Abstract
In this study, the safety and functionality of using citrus juice processing waste (CJPW) was confirmed. Large quantities of CJPW are generated on Jeju Island and cause environmental problems. CJPW extract (2,000 mg/kg) was administered intragastrically to male and female Sprague-Dawley (SD) rats for 14 days and the rats were analyzed. No general signs of toxicity were observed in SD rats administered CJPW extract. Feed intake did not differ between experimental and control animals. However, male and female rats administered CJPW extract had greater weight gain (102.9±5.53% and 114.15±6.89%, respectively) compared with the control animals. Higher weight gain was found in male and female experimental animals, but the difference was only significant in female animals. Serum analyses indicated that total protein and albumin, indicators of nutritional status, were significantly increased in animals administered CJPW. Further, serum glucose values were lower in male and female rats treated with CJPW (91.6±9.02% and 69.9±4.11%, respectively) compared with the controls; again, the difference was significant only for female animals. From the results of this study, CJPW can be considered as a safe material that does not induce toxicity in experimental animals. Therefore, CJPW is a potential raw material that can be used as a supplement in animal feed to help improve weight gain and achieve serum glucose con-trol.
Keywords: citrus juice processing waste, feed supplement, growth rate, safety evaluation, serum glucose
INTRODUCTION
Citrus is the world’s most popular fruit and is abundant in tropical, subtropical, and many other areas (Fernández-López et al., 2004). Citrus fruits contain flavonoids, carotenoids, phenylpropanoids, limonoids, and more than 60 physiologically active substances (Mouly et al., 1994; Jeong et al., 1997). The primary flavonoid compounds are naringin and hesperidin; these compounds have antioxidant, antinflammatory, antiviral, anticancer, immunity-enhancing, and capillary-strengthening properties (Kawaguchi et al., 1997; Sohn and Kim, 1998; Meiyanto et al., 2012; Roohbakhsh et al., 2015). They have been traditionally used as a medicinal ingredient due to its pharmacological effects (Arias and Ramón-Laca, 2005). In Korea, citrus fruit is the most processed food on Jeju Island.
Citrus production continues to increase; Korea’s annual production is approximately 600,000 tons (Ji et al., 2021). More than 30% of the produced citrus fruits are processed to produce citrus juice. However, after the production of citrus juice, a large amount of waste is generated, including peels, segments, and seeds (Ahn et al., 2007; Leporini et al., 2020). The generated citrus juice processing waste (CJPW) accounts for 50% to 60% of the total weight of citrus fruits, with 60 million tons generated worldwide and 50,000 to 70,000 tons generated annually on Jeju Island (Moon et al., 2018). This waste is an economic and environmental issue. Before 2013, organic waste resources, including CJPW, were discarded by ocean dumping. However, as the London Convention now prohibits such dumping, this has resulted in limitations to landfill and incineration capacities (Kim et al., 2014).
To solve the problems raised by these large amounts of CJPW, studies have attempted various methods to safely recycle CJPW without adversely affecting the environment. CJPW is characterized by a high content of naringin and hesperidin, but is also rich in other bioflavonoids and carotenoids (Kim et al., 2006; Andrad et al., 2007; Jeon et al., 2014). Further, CJPW contains a large proportion of dietary fiber, which is known to lower blood cholesterol (Khan et al., 2021). The waste also contains pharmacologically active substances effective in various adult diseases (Nawirska and Kwaśniewska, 2005). The functional dietary fiber and physiologically active compounds in CJPW can be used in the processing process to make health products (Fernández-López et al., 2004). Further, CJPW was used as a culture medium for mushrooms (Lee et al., 2007) and has been applied in natural dyes (Tayyab et al., 2020). In addition, a method was devised to use the citrus pulp in the manufacture of Korean paper, corrugated cardboard, tiles, and other similar products (Kim et al., 2007).
Although various studies are examining methods of recycling CJPW, the most effective way to treat mounts of waste is to use it as a feed supplement or substitute. A study in which CJPW was applied as a feed supplement was reported to enhance the nonspecific immunity of red seabream (Pagrus major), increase the collagen content in the bones, and reduce fish mortality (Song et al., 2013). It was reported that the content of highly unsaturated fat in meat was significantly increased when citrus pulp was added to the ostrich feed (Lanza et al., 2004). In addition, many studies have examined nutrition and quality after adding CJPW to livestock and bird feed (Yang et al., 2006; Jung et al., 2007; Yang et al., 2008). However, some studies have shown that CJPW promoted animal growth when used as a feed supplement, but other studies have found contrasting results (Bampidis and Robinson, 2006; Jwa and Yeo, 2015). In addition, few studies have examined animal safety and effects on blood and serological changes after CJPW ingestion. This study aimed to examine whether CJPW helped to promote animal growth and whether it was a safe material for use as a feed supplement, which is an efficient method of recycling CJPW. Therefore, this study investigated evaluated the effects of CJPW on safety, weight gain, blood, and serological changes in Sprague-Dawley (SD) rats.
MATERIALS AND METHODS
Preparation of CJPW extract
CJPW was provided and used by the Jeju Special Self-Governing Province Development Corporation from waste generated in the process of juicing after washing the entire fruit. The collected CJPW was dried at a low temperature (40°C) and then pulverized with a grinder to extract a sample. A dried CJPW sample (50 g) was immersed in 1 L of 50% ethanol and stirred at room temperature for 24 h. To collect the extracted material, the solution was filtered through a 0.45-μm bottle filter (Corning Bottle-Top Vacuum Filter System, Corning Inc., Corning, NY, USA). The ethanol was evaporated using a vacuum concentrator and the filtrate was lyophilized to prepare a powder. The powder was stored at −20°C and prepared for use as a suspension in sterile distilled water.
