Abstract
Background and Aims
Patient-reported outcome measures (PROMs) for children (aged ≤ 18 years) present methodological challenges. PROMs can be categorised by their diverse underlying conceptual bases, including functional, disability and health (FDH) status; quality of life (QoL); and health-related quality of life (HRQoL). Some PROMs are designed to be accompanied by preference weights. PROMs should account for childhood developmental differences by incorporating age-appropriate health/QoL domains, guidance on respondent type(s) and design. This systematic review aims to identify generic multidimensional childhood PROMs and synthesise their characteristics by conceptual basis, target age, measurement considerations, and the preference-based value sets that accompany them.
Methods
The study protocol was registered in the Prospective Register of Systematic Reviews (CRD42021230833), and reporting followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. We conducted systematic database searches for generic multidimensional childhood PROMs covering the period 2012–2020, which we combined with published PROMs identified by an earlier systematic review that covered the period 1992–2011. A second systematic database search identified preference-based value sets for generic multidimensional PROMs. The PROMs were categorised by conceptual basis (FDH status, QoL and HRQoL) and by target age (namely infants and pre-schoolers aged < 5 years, pre-adolescents aged 5–11, adolescents aged 12–18 and multi-age group coverage). Descriptive statistics assessed how PROM characteristics (domain coverage, respondent type and design) varied by conceptual basis and age categories. Involvement of children in PROM development and testing was assessed to understand content validity. Characteristics of value sets available for the childhood generic multidimensional PROMs were identified and compared.
Results
We identified 89 PROMs, including 110 versions: 52 FDH, 29 QoL, 12 HRQoL, nine QoL-FDH and eight HRQoL-FDH measures; 20 targeted infants and pre-schoolers, 29 pre-adolescents, 24 adolescents and 37 for multiple age groups. Domain coverage demonstrated development trajectories from observable FDH aspects in infancy through to personal independence and relationships during adolescence. PROMs targeting younger children relied more on informant report, were shorter and had fewer ordinal scale points. One-third of PROMs were developed following qualitative research or surveys with children or parents for concept elicitation. There were 21 preference-based value sets developed by 19 studies of ten generic multidimensional childhood PROMs: seven were based on adolescents’ stated preferences, seven were from adults from the perspective of or on behalf of the child, and seven were from adults adopting an adult’s perspective. Diverse preference elicitation methods were used to elicit values. Practices with respect to anchoring values on the utility scale also varied considerably. The range and distribution of values reflect these differences, resulting in value sets with notably different properties.
Conclusion
Identification and categorisation of generic multidimensional childhood PROMs and value sets by this review can aid the development, selection and interpretation of appropriate measures for clinical and population research and cost-effectiveness-based decision-making.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40273-021-01128-0.
Key Points for Decision Makers
| Our systematic review identified 110 versions of generic multidimensional patient-reported outcome measures (PROMs) for children (aged ≤ 18 years) spanning childhood age groups and conceptual bases of functional, disability and health status, quality of life and health-related quality of life. |
| A supplementary systematic review identified 21 preference-based value sets for ten PROMs designed to be accompanied by preference-based value sets. |
| Our catalogues of (1) PROMs categorised by target age group, conceptual bases and related characteristics (domain coverage, respondent type and design) and (2) value sets appraised for their development and statistical features can aid the development, selection and interpretation of appropriate childhood PROMs for clinical and population research and cost-effectiveness-based decision-making. |
Background
Patient-reported outcome measures (PROMs) enable direct assessment of health status, health-related quality of life (HRQoL) or quality of life (QoL) by patients or individuals [1–3]. They are used extensively as outcome instruments and can support patient-centred care [2, 4]. The use of PROMs in childhood populations (aged ≤ 18 years) presents methodological challenges compared to application in adults. One challenge is to account for age-based biopsychosocial developmental differences between children and adults and across children of different ages [5–7]. Distinguishing the age or age group of the target childhood population is important for content validity or concept coverage of the childhood PROM, its capacity for child self-report, appropriate design, and the culturally appropriate age for child self-report [2].
There are many ways that PROMs can be categorised. They can be divided into generic measures and disease or condition-specific measures, and further into domain-specific measures and multidimensional measures [4, 8]. Generic measures have the advantage of allowing comparisons across conditions and interventions. Multidimensional measures can be both generic and condition-specific and capture the multifaceted nature of concepts including health, QoL or HRQoL. PROMs can thus be further categorised by the concept(s) they seek to measure through their descriptive systems. There are multiple definitions of these concepts in the literature, with terms often used interchangeably [3, 9]. In this paper, we draw on the categories arising from a synthesis of definitions of functioning, disability, health, HRQoL and QoL used by the World Health Organization (WHO). In this taxonomy, a QoL/HRQoL measure can be distinguished from a functional, disability and health (FDH) measure by its reflection of the individual respondent’s perception, or subjective judgement of importance, of their assessed status [3]. Specifically, an FDH measure captures the ‘interactions among body structures and function, and activities and participation in the context of the environment and personal factors’, whilst QoL is ‘a person’s perception of [his/her] position in life’, where perception involves a subjective judgement over how the position relates to his/her goals, expectations, standards, enjoyment or concerns and not just a self-report of the position. HRQoL represents the individual’s ‘perception of his/her health and health-related states’ where ‘health’ represents a narrower domain than ‘position in life’ (p. 1086) [3].
PROMs can be accompanied by preference-based value sets which produce an overall index when applied to the multidimensional states generated by the descriptive systems [8]. PROMs accompanied by preference-based value sets are often referred to as multi-attribute utility instruments in the literature [10]. The value sets accompanying these measures use stated preference methods including standard gamble (SG) and time trade-off (TTO) to trade-off between a health state and mortality risk or life expectancy, respectively [11]. A key aspect of these methods is their ability to yield values that are anchored on a scale with 0 = dead and 1 = full health, as required for the use of values for estimating quality-adjusted life years (QALYs) in cost-utility analysis. Negative values are theoretically possible and represent health states considered worse than being dead. Discrete choice experiments (DCE) can also be used to elicit stated preferences for value sets for PROMs, although the resulting values lie on a latent scale unless accompanied by additional methods to allow anchoring at dead = 0 [12]. Decision makers around the world have shown a preference for country-specific value sets reflecting underlying differences in preferences of different populations [13–15].
There are considerable methodological challenges that arise in valuing childhood PROMs. Among them, there is contention around whose preferences are relevant, with some instruments offering both adult- and child-elicited value sets [16]. Eliciting values from children themselves may help to ensure that health services are relevant to their needs [17, 18], particularly when adults’ and children’s preferences differ [19]. However, there are practical and ethical issues surrounding the age at which children can be asked to participate in stated preference studies, especially where these include tasks that involve trade-offs with life expectancy. Both SG and TTO may impose high cognitive and/or emotional burden on children relative to DCE or best–worst scaling (BWS) tasks [17]. Where values are used to inform the allocation of healthcare resources financed primarily from taxation, a common normative position is that these should be based on the preferences of the adult general public [12, 20]. However, valuing childhood PROMs using adults’ preferences also raises issues. For example, it is unclear whether the respondent should be asked to think about themselves as a child, their child or another child [20].
Several systematic reviews of generic multidimensional childhood PROMs have been published since 2000 [3, 4, 21–26]. The most recent, by Janssens et al., identified 63 unique versions of PROMs (including eight accompanied by value sets) published between 1992 and 2011 [4]. Their review tabulated the characteristics of PROMs, including target age range, respondent type, domain coverage and several design features (e.g. number of items, response options and recall period) [4]. That review also mapped the domains of the PROMs onto the WHO’s International Classification of Functioning, Disability and Health, Child and Youth version (ICF-CY) framework [27], but noted the shortcoming of conflating the domains of FDH and QoL/HRQoL measures that have a different conceptual basis. It did not distinguish between types of informant response as recommended by the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) guideline, i.e. proxy if the informant infers the child’s perception of health/QoL states and observer if the informant focuses on observable traits without inferring perception [2]. Moreover, little attention was paid to differences in characteristics of measures across age groups, or the value set features. Fayed and colleagues distinguished between FDH and QoL/HRQoL measures; however, their search period was limited to 2004–2008 [3]. Cremeens et al. [21] and Grange et al. [24] limited their reviews to 3- to 8-year-olds and < 5 year olds, respectively, which hinders comparisons of PROM characteristics across all childhood age groups. Finally, Chen and Ratcliffe previously reported on the features of nine childhood PROMs accompanied by value sets, but this was not a systematic review [10]. These gaps in previous studies inform the aim and objectives of this systematic review.
Aim and Objectives
This systematic review aims to generate a comprehensive catalogue of generic multidimensional childhood PROMs that rely on child self- and/or informant-report (hereafter childhood PROMs for brevity in accordance with the ISPOR guideline [2]) and the value sets that accompany them. In doing so, we aim to inform the development, selection and interpretation of childhood PROMs for clinical and population research and cost-effectiveness-based decision-making. Accordingly, the objectives are to:
Systematically identify generic multidimensional childhood PROMs and preference-based value sets that accompany them.
Categorise the PROMs according to conceptual basis and target age group and evaluate their methodological features according to the ISPOR good research practice recommendations on the use of childhood PROMs [2].
Catalogue the value sets that exist for childhood PROMs, compare the methods that have been used to produce them and report the characteristics of the resulting value sets.
Methods
A pre-specified protocol outlining the systematic review methods was developed and registered with the Prospective Register of Systematic Reviews (CRD42021230833). For reporting purposes, the review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [28]. See the Supplementary Information for the PRISMA checklist for this paper (see the electronic supplementary material).
Data Sources and Study Selection
Two independent systematic database searches were conducted. The first aimed to identify primary development studies for generic multidimensional childhood PROMs published between 1 January 2012 and 16 October 2020. Studies identified in the search were pooled with those published between 1 January 1992 and 20 March 2012 identified in the earlier systematic review by Janssens et al. [4]. A second search was conducted without date limits on 18 February 2021 to identify preference-based value sets for generic multidimensional childhood PROMs. This was constructed around measures identified by the first search that reported the development of a value set or the objective to develop a value set or to inform economic evaluations. Developers of PROMs were also contacted to confirm whether, to the best of their knowledge, there was no missing published value set.
Both systematic searches covered seven academic databases (Medline, Embase, PsycInfo via Ovid, EconLit via Proquest, CINAHL via EBSCOHost, Scopus and Web of Science) and one grey literature database (PROQOLID). The Medline search strategy for the first and second searches are presented in Tables A and B in the Supplementary Information (see the electronic supplementary material). Searches were limited to English language texts. In both searches, references were imported into Endnote X9 and duplicates were removed. References were then exported into Covidence [29], where two researchers independently reviewed the titles and abstracts of identified articles. If an article received two approvals, it proceeded to the next stage, with disagreements referred to a third reviewer for the final assessment. Full text articles were independently reviewed by two reviewers with disagreements again referred to the third reviewer for final assessments.
The inclusion criteria for the systematic search of PROMs were as follows: studies reporting the development of a generic multidimensional childhood (aged ≤ 18 years) PROM and studies published in the English language. Conference abstracts with sufficient information (i.e. containing at least the acronym/name of the instrument(s), the aim/motivation of development, whether a general childhood population was targeted, and the multidimensionality of the instrument) were included alongside full text articles. Studies in adults only and animals were excluded, as were commentaries, single case reports and secondary application of PROMs. References of included studies were searched, including those published before 1 January 2012. The inclusion criteria for the systematic search to identify value sets were as follows: studies reporting the development of a value set for one or more generic multidimensional childhood PROM; the value set was anchored on a 0–1 scale required for the use of the values in QALY estimation; and studies (including full text articles and a user’s manual [no conference abstracts were identified]) published in the English language. Studies that only reported values for a sub-set of the health states described by a childhood PROM were excluded.
Data Extraction
For the first review that identified generic multidimensional childhood PROMs, data for each measure were extracted independently by two reviewers per article from a pool of eight reviewers, with disagreements resolved through discussion. For the second review that identified preference-based value sets, data for each value set were extracted by one reviewer, with a 20% check performed by a second reviewer. For the identified generic multidimensional childhood PROMs, the following characteristics were extracted according to proformas in Excel: acronym/name of the instrument, name of author(s)/developer(s), development year and country(ies), aim/motivation of development, target age range, background concept (e.g. definition of health/QoL adopted before commencing PROM development), development methods (e.g. focus groups with children for item selection), role of children in development, level of child’s perception captured, respondent type (e.g. child, proxy, observer), administration mode (e.g. by self, by interviewer), recall period, domains covered, number of items, response options and method for score generation (other than value set application).
For the identified value sets of the generic multidimensional childhood PROMs, the following characteristics were extracted: acronym/name of the instrument, name of author(s)/developer(s), valuation year and country(ies), methods for selecting valued health states, number of valued health states, sampling methods for valuation including target population, recruitment strategies, response rates, sample size, representativeness of sample and reasons for exclusion, and stated preference data collection methods. The latter included elicitation technique(s) (e.g. SG, TTO, DCE), the approach taken to anchoring at 0–1, characteristics of respondents, perspective and administration mode, and modelling methods. Finally, the properties of the value sets were examined (e.g. value range, proportion of health states with negative values). As some of these properties were not reported in the papers, we coded the value set algorithms based on the published results and calculated the index scores for all potential health states according to the classification system of the instruments.
Data Synthesis
Using a method similar to that of Fayed et al. [3], the generic multidimensional childhood PROMs identified were categorised by conceptual basis. The wording and response options for measure items were reviewed independently by two reviewers per article from a pool of eight reviewers. Measures with 75% or more of items that captured a child’s perception (i.e. relation to enjoyment, satisfaction, goals, expectations, standards or concerns) on health or broader position in life were labelled HRQoL or QoL measures, respectively. Measures with 25% or fewer items capturing a child’s perception were labelled FDH measures. Those with between 25 and 75% of items capturing a child’s perception were labelled hybrid HRQoL-FDH or QoL-FDH measures. Fayed and colleagues similarly described the hybrid nature of many measures: e.g. the General Health Questionnaire was classified as ‘FDH and QoL/HRQoL’, the HUI as ‘FDH (with one HRQoL/QoL attribute)’ and the KIDSCREEN as ‘HRQoL/QoL (with some functioning features)’. Instead of these mixed descriptions, this study specifies cut-off levels to allow for comparison across a wide range of measures.
Distinguishing between QoL and HRQoL was particularly challenging given the overlap between health and position in life. Indeed, Fayed et al. do not make this distinction in their results, grouping QoL and HRQoL under the same category [3]. A two-step pragmatic approach was taken: (1) refer to the stated aim/motivation of the study prior to measure development or use (e.g. some studies gave the definition of QoL or HRQoL and underlying constructs [30, 31]), and if still unclear, then (2) categorise as HRQoL measures if more than 50% of the domains cover body functioning, disabilities and daily activities (e.g. mobility, pain, vision, anxiety, depressive symptoms, chronic illness, dressing and grooming) that would constitute the concept of FDH as defined by Fayed et al. [3].
When step (1) in this pragmatic approach could not be applied (i.e. the study used conceptual labels without clearly defining them), we did not automatically apply the conceptual labels given to PROMs by the developer(s). Fayed et al. documented the discrepancies between the conceptual bases as labelled by developers and secondary applications of PROMs and those as classified by their review. For example, Fayed et al. noted that the HUI and the PedsQL were labelled as HRQoL/QoL measures in the primary and secondary literature but are more accurately labelled FDH measures [3]. In the current review, conceptual bases were similarly assigned according to extracted data rather than existing labels (unless clearly defined), but we did not document the discrepancies as was done by Fayed et al.
For measures that use informant responses, the informant type was classified according to the ISPOR guideline [2], namely proxy for QoL/HRQoL measures (since informants infer the child’s subjective perception) and observer for FDH measures (since no such inference is made). For hybrid QoL/HRQoL-FDH measures, the informant type was labelled hybrid proxy-observer.
Following the ISPOR good research practices guideline [2], we explored differences in measurement characteristics for PROMs targeted at different age groups. The ISPOR guideline’s age cut-offs were used to categorise the measures by their target age group: less than 5 years, covering infants, toddlers or pre-schoolers; 5–7 years for younger pre-adolescents; 8–11 years for older pre-adolescents; and 12–18 years for adolescents. We then explored how the characteristics of the measures (including range of domains covered, respondent and informant type, and other design features) varied by conceptual basis and target age. In cases where target ages were not clearly specified, inferences were made from the study aim and development methods to assign the target age category.
The level of content validity of each PROM was assessed according to whether children of the target age were involved in (1) the development of the measure, including qualitative research for domain and item elicitation, and (2) assessment of comprehension using cognitive interviews and/or pilot/feasibility studies. Note that appraisals of other psychometric properties such as construct validity and internal consistency are not reported here but in forthcoming work. Cultural issues present in the initial instrument development (as opposed to subsequent translations and secondary cross-cultural adaptations of the developed instrument) were similarly described.
For the review of value sets for generic multidimensional childhood PROMs, data were analysed using narrative synthesis. Summary tables were used to describe the bibliography/setting, sampling methods, preference data collection methods and statistical features of the identified value sets. Separate summaries of the modelling methods are presented for two categories of preference elicitation techniques: TTO, SG and rating scales (RS); DCEs and BWS.
Results
Search Results
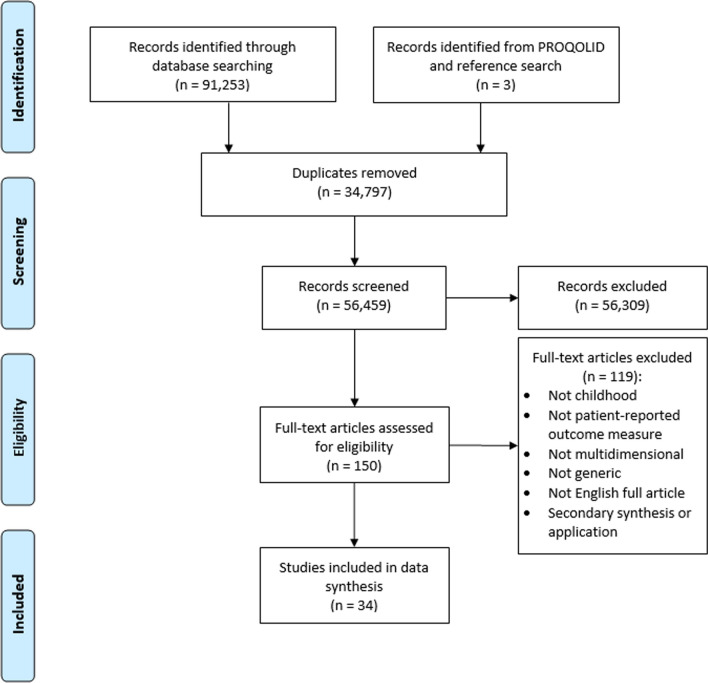
Figure 1 shows the PRISMA flow diagram for the first search that identified primary studies developing generic multidimensional childhood PROMs published between 1 January 2012 and 16 October 2020.
