i.e., 0.54 ± 0.04 or 1.06 ± 0.04 mM). Treatment of infected animals with various doses of Sbb (50, 100, 200, and 400 mg/kg) induced a strong leishmanicidal effect, as determined by the Stauber count (28); the ED50 and ED90 were approximately 38 and 164 mg of Sbb/kg, respectively.
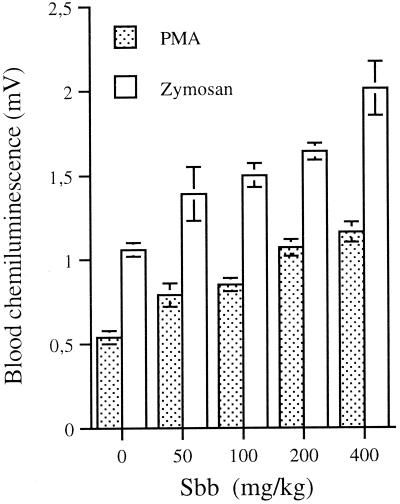
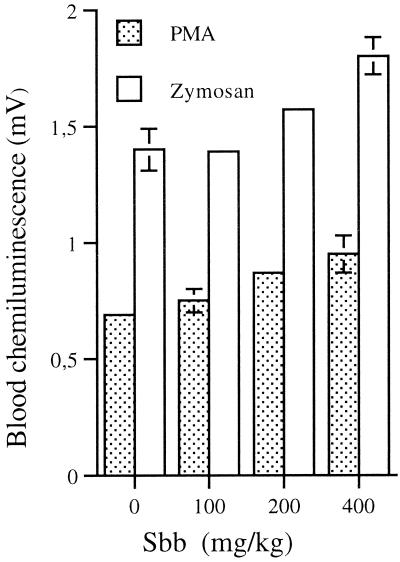
The in vivo effect of Sbb on the blood CL response of Leishmania-infected animals was evaluated next (Fig. 1). The CL response induced by PMA in the blood of animals treated with 50, 100, 200, or 400 mg of Sbb/kg was significantly enhanced by 46, 57, 98, or 114%, respectively, relative to that of the control (0.54 ± 0.04 mV). A similar priming effect of Sbb (P, <0.01) was also observed in the blood of animals treated with 50, 100, 200, or 400 mg of Sbb/kg after opsonized zymosan stimulation of the CL response: 31, 41, 64, or 89%, respectively, compared to the control (1.06 ± 0.04 mV). The CL response of the blood of uninfected mice measured in the absence of stimuli was approximately 0.8 mV and was not modified by treatment with Sbb (data not shown). The potentiating in vivo effect of Sbb on the CL response might be due to the decreased parasite burden or to an independent mechanism. To further clarify this point, the in vivo effect of Sbb on the CL response of the blood from uninfected mice was also studied. Treatment of mice with 100, 200, or 400 mg of Sbb/kg also primed (P, <0.01) the blood CL response induced by both PMA and zymosan (Fig. 2). However, a significant drug effect was observed with the highest Sbb concentrations (P, <0.05).
FIG. 1.
In vivo effect of Sbb on CL response of blood from L. infantum-infected mice. Mice were infected with Leishmania and treated in the absence (control) or presence of 50, 100, 200, or 400 mg of Sbb/kg. The CL of whole blood was induced in vitro with either PMA (1 μg/ml) or opsonized zymosan (0.5 mg/ml). Values are the means ± standard errors of the means of the peak CL obtained with at least six animals.
FIG. 2.
In vivo effect of Sbb on CL response of blood from uninfected mice. Uninfected mice were treated with 50, 100, 200, or 400 mg of Sbb/kg or saline (control), and the CL response of whole blood was induced with PMA (1 μg/ml) or opsonized zymosan (0.5 mg/ml). The peak CL response represents the means ± standard errors of the means obtained with six animals.