Animal and experimental procedures
Five-week-old female and male SD rats bred in specific-pathogen-free conditions were purchased from KOSA BIO Inc. (Seongnam, Korea). The animals were maintained in an environment with a 12-h light/dark cycle (8 am to 8 pm), constant temperature (23±3°C), and constant relative humidity (50±10%). The animals were regularly provided a standard diet (Altromin 1324, Altromin, Lage, Germany) and allowed access to water ad libitum. All procedures followed the Animal Experimental Ethics Regulations and the study was approved by the Animal Ethics Committee of the Catholic University of Pusan (no. CUP AEC 2020-002). After acclimation, the rats were divided into the control and CJPW groups. Five female and five male rats were included in each group. Toxicity studies using a citrus peel water extract showed no abnormalities in experimental animals at doses up to 4,000 mg/kg (Park et al., 2018). Therefore, an extract dose of 2,000 mg/kg was administered intragastrically daily in a volume of 10 mL/kg of sterile water to rats in the CJPW group. Rats in the control group received only sterile distilled water. Oral administration was continued for 14 days. The study was conducted with reference to the “Toxicity Test Standards for Drugs” from the Korean Ministry of Food and Drug Safety (Ministry of Government Legislation, 2017) and the Organization for Economic Cooperation and Development Guideline No. 407 (OECD, 2008).
Clinical symptoms, body weight, and feed intake
Animals were observed for 1 to 6 h after the first oral administration of the extract. Thereafter, all animals were observed for general and toxicological signs, decreased mobility, addiction, and death once daily at designated times during the study period. The body weight and feed intake were measured as described in a previous study (Lee et al., 2021).
Necropsy and organ weight measurement
Animals were fasted for 12 h before necropsy, and after anesthesia, blood was collected from the abdominal aorta, and gross organ abnormalities were recorded. The liver, lungs, heart, kidneys, spleen, and thymus were collected from all rats; the ovaries and uterus were collected from female rats and the testicles and prostate were collected from male rats. All organs were weighed after gross examination.
Analysis of blood and serum biochemistry
Blood from the abdominal aorta was collected into ethylenediaminetetraacetic acid tubes (BD Caribe, Ltd., Sumter, SC, USA), stirred on a roll mixer for 30 min, and analyzed for white blood cells, red blood cell (RBCs), hemoglobin, hematocrit (HCT), mean red blood cell volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration, and platelets (PLTs) using a blood analyzer (DxH500, Beckman Coulter, Inc., Brea, CA, USA).
Blood for serological analysis was collected into SST tubes, allowed to coagulate at room temperature (18∼20°C), and centrifuged at 2,500 rpm for 20 min. Serum was analyzed using a blood biochemical analyzer BT1500 (Biotecnica Instrument SpA, Rome, Italy). The parameters analyzed included total protein (TP), albumin (ALB), aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transferase (GGT), alkaline phosphatase (ALP), glucose, total cholesterol, triglyceride, total bilirubin, high-density lipoprotein cholesterol, urea (UA), and blood urea nitrogen (BUN).
Statistical analysis
Data were analyzed using SPSS version 25 (IBM Corp., Armonk, NY, USA). The data are expressed as the mean±standard deviation. Statistical analysis of body weight, feed intake, organ weight, blood, and serological values was performed using Student’s t-test to compare the control group and CJPW group. P-values of <0.05 were considered to indicate significant differences.
RESULTS
Clinical signs and death observations
After administration of CJPW extract, there were no changes in clinical signs, such as salivation, reduced mobility, and toxicity, as seen for the control rats. In addition, there were no drug-induced deaths among experimental or control rats (Table 1).
Table 1.
Clinical signs of Sprague-Dawley rats treated with citrus juice processing waste extract for 14 days
| Sex | Dose (mg/kg/d) |
Signs observed | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Clonic or tonic movement | Excessive grooming, repetitive circling | Self-mutilation | Salivation | Death | ||
| Male | 0 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| 2,000 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | |
| Female | 0 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| 2,000 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | |
Values are presented as number of animals with sign/total number of animals examined.
Body weight and feed intake
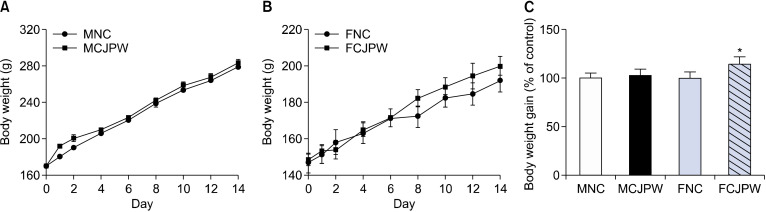
Animals in the CJPW and control groups showed normal body weight gain; typical gains were 50 g for females and 100 g for males over 14 days. Male rats gained 109.38±6.18 g in the control group and 112.50±6.76 g in the CJPW group; these gains were not significantly different. Female rats gained 45.38±7.25 g in the control group and 51.80±3.49 g in the CJPW group, which was a significant difference (Table 2). The percentage weight gain in CJPW-treated animals was 102.85±5.53% and 114.15±6.89% that of the control rats in males and females, respectively. Although the weight gain was increased by CJPW administration, the difference was only significant for females (P<0.05; Fig. 1). Feed intake in the control and CJPW groups was 23.68±0.93 g and 24.25±1.17 g, respectively, for male rats and 19.77±1.98 g and 17.37±0.96 g, respectively, for female rats. Feed intake tended to be lower in the CJPW group, but the difference was not significant (Table 2). These results seem show that the effect of weight gain after CJPW administration occurs regardless of dietary intake.
