Abstract
Background.
Synergy analysis provides a means of quantifying the complexity of neuromuscular control during gait. Prior studies have shown evidence of reduced neuromuscular complexity during gait in individuals with neurological disorders associated with stroke, cerebral palsy, and Parkinson’s disease.
Objective.
The purpose of this study was to investigate neuromuscular complexity during gait in individuals who experienced a prior traumatic brain injury (TBI) that resulted in chronic balance deficits.
Methods.
We measured and analyzed lower extremity electromyographic data during treadmill and overground walking for 44 individuals with residual balance deficits from a mild to moderate TBI at least 1 year prior. We also tested 20 unimpaired controls as a comparison. Muscle synergies were calculated for each limb using non-negative matrix factorization of the activation patterns for six leg muscles. We quantified neuromuscular complexity using Walk-DMC, a normalized metric of the total variance accounted for by a single synergy, in which a Walk-DMC score of 100 represents normal variance accounted for. We compared group-average synergy structures and inter-limb similarity using cosine similarity. We also quantified each individual’s gait and balance using the Sensory Organization Test, the Dynamic Gait Index, and the Six-Minute Walk Test.
Results.
Neuromuscular complexity was diminished for individuals with a prior TBI. Walk-DMC averaged 92.8 ± 12.3 for the TBI group during overground walking, which was significantly less than seen in controls (100.0 ± 10.0). Individuals with a prior TBI exhibited 13% slower overground walking speeds than controls and reduced performance on the Dynamic Gait Index (18.5 ± 4.7 out of 24). However, Walk-DMC measures were insufficient to stratify variations in assessments of gait and balance performance. Group-average synergy structures were similar between groups, although there were considerable between-group differences in the inter-limb similarity of the synergy activation vectors.
Conclusions.
Individuals with gait and balance deficits due to a prior TBI exhibit evidence of decreased neuromuscular complexity during gait. Our results suggest that individuals with TBI exhibit similar muscle synergy weightings as controls, but altered control of the temporal activation of these muscle weightings.
Keywords: Traumatic brain injury, muscle synergy, motor control, gait, electromyography, muscle coordination
Introduction
Chronic gait and balance abnormalities are often present among individuals who previously experienced a mild to moderate traumatic brain injury (TBI). For example, characteristic gait abnormalities in this heterogeneous population include reduced walking speed, shorter steps, and increased lateral sway, with varying severities.1–3 Characteristic deficits to standing balance include larger, slower, and more random oscillations of their center of pressure.4 As the recovery of walking and balance ability during rehabilitation tends to plateau at approximately six months,5 residual gait and balance abnormalities that persist past this time may be considered chronic. These chronic gait and balance abnormalities can contribute to a loss of mobility, increased risk of falling, and an overall decreased quality of life.6,7 Thus, an improved understanding of how TBI alters the neuromuscular control of gait and balance could inform targeted treatment and enhance functional recovery.
Quantitative electromyography (EMG) can provide insight into the neuromuscular control of gait by tracking subtle changes in lower limb muscle activity during walking. We have previously shown that individuals with chronic TBI exhibit characteristic changes in the temporal coordination of their lower extremity muscles while walking compared to unimpaired adults, and that these abnormal muscle activation patterns are associated with chronic gait deficits.8 However, this analysis examined the activation of each muscle independently and did not account for alterations to inter-muscular coordination, which may also contribute to gait abnormalities. For example, prior studies have found evidence that, compared to controls, individuals with TBI exhibit increased coactivation of their ankle muscles during the initial and late double support phases of gait,9 as well as increased coactivation of knee extensor and ankle plantar flexor muscles in all phases of gait except during single support.10 However, it is not fully understood how the inter-muscle coordination of muscles throughout the lower limbs may be altered in chronic TBI.
The neuromuscular control of multiple muscles during a task can be characterized by the complexity of the activation patterns. A motor control strategy that independently activates muscles can be described as more complex than a strategy that synergistically coactivates sets of muscles.11 Previous studies have shown that the complexity of motor control during gait is reduced for individuals with neurological disorders, including Parkinson’s disease,12 stroke,13,14 cerebral palsy,11,15,16 and incomplete spinal cord injury.17 Measures of complexity have also shown potential to distinguish responders to treatment,16,18 and may provide targets for rehabilitation.15,19 We are not aware of any prior studies evaluating the complexity of motor control in chronic TBI.
Muscle synergy analysis is a quantitative approach for characterizing the complexity of motor control. These analyses typically use matrix factorization algorithms to identify lower-dimensional patterns from experimental EMG data that can describe muscle coordination during tasks like walking. Such analyses reveal that a small number of coactivation patterns over groups of coactivated muscles, known as muscle synergies or modules, can effectively represent most of the variability within the EMG signals. Muscle synergy analyses can thus characterize complexity during gait by evaluating how well a small set of muscle synergies describes the original muscle activities.20,21 Thus, muscle synergy analyses can provide meaningful insight into complexity and inter-muscular coordination during walking.
Different motor control strategies may arise depending on the walking conditions. For example, there are many small biomechanical differences between walking on a treadmill and walking overground.22–24 Compared to treadmill walking, overground walking may require more flexible motor control strategies that can adapt to step-to-step internal and external perturbations.25 It is still unclear how the control of gait may vary for individuals with TBI during treadmill and overground walking.3,26–28 However, previous work has shown that rehabilitation outcome measures after gait training for individuals with TBI can be different when walking on a treadmill or when walking overground.29 Thus, the complexity of motor control may present differently during treadmill walking and overground walking.
It is unknown if the abnormal muscle activation patterns that individuals with chronic TBI exhibit during walking represent reduced complexity of motor control through increased synergistic muscle activation. Therefore, the purpose of this study was to compare how muscle synergies for individuals with chronic TBI differ from unimpaired individuals during gait. Acknowledging the potential differences in motor control strategies that may arise under different walking conditions, we examined muscle synergies during both treadmill walking and overground walking. We hypothesized that complexity of motor control during gait would be diminished in chronic TBI. We also hypothesized that measures of complexity would be related to clinical measures of gait and balance impairment.
Methods
Participants
We analyzed EMG and kinematic data from 44 adults with a balance disorder due to a mild-to-moderate traumatic brain injury (28 female; age: 53.4 ± 8.5 years, range: 28–64 years; time since injury: 6.3 ± 7.6 years, range: 1–33 years), as well as 20 control subjects (10 female; age: 25.3 ± 3.3 years). Participants were required to have had a non-penetrative injury that occurred at least one year prior, had previously participated in a focused physical rehabilitation program, and had reached a functional plateau in their recovery (as confirmed by a discharge note from their physical therapist). Data were collected during the baseline visit for a planned intervention study, where we also collected basic clinical assessments to describe functional ability, reported previously8 and described below. The study protocol was approved by the University of Wisconsin–Madison Health Sciences Institutional Review Board, and all subjects provided written informed consent before participating.
