Abstract
BACKGROUND
Human papillomavirus (HPV) is responsible for a growing proportion of oropharyngeal squamous cell carcinomas (OPSCCs) among men and White individuals. Whether similar trends apply to women, non‐Whites, and non‐oropharyngeal squamous cell carcinomas (non‐OPSCCs) is unknown.
METHODS
This is a cross‐sectional analysis combining 2 multi‐institutional case series of incident head and neck squamous cell carcinoma (HNSCC) cases. Incident HNSCCs from 1995 to 2012 were enrolled retrospectively using banked tumor samples and medical record abstraction. Incident HNSCCs from 2013 to 2019 were enrolled prospectively. The prevalence of tumor HPV biomarkers was tested over 3 time periods (1995‐2003, 2004‐2012, and 2013‐2019). Centralized testing was done for p16 immunohistochemistry (p16) and oncogenic HPV in situ hybridization (ISH).
RESULTS
A total of 1209 incident cases of HNSCC were included. Prevalence of p16‐ and ISH‐positive tumors increased significantly for oropharynx cancers over time. The majority were positive after 2013 for White patients (p16, 92%; P < .001; ISH 94%; P < .001), Black patients (p16, 72%; P = .021; ISH 67%; P = .011), and Hispanic patients (p16, 100%; P = .04; ISH 100%; P = .013). For women with OPSCC, the prevalence of p16‐ and ISH‐positive tumors increased significantly to 82% (P < .001) and 78% (P = .004), respectively. For non‐OPSCCs, there was increased p16 and ISH positivity overall with 24% p16 and 16% ISH positivity in the most recent time period (P < .001 for both).
CONCLUSIONS
The majority of OPSCCs in US tertiary care centers are now p16 and ISH positive for all sex and race groups. In some populations in the United States, 91% of OPSCCs are now caused by HPV. Few non‐OPSCCs are p16 and ISH positive.
Keywords: head and neck cancer, human papillomavirus, oropharyngeal cancer, prevalence, race, sex
Short abstract
This study evaluates the prevalence of p16 and in situ hybridization positivity in head and neck cancers over time. This study shows an increase in prevalence over time among women and non‐Whites, 2 groups that are understudied in the epidemiology of human papillomavirus.
Introduction
In recent decades, the epidemiology of head and neck cancer in the United States has drastically changed. There has been a significant decrease in the annual incidence of head and neck squamous cell carcinomas (HNSCCs) overall despite a significant increase in the incidence of oropharynx squamous cell cancers (OPSCCs). 1 , 2 , 3 , 4 The increased incidence of oropharynx cancers is driven by human papillomavirus (HPV) and has been predominantly observed among White individuals, men, and more recent birth cohorts. 5 , 6 An analogous increase has not been detected for other demographic groups such as women or non‐Whites, although the proportion of OPSCCs caused by HPV has increased significantly over time among women, 6 , 7 , 8 which may foreshadow future incidence shifts.
Early studies of incidence were composed mainly of men and White patients, thereby limiting examination of trends in prevalence to these groups. Recent research suggests HPV prevalence, when measured by either p16 or in situ hybridization (ISH), may be increasing among OPSCCs in non‐Whites; however, these trends were not statistically significant among Black and Hispanic individuals. 7 Given the growing similarity of sexual practices of people, independent of race, analogous increases in the prevalence of p16‐ and ISH‐positive tumors may be observed for OPSCCs across race groups in an adequately powered study. 9 Therefore, it remains important to determine whether the prevalence of HPV‐associated OPSCC is increasing over time among non‐Whites.
Furthermore, HPV appears to be responsible for a small proportion of nonoropharynx HNSCC tumors (nonoropharyngeal squamous cell carcinoma [non‐OPSCC]) as well. 10 , 11 , 12 Whether trends in prevalence observed in OPSCC are applicable to non‐OPSCC has not been explored. This study was designed to elucidate the prevalence of p16‐ and ISH‐positive tumors in HNSCC over the past 25 years with special attention to changes among women and non‐Whites.
Materials and Methods
This analysis consisted of 2 data sets. The first was a retrospective study of incident HNSCC cases diagnosed between 1995 and 2012 (retrospective PROVE study), designed to enrich for non‐Whites and female cases, as previously described. 7 The study included participants from the Sidney Kimmel Comprehensive Cancer Center at the Johns Hopkins Hospital (JHH) and the Helen Diller Family Comprehensive Cancer Center at the University of California San Francisco. Informed consent was waived by the institutional review boards. Demographic and clinical characteristics including age, sex, race, clinical staging, anatomic site, and alcohol/tobacco use were included.
The second data source was a prospective study of incident HNSCC diagnosed from 2013 to 2019 13 without prior radiation or head and neck cancers (prospective PROVE study). The prospective cohort added the Tisch Cancer Institute at Icahn School of Medicine at Mount Sinai to JHH and University of California San Francisco locations. The cohort was recruited from outpatient otolaryngology head and neck surgery clinics. All patients with incident HNSCC were eligible and approached. The study was approved by all sites' institutional review boards. Demographic characteristics (age, sex, race, tobacco, and alcohol use) were ascertained by computer assisted surveys completed by participants at the time of enrollment. Clinical characteristics (anatomic site, stage, and treatment) were prospectively ascertained by medical record abstraction.
