Abstract
BACKGROUND:
Boys with acute lymphoblastic leukemia (ALL) have historically experienced inferior survival compared to girls. We determined whether sex-based disparities persist with contemporary therapy and whether patterns of treatment failure vary by sex.
METHODS:
We included patients age 1–30.99 years enrolled on frontline COG trials between 2004–2014. Boys received an additional year of Maintenance therapy. We explored sex-based differences in the distribution of various prognosticators, event-free and overall survival (EFS, OS), and subcategories of relapse by site.
RESULTS:
We included 8,202 (54.4% male) B-ALL and 1,562 (74.3% male) T-ALL patients. There was no sex-based difference in central nervous system (CNS) status. Boys experienced inferior 5-year EFS and OS (EFS 84.6% ± 0.5% vs. 86.0% ± 0.6%; p=0.009; OS 91.3% ± 0.4% vs. 92.5% ± 0.4%; p=0.02). This was attributable to boys with B-ALL, who experienced inferior EFS [hazard ratio (HR) 1.2, 95% confidence interval (95CI) (1.1–1.3); p=0.004] and OS (HR 1.2, 95CI 1.0–1.4; p=0.046), after adjustment for prognosticators. Inferior B-ALL outcomes in boys were attributable to more relapses (5-year cumulative incidence 11.2%±0.5% vs. 9.6%±0.5%; p=0.001), particularly involving the CNS (4.2%±0.3% vs. 2.5%±0.3%; p<0.0001). There was no difference in isolated bone marrow relapses (5.4%±0.4% vs. 6.2%±0.4%; p=0.49). There were no sex-based differences in EFS or OS in T-ALL.
CONCLUSIONS:
Sex-based disparities in ALL persist, attributable to increased CNS relapses in boys with B-ALL. Studies of potential mechanisms are warranted. Improved strategies to identify and modify treatment for patients at highest risk of CNS relapse may have particular benefit for boys.
Keywords: Acute lymphoblastic leukemia, Childhood, Disparities, Sex, Survival
Precis:
Boys with childhood acute lymphoblastic leukemia (ALL) have historically experienced inferior outcomes compared to girls; whether these sex-based disparities persist in the context of modern therapy is unknown.
Even after adjusting for other disease prognosticators, boys continue to have worse outcomes, attributable mainly to increased rates of relapses involving the CNS among boys with B-ALL.
INTRODUCTION
Cure rates for children and adolescents with acute lymphoblastic leukemia (ALL) have dramatically increased over the last decades, with further improvements in outcomes seen even in the most recent time periods.1,2 Sex-based differences in ALL outcomes have been observed since the advent of curative therapy, with inferior survival among boys noted even in the 1960s and 1970s.2–8 Past studies have attributed this increased risk at least partially to a greater prevalence of adverse disease prognosticators in boys.
Modern ALL therapy now utilizes complex risk stratification systems which incorporate factors such as cytogenetics and sophisticated measures of early response.1,9 Therapy is intensified for patients known to be at higher risk of relapse. Whether sex-based disparities persist in the context of modern therapy is unknown. Our primary objective was therefore to determine whether ALL outcomes differed between boys and girls after adjustment for various prognosticators. Our secondary objective was to identify any sex-based difference in patterns of treatment failure, including types of relapse and death in remission.
METHODS
Study Patients
Between 2004 and 2014, children, adolescents, and young adults aged 1–30 years with newly diagnosed ALL were enrolled onto one of several Children’s Oncology Group (COG) clinical trials. After enrollment on a common classification protocol, patients enrolled on AALL0331 [NCI standard risk (SR) B-lymphoblastic leukemia (B-ALL), age >1 year and <10 years and initial white blood cell count (WBC) <50,000/μL; 2005–2010], AALL0232 [NCI high risk (HR) B-ALL, age 10–30 years or initial WBC ≥50,000/μL and any age; 2004–2011], or AALL0434 [T lymphoblastic leukemia (T-ALL), age 1–30 years; 2007–2014]. With the exception of those with Down Syndrome and infants diagnosed <1 year of age, all other patients enrolled onto one of these three clinical trials were included in this study. Two pilot studies of novel asparaginase agents or dosing were not included (AALL07P4, AALL08P1). All studies were approved by the NCI, the pediatric central institutional review board, and/or by the institutional review board of each participating center. Participating patients and/or a parent or guardian provided informed consent. Details of each clinical trial, including chemotherapy regimens, randomized treatment interventions, and outcomes, have been previously published (AALL0331 – NCT00103285; AALL0232 – NCT00075725; AALL0434 – NCT00408005).10–13
Induction therapy with either three (AALL0331) or four (AALL0232, AALL0434) drugs was followed by post-induction therapy, the intensity of which was dependent on risk stratification. The final phase of therapy, termed Maintenance, comprised a prolonged period of antimetabolite-based treatment with pulses of one dose of vincristine and 5-days of corticosteroid administered every 4 weeks. The duration of Maintenance therapy was sex-dependent, with boys receiving an additional year. Indications for cranial radiation differed by study. All patients with central nervous system (CNS) 3 status at diagnosis [≥5 WBCs/μL plus blasts with/without ≥10 red blood cells (RBCs)/μL or clinical signs of CNS disease] received 18 Gy of radiation. Among patients with NCI HR B-ALL, those with slow early response, KMT2A (MLL) rearrangements, and some patients pre-treated with steroids received prophylactic cranial radiation (12 Gy). Among patients with T-ALL, those with any of the following features received 12 Gy prophylactic cranial radiation: NCI HR disease, CNS2 status (<5 WBCs/μL and blasts with/without ≥10 RBCs/μL or ≥5 WBCs/μL plus blasts, with WBCs ≥5 times the number of RBCs), slow early response (≥5% blasts by morphology on the day 15 bone marrow), or end of induction minimal residual disease (MRD) ≥0.1%.