Fig. 1.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for database searches for studies developing generic multidimensional childhood patient-reported outcome measures published between 1 January 2012 and 16 October 2020
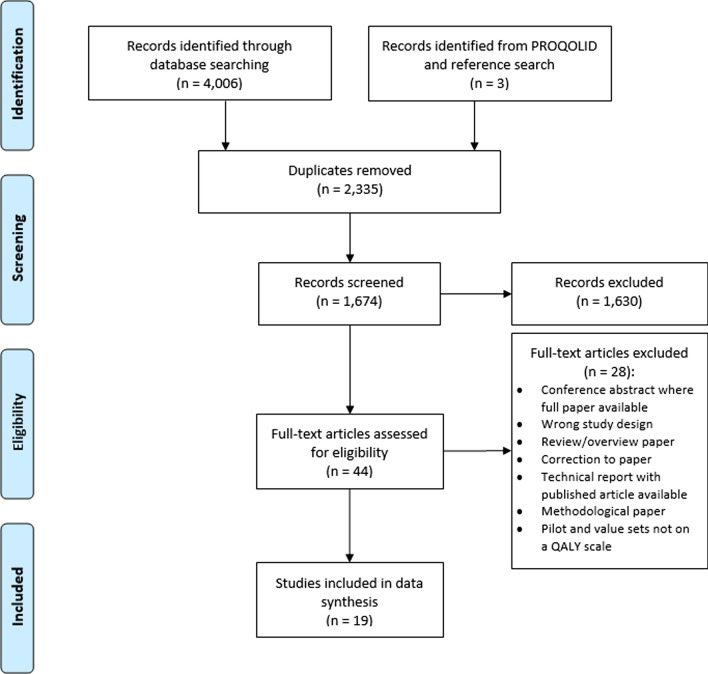
Thirty-four eligible studies were identified that described 26 PROMs. Sixty-three PROMs included in the review by Janssens et al. [4] that met the inclusion criteria were included to give 89 PROMs in total. Fourteen PROMs were accompanied or designed to be accompanied by preference-based value sets. Figure 2 shows the PRISMA flow diagram for the identification of value sets for these 14 PROMs. Nineteen studies were identified in total that described 21 value sets.
Fig. 2.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for database searches for valuation studies for generic multidimensional childhood PROMs identified by search strategies. PROM patient-reported outcome measure, QALY quality-adjusted life year
Overview of Generic Multidimensional Childhood Patient-Reported Outcome Measures (PROMs)
Table 1 provides an overview of the characteristics of the identified generic multidimensional childhood PROMs that are not accompanied by or designed to be accompanied by preference-based value sets, while Table 2 does so for PROMs designed to be accompanied by preference-based value sets. Some measures have several versions, and Table 1 includes all distinct versions, providing a total of 110 unique measures (for example, the PedsQL 4.0 Generic Core Scales are composed of age-specific modules for toddlers, young children, children and teens [32]). Of the 110 measures, 52 are FDH measures, 29 are QoL measures, 12 are HRQoL measures, nine are hybrid QoL-FDH measures, and eight are hybrid HRQoL-FDH measures. Seventeen measures (reflecting two versions each for HUI2 and HUI3 and two variants of the EQ-5D-Y) are designed to be accompanied by preference-based value sets. The measures were primarily developed in high-income countries (using the 2021 World Bank classifications [33]), with only seven (6%) developed in lower- or middle-income country (LMIC) settings as part of an international development process [34–40].
Table 1.
Summary of characteristics of included generic multidimensional childhood patient-reported outcome measures not designed to be accompanied by preference-based value setsa
| # | Acronym: name | Reference; country | Target age (years) | Perception captured (category)b | Respondent type | Administration mode | Recall period | Domains (number, n) | Items n | Response options [scoring method] |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ACHWM: Aboriginal Children's Health and Well-Being Measure | Young [48]; Canada | 8–18 | Yes (QoL) | Child | By staff; computer tablet | General | Spiritual; emotional; physical; mental (n = 4) | 65 | Frequency, degree of importance, yes/no (60 items); open-ended (5) [item average] |
| 2 | QUALIN: Infant's Quality of Life | Manificat [88, 106]; France | 0–3 | No (FDH) | Observer | By self | General | Psychomotor development; family environment; psychopathological; sociability (n = 4) | 33 | 5-point scale [item average] |
| 3 | AUQUEI Nursery: Child Pictured Self Report Nursery version | Manificat [88, 107]; France | 3–6 | Yes (QoL) | Child, aided by parents | By self | General | Family life; Social life; activities at school and leisure; health (n = 4) | 28 | 4-level pictorial happy-sad faces (26 items); open-ended (2) [item average] |
| 4 | AUQUEI Primary: AUQUEI for primary school child | Manificat (2002)c [88]; France | 6–11 | Yes (QoL) | Child, aided by parents | By self | General | Family life; social life; activities at school and leisure; health (n = 4) | 33 | 4-level pictorial happy-sad faces for satisfaction (31 items); open-ended (2) [item average] |
| 5 | OK.ado: Adolescent quality of life | Manificat (2002b) [61]; France | 11–18 | Yes (QoL) | Child | By self | General | Leisure and relationships; school; family; self-esteem (n = 4) | 34 | 5-point scale [item average] |
| 6 | CHAQ: Child Health Assessment Questionnaire | Singh [62]; US | 8–19 | No (FDH) | Child; Observer | By self | Past week | Dressing and grooming; arising; eating; walking; hygiene; reach; grip; activities. Also: aids/devices for functioning; activities needing assistance; VAS pain; VAS wellbeing (n = 8 + Aids/VAS) | 34 | 4-point scale for functioning (30 items); VAS (2); list of aids used (1); list of activities needing assistance (1) [highest item score in domain as domain score; domain average] |
| 7 | CHAQ Informant only | Singh [62] | 1–7 | No (FDH) | Observer | By self | Past week | See CHAQ | 34 | See CHAQ |
| 8 | CHAQ38: CHAQ revised version, 38 items | Lam [108]; Canada | 5–19 | No (FDH) | Child; observer | By self | Past week | Same domains as CHAQ plus 8 additional items on age-appropriate physical activities and challenges | 38 | 4- or 5-point scale [item average] |
| 9 | CHAQ38 Information only | Lam [108] | < 5 | No (FDH) | Observer | By self | Past week | See CHAQ38 | 38 | See CHAQ38 |
| 10 | CHIP-AE: Child Health and Illness Profile—Adolescent Edition | Starfield [7, 109]; US | 11–17 | No (FDH) | Child | By self | Past 4 weeks | Satisfaction; comfort; resilience; risk avoidance; achievement; disorders (n = 6) | 107; 46 disease-specific items | 5-point scale [item average per subdomain; subdomain average] |
| 11 | CHIP-CE CRF: Child Health and Illness Profile—Child Edition Child-Report Form | Riley [110]; US | 6–11 | No (FDH) | Child | By self | Past 4 weeks | Satisfaction; comfort; resilience; risk avoidance; achievement (n = 5) | 45 | 5 graduated circle responses with cartoons at beginning/end [item average per subdomain; subdomain average] |
| 12 | CHIP-CE PRF: CHIP-CE Parent-Report Form (long version) | Riley (2004b) [111]; US | 6–11 | No (FDH) | Observer | By self | Past 4 weeks | Satisfaction; comfort; resilience; risk avoidance; achievement (n = 5) | 76 | 5-point scale [item average per subdomain; subdomain average] |
| 13 | CHIP-CE PRF (short version) | Riley (2004b) [111]; US | 6–11 | No (FDH) | Observer | By self | Past 4 weeks | Satisfaction; comfort; resilience; risk avoidance; achievement (n = 5) | 45 | 5-point scale [item average per subdomain; subdomain average] |
| 14 | CHQ-PF50: Child Health Questionnaire Parent Form (long version) | Landgraf [112]; US | 5–18 | Some (HRQoL-FDH) | Proxy observer | By self; mail | Past 4 weeks; general for global health and family cohesion; compared to last year for change in health | Physical functioning; bodily pain; role functioning, physical; role, emotional; role, behavioural; general health perception; self-esteem; mental health; general behaviour; change in health; parental time impact; family activities; family cohesion (n = 13) | 50 | 4- to 6-point scale [item sum per domain] |
| 15 | CHQ-PF28: CHQ-PF (short version) | Raat [113]; US | 5–18 | Some (HRQoL-FDH) | Proxy-observer | By self | Past 4 weeks; general for global health and family cohesion; compared to last year for change in health | Physical functioning; bodily pain; role functioning, physical; role, emotional or behavioural; general health perception; self-esteem; mental health; general behaviour; change in health; parental emotional impact; parental time impact; family activities; family cohesion (n = 13) | 28 | 4- to 6-point scale [item sum per domain] |
| 16 | CHQ-CF87: CHQ Child Form (long version) | Landgraf [114]; US | 10–18 | Some (HRQoL-FDH) | Child | By self | Past 4 weeks; general for global health and family cohesion; compared to last year for change in health | Physical functioning; bodily pain; role functioning, physical; role, emotional; role, behavioural; general health perceptions; self-esteem; mental health; general behaviour; change in health; family activities; family cohesion (n = 12) | 87 | 4- to 6-point scale [item sum per domain] |
| 17 | CHQ-CF45: CHQ-CF (short version) | Landgraf [52]; US, The Netherlands | 10–18 | Some (HRQoL-FDH) | Child | By self: paper or online | Past 4 weeks; general for global health and family cohesion; compared to last year for change in health | Physical functioning; Bodily pain; role functioning, physical; role, emotional or behavioural; general health perceptions; self-esteem; mental health; getting along; global behaviour; change in health; family activities; family cohesion (n = 12) | 45 | 4- to 6-point scale [item sum per domain] |
| 18 | CHRS: Children's Health Ratings Scale | Maylath [115]; US | 9–12 | Yes (HRQoL) | Child, aided by staff; proxy | By self | Current; general | No domain structure but includes a range of questions on general health perceptions | 17 | 5-point scale [item sum] |
| 19 | CHSCS: Child's Health Self-Concept Scale | Hester [116]; US | 5–13 | Yes (HRQoL) | Child | By self, aided by staff before start | General | Psychosocial; physical health; healthiness; values; energy (n = 5) | 34 | 4-point scale: binary choice for positive or negative perception on health issue; 2-point scale on strength of perception [not stated] |
| 20 | ComQOL-S5: Comprehensive Quality of Life Scale—School version, 5th edition | Gullone [54]; Australia | 11–18 | Some (QoLd) | Child | By self | Past three months; general | Material wellbeing; health; productivity; intimacy; safety; place in community; emotional wellbeing (n = 7) | 21 | 5-point scale for frequency, importance and satisfaction [item sum weighted by importance score] |
| 21 | COOP Charts: Dartmouth Primary Care Cooperative Information Project Charts | Wasson [95]; US | 12–21 | Yes (HRQoL) | Child | By self | Past month | Physical fitness; emotional feelings; school work; social support; family communications; health habits (n = 6) | 6 | 5-point scale with descriptors and cartoons (picture-and-word chart) [item average] |
| 22 | CQoL: Child Quality of Life | Graham [117]; UK | 9–15 | Yes (QoL) | Child; proxy | By self | Past month | Activities; appearance; communication; continence; depression; discomfort; eating; family; friends; sight; mobility; school; self-care; sleep; worry; global QoL (n = 15) | 45 | 7-point scale [item average per subdomain; subdomain average] |
| 23 | DHP-A: Duke Health Profile—Adolescent version | Vo [118]; France | 12–19 | Yes (HRQoL) | Child | By self | Past week | Physical health; mental health; social health; general health; perceived health; self-esteem; anxiety; depression; pain; disability (n = 10) | 17 | 3-point scale [item sum per domain] |
| 24 | ExQoL: Exeter Quality of Life | Eiser [41]; UK | 6–12 | Yes (QoL) | Child | By self, supervised; computer | General | Symptoms; social wellbeing; School achievements; physical activity; worry; family relationships (n = 6) | 12 | 12 pictures, each rated twice using VAS—(1) ‘like me’; (2) ‘I would like to be’ [item average] |
| 25 | FSIIR Infants: Functional Status II Revised, long version, Infants | Stein [119]; US | 0–1 | No (FDH) | Observer | By interviewer | Past 2 weeks | General health; total functional status; responsiveness (n = 3) | 20 | 3-point scales for function difficulty and extent due to illness [item sum as proportion of maximum] |
| 26 | FSIIR Toddlers | Stein [119]; US | 1–2 | No (FDH) | Observer | By interviewer | Past 2 weeks | General health; total functional status; responsiveness (n = 3) | 28 | See FSIIR Infants |
| 27 | FSIIR Pre-schoolers | Stein [119]; US | 2–3 | No (FDH) | Observer | By interviewer | Past 2 weeks | General health; total functional status; activity (n = 3) | 38 | See FSIIR Infants |
| 28 | FSIIR School children | Stein [119]; US | 4–16 | No (FDH) | Observer | By interviewer | Past 2 weeks | General health; total functional status; Interpersonal functioning (n = 3) | 39 | See FSIIR Infants |
| 29 | FSIIR-14: FSIIR 14 items (short version) | Stein [119]; US | 0–16 | No (FDH) | Observer | By interviewer | Past2 weeks | No domain structure but includes a range of questions on general health | 14 | See FSIIR Infants |
| 30 | GCQ: Generic Children's Quality of Life | Collier [120]; UK | 6–14 | Yes (QoL) | Child; proxy | By self | General | General affect; peer relationships; attainments; relationships with parents; general satisfaction (n = 5) | 25 | 5-point scales for: perceived self and preferred self; based on story child characters [item sum] |
| 31 | HAY: How Are You | Le Coq [121]; The Netherlands | 8–12 | Yes (HRQoL) | Child | By self | Past week | Generic domains: physical; cognitive; social; physical complaints. asthma-specific domains (n = 5) | 32 | 4-point scale rating frequency, quality of performance and related feelings [item sum per subdomain as proportion of maximum] |
| 32 | HDQ Age 6: Health Dialogue Questionnaire for Age 6 | Holmström 2012) [122]; Sweden | 6 | Some (HRQoL-FDH) | Proxy-observer | By school nurse | Not stated | Physical; mental; social (n = 3) | 15 | 5-point scale [positive response as proportion of total questions] |
| 33 | HDQ Age 10 | Holmström (2012b) [123]; Sweden | 10 | Some (HRQoL-FDH) | Child, aided by parent | By school nurse | Not stated | Physical; mental; social (n = 3) | 15 | See HDQ Age 6 |
| 34 | HDQ Age 13 | Olofsson [124]; Sweden | 13 | Some (HRQoL-FDH) | Child, aided by parent | By school nurse | Not stated | Physical; mental; social (n = 3) | 15 | See HDQ Age 6 |
| 35 | HDQ Age 16 | Kristiansen [125]; Sweden | 16 | Some (HRQoL-FDH) | Child, aided by parent | By school nurse | Not stated | Physical; mental; social (n = 3) | 22 | See HDQ Age 6 |
| 36 | Healthy Pathways CRS: Healthy Pathways Child-Report Scales | Bevans [42]; US | 10–12 | Yes (HRQoL) | Child | Schools with computer access: by self; computer. No access: by self for 6th graders; by interviewer for 4th/5th; paper | Past 4 weeks | Comfort; energy; resilience; risk avoidance; subjective wellbeing; achievement (n = 6) | 88 | 5-point scale [item average per subdomain] |
| 37 | Healthy Pathways PRS: Healthy Pathways Parent-Report Scales | Bevans [92]; US | 10–12 | Yes (HRQoL) | Proxy | By self | Past 4 weeks | Comfort; energy; resilience; risk avoidance; wellbeing; achievement (n = 6) | 59 | 5-point scale [item average per subdomain] |
| 38 | ILC: Inventory of Life quality in Children and adolescents | Jozefiak (2016)e [126]; Germany | 10–16 | Yes (QoL) | Child | By self | Past week | Global QoL; school performance; family functioning; social integration; interests and hobbies; physical health; mental health (n = 7) | 7 | 5-point scale [item average] |
| 39 | ITQOL: Infant Toddler Quality of Life (long version) | Klassen [127]; Canada | 2 months–5 years | No (FDH) | Observer | By self; mail | Past 4 weeks; general for global health; compared to last year for change in health | Physical; growth and development; bodily pain; temperament/moods; general behaviour; getting along; general health perceptions; change in health; parental impact—emotion; time; mental; family cohesion; general health (n = 13) | 103 | 5-point scale except 4-point scale for parental time impact [item average per domain] |
| 40 | ITQOL SF47: ITQOL Short Form | Landgraf [128]; The Netherlands | 2 months–5 years | No (FDH) | Observer | By self | Past 4 weeks; general for global health; compared to last year for change in health | Physical; growth and development; bodily pain; temperament/moods; general behaviour; getting along; general health perceptions; change in health; parental impact—emotion; time; mental; family cohesion; general health (n = 13) | 47 | 5-point scale except for 4-point scale for parental time impact [item average per domain] |
| 41 | Kiddy-KINDL CQ: Kiddy-KINDL Children’s Questionnaire | Ravens-Sieberer (1998)f [89]; Germany | 4–6 | Some (QoL-FDH) | Child | By interviewer | Past week | Physical health; emotion; self-esteem; family; friends; school (n = 6) | 12 | 3-point scale [item average] |
| 42 | Kiddy-KINDL PQ: Kiddy-KINDL Parents’ Questionnaire | Ravens-Sieberer [89]; Germany | 3–6 | Some (QoL-FDH) | Proxy-observer | By self | Past week | Physical health; emotion; self-esteem; family; friends; school; child behaviour (n = 7) | 46 | 5-point scale [item average] |
| 43 | Kid-KINDL CQ: Kid-KINDL Children’s Questionnaire | Ravens-Sieberer [89]; Germany | 7–13 | Some (QoL-FDH) | Child | By self | Past week | Physical health; emotion; self-esteem; family; friends; school (n = 6) | 24 | 5-point scale [item average] |
| 44 | Kiddo-KINDL AQ: Kiddo-KINDL Adolescents’ Questionnaire | Ravens-Sieberer [89]; Germany | 14–17 | Some (QoL-FDH) | Child | By self | Past week | Physical health; emotion; self-esteem; family; friends; school (n = 6) | 24 | 5-point scale [item average] |
| 45 | Kid- & Kiddo-KINDL PQ: Kid- & Kiddo-KINDL Parents’ Questionnaire | Ravens-Sieberer [89]; Germany | 7–17 | Some (QoL-FDH) | Proxy-observer | By self | Past week | Physical health; emotion; self-esteem; family; friends; school (n = 6) | 24 | 5-point scale [item average] |
| 46 | KINDL CAT-SCREEN: KINDL Computer Assisted Touch version | Ravens-Sieberer [43]; Germany | 6–17 | Some (QoL-FDH) | Child | By self; computer assisted (visual and audio) | Past week | Physical health; emotion; self-esteem; family; friends; school (n = 6) | 24 | 5-point scale [item average] |
| 47 | KIDSCREEN-52 | Ravens-Sieberer (2003); [56, 57]; 13 European countriesg | 8–18 | Yes (QoL) | Child; proxy | By self | Past week | Physical wellbeing; psychological wellbeing; moods and emotions; self-perception; autonomy; parent relations and home life; social support and peers; school environment; social acceptance; financial resources (n = 10) | 52 | 5-point scale [Rasch score] |
