Abstract
As demonstrated by the Phase III clinical trial, MTN-020/ASPIRE, the monthly dapivirine vaginal ring is well tolerated and reduces the risk of HIV-1 as a woman-initiated prevention option. This analysis uses data from the follow-on MTN-032/ Assessment of ASPIRE and HOPE Adherence (AHA) qualitative study to understand how perceptions (or misperceptions) of ring efficacy may have influenced behavior during ASPIRE, and affected intention to use the ring in future ring projects, specifically HOPE, the planned open-label extension study. Single in-depth interviews (n = 98) and 12 focus group discussions (n = 89) were conducted with women at seven sites in Malawi, South Africa, Uganda and Zimbabwe. Eligibility included participation in the ASPIRE active arm, and ring use for ≥ 3 months or at least 1 month if seroconversion occurred. Interviews were audio-recorded, transcribed into English, coded in Dedoose and thematically analyzed. Demographic and behavioral questionnaire data were summarized in Stata. Most AHA participants perceived the ring to be effective, and described simply trusting it or having confidence in it because they, or other participants in risky situations, remained HIV-uninfected. Participants described ring efficacy after receiving ASPIRE results as a binary assessment: the ring worked or not. Many did not remember exact efficacy percentages because of lack of comprehension or memory but recalled key details about age differences. The majority expressed interest in future ring use. There is a need to investigate improved ways of explaining placebo-controlled trials and efficacy to women in Sub-Saharan Africa. Now that ring efficacy, is known, these benefits must be well communicated, and understood by end-users and key stakeholders. Engagement with end-users to construct effective messages and to develop tools to measure understanding of partial efficacy will be essential.
Keywords: Efficacy, Understanding, Perceptions, Dapivirine vaginal ring
Introduction
There are 5000 new HIV infections every day and 61% of these are in sub Saharan Africa [1]. Women, particularly young women under 25 have the highest prevalence and incidence rate of existing and new infections, putting them at the center of the epidemic [1]. Over the past decade, several clinical trials have been conducted in sub-Saharan Africa with the objective of identifying effective and acceptable HIV prevention options for women that could be comfortably integrated into their lives [2, 3]. Completed trials of oral or vaginal antiretroviral pre-exposure prophylaxis have shown that efficacy of these biomedical HIV prevention technologies largely depends on user adherence [3, 4]. For example, depending on adherence, oral PrEP efficacy has been shown to range from 44 to above 90% [5–7]. Related qualitative studies have revealed several factors affecting adherence, including but not limited to fear of side effects, stigma of taking ARVs and community myths and misconceptions [8]. For the dapivirine vaginal ring, results from two Phase III studies (MTN-020/ASPIRE; IPM-027/The Ring Study), two open-label extension studies (MTN-025/HOPE; IPM-032/DREAM), and modeling data have shown risk reduction of more than 50% with increased ring use [9–11]. PrEP, delivered as a daily oral tablet, is being rolled out in many countries in Africa, and the European Medicines Agency recently released a positive opinion of the monthly dapivirine vaginal ring, paving the way for future scale-up of this longer-acting technology [12].
It has been reported that participant acceptance (and its impact on subsequent use) of PrEP-based HIV prevention technologies depends in part on perceived efficacy, with higher perceived efficacy predicting greater acceptability [13, 14]. An individual’s expectations of the efficacy of HIV prevention technologies, whether in research or public health programs, can be shaped by messages that they receive from media, or discussions about the technology with peers, clinicians [13] or other healthcare providers. The MTN-032/Assessment of ASPIRE and HOPE Adherence (AHA) study was an exploratory qualitative study conducted in Malawi, South Africa, Uganda and Zimbabwe, designed to better understand women’s use of the monthly dapivirine ring during the placebo-controlled ASPIRE trial [3]. In this paper, we explored women’s narratives on their understanding of vaginal ring (VR) efficacy during ASPIRE (prior to knowing efficacy results) and after ASPIRE (after ASPIRE efficacy results were disseminated). The aim was to understand how these perceptions (or misperceptions) of ring efficacy may have influenced behavior during ASPIRE, and affected intention to use the ring in future ring projects, specifically HOPE, the planned open-label extension study. This analysis offers insight into both how study participants cognitively process messages about product efficacy, and how this impacts behavior.
Methods
Parent Study
The MTN-020/ASPIRE trial was a phase III, randomized, double-blind, placebo-controlled trial, which demonstrated the safety and efficacy of a monthly dapivirine vaginal ring for HIV prevention among healthy sexually active (defined as having vaginal intercourse at least once in the 3 months prior to screening), HIV un-infected women in sub-Saharan Africa [10]. A total of 2629 women were enrolled at 15 sites in Malawi, South Africa, Uganda and Zimbabwe between August 2012 and June 2015. Results of the ASPIRE study [9] were disseminated to participants via follow-up calls and dissemination meetings following public announcement in February 2016. After ASPIRE, participants at each of the 15 sites received approximately the same information regarding the results: that overall, the dapivirine vaginal ring raised no safety concerns and resulted in a 27% (95% confidence interval [CI], 1 to 46; P = 0.046) reduction in the rate of HIV acquisition compared to placebo and that those younger than age 25 had no significant reduction in HIV incidence (− 27%; 95% CI, − 133 to 31; P = 0.45). For women older than 21 there was a 56% (56%; 95% CI, 31 to 71; P < 0.001) reduction in the rate of HIV acquisition with an overall adherence rate of 70% [10].