Women and minorities were oversampled in the earlier but not the later study. Comparing the most recent time period with earlier time periods, there was a higher proportion of males (75% vs 64%) and White non‐Hispanics (73% vs 35%), respectively. The earlier study also sampled across tumor sites for more equitable inclusion of each tumor site, whereas the most recent period included all incident HNSCCs prospectively enrolled and represents the current oropharyngeal predominance among cases in these clinics. Given the enrichment by sex, race, and tumor site in the retrospective PROVE study, overall changes in the composition of HNSCC could not be explored. However, this oversampling provided the power to explore the role of HPV in these groups over time, which was our intended goal.
Tumor Testing
Centralized tumor testing was performed at JHH. This testing consisted of p16 immunohistochemistry (p16) (MTM Laboratories, Heidelberg, Germany) and ISH. ISH testing varied by time and study site and has been discussed previously. 14 In brief, GenPoint HPV16 DNA ISH (Dako, Carpinteria, California) and Ventana Inform HPV III Family 16 cocktail (Ventana Medical Systems, Tucson, Arizona) probes, oncogenic HPV DNA probes, were used on the retrospective cohorts and the prospective cohort until 2014. After 2014, RNA ISH testing with RNASCOPE (Advanced Cell Diagnostics, Hayward, California) was used. HPV testing was initially performed and, if negative and p16 positive, HPV high‐risk cocktail probes for high‐risk types were used. p16 positivity was defined as 70% or more of the tumor having a strong and diffuse nuclear or cytoplasmic staining pattern. 15 , 16 ISH positivity was defined as punctate hybridization signals localized to the tumor cell nuclei. 17 All histologic interpretation was performed centrally by head and neck pathologists blinded to other aspects of the study. A total of 1209 HNSCC participants with p16 or ISH test results were included in this analysis. This included 1202 participants (99.4%) with both p16 and ISH testing, 5 participants (0.4%) with only p16, and 2 participants (0.2%) with only ISH testing.
Statistical Analysis
Demographic characteristics were compared across groups using χ2 tests. Race and ethnicity were categorized as White, Black, Asian (all non‐Hispanic), or Hispanic (any race). For some analyses, HNSCC tumor sites were separated into OPSCC (including unknown primary) and non‐OPSCC (larynx, nasopharynx, oral cavity). Trends in prevalence across time periods (1995‐2003, 2004‐2012, and 2013‐2019) were tested for continuous change across these 3 periods and were analyzed using logistic regression to determine significance. p16 and ISH were analyzed separately. The average annual increases in p16 positivity and ISH positivity between 1995 and 2019 were analyzed separately using Poisson regression models with robust variance, for both OPSCC and non‐OPSCC groups. The AJCC Cancer Staging Manual 7th edition tumor and nodal stage guidelines were used for continuity across time periods. 18 Statistical significance was determined by 2‐sided P < .05. Stata version 16.1 (StataCorp, College Station, Texas) was used for analysis.
Results
The study population included 1209 incident cases of HNSCC (Table 1). Cases were similarly distributed over 3 time periods (31% vs 39% vs 29%). There were differences in the study population over time in sex, race, and tumor type (Table 1).
TABLE 1.
Characteristics of HNSCC Patients From 3 Tertiary Care Centers in the United States Between 1995 and 2019 by Time Period of Incident Cancer Diagnosis
| Characteristics | Retrospective Study | Prospective Study | P | |
|---|---|---|---|---|
| 1995‐2003 (n = 380), No. (%) | 2004‐2012 (n = 474), No. (%) | 2013‐2019 (n = 355), No. (%) | ||
| Study site | ||||
| JHH | 182 (48) | 245 (52) | 245 (69) | <.001 |
| UCSF | 198 (52) | 229 (48) | 69 (19) | |
| MSSM | — | — | 41 (12) | |
| Sex | ||||
| Men | 246 (65) | 300(63) | 271 (76) | <.001 |
| Women | 134 (35) | 134 (37) | 84 (24) | |
| Race | ||||
| White non‐Hispanic | 123 (32) | 188 (40) | 291 (82) | <.001 |
| Black non‐Hispanic | 138 (36) | 136 (29) | 37 (10) | |
| Hispanic | 43 (11) | 55 (11) | 13 (4) | |
