Abstract
Withania somnifera (ashwagandha) has been used traditionally as a remedy for insomnia and to enhance cognitive function. The effects of ashwagandha extract (AE, 35% withanolide glycosides, ShodenⓇ) on the expression levels of γ-aminobutyric acid (GABA)Aρ1 and histamine H3 receptors in Rattus norvegicus glioblastoma (C6) cell lines were studied using semiquantitative reverse transcriptase-polymerase chain reactions. The effects of AE on sleep onset and duration were studied in Swiss albino mice using the pentobarbital-induced sleep model. Furthermore, the effects on nonrapid eye movement (NREM) and rapid eye movement sleep patterns were studied in Wistar rats with electroencephalogram (EEG) to support the improvement in sleep quality. There was an increase in gene expression levels of GABAAρ1 receptor (1.38 and 1.94 folds) and histamine H3 (1.14 and 1.29 folds) receptors induced by AE at doses of 15 and 30 μg/mL compared to control. AE at doses of 10, 25, and 50 mg/kg body weight showed a significant decrease in time to sleep onset and increased total sleep duration in the pentobarbital-induced sleep model. At 50 mg/kg body weight dosage level, a 34% decrease (P<0.0001) in sleep onset time and 47% increase (P<0.0001) in sleep duration was observed. The EEG study showed significant improvement in alpha, beta, theta, delta, and gamma bands at doses of 10, 25, and 50 mg/kg body weight with delta waves showing increases of 30%, 46% (P<0.05), and 34%, respectively. The induction of sleep, GABA-mimetic action, NREM sleep, and the effects on slow-wave cycles support the calming property of AE in improving the quality of sleep.
Keywords: ashwagandha, brain waves, GABA mimetic, sleep, withanolide glycosides
INTRODUCTION
Sleep is a resting state of the body involving behavioral, physiological, and electrophysiological parameters. By recording the electrical activity of the brain (electroencephalogram, EEG) during sleep, it has been revealed that sleep is an active process that is tightly regulated. The necessity of sleep is also regulated and it depends on how long we stay awake. Moreover, the longer we stay awake, most times, the more intense our sleep becomes. Furthermore, the quantitative analysis of the sleep EEG resulted in the discovery of a slow-wave activity as a marker of sleep intensity, which closely reflects the homeostatic regulation of sleep.
The awake state is heterogeneous, being characterized by desynchronized EEG oscillations of low amplitude and mixed frequencies, and variable amounts of muscle activity. Active or motivated wakefulness is rich in theta (4∼9 Hz) and gamma (40∼300 Hz) EEG frequency ranges, whereas quiet wakefulness is characterized by slower EEG frequencies, including alpha (7∼15 Hz) and beta (8∼30 Hz). Using EEG and electromyogram recordings, it is possible to recognize two distinct sleep states: nonrapid eye movement (NREM) and rapid eye movement (REM)-that alternate cyclically across sleep. NREM sleep is characterized by high-amplitude low-frequency delta oscillations (0.5∼4.0 Hz) and spindles (bursts of 7∼15 Hz oscillations) in the EEG and low postural muscle tone. The EEG during REM sleep is dominated by theta and gamma oscillations, with a complete loss of muscle tone in axial postural muscles (REM muscle atonia) (Eban-Rothschild et al., 2018). Taken together, these observations have led to the aphorism that NREM sleep is characterized by an inactive brain in an active body, whereas REM sleep is characterized by an active brain in an inactive body (Purves et al., 2001).
Histamine is a neurotransmitter that is responsible for maintaining the awake state and is primarily produced in the tuberomammillary nucleus. It releases histamine, which binds to extrasynaptic receptors, by volume transmission. Histamine neurons also release the inhibitory neurotransmitter γ-aminobutyric acid (GABA). Several preclinical research reports have demonstrated that histamine, acting via H1 and H3 receptors, is essential for normal sleep/wake behavior. Traditionally, GABA has been viewed as a sleep-inducing neurotransmitter of which the most sleep-promoting populations are GABAergic.
Ashwagandha (Withania somnifera) is an annual evergreen shrub that has been widely studied for its adaptogenic properties. The present study was based on in vitro analyses to ascertain the hypothesis that ashwagandha extract (AE) increases the expression levels of GABA and histamine receptors in treated cells when compared to control cells, in a dose-dependent manner. Pentobarbital sleep-induction model with Swiss albino mice and diazepam positive control along with EEG brain wave were studied to confirm that hypothesis.
Scientifically, the activation of GABA and an increased endogenous histamine level are known to improve sleep. However, since in vitro efficacy on GABA and histamine may not translate to improved sleep in in vivo models due to unclear or multiple target sites and dependency on dose and duration of exposure, suitable animal models are warranted. Even though pentobarbital-induced sleep tests can effectively test sleep induction, a multidisciplinary approach incorporating neuronal activity by EEG gives a complete evaluation of sleep intensity (depth) and efficiency. The present study is an attempt to test the effects of AE on GABAAρ1/histamine H3 receptors and EEG changes in the rat brain and to hypothesize its effect on improving duration and depth of sleep.
MATERIALS AND METHODS
Materials
The study used 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), phosphate-buffered saline (PBS), Dulbecco’s modified Eagle’s medium (DMEM)-high glucose (HG), trypsin, antibiotics (penicillin, streptomycin, and amphotericin B), and picric acid were bought from HiMedia Laboratories, Pvt., Ltd. (Mumbai, India). Others are dimethyl sulfoxide (DMSO) (Finar Chemicals Pvt., Ltd., Ahmedabad, India), RNAIso (Takara Bio India Pvt., Ltd., New Delhi, India), reverse transcriptase (Thermo Fisher Scientific, Waltham, MA, USA), primers (Eurofins Scientific India Pvt., Ltd., Bangalore, India), polymerase chain reaction (PCR) master mix (Genei Laboratories Pvt., Ltd., Bangalore, India), carboxymethyl cellulose sodium (CMC) (SD Fine Chem Ltd., Mumbai, India), automated microplate reader (BioTek Instruments, Inc., Winooski, VT, USA), thermal cycler (Bio-Rad Laboratories, Hercules, CA, USA), gel electrophoresis unit (Chromous Biotech, Bengaluru, India), gel documentation system (Syngene Ingenius, Haryana, India), marathon M4 micromotor handpiece (SDE-H37L1 SMT, Saeyang Microtech Co., Ltd., Daegu, Korea), and dental acrylic resin (No. PSC10, Pyrax Polymars, Roorkee, India).
Cell line and cell culture
The C6 rat glioma-established cell line was procured from the National Center for Cell Sciences, Pune, India (Cat. No: NCCS/1911/2019-20; fibroblast; Rattus norvegicus sp. brain origin). The cell line was pretested for mycoplasma at the time of purchase. Furthermore, the stock cells were cultured in DMEM-HG supplemented with 10% inactivated fetal bovine serum (FBS), penicillin (100 IU/mL), streptomycin (100 μg/mL), and amphotericin B (5 μg/mL) in a humidified atmosphere of 5% CO2 at 37°C until confluent. All the aseptic techniques for handling and maintenance were followed while carrying out the experiments. The cells were dissociated with trypsin-ethylenediaminetetraacetic acid (EDTA) solution 1× (0.25% trypsin, 0.02% EDTA, Cat. No.: TCL-152, HiMedia Laboratories, Pvt., Ltd.). The seed cultures were grown in 25 cm2 culture flasks with a population density of 4,500 cells/cm2 and in their logarithmic growth phase. All experiments were carried out in 96 well microtiter plates. The subculture frequency was at confluency, the split ratio was 1:3, and the passage range was from 45 to 65.
Preparation of ashwagandha extract
The herb, ashwagandha (W. somnifera), was visually identified by a qualified botanist and a voucher specimen was kept with the herbarium ID: HERB-ED-22. Dried roots and leaves of ashwagandha were extracted using ethanol and water (70:30, v/v) and withanolide glycosides (WGs) were enriched using a proprietary process. The final hygroscopic pale yellow colored extract containing 35% WG was used as the test substance.