Table 2.
Weight gain and food intake in Sprague-Dawley rats orally administered citrus juice processing waste extract for 14 days
| Variable | Male dose (mg/kg) | Female dose (mg/kg) | |||
|---|---|---|---|---|---|
|
|
|
||||
| 0 | 2,000 | 0 | 2,000 | ||
| Body weight gain (g) | 109.38±6.18 | 112.50±6.76 | 45.38±7.25 | 51.80±3.49* | |
| Food intake (g/d) | 23.68±0.93 | 24.25±1.17 | 19.77±1.98 | 17.37±0.96 | |
Values are presented as mean±SD.
*Significant difference at P<0.05 vs. the control group.
Fig. 1.
Changes in body weight of Sprague-Dawley rats treated with citrus juice processing waste (CJPW) extract orally administered for 14 days. (A) Male group body weight change, (B) female group body weight change, and (C) weight gain rate in males and females compared with the control group. Weight gain rate data are expressed as [(treated animal final weight−initial weight)/(control animal final weight−initial weight)×100]. Data are presented as mean±SD (n=5). *P<0.05 vs. normal control group. MNC, males in the normal control group; MCJPW, males in the CJPW orally administered group; FNC, females in the normal control group; FCJPW, females in the CJPW orally administered group.
Necropsy and organ weight measurement
Rats were fasted for 12 h after the final treatment and then necropsied. No specific or abnormal findings related to the administration of CJPW extract or distilled water were observed. The organ weights of animals the control group and the CJPW group were within the standard range. However, the absolute weights of lung and thymus in male rats in the control group were 1.46±0.04 g and 0.67±0.03 g, respectively; significant increases were observed for the lung and thymus weights in the CJPW group at 1.55±0.18 g and 0.75±0.17 g, respectively (P<0.05). For female rats, a significantly higher absolute liver weight of 7.60±2.05 g was found in the CJPW group compared with 6.69±0.47 g in the control group (P<0.05). These results were analyzed in the same way in relative weight. The results of the other organs, including the spleen, heart, testis, prostate, ovary, and uterus, were similar to those in the control group (Table 3).
Table 3.
Absolute and relative weights of organs from Sprague-Dawley rats administered citrus juice processing waste extract orally for 14 days
| Organ | Male dose (mg/kg) | Female dose (mg/kg) | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| 0 | 2,000 | 0 | 2,000 | |||
| Liver | Absolute (g) | 9.50±0.34 | 9.88±0.76 | 6.69±0.47 | 7.60±2.05* | |
| Relative (%) | 3.41±0.12 | 3.49±0.27 | 3.48±0.24 | 3.80±1.03* | ||
| Spleen | Absolute (g) | 0.81±0.09 | 0.81±0.15 | 0.58±0.12 | 0.59±0.08 | |
| Relative (%) | 0.29±0.03 | 0.29±0.05 | 0.30±0.06 | 0.30±0.04 | ||
| Kidney-left | Absolute (g) | 1.18±0.10 | 1.18±0.13 | 0.74±0.09 | 0.81±0.11 | |
| Relative (%) | 0.44±0.04 | 0.46±0.05 | 0.40±0.06 | 0.41±0.07 | ||
| Kidney-right | Absolute (g) | 1.24±0.10 | 1.29±0.13 | 0.77±0.11 | 0.83±0.14 | |
| Relative (%) | 0.42±0.04 | 0.42±0.04 | 0.38±0.05 | 0.41±0.06 | ||
| Heart | Absolute (g) | 1.13±0.21 | 1.12±0.09 | 0.70±0.04 | 0.73±0.06 | |
| Relative (%) | 0.41±0.08 | 0.40±0.03 | 0.37±0.02 | 0.37±0.03 | ||
| Lung | Absolute (g) | 1.46±0.04 | 1.55±0.18* | 1.17±0.19 | 1.16±0.18 | |
| Relative (%) | 0.52±0.02 | 0.55±0.07* | 0.61±0.10 | 0.58±0.09 | ||
| Thymus | Absolute (g) | 0.67±0.03 | 0.75±0.17* | 0.52±0.07 | 0.47±0.07 | |
| Relative (%) | 0.24±0.01 | 0.26±0.06* | 0.27±0.04 | 0.24±0.04 | ||
| Testis-left | Absolute (g) | 1.49±0.15 | 1.58±0.16 | − | − | |
| Relative (%) | 0.55±0.05 | 0.60±0.05 | − | − | ||
| Testis-right | Absolute (g) | 1.54±0.13 | 1.60±0.13 | − | − | |
| Relative (%) | 0.54±0.05 | 0.56±0.06 | − | − | ||
| Prostate | Absolute (g) | 0.94±0.19 | 0.88±0.26 | − | − | |
| Relative (%) | 0.37±0.11 | 0.27±0.09 | − | − | ||
| Ovary-left | Absolute (g) | − | − | 0.11±0.07 | 0.10±0.06 | |
| Relative (%) | − | − | 0.06±0.04 | 0.05±0.03 | ||
| Ovary-right | Absolute (g) | − | − | 0.13±0.03 | 0.12±0.07 | |
| Relative (%) | − | − | 0.07±0.01 | 0.06±0.04 | ||
| Uterus | Absolute (g) | − | − | 0.60±0.19 | 0.57±0.11 | |
| Relative (%) | − | − | 0.31±0.10 | 0.23±0.05 | ||
Values are presented as mean±SD.