| Asian | 76 (20) | 95 (20) | 14 (4) | |
| Age, y | ||||
| Median (95% CI) | 56 (55‐57) | 58 (56‐59) | 59 (58‐60) | .015 |
| Anatomic site | ||||
| Oropharynx | 90 (24) | 145 (31) | 195 (55) | |
| Oral cavity | 103 (27) | 149 (31) | 109 (31) | <.001 |
| Nasopharynx | 59 (15) | 65 (14) | 3 (1) | |
| Larynx | 128 (34) | 115 (24) | 33 (9) | |
| Other HNSCC | 0 (0) | 0 (0) | 15 (4) | |
| Tumor stage | ||||
| T1 | 84 (24) | 130 (29) | 145 (42) | <.001 |
| T2 | 91 (26) | 128 (28) | 120 (35) | |
| T3 | 72 (20) | 80 (18) | 39 (12) | |
| T4 | 106 (30) | 116 (25) | 38 (11) | |
| Missing | 27 | 20 | 13 | |
| Nodal stage | ||||
| N0 | 161 (46) | 169 (38) | 125 (36) | <.001 |
| N1 | 52 (15) | 69 (15) | 59 (17) | |
| N2a‐c | 115 (33) | 188 (42) | 157 (46) | |
| N3 | 20 (6) | 24 (5) | 4 (1) | |
| Missing | 32 | 24 | 10 | |
| Metastatic stage | ||||
| M0 | 360 (98) | 452 (97) | 351 (99) | .10 |
| M1 | 9 (2) | 14 (3) | 3 (1) | |
| Unknown | 11 | 8 | 1 | |
| Tobacco use | ||||
| Never | 40 (19) | 117 (27) | 101 (32) | <.001 |
| Former | 73 (35) | 172 (39) | 170 (55) | |
| Current | 95 (46) | 147 (34) | 40 (13) | |
| Unknown | 172 | 38 | 44 | |
| Alcohol use | ||||
| Never | 55 (28) | 141 (33) | 8 (3) | <.001 |
| Former | 42 (21) | 82 (19) | 130 (51) | |
| Current | 102 (51) | 207 (48) | 117 (46) | |
| Unknown | 181 | 44 | 100 | |
Abbreviations: HNSCC, head and neck squamous cell carcinoma; JHH, Hopkins Hospital; MSSM, Mount Sinai Medical Center; UCSF, University of California San Francisco.
Overall Prevalence
Prevalence of both p16‐positive and ISH‐positive tumors over time was evaluated (Fig. 1). The most notable increase in p16 positivity and ISH positivity was in OPSCCs, as both p16 positivity (44% to 70% to 91%; P < .001; Fig. 1A) and ISH positivity (39% to 66% to 92%; P < .001; Fig. 1B) more than doubled across the 25 years to over 90% by the final time period. From 1995 to 2019, the average annual increase among OPSCC was 3% for p16 positivity and 4% for ISH positivity (each P < .0001, Supporting Table 2), after adjustment for tobacco, alcohol, and demographics.
Figure 1.
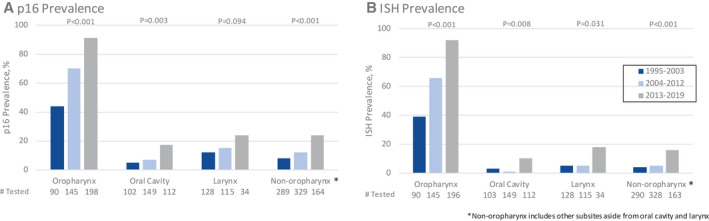
Prevalence of (A) p16‐positive and (B) in situ hybridization–positive head and neck squamous cell carcinomas from 3 tertiary care centers in the United States from 1995 to 2019 by tumor site and time period.
In non‐OPSCC sites, there were statistically significant increases observed in p16 (8% to 12% to 24%; P < .001; Fig. 1A) and ISH positivity (4% vs 5% vs 16%; P < .001; Fig. 1B), although most non‐OPSCCs remained negative for these biomarkers. Significant increases in p16 and ISH positivity over time were detected for each of the non‐OPSCC sites evaluated (Fig. 1A,B; Supporting Table 1). Between 1995 and 2019, the average annual increase among non‐OPSCC was 6% and 13% for p16 positivity and ISH positivity respectively (P = .009 and P = .001; Supporting Table 2), after adjustment for tobacco, alcohol, and demographics. Although p16 and ISH positivity was similar in the oropharynx, among non‐OPSCCs there was a discrepancy between the 2 modes of testing. p16 positivity was twice as common as ISH positivity for oral cavity and larynx tumors. Among non‐OPSCCs, larynx tumors had the highest prevalence of p16 (12% to 15% to 24%; P = .094) and ISH positivity (5% to 5% to 18%; P = .031).
Prevalence by Sex
Increasing prevalence of p16‐positive and ISH‐positive tumors were observed for both men and women (Table 2). Among OPSCCs, p16 and ISH positivity increased significantly over time, with HPV causing more than 90% of recent cases among men and more than 78% of cases among women in the most recent time period (Supporting Fig. 1A,B). A smaller but significant increase was observed with non‐OPSCCs. Among men with non‐OPSCC, p16 positivity increased from 13% in the earliest to 25% in the latest time period (P < .001). Similarly, ISH positivity increased from 3% to 16% (P < .001). Among women with non‐OPSCC, p16 positivity increased from 9% to 22% (P = .041) and ISH positivity increased from 6% to 15% (P = .096). In the later period, women approached parity with men for proportion of non‐OPSCC that were p16 or ISH positive.
TABLE 2.