Outcomes
Our primary outcomes were event-free and overall survival (EFS, OS). EFS was defined as time from study enrollment to first event (induction death, failure to attain complete remission, relapse, remission death, or the development of a second malignant neoplasm) or last follow-up. Overall survival (OS) was defined as the time from study enrollment to death from any cause or date of last follow-up if alive. Secondary outcomes included relapse, both overall and by site of relapse, induction death, death in remission (defined as any death while in complete remission), and second malignant neoplasms. Bone marrow involvement at relapse was determined through local morphology.
Covariates
Our key predictor of interest was biological sex. Additional covariates included age at diagnosis, presenting WBC, lineage (B-ALL vs. T-ALL), CNS status [CNS1 (no blasts) vs. CNS2 vs. CNS3], and end of induction MRD. Cytogenetics in B-ALL were categorized as favorable (ETV6-RUNX1 fusion or simultaneous trisomies of chromosomes 4, 10 and 17) vs. unfavorable (hypodiploidy with modal chromosome number <44 and/or DNA index <0.81, intrachromosomal amplification of chromosome 21 (iAMP21), KMT2A rearrangements, or BCR-ABL1 fusion)14–16 vs. neutral (all others).
Analyses
Differences in the distribution of covariates between boys vs. girls were examined using chi squared tests or t-tests as appropriate. Survival rates were estimated using the Kaplan-Meier method with standard errors of Peto et al.17,18 Survival curves were compared using the log-rank test. Cox proportional-hazards models were used for multivariable analyses of EFS and OS. The cumulative incidence function approach was used to determine the risk of relapse (with death considered a competing event) overall and by sex, which was compared using Gray’s test.19 Median time to relapse was compared by sex using t-tests. Statistical significance was defined as p<0.05. All analyses were performed using SAS® software version 9.4; SAS Institute, Cary, NC). All graphics were generated using R (http://www.R-project.org, version 2.13.1).
RESULTS
The study cohort comprised of 9,764 children, adolescents, and young adults with ALL, 5,624 (57.6%) of whom were male. Characteristics of the cohort are shown in Table 1. Boys were more likely to have T-ALL than girls (20.7% vs. 9.7%; p<0.0001). Among both patients with B-ALL and T-ALL, boys were older than girls at presentation (Table 1). Among patients with B-ALL, the median WBC did not differ by sex [boys: 9.5×109/L, [interquartile range (IQR) 4.1–29] vs. 8.9, IQR 3.9–29; p=0.16]. Among T-ALL patients, boys had a higher median WBC (79 ×109/L, IQR 21–230 vs. 59, IQR 16–203; p=0.04). CNS status did not differ by sex. For example, among patients with B-ALL, 10.3% of boys vs. 10.1% of girls presented with CNS2 disease, while 1.6% vs. 1.4% presented with CNS3 disease (p=0.72). Boys with B-ALL were more likely to have unfavorable cytogenetics than girls. This was due to small but statistically significant increases in the prevalence of specific cytogenetic lesions, including hypodiploidy and BCR-ABL1 (Supplemental Table 1). Among B-ALL patients, boys were more likely to have end induction MRD ≥0.01% than were girls (23.9% vs. 21.9%; p=0.04). Among T-ALL patients, there was no difference in rates of end of induction MRD ≥0.01% by sex. There was no difference in the proportion of children receiving cranial radiation among boys vs. girls with B-ALL (7.6% vs. 6.8%; p=0.15). Boys with T-ALL were however more likely to receive cranial radiation (68.1% vs. 61.3%; p=0.01), compared to girls.
Table 1.