Clinical Assessments
Clinical assessments were performed to assess the level of balance and gait impairment among the individuals with TBI. Computerized dynamic posturography assessed standing balance and was measured as the NeuroCom™ Sensory Organization Test30 composite score (ranging between 0–100). Walking ability was assessed using the Dynamic Gait Index (DGI),31 which consists of 8 tasks that include overground walking, changing gait speeds, walking with head turns, walking while turning, walking over and around obstacles, and stair climbing. Each task in the DGI was scored 0–3 by a trained physical therapist, where 3 indicates normal (max score of 24). DGI scores ≤ 19 have been linked to an increased risk for falls for individuals with TBI.32 Walking capacity was assessed using the Six-Minute Walk Test.33 Overground walking speed was recorded as the average speed for a subject to traverse a six-meter walkway, and treadmill walking speed was set to the highest speed at or below their overground walking speed in which the subject could walk comfortably on the treadmill.8
Electromyography
Surface EMG data (Trigno, Delsys Inc., Boston, MA) were collected bilaterally at 1926 Hz from six muscles (tibialis anterior, medial gastrocnemius, soleus, vastus lateralis, rectus femoris, medial hamstrings) during two 60-s walking trials. The first trial was walking on a treadmill (Bronze Basic Treadmill, PaceMaster, Logan, UT) at each subject’s preferred speed. Control subjects walked at 1.0 m/s to match the average treadmill speed of the individuals with TBI (1.0 ± 0.3 m/s). The second trial was level overground walking in a hallway at a comfortable, self-selected speed. During both trials, heel-strike events were detected using the accelerometers of two Trigno sensors (sampling at 148 Hz) positioned at the ankle over the Achilles tendons.8,34,35 We monitored the output of the EMG sensors during data collection in real-time, and if any sensors lost contact or developed an inappropriate level of noise, the trial was stopped, the sensor was reattached, and the trial was repeated. EMG sensors were placed over the target muscle bellies following the SENIAM guidelines for EMG preparation and placement.36 We also examined the output for each EMG sensor prior to the walking trials by having the participants perform simple exercises to verify we were recording the desired muscle activity.
We processed the EMG signals using a custom MATLAB script (R2018a, MathWorks Inc., Natick, MA). First, we bandpass filtered (10–500 Hz, 4th order Butterworth) and full-wave rectified the raw EMG data before low-pass filtering (10 Hz, 4th order Butterworth) to obtain linear envelopes of muscle activation.37,38 We then amplitude normalized individual EMG channels by their peak activation, such that all channels ranged from 0 to 1 in amplitude.39 Next, we divided the EMG data into strides (i.e., heel-strike to heel-strike) and time normalized the muscle activity to 101 points per stride (i.e., 0–100% of the gait cycle). EMG data for each stride were then concatenated using 100 points per stride (i.e., 0–99% of the gait cycle) to form a time series of stride-normalized muscle activity for each leg, which can better account for stride-by-stride variability in the synergy analysis.40
Synergy Analysis
We performed independent synergy analyses on the measured EMG patterns for each individual limb. Hence, for each limb and each trial, we combined the processed EMG data into a m × t matrix, where m is the number of muscles analyzed (6 in this study) and t is time (t = number of strides × 100 points per gait cycle). We then used non-negative matrix factorization (NNMF) to derive the muscle synergies (Wm×n) and the time-varying activation of those synergies (Cn×t) such that EMG = W × C + error. Here, W specifies the relative weighting of each muscle within a synergy, and n is the specified number of synergies extracted (we varied n from 1 to 5 in this study). We calculated the synergies using the NNMF function in MATLAB with the following parameters: 50 replicates, 1000 max iterations, 1 × 10−4 minimum threshold for convergence, and 1 × 10−6 threshold for completion.41 To quantify how accurately the derived muscle synergies described the original set of EMG signals, we then calculated the total variance accounted for (tVAF) by a given number of synergies (n) as:11,15,42,43
Synergy Complexity
We evaluated complexity of motor control using two methods. We first evaluated the number of muscle synergies required to describe at least 90% of the variance in the original EMG signals (i.e., tVAF ≥ 90%).39 A lower number of muscle synergies required represents less complexity in the EMG patterns.11,13,19 The number of muscle synergies required provides a coarse, ordinal measure, so we also evaluated the Dynamic Motor Control Index during Walking (Walk-DMC).11,37 Walk-DMC calculates the total variance accounted for by one synergy (tVAF1) as a z-score using the average and standard deviation of tVAF1 from a population of unimpaired controls:
Walk-DMC is scaled such that the average score of the unimpaired group is 100 ± 10. A lower Walk-DMC score (< 100) is interpreted as less complexity than normal in the EMG patterns.11
Group Synergy Structure and Similarity
We derived a set of synergy weights and activations to characterize the subject groups (i.e., individuals with TBI, controls) during both overground and treadmill walking. For each group, we pooled together the synergy weights W and corresponding activations C from each limb and then sorted them by W using k-means cluster analysis (randomly initialized, 1000 max iterations, 10 replicates), where k is the number of synergies analyzed (i.e., 1–5).11,44 If a limb had two synergy weights assigned to the same cluster, we calculated the cosine similarity (un-centered correlation coefficient)44,45 between each synergy weights vector and the cluster centroid vector , and the synergy weight vector with the highest similarity was matched to that cluster and the other synergy weights vector was assigned to the remaining cluster. We then determined the set of group synergy weights and activations as the average synergy weights and activations within each cluster.
To evaluate the inter-limb similarity of muscle synergies within subject groups, we calculated the cosine similarity between the synergy weights vectors of a cluster and the average synergy weights of that cluster.44,45 Cosine similarity can vary from 0 to 1, in which values closer to 1 indicate similar vectors. We also calculated the cosine similarity between synergy activations vectors of a cluster to the average synergy activations of that cluster. When comparing synergy activation vectors, we used each limb’s average activation across the gait cycle (101 points) rather than the limb’s activation across all strides, to reduce the similarity due to chance when comparing long vectors. When calculating the structures and similarities, we only considered n = 1:4 synergies, because we found that 4 synergies was the maximum number of synergies required to describe tVAF ≥ 90% for all of our subjects.
Statistical Analysis
We performed all statistical analyses using SPSS (v.25, IBM Corp., Armonk, NY), and an alpha level was set a priori to be p ≤ 0.05. To evaluate differences in the number of synergies required (ordinal data), a Mann-Whitney U Test compared group differences during treadmill and overground walking, and Wilcoxon signed-rank tests compared differences within groups between treadmill and overground walking. To evaluate differences in Walk-DMC, a two-way analysis of variance (ANOVA) tested for main effects and interactions between groups (TBI, control) and across walking conditions (treadmill, overground). When we found a significant main effect of group, post-hoc t-tests evaluated group differences with a Bonferroni correction to account for multiple comparisons. Shapiro-Wilk tests confirmed assumptions of normality. To evaluate differences in the inter-limb similarity of synergy structures, a series of Mann-Whitney U tests compared group differences in the cosine similarity of the weights and activations. Similarly, Wilcoxon signed-rank tests compared the cosine similarity between treadmill and overground walking. For these comparisons, we controlled for the false discovery rate using the Benjamini-Hochberg procedure.46 We also examined whether our measures of complexity may be related to an individual’s walking speed using Spearman’s rank-order correlation. We then examined the relationship between complexity and the clinical assessments of gait and balance using Spearman’s rank-order correlation.