| 48 | KIDSCREEN-27 | Ravens-Sieberer [58]; 13 European countriesg | 8–18 | Yes (QoL) | Child; proxy | By self | Past week | Physical wellbeing; psychological wellbeing; parent relations and autonomy; social support and peers; school environment (n = 5) | 27 | 5-point scale [Rasch score] |
| 49 | KIDSCREEN-10 | Ravens-Sieberer [59]; 13 European countriesg | 8–18 | Yes (QoL) | Child; proxy | By self | Past week | Physical activity; depressive moods and emotions; social and leisure time; relationship with parents; relationship with peers; cognitive and school performance (n = 6) | 10 | 5-point scale [Rasch score] |
| 50 | Kids-CAT: KIDSCREEN Computerized Adaptive Test | Devine (2015); Barthel [46, 129]; Germany | 7–17 | Yes (QoL) | Child | By self; computerized adaptive test | Past week | Physical wellbeing; psychological wellbeing; parent relations; social support and peers; school wellbeing; chronic-generic QoL (n = 6) | 42h | 5-point scale [Rasch score] |
| 51 | MCWBS: Multidimensional Child Well-Being Scale | Jiang [34]; China | 10–15 | No (FDH) | Child | By self, aided by staff before start | General | Physical; psychological; social; educational (n = 4) | 30 | 5-point scale [not stated] |
| 52 | Nordic QoLQ: Nordic Quality of Life Questionnaire for children | Lindström [130]; 5 Nordic countriesi | 12–18 | Some (QoLd) | Child and parent; proxy-observer | By self; mail | Past 3 months | External conditions; interpersonal conditions; psychological conditions (n = 3) | 25 | Unclear [item score dichotomised and averaged] |
| 53 | Nordic QoLQ Informant only | Lindström [130] | 2–11 | Some (QoLd) | Proxy-observer | By self; mail | Past three months | See Nordic QoLQ | 25 | See Nordic QoLQ |
| 54 | PedsQL GCS Toddler: Pediatric Quality of Life Inventory 4.0 Generic Core Scales, Toddler | Varni (2001)j [32]; US | 2–4 | No (FDH) | Observer | By self (paper); by interviewer (telephone) | Past month (past week for acute version) | Functioning: physical; emotional; social; school (n = 4) | 23 | 5-point scale [item average per domain] |
| 55 | PedsQL GCS Young Child | Varni [32]; US | 5–7 | No (FDH) | Child; observer | Child: by interviewer, informant: by self or interviewer (telephone) | Past month (past week for acute version) | Functioning: physical; emotional; social; school (n = 4) | 23 | Child: 3-level pictorial faces, Informant: 5-point scale [item average per domain] |
| 56 | PedsQL GCS Child | Varni [32]; US | 8–12 | No (FDH) | Child; observer | Child and informant: by self or interviewer (telephone) | Past month (past week for acute version) | Functioning: physical; emotional; social; school (n = 4) | 23 | 5-point scale [item average per domain] |
| 57 | PedsQL GCS Teen | Varni [32]; US | 13–18 | No (FDH) | Child; observer | Child and informant: by self or interviewer (telephone) | Past month (past week for acute version) | Functioning: physical; emotional; social; school (n = 4) | 23 | 5-point scale [item average per domain] |
| 58 | PedsQL SF15 Toddler: PedsQL 4.0 Short Form, Toddler | Chan (2005)j [131]; US | 2–4 | No (FDH) | Observer | By self (paper); by interviewer (telephone) | Past month (past week for acute version) | Functioning: physical; emotional; social; school (n = 4) | 23 | 5-point scale [item average per domain] |
| 59 | PedsQL SF15 Young Child | Chan [131]; US | 5–7 | No (FDH) | Child; observer | Child: by interviewer, informant: by self or interviewer (telephone) | Past month (past week for acute version) | Functioning: physical; emotional; social; school (n = 4) | 23 | Child: 3-level pictorial faces, Informant: 5-point scale [item average per domain] |
| 60 | PedsQL SF15 Child | Chan [131]; US | 8–12 | No (FDH) | Child; observer | Child and informant: by self or interviewer (telephone) | Past month (past week for acute version) | Functioning: physical; emotional; social; school (n = 4) | 23 | 5-point scale [item average per domain] |
| 61 | PedsQL SF15 Teen | Chan [131]; US | 13–18 | No (FDH) | Child; observer | Child and informant: by self or interviewer (telephone) | Past month (past week for acute version) | Functioning: physical; emotional; social; school (n = 4) | 23 | 5-point scale [item average per domain] |
| 62 | PedsQL Infant Scales (1–12 months) | Varni (2011)j [132]; US | 1–12 months | No (FDH) | Observer | By self; mail | Past month (past week for acute version) | Functioning: physical; emotional; social; cognitive; physical symptoms (n = 5) | 36 | 5-point scale [item average per domain] |
| 63 | PedsQL Infant Scales (1–24 months) | Varni [132]; US | 13–24 months | No (FDH) | Observer | By self; mail | Past month (past week for acute version) | Functioning: physical; emotional; social; cognitive; physical symptoms (n = 5) | 45 | 5-point scale [item average per domain] |
| 64 | P-MYMOP: Paediatric Measure Yourself Medical Outcomes Profile | Ishaque [133]; Australia | 7–11 | Yes (HRQoL) | Child, aided by parent | By self | Past week | Symptom 1; symptom 2; activity impairment; general wellbeing (child specifies symptoms and activity impairment affecting her most) (n = 4) | 4 | 7-point pictorial faces [item average] |
| 65 | PQ-LES-Q: Pediatric Quality of Life Enjoyment and Satisfaction Questionnaire | Endicott [63]; US | 6–17 | Yes (QoL) | Child | By self, unassisted as far as possible | Past week | Health; mood/feelings; school/learning; helping at home; getting along with friends; getting along with family; play/free time; getting things done; love/affection; getting/buying things; place you live at; paying attention; energy level; feelings about yourself; global QoL (n = 15) | 15 | 5-point scale [item sum as proportion of maximum] |
| 66 | PROMIS PIB: Patient-Reported Outcomes Measurement Information System—Pediatric Item Banks | Varni [44]; US | 8–17 | No (FDH) | Child; observer | By self; computer | Past week | Mobility; upper extremity; peer relationships; depressive symptoms; anxiety; anger; pain interference; lack of energy; tired; asthma impact (n = 10) | 165 | 5-point scale [Rasch score] |
| 67 | PROMIS PIB SF: PROMIS PIB Short Form | DeWalt [45]; US | 8–17 | No (FDH) | Child | By self; computer | Past week | Mobility; upper extremity; peer relationships; depressive symptoms; anxiety; anger; pain interference; fatigue (n = 8) | 64 | 5-point scale [Rasch score] |
| 68 | PROMIS PIB CAT: PROMIS PIB Computerized Adaptive Test | Varni [47]; US | 8–17 | No (FDH) | Child | By self; computer | Past week | Mobility; upper extremity; peer relationships; depressive symptoms; anxiety; anger; pain interference; fatigue (n = 8) | 5–12 items per domain | 5-point scale [Rasch score] |
| 69 | PROMIS PGH7: PROMIS Pediatric Global Health 7-item | Forrest [53]; US | 8–17 | No (FDH) | Child; observer | By self; online | General | General health; quality of life; physical health; mental health; sad; fun with friends; parents listen to ideas (n = 7) | 7 | 5-point scale [item sum] |
| 70 | PROMIS PGH7 Informant only | Forrest [53] | 5–7 | No (FDH) | Observer | By self; online | General | See PROMIS PGH7 | 7 | See PROMIS PGH7 |
| 71 | PROMIS EC: PROMIS Early Childhood | Blackwell [87]; US | 1–5 | No (FDH) | Observer | By self | Past week/month | (A) Global health. (B) Mental health: anger; anxiety; depressive symptoms; positive affect; engagement—curiosity; engagement—persistence; self-control—adaptability; Self-control—self-regulation. (C) Social relationships. (D) Physical health: physical activity; sleep health (n = 12) | 138 | Not yet developed |
| 72 | PWI-SC: Personal Wellbeing Index, School Children | Cummins [64]; Australia | 5–18 | Yes (QoL) | Child | By self or interviewer | Current | Standard of living; health; life achievement; personal relationships; personal safety; community-connectedness; future security; global QoL (n = 7) | 7 | 11-point scale (happiness) [item average] |
| 73 | PWI-SC8: PWI-SC, 8 items | Vaqué-Crusellas [134]; Spain | 10–12 | Yes (QoL) | Child | By self, in classroom with adult help available) | Current | Standard of living; health; life achievement; personal relationships; personal safety; community-connectedness; future security; food satisfaction (n = 8) | 8 | 11-point scale (happiness) [item average] |
| 74 | QLQC: Quality of Life Questionnaire for Children | Bouman [135]; The Netherlands | 8–12 | Yes (QoL) | Child; proxy | By self | Past 2 months | Physical: complaints; limitations; handicaps. Psychological: general wellbeing; cognitive; self-concept; anxious-depressed. social relations: parents (child-report only); siblings; peers; school; social conflicts; leisure-time activities (n = 13) | 81 | 3-point scale [item sum] |
| 75 | QLSI-C: Quality of Life Systemic Inventory for Children | Etienne [30]; Belgium | 8–12 | Yes (QoL) | Child | By interviewer | Current | Physical; emotional; cognitive; social; family functioning (n = 5) | 20 | Multi-component state-goal gap identification approachk [item average weighted by importance and rate of progress to goal] |
| 76 | QLSI-C Tablet: QLSI-C Tablet version | Touchèque [49]; Belgium | 8–12 | Yes (QoL) | Child | By self; tablet | Current | Physical; emotional; cognitive; social; family functioning (n = 5) | 20 | See QLSI-C |
| 77 | QoL-C: Quality of Life Scale for Children | Thompson [6]; UK | 4–9 | No (FDH) | Child; observer | Age 47: by interviewer, age 8–9: by self in class, informant: by self; mail | Today | Moving; looking after myself; doing usual activities; having pain; feeling worried, sad or unhappy; global health VAS (n = 5 + VAS) | 5 | 3-level pictorial faces; VAS [item sum] |
| 78 | QOLPAV: Quality of Life Profile: Adolescent Version | Raphael [136]; Canada | 15–18 | Yes (QoL) | Child | By staff in groups | Not stated | Being (physical, psychological, spiritual); belonging (physical, social, community); becoming (practical, leisure, growth) (n = 3) | 54 | 5-point scales: importance; enjoyment and satisfaction. Control and opportunity scores for context [item average per subdomain; subdomain average] |
| 79 | QOLQA: Quality of Life Questionnaire for Adolescents | Wang [35]; Japan; China | 12–15 | Yes (QoL) | Child | By self | General | Physical; psychological; independence; social relationship; environment (n = 5) | 70 | 5-point scale [item average per domain; domain average] |
| 80 | QOLQA-Taiwan: QOLQA Taiwanese version | Fuh [65]; Taiwan | 13–15 | Yes (QoL) | Child | By self | General | Family; residential environment; personal competence; social relationships; physical appearance; psychological wellbeing; pain (n = 7) | 38 | 5-point scale [item average per domain] |
| 81 | SLSS: Student Life Satisfaction Scale | Huebner [137]; US | 8–14 | Yes (QoL) | Child | By group, questions read by staff | Past several weeks | No domain structure but includes a range of questions on life satisfactionl | 7 | 4-point scale [item sum] |
| 82 | MSLSS: Multidimensional SLSS | Huebner [138]; US | 8–14 | Yes (QoL) | Child | By group, questions read by staff | General | Family; friends; school; living environment; self (n = 5) | 40 | 4-point scale [item average per domain] |
| 83 | MSLSS Brief | Seligson [55]; US | 11–14 | Yes (QoL) | Child | By self | General | Family; friends; school; living environment; self (n = 5) | 5 | 7-point scale. Importance rating scores obtained to weight each domain [item average per domain; domain average weighted by importance scores] |
| 84 | MSLSS-A: MSLSS Adolescent version | Gilligan [139]; US | 14–18 | Yes (QoL) | Child | By self | General | Family; same-sex friends; school; living environment; self; opposite-sex relationship (n = 6) | 53 | 6-point scale [item average per domain; domain average] |
| 85 | TACQOL: TNO-AZL questionnaire for Children's health-related Quality of Life | Vogels [31]; Theunissen [140]; The Netherlands | 8–15 | Yes (HRQoL) | Child; Proxy | By self | Past few weeks | Pain and symptoms; motor functioning; autonomy; cognitive functioning; social functioning; global positive emotional functioning; global negative emotional functioning (n = 7) | 53 | 3-point scale for severity; 4-point scale for child's emotional response to item; combined into 3-point ordinal scale with range 0–2m [item sum per domain] |
| 86 | TACQOL Informant only | See TACQOL | 6–7 | Yes (HRQoL) | Proxy | By self | Past few weeks | See TACQOL | 53 | See TACQOL |
| 87 | TAPQOL: TNO-AZL questionnaire for Preschool children's health-related Quality of Life | Fekkes [141]; The Netherlands | 1–5 | Yes (HRQoL) | Proxy | By self | Past 3 months | Sleeping; appetite; lung problems; stomach problems; skin problems; motor functioning; problem behaviour; social functioning; communication; positive mood; anxiety; liveliness (n = 12) | 43 | 3-point scale for severity; 4-point scale for child's emotional response to item; combined into 5-point ordinal scale with range 0–4n [item sum per domain] |
| 88 | TedQL | Lawford [142]; UK | 3–8 | Yes (QoL) | Child; proxy | Child: by interviewero; informant: by self | Current | Physical competence; peer acceptance; maternal acceptance; psychological functioning; cognitive functioning (n = 5) | 23 | Two 4-level scales: colour circles vs. linear scale. Circles took less time and more internally consistent [item average as proportion of maximum] |
| 89 | VSP-A: Vecu et Sante Percue de l'Adolescent | Simeoni [143]; France | 11–17 | Yes (HRQoL) | Child | By self | Past month | Psychological wellbeing; energy/vitality; friends; parents; leisure; school (n = 6) | 40 | 5-point scale [item average per domain] |
| 90 | WCHMP: Warwick Child Health and Morbidity Profile | Spencer [144]; UK | 0–5 | No (FDH) | Observer | By self or interviewer | Not stated | General health status; acute minor illness; behavioural; accident; acute significant illness; hospital admission; immunization; chronic illness; functional health; HRQoL (n = 10) | 10 | 4-point scale and free text for detail [no scoring] |
| 91 | WHOQOL-BREF | Izutsu [36]; Bangladesh | 11–18 | Yes (QoL) | Child | By interviewer (due to low literacy) | Past 2 weeks | Physical; psychological; social relationships; environmental; global QoL and health satisfaction (n = 4) | 26 | 5-point scale [item sum per domain] |
| 92 | YQoL-R: Youth Quality of Life instrument—Research version | Edwards (2002); Patrick [145, 146]; US | 12–18 | Yes (QoL) | Child | By self | General | Sense of self; social relationships; environment; general quality of life (n = 4) | 41 | 11-point scale [item sum per domain] |
| 93 | YQoL-S: YQoL—Surveillance version | Topolski [147]; US | 12–18 | Yes (QoL) | Child | By self | General; past month | Contextual; perceptual (n = 2) | 10 | 5-point scale for Contextual items; 11-point scale for perceptual [item sum for Perception domain] |
FDH functioning, disability and health, HRQoL health-related quality of life, QoL quality of life, VAS visual analogue scale
aMeasures arranged alphabetically; family of measures (e.g. QUALIN, AUQUEI Nursery, AUQUEI Primary, OK.ado) arranged together
bDenotes whether the measure items capture child respondent’s perception (i.e. his/her enjoyment, satisfaction, expectations, standards and concerns) on health, health-related aspect or position in life: ‘yes’ if more than 75% of items did; ‘no’ if less than 25% of items did; ‘some’ if between 25% and 75% of items did. ‘Yes’ implies that the measure is predominantly a QoL or HRQoL measure; ‘no’ predominantly an FDH measure; and ‘some’ a mix of QoL/HRQoL and FDH
cThis article is a review of AUQUEI by instrument developers. The primary development study is not available in English
dContains subjective QoL and objective position in life items. Though the latter do not capture perception, they are associated with QoL rather than health. Hence, the measure is categorised as QoL, not QoL-FDH
eThis article is a secondary application study of ILC in Norway. The primary development study was set in Germany and is not available in English
fSee also questionnaire forms on website: https://www.kindl.org/english/questionnaires/
gAustria, Germany, Switzerland, Spain, France, The Netherlands, Greece, Hungary, Ireland, Poland, Sweden, UK and Czech Republic
hDevine [46] reports that there are 155 items in total across item banks excluding chronic-generic QoL. Barthel [129] reports that the average number of items answered per child is around 42
iDenmark, Norway, Sweden, Finland and Iceland
jSee also questionnaire forms on website: https://www.pedsql.org/index.html
k(1) Adjust arrows on pictorial sundial to indicate the gap the between desired and current states for each item. (2) Indicate how quickly one is advancing or moving away from the goal. (3) Value the importance of each item on 7-point scale. (4) Four scores are produced for each item: (i) State score—distance between current and ideal states; (ii) Goal score—distance between goal and ideal state; (iii) Gap score (= QoL score)—distance between current state and goal weighted by speed of improvement and rank score; (iv) Rank score—importance of each item
l‘My life is going well’; ‘My life is just right’; ‘I would like to change many things’; ‘I wish I had a different kind of life’; ‘I have a good life’; ‘I have what I want in life’; ‘My life is better than most kinds’
mNo problem; problem without emotional response; problem with emotional response
nHas problem and child feels bad; has problem and feels 'quite bad'; has problem and feels 'not so good'; has problem and feels 'fine'; has no problem. For social functioning, problem behaviour, anxiety, positive mood and liveliness, only 3-point frequency scale used with score ranging 0–2
oChildren were interviewed using two medium-sized teddy bears (one which is good at the given activity and the other bad at the activity) and asked to rate how much they liked doing what the bears are doing
Table 2.