Characteristics of study cohort, stratified by sex and lineage
| B-lineage ALL | T-lineage ALL | |||||
|---|---|---|---|---|---|---|
| Boys (N=4,463) (N, %) | Girls (N=3,739) (N, %) | P value | Boys (N=1,161) (N, %) | Girls (N=401) (N, %) | P value | |
| Age at diagnosis (years) | <0.0001 | 0.04 | ||||
| <10 | 3311 (74.2) | 2855 (76.4) | 602 (51.8) | 237 (59.1) | ||
| 10–16 | 767 (17.2) | 671 (17.9) | 385 (33.2) | 112 (27.9) | ||
| ≥16 | 385 (8.6) | 213 (5.7) | 174 (15.0) | 52 (13.0) | ||
| WBC at presentation (×109/L) | 0.98 | 0.06 | ||||
| <50 | 3739 (83.8) | 3133 (83.8) | 473 (40.7) | 185 (46.1) | ||
| ≥50 | 722 (16.2) | 604 (16.2) | 688 (59.3) | 216 (53.9) | ||
| CNS status | 0.72 | 0.45 | ||||
| CNS1 | 3924 (88.1) | 3306 (88.5) | 839 (72.5) | 295 (74.0) | ||
| CNS2 | 461 (10.3) | 379 (10.1) | 236 (20.4) | 71 (17.8) | ||
| CNS3 | 71 (1.6) | 52 (1.4) | 83 (7.2) | 33 (8.3) | ||
| Cytogenetics | 0.05 | |||||
| Favorable | 1,842 (41.3) | 1,553 (41.5) | - | - | - | |
| Neutral | 2,320 (52.0) | 1,982 (53.0) | - | - | - | |
| Unfavorable | 301 (6.7) | 204 (5.5) | - | - | - | |
| End of induction BM MRD | 0.04 | 0.46 | ||||
| <0.01% | 3268 (76.1) | 2791 (78.1) | 659 (58.9) | 221 (56.8) | ||
| ≥0.01% | 1026 (23.9) | 784 (21.9) | 459 (41.1) | 168 (43.2) | ||
| Cranial radiation | 0.15 | 0.01 | ||||
| Yes | 339 (7.6) | 253 (6.8) | 791 (68.1) | 246 (61.3) | ||
| No | 4124 (92.4) | 3486 (93.2) | 370 (31.9) | 155 (38.7) | ||
BM – bone marrow; CNS – central nervous system; MRD – minimal residual disease; N – number; WBC – white blood cells
Study cohort outcomes are seen in Table 2. Among the entire cohort, including patients with both B- and T-ALL, boys experienced inferior 5-year EFS and OS as compared to girls (EFS 84.6% ± 0.5% vs. 86.0% ± 0.6%; p=0.009; OS 91.3% ± 0.4% vs. 92.5% ± 0.4%; p=0.02). This was predominantly attributable to patients with B-ALL (EFS 84.6% ± 0.6% in boys vs. 86.4% ± 0.6% in girls; p=0.003; OS 91.7% ± 0.4% vs. 92.8% ± 0.5%; p=0.047) (Figure 1). This disparity remained when adjusting for other covariates (Table 3). Boys experienced an approximately 20% increased hazard of an event [adjusted hazard ratio (aHR) 1.2, 95% confidence interval (95CI) 1.1–1.3; p=0.004] and of death (aHR 1.2, 95CI 1.0–1.4; p=0.046). No such sex-based disparity was seen among T-ALL patients (Tables 2 and 4, Figure 1). The increased risk seen in B-ALL boys was due to an increased risk of relapse [5-year cumulative incidence (CI) 11.2% ± 0.5% vs. 9.6% ± 0.5%; p=0.001]. This in turn was mainly attributable to an increase in relapses involving the CNS (5-year CI 4.2% ± 0.3% vs. 2.5% ± 0.3%, p<0.0001). Boys were at increased risk of both isolated CNS relapses and combined CNS and bone marrow relapses (Table 2). By contrast, there was no difference in the cumulative incidence of isolated BM relapses (5-year CI 5.4% ± 0.4% vs. 6.2% ± 0.4%; p=0.49). The median time to CNS relapse was later in boys vs. girls [2.5 years, IQR 1.7–3.7 years vs. 2.1 years, IQR 1.6–2.8 years; p=0.049). Characteristics of patients with B-ALL who suffered relapses involving the CNS are shown in Supplemental Table 2. Again, no sex-based differences were seen in T-ALL patients, either in overall relapse or in relapse by site.
Table 2.
Study cohort 5-year outcomes by sex, stratified by leukemia lineage (B vs. T)
| B-lineage ALL | T-lineage ALL | |||||
|---|---|---|---|---|---|---|
| Boys | Girls | P value | Boys | Girls | P value | |
| Event-free survival | 84.6% ± 0.6% | 86.4% ± 0.6% | 0.003 | 84.2% ± 1.1% | 82.7% ± 2.0% | 0.24 |
| Overall survival | 91.7% ± 0.4% | 92.8% ± 0.5% | 0.047 | 89.5% ± 1.0% | 89.7% ± 1.6% | 0.95 |
| Relapse | 11.2% ± 0.5% | 9.6% ± 0.5% | 0.001 | 9.2% ± 0.9% | 9.5% ± 1.5% | 0.83 |
| Isolated BM relapse | 5.4% ± 0.4% | 6.2% ± 0.4% | 0.49 | 3.4% ± 0.5% | 3.1% ± 0.9% | 0.74 |
| Relapse involving the CNS | 4.2% ± 0.3% | 2.5% ± 0.3% | <0.0001 | 4.1% ± 0.6% | 3.8% ± 1.0% | 0.64 |
| Isolated CNS relapse | 3.0% ± 0.3% | 1.8% ± 0.2% | 0.0001 | 2.7% ± 0.5% | 2.6% ± 0.8% | 0.85 |
| Combined CNS and BM relapse | 1.2% ± 0.2% | 0.8% ± 0.2% | 0.032 | 1.4% ± 0.4% | 1.3% ± 0.6% | 0.59 |
| Testicular relapse | 0.7% ± 0.1% | - | - | 0.0% | - | - |
| Death in remission | 0.7% ± 0.1% | 0.7% ± 0.1% | 0.995 | 0.9% ± 0.3% | 0.8% ± 0.4% | 0.84 |
| Second malignant neoplasm | 0.5% ± 0.1% | 0.6% ± 0.1% | 0.75 | 1.0% ± 0.3% | 0.5% ± 0.4% | 0.56 |
BM – bone marrow; CNS – central nervous system
Bolded values represent p<0.05
Figure 1.