Results
We examined data from 128 limbs (88 TBI, 40 controls). On average, each limb contributed 50.5 ± 6.0 individual strides (mean ± s.d.) on the treadmill (TBI: 51.6 ± 6.7 strides, controls: 48.0 ± 2.4 strides) and 51.3 ± 8.4 strides overground (TBI: 49.8 ± 9.7 strides, controls: 54.0 ± 3.7 strides). During our processing of the EMG data, we did not encounter any strides or signals that appeared unusable such that they would be dropped from our analysis.
Synergy Complexity
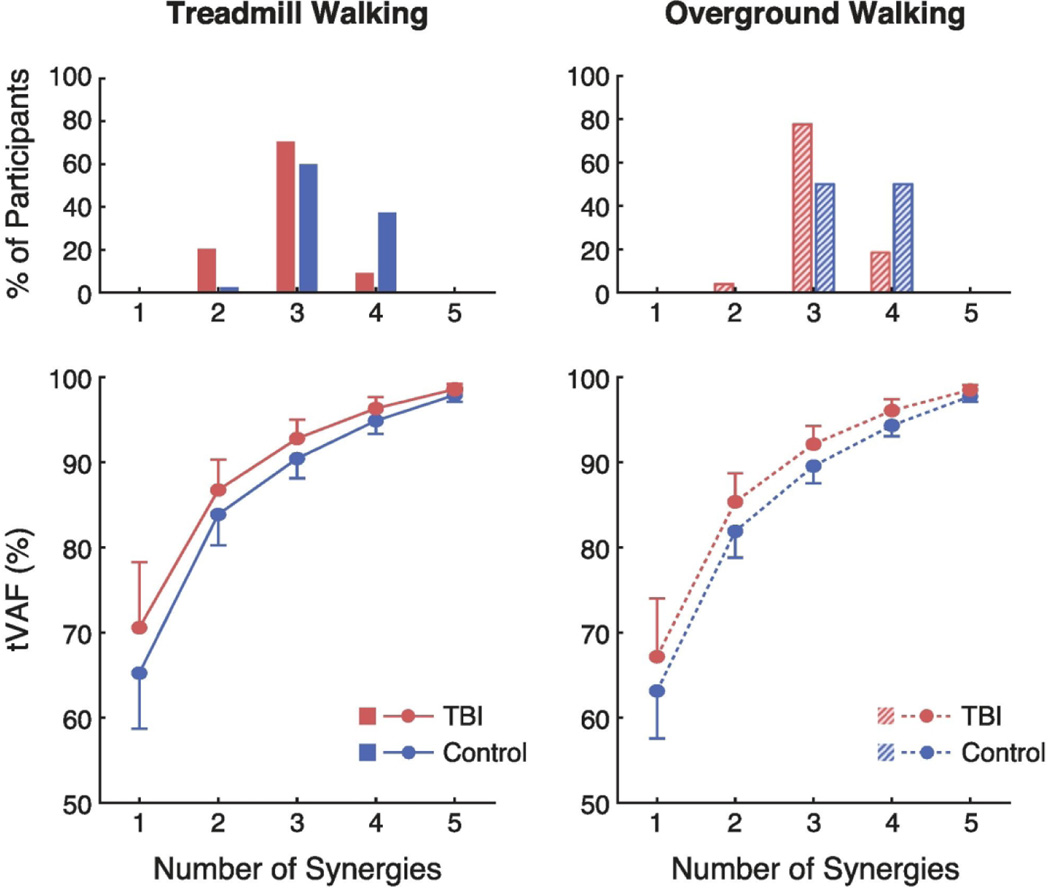
A smaller number of muscle synergies was sufficient to describe the variance in muscle activation during gait for the individuals with TBI compared to controls (Fig. 1). This was true during both treadmill (mean ± s.d.; TBI: 2.92 ± 0.54 synergies, controls: 3.35 ± 0.53) and overground walking (TBI: 3.14 ± 0.45, controls: 3.50 ± 0.51). The median number of synergies was significantly lower for individuals with TBI compared to controls during both treadmill walking (U = 2,445; z = 4.246; p < 0.001) and overground walking (U = 2,030; z = 3.649; p < 0.001). During treadmill walking, three synergies could describe over 90% of the total variance of muscle activity in 91% of the individuals with TBI, but only 63% of the control subjects. During overground walking, three synergies could describe over 90% of the total variance of muscle activity in 82% of the individuals with TBI, but only 50% of the control subjects. Compared to treadmill walking, overground walking significantly increased the median number of synergies required for individuals with TBI (z = 3.900; p < 0.001), but not for controls (z = 1.732; p = 0.083).
Fig. 1.
Fewer muscle synergies were needed to describe gait EMG activity for individuals with TBI when compared to controls. Top: Histogram of the number of muscle synergies required to account for at least 90% of the variance in the EMG data. Bottom: Mean (± standard deviation) total variance accounted for (tVAF) in the EMG data when using one to five synergies.
For the control group, one synergy accounted for 63.1% (± 5.6%) of the variance in muscle activity during overground walking and 65.2% (± 6.5%) of the variance in muscle activity during treadmill walking. For the individuals with TBI, one synergy accounted for 67.2% (± 6.8%) of variance in muscle activity during overground walking and 70.6% (± 7.7%) of the variance in muscle activity during treadmill walking.