To examine whether the potentiated CL response may result from the possible interaction of trace amounts of Sbb in the circulating blood of treated mice with the luminol assay, we investigated whether the presence of Sbb in the assays influenced the CL response. For this purpose, blood was collected from uninfected mice and was treated in vitro with 1 or 10 μg of Sbb/ml for 1 min prior to stimulation with PMA. The treatment failed to modify the PMA-induced CL response of blood relative to the control (data not shown). This control experiment suggests that the potentiating effect was not due to Sbb interaction with the luminol-enhanced CL assay. Taken together, these observations suggest that Sbb may modify the reactivity of phagocytes directly or indirectly.
In vitro effect of Sbb on the respiratory burst of phagocytes from healthy volunteers.
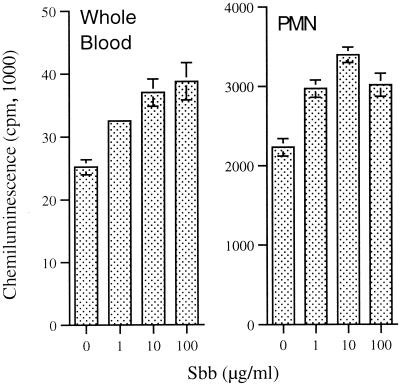
To further investigate the mode of action of Sbb, we analyzed the in vitro effects of Sbb on the CL response of human blood. Human blood was preferred to that of mice since it is suitable for the purification of large numbers of leukocytes for further investigation of drug effects in vitro. Among leukocytes, PMN were chosen for further study of the Sbb effect, since this cell type is a suitable model for clarifying the mode of action of drugs (11, 13, 24, 26). In addition, PMN were reported to ingest L. major in vivo and to control the parasite load (21). Treatment of human blood or purified PMN in vitro for 1 h in the presence of 1, 10, or 100 μg of Sbb/ml significantly (P, <0.01) enhanced the CL response induced by PMA (Fig. 3). The optimal potentiation of the CL response of PMN was obtained in the presence of 10 μg of Sbb/ml, whereas a higher concentration of the drug was less effective. The time course of cell treatment with 10 μg of Sbb/ml indicated that a significant drug potentiating effect required at least 30 min of PMN treatment.
FIG. 3.
In vitro effect of Sbb on CL responses of human blood and isolated PMN. Human blood or PMN were treated for 1 h in the absence (control) or presence of the indicated concentrations of Sbb. The CL response was induced with PMA (1 μg/ml) or zymosan (0.5 mg/ml). The peak CL response was measured by using a Packard Picolite luminometer and represents the means ± standard errors of the means of four experiments.
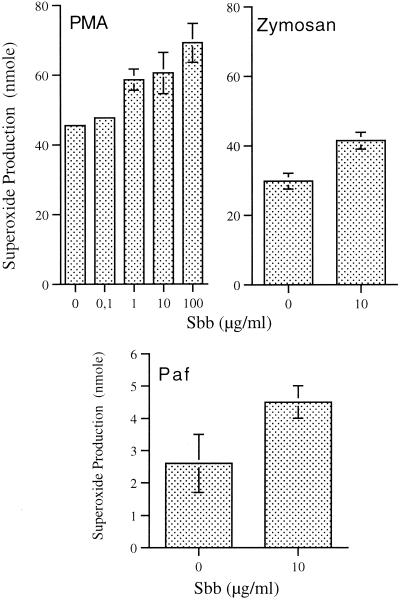
The luminol-enhanced CL response of stimulated PMN results from the combined stimulation of the production of ROS (respiratory burst) and the release of granular content (exocytosis). Peroxidases released during this process catalyze the oxidation of luminol in the presence of hydrogen peroxide and halide, a reaction in which light emission is enhanced. We determined whether the priming effects of Sbb were due to enhancement of the activity of the respiratory burst oxidase by measuring the generation of superoxide anion with the cytochrome c assay. To gain insight into the mode of action of Sbb, the PMN respiratory burst was also stimulated by various agents known to trigger different intracellular signaling pathways. Treatment of PMN with Sbb (0.1, 10, or 100 μg/ml) for 1 h induced a concentration-dependent increase in the production of superoxide induced by PMA (Fig. 4). The priming effect of Sbb (P, <0.05) was also observed in superoxide production induced by zymosan particles (Fig. 4) and the two chemoattractants PAF (Fig. 4) and fMLP (Fig. 5), although the latter two stimuli were less potent than PMA and zymosan.