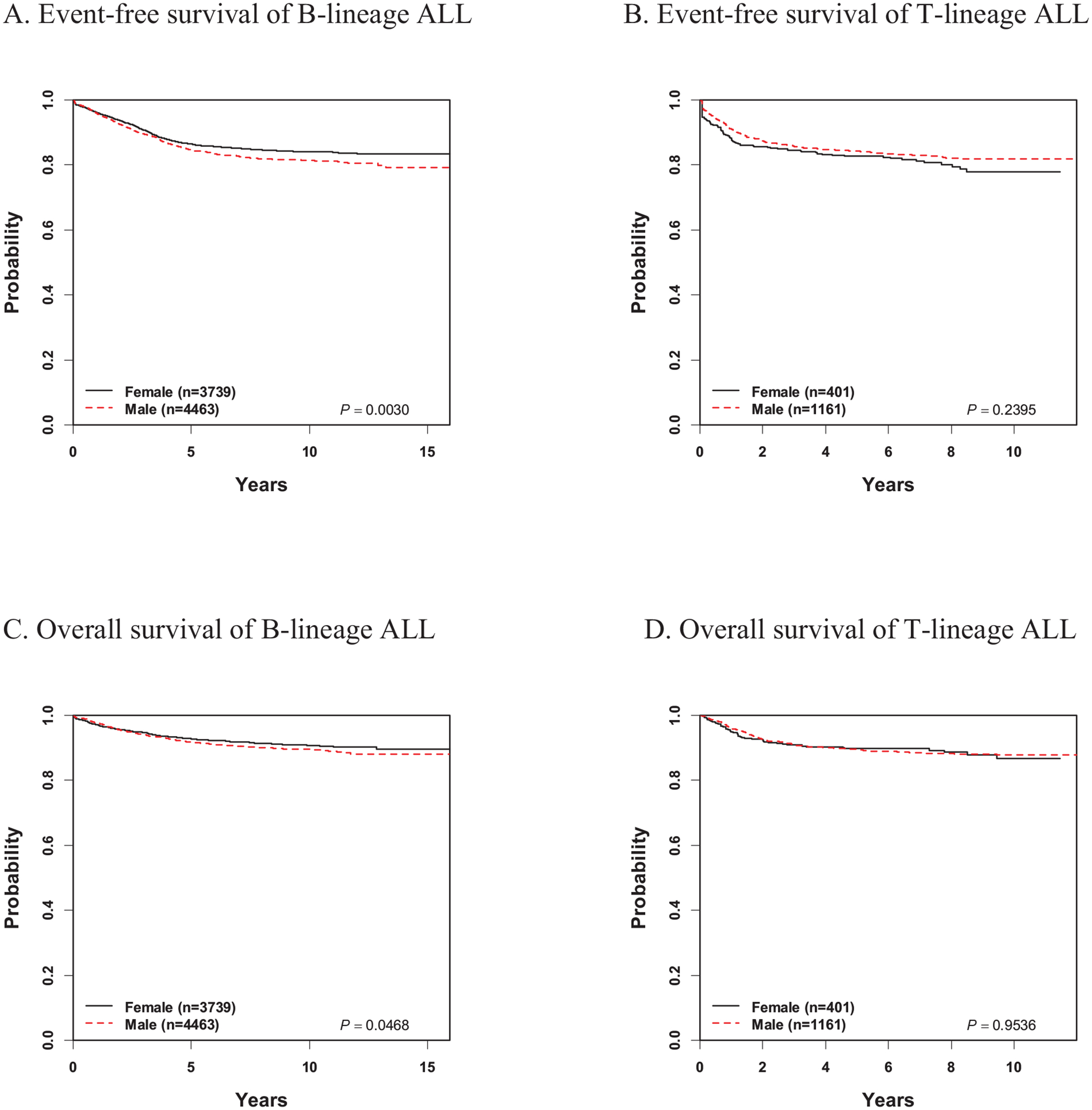
Event-free and overall survival of the study cohort by sex
Table 3.