Prevalence of p16‐Positive and ISH‐Positive Tumors From 3 Tertiary Care Centers in the United States Between 1995 and 2019 by Time Period, Sex, and Tumor Site
| p16, No. Tested (% Positive) | ISH, No. Tested (% Positive) | |||||||
|---|---|---|---|---|---|---|---|---|
| 1995‐2003 | 2004‐2012 | 2013‐2019 | P | 1995‐2003 | 2004‐2012 | 2013‐2019 | P | |
| Men | ||||||||
| All HNSCC | 245 (18) | 300 (31) | 270 (65) | <.001 | 246 (13) | 299 (26) | 269 (63) | <.001 |
| OPSCC | 66 (47) | 91 (74) | 161 (93) | <.001 | 66 (39) | 91 (71) | 161 (94) | <.001 |
| Non‐OPSCC | 179 (7) | 209 (12) | 109 (25) | <.001 | 180 (3) | 208 (6) | 108 (17) | <.001 |
| Women | ||||||||
| All HNSCC | 134 (14) | 174 (28) | 84 (45) | <.001 | 134 (12) | 174 (21) | 84 (39) | <.001 |
| OPSCC | 24 (38) | 54 (63) | 33 (82) | <.001 | 24 (38) | 54 (57) | 31 (77) | .004 |
| Non‐OPSCC | 110 (9) | 120 (12) | 51 (22) | .041 | 110 (6) | 120 (5) | 51 (16) | .10 |
| Men and women | ||||||||
| All HNSCC | 379 (17) | 474 (30) | 354 (60) | <.001 | 380 (12) | 473 (24) | 351 (58) | .001 |
| OPSCC | 90 (44) | 145 (70) | 194 (91) | <.001 | 90 (39) | 145 (66) | 192 (92) | .001 |
| Non‐OPSCC | 289 (8) | 329 (12) | 160 (24) | <.001 | 290 (4) | 328 (5) | 159 (16) | .001 |
Abbreviations: HNSCC, head and neck squamous cell carcinoma; OPSCC, oropharyngeal squamous cell carcinoma.
Prevalence by Race
A significant increase in prevalence of p16‐positive and ISH‐positive HNSCC tumors overall was observed for all races over the 3 time periods (Fig. 2). These increases were primarily in OPSCCs. A 176% or greater increase in ISH positivity was observed for all race groups between the first and third time period. The smallest significant change was seen in Black individuals, (13% to 36%), whereas White (15% to 61%) and Hispanic individuals (12% to 54%) had greater than a 330% increase in ISH‐positive OPSCC. Changes in p16 and ISH positivity for non‐OPSCCs were primarily among White patients (Fig. 2C,D).
Figure 2.
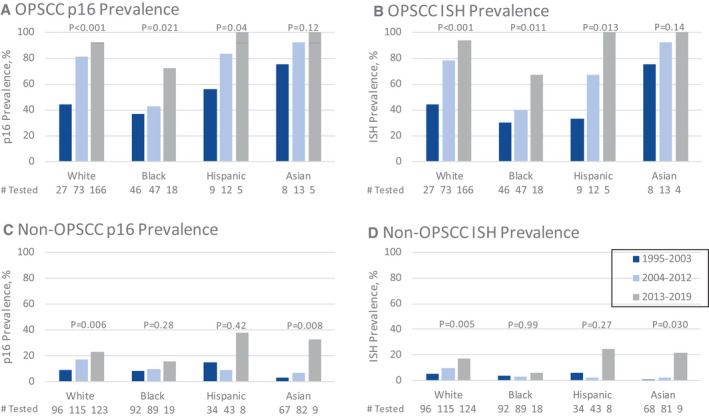
Prevalence of (A) p16‐positive OPSCC, (B) ISH‐positive OPSCC, (C) p16‐positive non‐OPSCC, and (D) ISH‐positive non‐OPSCC from 3 tertiary care centers in the United States from 1995 to 2019 by race and time period. ISH indicates in situ hybridization; non‐OPSCC, nonoropharyngeal squamous cell carcinomas; OPSCC, oropharyngeal squamous cell carcinoma.
White OPSCC patients had a significant increase in p16‐positive (44% to 81% to 92%; P < .001) and ISH‐positive tumors (44% to 78% to 94%; P < .001). p16 and ISH positivity increased among White patients with non‐OPSCC (9% to 17% to 23%; P = .007; 5% to 10% to 17%; P = .008).
Black individuals had a lower prevalence of p16‐positive and ISH‐positive tumors than White, but both markers increased significantly over time among Black individuals with OPSCC. Among 111 Black patients with OPSCC, there were statistically significant increases in the proportion of p16‐positive (37% to 43% to 72%; P = .021) and ISH‐positive tumors (30% to 40% to 67%; P = .011). Among 199 Black non‐OPSCC cases, prevalence of p16 doubled (8% to 10% to 16%; P = .28) but was not statistically different, perhaps due to few Black non‐OPSCC cases in the most recent period. The prevalence of ISH among Black non‐OPSCCs was stable (4% to 3% to 6%; P = .99).