Summary of characteristics of included generic multidimensional childhood patient-reported outcome measures designed to be accompanied by preference-based value setsa
| # | Acronym: name | Reference; country | Target age (years) | Perception captured (category)b | Respondent type | Administration mode | Recall period | Domains (number, n) | Items n | Response options [scoring method] |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 16D: 16-Dimensional Health-Related Measure | Apajasalo [18]; Finland | 12–15 | No (FDH) | Child; observer | By self or interviewer | Today | Mobility; vision; hearing; breathing; sleeping; eating; speech; excretion; school and hobbies; mental function; discomfort and symptoms; depression; distress; vitality; appearance; friends (n = 16) | 16 | 5-point scale [value set available] |
| 2 | 17D | Apajasalo [67]; Finland | 8–11 | No (FDH) | Child; observer | By interviewer | Today | Mobility; vision; hearing; breathing; sleeping; eating; speech; excretion; school and hobbies; mental function; discomfort and symptoms; depression; distress; vitality; appearance; friends; concentration (n = 17) | 17 | 5-point scale [value set available] |
| 3 | AHUM: Adolescent Health Utility Measure | Beusterien [78]; UK | 12–18 | No (FDH) | Child | By interviewer | Today | Self-care; pain; mobility; strenuous activities; self-image; health perceptions (n = 6) | 6 | 4- to 7-point scale [value set available] |
| 4 | AQoL-6D Adolescent: Assessment of Quality of Life, 6-Dimensional, Adolescent | Moodie [37]; Australia, New Zealand, Fiji, Tonga | 15–18 | No (FDH) | Child | By self | Past week | Physical ability; social and family relationships; mental health; coping; pain; vision, hearing and communication (n = 6) | 20 | 4- to 6-point scale [value set available] |
| 5 | CH-6D: Child Health—6 Dimensions | Kang [50]; South Korea | 7–12; 16–18 | No information | Child | By self; mobile | Not stated | Not stated (n = 6) | 6 | 3- to 4-point scale [value set planned/in development] |
| 6 | CHSCS-PS: Comprehensive Health Status Classification System—Preschool | Saigal [60]; Canada, Australia | 2–5 | No (FDH) | Observer (clinicians, parents) | By self | Past week | Vision; hearing; speech; mobility; dexterity; self-care; emotion; learning and remembering; thinking and problem solving; pain; behaviour; general health (n = 12) | 12 | 3- to 5-point scale [value set planned/in development] |
| 7 | HUI2: Health Utilities Index 2 | Torrance [75]; Canada | 8–18 | No (FDH) | Child | By self or interviewerc | General | Sensation; mobility; emotion; cognition; self-care; pain; fertility (n = 7) | 7 | 3- to 5-point scale [value set available] |
| 8 | HUI2 Informant only | Torrance [75] | 5–8 | No (FDH) | Observer | By self or interviewer | General | See HUI2 | 7 | See HUI2 |
| 9 | HUI3: Health Utilities Index 3 | Furlong [148]; Canada | 8–18 | No (FDH) | Child | By self or interviewerc | General | Vision; hearing; speech; ambulation/mobility; pain; dexterity; emotion; cognition (n = 8) | 8 | 5- to 6-point scale [value set available] |
| 10 | HUI3 Informant only | Furlong [148] | 5–8 | No (FDH) | Observer | By self or interviewer | General | See HUI3 | 8 | See HUI3 |
| 11 | CHU9D: Child Health Utility 9D | Stevens [84, 149]; UK | 7–11 | No (FDH) | Child; observer | By self | Today/last night | Worried; sad; annoyed; tired; pain; sleep; daily routine; school work/homework; able to join in activities (n = 9) | 9 | 5-point scale [value set available] |
| 12 | EQ-5D-Y: EuroQoL 5-Dimensional questionnaire for Youth | Wille [38]; 7 countriesd | 8–15 | No (FDH) | Child; observer | By self | Today | Mobility; self-care; usual activities; pain/discomfort; feeling worried/sad/unhappy; general health VAS (n = 5 + VAS) | 5 | 3-point scale + VAS [value set available] |
| 13 | EQ-5D-Y Proxy: EQ-5D-Y for informant | Gusi, Verstraete [40, 150]; Spain, South Africa | 3–7 | No (FDH) | Observer | By self (mail) or interviewer (telephone) | Today | See EQ-5D-Y | 5 | See EQ-5D-Y |
| 14 | EQ-5D-Y-5L: EQ-5D-Y, 5 levels | Kreimeier [151]; Germany, Spain, Sweden, UK | 8–15 | No (FDH) | Child | By self | Today | Mobility; self-care; usual activities; pain/discomfort; feeling worried/sad/unhappy; general health VAS (n = 5 + VAS) | 5 | 5-point scale + VAS [value set planned/in development] |
| 15 | IQI: Infant health-related Quality of life Instrument | Jabrayilov [51]; UK, New Zealand, Singapore | 0–1 | No (FDH) | Observer | By self; mobile | Today | Sleeping; feeding; breathing; stooling/poo; mood; skin; interaction (n = 7) | 7 | 4-point scale [value set available] |
| 16 | QWB: Quality of Well-Being scale | Kaplan [152, 153]; US | N/A | No (FDH) | Child; observer | by self or interviewer | Functional level: general, symptoms: past 3 days except today | Mobility; physical activity; social activity; symptom-problem complex (n = 4) | 61 | 2- to 4-point scale [value set available] |
| 17 | TANDI: Toddler and Infant health related quality of life instrument | Verstraete (2020b); (2020c) [39, 154]; South Africa | 0–3 | No (FDH) | Observer | By self; mail | Today | Movement; play; pain; relationships; communication; eating; general health VAS (n = 6) | 6 | 3-point scale [value set planned/in development] |
FDH functioning, disability and health, HRQoL health-related quality of life, QoL quality of life, VAS visual analogue scale, N/A Not applicable
aMeasures arranged alphabetically; family of measures (e.g. CHSCS-PS, HUI2 and HUI3) arranged together
bDenotes whether the measure items capture child respondent’s perception (i.e. his/her enjoyment, satisfaction, expectations, standards and concerns) on health, health-related aspect or position in life: ‘yes’ if more than 75% of items did; ‘no’ if less than 25% of items did; ‘some’ if between 25% and 75% of items did. ‘Yes’ implies that the measure is predominantly a QoL or HRQoL measure; ‘no’ predominantly an FDH measure; and ‘some’ a mix of QoL/HRQoL and FDH
cInterviewer-administered for age 8–12
dGermany, Spain, South Africa, Italy, Sweden, The Netherlands and the UK
Characteristics of Measure by Age Category
Age groups 5–7 and 8–11 were combined for the subsequent descriptions of measure characteristics because relatively few measures targeted these age groups. This generated three rather than four age categories for the subsequent descriptions. Overall, there were 20 measures that targeted children less than 5 years, 29 that targeted children aged 5–11 years and 24 that targeted adolescents aged 12–18 years. Thirty-seven measures covered multiple age groups. Table C in the Supplementary Information (see the electronic supplementary material) organises the included measures by the above four target age group categories.
Conceptual Basis
Figure 3 shows the number of identified PROMs by age category according to their conceptual basis. The proportion of measures designed to elicit the child’s perception of their status (i.e. QoL, HRQoL and QoL/HRQoL-FDH measures) varied between four out of 20 (20%) for the infants, toddlers or pre-schoolers category and 18 out of 24 (75%) for adolescents. Twenty of 37 (54%) measures with multi-age group coverage were designed to elicit the child’s perception of their status.
Fig. 3.
Number of identified generic multidimensional childhood PROMs by conceptual basis and age category. FDH functioning, disability and health, HRQoL health-related quality of life, PROM patient-reported outcome measure, QoL quality of life
Domain Coverage
Table 3 lists in alphabetical order the domains covered by generic multidimensional childhood PROMs stratified by age category and conceptual basis. Moreover, domains that are unique to each age category are presented in bold and underlined.
Table 3.
Domains covered by included patient-reported outcome measures by age category and conceptual basis. Domains unique to age category are highlighted in bold and underlined
| Age category | Underlying conceptual basis | Domains |
|---|---|---|
| Infants, toddlers or pre-schoolers (age < 5)a | FDH | Accident; activities; activities needing assistance; Acute minor illness; Acute significant illness; aids/devices for functioning; anger; anxiety; arising; behaviour; bodily pain; breathing; change in health over past over; chronic illness; cognitive functioning; communication; depressive symptoms; dexterity; dressing and grooming; eating; emotion; emotional functioning; Engagement—Curiosity; Engagement—Persistence; Family cohesion; Family environment; Feeding; general behaviour; general health; general health perceptions; getting along; global health; grip; Growth and development; hearing; Hospital admission; Hygiene; Immunisation; Interaction; Learning and remembering; mobility; mood; movement; pain; parental impact—emotion; Parental impact—General health; Parental impact—Mental; parental impact—time; physical activity; physical challenges; physical functioning; Physical symptoms; play; positive affect; Psychomotor development; Psychopathological elements; reach; relationships; Responsiveness; school functioning; self-care; Self-control—Adaptability; Self-control—Self-regulation; Skin; Sleep; Sociability; Social functioning; Social relationships; Speech; Stooling; Temperaments and moods; Thinking and problem solving; total functional status; VAS general health; VAS pain; VAS wellbeing; vision; walking |
| QoL/HRQoL-FDH | Child behaviour; emotion; family; friends; physical health; school; self-esteem | |
| QoL | Activities at school and leisure; family life; global health; social life | |
| HRQoL | Anxiety; Appetite; communication; Liveliness; Lung problems; motor functioning; positive mood; Problem behaviour; Skin problems; sleeping; social functioning; Stomach problems | |
| Pre-adolescents (age 5–11)b | FDH | Able to join activities; achievement; Annoyed; appearance; breathing; cognition; comfort; concentration; Daily routine; depression; dexterity; discomfort and symptoms; distress; doing usual activities; eating; emotion; emotional functioning; excretion; feeling worried, sad or unhappy; fertility; friends; fun with friends; general health; hearing; mental function; mental health; mobility; looking after myself; moving; pain; parents listen to ideas; physical functioning; physical health; QoL; resilience; risk avoidance; sad; satisfaction; school and hobbies; school functioning; school work; self-care; sensation; Sleeping; Social functioning; speech; tired; VAS global health; vision; vitality; worried |
| QoL/HRQoL-FDH | Emotion; family; friends; mental; physical; physical health; school; self-esteem; social | |
| QoL | Activities at school and leisure; anxious-depressed; cognitive; community connectedness; emotional; family functioning; family life; family relationships; Food satisfaction; future security; general wellbeing; global health; health; leisure-time activities; life achievement; personal relationships; personal safety; physical; physical activity; physical complaints; physical handicaps; physical limitations; relation with parents; relation with peers; Relation at school; Relation with siblings; school achievements; Self-concept; Social; Social conflicts; social life; social wellbeing; standard of living; symptoms; Worry | |
| HRQoL | Achievement; Activity impairment; asthma-specific; autonomy; cognitive; cognitive functioning; comfort; energy; general health perceptions; general wellbeing; global negative emotional functioning; global positive emotional functioning; healthiness; motor functioning; pain and symptoms; physical; physical complaints; physical health; psychosocial; resilience; risk avoidance; social; social functioning; symptoms; Values; wellbeing | |
| Adolescents (age 12–18)c | FDH | Achievement; appearance; breathing; comfort; coping; depression; discomfort and symptoms; Disorders; distress; eating; emotional functioning; excretion; friends; health perceptions; mental function; mental health; Physical ability; physical functioning; resilience; risk avoidance; satisfaction; school and hobbies; school functioning; self-image; sleeping; social and family relationships; social functioning Strenuous activities; vision, hearing and communications; vitality |
| QoL/HRQoL-FDH | Bodily pain; change in health; emotion; Emotional wellbeing; external conditions; family; family activities; family cohesion; friends; general behaviour; general health perceptions; getting along; global behaviour; health; interpersonal conditions; Intimacy; Material wellbeing; mental; mental health; physical; physical functioning; physical health; Place in community; productivity; psychological conditions; role functioning—behavioural; role functioning—emotional; role functioning—physical; school; Safety; self-esteem; social | |
| QoL | Becoming; Being; Belonging; Contextual factors to QoL; Environment; family; general QoL; global QoL and health satisfaction; Independence; leisure and relationships; living environment; Opposite-sex relationship; pain; Perceptual factors to QoL; Personal competence; physical; physical appearance; psychological; psychological wellbeing; residential environment; Same-sex friends; school; self; self-esteem; Sense of self; social relationship | |
| HRQoL | Anxiety; depression; Disability; emotional feelings; energy/vitality; Family communications; friends; general health; Health habits; leisure; mental health; pain; parents; perceived health; Physical fitness; physical health; psychological wellbeing; school; school work; self-esteem; Social health; social support | |
| Multi-age group coveraged | FDH | Activities; activities needing assistance; aids/devices for functioning; ambulation; anger; anxiety; arising; asthma impact; cognition; depressive symptoms; dexterity; dressing and grooming; eating; Educational; emotion; fatigue; feeling worried/sad/unhappy; fertility; fun with friends; general health; grip; hearing; hygiene; Interpersonal functioning; lack of energy; mental health; mobility; pain; pain interference; parents listen to ideas; peer relationships; physical; physical activity; physical challenges; physical health; psychological; QoL; sad; self-care; sensation; social; Social activity; speech; symptom-problem complex; tired; total functional status; Upper extremity; usual activities; VAS general health; VAS pain; VAS wellbeing; vision; walking |
| QoL/HRQoL-FDH | Bodily pain; change in health; emotion; external conditions; family; family activities; family cohesion; friends; general behaviour; general health perception; interpersonal conditions; mental health; parental impact—emotion; parental impact—time; physical functioning; physical health; psychological conditions; role functioning—behavioural; role functioning—emotional; role functioning—physical; school; self-esteem | |
| QoL | Activities; appearance; Attainments; autonomy; chronic-generic QoL; cognitive and school performance; cognitive functioning; communication; community connectedness; Continence; depression; depressive moods and emotions; discomfort; eating; emotional; energy level; family; family functioning; feelings about yourself; Financial resources; friends; future security; general affect; general satisfaction; getting along with friends; getting along with family; Getting/buying things; getting things done; global QoL; health; Helping at home; interests and hobbies; Life achievement; Life satisfaction; living environment; love/affection; maternal acceptance; mental; mental health; mobility; mood; moods and emotions; parent relationship; parent relations and home life; parent relations and autonomy; paying attention; peer acceptance; peer relationship; personal relationships; personal safety; physical; physical activity; Physical competence; physical health; physical wellbeing; place you live at; play/free time; Psychological functioning; psychological wellbeing; school; School environment; school performance; School wellbeing; self; self-care; self-perception; sight; sleep; social and leisure times; social acceptance; Social integration; social support and peers; Spiritual; standard of living; worry | |
| HRQoL | Autonomy; cognitive functioning; global negative emotional functioning; global positive emotional functioning; motor functioning; pain and symptoms; social functioning |
FDH functioning, disability and health, HRQoL health-related quality of life, QoL quality of life, VAS visual analogue scale
aVersions for this category (see Table 1 for full names of measures): QUALIN; AUQUEI Nursery; CHAQ38 Informant only; FSIIR Infants; FSIIR Toddlers; FSIIR Pre-schoolers; ITQOL; ITQOL SF47; Kiddy-KINDL CQ; Kiddy-KINDL PQ; PedsQL GCS Toddler; PedsQL SF15 Toddler; PedsQL Infant Scales (1–12 months); PedsQL Infant Scales (13–24 months); PROMIS EC; TAPQOL; WCHMP; CHSCS-PS; IQI; TANDI
bVersions for this category: AUQUEI Primary; CHIP-CE CRF; CHIP-CE PRF; CHIP-CE PRF (short-form); CHRS; CHSCS; ExQoL; HAY; HDQ Age 6; HDQ Age 10; Healthy Pathways CRS; Healthy Pathways PRS; Kid-KINDL CQ; PedsQL GCS Young Child; PedsQL GCS Child; PedsQL SF15 Young Child; PedsQL SF15 Child; P-MYMOP; PROMIS PGH7 Informant only; PWI-SC8; QLQC; QLSI-C; QLSI-C Tablet; QoL-C; TACQOL Informant only; 17D; HUI2 Informant only; HUI3 Informant only; CHU9D
cVersions for this category: OK.ado; CHIP-AE; CHQ-CF87; CHQ-CF45; ComQOL-S5; COOP Charts; DHP-A; HDQ Age 13; HDQ Age 16; Kiddo-KINDL AQ; Nordic QoLQ; PedsQL GCS Teen; PedsQL SF15 Teen; QOLPAV; QOLQA; QOLQA-Taiwan; MSLSS-A; VSP-A; WHOQOL-BREF; YQoL-R; YQoL-S; 16D; AHUM; AQoL-6D Adolescent
dVersions for this category: ACHWM; CHAQ; CHAQ Informant only; CHAQ38; CHQ-PF50; CHQ-PF28; CQoL; FSIIR School children; FSIIR-14; GCQ; ILC; Kid- & Kiddo-KINDL PQ; KINDL CAT-SCREEN; KIDSCREEN-52; KIDSCREEN-27; KIDSCREEN-10; Kids-CAT; MCWBS; Nordic QoLQ Informant only; PQ-LES-Q; PROMIS PIB; PROMIS PIB SF; PROMIS PIB CAT; PROMIS PGH7; PWI-SC; SLSS; MSLSS; MSLSS Brief; TACQOL; TedQL; CH-6D; EQ-5D-Y; EQ-5D-Y Proxy; EQ-5D-Y-5L; HUI2; HUI3; QWB
Thirty-one domains were unique to infants, toddlers or pre-schoolers, of which 25 were unique to FDH measures and six to HRQoL measures. There were ten unique domains for pre-adolescents, 25 for adolescents and 16 for the multi-age coverage category. The unique domains were concentrated in FDH measures (19 of 25; 76%) for infants, toddlers or pre-schoolers, and in QoL measures (16 of 25; 64%) for adolescents.
Respondent/Informant Type and Other Measure Characteristics
Table 4 summarises the respondent and informant types and design features of measures by age category.
Table 4.