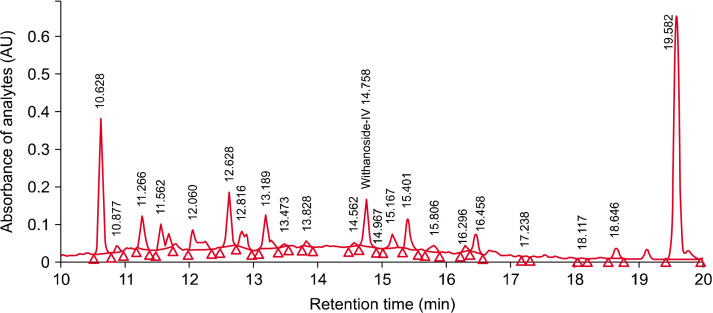
The analytical standardization procedures were carried out using high-performance liquid chromatography (HPLC) with photodiode array detector (Waters Corp., Milford, MA, USA) and C18 column of 250 mm×4.6 mm with 5-μ particle size. The procedure used a 40-min gradient solvent system with mobile phases of orthophosphoric acid in water and acetonitrile with a flow rate of 1.5 mL/min, column temperature was 25°C, injection volume of 20 μL, and a detection wavelength of 227 nm (Fig. 1). The method was validated for specificity, linearity, and repeatability with limit of detection 5 μg/L and limit of quantitation 10 μg/L. The quantitative determination of total WG was carried out using the U.S. Pharmacopeia reference standard of withanoside IV (CAS number 362472-81-9) by the HPLC method (Antony et al., 2020).
Fig. 1.
The high performance liquid chromatogram of ashwagandha extract.
Preparation of test and control samples for in vitro, in vivo, and EEG studies
For cytotoxicity studies, 10 mg (w/v) of AE was dissolved in DMEM with 2% inactivated FBS and volume was made up with DMEM to obtain a stock solution with a concentration of 10 mg/mL and sterilized by 0.22-μ syringe filtration. Serial two-fold dilutions were prepared from this solution to carry out cytotoxic studies (1,000, 500, 250, 125, 62.5, and 31.25 μg/mL). The negative control contained only cells cultured with growth media, for the positive control, GABA (1 μg/mL) was added to cell culture.
The treatment groups for the pentobarbital sleep-induction study received AE dissolved in 0.5% CMC at the dose levels of 5, 25, and 50 mg/kg body weight. The positive control was treated with diazepam (1 mg/kg body weight), while the negative control was treated with vehicle (0.5% CMC). Thirty minutes later, pentobarbital at 40 mg/kg body weight was orally administered by gavage daily to each mouse to induce sleep.
For the EEG study after the acclimatization, 18 animals were randomly divided into 3 groups comprising of six animals in each group. The animals were fasted overnight and were treated with 10 mg/kg of AE for groups I, II, and III with a dose mg/bodyweight of 10, 25, and 50, respectively.
Cytotoxicity study
The monolayer cell culture was trypsinized and the cell count was adjusted to 100,000 cells/mL using a medium containing 10% FBS. The diluted cell suspension (0.1 mL) was added to each well of 96 well microtiter plates. After 24 h of culture, when a partial monolayer was formed, the supernatant was flicked off and washed the monolayer once with the medium. Afterward, 100 μL of different test concentrations of the test substance was added onto the partial monolayer in microtiter plates. The plates were then incubated at 37°C for 3 days in 5% CO2 atmosphere and microscopic examination was carried out, while observations were noted at every 24 h interval. After 72 h, the drug solutions in the wells were discarded and 50 μL of MTT in PBS was added to each well. The plates were gently shaken and incubated for 3 h at 37°C in 5% CO2 atmosphere. The supernatant was removed, then 100 μL of the prepared DMSO solution was added and the plates were gently shaken to solubilize the formed formazan. The absorbance was measured using a microplate reader at a wavelength of 540 nm. The percentage growth inhibition was calculated and the concentration of test drug needed to inhibit cell growth by 50% (CTC50) values was generated from the dose-response curves for each cell line.
RNA isolation and cDNA synthesis
The trypsinized monolayer treated with the test substance was subjected to cell lysis by treating with TRI ReagentⓇ solution (Takara Bio Inc., Kusatsu, Japan). Chloroform was added to isolate the total RNA component from the samples and subjected to centrifugation. Out of the three distinct layers observed, the upper layer was collected in a fresh tube and an equal volume of isopropanol was added and incubated at −20°C for 10 min. Subsequently, an appropriate volume of ethanol was added to resuspend the pellet. After incubation and centrifugation, the pellet was air-dried and an appropriate volume of Tris-acetate-EDTA buffer was added (Takara Bio Inc.).
Amplification conditions
The thermocycler was configured to operate at 95°C for 5 min followed by 35 cycles of denaturation at 95°C for 30 s, annealing Tm for 30 s, and extension at 72°C for 45 s. This was followed by a final extension at 72°C for 10 min. The primers are specific for GABAAρ1 receptor and histamine H3-receptor (Table 1).
Table 1.
Primers for amplification
| Receptor | Strand | Primer (5’→3’) | Product size (bp) | |
|---|---|---|---|---|
| GABAAρ1 | Strand I synthesis | Oligo dT primer | 239 | |
| Strand II synthesis | Forward | TACAGCAGTACAGGCTGGTA | ||
| Reverse | TTGTAGGAGACGCGCGGCAT | |||
| Histamine H3 | Strand I synthesis | Oligo dT primer | 283 | |
| Strand II synthesis | Forward | TACAGTAGCTCTTCAGTCTG | ||
| Reverse | CCATCAGCATCTTCAGCATC | |||
The isolated total RNA was further used for the synthesis of cDNA molecules. These molecules were synthesized by priming with oligo (dT) primers followed by reverse transcriptase enzyme treatment according to the manufacturer’s protocol (Bio-Rad Laboratories, Hercules, CA, USA). The cDNA, thus, synthesized was used for PCR amplification of GABAAρ1, histamine receptor genes, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH, internal control).
Reverse transcriptase (RT)-PCR procedure
The mRNA expression levels of GABAAρ1 and histamine H3 receptors were determined using a semiquantitative RT-PCR. For the amplification of GABAAρ1 and histamine H3 receptor genes, 50 μL of the reaction mixture was subjected to PCR. The test cDNAs and the internal control, GAPDH (a housekeeping gene).
Gel electrophoresis and documentation
For the electrophoretic procedure, 1.5% agarose gels were prepared. After loading the PCR products, electrophoresis was carried out at 100 V. Visualization and image acquisition were carried out in a gel documentation system assisted with compatible software. The gel documentation system used from Syngene Bio Imaging (GeneSnap version 6.03, Syngene imaging software, Synoptics Ltd., Cambridge, UK).
Animal housing, care, and ethical treatment
Animal experiments were conducted in accordance with the guidelines of the Committee for the Purpose of Control and Supervision of Experiment on Animals and the study was approved by the Institutional Animal Ethics Committee of Jayamukhi College of Pharmacy, Telangana, India (JCP/IAEC/2021/01 and the date of approval is 1st March, 2021). Animals were housed in a standard stainless-steel cage [12×12×11 (inches)] having facilities for pelleted food and drinking water at a temperature of 22±3°C, relative humidity of 30% to 70%, 12-h light/dark cycle.
Pentobarbital sleep-induction study
Swiss albino male mice (in-house bred) weighing 25 to 30 g were selected for the study. All procedures involving animals were conducted humanely and were performed by or under the supervision of trained or experienced personnel.
The animals were randomized into five groups consisting of eight animals each. Each animal was given a random number and marked with picric acid. Stratified balanced randomization with sex as a trait using the software WinPepi version 11 (Abramson, 2011) was carried out by an independent statistician. Each cage had a maximum of three animals and was numbered distinctly to identify the groups. Emphatically, the randomization and allocation of animals to groups was carried out by a person who did not participate in the study. The test products were identified only by alphanumeric codes. The treatments were administered by the study veterinarian and the study outcomes were recorded by the study scientist. Group I served as the negative control; being treated with 0.5% CMC; Group II was the positive control; being treated with diazepam (1 mg/kg body weight) via intraperitoneal (IP) route, groups III, IV, and V were the treatment groups. All the groups except the negative and positive control groups were treated with test substances.