*Significant difference at P<0.05 vs. control group.
Blood analysis and serum biochemistry
Most hematological parameters in the CJPW group were in the standard ranges and similar to those in the control group (Table 4). However, in male rats, the RBC count was significantly increased from 6.41±0.16 in the control group to 7.01±0.24 in the CJPW group (P<0.01); further, in the CJPW group, HCT, and PLT were significantly increased (P<0.01), and MCV and MCH were significantly decreased (P<0.01 and P<0.05) compared with the control group. The RBC in females was 6.32±0.77 and 7.36±0.65 in the control and CJPW groups, respectively, which represented a significant increase in the CJPW group (P<0.05).
Table 4.
Hematological parameters of Sprague-Dawley rats administered citrus juice processing waste extract orally for 14 days
| Parameter | Male dose (mg/kg) | Female dose (mg/kg) | |||
|---|---|---|---|---|---|
|
|
|
||||
| 0 | 2,000 | 0 | 2,000 | ||
| WBC (×103/μL) | 7.81±0.98 | 7.99±1.36 | 7.46±0.55 | 7.81±0.84 | |
| RBC (×106/μL) | 6.41±0.16 | 7.01±0.24** | 6.32±0.77 | 7.36±0.65* | |
| HGB (g/dL) | 14.49±0.75 | 14.90±0.36 | 13.44±1.63 | 14.37±1.59 | |
| HCT (%) | 40.82±0.74 | 43.75±1.21** | 37.08±4.71 | 36.97±4.32 | |
| MCV (fL) | 63.94±0.74 | 62.40±1.07* | 58.66±0.87 | 57.68±0.52 | |
| MCH (pg) | 22.38±0.48 | 21.25±0.57** | 21.28±0.34 | 20.82±0.63 | |
| MCHC (g/dL) | 35.02±0.86 | 34.05±0.47 | 36.26±0.38 | 36.08±1.03 | |
| PLT (×103/μL) | 637.68±38.38 | 746.95±30.20** | 672.80±35.05 | 668.52±18.26 | |
Values are presented as mean±SD (n=5).
Significant differences at *P<0.05 and **P<0.01 vs. control group.
WBC, white blood cell; RBC, red blood cell; HGB, hemoglobin; HCT, hematocrit; MCV, mean red blood cell volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; PLT, platelet.
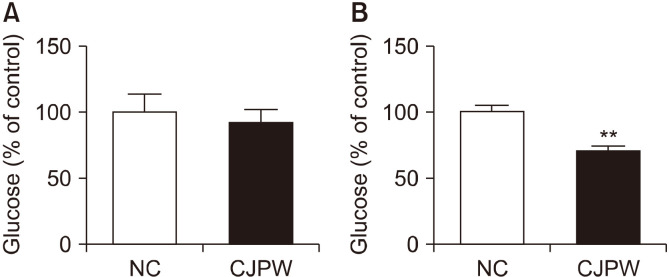
All serological analyses were within the normal ranges. However, the male rats that received CJPW had significantly increased levels of TP and ALB (P<0.01) but significantly decreased levels of BUN (P<0.05). Slightly different results were seen for female rats in the CJPW group, where TP, ALB, and BUN levels were all increased (P<0.01 and P<0.05) (Table 5). Further, conversion of the serum glucose value into a percentage relative to the control group revealed that the serum glucose level in the CJPW group was lower in males and females than in the control group (91.58±9.02% and 69.94±4.11%, respectively) (Fig. 2); however, the decrease was only significant in female rats (P<0.01).
Table 5.
Serum biochemical parameters of Sprague-Dawley rats administered citrus juice processing waste extract orally for 14 days
| Parameter | Male dose (mg/kg) | Female dose (mg/kg) | |||
|---|---|---|---|---|---|
|
|
|
||||
| 0 | 2,000 | 0 | 2,000 | ||
| TP (g/dL) | 5.94±0.21 | 6.52±0.26** | 5.51±0.29 | 5.93±0.28* | |
| ALB (g/dL) | 3.53±0.12 | 3.81±0.09** | 3.32±0.20 | 3.61±0.19* | |
| AST (U/L) | 121.26±7.55 | 126.90±3.97 | 108.68±17.22 | 120.77±16.29 | |
| ALT (U/L) | 53.20±4.21 | 54.00±3.39 | 38.00±7.78 | 35.60±8.44 | |
| GGT (U/L) | 2.18±0.59 | 1.91±1.12 | 2.35±0.66 | 2.28±1.09 | |
| ALP (U/L) | 387.96±30.90 | 378.22±27.57 | 180.32±20.70 | 176.82±18.02 | |
| GLU (mg/dL) | 118.45±16.60 | 108.48±11.94 | 188.46±9.22 | 131.80±8.65** | |
| CHO (mg/dL) | 79.40±5.89 | 83.75±8.27 | 78.70±7.28 | 74.80±5.41 | |
| TG (mg/dL) | 50.93±11.66 | 50.72±12.11 | 33.49±3.02 | 34.02±5.84 | |
| TB (mg/dL) | 0.83±0.04 | 0.83±0.05 | 0.79±0.06 | 0.79±0.06 | |
| HDL (mg/dL) | 49.61±6.89 | 54.10±4.00 | 57.68±7.59 | 58.86±3.68 | |
| UA (mg/dL) | 1.49±0.37 | 2.02±0.68 | 1.13±0.38 | 1.17±0.24 | |
| BUN (mg/dL) | 24.49±1.97 | 20.84±2.45* | 18.44±1.71 | 22.38±3.27* | |
Values are expressed as mean±SD (n=5).