AHA
During the first phase of MTN-032/AHA study, 187 former ASPIRE participants were randomly selected at seven of the 15 sites in Malawi, South Africa, Uganda and Zimbabwe to participate in this qualitative follow-on study. Out of the 187 participants enrolled, 9 had seroconverted during the ASPIRE, 3 from Malawi and 6 from South Africa. Data was collected from June 2016 to October 2016 from in-depth interviews (IDI) and focus group discussions (FGD). Using both qualitative methods offered complementary perspectives on both personal experiences and normative attitudes. During the AHA study, participants were presented with retrospective “objective” [biological] adherence data derived from dapivirine levels measured in plasma and returned rings to explore reasons for ring use or nonuse. Data from the second phase of AHA, exploring MTN-025/HOPE trial (the open-label extension study) will be presented elsewhere.
Study Participants
AHA participants were recruited from amongst those who had been in the active product arm of the ASPIRE study and had provided permission to be contacted after the study had ended. Active arm participants were selected so that their individual-level adherence data could be discussed since objective adherence data could not be obtained from the placebo rings. Participants were required to have had adherence data available from at least three quarterly visits for those who did not acquire HIV, and from at least one visit for those who did acquire HIV. Recruitment for IDIs was stratified by plasma adherence level at month 3 (< 95 pg/ mL, > 95 pg/ mL) and by age group at time of ASPIRE enrollment (18–21 years, 22–45 years). FGD recruitment was stratified by age group only. Within each recruitment group, participants were randomly selected and contacted sequentially from a list generated by the data management center, until the accrual target was met. Across the seven research sites in Lilongwe, Malawi; Durban and Johannesburg, South Africa; Kampala, Uganda; and Harare, Zimbabwe; a total of 98 women took part in IDIs and 89 participated in one of 12 FGDs (Fig. 1). Details of screening and enrollment numbers by type of interview and age group are shown in Fig. 1*. Data are presented as direct quotes from translated transcripts including pseudonym, type of interview, city of origin and/or country.
Fig. 1.
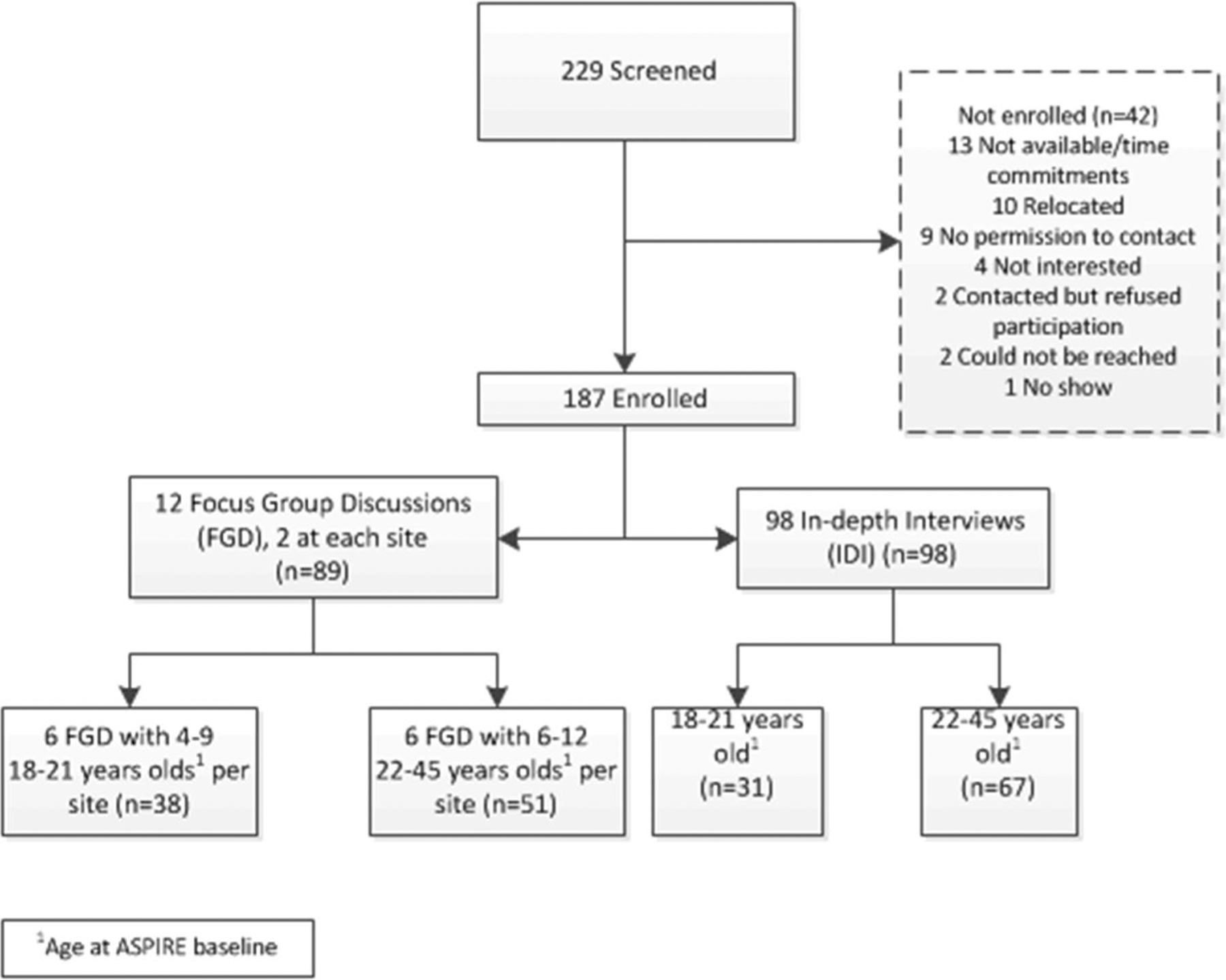
Screening and enrollment for AHA phase 1. *Figure developed by Montgomery et al. for study primary paper [15]
Procedures
Interviews using semi-structured topic guides were conducted by trained female social scientists/interviewers fluent in the local languages spoken by the participants; Chichewa in Malawi, Luganda in Uganda, Shona in Zimbabwe, and Xhosa, Zulu and/or English in South Africa. Participants were asked during IDIs and FGDs to share about their experiences during ASPIRE and to explain in their own words what the ASPIRE results meant to them in order to capture their level of understanding as well as their reactions to the efficacy results. IDIs and FGDs were audio-recorded and translated into English transcripts. The English transcripts were reviewed by social scientists fluent in both local and English languages for accuracy, with quality control conducted by the data management centre.
Data Analysis
Coding of translated interview transcripts was conducted in Dedoose software by a team of six analysts, using a code-book developed iteratively using the research objectives, interview guides, and themes tracked throughout data collection. Inter-coder consistency was confirmed for 10% of transcripts with a mean kappa score of 0.77 using a function of the software called the Dedoose Training Center. The analysis team met weekly for approximately 6 months to discuss coding questions, emerging themes, and to reach consensus on coding issues and interpretation of findings.