The treatment groups received a single acute dose of test substances at the dose levels of 5, 25, and 50 mg/kg body weight and the positive control was treated with diazepam (1 mg/kg body weight), while the negative control was treated with the vehicle (i.e., 0.5% CMC). Thirty minutes later, 40 mg/kg body weight of pentobarbital was administered to each mouse to induce sleep. The onset time of sleep was recorded for all the animals and the time when the animals show no mobility is taken as the time of onset of sleep as mentioned by Kobayashi et al. (2016). After the induction of sleep, mice were placed in the inverted position and when sedation was over, the time point when each mouse returned to its normal posture was noted. The time interval between dosing and the start of sleep was recorded as latency time.
EEG study
In-house bred albino Wistar rats of both sexes with a bodyweight of 195 to 225 g were selected. The rats were randomized using the randomization function in Graphpad.com. The randomization was done by an independent statistician and six rats were allocated to three groups with each cage having a maximum of three rats. Each rat served as its own control. The feeding and administration of treatments were carried out by the study veterinarian. Furthermore, the EEG recording and observations were made by the principal investigator.
A fine cut was made on the cleanly shaved and sterilized scalp of the rats using a scalpel blade from the midline and the scalp was partially removed. This helped to locate the periosteal connective tissue which was adhering to the bone and was scrapped using the blunt edge of a scalpel blade. This was to ensure that the bleeding from the capillaries was under control. Then the bone surface was disinfected with hydrogen peroxide to make the cranial suture visible. At this point, the bregma and lambda areas were noted in the skull. Electrode implantation was then carried out with respect to bregma by marking with a pencil or marker pen on the surface of the skull. Stainless-steel screws of 2 mm diameter and 1 mm head size were fixed on the frontal [anterior-posterior (AP): 2.0 mm; medial-lateral (ML): +2.00 mm and −2.00 mm, from bregma] and parietal region (AP: −2.00 mm; ML: +2.00 mm and −2.00 mm, from bregma) and fixed to the bones to record EEG from frontal and parietal cortices. The screw fixed laterally to the midline of the nasal bone acted as the ground electrode. Three wires of 100 nm thickness were fixed to the nine-pin connector and the screws by soldering and fixing with dental acrylic resin. Any extra incision on the rat head was closed by suturing. Holes in the skull were washed with sterile saline by using an injection syringe. All of these operations were carried out while the animal was in a stereotaxic instrument.
Anesthesia was achieved using ketamine (72 mg/kg, IP) and xylazine (8.0 mg/kg, IP). To prevent any respiratory distress, atropine (8 mg/kg, IP) was given. Iodine-povidone solution was applied to control possible postoperative infections. After removing the stereotaxic instrument, the animals were injected with dexamethasone (1.5 mg/kg) to reduce brain inflammation. In addition, the iodine-povidone solution was applied and steroids were injected for 4 to 5 days to recover completely from the operative procedures. For the first three days after surgery animals were given a liquid diet twice a day using a syringe. After 7 to 9 days they were ready for experimentation.
Before starting the experiment, animals were made to acclimatize to a wooden EEG recording chamber [12×12×11 (inches)]. The rats were conscious throughout the study. The frequency bands used for the analysis were <4 Hz (delta band), 4 to 8 Hz (theta band), 8 to 14 Hz (alpha band), 14 to 30 Hz (beta band), and >30 Hz (gamma band). The brain activity was recorded at the baseline with the vehicle before the test extract was administered. Actively moving rats generated many artifacts during the EEG recording time. This was the very reason considered to record and to retain the wires with electrode data collected for only 20 to 30 min after the administration of AE. For the baseline, only the vehicle was used (i.e., 1% tragacanth suspension) to record various waves between 9 a.m. to 2 p.m. Indian Standard Time through a two-channel Biopac MP45 data acquisition unit and processed with the use of software AcqKnowledge 4.4 (Biopac Systems Inc., Goleta, CA, USA) by using high pass 0.5 Hz and low pass 100 Hz filters, with signals processed at 200 Hz (200 samples/s) sampling rate. All five bands of EEG were manually selected for 4 s epoch employing standard criteria for rats; this was executed in the offline mode. EEG spectral of 0 to 100 Hz was accessed by using fast Fourier transforms.
Baseline signals were recorded for 10 min and at the end of the recordings, AEs were administered and recording of the EEG was initiated after 45 min and EEG was recorded for the next 15 min. The spectrogram of EEG signals was calculated using the parameter: run between 0.5 and 100 Hz with channel sampling rate at 200 samples/s. The components of the analysis, included gamma, delta, theta, beta, and alpha power.
Statistical analysis
Fold change was calculated for the receptor expression study based on the band intensity. Since a semiquantitative PCR technique was used, statistical tests were not performed for the study on receptor gene expression. The outcome measures studied in the pentobarbital sleep-induction experiment were sleep latency and sleep duration. The time when the animal lost righting reflex was considered as the onset of sleep and the time when the animal became mobile was considered as the wake-up time. The data were analyzed by one-way ANOVA followed by the Dunnet test with P<0.05 considered as being significant. The values were expressed in mean±standard deviation or standard error of mean. Statistical analysis was carried out using PROC MIXED procedure in SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) by considering duration and onset time as independent variables and treatment as dependent variable. Bonferroni test was used for multiple comparisons of the columns.
The studied outcome measures in the electrophysiological experiments were a change in the EEG signals of the alpha, beta, gamma, theta, and delta bands. The Wilcoxon test was applied to determine probability in power spectra of each frequency band and analyzed separately. Statistical calculations were carried out using Graphpad Prism (version 5, GraphPad Software, San Diego, CA, USA). EEG data were analyzed using paired t-test and the value P<0.05 was considered as a significant value.
RESULTS
In vitro gene expression study
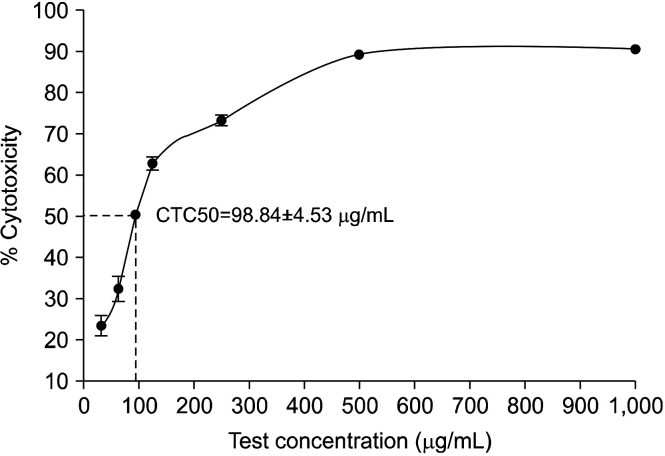
Cytotoxicity study: The CTC50 (median cytotoxicity inhibition) was found to be 98.84±4.53 μg/mL. Thus, test sample concentrations of 15 and 30 μg/mL were considered as consensus concentrations for the subsequent experiments (Fig. 2).
Fig. 2.
Cytotoxic inhibition (%) of ashwagandha extract on C6 cell line by MTT assay. CTC50, median cytotoxicity inhibition; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
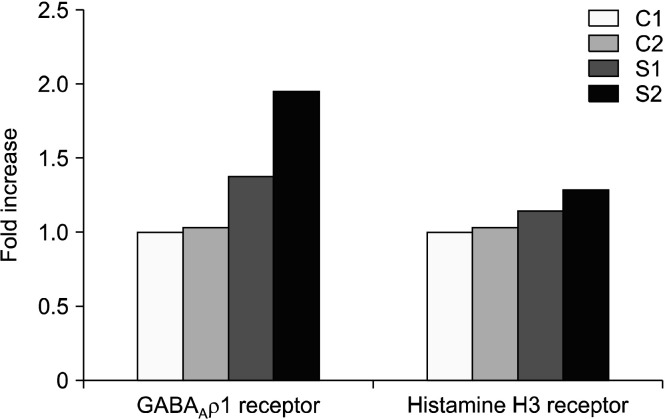
GABA and histamine receptor gene expression: The quantifications of gene expression for both of GABAAρ1 and histamine receptors were expressed as fold increase, such that the control cells had a value of 1.00 fold for both GABAAρ1 and histamine receptor expression. The positive control GABA (1 μg/mL) had a value of 1.03 fold for GABAAρ1 receptor, while 1.01 fold increase for the histamine H3 receptor. AE at 15 and 30 μg/mL had an increase of 1.38 and 1.94 folds, respectively for GABAAρ1 receptor with an increase of 1.14 and 1.29 folds, respectively for histamine H3 receptor (Fig. 3).