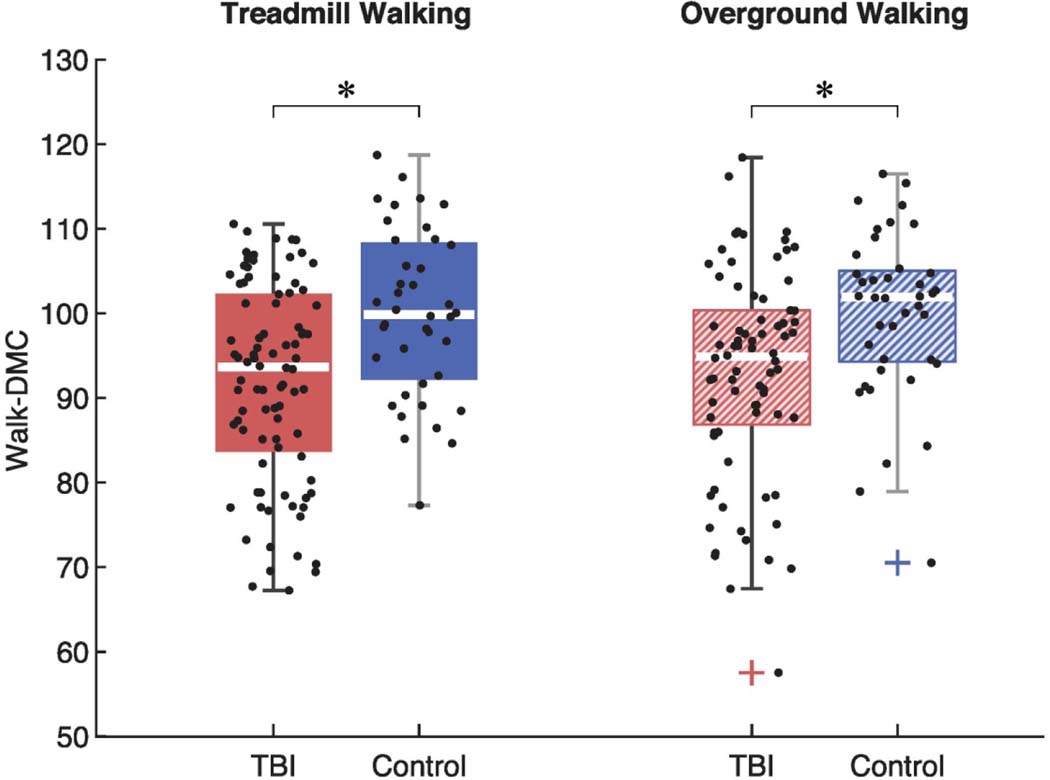
Walk-DMC was reduced for the individuals with TBI compared to controls (Fig. 2). This was true during both treadmill walking (mean ± s.d.; TBI: 91.9 ± 11.7, controls: 100.0 ± 10.0) and overground walking (TBI: 92.8 ± 12.3, controls: 100.0 ± 10.0). During treadmill walking, 39% of the individuals with TBI had a Walk-DMC < 90 (i.e., greater than one standard deviation below the mean Walk-DMC for controls). During overground walking, 30% of the TBI group had a Walk-DMC < 90. The two-way ANOVA revealed a significant main effect of group on Walk-DMC (p = 0.001; 𝜂2𝑝 = 0.987), indicating a significant difference in Walk-DMC between individuals with TBI and controls across walking conditions. The two-way ANOVA found no significant main effect of walking conditions (p = 0.972), indicating that Walk-DMC was not significantly different between treadmill or overground walking across all subjects. The two-way ANOVA did not reveal any significant interaction effects (p = 0.972). Post-hoc comparisons found that during treadmill walking, the mean Walk-DMC for individuals with TBI was 8% lower than the controls (p < 0.001, Hedges’ g = 0.72). Similarly, during overground walking, the average Walk-DMC index for individuals with TBI was 7% lower than the controls (p = 0.002, Hedges’ g = 0.61).
Fig. 2.
Complexity of motor control during gait was significantly reduced for individuals with TBI compared to controls, as measured by the Dynamic Motor Control Index during Walking (Walk-DMC). The middle lines of each box plot represent the medians. Asterisks (*) indicate significant differences between groups. Plus signs (+) indicate outliers.
Synergy Structure and Similarity
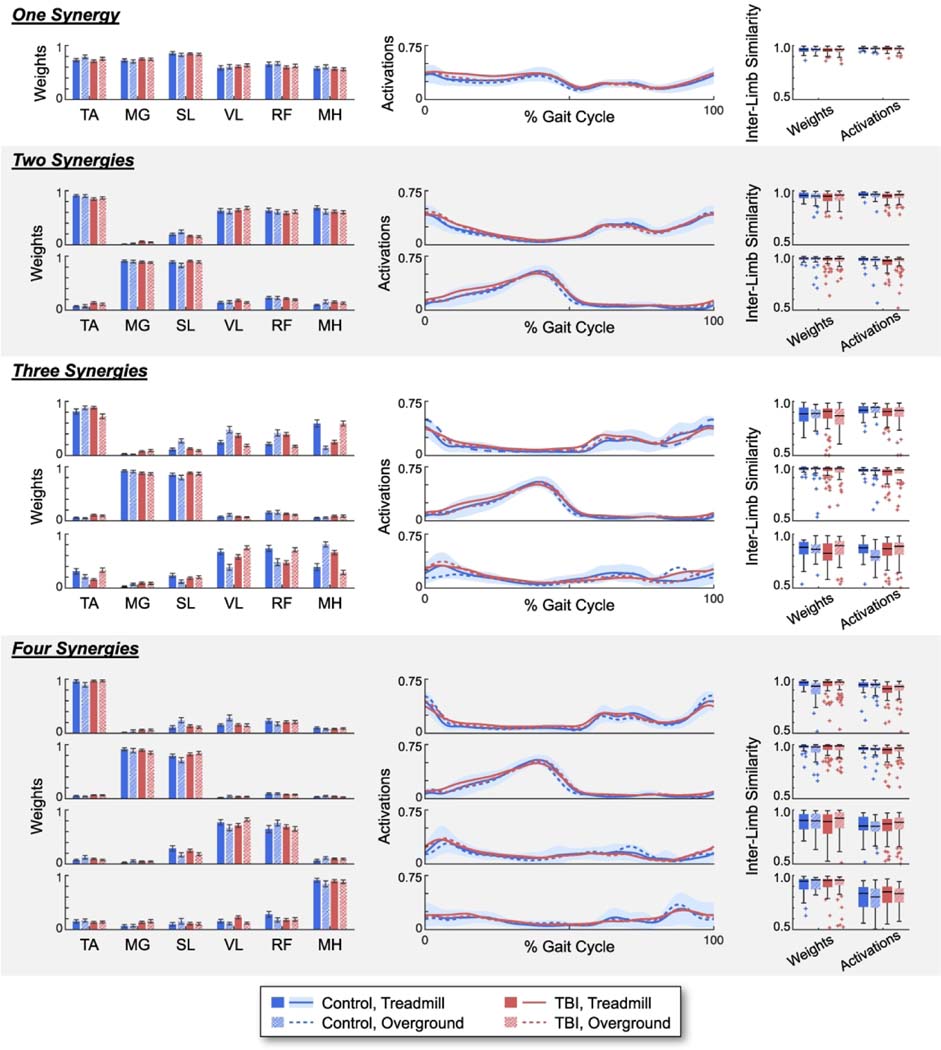
The group average synergy weights revealed a consistent organization of lower limb muscles for all groups and walking conditions (Fig. 3). For example, the plantar flexors (medial gastrocnemius and soleus) dominated the second vector of synergy weights for the two-synergy solution for both individuals with TBI and controls during both treadmill and overground walking. The synergy weights vectors also revealed consistent patterns as the number of muscle synergies examined increased from one to four. For example, the two-synergy solution, the three-synergy solution, and the four-synergy solution all identified synergy weights vectors dominated by the plantar flexors and a synergy weights vector dominated by the dorsiflexors (tibialis anterior). The four-synergy solution identified a third vector dominated by quadriceps muscles (vastus lateralis and rectus femoris), and a fourth vector dominated by the medial hamstrings. Variability between group weightings was most prominent for the three-synergy solution. For example, although the quadriceps and hamstrings dominated the third vector of synergy weights, the magnitudes of these weightings were inconsistent between groups and walking conditions.