Frequencies of alternative characteristics of measures by target age category
| Target age category | |||||
|---|---|---|---|---|---|
| Age < 5 N = 20 |
Age 5–11 N = 29 |
Age 12–18 N = 24 |
Multi-age N = 37 |
Total N = 110 |
|
| Respondent and informanta type—number of measures (%) | |||||
| Designed primarily for child report | 1 (2.3) | 9 (20.5) | 18 (40.9) | 16 (36.4) | 44 (40.0) |
| Child report, aided by adult (e.g. parent) | 1 (16.7) | 3 (50.0) | 2 (33.3) | 0 (0.0) | 6 (5.5) |
| Compatible with child and proxyb | 0 (0.0) | 2 (20.0) | 1 (10.0) | 7 (70.0) | 10 (9.1) |
| Compatible with child and observer | 0 (0.0) | 7 (43.8) | 3 (18.8) | 6 (37.5) | 16 (14.5) |
| Compatible with proxy report onlyb | 2 (22.2) | 3 (33.3) | 0 (0.0) | 4 (44.4) | 9 (8.2) |
| Compatible with observer report only | 16 (64.0) | 5 (20.0) | 0 (0.0) | 4 (16.0) | 25 (22.7) |
| Administration mode—number of measures (%) | |||||
| Compatible with child self-report | |||||
| By self, non-electronic | 0 (0.0) | 8 (21.1) | 15 (39.5) | 15 (39.5) | 38 (50.0) |
| By self, electronic | 0 (0.0) | 3 (27.3) | 1 (9.1) | 7 (63.6) | 11 (14.5) |
| By self or staff/interviewer | 0 (0.0) | 2 (28.6) | 3 (42.9) | 2 (28.6) | 7 (9.2) |
| By group or aided | 1 (14.3) | 2 (28.6) | 1 (14.3) | 3 (42.9) | 7 (9.2) |
| By staff/interviewer | 1 (7.7) | 6 (46.2) | 4 (30.8) | 2c (15.4) | 13 (17.1) |
| Compatible only with informant report | |||||
| By self, non-electronic | 11 (50.0) | 6 (27.3) | 0 (0.0) | 5 (22.7) | 22 (64.7) |
| By self, electronic | 1 (50.0) | 1 (50.0) | 0 (0.0) | 0 (0.0) | 2 (5.9) |
| By self or staff/interviewer | 3 (75.0) | 0 (0.0) | 0 (0.0) | 1 (25.0) | 4 (11.8) |
| By staff/interviewer | 3 (50.0) | 1 (16.7) | 0 (0.0) | 2 (33.3) | 6 (17.6) |
| Recall period—number of measures (%) | |||||
| Compatible with child self-report | |||||
| Current/Today | 0 (0.0) | 7 (53.8) | 2 (15.4) | 4 (30.8) | 13 (17.1) |
| General | 1 (5.3) | 3 (15.8) | 6 (31.6) | 9 (47.4) | 19 (25.0) |
| Less than month (4 weeks) | 1 (4.8) | 3 (14.3) | 4 (19.0) | 13 (61.9) | 21 (27.6) |
| Month (4 weeks) or more | 0 (0.0) | 7 (38.9) | 9 (50.0) | 2 (11.1) | 18 (23.7) |
| Unclear | 0 (0.0) | 1 (20.0) | 3 (60.0) | 1 (20.0) | 5 (6.6) |
| Compatible only with informant report | |||||
| Current/Today | 2 (66.7) | 0 (0.0) | 0 (0.0) | 1 (33.3) | 3 (8.8) |
| General | 1 (25.0) | 3 (75.0) | 0 (0.0) | 0 (0.0) | 4 (11.8) |
| Less than month (4 weeks) | 7 (58.3) | 1 (8.3) | 0 (0.0) | 4 (33.3) | 12 (35.3) |
| Month (4 weeks) or more | 7 (53.8) | 3 (23.1) | 0 (0.0) | 3 (23.1) | 13 (38.2) |
| Unclear | 1 (50.0) | 1 (50.0) | 0 (0.0) | 0 (0.0) | 2 (5.9) |
| Number of domains—number of measures (%) | |||||
| Compatible with child self-report | |||||
| With ≤ 5 domains | 1 (2.9) | 13 (38.2) | 11 (32.4) | 9 (26.5) | 34 (44.7) |
| With > 5 domains | 1 (2.4) | 8 (19.0) | 13 (31.0) | 20 (47.6) | 42 (55.3) |
| Compatible only with informant report | |||||
| With ≤ 5 domains | 8 (57.1) | 3 (21.4) | 0 (0.0) | 3 (21.4) | 14 (41.2) |
| With > 5 domains | 10 (50.0) | 5 (25.0) | 0 (0.0) | 5 (25.0) | 20 (58.8) |
| Number of items—number of measures (%) | |||||
| Compatible with child self-report | |||||
| With ≤ 30 items | 2 (4.2) | 15 (31.3) | 14 (29.2) | 17 (35.4) | 48 (63.2) |
| With > 30 items | 0 (0.0) | 6 (21.4) | 10 (35.7) | 12 (42.9) | 28 (36.8) |
| Compatible only with informant report | |||||
| With ≤ 30 items | 8 (47.1) | 4 (23.5) | 0 (0.0) | 5 (29.4) | 17 (50.0) |
| With > 30 items | 10 (58.8) | 4 (23.5) | 0 (0.0) | 3 (17.6) | 17 (50.0) |
| Response optiond | |||||
| Compatible with child self-report | |||||
| With < 5 points on scale | 1 (6.3) | 6 (37.5) | 1 (6.3) | 8 (50.0) | 16 (21.1) |
| With ≥ 5 points on scale | 1 (1.9) | 11 (20.4) | 24 (44.4) | 18 (33.3) | 54 (71.1) |
| Non-point option/uncleare | 0 (0.0) | 5 (83.3) | 1 (16.7) | 0 (0.0) | 6 (7.9) |
| Compatible only with informant report | |||||
| With < 5 points on scale | 7 (58.3) | 1 (8.3) | 0 (0.0) | 4 (33.3) | 12 (35.3) |
| With ≥ 5 points on scale | 10 (50.0) | 7 (35.0) | 0 (0.0) | 3 (15.0) | 20 (58.8) |
| Non-point option/unclear | 1 (50.0) | 0 (0.0) | 0 (0.0) | 1 (50.0) | 2 (6.1) |
| Scoring method | |||||
| Unweighted sum/average/proportion | 15 (19.2) | 22 (28.2) | 20 (25.6) | 21 (26.9) | 78 (70.9) |
| Rasch score | 0 (0.0) | 0 (0.0) | 0 (0.0) | 7 (100.0) | 7 (6.4) |
| Weighted sum/average/proportion | 0 (0.0) | 2 (50.0) | 1 (25.0) | 1 (25.0) | 4 (3.6) |
| Value set application | 3 (17.6) | 4 (23.5) | 3 (17.6) | 7 (41.2) | 17 (15.5) |
| Unclear | 2 (50.0) | 1 (25.0) | 0 (0.0) | 1 (25.0) | 4 (3.6) |
FDH functioning, disability and health, HRQoL health-related quality of life, QoL quality of life
aInformant responds to the questionnaire instead of child self-report. Two informant types are proxy if the informant infers the child’s perception on health/QoL items and observer if not
bApplicable to both child and proxy after changing question wording from first- to third-person reference; includes versions of QoL/HRQoL-FDH measures that require informant to be both proxy and observer
cOne version was administered by staff using computer tablet
dEleven measures used pictorial/narrative aid: one for age < 5; seven for age 5–11; one for age 12–18; two for multi-age
eExamples of non-point option are visual analogue scale and sundial approach in QLSI-C
Measures targeting younger age groups were more likely to rely on informant report. For example, of the 25 measures compatible with observer report only, 16 (64%) targeted children aged less than 5, and none targeted adolescents. Of the 44 measures designed primarily for child report, 18 (41%) targeted adolescents, and one (2%) targeted children aged less than 5.
Of 76 measures compatible with child self-report, 49 (65%) were designed for self-administration using non-electronic or electronic modes of data collection. Overall, there were 14 measures (13%) that used electronic data collection from children or informants: seven using computers at study locations [41–47], four using tablets or mobiles [48–51] and three via the internet from non-study locations [52, 53].
Shorter recall periods were used for younger age groups. Of 13 measures compatible with child self-report that had a recall period of current/today, seven (54%) targeted children aged 5–11, while two (15%) targeted adolescents.
The numbers of domains and items tended to be lower for measures targeting younger age groups. Of 42 measures compatible with child self-report that had more than five domains, 13 (31%) targeted adolescents and eight (19%) targeted children aged 5–11. Of 28 measures compatible with child self-report that had more than 30 items, ten (36%) targeted adolescents and six (21%) targeted children aged 5–11. Two measures used computerized adaptive tests [46, 47] that tailored the number of items according to respondents’ previous answers.
Most measures (102 of 110; 93%) used some form of ordinal scale with a pre-defined number of response points (e.g. Likert scale). Six measures used a visual analogue scale (VAS) alongside a Likert scale. Eleven measures used pictorial or narrative aids for child self-reports.
The vast majority of measures without value sets (78 of 93; 84%) used unweighted sums, averages or proportion of maximum scores as scoring methods. Four measures without value sets elicited importance scores from children, with items weighted to produce the overall QoL score [30, 49, 54, 55]. The KIDSCREEN and PROMIS groups of measures used item response theory scoring [44–47, 56–59].
Content Validity of Measures
Table D in the Supplementary Information (see the electronic supplementary material) describes the background concepts and development methods for measures, including whether and how children (or parents/primary caregivers in case of infants, toddlers or pre-schoolers) were involved in the development and pilot-testing processes. The development of 21 of the 110 measures (19%) involved qualitative research with or surveys of children for domain and item elicitation. Nine measures were based on adapting existing adult measures: one conducted focus groups with adolescents [37]; two cognitive interviews [6, 38]; one feasibility testing [60]; and five made little or no mention of children’s involvement [54, 61–64]. Twenty-three measures reported no involvement of children, relying on clinical and/or research expertise for domain/item elicitation, using statistical techniques for item selection (e.g. for short-form development), or provided little detail on development. Among measures with multi-age group coverage, nine of the 37 measures (24%) conducted qualitative research with children.
Seven measures addressed cultural issues in initial measure development. The ACHWM aimed to develop a culturally appropriate model of health and wellbeing for Aboriginal communities in Canada [48]. The QOLQA and QOLQA-Taiwan aimed to develop measures appropriate for Asian adolescents [35, 65]. The development of the IQI involved interviews with parents of infants aged 0–3 years from New Zealand, Singapore and the UK to assess cross-cultural interpretability [51]. Izutsu et al. adapted the existing English version of the WHOQOL-BREF for Bangladeshi adolescents and identified the need for interviewer administration due to low literacy rates in this context [36]. Finally, the KIDSCREEN measures were developed across 13 European countries specifically for cross-cultural relevance [57]; while the development of the EQ-5D-Y similarly included seven countries [38].
Characteristics of Preference-Based Value Sets for Childhood PROMs
The characteristics of 21 preference-based value sets developed by 19 studies of ten generic multidimensional childhood PROMs are summarised in Table 5. The CH-6D, CHSCS-PS, EQ-5D-Y-5L and TANDI, which have been designed as generic multidimensional childhood PROMs with the accompaniment of a value set as an objective, did not have value sets publicly available at the time of the review. The 21 value sets were predominately developed in high-income countries with the exception of those developed for Fiji and Tonga (AQoL-6D Adolescents) [37] and China (CHU9D) [66]. BWS and DCEs increased in frequency of use in recent valuation studies, with TTO and SG techniques largely utilised at least a decade ago, unless used for the specific purpose of anchoring the DCE/BWS to the QALY scale. The respondent types from which the value sets were derived differ across measures, ranging from school children and students (16D, AQoL-6D, CHU9D [Australia and China]) through to parents/caregivers (17D, HUI2, IQI), with general population adult samples forming the most prevalent source of values overall (AHUHM, CHU9D [UK and The Netherlands], EQ-5D-Y, HUI2, HUI3, QWB, IQI).
Table 5.
An overview of country-specific value sets for generic multidimensional childhood PROMsa
| Bibliography/setting | Sampling methods | Preference data collection | Value set statistical features | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Acronymb | Valuation reference | Valuation country | Valuation population | Sample size | Elicitation technique | Administration mode | Perspective | Rank order of domains (most to least important)c | Value range | Negative values (%)d |
| 16D | Apajasalo [18] | Finland | School children in Helsinki aged 12–15 | 213 | RS | By self | Adolescent’s own health | Breathing (most important); physical appearance (least important) | Not reported | 0 |
| 17D | Apajasalo [67] | Finland | Parents of children in the study: school children, survivors of organ donation and patients with skeletal dysplasia aged 8–11 | 115 | RS | By interviewer | Adult rates on behalf of child | Mobility > breathing > school and hobbies > friends > hearing > vision > eating > elimination > vitality > sleeping > anxiety > discomfort and symptoms > learning and memory > ability to concentrate > depression > speech > physical appearance | Not reported | 0 |
| AHUM | Beusterien [78] | UK | UK general population—adults aged ≥ 18 | 312 | TTO | By interviewer | Adult’s own health | Not provided | 0.419 to 1 | 0 |
| AQoL-6D Adolescent | Moodie [37] | Australia, Fiji, NZ, Tonga | Students (adolescents) from four countries | 279 | TTO | Classroom based | Adolescent’s own health | Not provided | Australia: 0.072–1; Fiji: 0.094–1; NZ: 0.053–1; Tonga: 0.068–1 | 0 |
| CHU9D | Stevens [79] | UK | UK general population (mean age 49) | 300 | SG | By interviewer | Adult’s own health | Pain > activities > daily routine > sleep > sad > work > tired > annoyed > worry | 0.337 to 1 | 0 |
| Ratcliffe [69] | Australia | Convenient sample of students aged 18–29 (Flinders University, Faculty of Medicine, Nursing and Health Sciences) | 38 | TTO | By interviewer | Young adult’s own health | Sad > pain > annoyed > work > sleep > daily routine > worried > activities > tired [by utility range] | − 0.1059 to 1 | 0.19 | |
| Ratcliffe [74] | Community based sample of adolescents aged 11–17 (online panel) | 1982 | BWS | By self; online | Adolescent’s own health | |||||
| Chen [66] | China | Students in Nanjing City, China (BWS); undergraduate students from Nanjing Medical University (TTO) | 902 (BWS); 38 (TTO) | BWS/TTO | By self (BWS); by interviewer (TTO) | Adolescent’s/young adult’s own health | Activities > tired > worried > work > sad> sleep > daily routine > pain > annoyed [by utility range] | 0.0563 to 1 | 0 | |
| Rowen [72] | The Netherlands | General adult population in The Netherlands | 1276 | DCETTO | By self; online | Adult’s own health | Pain > sleep > activities > daily routine > worry > sad > tired > annoyed > work | − 0.568 to 1 | 5.17 | |
| EQ-5D-Y | Craig [68] | US | US general adult population aged ≥ 18 | 4155 | Paired comparisons (DCE) | By self; online | Adult values hypothetical scenario: age of child described (7 or 10); duration of loss in child health | [Based on changes from level 2 to 3] pain/discomfort > anxiety/depression > usual activities > mobility > self-care | Unclear | Unclear |
| Rupel [70] | Slovenia | Online panel adults (aged ≥ 18) across the country (DCE); non-probability purposive non-representative adult sample (aged ≥ 18) in one region (cTTO) | 1074 (DCE); 202 (cTTO) | DCE/cTTO | By self (online); By interviewer (face-to-face) | Adult takes perspective of 10-year-old child | Pain/discomfort > anxiety/depression > usual activities > mobility > self-care | − 0.691 to 1 | 20.60 | |
| Shiroiwa [71] | Japan | Japanese general adult population (aged 20–79) | 1,047 | DCE/cTTO | By interviewer; computer | Adult takes perspective of 10-year-old child | Pain/discomfort > worried/sad/unhappy > usual activities > mobility > looking after myself | 0.288 to 1 | 0 | |
| HUI2 | Torrance [75] | Canada | Parents of school-aged children from general population | 194 | SG/RS | By interviewer | Adult takes perspective of 10-year-old child: imagine living in health state until death at age 70; choose for child, not parent/family | Pain > emotion > mobility > sensation > cognition > self-care > fertility | − 0.025 to 1 | 0.26 |
| McCabe [76] | UK | UK general adult population | 175 | SG/RS | By interviewer | Adult takes perspective of 10-year-old child: imagine living in health state until death at age 70 | Pain > sensation > emotion > cognition > self-care > mobility | − 0.08 to 1 | 0.28 | |
| HUI3 | Feeny [80] | Canada | General adult population aged ≥ 16 | 256 | SG/RS | By interviewer | Adult’s own health | [Based on most impaired level per domain] cognition > emotion > pain > dexterity > ambulation > hearing = vision > speech | − 0.359 to 1 | 77.69e |
| Noto [81] | Japan | General adult population (aged 20–79) from 5 cities | 774 | SG/RS | By interviewer | Adult’s own health | [Based on most impaired level per domain] cognition > emotion > ambulation = vision > dexterity > speech > pain > hearing | − 0.002 to 1 | 0.01 | |
| Le Galès [82] | France | French general adult population aged 20–65 not suffering from any major chronic/incapacitating illness | 365 | SG/RS | By interviewer | Adult’s own health | Unclear | Unclear whether death is anchored at 0 | ||
| QWB | Seiber [83] | USA | US adults (aged 18–85) from primary care and 2 college campuses in San Diego | 430 | RS | By interviewer | Adult’s own health | Calculation taking into account symptoms and activities | 0.09–1 | 0 |
| IQI | Krabbe [77] | China-HK, UK, US | General adult population and primary caregivers of infants and toddlers (0–3 years) | 2,638 | DCE | By self; online | Adult chooses for infant | Breathing > sleeping > feeding > mood > interaction > skin > stooling | 0.015–1 | 0 |
BWS best–worst scaling, cTTO composite time trade-off, DCE discrete choice experiment, DCETTO DCE anchored with TTO, HK Hong Kong, NZ New Zealand, PROM patient-reported outcome measure, RS rating scale, SG standard gamble, TTO time trade-off
aThere are 19 studies: one study included four value sets [37], and two studies had one value set [69, 74]; thus there are 21 value sets from the 19 studies
bSee Table 1 for full name of instruments
cExamined by the authors of this review based on how the value sets were developed and whether sufficient information was reported; it should be noted that there could not be a consistent way to examine all value sets
dCalculated by authors for all potential health states
eBased on simulation of publicly available Canadian HUI3 algorithm; however, separate communication with the developers suggests this may be an overestimate
The size of the samples from which the value sets were derived differed markedly between valuation studies, ranging from 115 (17D, parents of children [67]) to 4155 (EQ-5D-Y US value set, adults [68]). Some studies conducted complementary TTO experiments to assist with anchoring [66, 69–71] or used a particular type of DCE that includes a life year attribute (which is commonly referred to as DCETTO) [72] to assist with anchoring. Studies using BWS and DCE generally used larger sample sizes than more traditional approaches to health state valuation such as RS, TTO and SG (supplementary Figure S1, see the electronic supplementary material) [73]. Studies employing DCE and BWS approaches in relatively large samples also tended to be administered in self-complete online surveys. In contrast, TTO and SG were more likely to be administered in smaller samples and in an interview format due to their greater complexity and iterative nature [73].
Seven value sets were elicited from adolescents using RS for the 16D [18], TTO for AQoL-6D Adolescents (four value sets) [37] and BWS for CHU9D [66, 74]. One study elicited values from young adults to anchor the Ratcliffe 2015 value set for the CHU9D [74]. Amongst the remaining studies that sampled adult populations, five studies eliciting country-specific value sets for the EQ-5D-Y or HUI2 asked adult respondents to express their preferences from the perspective of a child aged 7 and 10 years [68] or 10 years [70, 71, 75, 76]. The HUI2 value sets [75, 76] added additional context by specifically asking the adult to imagine the child living in the health state for the remainder of their life expectancy, with death at age 70. Two value sets were elicited from adults on behalf of the child [67, 77]. The remaining seven studies [72, 78–83] elicited health state preferences from adults who were asked to value health states from the perspective of their own health.
There were noticeable variations in the features of the value sets depending on the setting and valuation protocols adopted. For example, there are several value sets for the CHU9D, including the UK general adult population value set based on SG [79], the Dutch general adult population value set based on the DCETTO [72] and Australian [74] and Chinese [66] adolescent value sets based on BWS. Variation in the rank order of the most important domains is evident and illustrated in Table 5, with adolescents in China ranking the ability to join in activities and tired domains as most important, adolescents in Australia placing greater importance on mental health domains (e.g. sad, annoyed) and adults in both the UK and the Netherlands placing greater importance on physical health domains (e.g. pain and sleep). However, these differences may be due to the differences in valuation protocol, the sample characteristics and differences in preferences between countries
The range of health state values and the percentage of negative health state values (health states considered worse than being dead) differs across value sets within measures (Fig. 4 shows the value range by perspective, and supplementary Figure S2 shows the range by elicitation method). For example, the worst health state described by the CHU9D had a value of 0.337 in the UK general population value set, in contrast to the Australian [69], Chinese [66] and The Netherlands [72] value sets, where the worst health state had values of −0.1059, 0.0563 and −0.568, respectively. The UK general population and Chinese adolescent CHU9D value sets have no health states valued at worse than being dead, whereas the Australian adolescent and The Netherlands general population value sets have 0.19 and 5.17% of health states, respectively, valued at worse than being dead.
Fig. 4.
Preference-based index value range by perspective
Similarly, when drawing comparisons between different instruments, marked differences are evident in the range of values for value sets and in the proportions of health states that are valued at worse than being dead, with most studies reporting no negative health state values [18, 37, 66, 67, 71, 77, 79, 83]. However, some studies report relatively high proportions of health states valued at worse than being dead: e.g. 21% for the Slovenian EQ-5D-Y value set [70], whilst the publicly available Canadian algorithm for the HUI3 suggests that as many as 78% of health states are valued as worse than being dead [80]. The latter’s value range and proportion of negative values is noticeably greater than all other value sets. A potential reason for the high proportion could be the multiplicative function used for HUI3 valuation tasks as compared to the more common additive functional form used by other measures (supplementary Figs. S3a and S3b). More broadly, differences in modelling approaches can be as important as differences in the stated-preference methods used in determining the overall characteristics of value sets. Supplementary Tables E and F contain further detail on the valuation tasks and modelling methods for studies using two classes of methods for value set development (RS, TTO and SG [Table E, see the electronic supplementary material]; and DCE and BWS [Table F, see the electronic supplementary material]).