Univariate and multivariable predictors of event-free and overall survival among patients with B-ALL
| Event-free Survival | Overall Survival | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariable | Univariate | Multivariable | |||||
| HR (95CI) | P value | HR (95CI) | P value | HR (95CI) | P value | HR (95CI) | P value | |
| Age at diagnosis (years) | ||||||||
| <10 | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| ≥10 | 2.7 (2.4–2.9) | <0.0001 | 1.7 (1.5–1.9) | <0.0001 | 4.1 (3.6–4.8) | <0.0001 | 2.5 (2.2–3.0) | <0.0001 |
| Sex | ||||||||
| Female | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Male | 1.2 (1.1–1.3) | 0.003 | 1.2 (1.1–1.3) | 0.004 | 1.2 (1.0–1.3) | 0.047 | 1.2 (1.0–1.4) | 0.046 |
| WBC at presentation (×109/L) | ||||||||
| <50 | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| ≥50 | 2.1 (1.9–2.4) | <0.0001 | 1.6 (1.4–1.8) | <0.0001 | 2.3 (2.0–2.7) | <0.0001 | 1.5 (1.3–1.8) | <0.0001 |
| CNS status | ||||||||
| CNS1 | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| CNS2 | 1.8 (1.5–2.0) | <0.001 | 1.4 (1.2–1.6) | <0.0001 | 1.9 (1.6–2.3) | <0.0001 | 1.4 (1.1–1.7) | 0.002 |
| CNS3 | 1.7 (1.1–2.5) | 0.008 | 0.9 (0.6–1.5) | 0.82 | 2.2 (1.4–3.4) | 0.0006 | 1.0 (0.6–1.8) | 0.91 |
| Cytogenetics | ||||||||
| Favorable | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Neutral | 2.8 (2.5–3.3) | <0.0001 | 2.1 (1.8–2.5) | <0.0001 | 4.1 (3.3–5.1) | <0.0001 | 2.9 (2.3–3.8) | <0.0001 |
| Unfavorable | 6.9 (5.7–8.2) | <0.0001 | 3.6 (2.9–4.5) | <0.0001 | 11.8 (9.3–15.2) | <0.0001 | 5.8 (4.3–7.9) | <0.0001 |
| End of induction BM MRD | ||||||||
| <0.01% | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| ≥0.01% | 3.7 (3.3–4.1) | <0.0001 | 2.9 (2.5–3.2) | <0.0001 | 4.3 (3.7–5.0) | <0.0001 | 2.9 (2.5–3.4) | <0.0001 |
95CI – 95% confidence interval; BM – bone marrow; CNS – central nervous system; HR – hazard ratio; MRD – minimal residual disease; WBC – white blood cells
Table 4.
Univariate and multivariable predictors of event-free and overall survival among patients with T-ALL
| Event-free Survival | Overall Survival | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariable | Univariate | Multivariable | |||||
| HR (95CI) | P value | HR (95CI) | P value | HR (95CI) | P value | HR (95CI) | P value | |
| Age at diagnosis (years) | ||||||||
| <10 | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| ≥10 | 1.0 (0.8–1.3) | 0.85 | 1.0 (0.8–1.3) | 0.86 | 1.4 (1.1–1.9) | 0.01 | 1.4 (1.0–1.9) | 0.04 |
| Sex | ||||||||
| Female | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Male | 1.2 (0.9–1.5) | 0.24 | 0.9 (0.7–1.1) | 0.29 | 1.0 (1.4–0.7) | 0.95 | 1.1 (0.7–1.5) | 0.73 |
| WBC at presentation (×109/L) | ||||||||
| <50 | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| ≥50 | 1.5 (1.1–1.9) | 0.003 | 1.5 (1.2–2.0) | 0.003 | 1.7 (1.2–2.3) | 0.001 | 1.9 (1.3–2.7) | 0.0004 |
| CNS status | ||||||||
| CNS1 | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| CNS2 | 1.2 (0.9–1.6) | 0.35 | 1.1 (0.8–1.5) | 0.71 | 1.1 (0.75–1.6) | 0.63 | 0.9 (0.6–1.4) | 0.72 |
| CNS3 | 2.0 (1.4–2.9) | 0.0002 | 1.9 (1.3–2.9) | 0.0008 | 1.7 (1.1–2.8) | 0.02 | 1.5 (0.9–2.5) | 0.14 |
| End of induction BM MRD | ||||||||
| <0.01% | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| ≥0.01% | 2.4 (1.9–3.1) | <0.0001 | 2.5 (2.0–3.2) | <0.0001 | 2.3 (1.7–3.1) | <0.0001 | 2.4 (1.7–3.3) | <0.0001 |
95CI – 95% confidence interval; BM – bone marrow; CNS – central nervous system; HR – hazard ratio; MRD – minimal residual disease; WBC – white blood cells
There was no difference in the rate of induction death either among patients with B-ALL [boys 33/4,463 (0.7%) vs. girls 40/3,739 (1.1%); p=0.11] or with T-ALL [boys 5/1,161 (0.4%) vs. girls 2/401 (0.5%); p=1.00]. There was no difference in the cumulative incidence of either death in remission or second malignant neoplasms among children with either B-ALL or T-ALL (Table 2).
DISCUSSION
Among this group of almost 10,000 children, adolescents, and young adults with ALL diagnosed between 2004 and 2014, boys continued to have inferior EFS and OS as compared to girls. The sex-based outcome disparities were restricted to those with B-ALL and were not seen among patients with T-ALL. B-ALL outcome disparities were attributable to higher rates among boys of relapses involving the CNS. Neither isolated bone marrow relapses, induction deaths, deaths in remission, nor second malignant neoplasms varied by sex.