Fig. 3.
Group average muscle synergy weights and activations using one to four synergies, as well as the inter-limb similarity of the muscle synergy weights and activations within each group. Synergy weights for each muscle are plotted as mean ± standard error. Synergy activations plotted as the mean activation over the gait cycle. The shaded bar represents the standard deviation for the controls during treadmill walking. Inter-limb similarity plotted as boxplots of the cosine similarity between individual synergy weights and activations and the group average.
TA = tibialis anterior; MG = medial gastrocnemius; SL = soleus; VL = vastus lateralis; RF = rectus femoris; MH = medial hamstrings.
The inter-limb similarities between individual limb synergy weights and group average synergy weights were high (i.e., average cosine similarities > 0.80) (Fig. 3). The one-synergy solution had the most consistent inter-limb similarity across all subjects and walking conditions, with an inter-quartile range (IQR) of 0.03, compared to n=2:4 synergies with an IQR of 0.05, 0.14, and 0.09, respectively. There were no significant differences in inter-limb similarity between the individuals with TBI and the controls for n=1:4 synergies, except for the first vector of the four-synergy solution. Here, we found that the controls had significantly less inter-limb similarity than the individuals with TBI during overground walking, although both groups were still highly similar to their group averages (TBI: median = 0.97, IQR = 0.04; controls: median = 0.93, IQR = 0.10; p < 0.001). We also found no significant differences in inter-limb similarity between the overground and walking conditions for both subject groups.
The group average synergy activations exhibited comparable activation patterns across the gait cycle for all groups and walking conditions (Fig. 3). Throughout the majority of the gait cycle, the average synergy activation patterns for the individuals with TBI fell within one standard deviation of the average synergy activation patterns for the controls. When the weightings within a synergy weight vector favored a single muscle, the corresponding synergy activation pattern over the gait cycle resembled a typical healthy muscle activation pattern for that muscle (For a visual description of typical muscle activation patterns during gait, the authors recommend the books Whittle’s Gait Analysis47 and Observational Gait Analysis48). For example, the first weighting vector of the four-synergy solution was dominated by the tibialis anterior muscle and the corresponding activation pattern closely resembled a typical healthy muscle activation pattern of the tibialis anterior over the gait cycle.
The inter-limb similarity between individual limb synergy activations and group average synergy activations were also relatively high (i.e., average cosine similarities > 0.78) (Fig. 3). The one-synergy solution had the most consistent inter-limb similarity across all subjects and walking conditions, with an inter-quartile range (IQR) of 0.02, compared to n=2:4 synergies with an IQR of 0.04, 0.10, and 0.12, respectively. The individuals with TBI had significantly less inter-limb similarity than controls during treadmill walking for the two-synergy solution (vectors 1 and 2, p’s ≤ 0.001), the three-synergy solution (vector 2, p = 0.005), and the four-synergy solution (vector 1 and 2, p ≤ 0.001), and during overground walking for the three-synergy solution (vector 1, p < 0.001). However, the controls had less inter-limb similarity than individuals with TBI during overground walking for the three-synergy solution (vector 3, p < 0.001). There were generally no significant differences in inter-limb similarity between walking conditions, except the controls exhibited significantly less similarity during overground walking than treadmill walking for the three-synergy solution (vector 3, p < 0.001) and the individuals with TBI exhibited less similarity during treadmill walking than overground walking for the two-synergy solution (vector 2, p = 0.003).
Clinical Assessments
The individuals with TBI walked at a mean speed of 1.07 ± 0.24 m/s when walking overground and at 1.00 ± 0.26 m/s when walking on the treadmill. There were no significant correlations between our measures of complexity and walking speed for the individuals with TBI and controls during treadmill (rs: −0.04–0.10; p: 0.39–0.84) or overground walking (rs: 0.08–0.17; p: 0.15–0.47). The individuals with TBI had a mean composite score of 39.3 ± 16.5 on the Sensory Organization Test, a mean score of 18.5 ± 4.7 on the Dynamic Gait Index, and a mean distance of 385.9 ± 78.0 m on the Six-Minute Walk Test. We found no significant correlations between the measures of complexity and the Sensory Organization Test (rs: −0.03–0.11; p: 0.33–0.82), Dynamic Gait Index (rs: −0.08–0.03; p: 0.48–0.92), or the Six-Minute Walk Test (rs: −0.13–0.07; p: 0.26–0.60).
Discussion
In this study, we investigated how muscle synergies for individuals with chronic TBI differ from unimpaired individuals during treadmill and overground walking. As we hypothesized, the synergy analysis suggested a reduced complexity of motor control in individuals with a prior TBI. However, contrary to our second hypothesis, the synergy metrics were insufficient to delineate variable levels of gait and balance impairment among the chronic TBI individuals. Muscle groups identified by the synergy analysis were generally similar between individuals with TBI and unimpaired controls. These data add to a growing body of evidence suggesting that brain injury reduces the complexity of muscle activation patterns underlying gait,12,13,15,17,49,50 and hence may result from a change in the use of cortical activity to modulate the rhythmic muscle activation patterns underlying walking.