Discussion
This systematic review has generated an up-to-date and comprehensive catalogue of generic multidimensional childhood PROMs and the value sets that accompany them. The outputs can be used to inform the development, selection and interpretation of measures and value sets across a range of research contexts, including research designed to inform patient-centred care and research designed to inform cost-effectiveness-based decision-making. Specifically, the description provided of PROM characteristics such as target age range and domain coverage—alongside evidence on their psychometric performance explored in a forthcoming systematic review—should aid decisions around measure selection and, where appropriate measures are currently not available, measure development. Likewise, the granulated descriptions and categorisations of measures and value sets should aid interpretation of the outputs produced by measures across a range of methodological characteristics.
The review is the first to comprehensively catalogue generic multidimensional childhood PROMs according to the conceptual basis using definitions identified and outlined by the author team. It hence builds on the previous work by Fayed et al., who highlighted ways in which the terms health status, QoL and HRQoL are often used interchangeably in the literature despite important conceptual differences [3]. This is similarly noted by Karimi and Brazier [9], although their suggestion that the term HRQoL be reserved only to describe health utilities does not distinguish between measurement (individual perception incorporated into the descriptive systems of HRQoL measures) and valuation (societal preferences used to derive value sets) considerations. The latter may be appropriate for informing societal resource allocation decisions, while the former is more suited to informing clinical decisions for individuals [84], although the dichotomy is not absolute. Therefore, appropriate selection of childhood PROMs for research and decision making necessitates careful consideration of the context within which they are to be used.
The ISPOR guidelines for application of childhood PROMs recommend end users consider the applicability of characteristics of measures across children of different ages [2]. For example, a key consideration is the health/QoL domain coverage of PROMs targeted at particular age groups. This review describes the methods adopted to elicit relevant domains and items from children of different ages or their parents/caregivers and updates the content validity assessment conducted by Janssens et al. [85]. Moreover, our review catalogues the resulting domains by conceptual basis and target age group, highlighting those that are unique to each category and aiding instrument selection by the end user’s target childhood population and domains of interest. Previous reviews have listed domain coverage, but only for individual instruments and not by conceptual and age categories [4, 10, 22, 25, 26]. The review identifies unique domains across developmental stages from infancy to adolescence, with a focus on observable FDH aspects (e.g. responsiveness, appetite) during the first 5 years of life replaced by increased focus on self-perception of activities and immediate relations during pre-adolescence (e.g. activity impairment, relation with siblings), followed by heightened awareness of personal independence and wider relations during adolescence (e.g. personal competence, opposite-sex relationships).
The emphasis on age-specific content should be balanced by the ability to generate outcomes that are comparable across childhood age groups and between children and adults; the latter is particularly important for research informing life-course healthcare decision-making [86]. The development of early childhood (age 1–5 years) versions of PROMIS sought to balance the two priorities by eliciting relevant domains and items from parents while minimising changes to the recall period and question wording of adult PROMIS measures [87]. Likewise, we identified nine measures that adapted existing adult measures, three after conducting qualitative research or cognitive interviews with children [6, 37, 38]. Significantly, two of these measures that were adapted with childhood input—QoL-C for age 4–9 years [6] and EQ-5D-Y for age 8–15 years [38]—are based on the EQ-5D, the reference case outcome measure for cost-utility analyses in the UK [13].
Another key age-dependent characteristic is the feasibility of child self-report, which is clearly dependent on not only chronological age, but also on children’s cognitive capacity and their educational abilities (reading, comprehension and writing skills). Unsurprisingly, only two of 20 measures targeting children aged less than 5 provide for self-report [88, 89], compared to 21 of 29 measures targeting pre-adolescents and all measures targeting adolescents. Where informant reports are used, this review distinguishes between proxy and observer report depending on the extent to which the informant is asked to infer a subjective perception over health/QoL items for the child. Hence, there is close overlap between the conceptual basis of the measure and informant type. This distinction is recommended by the ISPOR guideline [2], but is yet to be widely implemented. Janssens et al., for example, did not include the observer/proxy distinction in their discussion (p. 325) [4]. Moreover, based on empirical findings that child–informant agreement is poorer for subjective health/QoL constructs (e.g. emotion, pain) than observable ones (e.g. mobility, self-care) [90, 91], the ISPOR guideline recommends instruments involving observer report over those involving proxy report where informant report is found necessary (p. 470) [2]. Of the 61 identified measures compatible with informant report, 19 (31%) involved proxies; of the 33 measures compatible only with informant report (i.e. not designed for child self-report), nine (27%) involved proxies. This suggests that many existing measures do not adhere to the ISPOR recommendation, although more recently developed measures for infants and early childhood specifically cite this recommendation as the reason for including only observable domains [39, 87]. A key rationale for incorporating informant (specifically parent) report is that informants often have vital insights into the child’s health needs and medical history and hence this facilitates healthcare decision-making [2, 92]. Future research should examine to what extent this decision-making is impaired by limiting the informant focus to observable domains.
Incorporating age-appropriate instrument design is another ISPOR good research practice principle [2]. Electronic data collection is recommended as the preferred administration mode, particularly in pre-adolescent age groups where self-report is possible [2, 21, 93]. Accordingly, three measures specifically targeting pre-adolescents incorporated electronic data collection in self-reports [41, 42, 49] as did another seven with multi-age group coverage including pre-adolescents [43–47, 50, 53]. The ISPOR guideline notes the difficulties children face in comprehending extended periods and recommends a recall period of 24 h or less. A recent systematic review by Coombes et al. on the design of childhood PROMs in primary studies similarly recommends a recall period of 48 h or less for children aged 5–7 years [94]. These recommendations were applied (i.e. recall period of current/today or general) by half of the measures in our review that elicit pre-adolescent self-reports. The other half contains recall periods ranging from the past week to the past 2 months. Concerning instrument length, relatively few instrument developers were concerned about the administrative burden of long instruments and used this as a rationale for development of short forms [53, 55, 58, 95]. In all, the number of instruments adhering to design recommendations is generally low.
For childhood PROMs accompanied by preference-based value sets, a key challenge lies in how to elicit societal preference weights. This review reveals an increasing trend towards using ordinal approaches (e.g. DCE and BWS) in health state valuation. These approaches (in particular BWS) have different cognitive demands to iterative approaches and may be more suitable for children to self-complete [96]. Given death is normally not included in the hypothetical choice tasks, this also aligns with ethical considerations for children. It enables preferences of children to be directly elicited and has been adopted by the studies that derived value sets for the CHU9D in Australia and China [66, 74]. However, given the raw values from these ordinal tasks are estimated on a latent utility scale, there is a need to have a standalone valuation survey using traditional direct approaches to health state valuation (e.g. TTO or SG) with (normally) a small convenience sample to facilitate the re-scaling exercise. This enables the value sets to produce cardinal health state values compatible with the 0–1 (dead to full health) QALY scale (for a comprehensive overview, see [73, 97]). For the most recently published EQ-5D-Y value sets, a similar approach was used, but instead of using BWS, a DCE was adopted in the main valuation task [70, 71].
This review identified that the majority of value sets (14/21 value sets) were derived using adult samples; in half of these, the adult was asked to take the perspective of a child, and half were from the adult’s own perspective. Decisions about the appropriate perspective to adopt when developing value sets for economic evaluation need to consider the preference of health technology assessment (HTA) agencies or decision makers in each country. There are currently notable differences in the approaches taken for each country. For example, CHU9D value sets developed in the UK and The Netherlands are both based on the perspective and preference of the general public (aged ≥ 18 years) [72, 79]. The SG was used for the UK value set [79], whilst a DCETTO was used for The Netherlands value set [72]. The DCETTO does not require a separate task or data manipulation for states considered worse than dead [12, 98]; however, this approach was developed for adults, and may not be suitable for children and adolescents given ethical concerns about the inclusion of death. Notably, this issue also applies to the TTO. It will be important for future value set generation that the methods align to decision maker preferences as results will differ depending on approach taken. This also means that caution is required when comparing values generated across country value sets. Comparability of utilities for children with utilities generated for adults will also need to be considered by decision makers. The differences in perspectives used and normative judgments around appropriate perspectives will need to be balanced with considerations of comparability.
The review has also identified a trend over the last decade towards DCE and BWS exercises involving relatively larger samples and a reliance on online surveys. Whilst there are considerable practical advantages with online sampling, care is needed to ensure the quality of responses obtained, with protocols such as those developed by the EuroQol Group playing a critical role in quality assurance [99]. Qualitative studies such as ‘think aloud’ also provide information to understand participant decisions in the absence of a face-to-face interaction [100].
Across all categories of PROMs, regardless of the construct (or concept) being measured or target age(s), a noticeable feature is the scarcity of measures developed in LMIC settings (though this could be partly related to the reviews’ sole inclusion of English language studies). Only five LMICs were covered by PROM development and only three by value set development. A previous systematic review of studies applying generic childhood PROMs accompanied by value sets found a similar scarcity, with only 8% of samples coming from LMICs [16] despite the substantially higher childhood disease burdens in these settings [101]. Further development of PROMs and value sets relevant to LMICs is a pressing research need, particularly when many characteristics of PROMs are contingent upon local contexts (e.g. low literacy rates hindering self-administration [36]) and when national HTA agencies demand value sets derived from samples relevant to local decision-making [13].
Strengths and Limitations
The contributions and strengths of this systematic review relative to previous reviews of childhood PROMs have been discussed under specific themes above (e.g. categorising PROMs by conceptual basis and target age group). There are nonetheless limitations warranting further research as well as caveats that should be borne in mind by readers. First, in contrast to previous reviews [21, 22, 24–26, 102], this review did not assess the psychometric properties of identified PROMs. Assessment of psychometric properties is recommended for PROM selection in research and decision-making [2]. In common with the work conducted by Janssens et al. [85], a separate systematic search and synthesis of the psychometric properties of the PROMs identified by this review is planned. This review provides partial evidence of the content validity of PROMs (Table D, see the electronic supplementary material), which will be combined with evidence of other aspects of content validity such as relevance and comprehensibility, as well as evidence for other psychometric properties [103]. Second, the research is constrained by a lack of consensus surrounding terms that describe the conceptual bases underpinning PROMs [9]. Hence, the methods used in this work to establish the categories of FDH, QoL and HRQoL measures [3], and the cut-off levels for hybrid categories, may be disputed. That said, a sensitivity analysis that changed the cut-off levels required for the proportion of items within measures that captured a child’s perception for them to be labelled QoL/HRQoL-FDH hybrid measures (from 25–75% to 33–67%) altered the label of only one measure (Kiddy-KINDL parent questionnaire [89]), and this had minimal impact on subsequent analyses.
Third, this review covered generic multidimensional childhood PROMs only and did not cover multidimensional childhood PROMs developed for specific conditions. Future research could usefully generate a parallel catalogue of condition-specific multidimensional childhood PROMs to inform research in specific clinical areas [104]. Fourth, the delineation of age and age categories throughout the paper is framed in chronological terms. Consideration of developmental age or markers of reading or educational ability could inform the selection of multidimensional childhood PROMs in some contexts, but this would require analyses of individual-level data from studies that also report developmental or educational outcomes. A final caveat is that this review does not report overall scores on the methodological or reporting quality of included studies through, for example, the Checklist for Reporting Valuation Studies (CREATE) score for valuation studies [105]. This was because CREATE was not developed to encompass values for childhood PROMs and misses key additional elements of the methodological choices required in this context. For PROM development studies, the planned systematic review of psychometric properties of measures will apply the Consensus-based Standards for the selection of health status Measurement Instruments (COSMIN) checklist to assess their methodological quality [103].
Conclusion
The catalogue of generic multidimensional childhood PROMs generated by this systematic review creates a valuable resource for researchers, practitioners and policy makers in selecting the most appropriate measure(s) for application within childhood populations according to their conceptual basis, target age, design and needs of the end user. This information should be viewed in conjunction with evidence surrounding the psychometric performance of measures to be presented in the follow-up systematic review. The description of PROM characteristics should inform decisions about the need to develop measures with alternative features or targeted at particular age groups. Moreover, the identified value sets covering all childhood age groups can be used to inform cost-utility analyses of childhood interventions. However, many methodological questions remain regarding the methods to use in valuing childhood PROMs, and this is currently a very active area of research. The availability of childhood PROMs and the value sets that accompany them have important roles in informing, respectively, individualised care and cost-effectiveness-based decision-making.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Funding
This research has been funded by the Australian Government’s Medical Research Future Fund under Grants MRF1200816 and MRF1199902. SP receives support as a National Institute for Health Research (NIHR) Senior Investigator (NF-SI-0616-10103) and from the NIHR Applied Research Collaboration Oxford and Thames Valley. CH is funded by an NIHR Doctoral Research Fellowship (NIHR300684). The views expressed are those of the authors and not necessarily those of the Australian Government, the NIHR or the Department of Health and Social Care in England.
Conflict of interest
ND and BM are members of the EuroQol Group, which developed the EQ-5D-Y, one of the measures included in review. Other authors have no conflict of interest to declare.
Ethical approval
Not applicable.
Availability of data and material
The Excel files of extracted data are available from the corresponding authors on reasonable request.
Code availability
The code used in this study is available from the authors upon reasonable request.
Consent for publication
The authors, jointly and severally, give the publisher the permission to publish the work.
Consent to participate
Not applicable.
Author contributions
Conceptualisation and methodology: all co-authors. Database search, study selection and data extraction: SP, JK, EH, MH, KK, SD, NR, CH and SS for identification of patient-reported outcome measures; LF, GC and ND for preference-based value sets. Data synthesis: SP, JK, EH, MH, KK, SD, CH and SS for identification of patient-reported outcome measures; LF, GC, ND, CB, KD, AH, BM and JR for preference-based value sets. Writing original draft: SP, JK, EH, MH, SD, GC, ND, CB, BM and JR. Draft review and editing: all co-authors.
Footnotes
The original Online version of this article was revised: The co-author's name has been incorrectly published.
Joseph Kwon and Louise Freijser: Joint first authors.
Julie Ratcliffe and Stavros Petrou: Joint senior authors.
Change history
2/14/2022
A Correction to this paper has been published: 10.1007/s40273-022-01135-9
Contributor Information
Joseph Kwon, Email: jkwon6@sheffield.ac.uk.
Louise Freijser, Email: louise.freijser@unimelb.edu.au.
Elisabeth Huynh, Email: elisabeth.huynh@anu.edu.au.
Martin Howell, Email: martin.howell@sydney.edu.au.
Gang Chen, Email: gang.chen@monash.edu.
Kamran Khan, Email: k.a.khan@warwick.ac.uk.
Shahd Daher, Email: shahd.daher@phc.ox.ac.uk.
Nia Roberts, Email: nia.roberts@bodleian.ox.ac.uk.
Conrad Harrison, Email: conrad.harrison@balliol.ox.ac.uk.
Sarah Smith, Email: sarah.smith@lshtm.ac.uk.
Nancy Devlin, Email: nancy.devlin@unimelb.edu.au.
Kirsten Howard, Email: kirsten.howard@sydney.edu.au.
Emily Lancsar, Email: emily.lancsar@anu.edu.au.
Cate Bailey, Email: cate.bailey@unimelb.edu.au.
Jonathan Craig, Email: jonathan.craig@flinders.edu.au.
Kim Dalziel, Email: kim.dalziel@unimelb.edu.au.
Alison Hayes, Email: alison.hayes@sydney.edu.au.
Brendan Mulhern, Email: brendan.mulhern@chere.uts.edu.au.
Germaine Wong, Email: germaine.wong@health.nws.gov.au.
Julie Ratcliffe, Email: julie.ratcliffe@flinders.edu.au.
Stavros Petrou, Email: stavros.petrou@phc.ox.ac.uk.
References
- 1.Johnston BC, Patrick DL, Devji T, Maxwell LJ, Bingham III CO, Beaton D, et al. Patient-reported outcomes. In: Higgins JPT TJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane handbook for systematic reviews of interventions version 62 (updated February 2021): Cochrane; 2021.
- 2.Matza LS, Patrick DL, Riley AW, Alexander JJ, Rajmil L, Pleil AM, et al. Pediatric patient-reported outcome instruments for research to support medical product labeling: report of the ISPOR PRO good research practices for the assessment of children and adolescents task force. Value Health. 2013;16(4):461–479. doi: 10.1016/j.jval.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Fayed N, De Camargo OK, Kerr E, Rosenbaum P, Dubey A, Bostan C, et al. Generic patient-reported outcomes in child health research: a review of conceptual content using World Health Organization definitions. Dev Med Child Neurol. 2012;54(12):1085–1095. doi: 10.1111/j.1469-8749.2012.04393.x. [DOI] [PubMed] [Google Scholar]
- 4.Janssens A, Coon JT, Rogers M, Allen K, Green C, Jenkinson C, et al. A systematic review of generic multidimensional patient-reported outcome measures for children, part I: descriptive characteristics. Value Health. 2015;18(2):315–333. doi: 10.1016/j.jval.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Petrou S. Methodological issues raised by preference-based approaches to measuring the health status of children. Health Econ. 2003;12(8):697–702. doi: 10.1002/hec.775. [DOI] [PubMed] [Google Scholar]
- 6.Thompson HL, Reville M-C, Price A, Reynolds L, Rodgers L, Ford T. The quality of life scale for children (QOL-C) J Child Serv. 2014;9(1):4–17. doi: 10.1108/JCS-05-2013-0019. [DOI] [Google Scholar]
- 7.Starfield B, Bergner M, Ensminger M, Riley A, Ryan S, Green B, et al. Adolescent health status measurement: development of the Child Health and Illness Profile. Pediatrics. 1993;91(2):430–435. doi: 10.1542/peds.91.2.430. [DOI] [PubMed] [Google Scholar]
- 8.Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 2015. [Google Scholar]
- 9.Karimi M, Brazier J. Health, health-related quality of life, and quality of life: what is the difference? Pharmacoeconomics. 2016;34(7):645–649. doi: 10.1007/s40273-016-0389-9. [DOI] [PubMed] [Google Scholar]
- 10.Chen G, Ratcliffe J. A review of the development and application of generic multi-attribute utility instruments for paediatric populations. Pharmacoeconomics. 2015;33(10):1013–1028. doi: 10.1007/s40273-015-0286-7. [DOI] [PubMed] [Google Scholar]
- 11.Torrance GW. Measurement of health state utilities for economic appraisal: a review. J Health Econ. 1986;5(1):1–30. doi: 10.1016/0167-6296(86)90020-2. [DOI] [PubMed] [Google Scholar]
- 12.Bansback N, Brazier J, Tsuchiya A, Anis A. Using a discrete choice experiment to estimate health state utility values. J Health Econ. 2012;31(1):306–318. doi: 10.1016/j.jhealeco.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 13.National Institute for Health and Care Excellence. Guide to the methods of technology appraisal 2013. In: Excellence NIfHaC, editor. PMG92013. [PubMed]
- 14.Pharmaceutical Benefits Advisory Committee. Guidelines for preparing submissions to the Pharmaceutical Benefits Advisory Committee (Version 5). In: Pharmaceutical Benefits Advisory Committee DoH, editor. 2016.