Inferior outcomes among boys have been noted for decades, and have been at least partially attributable to imbalances in various prognosticators.2–8 Early on, Baumer et al. described differences in age at diagnosis, with boys presenting at older ages, while multiple groups noted the higher incidence of T-ALL among boys vs. girls.3,5,7,8 Whether these imbalances fully account for this disparity has been less clear. Using a cohort of 2,055 children treated on St. Jude Children’s Research Hospital (SJCRH) ALL trials between 1962–1994, Pui et al. found that stratification by variables such as immunophenotype and cytogenetics abrogated sex-based disparities.5 Interestingly, among this cohort, disparities were also restricted to children with B-ALL and not T-ALL, mirroring our findings. In contrast, using population-based registry-derived cohorts, Holmes et al. found that adjustment for immunophenotype and age did not impact upon mortality disparities, with boys continuing to experience a 15% higher risk of death.8 In our earlier report of over 20,000 children, adolescents and young adults treated on COG trials between 1990 and 2005, sex was a significant prognostic factor in multivariable analysis with girls having a 15–20% lower risk of death than boys.2 Among more contemporary protocols, sex-based disparities have not been seen, though these cohorts were generally of smaller sample size than that of the current study, and thus may not have been adequately powered to detect smaller differences.20–22 In our current cohort of almost 10,000 patients, male sex retained independent adverse prognostic impact even after adjustment for modern risk classification variables which showed small but significant differences according to sex (e.g. adverse cytogenetics and end induction MRD in B-ALL). Indeed, male sex was associated with similar magnitudes of increased risk both in univariate and multivariable analyses.
Few contemporaneous studies have examined these disparities in further detail by, for example, studying specific causes of treatment failure. Older cohorts treated on Children’s Cancer Study Group (CCG) or SJCRH trials noted an increased risk of bone marrow relapses among boys but no excess risk of CNS relapses, in direct contrast to our findings.4,5 Strategies to prevent CNS relapse in childhood ALL have evolved dramatically, moving from the addition of prophylactic radiation to replacing radiation with increased use of chemotherapeutic agents which reach the CNS, including intensive, prolonged intrathecal chemotherapy. The impact of different CNS strategies may well differ by sex. The introduction of prophylactic radiation on CCG trials in the 1970s was shown to have more benefit among boys vs. girls.4 Similarly, the elimination of cranial irradiation among standard risk patients on the Dana-Farber Cancer Institute (DFCI) 87-01 trial resulted in a substantial increase in CNS relapses among boys but not among girls.23 Historically, prophylactic cranial radiation has been administered more frequently to patients with T-ALL vs. B-ALL;24 this may partially explain the lack of sex-based disparities among children with T-ALL seen in our cohort as approximately 90% of the AALL0434 patients received cranial irradiation.13 As rates of cranial radiation decrease further in T-ALL, continued monitoring of sex-based disparities is warranted, though the addition of newer additional CNS-directed therapies such as nelarabine may mitigate this impact.13 Analyses of this subpopulation of patients treated on protocols which have entirely eliminated radiation would also be of use.22
Though mechanisms underlying sex-based differences in CNS relapse rates are unclear, several hypotheses are possible. First, the strength and permeability of the blood brain barrier has been shown to vary between sexes, which may impact CNS penetration of chemotherapeutic agents, the probability of CNS leukemia involvement at levels below that detectable by conventional techniques, or both.25 Second, though not a consistent finding, clearance of glucocorticoids has been found to be higher among males vs. females.26,27 This may explain both higher rates of relapse and lower rates of osteonecrosis among the former.28 Third, the dosing of intrathecal chemotherapy is based on age-dependent volumes of cerebrospinal fluid CSF), and not body surface area, as CSF volume increases at a more rapid rate than body surface and reaches near adult values after three years of age.29 Age-related dosing has thus been associated with increased therapeutic effect and reduced neurotoxicity.30 Sex-based differences in CSF volume however have not been rigorously evaluated, though recent studies have suggested that adult females may have lower CSF volumes.31 Lower CSF volumes among girls as compared to age-matched boys would result in higher concentrations of intrathecal chemotherapy among the former. Finally, boys in our cohort received an extra year of Maintenance therapy as compared to girls, including four extra intrathecal treatments. Though pre-symptomatic detection of CNS relapse may therefore be more likely in boys vs. girls during that year, the median time to CNS relapse was longer among boys, indicating that our finding is not artefactual and instead reflects a true increase in risk.
Perhaps the most important remaining question is how the demonstrated sex-based outcome disparities can be abolished. Previous attempts to improve the outcomes of boys, including surveillance testicular biopsies, have not been successful. Longer treatment durations for boys have also been unsuccessful in eliminating sex-based outcome disparities. For example, an additional year of Maintenance for boys in the ALL-BFM 95 study did not improve outcomes compared to historical controls; in both cohorts, boys experienced slightly inferior outcomes to girls.32 Overall, the rationale for continuing to expose boys to longer durations of therapy is weak.6 High overall cure rates represent an additional challenge in addressing small absolute differences in outcomes between sexes. For example, though the incidence of CNS relapse in boys was 70% higher than that in girls, intensification of CNS therapy in the former through cranial radiation, extra intrathecal chemotherapy, or high-dose methotrexate would result in the unnecessary exposure of large number of boys to additional treatment with consequent late effects for the possibility of preventing a comparably small number of relapses. Given this, two other strategies are possible. Should sex-based differences in CSF volume be identified, sex-based intrathecal chemotherapy dosing may be warranted and effective. Second, improvements in the ability to predict CNS relapse, regardless of sex, may be of particular benefit to boys. Recently, several groups have demonstrated that detection of low levels of CNS leukemia at initial diagnosis through techniques such as flow cytometry and next generation sequencing may better identify patients at highest risk for subsequent CNS relapse, and thus targets for therapy intensification.33–35 These more sensitive techniques may well identify a larger number of boys at risk than girls.