There is evidence of reduced motor complexity during gait following a number of pathologies that affect brain function. For example, the number of synergies needed to characterize muscle activity is reduced in populations with stroke,13 cerebral palsy,11 incomplete spinal cord injury,17 and Parkinson’s disease.12 All of the TBI subjects in this study acquired a brain injury as an adult, which is believed to impair sensory integration2,51,52 and thereby contribute to balance deficits often seen in this population. The control of walking involves rhythmic neural activity at the spinal cord that is supplemented with cortically modulated descending signals that are believed to enhance robustness to internal and external perturbations.53 It is thought that muscle synergy weightings are established primarily at the spinal level.21,54 As we observed reduced complexity in a population with TBI, and that differences in synergy structures were found in the temporal synergy activation vectors, this supports the notion that diminished complexity may primarily emerge from disruption of the cortically modulated descending signals.12 This also may explain in part why diminished complexity appears to reflect a control strategy present in early development before higher level control of gait is established.11,55
The measures of complexity for the individuals with TBI were not related to walking speed or select clinical assessments of balance and gait. This result is in contrast to a prior large-scale study of cerebral palsy (n=633), which reported a modest correlation between Walk-DMC and walking speed in children.11 However, there are many possible factors that could influence an association between walking speed and complexity of motor control. For example, this relationship might depend on the characteristics of the specific clinical population studied, which may have very different mechanisms underlying their impaired gait and motor control. Within TBI populations, a relationship between complexity and walking speed or clinical assessments may also depend on the specific type of brain injury (e.g., a localized lesion within the brain). It is also possible that many more mild-to-moderate TBI subjects would be needed to observe this relationship in a population of TBI, and relatively small sample sizes may explain why prior studies did not find a relationship between complexity and gait performance. For example, past smaller studies evaluating complexity, such as in individuals with Parkinson’s disease12 (n=15) and incomplete spinal cord injury17 (n=8), did not find significant correlations between complexity and gait performance, but a larger study of complexity in individuals with stroke13 (n=55) did find that complexity was related to walking speed and gait parameters. We note that we previously did observe a relationship between abnormal temporal EMG patterns and the Dynamic Gait Index,8 which is a battery of locomotor tasks that challenge walking balance under altered physical and sensory conditions. Hence, abnormal muscle timing, rather than synergistic muscle activations, may be a more sensitive indicator of walking performance.
Group average comparisons of the synergy structures (i.e., synergy weights and activations) did not explain the reduced complexity in individuals with TBI. The inter-limb similarity of the group average synergy structures between the individuals with TBI and controls was relatively high, even between treadmill and overground walking. Prior studies56,57 have considered synergy structures to be “similar” when cosine similarity values are > 0.80, which was true for the majority of our subjects. We previously found that muscle activation in the individuals with TBI were most disrupted in the tibialis anterior, medial gastrocnemius, and rectus femoris,8 however we did not observe any characteristic group differences in the muscle synergy weightings surrounding these muscles. In fact, the synergy weighting vectors for n=2:4 always included a first weighting vector dominated by activity in the tibialis anterior and second weighting vector dominated by activity in the medial gastrocnemius. Inter-limb similarity was largely consistent between groups for the synergy weighting vectors, but more between-group differences appeared for the synergy activation vectors, which may explain why inter-joint coordination is altered for individuals with TBI.58 Taken together, our results suggest that individuals with TBI have access to similar muscle weighting vectors as the controls, but altered control of the temporal activation of these muscle weightings, as has been observed for persons with Parkinson’s disease.12
The differences in motor complexity during treadmill and overground walking were dependent on the complexity metric used. We found that Walk-DMC was similar between treadmill and overground walking for both the TBI group and controls. This metric suggests that, on average, individuals with TBI might use a similar strategy for the neuromuscular control of gait during treadmill and overground walking. However, we also found that the median number of synergies required to describe the variance in EMG during overground walking was greater than the median number of synergies during treadmill walking for only the TBI group. This metric suggests that individuals with a TBI used a more complex motor control strategy during overground walking than during treadmill walking. This is also a reasonable conclusion, as overground walking may require more flexible motor control strategies that adapt to step-to-step internal and external perturbations.25 This may explain why individuals with a TBI can have different gait rehabilitation outcomes when walking on a treadmill or walking overground.29 Given that there are many small biomechanical differences between walking on a treadmill and walking overground,22–24 there may also be subtle individual differences in motor control that may not readily appear in group-average comparisons of synergy structures or complexity. Future work should assess the sensitivity of these complexity metrics under different walking conditions. Regardless, both metrics were sensitive enough to differentiate complexity between the individuals with TBI and controls, and both may have utility for assessing neuromuscular complexity in other clinical populations.
There are several important limitations to consider when interpreting our results. First, the gait and balance deficits that accompany individuals with TBI vary considerably in presentation and severity.59 This contributes substantial variability in walking patterns between subjects, which may interfere with synergy extraction. Group average comparisons are therefore limited, as individual differences in muscle synergies are likely not accounted for. This heterogeneity has made characterizations of gait disorders for individuals with TBI challenging.27,59 Second, the TBI group was on average older than the controls (+28 years, although the ages spanned a range of 28–64 years), and thus we cannot exclude the possibility that age-differences may account for some of our observed synergy differences between the TBI and control groups. For example, age-related differences in Walk-DMC have been reported for children with cerebral palsy.11,16,37 However, in a previous study of these participants we found that the age of the TBI subjects was not associated with their measures of balance and gait,8 suggesting that the group differences we observed were not solely explained by their differences in age. In addition, a prior study found that age-related changes in muscle activation patterns of adults generally only emerge at faster speeds,60 whereas the current study used speeds that would be classified as slow and normal in healthy controls. Third, the relative consistency in the synergy weighting vectors could in part reflect the mechanical demands of walking, which may tightly couple the interaction of lower extremity muscles.20,21 We included six representative muscles in our analysis for each limb. It is possible to identify additional synergy weightings if we had chosen different muscles or increased the number of muscles considered. Additionally, our clusters were solely based on the synergy weightings, and our conclusions may be different if our clusters were based on the combination of weights and activations.61 Thus, we took caution not to over-interpret the specific composition of the weightings extracted but focused on their relative consistency between groups. We also did not examine intra-subject consistency (i.e., stride-to-stride variability) of muscle synergies which may nuance our group characterizations.
In summary, we have shown that individuals who have experienced a prior TBI exhibit decreased neuromuscular complexity during gait. There were no characteristic changes in the grouping of muscle activation patterns, and measures of reduced complexity were insufficient to stratify variations in gait and balance performance. Similar to treatment for children with cerebral palsy,15,16 measures of complexity may also serve as a clinical tool to track changes in muscle coordination during rehabilitation of TBI and possibly identify potential responders to treatment.
Acknowledgements
We would like to thank the staff of the Tactile Communication and Neurorehabilitation Laboratory, Holly Schoenberg, Michael Schmidt, Kristen Rasske, Emily Keuler, Isaac Loegering, and John Zunker for assistance with the gait testing. We also thank Prof. Katherine Steele for her help with the synergy analysis and feedback regarding this manuscript. Development of the TBI training and testing methods used in this study were supported by the Department of Defense [W81XWH-13–1-0081]. SAA’s participation in the EMG collection and analysis was supported by a National Institutes of Health training grant [R25GM08325].