Significant difference at *P<0.05 and **P<0.01 vs. control group.
TP, total protein; ALB, albumin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma-glutamyl transferase; ALP, alkaline phosphatase; GLU, glucose; CHO, total cholesterol; TG, triglyceride; TB, total bilirubin; HDL, high-density lipoprotein cholesterol; UA, urea; BUN, blood urea nitrogen.
Fig. 2.
Serum glucose levels of Sprague-Dawley rats treated with citrus juice processing waste (CJPW) extract for 14 days compared with those of the control group. (A) Male experimental animals and (B) female experimental animals. Serum glucose levels (%) were significantly decreased in treated female rats. The data are expressed as (the glucose level in treated rats/glucose level in control rats×100). Data are mean±SD (n=5). **P<0.01 vs. control group. NC, normal control animals; CJPW, CJPW orally administered animals.
DISCUSSION
The study examined the use of CJPW as a feed or feed supplement as a means for efficiently recycling the 50,000 to 70,000 tons of waste produced annually. CJPW extract was administered to rats for 14 days. The general signs, feed intake, weight gain, changes in organ weights, blood hematology, and serum biochemical analysis were used to assess the efficacy and toxicity of the extract.
No deaths were recorded during the study. There were no changes in clinical signs, such as decreased motility and intoxication, that may occur during oral drug administration. Furthermore, salivation was observed after the administration of a hot-water extract powder of Oplopanax elatus Nakai, but this effect was not induced by the citrus extract (Yoo et al., 2019).
Body weight change was recorded as a measure of toxicity (Bailey et al., 2004). Body weights were higher in rats treated with CJPW compared with the control rats. The rate of weight gain was greater in female rats than in male rats. A study in which citrus peel extract was administered repeatedly for 90 days revealed a tendency to increase body weight of animals in the CJPW group (Park et al., 2018). Consistent with the results of this study, it was considered that the citrus waste extract increased the weight gain in the experimental animals. Differences in body weights between female and male animals are typical for many species. Resolving sex-based differences in various medical fields has emerged as a priority topic in metabolic diseases (Mauvais-Jarvis et al., 2017; Rich-Edwards et al., 2018). Variations in metabolism, gene expression, anatomical structure, and stress response can be reflected in sex differences (Tower et al., 2020). It is known that there are sex-related differences in energy storage, adipose tissue distribution, and functional areas (Karastergiou et al., 2013; Kautzky-Willer et al., 2016; Link and Reue, 2017). In addition, the most prominent differences between sexes occur as the result of metabolic responses to sex hormone secretion (Tramunt et al., 2020). CJPW indicates a larger weight gain in female rats than in male rats, likely as a result of one or more of these factors.
Differences in weight gain were not due to differences in feed intake. No difference in food consumption between the control and CJPW groups was found. The increase in body weight of the experimental animals is thus judged to be an effect of CJPW administration.
Changes in the weight of major organs are used as sensitive indicators of chemical toxicity (Bailey et al., 2004). The organ weights for all animals in the study were greater than those in the baseline data (Kang et al., 2001; Okamura et al., 2011). These latter data were reported from 7-week-old rats; higher organ weights were expected for the present study because animals were 8 weeks old at the time of necropsy.
RBC levels were significantly higher in female and male rats administered CJPW. In addition, MCV and MCH decreased significantly in CJPW-treated male rats, and HCT and PLT increased significantly. According to the study results on the hematological analysis of experimental animals, the aforementioned difference suggests that it is not an abnormal symptom caused by CJPW administration (Kang et al., 2004; Petterino and Argentino-Storino, 2006).
The levels of serum liver biomarker enzymes (AST, ALT, and ALP) are widely used as sensitive markers to assess liver toxicity (Mukinda and Syce, 2007). An increase in AST indicates extensive tissue necrosis and liver cell damage, ALT is used as an indicator for hepatomegaly, and ALP is used to confirm biliary obstruction (Ozdil et al., 2010; Han et al., 2011). Indices of liver damage and drug-related liver abnormalities, such as AST, ALT, ALP, and GGT, were normal in treated animals. Over the 14 days of the experiment, rats in the CJPW had significant increases in TP, an indicator of overall nutritional status, and ALB, an indicator of nutritional status. CJPW was not toxic to the liver and was judged to be beneficial to nutritional status.
The kidneys regulate excretion and acid-base homeostasis. These organs excrete wastes and regulate the excretion of UA and electrolytes (Ferguson and Waikar, 2012). BUN values were significantly lower in male rats treated with CJPW, but were increased in female rats. However, BUN values were within the normal range, and no abnormalities were seen in UA results. Renal function was thus not affected. In addition, serum glucose levels were lower in CJPW-treated rats than in controls, although the difference was only significant in females. This tendency for lower serum glucose levels was similar that found studies reporting that citrus hesperidin and naringin reduced serum glucose in diabetic rats and that citrus peel extract helped reduce the risk of hyperglycemia (Mahmoud et al., 2012; Ashraf et al., 2017). Flavonoids in CJPW may help reduce serum glucose. A significant difference in some serum biochemical results was seen; however, all values were within the normal range. Differences in serology are not considered evidence of toxicity in treated rats (Wolford et al., 1986; Han et al., 2010).