FIG. 4.
Effect of Sbb on PMA-, zymosan-, and PAF-induced production of superoxide by PMN. Suspensions of PMN were incubated with 1, 10, or 100 μg of Sbb/ml for 1 h before stimulation with PMA (0.1 μg/ml), zymosan (0.5 mg/ml), or PAF (10 μM) for 10 min. Results represent the total amount of superoxide generated per 106 PMN (means of four experiments ± standard errors of the means).
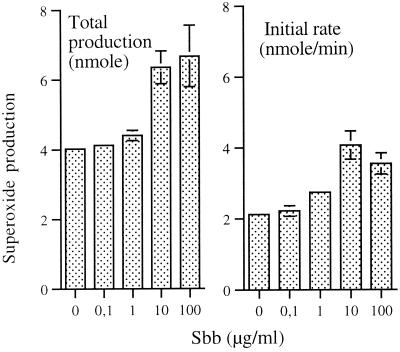
FIG. 5.
Effect of Sbb on fMLP-induced production of superoxide by PMN. PMN were treated in the absence (control) or presence of the indicated concentrations of Sbb for 1 h and stimulated with 0.1 μM fMLP. Results represent the initial rate of production and the total production of superoxide (means of four experiments ± standard errors of the means).
The stimulation of the PMN respiratory burst by chemoattractants is rapid and constant for a few minutes (initial rate) and is achieved within approximately 10 min. Any potentiation of the respiratory burst may result from priming of the biochemical process involved in the activation of the superoxide-generating NADPH oxidase and/or inhibition of the terminating process. Priming agents may modify one or both of these parameters, as suggested by comparison of the initial rate of production and the total production of superoxide (11, 25). Low concentrations of Sbb (1 and 10 μg/ml) potentiated both parameters of the respiratory burst (Fig. 5). However, Sbb was more active in priming the initial rate of superoxide generation (linear part of the response) relative to the total amount of superoxide generated. These observations suggest that the biochemical alterations induced by Sbb might predominantly affect the stimulatory rather than the terminating biochemical process.
DISCUSSION
This study provides evidence that Sbb has an in vivo protective effect against the down-regulation of the phagocyte respiratory burst mediated by L. infantum. This property is associated with Sbb's ability to induce a dose-dependent priming of ROS formation in the whole blood of infected and uninfected animals. The biochemical mechanism of this priming remains to be elucidated. However, the data suggest that at least two main processes may be involved. First, it has been previously shown that Sbb interacts directly with Leishmania and inhibits its survival (6). The decrease in parasite burden may consequently reduce the down-regulation of phagocyte killing activity, which has been shown to be mediated in part by Leishmania antigens (2). A second mechanism is suggested by our data. The enhancement of the oxygen-dependent defense activity of phagocytes in uninfected mice by Sbb indicates that Sbb may directly or indirectly alter the state of phagocyte activation, independent of the parasite burden. This novel property of Sbb is further supported by the observation that low concentrations of the drug (1 to 10 μg/ml) induce increases in ROS formation in normal human blood and by isolated PMN in vitro. This potentiation also affects the production of superoxide, indicating that Sbb enhanced the activity of NADPH oxidase, the enzyme that generates superoxide.