For this paper, data that were included in the analysis included transcript excerpts that were coded as EFFICACY, SEX, HOPE, FUTURE USE, RESULTS, and/or RISK, as defined in Table 1. Data from coded excerpts were summarized by the lead authors and vetted among analysts and co-authors from all collaborating institutions until consensus on key findings was reached.
Table 1.
Analytic codes and definitions used in this analysis
| Code | Definition |
|---|---|
| EFFICACY | Apply to discussions of product efficacy, either actual, perceived, or desired. Should be used to capture discussions about how participants think the ring works to prevent HIV |
| SEX | Anything about sexuality, sex practices (oral, vaginal or anal) or behaviors, including how the ring affected the sexual experience. Include comments about experience of discomfort or pain during sex and any mention of participant or partner either feeling or not feeling the ring during sex. Any comments about experience of pleasure during sex, either positive or negative. Any change in sexual practices as a result of ring use (different position, avoiding oral or digital sex, etc.). Applies to either woman’s or man’s experience |
| HOPE | Anything about HOPE trial including willingness to join, feelings or worries about the study |
| FUTURE USE | Code any discussion regarding willingness or plans to use (or not) product in the future, in general not specific to any study |
| RESULTS | Any discussion about understanding of ASPIRE study results |
| RISK | Any discussion of risk behavior or situations, including sexual risk, multiple partners, partner has other partners, other risk behavior (drugs/alcohol, pregnancy risk) or risk in general (i.e. having a reckless behavior, or being in a risky situation). Includes perceived lack of risk and discussions of risk perception. Also includes discussion about HIV worries, including what influenced worry and timeline or changes in level of worry (before ASPIRE, after, etc.) |
Results
Participant Demographics
Demographics of women who participated in the AHA phase one study have been presented previously [15]. Briefly, the average age of participants was 27 years, 62% were unmarried and less than half (48%) had completed secondary school education. More than half of the women did not know if their sexual partner had other sexual partners and 37% of the women did not know the HIV status of their sexual partners (Table 2).
Table 2.
Participant characteristics (developed for study primary paper [15]
| All sites (n = 187) *Statistics are N (%) | |
|---|---|
| Country of enrollment | |
| South Africa | 93 (50%) |
| Zimbabwe | 31 (17%) |
| Uganda | 29 (16%) |
| Malawi | 34 (18%) |
| Age at time of 032 interview, yearsa | 27 (28, 19–48) |
| Age at time of 020 baselinea | 24 (25, 18–44) |
| 18–21 | 69 (37%) |
| 22–45 | 118 (63%) |
| Completed secondary school or more | 48% |
| Earns own income | 109 (58%) |
| SES scoreb | |
| Low (lowest 40%) | 75 (40%) |
| Middle | 66 (35%) |
| High (highest 20%) | 46 (25%) |
| Marital status | |
| Legally | 9 (5%) |
| Traditionally | 56 (33%) |
| Unmarried | 107 (62%) |
| Same partner as when exited ASPIRE | 135 (78%) |
| Currently living with partner | 91 (53%) |
| Primary sex partner has other sex partners | 32 (19%) |
| Don’t know | 99 (58%) |
| Primary partner’s HIV status | |
| HIV positive | 10 (6%) |
| HIV negative | 98 (57%) |
| Participant does not know | 64 (37%) |
Median (mean, min–max)
An SES indicator variable was created using principal component analysis (PAC) pf 10 demographic assets from the demographic case report from including: home ownership, number of rooms in household; household assets of electricity, radio, television, non-mobile telephone, refrigerator; toilet facilities; and drinking water sources. A tri-level categorical variable (lowest 40%; middle 40%, and highest 20%) was created based on the first eigenvalue and the SAS-generated PRINI score
Qualitative findings are presented below categorized by thematic area to provide in-depth insights of women’s experience with the concept of whether the ring worked or not during ASPIRE when results were unknown, and after ASPIRE when results were known.