Fig. 3.
Semiquantitative gene expression level of GABAAρ1 and histamine H3 receptors for fold increase, the relative level of GABA and histamine gene expression is normalized to GAPDH. The result was calculated with software from Alpha view SA (version 3.3.1, Cell Biosciences Inc., Victoria, VIC, Australia). C1, negative control (cell control); C2, positive control GABA (1 mg/mL); S1, ashwagandha extract at 15 μg/mL; S2, ashwagan-dha extract at 30 μg/mL; GABA, γ-aminobutyric acid; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Pentobarbital sleep-induction study
The baseline body weight of mice (n=8) in group I was 27.19±0.19 g, group II (n=8) was 27.24±0.16 g, group III (n=8) was 27.28±0.24 g, group IV (n=8) was 27.43±0.19 g, and group V (n=8) was 27.28±0.18 g. There was a 9.4% decrease in onset to sleep with the low dose, 20.2% decrease with the mid-dose, 33.9% decrease with the high dose, and 52.7% decrease with diazepam when compared to the negative control. The duration of sleep increased by 11.9% with the low dose, 27.8% with the mid-dose, 46.9% with the high dose, and 70.5% with diazepam (data not shown). AE at low, mid-, and high doses significantly (P=0.002 for low dose and P<0.0001 for mid- and high) decreased the latency time to sleep than in negative control. The duration of sleep was significantly (P=0.002 for low dose and P<0.0001 for mid- and high) increased with AE at low, mid-, and high doses as compared to negative control (Table 2).
Table 2.
Effect of ashwagandha extract on the onset time to sleep and duration of sleep using the pentobarbital sleep-induction model
| Group | Onset time to sleep (latency time, min) | Duration of sleep (min) |
|---|---|---|
| Control | 53.13±1.62 | 59.75±1.11 |
| Positive control (diazepam) | 25.13±0.48* | 101.88±1.22* |
| Ashwagandha extract (low dose) | 48.13±0.55#§ | 66.88±1.09#§ |
| Ashwagandha extract (mid-dose) | 42.38±0.56*§† | 76.38±1.13*§‡ |
| Ashwagandha extract (high dose) | 35.13±0.35*§†∥ | 87.75±1.47*§†∥ |
Data are presented as mean±SEM.
*P<0.0001 and #P=0.002 compared with control.
§P<0.0001 compared with positive control.
†P<0.0001 and ‡P=0.0003 compared with ashwagandha low dose.
∥P<0.0001 compared with ashwagandha mid-dose.
In vivo EEG study
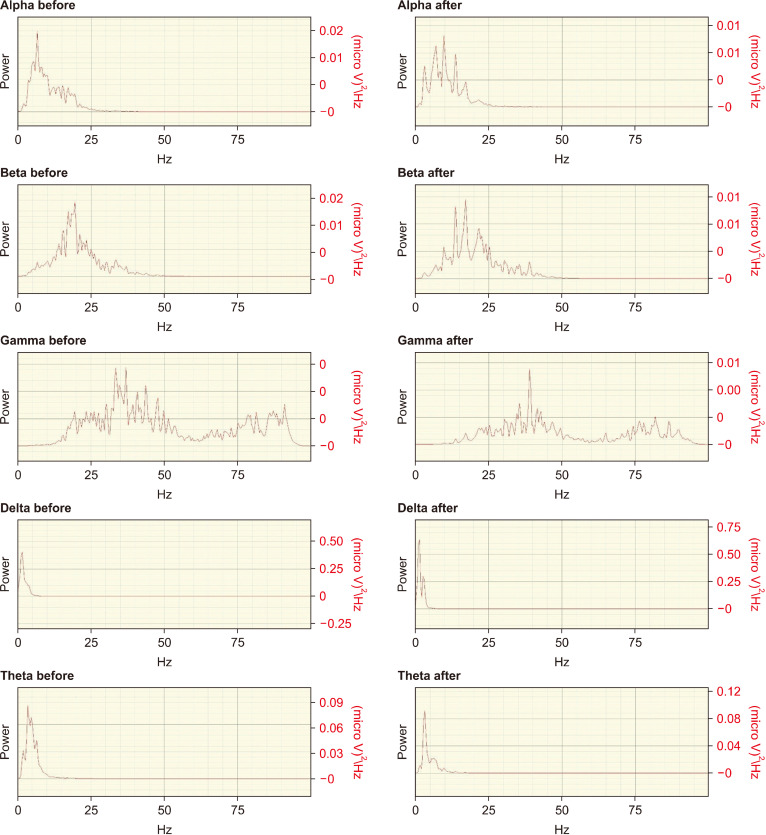
AE caused noticeable changes in the electrophysiological properties of the rat brain with all three doses of the extract. At a dose of 10 mg and 25 mg/kg body weight, there was a significant increase (29.6% and 45.9%, respectively, P<0.05) in the activity of delta band and an increase of 33.7% (P>0.05) with 50 mg/kg body weight (Fig. 4 and Table 3). The theta band was 2.5% in high dose, 7.0% in mid-, and 4.9% in low dose. Similarly, gamma-band activity was increased by 11.1% in low dose, 14.5% in mid-, and 4.5% in high dose. The beta band was significantly (P<0.05) increased by 13.8% in the low dose group and insignificantly (P>0.05) increased by 17.15 and 15.71 for mid- and high doses, respectively. Furthermore, the alpha band activity diminished physiologically in the low and mid-dose ranges (−65.5% and −66.8%), while at 50 mg/kg it slightly increased by 1.1% (Table 3).
Fig. 4.
Electroencephalograph of rats before and after dosing with ashwagandha extract (50 mg/kg body weight). Signal acquisition was carried out with BIOPAC MP-45 data acquisition unit and AcqKnowledge software (version 4.4, Biopac Systems, Inc.). Signals were filtered between 0.1 and 100.0 Hz and sampled at 200 Hz. The x-axis represents frequency in Hz, while the y-axis represents power in micro V2/Hz.
Table 3.
Quantitative electroencephalograph data of rats before and after dosing with ashwagandha extract
| Time point | EEG band | Power (micro V2/Hz) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| 50 mg/kg | 25 mg/kg | 10 mg/kg | ||||||||||
|
|
|
|
||||||||||
| Mean±SD | % Change | P-value | Mean±SD | % Change | P-value | Mean±SD | % Change | P-value | ||||
| Baseline value | Gamma | 4.67±3.91 | 4.49 | 0.5313 | 4.54±12.66 | 14.53 | 0.0625 | 4.40±5.65 | 11.13 | 0.0938 | ||
| Post treatment | Gamma | 4.88±5.08 | 5.20±5.91 | 4.89±4.79 | ||||||||
| Baseline value | Delta | 1.63±2.26 | 33.74 | 0.4468 | 2.60±3.35 | 29.61 | 0.0313* | 2.05±2.89 | 45.85 | 0.0313* | ||
| Post treatment | Delta | 2.18±1.86 | 3.37±4.08 | 2.99±3.45 | ||||||||
| Baseline value | Theta | 6.92±7.82 | 2.45 | 0.6427 | 6.73±6.45 | 6.98 | 0.1563 | 7.49±8.39 | 4.93 | 0.3125 | ||
| Post treatment | Theta | 7.09±7.50 | 7.20±7.02 | 7.86±8.60 | ||||||||
| Baseline value | Beta | 3.50±4.18 | 15.71 | 0.057 | 3.79±4.31 | 17.15 | 0.0625 | 3.92±4.52 | 13.77 | 0.0313* | ||
| Post treatment | Beta | 4.05±4.86 | 4.44±4.04 | 4.46±3.95 | ||||||||
| Baseline value | Alpha | 9.02±3.77 | 1.10 | 0.6898 | 8.56±8.61 | 66.83 | 0.0938 | 9.17±2.93 | 65.53 | 0.2188 | ||
| Post treatment | Alpha | 9.12±3.27 | 2.84±2.91 | 3.16±3.79 | ||||||||
Data extracted with AcqKnowledge software version 4.4 (Biopac Systems, Inc.).