Interestingly, the Sbb priming effect occurred at low drug concentrations similar to those measured in the plasma of patients (5, 9). Furthermore, priming improves the PMN response induced by various stimuli (fMLP, PAF, and opsonized zymosan) known to stimulate specific membrane receptors which are also present on monocytes and macrophages. This observation suggests that the drug may also potentiate receptor-mediated immune responses. In this context, a third mechanism may be also considered; Sbb may enhance the production of cytokines such as interleukin 3 and gamma interferon, which have been shown to induce antileishmanial activity via an enhancement of the oxidative capacity of macrophages (16). Although the relative contributions of the mechanisms proposed above remain to be quantified, the potentiation of ROS formation described here suggests that Sbb may enhance the killing activities of phagocytes and the intracellular suppression of L. infantum. However, it should be noted that increased ROS formation may also have deleterious effects, particularly in some pathologies. In AIDS, ROS production by PMN was found to enhance virus replication in patient monocytes (15). Consequently, one may speculate that the enhancement of viral replication might further decrease the cell-mediated immune response. This may contribute in part to the failure of Sbb treatment in AIDS patients.
The mechanism of PMN priming by Sbb is not known. This priming affects the respiratory burst induced by proinflammatory agents, such as PAF or fMLP, and thus mimics the effects of other drugs that were previously described for human PMN (11, 25). One of these, staurosporine, is a bacterial alkaloid known as a potent inhibitor of PKC. This agent also stimulates G protein (19) and phospholipase D (PLD) activity (19, 24) and primes fMLP-induced PLD activity in a manner that is correlated with the respiratory burst of PMN (26). Another agent, okadaic acid, an inhibitor of protein phosphatase 2A, also primes PLD activity (13) and respiratory bursts (12). In contrast to the latter two agents, Sbb also potentiated the PMN respiratory burst induced by PMA, a direct activator of PKC. This suggests that Sbb may enhance PKC activity or downstream signaling events, such as phosphorylation of a major oxidase component, p47phox, as previously shown with staurosporine (10). Furthermore, the observation that Sbb has a greater priming effect on the rate of induction than on the total amount of superoxide induced by fMLP suggests that the biochemical alteration induced by Sbb may preferentially affect early events of the signaling cascade involved in the production of second messengers by chemoattractant receptors. Among these, PLD activity may play a major role since it was shown to be correlated with the primed respiratory burst of PMN (26). Whether Sbb enhances the generation of ROS and nitric oxide by macrophages is a major point to be studied in order to understand the mechanism of Sbb's leishmanicidal effect.
In conclusion, Sbb induced a protective in vivo effect against the down-regulation of phagocyte defense activity mediated by Leishmania. This novel property appears to be mediated in part by a direct interaction of Sbb with phagocytes, resulting in a priming of their ability to generate ROS in response to various stimuli. This effect may contribute to the drug's leishmanicidal property.
ACKNOWLEDGMENTS
This work is support by Sidaction (Fondation pour la Recherche Médicale).
We thank the staff of the blood bank of the St. Vincent Hospital for providing blood samples.
REFERENCES
- 1.Allen R C. Phagocytic leukocyte oxygenation activities and chemiluminescence: a kinetic approach to analysis. Methods Enzymol. 1986;133:449–493. doi: 10.1016/0076-6879(86)33085-4. [DOI] [PubMed] [Google Scholar]