- 15.Canadian Agency for Drugs and Technologies in Health. Guidelines for the economic evaluation of health technologies: Canada 4th Edition. In: Guidelines CMa, editor. 2017.
- 16.Kwon J, Kim SW, Ungar WJ, Tsiplova K, Madan J, Petrou S. Patterns, trends and methodological associations in the measurement and valuation of childhood health utilities. Qual Life Res. 2019;28(7):1705–1724. doi: 10.1007/s11136-019-02121-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ratcliffe J, Flynn T, Terlich F, Stevens K, Brazier J, Sawyer M. Developing adolescent-specific health state values for economic evaluation: an application of profile case best-worst scaling to the Child Health Utility 9D. Pharmacoeconomics. 2012;30(8):713–727. doi: 10.2165/11597900-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 18.Apajasalo M, Sintonen H, Holmberg C, Sinkkonen J, Aalberg V, Pihko H, et al. Quality of life in early adolescence: a sixteen-dimensional health-related measure (16D) Qual Life Res. 1996;5(2):205–211. doi: 10.1007/BF00434742. [DOI] [PubMed] [Google Scholar]
- 19.Ratcliffe J, Stevens K, Flynn T, Brazier J, Sawyer MG. Whose values in health? An empirical comparison of the application of adolescent and adult values for the CHU-9D and AQOL-6D in the Australian adolescent general population. Value Health. 2012;15(5):730–736. doi: 10.1016/j.jval.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Rowen D, Rivero-Arias O, Devlin N, Ratcliffe J. Review of valuation methods of preference-based measures of health for economic evaluation in child and adolescent populations: where are we now and where are we going? Pharmacoeconomics. 2020;38(4):325–340. doi: 10.1007/s40273-019-00873-7. [DOI] [PubMed] [Google Scholar]
- 21.Cremeens J, Eiser C, Blades M. Characteristics of health-related self-report measures for children aged three to eight years: a review of the literature. Qual Life Res. 2006;15(4):739–754. doi: 10.1007/s11136-005-4184-x. [DOI] [PubMed] [Google Scholar]
- 22.Davis E, Waters E, Mackinnon A, Reddihough D, Graham HK, Mehmet-Radji O, et al. Paediatric quality of life instruments: a review of the impact of the conceptual framework on outcomes. Dev Med Child Neurol. 2006;48(4):311–318. doi: 10.1017/S0012162206000673. [DOI] [PubMed] [Google Scholar]
- 23.Eiser C, Morse R. A review of measures of quality of life for children with chronic illness. Arch Dis Child. 2001;84(3):205–211. doi: 10.1136/adc.84.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grange A, Bekker H, Noyes J, Langley P. Adequacy of health-related quality of life measures in children under 5 years old: systematic review. J Adv Nurs. 2007;59(3):197–220. doi: 10.1111/j.1365-2648.2007.04333.x. [DOI] [PubMed] [Google Scholar]
- 25.Harding L. Children's quality of life assessments: a review of generic and health related quality of life measures completed by children and adolescents. Clin Psychol Psychotherapy. 2001;8(2):79–96. doi: 10.1002/cpp.275. [DOI] [Google Scholar]
- 26.Solans M, Pane S, Estrada MD, Serra-Sutton V, Berra S, Herdman M, et al. Health-related quality of life measurement in children and adolescents: a systematic review of generic and disease-specific instruments. Value Health. 2008;11(4):742–764. doi: 10.1111/j.1524-4733.2007.00293.x. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization . International classification of functioning, disability, and health: children and youth version: ICF-CY. World Health Organization; 2007. [Google Scholar]
- 28.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 29.Veritas Health Innovation. Covidence systematic review software. Melbourne.
- 30.Etienne A-M, Dupuis G, Spitz E, Lemetayer F, Missotten P. The gap concept as a quality of life measure: validation study of the Child Quality of Life Systemic Inventory. Soc Indic Res. 2011;100(2):241–257. doi: 10.1007/s11205-010-9614-7. [DOI] [Google Scholar]
- 31.Vogels T, Verrips G, Verloove-Vanhorick S, Fekkes M, Kamphuis R, Koopman H, et al. Measuring health-related quality of life in children: the development of the TACQOL parent form. Qual Life Res. 1998;7(5):457–465. doi: 10.1023/A:1008848218806. [DOI] [PubMed] [Google Scholar]
- 32.Varni JW, Seid M, Kurtin PS. PedsQL™ 4.0: reliability and validity of the Pediatric Quality of Life Inventory™ Version 4.0 Generic Core Scales in healthy and patient populations. Med Care. 2001;39:800–812. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 33.World Bank. Data: World Bank Country and Lending Groups 2021 [31.5.2021]. Available from: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups.
- 34.Jiang S, Ngai SS-Y. Assessing multiple domains of child well-being: Preliminary development and validation of the multidimensional child well-being scale (MCWBS). Curr Psychol. 2020:1–12.
- 35.Wang X, Matsuda N, Ma H, Shinfuku N. Comparative study of quality of life between the Chinese and Japanese adolescent populations. Psychiatry Clin Neurosci. 2000;54(2):147–152. doi: 10.1046/j.1440-1819.2000.00650.x. [DOI] [PubMed] [Google Scholar]
- 36.Izutsu T, Tsutsumi A, Islam MA, Matsuo Y, Yamada HS, Kurita H, et al. Validity and reliability of the Bangla version of WHOQOL-BREF on an adolescent population in Bangladesh. Qual Life Res. 2005;14(7):1783–1789. doi: 10.1007/s11136-005-1744-z. [DOI] [PubMed] [Google Scholar]
- 37.Moodie M, Richardson J, Rankin B, Iezzi A, Sinha K. Predicting time trade-off health state valuations of adolescents in four Pacific countries using the Assessment of Quality-of-Life (AQoL-6D) instrument. Value Health. 2010;13(8):1014–1027. doi: 10.1111/j.1524-4733.2010.00780.x. [DOI] [PubMed] [Google Scholar]
- 38.Wille N, Badia X, Bonsel G, Burström K, Cavrini G, Devlin N, et al. Development of the EQ-5D-Y: a child-friendly version of the EQ-5D. Qual Life Res. 2010;19(6):875–886. doi: 10.1007/s11136-010-9648-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verstraete J, Ramma L, Jelsma J. Item generation for a proxy health related quality of life measure in very young children. Health Qual Life Outcomes. 2020;18(1):1–15. doi: 10.1186/s12955-019-1245-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verstraete J, Lloyd A, Scott D, Jelsma J. How does the EQ-5D-Y proxy version 1 perform in 3, 4 and 5-year-old children? Health Qual Life Outcomes. 2020;18:1–10. doi: 10.1186/s12955-019-1245-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eiser C, Vance Y, Seamark D. The development of a theoretically driven generic measure of quality of life for children aged 6–12 years: a preliminary report. Child Care Health Dev. 2000;26(6):445–456. doi: 10.1046/j.1365-2214.2000.00177.x. [DOI] [PubMed] [Google Scholar]
- 42.Bevans KB, Riley AW, Forrest CB. Development of the healthy pathways child-report scales. Qual Life Res. 2010;19(8):1195–1214. doi: 10.1007/s11136-010-9687-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ravens-Sieberer U, Heilmann M, Walleser S. Assessment of quality of life in young children with a Computer Assisted Touch Screen Program (CAT-Screen)-reliability, validity and feasibility. Qual Life Res. 2000:298.
- 44.Varni JW, Thissen D, Stucky BD, Liu Y, Gorder H, Irwin DE, et al. PROMIS® Parent Proxy Report Scales: an item response theory analysis of the parent proxy report item banks. Qual Life Res. 2012;21(7):1223–1240. doi: 10.1007/s11136-011-0025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DeWalt DA, Gross HE, Gipson DS, Selewski DT, DeWitt EM, Dampier CD, et al. PROMIS® pediatric self-report scales distinguish subgroups of children within and across six common pediatric chronic health conditions. Qual Life Res. 2015;24(9):2195–2208. doi: 10.1007/s11136-015-0953-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Devine J, Otto C, Rose M, Barthel D, Fischer F, Mülhan H, et al. A new computerized adaptive test advancing the measurement of health-related quality of life (HRQoL) in children: the Kids-CAT. Qual Life Res. 2015;24(4):871–884. doi: 10.1007/s11136-014-0812-7. [DOI] [PubMed] [Google Scholar]
- 47.Varni JW, Magnus B, Stucky BD, Liu Y, Quinn H, Thissen D, et al. Psychometric properties of the PROMIS® pediatric scales: precision, stability, and comparison of different scoring and administration options. Qual Life Res. 2014;23(4):1233–1243. doi: 10.1007/s11136-013-0544-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Young NL, Wabano MJ, Burke TA, Ritchie SD, Mishibinijima D, Corbiere RG. A process for creating the Aboriginal Children’s Health and Well-being Measure (ACHWM) Can J Public Health. 2013;104(2):e136–e141. doi: 10.1007/BF03405677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Touchèque M, Etienne A-M, Missotten P, Dupuis G. A comparison of a tablet version of the Quality of Life Systemic Inventory for Children (QLSI-C) to the standard paper version. Psychol Assess. 2016;28(6):780. doi: 10.1037/pas0000234. [DOI] [PubMed] [Google Scholar]
- 50.Kang E. Validity of Child Health-6 Dimension (Ch-6d) for adolescents. Value Health. 2016;19(7):A854. doi: 10.1016/j.jval.2016.08.458. [DOI] [Google Scholar]
- 51.Jabrayilov R, van Asselt AD, Vermeulen KM, Volger S, Detzel P, Dainelli L, et al. A descriptive system for the Infant health-related Quality of life Instrument (IQI): measuring health with a mobile app. PLoS ONE. 2018;13(8):e0203276. doi: 10.1371/journal.pone.0203276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Landgraf JM, van Grieken A, Raat H. Giving voice to the child perspective: psychometrics and relative precision findings for the Child Health Questionnaire self-report short form (CHQ-CF45) Qual Life Res. 2018;27(8):2165–2176. doi: 10.1007/s11136-018-1873-9. [DOI] [PubMed] [Google Scholar]
- 53.Forrest CB, Bevans KB, Pratiwadi R, Moon J, Teneralli RE, Minton JM, et al. Development of the PROMIS® pediatric global health (PGH-7) measure. Qual Life Res. 2014;23(4):1221–1231. doi: 10.1007/s11136-013-0581-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gullone E, Cummins RA. The Comprehensive Quality of Life Scale: a psychometric evaluation with an adolescent sample. Behav Change. 1999;16(2):127–139. doi: 10.1375/bech.16.2.127. [DOI] [Google Scholar]
- 55.Seligson JL, Huebner ES, Valois RF. Preliminary validation of the brief multidimensional students' life satisfaction scale (BMSLSS) Soc Indic Res. 2003;61(2):121–145. doi: 10.1023/A:1021326822957. [DOI] [Google Scholar]
- 56.Ravens-Sieberer U, Erhart M, Power M, Auquier P, Cloetta B, Hagquist C, et al. # 1793-C/item-response-theory analyses of child and adolescent self-report quality of life data: the European Cross Cultural Research Instrument Kidscreen. Qual Life Res. 2003:722.
- 57.Ravens-Sieberer U, Gosch A, Rajmil L, Erhart M, Bruil J, Power M, et al. The KIDSCREEN-52 quality of life measure for children and adolescents: psychometric results from a cross-cultural survey in 13 European countries. Value Health. 2008;11(4):645–658. doi: 10.1111/j.1524-4733.2007.00291.x. [DOI] [PubMed] [Google Scholar]
- 58.Ravens-Sieberer U, Auquier P, Erhart M, Gosch A, Rajmil L, Bruil J, et al. The KIDSCREEN-27 quality of life measure for children and adolescents: psychometric results from a cross-cultural survey in 13 European countries. Qual Life Res. 2007;16(8):1347–1356. doi: 10.1007/s11136-007-9240-2. [DOI] [PubMed] [Google Scholar]
- 59.Ravens-Sieberer U, Erhart M, Rajmil L, Herdman M, Auquier P, Bruil J, et al. Reliability, construct and criterion validity of the KIDSCREEN-10 score: a short measure for children and adolescents’ well-being and health-related quality of life. Qual Life Res. 2010;19(10):1487–1500. doi: 10.1007/s11136-010-9706-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saigal S, Rosenbaum P, Stoskopf B, Hoult L, Furlong W, Feeny D, et al. Development, reliability and validity of a new measure of overall health for pre-school children. Qual Life Res. 2005;14(1):243–252. doi: 10.1007/s11136-004-4228-7. [DOI] [PubMed] [Google Scholar]
- 61.Manificat S, Dazord A. Assessing adolescent's quality of life: validation of a new questionnaire. Qual Life Newsl. 2002;28:2–3. [Google Scholar]
- 62.Singh G, Athreya BH, Fries JF, Goldsmith DP. Measurement of health status in children with juvenile rheumatoid arthritis. Arthritis Rheum. 1994;37(12):1761–1769. doi: 10.1002/art.1780371209. [DOI] [PubMed] [Google Scholar]
- 63.Endicott J, Nee J, Yang R, Wohlberg C. Pediatric quality of life enjoyment and satisfaction questionnaire (PQ-LES-Q): reliability and validity. J Am Acad Child Adolesc Psychiatry. 2006;45(4):401–407. doi: 10.1097/01.chi.0000198590.38325.81. [DOI] [PubMed] [Google Scholar]
- 64.Cummins RA, Lau AL. Personal Wellbeing Index—school children. Melbourne: School of Psychology, Deakin University; 2005. [Google Scholar]
- 65.Fuh J, Wang S, Lu S, Juang K. Assessing quality of life for adolescents in Taiwan. Psychiatry Clin Neurosci. 2005;59(1):11–18. doi: 10.1111/j.1323-1316.2005.01306.x. [DOI] [PubMed] [Google Scholar]
- 66.Chen G, Xu F, Huynh E, Wang Z, Stevens K, Ratcliffe J. Scoring the Child Health Utility 9D instrument: estimation of a Chinese child and adolescent-specific tariff. Qual Life Res. 2019;28(1):163–176. doi: 10.1007/s11136-018-2032-z. [DOI] [PubMed] [Google Scholar]
- 67.Apajasalo M, Rautonen J, Holmberg C, Sinkkonen J, Aalberg V, Pihko H, et al. Quality of life in pre-adolescence: a 17-dimensional health-related measure (17D) Qual Life Res. 1996;5(6):532–538. doi: 10.1007/BF00439227. [DOI] [PubMed] [Google Scholar]
- 68.Craig BM, Greiner W, Brown DS, Reeve BB. Valuation of child health-related quality of life in the United States. Health Econ. 2016;25(6):768–777. doi: 10.1002/hec.3184. [DOI] [PubMed] [Google Scholar]
- 69.Ratcliffe J, Chen G, Stevens K, Bradley S, Couzner L, Brazier J, et al. Valuing Child Health Utility 9D health states with young adults: insights from a time trade off study. Appl Health Econ Health Policy. 2015;13(5):485–492. doi: 10.1007/s40258-015-0184-3. [DOI] [PubMed] [Google Scholar]
- 70.Rupel VP, Ogorevc M. EQ-5D-Y value set for Slovenia. Pharmacoeconomics. 2021;39(4):463–471. doi: 10.1007/s40273-020-00994-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shiroiwa T, Ikeda S, Noto S, Fukuda T, Stolk E. Valuation survey of EQ-5D-Y Based on the international common protocol: development of a value set in Japan. Med Decis Mak. 2021;41(5):597–606. doi: 10.1177/0272989X211001859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rowen D, Mulhern B, Stevens K, Vermaire JH. Estimating a Dutch value set for the pediatric preference-based CHU9D using a discrete choice experiment with duration. Value Health. 2018;21(10):1234–1242. doi: 10.1016/j.jval.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 73.Brazier J, Ratcliffe J, Saloman J, Tsuchiya A. Measuring and valuing health benefits for economic evaluation. Oxford: Oxford University Press; 2017. [Google Scholar]
- 74.Ratcliffe J, Huynh E, Chen G, Stevens K, Swait J, Brazier J, et al. Valuing the Child Health Utility 9D: using profile case best worst scaling methods to develop a new adolescent specific scoring algorithm. Soc Sci Med. 2016;157:48–59. doi: 10.1016/j.socscimed.2016.03.042. [DOI] [PubMed] [Google Scholar]
- 75.Torrance GW, Feeny DH, Furlong WJ, Barr RD, Zhang Y, Wang Q. Multiattribute utility function for a comprehensive health status classification system: Health Utilities Index Mark 2. Med Care. 1996;34:702–722. doi: 10.1097/00005650-199607000-00004. [DOI] [PubMed] [Google Scholar]
- 76.McCabe C, Stevens K, Roberts J, Brazier J. Health state values for the HUI 2 descriptive system: results from a UK survey. Health Econ. 2005;14(3):231–244. doi: 10.1002/hec.925. [DOI] [PubMed] [Google Scholar]
- 77.Krabbe PF, Jabrayilov R, Detzel P, Dainelli L, Vermeulen KM, van Asselt AD. A two-step procedure to generate utilities for the Infant health-related Quality of life Instrument (IQI) PLoS ONE. 2020;15(4):e0230852. doi: 10.1371/journal.pone.0230852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Beusterien KM, Yeung J-E, Pang F, Brazier J. Development of the multi-attribute adolescent health utility measure (AHUM) Health Qual Life Outcomes. 2012;10(1):1–9. doi: 10.1186/1477-7525-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stevens K. Valuation of the child health utility 9D index. Pharmacoeconomics. 2012;30(8):729–747. doi: 10.2165/11599120-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 80.Feeny D, Furlong W, Torrance GW, Goldsmith CH, Zhu Z, DePauw S, et al. Multiattribute and single-attribute utility functions for the health utilities index mark 3 system. Med Care. 2002;40(2):113–128. doi: 10.1097/00005650-200202000-00006. [DOI] [PubMed] [Google Scholar]
- 81.Noto S, Uemura T. Japanese health utilities index mark 3 (HUI3): measurement properties in a community sample. J Patient-Rep Outcomes. 2020;4(1):9. doi: 10.1186/s41687-020-0175-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Le Galès C, Buron C, Costet N, Rosman S, Slama PG. Development of a preference-weighted health status classification system in France: the Health Utilities Index 3. Health Care Manag Sci. 2002;5(1):41–51. doi: 10.1023/A:1013201102918. [DOI] [PubMed] [Google Scholar]
- 83.Seiber WJ, Groessl EJ, David KM, Ganiats TG, Kaplan RM. Quality of well being self-administered (QWB-SA) scale. San Diego: Health Services Research Center, University of California; 2008. [Google Scholar]
- 84.Stevens K. Developing a descriptive system for a new preference-based measure of health-related quality of life for children. Qual Life Res. 2009;18(8):1105–1113. doi: 10.1007/s11136-009-9524-9. [DOI] [PubMed] [Google Scholar]