References
- 1.Basford JR, Chou LS, Kaufman KR, et al. An assessment of gait and balance deficits after traumatic brain injury. Archives of Physical Medicine and Rehabilitation. 2003;84:343–349. [DOI] [PubMed] [Google Scholar]
- 2.Kaufman KR, Brey RH, Chou L-S, Rabatin A, Brown AW, Basford JR. Comparison of subjective and objective measurements of balance disorders following traumatic brain injury. Medical Engineering & Physics. 2006;28:234–239. [DOI] [PubMed] [Google Scholar]
- 3.Vasudevan EVL, Glass RN, Packel AT. Effects of Traumatic Brain Injury on Locomotor Adaptation. Journal of Neurologic Physical Therapy. 2014;38:172–182. [DOI] [PubMed] [Google Scholar]
- 4.Degani AM, Santos MM, Leonard CT, et al. The effects of mild traumatic brain injury on postural control. Brain Injury. 2017;31:49–56. [DOI] [PubMed] [Google Scholar]
- 5.Bland DC, Zampieri C, Damiano DL. Effectiveness of physical therapy for improving gait and balance in individuals with traumatic brain injury: a systematic review. Brain injury : [BI]. 2011;25:664–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCain K, Shearin S. A Clinical Framework for Functional Recovery in a Person With Chronic Traumatic Brain Injury. Journal of Neurologic Physical Therapy. 2017;41:173–181. [DOI] [PubMed] [Google Scholar]
- 7.Thurman DJ, Alverson C, Dunn KA, Guerrero J, Sniezek JE. Traumatic brain injury in the United States: A public health perspective. The Journal of head trauma rehabilitation. 1999;14:602–15. [DOI] [PubMed] [Google Scholar]
- 8.Acuña SA, Tyler ME, Danilov YP, Thelen DG. Abnormal muscle activation patterns are associated with chronic gait deficits following traumatic brain injury. Gait & Posture. 2018;62:510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chow JW, Yablon SA, Stokic DS. Coactivation of ankle muscles during stance phase of gait in patients with lower limb hypertonia after acquired brain injury. Clinical Neurophysiology. 2012;123:1599–1605. [DOI] [PubMed] [Google Scholar]
- 10.Chow JW, Yablon SA, Stokic DS. Intrathecal baclofen bolus reduces exaggerated extensor coactivation during pre-swing and early-swing of gait after acquired brain injury. Clinical Neurophysiology. 2017;128:725–733. [DOI] [PubMed] [Google Scholar]
- 11.Steele KM, Rozumalski A, Schwartz MH. Muscle synergies and complexity of neuromuscular control during gait in cerebral palsy. Developmental Medicine & Child Neurology. 2015;57:1176–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez KL, Roemmich RT, Cam B, Fregly BJ, Hass CJ. Persons with Parkinson’s disease exhibit decreased neuromuscular complexity during gait. Clinical Neurophysiology. 2013;124:1390–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark DJ, Ting LH, Zajac FE, Neptune RR, Kautz SA. Merging of Healthy Motor Modules Predicts Reduced Locomotor Performance and Muscle Coordination Complexity Post-Stroke. Journal of Neurophysiology. 2010;103:844–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bowden MG, Clark DJ, Kautz SA. Evaluation of Abnormal Synergy Patterns Poststroke: Relationship of the Fugl-Meyer Assessment to Hemiparetic Locomotion. Neurorehabilitation and Neural Repair. 2010;24:328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shuman BR, Goudriaan M, Desloovere K, Schwartz MH, Steele KM. Muscle synergies demonstrate only minimal changes after treatment in cerebral palsy. Journal of NeuroEngineering and Rehabilitation. 2019;16:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz MH, Rozumalski A, Steele KM. Dynamic motor control is associated with treatment outcomes for children with cerebral palsy. Developmental Medicine and Child Neurology. 2016;58:1139–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pérez-Nombela S, Barroso F, Torricelli D, et al. Modular control of gait after incomplete spinal cord injury: differences between sides. Spinal Cord. 2017;55:79–86. [DOI] [PubMed] [Google Scholar]
- 18.Allen JL, McKay JL, Sawers A, Hackney ME, Ting LH. Increased neuromuscular consistency in gait and balance after partnered, dance-based rehabilitation in Parkinson’s disease. Journal of Neurophysiology. 2017;118:363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Routson RL, Clark DJ, Bowden MG, Kautz SA, Neptune RR. The influence of locomotor rehabilitation on module quality and post-stroke hemiparetic walking performance. Gait and Posture. 2013;38:511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tresch MC, Jarc A. The case for and against muscle synergies. Current Opinion in Neurobiology. 2009;19:601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ting LH, Chiel HJ, Trumbower RD, et al. Neuromechanical Principles Underlying Movement Modularity and Their Implications for Rehabilitation. Neuron. 2015;86:38–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayes HB, Chvatal SA, French MA, Ting LH, Trumbower RD. Neuromuscular constraints on muscle coordination during overground walking in persons with chronic incomplete spinal cord injury. Clinical Neurophysiology. 2014;125:2024–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kautz SA, Bowden MG, Clark DJ, Neptune RR. Comparison of Motor Control Deficits During Treadmill and Overground Walking Poststroke. Neurorehabilitation and Neural Repair. 2011;25:756–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee SJ, Hidler J. Biomechanics of overground vs. treadmill walking in healthy individuals. Journal of Applied Physiology. 2008;104:747–755. [DOI] [PubMed] [Google Scholar]
- 25.Chvatal SA, Ting LH. Voluntary and Reactive Recruitment of Locomotor Muscle Synergies during Perturbed Walking. The Journal of Neuroscience. 2012;32:12237 LP – 12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katz-Leurer M, Rotem H, Keren O, Meyer S. The immediate effect of treadmill walking on step variability in boys with a history of severe traumatic brain injury and typically-developed controls. Dev Neurorehabil. 2010;13:170–174. [DOI] [PubMed] [Google Scholar]
- 27.Williams G, Galna B, Morris ME, Olver J. Spatiotemporal deficits and kinematic classification of gait following a traumatic brain injury: a systematic review. The Journal of head trauma rehabilitation. 2010;25:366–74. [DOI] [PubMed] [Google Scholar]
- 28.Buster T, Burnfield J, Taylor AP, Stergiou N. Lower Extremity Kinematics During Walking and Elliptical Training in Individuals With and Without Traumatic Brain Injury Lower Extremity Kinematics During Walking and Elliptical Training in Individuals With and Without Traumatic Brain Injury. Journal of neurologic physical therapy : JNPT. 2013;37:176–86. [DOI] [PubMed] [Google Scholar]
- 29.Brown TH, Mount J, Rouland BL, Kautz KA, Barnes RM, Kim J. Body Weight-Supported Treadmill Training Versus Conventional Gait Training for People With Chronic Traumatic Brain Injury. The Journal of Head Trauma Rehabilitation. 2005;20. Available at: https://journals.lww.com/headtraumarehab/Fulltext/2005/09000/Body_Weight_Supported_Treadmill_Training_Versus.2.aspx. [DOI] [PubMed] [Google Scholar]