- 2.al-Mofleh I A, Mahmoud A A, al-Khuwaiter A S, al-Tuwaijri A S. Effect of Leishmania major on luminol-dependent chemiluminescence of human whole blood phagocytosis. Trop Med Parasitol. 1989;40:279–284. [PubMed] [Google Scholar]
- 3.al Tuwaijri A S, al Mofleh I A, Mahmoud A A. Effect of Leishmania major on human polymorphonuclear leucocyte function in vitro. J Med Microbiol. 1990;32:189–193. doi: 10.1099/00222615-32-3-189. [DOI] [PubMed] [Google Scholar]
- 4.Babior B M. Oxygen-dependent microbial killing by phagocytes (first of two parts) N Engl J Med. 1978;298:659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- 5.Berman J D, Melby P C, Neva F A. Concentration of Pentostam in human breast milk. Trans R Soc Trop Med Hyg. 1989;83:784–785. doi: 10.1016/0035-9203(89)90327-1. [DOI] [PubMed] [Google Scholar]
- 6.Berman J D, Waddell D, Hanson B D. Biochemical mechanisms of the antileishmanial activity of sodium stibogluconate. Antimicrob Agents Chemother. 1985;27:916–920. doi: 10.1128/aac.27.6.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bokoch G M. Chemoattractant signaling and leukocyte activation. Blood. 1995;86:1649–1660. [PubMed] [Google Scholar]
- 8.Carvalho E M, Bacellar O, Barral A, Badaro R, Johnson W D., Jr Antigen-specific immunosuppression in visceral leishmaniasis is cell mediated. J Clin Investig. 1989;83:860–864. doi: 10.1172/JCI113969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chulay J D, Fleckenstein L, Smith D H. Pharmacokinetics of antimony during treatment of visceral leishmaniasis with sodium stibogluconate or meglumine antimoniate. Trans R Soc Trop Med Hyg. 1988;82:69–72. [PubMed] [Google Scholar]
- 10.Combadiere C, el Benna J, Pedruzzi E, Hakim J, Perianin A. Stimulation of the human neutrophil respiratory burst by formyl peptides is primed by a protein kinase inhibitor, staurosporine. Blood. 1993;82:2890–2898. [PubMed] [Google Scholar]
- 11.Combadiere C, Hakim J, Giroud J P, Perianin A. Staurosporine, a protein kinase inhibitor, up-regulates the stimulation of human neutrophil respiratory burst by N-formyl peptides and platelet activating factor. Biochem Biophys Res Commun. 1990;168:65–70. doi: 10.1016/0006-291x(90)91675-i. [DOI] [PubMed] [Google Scholar]
- 12.Djerdjouri B, Combadiere C, Pedruzzi E, Hakim J, Perianin A. Contrasting effects of calyculin A and okadaic acid on the respiratory burst of human neutrophils. Eur J Pharmacol. 1995;288:193–200. doi: 10.1016/0922-4106(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 13.Djerdjouri B, Pedruzzi E, Hakim J, Perianin A. Okadaic acid, an inhibitor of type 1 and type 2A phosphatases, modulates the activation of phospholipase D in formyl peptide- and mastoparan-stimulated human neutrophils. Biochem Biophys Res Commun. 1994;205:1481–1487. doi: 10.1006/bbrc.1994.2832. [DOI] [PubMed] [Google Scholar]
- 14.Hallett M B, Lloyds D. Neutrophil priming: the cellular signals that say 'amber' but not 'green.' Immunol. Today. 1995;16:264–268. doi: 10.1016/0167-5699(95)80178-2. [DOI] [PubMed] [Google Scholar]
- 15.Ho J, He S, Hu A, Geng J, Basile F, Almeida M, Saito A, Laurence J, Johnson W J. Neutrophils from human immunodeficiency virus (HIV)-seronegative donors induce HIV replication from HIV-positive patients' mononuclear cells and cell lines: an in vitro model of HIV transmission facilitated by Chlamydia trachomatis. J Exp Med. 1995;181:1493–1505. [PMC free article] [PubMed] [Google Scholar]