Overall, the rats administered CJPW displayed similar responses to rats administered distilled water. However, weight gain after extract administration was larger, and the high sugar content of the extract did not increase serum glucose. CJPW did not cause blood or serological abnormalities. Therefore, CJPW is considered nontoxic to animals, and its potential as a feed supplement that can help animals gain weight and control serum glucose was confirmed. These results suggest that CJPW can be used as a feed supplement, which may help to solve environmental problems and disposal costs caused by waste resources in Jeju Island. However, to apply CJPW as a feed supplement, follow-up animal studies are required to confirm weight gain, blood, and serological changes after feeding livestock (cow and pig).
ACKNOWLEDGEMENTS
This study was supported by the Industrialization of Organic Resources in Jeju Special Self-Governing Province and Brain Busan 21 Plus Project.
Footnotes
FUNDING
None.
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Concept and design: YHL, KSC. Analysis and interpreta-tion: YHL, YMH. Data collection: YHL, YMH. Writing the article: YHL. Critical revision of the article: KSC. Final approval of the article: all authors. Statistical analysis: YHL. Obtained funding: YHJ. Overall responsibility: KSC.
REFERENCES
- Ahn MS, Seo MS, Kim HJ. A study on the antioxidative and anti-microbial activities of the Citrus unshju peel extracts. Korean J Food Cult. 2007;22:454–461. [Google Scholar]
- Arias BA, Ramón-Laca L. Pharmacological properties of citrus and their ancient and medieval uses in the Mediterranean region. J Ethnopharmacol. 2005;97:89–95. doi: 10.1016/j.jep.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Ashraf H, Butt MS, Iqbal MJ, Suleria HAR. Citrus peel extract and powder attenuate hypercholesterolemia and hyperglycemia using rodent experimental modeling. Asian Pac J Trop Biomed. 2017;7:870–880. doi: 10.1016/j.apjtb.2017.09.012. [DOI] [Google Scholar]
- Bailey SA, Zidell RH, Perry RW. Relationships between organ weight and body/brain weight in the rat: what is the best analytical endpoint? Toxicol Pathol. 2004;32:448–466. doi: 10.1080/01926230490465874. [DOI] [PubMed] [Google Scholar]
- Bampidis VA, Robinson PH. Citrus by-products as ruminant feeds: a review. Anim Feed Sci Technol. 2006;128:175–217. doi: 10.1016/j.anifeedsci.2005.12.002. [DOI] [Google Scholar]
- Ferguson MA, Waikar SS. Established and emerging markers of kidney function. Clin Chem. 2012;58:680–689. doi: 10.1373/clinchem.2011.167494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-López J, Fernández-Ginés JM, Aleson-Carbonell L, Sendra E, Sayas-Barberá E, Pérez-Alvarez JA. Application of functional citrus by-products to meat products. Trends Food Sci Technol. 2004;15:176–185. doi: 10.1016/j.tifs.2003.08.007. [DOI] [Google Scholar]
- Han YD, Song SY, Lee JH, Lee DS, Yoon HC. Multienzyme-modified biosensing surface for the electrochemical analysis of aspartate transaminase and alanine transaminase in human plasma. Anal Bioanal Chem. 2011;400:797–805. doi: 10.1007/s00216-011-4797-6. [DOI] [PubMed] [Google Scholar]
- Han ZZ, Xu HD, Kim KH, Ahn TH, Bae JS, Lee JY, et al. Reference data of the main physiological parameters in control Sprague-Dawley rats from preclinical toxicity studies. Lab Anim Res. 2010;26:153–164. doi: 10.5625/lar.2010.26.2.153. [DOI] [Google Scholar]
- Jeong WS, Park SW, Chung SK. The antioxidative activity of Korean Citrus unshiu peels. Food Sci Biotechnol. 1997;6:292–296. [Google Scholar]
- Jeon HJ, Yu SN, Kim SH, Park SK, Choi HD, Kim KY, et al. Anti-obesity effect of citrus peel extract fermented with Aspergillus oryzae. J Life Sci. 2014;24:827–836. doi: 10.5352/JLS.2014.24.8.827. [DOI] [Google Scholar]
- Ji SC, Dai Q, Cho SH. Dietary substitution effect of Saccharina japonica with residues of citrus juice production in formulated diets on the growth, body composition and air exposure of juvenile abalone (Haliotis discus, Reeve 1846) Aquaculture. 2021;545:737165. doi: 10.1016/j.aquaculture.2021.737165. https://doi.org/10.1016/j.aquaculture.2021.737165. [DOI] [Google Scholar]
- Jung IC, Yang SJ, Moon YH. Feeding effects of citrus by-product TMR forage on the nutritional composition and palatability of Hanwoo loin. J Korean Soc Food Sci Nutr. 2007;36:578–583. doi: 10.3746/jkfn.2007.36.5.578. [DOI] [Google Scholar]
- Jwa MS, Yeo IK. Effects of dietary supplementation with citrus pomace and Ecklonia cava residue on the physiological changes and growth of disk abalone, Haliotis discus discus. J Fish Pathol. 2015;28:53–62. doi: 10.7847/jfp.2015.28.1.053. [DOI] [Google Scholar]