During ASPIRE: Perceptions of Ring Efficacy
Overall, the majority of women at all sites in this qualitative study said that they perceived the ring to be effective while they were in ASPIRE, and that they trusted that the ring protected them from HIV infection. Some simply trusted that the ring would work and believed in it even when they knew it was investigational and the study was blinded. Others had a personal experience of not being infected despite potential exposure, or heard stories from others that reinforced confidence that the ring was protective. There were some women, however, who were hoping that the ring would be found to be protective but were less confident in the efficacy and were waiting on the results to confirm protection.
Simply Trusting
Many participants had a straightforward confidence that the ring provided protection from HIV. As IDI_5002 participant from Zimbabwe explained, “I just had the feeling that it is going to finish the AIDS…, I would think I was not going to contract HIV.” These perceptions of the ring being effective continued even when study staff informed and reminded participants that the effectiveness of the ring was being tested in the research, as acknowledged by IDI_5004 participant from Zimbabwe, “I just had confidence in it, despite what we had been told that it was not yet in use. But in my mind, I was convinced it was effective. I just believed it could work. I had the faith that it would work. Just that.”
Some of the women believed that their risk of acquiring HIV was reduced specifically because of their faith that the ring works. Like IDI_3014 participant from Durban, South Africa who explained that, “…Yes I trust it will help me. If you believe that something will help you then it will help you…That is why I am also saying that I trusted the ring.” For others, their faith in ring efficacy stemmed from their desire for it to be true, which came from an acknowledgment of their level of risk of acquiring HIV from either partners or, in the case of rape, strangers. This was expressed by IDI_5001 participant from Zimbabwe regarding her husband:
I did not know whether I was using a placebo or an active ring…but with that ring use only I had this belief that because my husband does not allow us to use condoms, he doesn’t allow, so I was just feeling that I am now safe. I don’t know this feeling that I just had. I just felt that things were ok for me. It was just coming from within.
Similarly, this woman from FGD # 202 in Johannesburg, South Africa expressed her confidence in protection in the case of rape: “I believe in the ring. As women our lives are at risk. While walking on the street we might get raped. It does not matter if you are not wearing a condom because the ring will protect you and it will make change in the next generation”.
Personal Experience or External Cues Confirming Belief in Efficacy
Some participants specifically noted their lack of infection as confirmation that the ring was protected. This was noted by IDI_3023 participant from Durban, South Africa who said “…Just like I said when I told you my story, I was with someone who was sick [referring to her HIV infected sexual partner]. I did think that it worked…In fact, I was sure that the ring worked.” Some other participants looked to other participants’ experiences to confirm their belief in ring efficacy. IDI_6002 participant from Uganda described how her perception of efficacy was based on her interpretation of a fellow participant’s experience staying HIV negative while in the study:
…I heard one of them say that their husband got HIV, but when she came for a blood test she was negative and she said that she was waiting for the following months to have another blood test. But in the following months I didn’t ask her what her results were but I continued to see her here in this research. But I think she didn’t get HIV…I became encouraged and I was happy that it could be working.
Waiting for Results to Confirm Efficacy
Some of the women, however, were less sure about efficacy and wanted assurance that the ring would protect them. They hoped, however, like this participant, that they would receive positive news from the researchers that the ring provides protection from HIV infection, like narrated by this IDI_2008 participant from Johannesburg, South Africa:
I am just waiting for the ASPIRE team to call me and tell me that the ring can protect me from HIV, not that it might protect me. That’s what I am wishing for that they would tell me that this ring would protect me from getting HIV.
During ASPIRE: Perception of Efficacy Changing Sexual Behavior
Some women across the study sites reported that perceptions of the ring’s efficacy may have led them and other women in the study to engage in higher-risk sexual behaviors including having multiple partners, or having condomless sex, because they perceived that the ring was protecting them from HIV infection. Like IDI_6003 participant from Uganda, whose perception of ring efficacy led her to stop worrying about using condoms:
Okay …In the beginning it happened ‘I protect myself [being faithful to her partner] but this husband of mine! Won’t he infect me!’ But then later I was like ‘Aaahh…after all I have a vaginal ring’ and I forgot everything about it and stopped worrying…I knew that they [health workers] had helped me prevent HIV… Because if I have it inserted, even when he comes in any state [partner having other partners and not using condoms] I am confident that the vaginal ring is there, I would not be so worried…it prevents HIV…
FGD_601 participants from Uganda described a similar change in behavior about her fellow participants, yet she acknowledges that this is risky due to the placebo-controlled aspect of the trial:
…during the recent study of the use of the ring, most people interpreted it to mean that they are protected and so free to have sex without any protection. That is what I think…they think that they now have the medicated rings that can protect them and so they have sex without protection. And yet you may come back here to the health facility and they tell you that you took a non-medicated ring.