Hispanic and Asian patients also increased in p16 positivity and ISH positivity over time (Fig. 2). Among 26 Hispanics with OPSCC, there was a marginally significant increase in prevalence of p16 positivity, with 100% of tumors positive in the most recent period (56% to 83% to 100%; P = .04), and a significant increase in ISH positivity (33% to 67% to 100%; P = .013). In 85 non‐OPSCC tumors among Hispanics, increases in prevalence of p16‐positive tumors (15% to 30% to 38%; P = .42) and ISH‐positive tumors (6% to 2% to 25%; P = .27) were observed but were nonsignificant. For the 26 Asian patients with OPSCC, there was a nonsignificant increase of p16 positivity (75% to 92% to 100%; P = .12) and ISH positivity (75% to 92% to 100%; P = .14). In 158 non‐OPSCCs among Asians, there were significant increases in the prevalence of p16 and ISH positivity (3% to 7% to 33%; P = .008; 1% to 2% to 22%; P = .03).
Discussion
This analysis shows a notable increase in the prevalence of p16‐ and ISH‐positive OPSCCs among all sex and race groups over a 25‐year period. This trend among Black and Hispanic patients has not been shown previously. Although the increasing proportion of cancers caused by HPV is partially explained by decreasing tobacco‐related HNSCC, it may also represent increases in incidence of HPV‐associated OPSCCs among women and non‐White patients. The increase in IRR in p16 positivity and ISH positivity in our study, while controlling for smoking and drinking, bolster this possibility. The large increase in prevalence of HPV‐associated OPSCCs in women has not been previously shown in epidemiologic studies. The observed increases in prevalence of p16‐ and ISH‐positive non‐OPSCCs have similarly not previously been detected. This large study evaluates p16 and ISH prevalence in HNSCCs from tertiary care centers in the United States during the past 25 years. Because of the inherent demographic differences between the 2 source populations, conclusions about the changes in prevalence across time periods are best examined within specific gender and racial groups. This is the first study to show that the majority of OPSCC among all race and sex groups are now caused by HPV and suggests that more than 90% of OPSCCs in the United States are now caused by HPV.
Few studies have systematically evaluated the prevalence of HPV‐related OPSCC tumors among Black patients in the United States. Our findings are consistent with evidence from prior studies that the prevalence of HPV‐associated tumors remains lower among Black individuals. 19 , 20 , 21 Prior studies showed that the majority of HNSCC among Black patients were HPV negative. In this study, we show for the first time that the majority of OPSCC among Black patients are p16 positive and ISH positive, with proportions (~70%) analogous to those of White patients in the last decade. These changes are consistent with decreases in tobacco use and changes in sexual behavior in the United States. 22 , 23 , 24 , 25 , 26 , 27 In the most recent period, the majority of OPSCCs are now HPV related (by p16 and ISH) across all race groups.
This study underscores the dominance of HPV as a primary cause of OPSCC in the United States. This proportion is higher than earlier studies, consistent with the temporal trends shown in this study. 28 , 29 , 30 For White, Hispanic, and Asian patients, the probability of contemporary OPSCC in the United States being HPV associated is greater than 90%, whereas for Black patients it is roughly 70%.
These trends are not surprising as HPV‐associated oropharynx cancer is a consequence of HPV infection transmitted by oral sex. 31 Although there are statistical differences in sexual practices by race, 32 the practice of oral sex has increased among all racial groups in the United States, and the differences in sexual behavior are more modest 33 , 34 than the observed differences in incidence of OPSCC by race. 9 , 35 Recent research shows important changes related to race and HPV risk factors including increases in oral sexual partners among Black individuals when compared to other races, 36 increased oral sex by younger birth cohorts, 35 a higher rate of cervical HPV infection among Black women, 37 and decreased adherence to vaccination guidelines. 38 These factors suggest increased exposure to HPV among Black individuals in the future. This increased exposure, and the increasing prevalence of HPV‐positive tumors among Black patients in this study, adds to previous studies portending increases in incidence of HPV‐associated OPSCCs among Black individuals in the United States. 21 Similarly, increased oral sex and prevalence of p16‐ and ISH‐positive OPSCC tumors among non‐White groups portends an increase in incidence of HPV‐associated OPSCCs. Vaccination will decrease the incidence of HPV‐associated OPSCC only after 2045, because of the lack of vaccination in older age cohorts. 39
Another notable finding of this study was the increase in p16‐positive and ISH‐positive non‐OPSCCs. Neither has previously been detected, likely due to their low prevalence and the lack of prospective studies in the last 10 years. 40 Among non‐OPSCCs, p16 and ISH were often discordant, highlighting that p16 is likely a poor marker for HPV in non‐OPSCC. 41 , 42 In OPSCCs, p16 is an acceptable surrogate and predicts ISH positivity, but the same is not true for non‐OPSCCs. 43 , 44 It is plausible, though unlikely, that p16 positivity in non‐OPSCCs indicates the presence of HPV oncogenic types not accounted for by ISH. The primary HPV types found in PCR studies of head and neck cancers are detected in the ISH pooled assay. 17 Therefore, p16 positivity in these cases is likely true but not reflective of HPV as the etiology in a substantial proportion of non‐OPSCCs. 13 When tumors are ISH positive, it is unclear whether HPV is a cofactor, rather than the etiologic agent for non‐OPSCCs, or whether HPV is present but nononcogenic in these cancers. Here, p16 may reflect the biology of the tumor and protein upregulation due to reasons other than downstream E6 or E7 oncogenesis and represents a focus of future research. Nevertheless, HPV based on ISH is increasing in non‐OPSCCs among White individuals and Asians, which may portend an increasing burden of incident HPV‐related cancers in the future.