*P<0.05 by Wilcoxon test.
EEG, electroencephalogram.
DISCUSSION
The GABA-mimetic activity of ashwagandha root extract has been shown for several decades (Mehta et al., 1991) with a recent study identifying that GABAAρ1 receptors showed 27 times more affinity for an aqueous W. somnifera than GABAA receptors. The GABAergic activity of the aqueous W. somnifera was not mediated by GABA as the root extract was not reported to possess a significant presence of GABA. Withaferin A and withanolide A did not activate GABAA nor GABAAρ1 receptors, suggesting that other constituent(s) in W. somnifera may be responsible for GABAA receptor-mediated responses (Candelario et al., 2015). Biochemical studies by Kulkarni and George (1996) suggested that the AE contained an ingredient, which had a GABA-mimetic activity. These GABA-mimetic and adaptogenic effects likely play a significant role in the long-standing history of using ashwagandha for the treatment of anxiety, neurobehavioral, and sleep disorders.
GABA is present in W. somnifera leaves (16.74 mg/g of the dry weight of W. somnifera aqueous extracts), while it is only detected in trace amounts in root aqueous extracts (Chatterjee et al., 2010). Aqueous and methanol extracts, both known to produce GABA-mimetic actions, suggest that at least one of the withanolide derivatives is responsible for the effects on GABA receptors (Candelario et al., 2015). WG which is present in AE at a high concentration might act through these sites. Thus, providing a better activity of GABA receptors leading to a sound, good, and enough sleep.
Recent studies have found that GABA acts on the extrasynaptic GABAA receptors and balances the excitatory effect of histamine (Abdurakhmanova et al., 2020). Hence, in this work, GABA was considered as a positive control, since we are studying the modulatory effect of the test substance on GABA and histamine receptors. The modulation by H3Rs of GABA released in the striatum has also been studied using electrophysiological methods. H3R activation presynaptically modulates the release of the main neurotransmitters, namely: glutamate and GABA, as well as the release of neuromodulators, dopamine and serotonin (5-hydroxytryptamine, 5-HT), and regulates the release of histamine. Histamine can modify the transport of GABA in synaptosomes and the modification depends on both the source of histamine (exogenous or endogenous) and its concentration.
Hitherto, five structurally related families of subunits of GABA receptor have been identified, viz: α, β, γ, δ, and ρ. The precise combination of protein subunits determines the physiological and pharmacological properties of a GABAA receptor (Lüddens et al., 1995; Stephenson 1995; Johnston, 1996). Coexpression studies have demonstrated that a channel with the properties of native receptors requires the combination of at least one of α, β, and γ subunits (Pritchett et al., 1989). While most native GABAA receptors are susceptible to modulation by barbiturates and neurosteroids, many are insensitive to benzodiazepines. Sensitivity to benzodiazepines requires the presence of the γ2 subunit (Pritchett et al., 1989). Furthermore, the pharmacological influence of benzodiazepines depends on the component type of α subunit.
The barbiturates, particularly pentobarbital, increase the Cl− channel opening duration at the GABAA receptor level. At higher concentrations, it directly opens GABAA receptor-associated chloride channels in the absence of GABA. Benzodiazepines binding at their specific site enhance the action of GABA at the GABAA receptor level by increasing the frequency of Cl− channel opening; however, with little effect on the channel opening time or channel conductance (Sieghart, 1995). Electrophysiological experiments have showed that sedative benzodiazepines are inactive at the GABAA receptor complex in the absence of GABA, but enhance the frequency of GABA-induced opening of the chloride channels (Study and Barker, 1981). In rats, pentobarbital shortens sleep latency, increases pre-REM sleep, a transitional state between NREM and REM sleep, and decreases REM sleep (Gottesmann et al., 1998; Lancel and Steiger, 1999). Again, in rats, after acute systemic administration of benzodiazepines, such as flurazepam, diazepam, triazolam, or midazolam, sleep latency decreases dose-dependently and increases NREM sleep and pre-REM sleep.
Upon activation by GABA or an appropriate analog (GABAA agonist), the membrane conductance for anions, mainly chloride ions, increases. Owing to the fact that the concentrations of chloride ions within the neurons are generally low, GABAergic transmission usually produces a slight, short-lasting hyperpolarization and, thus, reduced excitability of the recipient neuron. In addition to GABA recognition sites, GABAA receptors are endowed with several other binding sites, including those for barbiturates, benzodiazepines, and anesthetic steroids. The binding of an appropriate ligand induces a conformational change and thereby enhances (agonistic modulators) or attenuates (inverse agonistic modulators) GABA-evoked chloride ion flux or inhibits the action of agonistic modulators (antagonistic modulators). In most brain regions, GABAA receptors outnumber GABAB receptors (Young and Chu, 1990). Emphatically, GABAA receptors constitute the fast-acting ligand-gated anion channels.
Various steroid hormones modulate GABAA-mediated transmission, probably through unique steroid-binding sites that are distinct from those for barbiturates and benzodiazepines (Deutsch et al., 1992; Macdonald and Olsen, 1994; Olsen and Sapp, 1995; Sieghart, 1995; Rupprecht, 1997). At low nanomolar concentrations, these steroids augment GABA-induced GABAA receptor currents by increasing both the frequency and duration of the opening of chloride channels, equivalent to the activity of barbiturates and diazepam. Similar to barbiturates, at high concentrations, they are able to directly activate GABAA receptors in the absence of GABA (Callachan et al., 1987; Harrison et al., 1987; Peters et al., 1988). Since sleep duration was more for ashwagandha, it is likely that it activates more sites/subunits of the receptors. Considering that activation by diazepam depends on the presence of GABA, it is all the more likely that diazepam is not as effective as barbiturates if the production and metabolism of GABA do not favor this. Sleep-related processes are associated with the liberation of GABA in specific brain regions. Thus, an alternative and obvious explanation for the different effects of GABAA agonists and benzodiazepines is that the former stimulate GABAA receptors throughout the brain, while benzodiazepines potentiate the action of selectively released GABA on GABAA receptors (Lancel and Steiger, 1999).
Benzodiazepines are analgesic drugs that act through GABA neurotransmitter as natural sleep does. Many of them increase total sleep time and reduce sleep onset latency, while failing to improve the sleep quality (Stone, 1979) and reducing later stages of slow-wave sleep (SWS) (Livezey et al., 1985). It was purported that benzodiazepine increased only the lighter stage of SWS (SWS1), indicating that midazolam-induced anesthesia increased SWS1 by 158% (Radulovacki et al., 1984). Meanwhile, diazepam, another benzodiazepine-based drug, also increased SWS1 by 255% and reduced SWS latency by 92%. In addition, Wedzicha and colleagues (1988) stated that diazepam improved sleep duration in patients with chronic airflow obstruction. However, the usage potentially results in adverse effects, including hypothermia, tachycardia, hypertension, and partial memory impairment (Coenen and van Luijtelaar, 1997; Mailliet et al., 2001).
One of the most striking differences between wake and sleep is the EEG activity. During wakefulness the EEG is characterized by relatively low voltage, mixed-frequency activity, with alpha and beta (typically 18∼25 Hz) frequencies dominating (Avidan, 2005). During relaxed wakefulness with eye closure, alpha rhythm (8∼13 Hz) is very prominent, particularly in the occipital EEG derivations. For instance, in adults and older children, sleep onset begins with NREM sleep. Basically, NREM sleep is further divided into four stages: 1 to 4; AASM uses the terms N for NREM sleep stages and R for REM sleep stages. However, N1 and N2 are used instead of stages 1 and 2, while N3 is used to indicate the sum of stages 3 and 4 (often called SWS) which approximates a continuum of sleep depth. Stage 1 is the lightest and stage 4 the deepest sleep with arousal thresholds being correspondingly lowest in stage 1 and highest in stage 4 NREM sleep (Carskadon and Dement, 2011). Stage 1 NREM sleep is characterized by a low voltage mixed-frequency EEG background pattern with theta activity dominating. Stage 2 NREM sleep is characterized by two landmark EEG waveforms, namely: sleep spindles and K-complexes. Stages 3 and 4 NREM sleep are easily recognized by the appearance and predominance of slow, high-voltage delta waves (0.5∼2.0 Hz). When combined, stages 3 and 4 NREM sleep are termed SWS.