- 85.Janssens A, Rogers M, Coon JT, Allen K, Green C, Jenkinson C, et al. A systematic review of generic multidimensional patient-reported outcome measures for children, part II: evaluation of psychometric performance of English-language versions in a general population. Value Health. 2015;18(2):334–345. doi: 10.1016/j.jval.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 86.Brennan A, Akehurst R. Modelling in health economic evaluation. Pharmacoeconomics. 2000;17(5):445–459. doi: 10.2165/00019053-200017050-00004. [DOI] [PubMed] [Google Scholar]
- 87.Blackwell CK, Wakschlag L, Krogh-Jespersen S, Buss KA, Luby J, Bevans K, et al. Pragmatic health assessment in early childhood: the PROMIS® of developmentally based measurement for pediatric psychology. J Pediatr Psychol. 2020;45(3):311–318. doi: 10.1093/jpepsy/jsz094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Manificat S, Dazord A. Infant, child and adolescent quality of life: surveys performed in a European context. Expert Rev Pharmacoecon Outcomes Res. 2002;2(6):589–596. doi: 10.1586/14737167.2.6.589. [DOI] [PubMed] [Google Scholar]
- 89.Ravens-Sieberer U, Bullinger M. Assessing health-related quality of life in chronically ill children with the German KINDL: first psychometric and content analytical results. Qual Life Res. 1998;7(5):399–407. doi: 10.1023/A:1008853819715. [DOI] [PubMed] [Google Scholar]
- 90.Eiser C, Morse R. Can parents rate their child's health-related quality of life? Results of a systematic review. Qual Life Res. 2001;10(4):347–357. doi: 10.1023/A:1012253723272. [DOI] [PubMed] [Google Scholar]
- 91.Khadka J, Kwon J, Petrou S, Lancsar E, Ratcliffe J. Mind the (inter-rater) gap an investigation of self-reported versus proxy-reported assessments in the derivation of childhood utility values for economic evaluation: a systematic review. Soc Sci Med. 2019;240:112543. doi: 10.1016/j.socscimed.2019.112543. [DOI] [PubMed] [Google Scholar]
- 92.Bevans KB, Riley AW, Forrest CB. Development of the healthy pathways parent-report scales. Qual Life Res. 2012;21(10):1755–1770. doi: 10.1007/s11136-012-0111-0. [DOI] [PubMed] [Google Scholar]
- 93.Coons SJ, Gwaltney CJ, Hays RD, Lundy JJ, Sloan JA, Revicki DA, et al. Recommendations on evidence needed to support measurement equivalence between electronic and paper-based patient-reported outcome (PRO) measures: ISPOR ePRO Good Research Practices Task Force report. Value Health. 2009;12(4):419–429. doi: 10.1111/j.1524-4733.2008.00470.x. [DOI] [PubMed] [Google Scholar]
- 94.Coombes L, Bristowe K, Ellis-Smith C, Fraser LK, Downing J, et al. Enhancing validity, reliability and participation in self-reported health outcome measurement for children and young people: a systematic review of recall period, response scale format, and administration modality. Qual Life Res. 2021;30:1803–1832. doi: 10.1007/s11136-021-02814-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wasson JW, Kairys SW, Nelson EC, Kalishman N, Baribeau P. A short survey for assessing health and social problems of adolescents. J Fam Pract. 1994;38(5):489–495. [PubMed] [Google Scholar]
- 96.Ratcliffe J, Couzner L, Flynn T, Sawyer M, Stevens K, Brazier J, et al. Valuing child health utility 9D health states with a young adolescent sample. Appl Health Econ Health Policy. 2011;9(1):15–27. doi: 10.2165/11536960-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 97.Rowen D, Brazier J, Van Hout B. A comparison of methods for converting DCE values onto the full health-dead QALY scale. Med Decis Mak. 2015;35(3):328–340. doi: 10.1177/0272989X14559542. [DOI] [PubMed] [Google Scholar]
- 98.Viney R, Norman R, Brazier J, Cronin P, King MT, Ratcliffe J, et al. An Australian discrete choice experiment to value EQ-5D health states. Health Econ. 2014;23(6):729–742. doi: 10.1002/hec.2953. [DOI] [PubMed] [Google Scholar]
- 99.Ramos-Goñi JM, Oppe M, Stolk E, Shah K, Kreimeier S, Rivero-Arias O, et al. International valuation protocol for the EQ-5D-Y-3L. Pharmacoeconomics. 2020;38(7):653–663. doi: 10.1007/s40273-020-00909-3. [DOI] [PubMed] [Google Scholar]
- 100.Rogers HJ, Marshman Z, Rodd H, Rowen D. Discrete choice experiments or best-worst scaling? A qualitative study to determine the suitability of preference elicitation tasks in research with children and young people. J Patient-Rep Outcomes. 2021;5(1):1–11. doi: 10.1186/s41687-021-00302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ungar WJ, Zur RM. Optimizing treatment for children in the developing world. Berlin: Springer; 2015. Health economic evaluation for improving child health in low-and middle-income countries; pp. 213–224. [Google Scholar]
- 102.Rowen D, Keetharuth AD, Poku E, Wong R, Pennington B, Wailoo A. A review of the psychometric performance of selected child and adolescent preference-based measures used to produce utilities for child and adolescent health. Value Health. 2020;24:443–460. doi: 10.1016/j.jval.2020.09.012. [DOI] [PubMed] [Google Scholar]
- 103.Mokkink LB, Terwee CB, Patrick DL, Alonso J, Stratford PW, Knol DL, et al. The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: an international Delphi study. Qual Life Res. 2010;19(4):539–549. doi: 10.1007/s11136-010-9606-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Oluboyede Y, Robinson T. Measuring weight-specific quality of life in adolescents: an examination of the concurrent validity and test-retest reliability of the WAItE. Value Health. 2019;22(3):348–354. doi: 10.1016/j.jval.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 105.Xie F, Pickard AS, Krabbe PF, Revicki D, Viney R, Devlin N, et al. A checklist for reporting valuation studies of multi-attribute utility-based instruments (CREATE) Pharmacoeconomics. 2015;33(8):867–877. doi: 10.1007/s40273-015-0292-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Manificat S, Dazord A, Langue J, Danjou G, Bauche P, Bovet F, et al. A new instrument to evaluate infant quality of life. Qual Life Newsl. 1999;7(8–7):8. [Google Scholar]
- 107.Manificat S, Dazord A. Children's quality of life assessment: preliminary results obtained with the AUQUEI questionnaire. Qual Life Newsl. 1998;19:2–3. [Google Scholar]
- 108.Lam C, Young N, Marwaha J, McLimont M, Feldman BM. Revised versions of the Childhood Health Assessment Questionnaire (CHAQ) are more sensitive and suffer less from a ceiling effect. Arthritis Care Res. 2004;51(6):881–889. doi: 10.1002/art.20820. [DOI] [PubMed] [Google Scholar]
- 109.Starfield B, Riley AW, Green BF, Ensminger ME, Ryan SA, Kelleher K, et al. The adolescent child health and illness profile: a population-based measure of health. Med Care. 1995;33:553–566. doi: 10.1097/00005650-199505000-00008. [DOI] [PubMed] [Google Scholar]
- 110.Riley AW, Forrest CB, Rebok GW, Starfield B, Green BF, Robertson JA, et al. The child report form of the CHIP-child edition: reliability and validity. Med Care. 2004;42:221–231. doi: 10.1097/01.mlr.0000114910.46921.73. [DOI] [PubMed] [Google Scholar]
- 111.Riley AW, Forrest CB, Starfield B, Rebok GW, Robertson JA, Green BF. The parent report form of the CHIP-Child edition: reliability and validity. Med Care. 2004;42:210–220. doi: 10.1097/01.mlr.0000114909.33878.ca. [DOI] [PubMed] [Google Scholar]
- 112.Landgraf J, Maunsell E, Speechley KN, Bullinger M, Campbell S, Abetz L, et al. Canadian-French, German and UK versions of the Child Health Questionnaire: methodology and preliminary item scaling results. Qual Life Res. 1998;7(5):433–445. doi: 10.1023/A:1008810004694. [DOI] [PubMed] [Google Scholar]
- 113.Raat H, Botterweck AM, Landgraf JM, Hoogeveen WC, Essink-Bot M-L. Reliability and validity of the short form of the child health questionnaire for parents (CHQ-PF28) in large random school based and general population samples. J Epidemiol Community Health. 2005;59(1):75–82. doi: 10.1136/jech.2003.012914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Landgraf JM, Abetz LN. Functional status and well-being of children representing three cultural groups: initial self-reports using the CHQ-CF87. Psychol Health. 1997;12(6):839–854. doi: 10.1080/08870449708406744. [DOI] [Google Scholar]
- 115.Maylath NS. Development of the Children's Health Ratings Scale. Health Educ Q. 1990;17(1):89–97. doi: 10.1177/109019819001700109. [DOI] [PubMed] [Google Scholar]
- 116.Hester NO. Child's health self-concept scale: its development and psychometric properties. Adv Nurs Sci. 1984;7(1):45–55. doi: 10.1097/00012272-198410000-00006. [DOI] [PubMed] [Google Scholar]
- 117.Graham P, Stevenson J, Flynn D. A new measure of health-related quality of life for children: preliminary findings. Psychol Health. 1997;12(5):655–665. doi: 10.1080/08870449708407412. [DOI] [Google Scholar]
- 118.Vo TXH, Guillemin F, Deschamps J-P. Psychometric properties of the DUKE Health Profile-adolescent version (DHP-A): a generic instrument for adolescents. Qual Life Res. 2005;14(10):2229–2234. doi: 10.1007/s11136-005-7021-3. [DOI] [PubMed] [Google Scholar]
- 119.Stein RE, Jessop DJ. Functional status II (R): a measure of child health status. Med Care. 1990;28:1041–1055. doi: 10.1097/00005650-199011000-00006. [DOI] [PubMed] [Google Scholar]
- 120.Collier J, MacKinley D. Developing a generic child quality of life measure. Health Psychol Update. 1997:12–6.
- 121.le Coq EM, Colland VT, Boeke AJP, Bezemer DP, van Eijk JTM. Reproducibility, construct validity, and responsiveness of the “How Are You?”(HAY), a self-report quality of life questionnaire for children with asthma. J Asthma. 2000;37(1):43–58. doi: 10.3109/02770900009055427. [DOI] [PubMed] [Google Scholar]
- 122.Holmström MR, Olofsson N, Kristiansen L, Asplund K. Health among 6-year-old children in a Swedish county: based on the Health dialogue. Vulnerable Groups Incl. 2012;3(1):8416. doi: 10.3402/vgi.v3i0.8416. [DOI] [Google Scholar]
- 123.Holmström MR, Olofsson N, Asplund K, Kristiansen L. Exploring the development of school children's health. Br J School Nurs. 2012;7(4):189–197. doi: 10.12968/bjsn.2012.7.4.189. [DOI] [Google Scholar]
- 124.Olofsson N, Rising Holmström M, Kristiansen L. Assessing the construct validity and reliability of school health records of the ‘Health Dialogue Questionnaire,’ in 7th Grade in Compulsory School. MOJ Public Health. 2015;2(1). [DOI] [PMC free article] [PubMed]
- 125.Kristiansen L, Holmstrom MR, Olofsson N. Assessing the construct validity and reliability of school health records using the ‘Health Dialogue Questionnaire’ in the eleventh grade. AIMS Public Health. 2016;3(3):470. doi: 10.3934/publichealth.2016.3.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jozefiak T, Wallander JL. Perceived family functioning, adolescent psychopathology and quality of life in the general population: a 6-month follow-up study. Qual Life Res. 2016;25(4):959–967. doi: 10.1007/s11136-015-1138-9. [DOI] [PubMed] [Google Scholar]
- 127.Klassen AF, Landgraf JM, Lee SK, Barer M, Raina P, Chan HW, et al. Health related quality of life in 3 and 4 year old children and their parents: preliminary findings about a new questionnaire. Health Qual Life Outcomes. 2003;1(1):1–11. doi: 10.1186/1477-7525-1-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Landgraf JM, Vogel I, Oostenbrink R, van Baar ME, Raat H. Parent-reported health outcomes in infants/toddlers: measurement properties and clinical validity of the ITQOL-SF47. Qual Life Res. 2013;22(3):635–646. doi: 10.1007/s11136-012-0177-8. [DOI] [PubMed] [Google Scholar]
- 129.Barthel D, Fischer K, Nolte S, Otto C, Meyrose A-K, Reisinger S, et al. Implementation of the Kids-CAT in clinical settings: a newly developed computer-adaptive test to facilitate the assessment of patient-reported outcomes of children and adolescents in clinical practice in Germany. Qual Life Res. 2016;25(3):585–594. doi: 10.1007/s11136-015-1219-9. [DOI] [PubMed] [Google Scholar]
- 130.Lindström B, Köhler L. Youth, disability and quality of life. Pediatrician. 1991. [PubMed]
- 131.Chan KS, Mangione-Smith R, Burwinkle TM, Rosen M, Varni JW. The PedsQL™: reliability and validity of the short-form generic core scales and asthma module. Med Care. 2005;43:256–265. doi: 10.1097/00005650-200503000-00008. [DOI] [PubMed] [Google Scholar]
- 132.Varni JW, Limbers CA, Neighbors K, Schulz K, Lieu JE, Heffer RW, et al. The PedsQL™ Infant Scales: feasibility, internal consistency reliability, and validity in healthy and ill infants. Qual Life Res. 2011;20(1):45–55. doi: 10.1007/s11136-010-9730-5. [DOI] [PubMed] [Google Scholar]
- 133.Ishaque S, Roberts R, Karnon J, Thomas D, Salter A, editors. Adaptation/content validation of measure yourself medical outcomes profile (MYMOP) questionnaire for 7–11 year old children. Quality of life research. Dordrectht: Springer; 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vaqué-Crusellas C, González M, Casas F. Does satisfaction with food matter? Testing the personal well-being index-school children (PWI-SC) with an additional item on satisfaction with food on a sample of 10 to 12-year-olds. Child Indic Res. 2015;8(4):961–973. doi: 10.1007/s12187-015-9301-y. [DOI] [Google Scholar]
- 135.Bouman NH, Koot HM, Van Gils AP, Verhulst FC. Development of a health-related quality of life instrument for children: the quality of life questionnaire for children. Psychol Health. 1999;14(5):829–846. doi: 10.1080/08870449908407350. [DOI] [Google Scholar]
- 136.Raphael D, Rukholm E, Brown I, Hill-Bailey P, Donato E. The quality of life profile—adolescent version: background, description, and initial validation. J Adolesc Health. 1996;19(5):366–375. doi: 10.1016/S1054-139X(96)00080-8. [DOI] [PubMed] [Google Scholar]
- 137.Huebner ES. Initial development of the student's life satisfaction scale. Sch Psychol Int. 1991;12(3):231–240. doi: 10.1177/0143034391123010. [DOI] [Google Scholar]
- 138.Huebner ES. Preliminary development and validation of a multidimensional life satisfaction scale for children. Psychol Assess. 1994;6(2):149. doi: 10.1037/1040-3590.6.2.149. [DOI] [Google Scholar]
- 139.Gilligan TD, Huebner S. Initial development and validation of the multidimensional students’ life satisfaction scale–adolescent version. Appl Res Qual Life. 2007;2(1):1–16. doi: 10.1007/s11482-007-9026-2. [DOI] [Google Scholar]
- 140.Theunissen NC, Vogels TG, Koopman HM, Verrips GH, Zwinderman KA, Verloove-Vanhorick SP, et al. The proxy problem: child report versus parent report in health-related quality of life research. Qual Life Res. 1998;7(5):387–397. doi: 10.1023/A:1008801802877. [DOI] [PubMed] [Google Scholar]
- 141.Fekkes M, Theunissen N, Brugman E, Veen S, Verrips E, Koopman H, et al. Development and psychometric evaluation of the TAPQOL: a health-related quality of life instrument for 1–5-year-old children. Qual Life Res. 2000;9(8):961–972. doi: 10.1023/A:1008981603178. [DOI] [PubMed] [Google Scholar]
- 142.Lawford J, Volavka N, Eiser C. A generic measure of quality of life for children aged 3–8 years: results of two preliminary studies. Pediatr Rehabil. 2001;4(4):197–207. doi: 10.1080/13638490210124033. [DOI] [PubMed] [Google Scholar]
- 143.Simeoni M, Auquier P, Antoniotti S, Sapin C, San MJ. Validation of a French health-related quality of life instrument for adolescents: the VSP-A. Qual Life Res. 2000;9(4):393–403. doi: 10.1023/A:1008957104322. [DOI] [PubMed] [Google Scholar]
- 144.Spencer PN, Coe C. The development and validation of a measure off parent-reported child health and morbidity: the Warwick Child Health and Morbidity Profile. Child Care Health Dev. 1996;22(6):367–379. doi: 10.1111/j.1365-2214.1996.tb00439.x. [DOI] [PubMed] [Google Scholar]
- 145.Edwards TC, Huebner CE, Connell FA, Patrick DL. Adolescent quality of life, part I: conceptual and measurement model. J Adolesc. 2002;25(3):275–286. doi: 10.1006/jado.2002.0470. [DOI] [PubMed] [Google Scholar]
- 146.Patrick DL, Edwards TC, Topolski TD. Adolescent quality of life, part II: initial validation of a new instrument. J Adolesc. 2002;25(3):287–300. doi: 10.1006/jado.2002.0471. [DOI] [PubMed] [Google Scholar]
- 147.Topolski TD, Patrick DL, Edwards TC, Huebner CE, Connell FA, Mount KK. Quality of life and health-risk behaviors among adolescents. J Adolesc Health. 2001;29(6):426–435. doi: 10.1016/S1054-139X(01)00305-6. [DOI] [PubMed] [Google Scholar]
- 148.Furlong WJ, Feeny DH, Torrance GW, Barr RD. The Health Utilities Index (HUI®) system for assessing health-related quality of life in clinical studies. Ann Med. 2001;33(5):375–384. doi: 10.3109/07853890109002092. [DOI] [PubMed] [Google Scholar]
- 149.Stevens K. Assessing the performance of a new generic measure of health-related quality of life for children and refining it for use in health state valuation. Appl Health Econ Health Policy. 2011;9(3):157–169. doi: 10.2165/11587350-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 150.Gusi N, Perez-Sousa M, Gozalo-Delgado M, Olivares P. Validity and reliability of the Spanish EQ-5D-Y proxy version. Anales de Pediatría (English Edition). 2014;81(4):212–219. doi: 10.1016/j.anpede.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 151.Kreimeier S, Åström M, Burström K, Egmar A-C, Gusi N, Herdman M, et al. EQ-5D-Y-5L: developing a revised EQ-5D-Y with increased response categories. Qual Life Res. 2019;28(7):1951–1961. doi: 10.1007/s11136-019-02115-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kaplan RM, Bush JW, Berry CC. Health status: types of validity and the index of well-being. Health Serv Res. 1976;11(4):478–507. [PMC free article] [PubMed] [Google Scholar]
- 153.Kaplan RM, Sieber WJ, Ganiats TG. The quality of well-being scale: comparison of the interviewer-administered version with a self-administered questionnaire. Psychol Health. 1997;12(6):783–791. doi: 10.1080/08870449708406739. [DOI] [Google Scholar]
- 154.Verstraete J, Ramma L, Jelsma J. Validity and reliability testing of the Toddler and Infant (TANDI) health related quality of life instrument for very young children. J Patient-Rep Outcomes. 2020;4(1):1–14. doi: 10.1186/s41687-020-00251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.