Detection of HPV in larynx and nasopharynx cancers raises questions regarding the biological plausibility of HPV infection in these sites and requisite sexual exposures; it is unknown whether a form of viral field cancerization in these cases is possible. Of the non‐OPSCC anatomic sites, the nasopharynx and larynx have the largest proportion of ISH‐positive tumors, possibly reflecting the increased lymphoid tissue of these areas which are contiguous with Waldeyer ring. Of nonoropharyngeal sites, the sinonasal tract has the highest prevalence of HPV. 45 , 46 Tumor testing of non‐OPSCCs remains discouraged. 40 , 43 , 44 The prognostic benefits of p16 and ISH positivity have not been shown consistently for non‐OPSCCs. 47 , 48 , 49
This study has several limitations. The earlier cohorts oversampled for women and minorities. Therefore, the proportion of women and minorities across the time periods was lower in the most recent group. It is also possible that degradation of RNA or DNA in samples over time reduces detection of the assays employed and may affect prevalence. 17 Certain anatomical sites had low enrollment numbers in the prospective cohort; thus, their high percentage of p16‐ and ISH‐positivity may be due to chance. The prospective portion of this study may also be subject to selection bias, as the 355 patients enrolled is a fraction of the incident HNSCC seen at each site over 6 years. Furthermore, a majority (69%) of the prospectively enrolled patients came from a single site (JHH), also limiting the study's generalizability.
The strengths of this study include the focus on women and minorities, a long‐time span, and a study of the years after 2012, which have not yet been well characterized. In addition, this was a multicenter study with centralized gold standard tumor testing and interpretation by expert head and neck pathologists.
In conclusion, p16‐ and ISH‐positive HNSCC are increasing over time among all sex and race groups. Consequently, HPV is likely now responsible for the majority of oropharyngeal cancers in the United States independent of sex or race. Additionally, p16‐positive and ISH‐positive non‐OPSCCs are rarer than in the oropharynx, yet for the first time they are shown to be increasing in specific groups.
Funding Support
Nicholas Scott‐Wittenborn was supported by the National Institue of Deafness and Other Communication Disorders, grant #5T32DC000027‐2; William H. Westra was supported by the National Institute of Dental and Craniofacial Research, grant #P50DE019032; and Gypsyamber D'Souza was supported by the National Institute of Dental and Craniofacial Research, grant #R35DE026631.
Conflict of Interest Disclosures
William R. Ryan is advisor for Olympus and Medtronic and a consultant for Rakuten Medical. Patrick K. Ha is on the advisory boards of Rakuten Medical and Loxo Oncology and received educational funding from Stryker, Medtronic, Axogen, and Johnson & Johnson.
Author Contributions
Nicholas Scott‐Wittenborn: Investigation, revision, analysis, and drafting. Gypsyamber D'Souza: Conceptualization, administration, supervision, investigation, drafting, and revision. Sakshi Tewari: Investigation, revision, and analysis. Lisa Rooper: Investigation and revision. Tanya Troy: Administration, investigation, and revision. Virginia Drake: Investigation and revision. Elaine O. Bigelow: Investigation and revision. Melina J. Windon: Investigation and revision. William R. Ryan: Investigation and revision; Patrick K. Ha: Investigation and revision. Ana P. Kiess: Investigation and revision; Brett Miles: Investigation and revision; William H. Westra: Investigation and revision. Wojciech K. Mydlarz: Investigation and revision. David W. Eisele: Revision. Carole Fakhry: Conceptualization, supervision, investigation, drafting, and revision.