Strengths of our study include its large sample size, uniform treatment, and the availability of detailed information on prognosticators, including centrally reviewed cytogenetics and centrally determined MRD. Several limitations also merit note. While our multivariable models accounted for imbalance in prognosticators between sexes, we cannot rule out a degree of residual confounding. In addition, while modern therapy of newly diagnosed childhood ALL remains mainly unchanged from that received by the study cohort, novel immunotherapies have been incorporated into the treatment of relapsed disease.36–38 As the role of immunotherapy in first line therapy is investigated,39 continued monitoring of sex-based disparities is justified.
In conclusion, despite increases in overall cure rates, boys with ALL continue to experience small but significant decreases in outcome as compared to girls. This disparity is not accounted for by imbalances in risk factors, and is attributable to higher rates of CNS relapse among boys with B-ALL despite an additional year of maintenance therapy. Continued monitoring of sex-based disparities is warranted as fewer children undergo prophylactic cranial irradiation and as immunotherapies are incorporated into treatment regimens. Improved strategies to identify patients at highest risk of CNS relapse may have particular benefit for boys.
Supplementary Material
Funding Statement:
This study was supported by the Children’s Oncology Group, and the National Cancer Institute of the National Institutes of Health under award numbers U10CA098543, U10CA098413, U10CA180886, and U10CA180899, and by St. Baldrick’s Foundation.
Footnotes
Conflict of Interest Statement: The authors acknowledge no relevant conflicts of interest.
Publisher's Disclaimer: DISCLAIMER
Publisher's Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in children. New Engl J Med 2015; 373: 1541–2. [DOI] [PubMed] [Google Scholar]
- 2.Hunger SP, Lu X, Devidas M, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children’s oncology group. J Clin Oncol 2012; 30(4): 1663–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumer JH, Mott MG. Sex and prognosis in childhood acute lymphoblastic lymphoma. Lancet 1978: 522–3. [DOI] [PubMed] [Google Scholar]
- 4.Sather H, Miller D, Nesbit M, Heyn R, Hammond D. Differences in prognosis for boys and girls with acute lymphoblastic leukemia. Lancet 1981; 8233: 739–43. [DOI] [PubMed] [Google Scholar]
- 5.Pui CH, Boyett JM, Relling MV, et al. Sex differences in prognosis for children with acute lymphoblastic leukemia. J Clin Oncol 1999; 17(3): 818–24. [DOI] [PubMed] [Google Scholar]
- 6.Teachey DT, Hunger SP, Loh ML. Optimizing therapy in the modern age: Differences in length of maintenance therapy in acute lymphoblastic leukemia. Blood 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang L, Bhatia S, Gomez SL. Differential inequality trends over time in survival among U.S. children with acute lymphoblastic leukemia by race/ethnicity, age at diagnosis, and sex. Cancer Epidemiol Biomarkers Prev 2015; 24: 1781–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Homes L Jr, Hossain J, des Vignes-Kendrick M, Opara F. Sex variability in pediatric leukemia survival: Large cohort evidence. Int Scholarly Res Network Oncol 2012; 2012: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loh ML, DelRocco N, Borowitz MJ, et al. Enhanced risk stratification of 21,178 children, adolescents, and young adults with acute lymphoblastic leukemia (ALL) incorporating white blood count (WBC), age, and minimal residual disease (MRD) at day 8 and 29 as continous variables: A Children’s Oncology Group (COG) report. Blood 2020; 136: 39–40. [Google Scholar]
- 10.Mattano LA, Devidas M, Maloney KW, et al. Favorable trisomies and ETV6-RUNX1 predict cure in low-risk B-cell acute lymphoblastic leukemia: Results from Children’s Oncology Group trial AALL0331. J Clin Oncol 2021; 14: 1540–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maloney KW, Devidas M, Wang C, et al. Outcome in children with standard-risk B-cell acute lymphoblastic leukemia: Results of Children’s Oncology Group Trial AALL0331. J Clin Oncol 2020; 38(6): 602–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larsen EC, Devidas M, Chen S, et al. Dexamethasone and High-Dose Methotrexate Improve Outcome for Children and Young Adults With High-Risk B-Acute Lymphoblastic Leukemia: A Report From Children’s Oncology Group Study AALL0232. J Clin Oncol 2016; 34(20): 2380–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winter SS, Dunsmore KP, Devidas M, et al. Improved survival for children and young adults with T-lineage acute lymphoblastic leukemi: Results from the Children’s Oncology Group AALL0434 methotrexate randomization. J Clin Oncol 2018; 36(29): 2926–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schultz KR, Pullen DJ, Sather HN, et al. Risk- and response-based classification of childhood B-precursor acute lymphoblastic leukemia: a combined analysis of prognostic markers from the Pediatric Oncology Group (POG) and Children’s Cancer Group (CCG). Blood 2007; 109(3): 926–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borowitz MJ, Wood BL, Devidas M, et al. Prognostic significance of minimal residual disease in high risk B-ALL: a report from Children’s Oncology Group study AALL0232. Blood 2015; 126(8): 964–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heerema NA, Carroll AJ, Devidas M, et al. Intrachromosomal amplification of chromosome 21 is associated with inferior outcomes in children with acute lymphoblastic leukemia treated in contemporary standard-risk Children’s Oncology Group Studies: A report from the Children’s Oncology Group. J Clin Oncol 2013; 31(27): 3397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–81. [Google Scholar]