After ASPIRE: Recall, Understanding and Reactions to ASPIRE Results
Reactions to the ASPIRE results were primarily positive, although participants’ understanding and recall of the results ranged widely, as described in the sections below.
Participants’ Understanding and/or Recall of the ASPIRE Results
Women’s understanding and level of recall of the results varied, with only a few being able to explain the level of efficacy offered by the ring in terms of percentages of efficacy and/or risk reduction. Participants generally described the ASPIRE results as a simple, binary assessment of whether the ring worked or it did not, with most expressing happiness and validation that the ring did work.
Some participants had a recall challenge, being unable to remember what they had been told about ring efficacy. For example, when asked how much the ring protected, IDI_3001 participant from Durban, South Africa stated, “…I don’t remember…No, I didn’t hear”, but she added that she was happy about the results. Another participant from Zimbabwe [IDI_5002] also did not remember specifics but remembered low protection: “I was not yet sure even up to now I am not yet sure, but at one time when the results came we were told that ASPIRE succeeded but the percentage of the level of protection was very low.” Others described aspects of the results, like this participant from FGD 601 who recalled the age differences: “What I heard was that older women used the vaginal ring better than younger women”. And some participants, like IDI_3002 from Durban, South Africa, likened the results to performance, saying “I heard that results came out and we passed.”
A few participants were able to describe their understanding of ring efficacy using percentages or figures, however there was a wide range of efficacy mentioned from 20 to 100%. IDI_6002 participant from Uganda came up with some inflated numbers, acknowledging that she couldn’t remember:
They are still testing it to see that it’s 100% but it works…I think like 85% or 70%. But I can’t remember the right percentages that we were told in the meetings we held because we also sometime come to these meeting[s] but we be having some other pressing issues and I can’t remember this…
When asked what she would tell a friend regarding the ring results, she astutely explained the difficulty with explaining efficacy results to others:
…I can tell them that there has been an introduced method of reducing on the risks of getting HIV in women. …It’s hard to tell someone about the guaranteed safety and it’s hard to explain to some that you get some/partial protection from getting HIV.
Only one participant, IDI_7001 from Malawi, was able to break down what the percentages meant:
I can also say that with this ring, for every ten [participants], three were being protected from HIV, whereas people aged from 18 to 25 years were not protected from HIV, my thoughts are that these people could not be protected because they were not using the ring properly, but the other three were wearing it well.
Participants’ Recall of Their Reactions to the Results
Participants reacted to the results by expressing their feelings, by explaining what their expectations of the results had been, and what the results meant to them.
Participants expressed feelings of happiness and validation that the ring they used, and their trial participation, was successful. For example, an FGD_601 participant from Uganda said, “…I feel good because we received exactly what we wanted, we were promised to benefit, we are glad that they told us that we used the rings effectively and we shall benefit from the results since the ring worked”. Similarly, IDI_3001 from Durban, South Africa said, “What I heard, and what made me happy is that the ring that we were using had been successful. That made me very happy. I know that it would have been sad for me that I used the ring so well, I followed all the teachings, but it did not work. So I was happy.”
However, some of the participants were sad that the ring offered little protection because they had expected the level of HIV risk reduction offered by the ring would be higher. One example of a participant expressing sadness included IDI_1004:
“Interviewer (I): How does is it make you feel that it (the ring) protects with 31%?
Respondent (R): It is making me feel sad.
I: Why?
R: 31% is too low, it would have been better if it was 70 to 80%”
In addition to emotional reactions, participants reacted to the results by expressing what their expectations had been. Like this participant, IDI_1001, from Durban, South Africa who expressed what she expected to hear from the results based on her perception of ring use during ASPIRE as indicated in her discussion with the interviewer:
I: “You said you feel disappointed by these results? …
R: Yes, I am…
I: Why?
R: I didn’t expect these results…
I: What sort of results were you expecting?
R: I expected results that were going to make me overjoyed like 85% success… everyone was saying they are using it, and of course in the beginning some did disclose that they were not using the ring, but that didn’t sit well with some of us. We ended up reporting them, we even had a meeting with sis Lungi…. After that no one came out as a non-consistent user of the product perhaps that came as a result of the fact that we had to report this issue…”
AFTER ASPIRE: Impact of Understanding of Ring Efficacy on Future Ring Use Intent
The majority of participants expressed their intention to use the ring in future, particularly in the HOPE study. Reasons for wanting to use the ring were based on the fact that the ASPIRE results revealed that the ring provided some level of HIV risk reduction. Furthermore, participants expressed that their ring adherence would improve in HOPE due to both their knowledge of ring efficacy and knowledge they would be using an active product. Like IDI_7017 participant from Malawi expressed, “I know that, in that study, the ring shall be medicated… I can be very happy because I know by using it, I shall be protected.”