Supporting information
Supplementary Material
Scott‐Wittenborn N, D'Souza G, Tewari S, Rooper L, Troy T, Drake V, Bigelow EO, Windon MJ, Ryan WR, Ha PK, Kiess AP, Miles B, Westra WH, Mydlarz WK, Eisele DW, Fakhry C. Prevalence of human papillomavirus in head and neck cancers at tertiary care centers in the United States over time. Cancer. 2022. 10.1002/cncr.34124
References
- 1. Chaturvedi AK, Anderson WF, Lortet‐Tieulent J, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol. 2013;31:4550‐4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ryerson AB, Peters ES, Coughlin SS, et al. Burden of potentially human papillomavirus‐associated cancers of the oropharynx and oral cavity in the US, 1998‐2003. Cancer. 2008;113(suppl):2901‐2909. [DOI] [PubMed] [Google Scholar]
- 3. Fakhry C, Krapcho M, Eisele DW, D'Souza G. Head and neck squamous cell cancers in the United States are rare and the risk now is higher among White individuals compared with Black individuals. Cancer. 2018;124:2125‐2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Patel MA, Blackford AL, Rettig EM, Richmon JD, Eisele DW, Fakhry C. Rising population of survivors of oral squamous cell cancer in the United States. Cancer. 2016;122:1380‐1387. [DOI] [PubMed] [Google Scholar]
- 5. Rettig EM, D'Souza G. Epidemiology of head and neck cancer. Surg Oncol Clin N Am. 2015;24:379‐396. [DOI] [PubMed] [Google Scholar]
- 6. Gillison ML, Chaturvedi AK, Anderson WF, Fakhry C. Epidemiology of human papillomavirus‐positive head and neck squamous cell carcinoma. J Clin Oncol. 2015;33:3235‐3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. D'Souza G, Westra WH, Wang SJ, et al. Differences in the prevalence of human papillomavirus (HPV) in head and neck squamous cell cancers by sex, race, anatomic tumor site, and HPV detection method. JAMA Oncol. 2017;3:169‐177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weatherspoon DJ, Chattopadhyay A, Boroumand S, Garcia I. Oral cavity and oropharyngeal cancer incidence trends and disparities in the United States: 2000‐2010. Cancer Epidemiol. 2015;39:497‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. D'Souza G, Cullen K, Bowie J, Thorpe R, Fakhry C. Differences in oral sexual behaviors by gender, age, and race explain observed differences in prevalence of oral human papillomavirus infection. PLoS One. 2014;9:e86023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lingen MW, Xiao W, Schmitt A, et al. Low etiologic fraction for high‐risk human papillomavirus in oral cavity squamous cell carcinomas. Oral Oncol. 2013;49:1‐8. [DOI] [PubMed] [Google Scholar]
- 11. Krüger M, Pabst AM, Walter C, et al. The prevalence of human papilloma virus (HPV) infections in oral squamous cell carcinomas: a retrospective analysis of 88 patients and literature overview. J Maxillofac Surg. 2014;42:1506‐1514. [DOI] [PubMed] [Google Scholar]
- 12. Mehanna H, Beech T, Nicholson T, et al. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer—systematic review and meta‐analysis of trends by time and region. Head Neck. 2013;35:747‐755. [DOI] [PubMed] [Google Scholar]
- 13. Windon MJ, D'Souza G, Waterboer T, et al. Risk factors for human papillomavirus‐positive nonoropharyngeal squamous cell carcinoma. Head Neck. 2020;42:1954‐1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rooper LM, Windon MJ, Hernandez T, et al. HPV‐positive squamous cell carcinoma of the larynx, oral cavity, and hypopharynx: clinicopathologic characterization with recognition of a novel warty variant. Am J Surg Pathol. 2020;44:691‐702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lewis JS, Chernock RD, Ma X‐J, et al. Partial p16 staining in oropharyngeal squamous cell carcinoma: extent and pattern correlate with human papillomavirus RNA status. Mod Pathol. 2012;25:1212‐1220. [DOI] [PubMed] [Google Scholar]
- 16. Begum S, Gillison ML, Ansari‐Lari MA, Shah K, Westra WH. Detection of human papillomavirus in cervical lymph nodes. A highly effective strategy for localizing site of tumor origin. Clin Cancer Res. 2003;9:6469‐6475. [PubMed] [Google Scholar]
- 17. Bishop JA, Ma XJ, Wang H, et al. Detection of transcriptionally active high‐risk HPV in patients with head and neck squamous cell carcinoma as visualized by a novel E6/E7 mRNA in situ hybridization method. Am J Surg Pathol. 2012;36:1874‐1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Edge SB, Byrd DR, Carducci MA, Compton CC, Fritz A, Greene F. AJCC Cancer Staging Manual. Vol 649. Springer; 2010. [Google Scholar]
- 19. Settle K, Posner MR, Schumaker LM, et al. Racial survival disparity in head and neck cancer results from low prevalence of human papillomavirus infection in Black oropharyngeal cancer patients. Cancer Prev Res (Phila). 2009;2:776‐781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chernock RD, Zhang Q, El‐Mofty SK, Thorstad WL, Lewis JS Jr. Human papillomavirus‐related squamous cell carcinoma of the oropharynx: a comparative study in Whites and African Americans. Arch Otolaryngol Head Neck Surg. 2011;137:163‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zandberg DP, Liu S, Goloubeva OG, Schumaker LM, Cullen KJ. Emergence of HPV16‐positive oropharyngeal cancer in Black patients over time: University of Maryland 1992‐2007. Cancer Prev Res (Phila). 2015;8:12‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Creamer MR, Wang TW, Babb S, et al. Tobacco product use and cessation indicators among adults—United States, 2018. MMWR Surveill Summ. 2019;68:1013‐1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mosher WD, Chandra A, Jones J. Sexual behavior and selected health measures: men and women 15‐44 years of age, United States, 2002. Adv Data. 2005;1‐55. [PubMed] [Google Scholar]