- 30.Wrisley DM, Stephens MJ, Mosley S, Wojnowski A, Duffy J, Burkard R. Learning Effects of Repetitive Administrations of the Sensory Organization Test in Healthy Young Adults. Archives of Physical Medicine and Rehabilitation. 2007;88:1049–1054. [DOI] [PubMed] [Google Scholar]
- 31.Herman T, Inbar-Borovsky N, Brozgol M, Giladi N, Hausdorff JM. The Dynamic Gait Index in healthy older adults: the role of stair climbing, fear of falling and gender. Gait & posture. 2009;29:237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Medley A, Thompson M, French J. Predicting the probability of falls in community dwelling persons with brain injury: a pilot study. Brain injury. 2006;20:1403–8. [DOI] [PubMed] [Google Scholar]
- 33.van Loo MA, Moseley AM, Bosman JM, de Bie RA, Hassett L. Test-re-test reliability of walking speed, step length and step width measurement after traumatic brain injury: a pilot study. Brain injury. 2004;18:1041–1048. [DOI] [PubMed] [Google Scholar]
- 34.Meinert I, Brown N, Alt W. Effect of footwear modifications on oscillations at the Achilles tendon during running on a treadmill and over ground: A cross-sectional study. PLoS ONE. 2016;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grech C, Formosa C, Gatt A. Shock attenuation properties at heel strike: Implications for the clinical management of the cavus foot. Journal of Orthopaedics. 2016;13:148–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. Journal of Electromyography and Kinesiology. 2000;10:361–374. [DOI] [PubMed] [Google Scholar]
- 37.Shuman BR, Goudriaan M, Desloovere K, Schwartz MH, Steele KM. Associations between muscle synergies and treatment outcomes in cerebral palsy are robust across clinical centers. Archives of Physical Medicine and Rehabilitation. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anon. Standards for Reporting EMG data. Journal of Electromyography and Kinesiology. 1999;9:III–IV. [Google Scholar]
- 39.Shuman BR, Schwartz MH, Steele KM. Electromyography Data Processing Impacts Muscle Synergies during Gait for Unimpaired Children and Children with Cerebral Palsy. Frontiers in computational neuroscience. 2017;11:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oliveira AS, Gizzi L, Farina D, Kersting UG. Motor modules of human locomotion: influence of EMG averaging, concatenation, and number of step cycles. Frontiers in Human Neuroscience. 2014;8:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shuman B, Goudriaan M, Bar-On L, Schwartz MH, Desloovere K, Steele KM. Repeatability of muscle synergies within and between days for typically developing children and children with cerebral palsy. Gait and Posture. 2016;45:127–132. [DOI] [PubMed] [Google Scholar]
- 42.de Rugy A, Loeb GE, Carroll TJ. Are muscle synergies useful for neural control? Frontiers in Computational Neuroscience. 2013;7:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Torres-Oviedo G, Macpherson JM, Ting LH. Muscle Synergy Organization Is Robust Across a Variety of Postural Perturbations. Journal of Neurophysiology. 2006;96:1530–1546. [DOI] [PubMed] [Google Scholar]
- 44.Rimini D, Agostini V, Knaflitz M. Intra-Subject Consistency during Locomotion: Similarity in Shared and Subject-Specific Muscle Synergies. Frontiers in Human Neuroscience. 2017;11:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steele KM, Munger ME, Peters KM, Shuman BR, Schwartz MH. Repeatability of electromyography recordings and muscle synergies during gait among children with cerebral palsy. Gait & Posture. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. Series B (Methodological). 1995;57:289–300. [Google Scholar]
- 47.Whittle MW. Whittle’s Gait Analysis. (Levine D, Richards J, Whittle MW, eds.). Elsevier; 2012. Available at: https://www.elsevier.com/books/whittles-gait-analysis/levine/978-0-7020-4265-2. [Google Scholar]
- 48.Pathokinesiology Service & Physical Therapy Dept. RLANRC. Observational gait analysis. Downey, CA: Los Amigos Research and Education Institute, Inc.; 2001. Available at: https://search.library.wisc.edu/catalog/999931728502121. [Google Scholar]
- 49.Cheung VCK, Turolla A, Agostini M, et al. Muscle synergy patterns as physiological markers of motor cortical damage. Proceedings of the National Academy of Sciences. 2012;109:14652–14656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goudriaan M, Shuman BR, Steele KM, et al. Non-neural Muscle Weakness Has Limited Influence on Complexity of Motor Control during Gait. Frontiers in Human Neuroscience. 2018;12:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pickett TC, Radfar-Baublitz LS, McDonald SD, Walker WC, Cifu DX. Objectively assessing balance deficits after TBI: Role of computerized posturography. Journal of rehabilitation research and development. 2007;44:983–990. [DOI] [PubMed] [Google Scholar]
- 52.Lin L-F, Liou T-H, Hu C-J, et al. Balance function and sensory integration after mild traumatic brain injury. Brain Injury. 2015;29:41–46. [DOI] [PubMed] [Google Scholar]
- 53.Ijspeert AJ. Central pattern generators for locomotion control in animals and robots: A review. Neural Networks. 2008;21:642–653. [DOI] [PubMed] [Google Scholar]
- 54.Giszter S, Patil V, Hart C. Primitives, premotor drives, and pattern generation: a combined computational and neuroethological perspective. In: Cisek P, Drew T, Kalaska JFBT-P in BR, eds. Computational Neuroscience: Theoretical Insights into Brain Function.Vol 165. Elsevier; 2007:323–346. [DOI] [PubMed] [Google Scholar]
- 55.Ivanenko YP, Dominici N, Cappellini G, et al. Changes in the Spinal Segmental Motor Output for Stepping during Development from Infant to Adult. The Journal of Neuroscience. 2013;33:3025 LP – 3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saito A, Tomita A, Ando R, Watanabe K, Akima H. Similarity of muscle synergies extracted from the lower limb including the deep muscles between level and uphill treadmill walking. Gait and Posture. 2018;59:134–139. [DOI] [PubMed] [Google Scholar]
- 57.Oliveira AS, Silva PB, Lund ME, Kersting UG, Farina D. Fast changes in direction during human locomotion are executed by impulsive activation of motor modules. Neuroscience. 2013;228:283–293. [DOI] [PubMed] [Google Scholar]
- 58.Austin HM, Balendra N, Langenderfer JE, Ustinova KI. Decomposition of leg movements during overground walking in individuals with traumatic brain injury. Brain Injury. 2018;32:739–746. [DOI] [PubMed] [Google Scholar]
- 59.Williams G, Lai D, Schache A, Morris ME. Classification of Gait Disorders Following Traumatic Brain Injury. Journal of Head Trauma Rehabilitation. 2015;30:E13––E23. [DOI] [PubMed] [Google Scholar]
- 60.Schmitz A, Silder A, Heiderscheit B, Mahoney J, Thelen DG. Differences in lower-extremity muscular activation during walking between healthy older and young adults. Journal of Electromyography and Kinesiology. 2009;19:1085–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.d’Avella A, Ivanenko Y, Lacquaniti F. Muscle synergies in cerebral palsy and variability: challenges and opportunities. Dev Med Child Neurol. 2021. [DOI] [PubMed] [Google Scholar]