Some participants described how they were interested in using the ring moving forward because they had used the ring before, had gained trust in it, and believed it had been preventing them from acquiring HIV already. A woman from Uganda [IDI_6001] explained: “I would like to participate because I never got any problem while participating in the first study and I like all that was going on because it was all helping me out.” Another respondent from Zimbabwe [IDI_5004] expanded on that idea of trust and added that the results, however imperfect, are a motivating factor to improve the way she uses the ring in HOPE, now that she knows it provides some level of protection:
I would like to join HOPE because I want to improve the way I was using the ring because now I know why it is important to use it, back then I was not satisfied because I thought, maybe it doesn’t work or maybe it will work, now I am satisfied, at least 23% is better than nothing.
Coupled with participants’ understanding of ring efficacy, participants were interested in joining HOPE since the ring would have the active drug and not be a placebo. This was also noted as a motivating factor to improve ring adherence in HOPE. For example, a participant from Durban, South Africa said, “Like I said before, I love the ring. So first thing that would make me want to use the ring is that I know that the ring I would be using is the ring that has umuthi [medicine]. The thing is that in ASPIRE, I was able to use the ring as directed although I did not know if the ring had muthi or not. Now that I would know that the ring I am using has muthi, then that will make me use it even better…”.
Discussion
In our analysis of data from IDIs and FGDs with 187 former MTN-020/ASPIRE participants from Malawi, South Africa, Uganda and Zimbabwe, we found that overall, the majority of women believed that the dapivirine vaginal ring provided protection from HIV during the placebo-controlled trial, and this may have impacted both their adherence and risk-taking behavior. After the study results were disseminated, participant’s recall and understanding of the results varied, but were generally described in binary terms—either the ring worked, or it did not—with most expressing happiness and feelings of validation that the ring worked. Due to this perception of ring efficacy, most participants expressed an intention to use the ring in the future open-label extension trial, MTN-025/HOPE.
For these participants, there seemed to be an overconfidence in their ability to know whether the ring worked during ASPIRE, a concept known as “preventive misconception”, which refers to a belief that investigational prevention methods are effective [16]. During the trial, women theoretically received consistent information from study staff regarding the investigational nature of the ring and the placebo-controlled study design. Yet, women individually processed this information differently and did not seem to retain or fully comprehend the specifics, leading some participants to decide to believe that the ring was protecting them. It is also likely that participant perceptions of ring efficacy were influenced by their peers, partners, or other community members [17–20]. Nevertheless, this preventive misconception has important implications for how well the informed consent process or health education and counseling was working, since some women may have engaged in higher-risk sexual behaviors believing that they were protected, even after being reminded by study staff that they couldn’t know that for sure. Preventive misconception has been noted in other microbicide and vaccine research in similar settings, mostly in the context of willingness to join clinical studies [16, 21, 22]. There is limited research on how to address these beliefs, however, one study that evaluated an intervention to reduce preventive misconception among adolescents in HIV vaccine trials found that using a brochure during the informed consent process with “two-sided persuasive messaging” improved participants’ understanding of the concepts around randomization and placebo [23]. Interventions like this may be worth investigating for other types of future HIV prevention clinical trials as well.
The results also suggest that participants’ understanding of the statistical results about ring efficacy from ASPIRE were oversimplified. As noted above, participants individually processed this information and had varied understanding and recall of the ASPIRE results when asked about it during MTN-032/AHA. Most participants did not remember the level of estimated efficacy in terms of the percentages that were presented to them during the results dissemination, or they had over-inflated memories of the percentages. Some women’s inability to accurately remember the HIV risk reduction offered by the ring could be attributed to recall bias because this study was conducted after ASPIRE. Alternatively, the discrepancy could be due to the way the efficacy of the ring message was framed and communicated to participants during results dissemination. Explaining safety and partial efficacy is challenging, as noted by one of the participants from Uganda when asked how she would explain the results to a friend. Stadler et al. noted that challenges remain in messaging and message-framing on how to communicate facts about PrEP efficacy to trial participants, which then shape an individual’s expectations of PrEP efficacy [24]. It is critical that perceptions of efficacy and preventive misconception are explored during or prior to trials to inform message-framing that will allow participants to understand the risks of using an investigational product.
Difficulty explaining efficacy to potential end-users has been noted in HIV prevention efforts regarding oral PrEP implementation [13]. PrEP roll-out activities have even discussed the difficulty in explaining PrEP efficacy and partial efficacy to healthcare providers and clinicians [25]. Given the difficulty in explaining and understanding partial efficacy, and the fact that the ring is an important HIV prevention method that will provide women with an alternative option for managing their sexual health beyond oral PrEP, more research on the most effective strategy to frame ring efficacy is needed [8, 26, 27]. The findings in this paper emphasize the need for adaptation of efficacy messages that fit the culture and/or the setting where the ring will be used. This need is particularly time-sensitive given the recent positive opinion of the dapivirine vaginal ring from the European Medicines Agency [12], which is the first step toward regulatory approval and its rollout in global HIV endemic settings.