- 24. Brawley OW. Oropharyngeal cancer, race, and the human papillomavirus. Cancer Prev Res (Phila). 2009;2:769‐772. [DOI] [PubMed] [Google Scholar]
- 25. Billy JO, Tanfer K, Grady WR, Klepinger DH. The sexual behavior of men in the United States. Fam Plann Perspect. 1993;25:52‐60. [PubMed] [Google Scholar]
- 26. Prinstein MJ, Meade CS, Cohen GL. Adolescent oral sex, peer popularity, and perceptions of best friends' sexual behavior. J Pediatr Psychol. 2003;28:243‐249. [DOI] [PubMed] [Google Scholar]
- 27. Laumann EO. The Social Organization of Sexuality: Sexual Practices in the United States. University of Chicago Press; 2000. [Google Scholar]
- 28. Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294‐4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stein AP, Saha S, Kraninger JL, et al. Prevalence of human papillomavirus in oropharyngeal cancer: a systematic review. Cancer J. 2015;21:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Haeggblom L, Ramqvist T, Tommasino M, Dalianis T, Näsman A. Time to change perspectives on HPV in oropharyngeal cancer. A systematic review of HPV prevalence per oropharyngeal sub‐site the last 3 years. Papillomavirus Res. 2017;4:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Drake VE, Fakhry C, Windon MJ, et al. Timing, number, and type of sexual partners associated with risk of oropharyngeal cancer. Cancer. 2021;127:1029‐1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Quadagno D, Sly DF, Harrison DF, Eberstein IW, Soler HR. Ethnic differences in sexual decisions and sexual behavior. Arch Sex Behav. 1998;27:57‐75. [DOI] [PubMed] [Google Scholar]
- 33. Wells BE, Twenge JM. Changes in young people's sexual behavior and attitudes, 1943‐1999: a cross‐temporal meta‐analysis. Rev Gen Psychol. 2005;9:249‐261. [Google Scholar]
- 34. Auslander BA, Biro FM, Succop PA, Short MB, Rosenthal SL. Racial/ethnic differences in patterns of sexual behavior and STI risk among sexually experienced adolescent girls. J Pediatr Adolesc Gynecol. 2009;22:33‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. D'Souza G, Agrawal Y, Halpern J, Bodison S, Gillison ML. Oral sexual behaviors associated with prevalent oral human papillomavirus infection. J Infect Dis. 2009;199:1263‐1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Habel MA, Leichliter JS, Dittus PJ, Spicknall IH, Aral SO. Heterosexual anal and oral sex in adolescents and adults in the United States, 2011‐2015. Sex Transm Dis. 2018;45:775‐782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lin L, Benard VB, Greek A, Hawkins NA, Roland KB, Saraiya M. Racial and ethnic differences in human papillomavirus positivity and risk factors among low‐income women in federally qualified health centers in the United States. Prev Med. 2015;81:258‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Montealegre JR, Varier I, Bracamontes CG, et al. Racial/ethnic variation in the prevalence of vaccine‐related human papillomavirus genotypes. Ethn Health. 2019;24:804‐815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang Y, Fakhry C, D'Souza G. Projected association of human papillomavirus vaccination with oropharynx cancer incidence in the US, 2020‐2045. JAMA Oncol. 2021;7:e212907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schmitt NC. HPV in non‐oropharyngeal head and neck cancer: does it matter? Ann Transl Med. 2020;8:1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Palve V, Bagwan J, Krishnan NM, et al. Detection of high‐risk human papillomavirus in oral cavity squamous cell carcinoma using multiple analytes and their role in patient survival. J Glob Oncol. 2018;4:1‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hernandez BY, Rahman M, Lynch CF, et al. p16(INK4A) expression in invasive laryngeal cancer. Papillomavirus Res. 2016;2:52‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fakhry C, Lacchetti C, Perez‐Ordonez B. Human papillomavirus testing in head and neck carcinomas: ASCO clinical practice guideline endorsement summary of the CAP guideline. J Oncol Pract. 2018;14:613‐617. [DOI] [PubMed] [Google Scholar]
- 44. Lewis JS Jr, Beadle B, Bishop JA, et al. Human papillomavirus testing in head and neck carcinomas: guideline from the College of American Pathologists. Arch Pathol Lab Med. 2018;142:559‐597. [DOI] [PubMed] [Google Scholar]
- 45. Bishop JA, Westra WH. Human papillomavirus‐related multiphenotypic sinonasal carcinoma: an emerging tumor type with a unique microscopic appearance and a paradoxical clinical behaviour. Oral Oncol. 2018;87:17‐20. [DOI] [PubMed] [Google Scholar]
- 46. Bishop JA, Andreasen S, Hang JF, et al. HPV‐related multiphenotypic sinonasal carcinoma: an expanded series of 49 cases of the tumor formerly known as HPV‐related carcinoma with adenoid cystic carcinoma‐like features. Am J Surg Pathol. 2017;41:1690‐1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Meshman J, Wang PC, Chin R, et al. Prognostic significance of p16 in squamous cell carcinoma of the larynx and hypopharynx. Am J Otolaryngol. 2017;38:31‐37. [DOI] [PubMed] [Google Scholar]
- 48. Fakhry C, Westra WH, Wang SJ, et al. The prognostic role of sex, race, and human papillomavirus in oropharyngeal and nonoropharyngeal head and neck squamous cell cancer. Cancer. 2017;123:1566‐1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. D'Souza G, Anantharaman D, Gheit T, et al. Effect of HPV on head and neck cancer patient survival, by region and tumor site: a comparison of 1362 cases across three continents. Oral Oncol. 2016;62:20‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