- Kang BH, Kim IH, Kim YB, Kim YH, Lee HS, Ha CS. Reference values of organ weights in Sprague-Dawley rats. Korean J Vet Pathol. 2001;5:39–42. [Google Scholar]
- Kang BH, Kim YB, Lee HS, Kim YH, Im WJ, Ha CS. Background data on hematology, blood biochemistry and organ weights for 2 weeks and 4 weeks repeated-dose toxicity studies using Sprague-Dawley (SD) rats. Korean J Lab Anim Sci. 2004;20:134–140. [Google Scholar]
- Karastergiou K, Fried SK, Xie H, Lee MJ, Divoux A, Rosencrantz MA, et al. Distinct developmental signatures of human abdom-inal and gluteal subcutaneous adipose tissue depots. J Clin Endocrinol Metab. 2013;98:362–371. doi: 10.1210/jc.2012-2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kautzky-Willer A, Harreiter J, Pacini G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr Rev. 2016;37:278–316. doi: 10.1210/er.2015-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi K, Mizuno T, Aida K, Uchino K. Hesperidin as an inhibitor of lipases from porcine pancreas and Pseudomonas. Biosci Biotechnol Biochem. 1997;61:102–104. doi: 10.1271/bbb.61.102. [DOI] [PubMed] [Google Scholar]
- Khan UM, Sameen A, Aadil RM, Shahid M, Sezen S, Zarrabi A, et al. Citrus genus and its waste utilization: a review on health-promoting activities and industrial application. Evid Based Complement Alternat Med. 2021;2021:2488804. doi: 10.1155/2021/2488804. https://doi.org/10.1155/2021/2488804. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kim HG, Lim HA, Kim SY, Kang SS, Lee HY, Yun PY. Develop-ment of functional Hanji added citrus peel (I)-Hanji added Korean citrus peel-. J Korea TAPPI. 2007;39:38–47. [Google Scholar]
- Kim JW, Jeon YJ, Lee JH, Lee SC. Effect of far-infrared irradiation and heat treatment on the antioxidant activity of extracts from citrus pomaces. J Korean Soc Appl Biol Chem. 2006;49:60–64. [Google Scholar]
- Kim K, Kim H, Lim H. Research on the dyeability and functional property of citrus peel extract as a natural dye. Res J Costume Cult. 2014;22:431–439. doi: 10.7741/rjcc.2014.22.3.431. [DOI] [Google Scholar]
- Lanza M, Fasone V, Galofaro V, Barbagallo D, Bella M, Pennisi P. Citrus pulp as an ingredient in ostrich diet: effects on meat quality. Meat Sci. 2004;68:269–275. doi: 10.1016/j.meatsci.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Lee CH, Yang MH, Park SR, Kang YJ. Major components of mushroom mycelia cultivated with citrus juice processing wastes. Korean J Food Sci Technol. 2007;39:128–132. [Google Scholar]
- Lee YH, Yeo MH, Yoon SA, Hyun HB, Ham YM, Jung YH, et al. Effects of Sargassum horneri and Ulva australis extracts on body weight and serum glucose levels of Sprague-Dawley rats. Prev Nutr Food Sci. 2021;26:307–314. doi: 10.3746/pnf.2021.26.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leporini M, Loizzo MR, Sicari V, Pellicanò TM, Reitano A, Dugay A, et al. Citrus × Clementina hort. Juice enriched with its by-products (peels and leaves): chemical composition, in vitro bioactivity, and impact of processing. Antioxidants. 2020;9:298. doi: 10.3390/antiox9040298. https://doi.org/10.3390/antiox9040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link JC, Reue K. Genetic basis for sex differences in obesity and lipid metabolism. Annu Rev Nutr. 2017;37:225–245. doi: 10.1146/annurev-nutr-071816-064827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud AM, Ashour MB, Abdel-Moneim A, Ahmed OM. Hesperidin and naringin attenuate hyperglycemia-mediated oxidative stress and proinflammatory cytokine production in high fat fed/streptozotocin-induced type 2 diabetic rats. J Diabetes Complications. 2012;26:483–490. doi: 10.1016/j.jdiacomp.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Mauvais-Jarvis F, Arnold AP, Reue K. A guide for the design of pre-clinical studies on sex differences in metabolism. Cell Metab. 2017;25:1216–1230. doi: 10.1016/j.cmet.2017.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiyanto E, Hermawan A, Anindyajati Natural products for cancer-targeted therapy: citrus flavonoids as potent chemopreventive agents. Asian Pac J Cancer Prev. 2012;13:427–436. doi: 10.7314/APJCP.2012.13.2.427. [DOI] [PubMed] [Google Scholar]
- Ministry of Government Legislation, author. Guidelines for toxicity study of pharmaceuticals. 2017. [cited 2017 Aug 30]. Available from: https://www.law.go.kr/admRulSc.do?menuId=5&subMenuId=41&tabMenuId=183&query=%EC%9D%98%EC%95%BD%ED%92%88%EB%93%B1%EC%9D%98%20%EB%8F%85%EC%84%B1%EC%8B%9C%ED%97%98%EA%B8%B0%EC%A4%80#liBgcolor0 .