Finally, while most participants expressed an intention to join the open label extension trial, HOPE, and noted plans to improve their adherence to the now known effective and active-product ring, actual enrollment numbers showed that a little over half of ASPIRE participants joined HOPE [9]. Some reasons for the decreased enrollment in HOPE could be due to acquisition of HIV in the time between ASPIRE and HOPE, or other changes in circumstances such as pregnancy, or reduced perceived risk and thus reduced interest in the product. Taking a conservative position, however, it is likely that some participants chose not to join HOPE, or joined but chose not to use the ring during HOPE due to their concerns about the ring conferring only partial efficacy. Drawing from the Health Belief Model (HBM), which asserts that the motivation for people to take action to promote or prevent disease is based on factors such as how strongly they believe that they are susceptible to the disease in question and whether the suggested health intervention is of benefit [28], one could argue that a perception of the efficacy of the ring would likely play an important role in whether or not an individual would use the product in the future. Marshall et al., explained the “benefit versus risk” of medicine taking: medication-taking behaviour can be influenced by a benefit–risk analysis in which patients weigh the benefits of medication against the risks associated with medication such as adverse symptoms. When a product is being tested in a trial, some of its benefits and risks are unknown, highlighting the importance of understanding how each person’s conceptualization of product efficacy might contribute to their product acceptance and adherence behaviour [29].
The reader should consider several limitations to this analysis. Understanding of ring efficacy described in these analyses may have been reflective of the way participant comprehension was explored by different interviewers at different sites. Some interviewers used probes to help participants explain their understanding of efficacy while other interviewers repeated the percentages of efficacy and then asked what these percentages meant to the women. The style of questioning, and mode of presentation in the in-depth interviews may have affected participant’s understanding of ring efficacy and their descriptions of subsequent interest in future ring adherence and/or use. The tone of that discussion may also have affected the interpretation of the results presented in this paper. Participants were interviewed several months after they received the ASPIRE results of ring efficacy so recall bias may have been a challenge, and indeed, several women said that they had forgotten what they were told about ring efficacy. Furthermore, perception of what they thought about the efficacy of the ring during ASPIRE may have been influenced by updated knowledge of ring efficacy heard during ASPIRE results presentation, even if they only remembered that it was found to be partially effective. Finally, the study sample was selected from among those who were using active rings, and although blinded during ASPIRE, they were told about their assignment between participation in ASPIRE and AHA. Knowledge of which ring they used may have biased their perspectives about efficacy.
Conclusions
The findings from this qualitative sub-study of the ASPIRE Phase III clinical trial of the monthly dapivirine vaginal ring suggest that there is a need to investigate improved and suitable ways of explaining placebo-controlled trials and efficacy to women in Sub-Saharan Africa (where clinical trials are conducted and where novel HIV prevention products are needed and will be rolled out). The benefits of the ring are now known, and the EMA positive opinion confirms the imperative that those benefits are well communicated and understood by potential end-users, health care providers, and community members. Engagement with end-users to construct effective messages and to develop tools to measure understanding of partial efficacy will be an essential part of this communication process.
Funding
The MTN-032 study was designed and implemented by the Microbicide Trials Network (MTN) funded by the National Institute of Allergy and Infectious Diseases through individual grants (UM1AI068633, UM1AI068615 and UM1AI106707), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health, all components of the U.S. National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Conflict of interest
The primary author implemented the study at the Kampala-Uganda site as the Investigator of Record through the Microbicide Trials Network (MTN). The study was funded by the National Institute of Allergy and Infectious Diseases through individual grants (UM1AI068633, UM1AI068615 and UM1AI106707), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health, all components of the U.S. National Institutes of Health (NIH). The primary author received salary support during the implementation of this study.
Footnotes
Ethical Approval This study was approved at the various study sites by all regulatory/ethics committees in each respective country. At Makerere University-Johns Hopkins University (MUJHU) in Uganda where the primary author conducted the study, ethical approval was received from Joint Clinical Research (JCRC) ethics review committee, Uganda National Council for Science and Technology (UNCST) and Johns Hopkins Medicine IRB. All study participants were consented for participation in the study, dissemination and publication of the study findings.