- 16.Ho J L, Reed S G, Sobel J, Arruda S, He S H, Wick E A, Grabstein K H. Interleukin-3 induces antimicrobial activity against Leishmania amazonensis and Trypanosoma cruzi and tumoricidal activity in human peripheral blood-derived macrophages. Infect Immun. 1992;60:1984–1993. doi: 10.1128/iai.60.5.1984-1993.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho M, Koech D K, Iha D W, Bryceson A D. Immunosuppression in Kenyan visceral leishmaniasis. Clin Exp Immunol. 1983;51:207–214. [PMC free article] [PubMed] [Google Scholar]
- 18.Huu T P, Dumez Y, Marquetty C, Durandy A, Boue J, Hakim J. Prenatal diagnosis of chronic granulomatous disease (CGD) in four high risk male fetuses. Prenatal Diagn. 1987;7:253–260. doi: 10.1002/pd.1970070405. [DOI] [PubMed] [Google Scholar]
- 19.Kanaho Y, Takahashi K, Tomita U, Iiri T, Katada T, Ui M, Nozawa Y. A protein kinase C inhibitor, staurosporine, activates phospholipase D via a pertussis toxin-sensitive GTP-binding protein in rabbit peritoneal neutrophils. J Biol Chem. 1992;267:23554–23559. [PubMed] [Google Scholar]
- 20.Liew F Y, Millott S, Parkinson C, Palmer R M, Moncada S. Macrophage killing of Leishmania parasite in vivo is mediated by nitric oxide from l-arginine. J Immunol. 1990;144:4794–4797. [PubMed] [Google Scholar]
- 21.Lima G M, Vallochi A L, Silva U R, Bevilacqua E M, Kiffer M M, Abrahamsohn I A. The role of polymorphonuclear leukocytes in the resistance to cutaneous leishmaniasis. Immunol Lett. 1998;64:145–151. doi: 10.1016/s0165-2478(98)00099-6. [DOI] [PubMed] [Google Scholar]
- 22.Murray H W, Nathan C F. Macrophage microbicidal mechanisms in vivo: reactive nitrogen versus oxygen intermediates in the killing of intracellular visceral Leishmania donovani. J Exp Med. 1999;189:741–746. doi: 10.1084/jem.189.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paul M, Durand R, Fessi H, Rivollet D, Houin R, Astier A, Deniau M. Activity of a new liposomal formulation of amphotericin B against two strains of Leishmania infantum in a murine model. Antimicrob Agents Chemother. 1997;41:1731–1734. doi: 10.1128/aac.41.8.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perianin A, Combadiere C, Pedruzzi E, Djerdjouri B, Hakim J. Staurosporine stimulates phospholipase D activation in human polymorphonuclear leukocytes. FEBS Lett. 1993;315:33–37. doi: 10.1016/0014-5793(93)81127-l. [DOI] [PubMed] [Google Scholar]
- 25.Perianin A, Pedruzzi E, Hakim J. W-7, a calmodulin antagonist, primes the stimulation of human neutrophil respiratory burst by formyl peptides and platelet-activating factor. FEBS Lett. 1994;342:135–138. doi: 10.1016/0014-5793(94)80487-7. [DOI] [PubMed] [Google Scholar]
- 26.Rais S, Pedruzzi E, Dang M C, Giroud J P, Hakim J, Perianin A. Priming of phosphatidic acid production by staurosporine in f-Met-Leu-Phe-stimulated human neutrophils—correlation with respiratory burst. Cell Signal. 1998;10:121–129. doi: 10.1016/s0898-6568(97)00116-2. [DOI] [PubMed] [Google Scholar]
- 27.Scott P. The role of TH1 and TH2 cells in experimental cutaneous leishmaniasis. Exp Parasitol. 1989;68:369–372. doi: 10.1016/0014-4894(89)90120-3. [DOI] [PubMed] [Google Scholar]
- 28.Stauber L A, Franchino E M, Grun J. An eight day method for screening compounds against Leishmania donovani in golden hamster. J Protozool. 1958;5:269–273. [Google Scholar]
- 29.Tono-oka T, Matsumoto T, Ueno N, Yashiki N, Matsumoto S. Chemiluminescence of whole blood. II. Application to clinical examination of phagocytic functions of whole blood from various types of disease. Clin Immunol Immunopathol. 1983;29:333–340. doi: 10.1016/0090-1229(83)90036-3. [DOI] [PubMed] [Google Scholar]
- 30.Vouldoukis I, Riveros-Moreno V, Dugas B, Ouaaz F, Becherel P, Debre P, Moncada S, Mossalayi M D. The killing of Leishmania major by human macrophages is mediated by nitric oxide induced after ligation of the Fc epsilon RII/CD23 surface antigen. Proc Natl Acad Sci USA. 1995;92:7804–7808. doi: 10.1073/pnas.92.17.7804. [DOI] [PMC free article] [PubMed] [Google Scholar]