- Moon MJ, Kam SK, Lee MG. Removal of Cu and Pb ions from aque-ous solution by waste citrus peel-based activated carbon. J Environ Sci Int. 2018;27:401–410. doi: 10.5322/JESI.2018.27.6.401. [DOI] [Google Scholar]
- Mouly PP, Arzouyan CR, Gaydou EM, Estienne JM. Differentiation of citrus juices by factorial discriminant analysis using liquid chromatography of flavanone glycosides. J Agric Food Chem. 1994;42:70–79. doi: 10.1021/jf00037a011. [DOI] [Google Scholar]
- Mukinda JT, Syce JA. Acute and chronic toxicity of the aqueous extract of Artemisia afra in rodents. J Ethnopharmacol. 2007;112:138–144. doi: 10.1016/j.jep.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Nawirska A, Kwaśniewska M. Dietary fibre fractions from fruit and vegetable processing waste. Food Chem. 2005;91:221–225. doi: 10.1016/j.foodchem.2003.10.005. [DOI] [Google Scholar]
- OECD, author. Test no. 407: repeated dose 28-day oral toxicity study in rodents. 2008. [cited 2021 Mar 9]. Available from: https://www.oecd-ilibrary.org/environment/test-no-407-repeated-dose-28-day-oral-toxicity-study-in-rodents_9789264070684-en .
- Okamura T, Suzuki S, Ogawa T, Kobayashi J, Kusuoka O, Hatayama K, et al. Background data for general toxicology parameters in RccHan:WIST rats at 8, 10, 19 and 32 weeks of age. J Toxicol Pathol. 2011;24:195–205. doi: 10.1293/tox.24.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdil B, Kece C, Cosar A, Akkiz H, Sandikci M. Potential benefits of combined N-acetylcysteine and ciprofloxacin therapy in partial biliary obstruction. J Clin Pharmacol. 2010;50:1414–1419. doi: 10.1177/0091270010361257. [DOI] [PubMed] [Google Scholar]
- Park H, Hwang YH, Choi JG, Ma JY. In vitro and in vivo evaluation of systemic and genetic toxicity of Citrus unshiu peel. J Ethnopharmacol. 2018;215:120–123. doi: 10.1016/j.jep.2017.12.029. [DOI] [PubMed] [Google Scholar]
- Petterino C, Argentino-Storino A. Clinical chemistry and haematology historical data in control Sprague-Dawley rats from pre-clinical toxicity studies. Exp Toxicol Pathol. 2006;57:213–219. doi: 10.1016/j.etp.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Rich-Edwards JW, Kaiser UB, Chen GL, Manson JE, Goldstein JM. Sex and gender differences research design for basic, clinical, and population studies: essentials for investigators. Endocr Rev. 2018;39:424–439. doi: 10.1210/er.2017-00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roohbakhsh A, Parhiz H, Soltani F, Rezaee R, Iranshahi M. Mo-lecular mechanisms behind the biological effects of hesperidin and hesperetin for the prevention of cancer and cardiovascular diseases. Life Sci. 2015;124:64–74. doi: 10.1016/j.lfs.2014.12.030. [DOI] [PubMed] [Google Scholar]
- Sohn JS, Kim MK. Effects of hesperidin and naringin on antioxi-dative capacity in the rat. Korean J Nutr. 1998;31:687–696. [Google Scholar]
- Song JW, Park SH, Lee CR, Lee KJ. Effects of dietary supplementation of a citrus by-product on growth performance, innate immunity and tolerance of low water temperature in red seabream Pagrus major. Korean J Fish Aquat Sci. 2013;46:399–406. doi: 10.5657/KFAS.2013.0399. [DOI] [Google Scholar]
- Tayyab N, Sayed RY, Faisal R, Wang W, Javeed AA, Mudassar A, et al. Dyeing and colour fastness of natural dye from Citrus aurantium on lyocell fabric. Ind Textila. 2020;71:350–356. doi: 10.35530/IT.071.04.1686. [DOI] [Google Scholar]
- Tower J, Pomatto LCD, Davies KJA. Sex differences in the response to oxidative and proteolytic stress. Redox Biol. 2020;31:101488. doi: 10.1016/j.redox.2020.101488. https://doi.org/10.1016/j.redox.2020.101488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramunt B, Smati S, Grandgeorge N, Lenfant F, Arnal JF, Montagner A, et al. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia. 2020;63:453–461. doi: 10.1007/s00125-019-05040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolford ST, Schroer RA, Gohs FX, Gallo PP, Brodeck M, Falk HB, et al. Reference range data base for serum chemistry and hematology values in laboratory animals. J Toxicol Environ Health. 1986;18:161–188. doi: 10.1080/15287398609530859. [DOI] [PubMed] [Google Scholar]
- Yang SJ, Jung IC, Moon YH. Effects of feeding citrus by products on nutritional components of Korean native chickens. J Life Sci. 2008;18:1369–1376. doi: 10.5352/JLS.2008.18.10.1369. [DOI] [Google Scholar]
- Yang SJ, Kang CH, Yang JB, Jung IC, Moon YH. Effect of feeding dietary tangerine byproduct for a long time on chemical compositions of loin for crossbred pig. J East Asian Soc Diet Life. 2006;16:186–191. [Google Scholar]
- Yoo NH, Kwon Y, Chun HS, An KS, Kim HJ, Ryu HY, et al. Single oral dose toxicity test and four weeks repeated oral dose de-termination test of Oplopanax elatus (Nakai) Nakai hydrothermal extract powder in Sprague-Dawley rats. Korean J Pharmacogn. 2019;50:205–218. [Google Scholar]