Despite the similarities in EEG recordings obtained in REM sleep and wakefulness, the two conditions are categorically not equivalent brain states. The EEG recording of REM sleep is very different from that of NREM sleep and it is characterized by low voltage, mixed-frequency (mostly theta) activity similar to that of wakefulness. Alpha activity is prominent in REM sleep, but is typically 1∼2 Hz slower than seen during wakefulness (Hori et al., 2001). The data from the study shows that power increases in all bands in a dose-dependent manner except in the alpha band which may be due to animals entering a state of torpor. This pattern is different from the EEG pattern of classical benzodiazepines which attenuates low-frequency EEG activity and suppresses REM sleep. EEG profile seems to be controlled by different neuronal circuits and unlike sedative drugs, ashwagandha intensifies the physiological pattern of sleep. The GABAA agonists are found to enhance low-frequency (<10 Hz) EEG activity during NREM sleep and high-frequency (>10 Hz) activity during REM sleep in a dose-related fashion. Ashwagandha being a GABAA agonist produces progressive hyperpolarization of membrane potential which switches the state of thalamocortical neurons from the “relay mode”, characteristic of wakefulness and REMS into a “spindle mode” and ultimately into a “delta mode”. Even though the power spectrum analysis cannot directly relate the duration of either NREM or REM sleep, the increase in the wave intensity as a result of progressive hyperpolarization signals a shorter time to sleep onset and a faster switch from REM to NREM sleep with a reduced NREM latency. The normal wake cycle demands a smooth transition back to REM sleep requiring a simultaneous increase in the intensity of all brain waves. This explains the increase in beta and gamma waves of REM sleep. Contrary to the above is the reduction in the alpha waves which is mostly due to the torpor state exhibited by animals during sleep. The total of the high power and high-intensity wave characteristics is defined as deep sleep in the experimental animals.
Analysis of the EEG recordings shows that rodents are mainly in NREM sleep and that REM sleep is reduced or not present (Kräuchi and de Boer, 2011). The passage into sleep is a transition from wakefulness into the stage called SWS. SWS and its characteristic electrical pattern of brain waves in the frequency range of 0.7 to 4.0 Hz is interrupted by periods of sleep characterized by REM sleep. Wakefulness, sleep, and torpor are likely not mutually exclusive states, but sleep and torpor are continuous and share important similarities in terms of their electrophysiological characteristics.
Mammalian sleep consists of periods of REM and non-REM sleep. In animals, like rats, the transition from NREM to REM sleep, called pre-REM sleep can easily be recognized by the occurrence of long-lasting, high-amplitude spindle-like EEG signals on a background of theta activity (Gottesmann, 1996). In contrast to NREM sleep, REM sleep is an activated brain state and it is associated with dreams. As awakening thresholds rise with the increased occurrence of slow waves (Blake and Gerard, 1937; Frederickson and Rechtschaffen, 1978; Grahnstedt and Ursin, 1980), spectral power in the slow-wave frequencies (slow-wave activity) is commonly used as an index for NREM sleep intensity. Since the recording of REMs is difficult in small animals, their REM sleep criteria include a combination of wake-like EEG with flat electromyogram indicating muscle atonia.
The predominant state of sleep, NREM sleep, is characterized by salient signals in the EEG, particularly spindles and slow waves. Spindles, which constitute the landmark of sleep onset, last from 0.5 to 2.0 s and are composed of sigma frequencies (11∼16 Hz) with waxing and waning amplitude. On the other hand, slow waves are high-amplitude waves in the delta frequency range (1∼4 Hz). It is accompanied by typical REMs, a very low muscle tone, and EEG signals that are reminiscent of those observed during wakefulness [low voltage, fast activity in primates, and regular theta activity (4∼9 Hz)] in rodents. Non-REM and REM sleep stages alternate, giving rise to NREM/REM cycles of about 90 min in adult humans and of a much shorter duration in smaller species (Borbély and Neuhaus, 1979; Feinberg and Floyd, 1979). Our results with AE revealed that the GABAA-selective compounds all shared a dose-dependent EEG spectral signature comprised of all band power elevation.
Sleep deprivation, being the most powerful method to promote sleep (Borbély et al., 1981), results in an increase of SWS and REM sleep. Sleep-EEG power increases in the lower frequency range and decreases in the higher frequency range. One should expect that ideal hypnotics share the effects of sleep deprivation (Steiger, 2010). However, after benzodiazepines and nonbenzodiazepine hypnotics as well, opposite changes of sleep EEG occur. The power of sleep EEG in the lower frequency range, as well as in the higher frequency range increases.
GABA is known to modulate the amplitude and frequency of gamma oscillations (Whittington et al., 2000b) and the frequency of gamma oscillations is controlled by the duration of individual inhibitory synaptic events. Additionally, its power is affected by the amplitude of the GABAA synaptic response. In fact, drugs that affect these parameters of inhibition include many sedative/hypnotic or general anesthetic drugs (Whittington et al., 2000b). Diazepam has been shown to predominantly increase the amplitude of gamma oscillations and decrease their frequency (Whittington et al., 2000a), but not to disrupt the synchrony or rhythmicity of gamma oscillations (Faulkner et al., 1998). Furthermore, a frequency reduction is seen for the hippocampal theta rhythm. Thus, GABA modulators, such as the drugs and AE used in this study, can increase gamma power and shift the frequency of gamma oscillations to lower frequencies in the beta range. This could actually represent modified gamma activity (Faulkner et al., 1999; Whittington et al., 2000b). GABAA receptor modulators increase gamma activity, however, by decreasing the gamma frequencies at the same time would cause an increase in beta activity.
Under drug-free conditions, EEG beta and gamma-band power elevation traditionally has been associated with increased arousal (Brown et al., 2012), as well as higher cognitive functions, such as working memory (Tallon-Baudry et al., 2004) and perception (Rodriguez et al., 1999). The EEG changes produced by benzodiazepine drugs, thus, present an apparent paradox in that EEG beta and gamma-band power enhancement is observed in the face of psycho-pharmacologically driven over sedation.
Beta-band power elevation is well-established for benzodiazepines at behaviorally relevant doses both preclinically and clinically. Similarly, gamma-band elevation in the 30 to 50 Hz range, although not as widely established in the literature, has been demonstrated with diazepam in rodent EEG (Krijzer et al., 1993; van Lier et al., 2004), and was readily detected here for lorazepam over the same dose range affecting beta-band activity. AE, used in this study, is in line with the conclusions already made with benzodiazepines.
Even though sleep is predicted to have a reduction of neuronal activity microelectrode studies have shown, however, that almost all units in the cortex, hypothalamus, hippocampus, and brain stem discharge more rapidly during sleep. During NREM sleep theta and delta waves are predominant. REM is likely an intermediary step between SWS and wakefulness that has waves similar to waking up. Categorically, REM is a more activated brain state than SWS. Without REM and in the absence of external stimulus, the brain might stay in Stage N3 coma continuously until starvation and dehydration cause death. REM may be a process for combating the activity disarray occurring in SWS by increasing ordered unit activity and promoting regional (and perhaps interfrequency) coherences. REM and dreaming could be a self-start mode to reconstruct and sustain consciousness after that capability has been wiped out in Stage IV (N3). In other words, REM is the brain’s way of “booting up” its consciousness in the absence of an “external trigger”. In REM, almost all neurons in all parts of the brain increase firing rate compared with SWS and often compared with alert wakefulness (Evarts, 1967; Hobson and McCarley, 1971). Since firing rate is generally considered indicative of biologically significant information processing, explains the need for energy increase in all bands. Drug-induced sleep/sedation increases only alpha and delta waves, but decreases beta waves.
The modulation by H3 receptors of GABA release in the striatum has been studied based on electrophysiological methods. H3R activation pre-synaptically modulates the release of the main neurotransmitters, glutamate, and GABA, as well as the release of the neuromodulators dopamine and 5-HT. The increased expression of GABA and histamine receptors in the current study could be taken as an indirect indication of an increase in GABA or GABA agonists (Márquez-Gómez et al., 2016). Direct measurement of GABA released into the postsynaptic nerve terminal along with knockout mice models could provide a better understanding of the interactions of GABA and histamine receptors with AE.