- 18.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer 1977; 35(1): 1–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med 2007; 26: 2389–430. [DOI] [PubMed] [Google Scholar]
- 20.Vrooman LM, Blonquist TM, Harris MH, et al. Refining risk classification in childhood B acute lymphoblastic leukemia: results of DFCI ALL Consortium Protocol 05–001. Blood Adv 2018; 2(12): 1449–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toft N, Birgens H, Abrahamsson J, et al. Results of NOPHO ALL2008 treatment for patients aged 1–45 years with acute lymphoblastic leukemia. Leukemia 2018; 32: 606–15. [DOI] [PubMed] [Google Scholar]
- 22.Jeha S, Pei D, Choi J, et al. Improved CNS control of childhood acute lymphoblastic leukemia without cranial irradiation: St Jude Total Therapy Study 16. J Clin Oncol 2019; 35: 3377–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leclerc JM, Billett AL, Gelber RD, et al. Treatment of childhood acute lymphoblastic leukemia: results of Dana-Farber ALL Consortium Protocol 87–01. J Clin Oncol 2002; 20(1): 237–46. [DOI] [PubMed] [Google Scholar]
- 24.Vora A, Andreano A, Pui CH, et al. Influence of cranial radiotherapy on outcome in children with acute lymphoblastic leukemia treated with contemporary therapy. J Clin Oncol 2016; 34(9): 919–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weber CM, Clyne AM. Sex differences in the blood-brain barrier and neurodegenerative diseases. APL Bioeng 2021; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ayyar VS, DuBois DC, Nakamura T, Almon RR, Jusko WJ. Modeling corticosteroid pharmacokinetics and pharmacodynamics, Part II: Sex differences in methylprednisolone pharmacokinetics and corticosterone suppression. J Pharmacol Exp Ther 2019; 370(2): 327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song D, Sun L, DuBois DC, Almon RR, Meng S, Jusko WJ. Physiologically based pharmacokinetics of dexamethasone in rats. Drug Metab Dispos 2020; 48(9): 811–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barzilai-Birenboim S, Yacobovich J, Zalcberg Y, et al. Bone pain at leukemia diagnosis and other risk factors for symptomatic osteonecrosis in children with acute lymphoblastic leukemia. Pediatr Blood Cancer 2021; 68. [DOI] [PubMed] [Google Scholar]
- 29.Triarico S, Maurizi P, Mastrangelo S, Attina G, Capozza MA, Ruggiero A. Improving the brain delivery of chemotherapeutic drugs in childhood brain tumors. Cancers 2019; 11(824). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bleyer AW. Clinical pharmacology of intrathecal methotrexate. II. An improved dosage regimen derived from age-related pharmacokinetics. Cancer Treat Rep 1977; 61: 1419–25. [PubMed] [Google Scholar]
- 31.Podgorski P, Bladowska J, Sasiadek M, Zimny A. Novel volumetric and surface-based magnetic resonance indices of the aging brain - Does male and female brain age in the same way? Front Neurol 2021; 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moricke A, Reiter A, Zimmerman M, et al. Risk-adjusted therapy of acute lymphoblastic leukemia can decrease treatment burden and improve survival: Treatment results of 2169 unselected pediatric and adolescent patients enrolled in the trial ALL-BFM 95. Blood 2008; 111: 4477–89. [DOI] [PubMed] [Google Scholar]
- 33.Thastrup M, Marquart HV, Levinsen M, et al. Flow cytometric detection of leukemia blasts in cerebrospinal fluid predicts risk of relapse in childhood acute lymphoblastic leukemia: a Nordic Society of Pediatric Hematology and Oncology study. Leukemia 2020; 34(2): 336–46. [DOI] [PubMed] [Google Scholar]
- 34.Shalabi H, Yuan CM, Kulshreshtha A, et al. Disease detection methodologies in relapsed B-cell acute lymphoblastic leukemia: Opportunities for improvement. Pediatr Blood Cancer 2020; 67(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi JK, Jeha S, Zheng J, Carlton V, Faham M, Pui CH. Assessment of central nervous system involvement in pediatric acute lymphoblastic leukemia patients using next-generation sequencing method. Blood 2015; 126(23). [Google Scholar]
- 36.Maude SL, Teachey DT, Porter DL, Grupp SA. CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Blood 2015; 125(26): 4017–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown PA, Ji L, Xu X, et al. Effect of postreinduction therapy consolidation with blinatumomab vs chemotherapy on disease-free survival in children, adolescents and young adults with first relapse of B-cell acute lymphoblastic leukemia: A randomized clinical trial. JAMA 2021; 325(9): 833–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Locatelli F, Zugmaier G, Rizzari C, et al. Effect of blinatumomab vs chemotherapy on event-free survival among children with high-risk first-relapse B-cell acute lymphoblastic leukemia: A randomized clinical trial. JAMA 2021; 325(9): 843–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McNeer JL, Rau RE, Gupta S, Maude SL, O’Brien MM. Cutting to the front of the line: Immunotherapy for childhood acute lymphoblastic leukemia. Am Soc Clin Oncol Educ Book 2020; 40: e132–e43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