References
- 1.UNAIDS D. Update AE. Geneva: Joint United Nations Programme on HIV. AIDS. 2019. [Google Scholar]
- 2.Eakle R, Bourne A, Jarrett C, Stadler J, Larson H. Motivations and barriers to uptake and use of female-initiated, biomedical HIV prevention products in sub-Saharan Africa: an adapted meta-ethnography. BMC Public Health. 2017;17(1):968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baeten JM, Palanee-Phillips T, Brown ER, Schwartz K, Soto-Torres LE, Govender V, et al. Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. N Engl J Med. 2016;375(22):2121–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372(6):509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367(5):423–34. [DOI] [PubMed] [Google Scholar]
- 7.Choopanya K, Martin M, Suntharasamai P, Sangkum U, Mock PA, Leethochawalit M, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381(9883):2083–90. [DOI] [PubMed] [Google Scholar]
- 8.van der Straten A, Stadler J, Montgomery E, Hartmann M, Magazi B, Mathebula F, et al. Women’s experiences with oral and vaginal pre-exposure prophylaxis: the VOICE-C qualitative study in Johannesburg, South Africa. PloS One. 2014;9(2):e89118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baeten J, Palanee-Phillips T, Mgodi N, Ramjee G, Gati B, Mhlanga F, et al. High adherence and sustained impact on HIV-1 incidence: final results of an open-label extension trial of the dapivirine vaginal ring. J Int AIDS Soc. 2019;22:29. [Google Scholar]
- 10.Baeten JM, Palanee-Phillips T, Brown ER, Schwartz K, Soto-Torres LE, Govender V, et al. Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. N Engl J Med. 2016;375:2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nel A, Bekker L-G, Bukusi E, Hellstrӧm E, Kotze P, Louw C, et al. Safety, acceptability and adherence of dapivirine vaginal ring in a microbicide clinical trial conducted in multiple countries in Sub-Saharan Africa. PloS One. 2016;11(3):e0147743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. European Medicines Agency (EMA) approval of the dapivirine ring for HIV prevention for women in high HIV burden settings. Geneva: WHO; 2020. [Google Scholar]
- 13.Underhill K, Morrow KM, Colleran C, Calabrese SK, Operario D, Salovey P, et al. Explaining the efficacy of pre-exposure prophylaxis (PrEP) for HIV prevention: a qualitative study of message framing and messaging preferences among US men who have sex with men. AIDS Behav. 2016;20(7):1514–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koblin BA, Mansergh G, Frye V, Tieu HV, Hoover DR, Bonner S, et al. Condom-use decision making in the context of hypothetical pre-exposure prophylaxis efficacy among substance-using men who have sex with men: project MIX. JAIDS J Acquir Immun Defic Syndr. 2011;58(3):319–27. [DOI] [PubMed] [Google Scholar]
- 15.Montgomery ET, Stadler J, Naidoo S, Katz AW, Laborde N, Garcia M, et al. Reasons for nonadherence to the dapivirine vaginal ring: narrative explanations of objective drug-level results. AIDS. 2018;32(11):1517–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woodsong C, Alleman P, Musara P, Chandipwisa A, Chirenje M, Martinson F, et al. Preventive misconception as a motivation for participation and adherence in microbicide trials: evidence from female participants and male partners in Malawi and Zimbabwe. AIDS Behav. 2012;16(3):785–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katz AW, Naidoo K, Reddy K, Chitukuta M, Nabukeera J, Siva S, et al. The power of the shared experience: MTN-020/ASPIRE trial participants’ descriptions of peer influence on acceptability of and adherence to the dapivirine vaginal ring for HIV prevention. AIDS Behav. 2020;24:2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montgomery ET, van der Straten A, Chidanyika A, Chipato T, Jaffar S, Padian N. The importance of male partner involvement for women’s acceptability and adherence to female-initiated HIV prevention methods in Zimbabwe. AIDS Behav. 2011;15(5):959–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montgomery ET, van der Straten A, Stadler J, Hartmann M, Magazi B, Mathebula F, et al. Male partner influence on women’s HIV prevention trial participation and use of pre-exposure prophylaxis: the importance of “understanding.” AIDS Behav. 2015;19(5):784–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chitukuta M, Duby Z, Katz A, Nakyanzi T, Reddy K, Palanee-Phillips T, et al. Negative rumours about a vaginal ring for HIV-1 prevention in sub-Saharan Africa. Cult Health Sex. 2019;21(11):1209–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dellar RC, Karim QA, Mansoor LE, Grobler A, Humphries H, Werner L, et al. The preventive misconception: experiences from CAPRISA 004. AIDS Behav. 2014;18(9):1746–52. [DOI] [PubMed] [Google Scholar]
- 22.Ott MA, Alexander AB, Lally M, Steever JB, Zimet GD, the Adolescent Medicine Trials Network (ATN). Preventive misconception and adolescents’ knowledge about HIV vaccine trials. J Med Ethics. 2013;39(12):765–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lally M, Goldsworthy R, Sarr M, Kahn J, Brown L, Peralta L, et al. Evaluation of an intervention among adolescents to reduce preventive misconception in HIV vaccine clinical trials. J Adolesc Health. 2014;55(2):254–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stadler J, Saethre E. Blockage and flow: intimate experiences of condoms and microbicides in a South African clinical trial. Cult Health Sex. 2011;13(1):31–44. [DOI] [PubMed] [Google Scholar]
- 25.Young I, Valiotis G. Strategies to support HIV literacy in the roll-out of pre-exposure prophylaxis in Scotland: findings from qualitative research with clinical and community practitioners. BMJ Open. 2020;10(4):e033849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Straten A, Montgomery ET, Musara P, Etima J, Naidoo S, Laborde N, et al. Disclosure of pharmacokinetic drug results to understand nonadherence: results from a qualitative study. AIDS (London, England). 2015;29(16):2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubincam C, Newman PA, Atujuna M, Bekker L-G. ‘Why would you promote something that is less percent safer than a condom?’: Perspectives on partially effective HIV prevention technologies among key populations in South Africa. SAHARA-J J Soc Asp HIV/AIDS. 2018;15(1):179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tarkang EE, Zotor FB. Application of the Health Belief Model (HBM) in HIV prevention: a literature review. Central Afr J Public Health. 2015;1(1):1–8. [Google Scholar]
- 29.Marshall VK, Given BA. Factors associated with medication beliefs in patients with cancer: an integrative review. Oncol Nurs Forum. 2018;45:508. [DOI] [PubMed] [Google Scholar]