The available conventional therapies of insomnia are known to develop drug dependency and exert side effects. However, AE, with sleep-inducing potential, is well tolerated, improves sleep quality, and sleep onset latency. It could be a potential candidate for the treatment of insomnia.
ACKNOWLEDGEMENTS
The authors acknowledge in-kind support for ashwagandha extract (Shoden) from Arjuna Natural (P) Ltd., India.
Footnotes
FUNDING
This research was supported by the research program (No. JCP/2021/BR-1) of Jayamukhi College of Pharmacy, Telangana, India.
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Conceptualization: SVM. Methodology: SVM. Validation: SVM. Formal analysis: SVM. Investigation: SVM. Resources: SVM. Data curation: SNF. Writing-original draft preparation: SVM. Writing-review and editing: SNF, RM. Visualization: RM. Supervision: RM. Project administration: RM. Funding acquisition: SVM. All authors have read and agreed to the published version of the manuscript.
REFERENCES
- 1.Abdurakhmanova S, Grotell M, Kauhanen J, Linden AM, Korpi ER, Panula P. Increased sensitivity of mice lacking extrasynaptic δ-containing GABAA receptors to histamine receptor 3 antagonists. Front Pharmacol. 2020;11:594. doi: 10.3389/fphar.2020.00594. https://doi.org/10.3389/fphar.2020.00594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abramson JH. WINPEPI updated: computer programs for epidemiologists, and their teaching potential. Epidemiol Perspect Innov. 2011;8:1. doi: 10.1186/1742-5573-8-1. https://doi.org/10.1186/1742-5573-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antony B, Benny M, Kuruvilla BT, Sebastian A, Pillai AAA, Joseph B, et al. Development and validation of an RP-HPLC method for the simultaneous determination of total withanolide glycosides and Withaferin A in Withania somnifera (Ashwagandha) Curr Chromatogr. 2020;7:106–120. doi: 10.2174/2213240607999200813194349. [DOI] [Google Scholar]
- 4.Avidan AY. Recognition of sleep stages and adult scoring technique. Atlas Sleep Med. 2005;2005:95–121. doi: 10.1016/B978-0-7506-7398-3.50007-3. [DOI] [Google Scholar]
- 5.Blake H, Gerard RW. Brain potentials during sleep. Am J Physiol. 1937;119:692–703. doi: 10.1152/ajplegacy.1937.119.4.692. [DOI] [Google Scholar]
- 6.Borbély AA, Baumann F, Brandeis D, Strauch I, Lehmann D. Sleep deprivation: effect on sleep stages and EEG power density in man. Electroencephalogr Clin Neurophysiol. 1981;51:483–495. doi: 10.1016/0013-4694(81)90225-X. [DOI] [PubMed] [Google Scholar]
- 7.Borbély AA, Neuhaus HU. Sleep-deprivation: effects on sleep and EEG in the rat. J Comp Physiol. 1979;133:71–87. doi: 10.1007/BF00663111. [DOI] [Google Scholar]
- 8.Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Physiol Rev. 2012;92:1087–1187. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Callachan H, Cottrell GA, Hather NY, Lambert JJ, Nooney JM, Peters JA. Modulation of the GABAA receptor by progesterone metabolites. Proc R Soc Lond B Biol Sci. 1987;231:359–369. doi: 10.1098/rspb.1987.0049. [DOI] [PubMed] [Google Scholar]
- 10.Candelario M, Cuellar E, Reyes-Ruiz JM, Darabedian N, Feimeng Z, Miledi R, et al. Direct evidence for GABAergic activity of Withania somnifera on mammalian ionotropic GABAA and GABAr receptors. J Ethnopharmacol. 2015;171:264–272. doi: 10.1016/j.jep.2015.05.058. [DOI] [PubMed] [Google Scholar]
- 11.Carskadon MA, Dement WC. Normal human sleep: an overview. In: Kryger MH, Dement WC, Roth T, editors. Principles and Practice of Sleep Medicine. Elsevier, Inc.; Philadelphia, PA, USA: 2011. pp. 15–24. [DOI] [Google Scholar]
- 12.Chatterjee S, ivastava S, Sr, Khalid A, Singh N, Sangwan RS, Sidhu OP, et al. Comprehensive metabolic fingerprinting of Withania somnifera leaf and root extracts. Phytochemistry. 2010;71:1085–1094. doi: 10.1016/j.phytochem.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Coenen AM, van Luijtelaar EL. Effects of benzodiazepines, sleep and sleep deprivation on vigilance and memory. Acta Neurol Belg. 1997;97:123–129. [PubMed] [Google Scholar]
- 14.Deutsch SI, Mastropaolo J, Hitri A. GABA-active steroids: endogenous modulators of GABA-gated chloride ion conductance. Clin Neuropharmacol. 1992;15:352–364. doi: 10.1097/00002826-199210000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Eban-Rothschild A, Appelbaum L, de Lecea L. Neuronal mechanisms for sleep/wake regulation and modulatory drive. Neuropsychopharmacology. 2018;43:937–952. doi: 10.1038/npp.2017.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evarts EV. Unit activity in sleep and wakefulness. In: Quarton GC, Melnechuk T, Schmitt FO, editors. The Neurosciences. A Study Program. Rockefeller University Press; New York, NY, USA: 1967. pp. 545–556. [Google Scholar]
- 17.Faulkner HJ, Traub RD, Whittington MA. Anaesthetic/amnesic agents disrupt beta frequency oscillations associated with potentiation of excitatory synaptic potentials in the rat hippocampal slice. Br J Pharmacol. 1999;128:1813–1825. doi: 10.1038/sj.bjp.0702948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faulkner HJ, Traub RD, Whittington MA. Disruption of synchronous gamma oscillations in the rat hippocampal slice: a common mechanism of anaesthetic drug action. Br J Pharmacol. 1998;125:483–492. doi: 10.1038/sj.bjp.0702113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feinberg I, Floyd TC. Systematic trends across the night in human sleep cycles. Psychophysiology. 1979;16:283–291. doi: 10.1111/j.1469-8986.1979.tb02991.x. [DOI] [PubMed] [Google Scholar]
- 20.Frederickson CJ, Rechtschaffen A. Effects of sleep deprivation on awakening thresholds and sensory evoked potentials in the rat. Sleep. 1978;1:69–82. [PubMed] [Google Scholar]
- 21.Gottesmann C, Gandolfo G, Arnaud C, Gauthier P. The intermediate stage and paradoxical sleep in the rat: influence of three generations of hypnotics. Eur J Neurosci. 1998;10:409–414. doi: 10.1046/j.1460-9568.1998.00069.x. [DOI] [PubMed] [Google Scholar]
- 22.Gottesmann C. The transition from slow-wave sleep to paradoxical sleep: evolving facts and concepts of the neurophysiological processes underlying the intermediate stage of sleep. Neurosci Biobehav Rev. 1996;20:367–387. doi: 10.1016/0149-7634(95)00055-0. [DOI] [PubMed] [Google Scholar]
- 23.Grahnstedt S, Ursin R. Awakening thresholds for electrical brain stimulation in five sleep-waking stages in the cat. Electroencephalogr Clin Neurophysiol. 1980;48:222–229. doi: 10.1016/0013-4694(80)90307-7. [DOI] [PubMed] [Google Scholar]
- 24.Harrison NL, Majewska MD, Harrington JW, Barker JL. Structure-activity relationships for steroid interaction with the gamma-aminobutyric acidA receptor complex. J Pharmacol Exp Ther. 1987;241:346–353. [PubMed] [Google Scholar]
- 25.Hobson JA, McCarley RW. Cortical unit activity in sleep and waking. Electroencephalogr Clin Neurophysiol. 1971;30:97–112. doi: 10.1016/0013-4694(71)90271-9. [DOI] [PubMed] [Google Scholar]
- 26.Hori T, Sugita Y, Koga E, Shirakawa S, Inoue K, Uchida S, et al. Proposed supplements and amendments to 'A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects', the Rechtschaffen & Kales (1968) standard. Psychiatry Clin Neurosci. 2001;55:305–310. doi: 10.1046/j.1440-1819.2001.00810.x. [DOI] [PubMed] [Google Scholar]
- 27.Johnston GA. GABAA receptor pharmacology. Pharmacol Ther. 1996;69:173–198. doi: 10.1016/0163-7258(95)02043-8. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi Y, Takemoto H, Fua Z, Shimizu E, Kinjo Y. Enhancement of pentobarbital-induced sleep by the vaporized essential oil of Citrus keraji var. kabuchii and its characteristic component, γ-terpinene. Nat Prod Commun. 2016;11:1175–1178. doi: 10.1177/1934578X1601100836. [DOI] [PubMed] [Google Scholar]
- 29.Kräuchi K, de Boer T. Body temperatures, sleep, and hibernation. In: Kryger M, Dement WC, Roth T, editors. Principles and Practice of Sleep Medicine. Elsevier, Inc.; Philadelphia, PA, USA: 2011. pp. 323–334. [DOI] [Google Scholar]
- 30.Krijzer F, Koopman P, Olivier B. Classification of psychotropic drugs based on pharmaco-electrocorticographic studies in vigilance-controlled rats. Neuropsychobiology. 1993;28:122–137. doi: 10.1159/000119015. [DOI] [PubMed] [Google Scholar]
- 31.Kulkarni SK, George B. Anticonvulsant action of Withania somnifera (Aswaganda) root extract against pentylenetetrazol-induced kindling in mice. Phytother Res. 1996;10:447–449. doi: 10.1002/(SICI)1099-1573(199608)10:5<447::AID-PTR869>3.0.CO;2-M. [DOI] [Google Scholar]
- 32.Lancel M, Steiger A. Sleep and its modulation by drugs that affect GABAA receptor function. Angew Chem Int Ed Engl. 1999;38:2852–2864. doi: 10.1002/(SICI)1521-3773(19991004)38:19<2852::AID-ANIE2852>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 33.Livezey GT, Radulovacki M, Isaac L, Marczynski TJ. Prenatal exposure to diazepam results in enduring reductions in brain receptors and deep slow wave sleep. Brain Res. 1985;334:361–365. doi: 10.1016/0006-8993(85)90233-1. [DOI] [PubMed] [Google Scholar]
- 34.Lüddens H, Korpi ER, Seeburg PH. GABAA/benzodiazepine receptor heterogeneity: neurophysiological implications. Neuropharmacology. 1995;34:245–254. doi: 10.1016/0028-3908(94)00158-O. [DOI] [PubMed] [Google Scholar]
- 35.Macdonald RL, Olsen RW. GABAA receptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- 36.Mailliet F, Galloux P, Poisson D. Comparative effects of melatonin, zolpidem and diazepam on sleep, body temperature, blood pressure and heart rate measured by radiotelemetry in Wistar rats. Psychopharmacology. 2001;156:417–426. doi: 10.1007/s002130100769. [DOI] [PubMed] [Google Scholar]
- 37.Márquez-Gómez R, García-Gálvez AM, Morales-Figueroa GE, Arias-Montaño JA. Modulation by histamine H3 receptors of neurotransmitter release in the basal ganglia. In: Blandina P, Passani MB, editors. Histamine Receptors. Humana Press; Totowa, NJ, USA: 2016. pp. 265–293. [DOI] [Google Scholar]
- 38.Mehta AK, Binkley P, Gandhi SS, Ticku MK. Pharmacological effects of Withania somnifera root extract on GABAA receptor complex. Indian J Med Res. 1991;94:312–315. [PubMed] [Google Scholar]
- 39.Peters JA, Kirkness EF, Callachan H, Lambert JJ, Turner AJ. Modulation of the GABAA receptor by depressant barbiturates and pregnane steroids. Br J Pharmacol. 1988;94:1257–1269. doi: 10.1111/j.1476-5381.1988.tb11646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pritchett DB, Sontheimer H, Shivers BD, Ymer S, Kettenmann H, Schofield PR, et al. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature. 1989;338:582–585. doi: 10.1038/338582a0. [DOI] [PubMed] [Google Scholar]
- 41.Purves D, Augustine GJ, Fitzpatrick D, Katz LC, LaMantia AS, McNamara JO, et al. Neuroscience. 3rd ed. Sinauer Associates; Sunderland, MA, USA: 2001. p. 671. [Google Scholar]
- 42.Olsen RW, Sapp DW. Neuroactive steroid modulation of GABAA receptors. Adv Biochem Psychopharmacol. 1995;48:57–74. [PubMed] [Google Scholar]
- 43.Radulovacki M, eckovic G, Sr, Zak R, Zahrebelski G. Diazepam and midazolam increase light slow-wave sleep (SWS1) and decrease wakefulness in rats. Brain Res. 1984;303:194–196. doi: 10.1016/0006-8993(84)90229-4. [DOI] [PubMed] [Google Scholar]
- 44.Rodriguez E, George N, Lachaux JP, Martinerie J, Renault B, Varela FJ. Perception's shadow: long-distance synchronization of human brain activity. Nature. 1999;397:430–433. doi: 10.1038/17120. [DOI] [PubMed] [Google Scholar]
- 45.Rupprecht R. The neuropsychopharmacological potential of neuroactive steroids. J Psychiatr Res. 1997;31:297–314. doi: 10.1016/S0022-3956(96)00060-X. [DOI] [PubMed] [Google Scholar]
- 46.Sieghart W. Structure and pharmacology of gamma-aminobutyric acidA receptor subtypes. Pharmacol Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- 47.Steiger A. Sleep and its modulation by substances that affect GABAA receptor function. In: Monti JM, Pandi-Perumal SR, Möhler H, editors. GABA and Sleep: Molecular, Functional and Clinical Aspects. Springer; New York, NY, USA: 2010. pp. 121–146. [DOI] [Google Scholar]
- 48.Stephenson FA. The GABAA receptors. Biochem J. 1995;310:1–9. doi: 10.1042/bj3100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stone BM. Diazepam and its hydroxylated metabolites: studies on sleep in healthy man. Br J Clin Pharmacol. 1979;8:57S–16S. doi: 10.1111/j.1365-2125.1979.tb00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Study RE, Barker JL. Diazepam and (-)-pentobarbital: fluctuation analysis reveals different mechanisms for potentiation of gamma-aminobutyric acid responses in cultured central neurons. Proc Natl Acad Sci USA. 1981;78:7180–7184. doi: 10.1073/pnas.78.11.7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tallon-Baudry C, Mandon S, Freiwald WA, Kreiter AK. Oscillatory synchrony in the monkey temporal lobe correlates with performance in a visual short-term memory task. Cereb Cortex. 2004;14:713–720. doi: 10.1093/cercor/bhh031. [DOI] [PubMed] [Google Scholar]
- 52.van Lier H, Drinkenburg WH, van Eeten YJ, Coenen AM. Effects of diazepam and zolpidem on EEG beta frequencies are behavior-specific in rats. Neuropharmacology. 2004;47:163–174. doi: 10.1016/j.neuropharm.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 53.Wedzicha JA, Wallis PJ, Ingram DA, Empey DW. Effect of diazepam on sleep in patients with chronic airflow obstruction. Thorax. 1988;43:729–730. doi: 10.1136/thx.43.9.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Whittington MA, Faulkner HJ, Doheny HC, Traub RD. Neuronal fast oscillations as a target site for psychoactive drugs. Pharmacol Ther. 2000a;86:171–190. doi: 10.1016/S0163-7258(00)00038-3. [DOI] [PubMed] [Google Scholar]
- 55.Whittington MA, Traub RD, Kopell N, Ermentrout B, Buhl EH. Inhibition-based rhythms: experimental and mathematical observations on network dynamics. Int J Psychophysiol. 2000b;38:315–336. doi: 10.1016/S0167-8760(00)00173-2. [DOI] [PubMed] [Google Scholar]
- 56.Young AB, Chu D. Distribution of GABAA and GABAB receptors in mammalian brain: potential targets for drug development. Drug Dev Res. 1990;21:161–167. doi: 10.1002/ddr.430210303. [DOI] [Google Scholar]