Abstract
Background
Household contact investigation is an important strategy to identify individuals with tuberculosis (TB) exposure, infection, and disease, including those who may benefit from tuberculosis preventive therapy (TPT). Data in children exposed to rifampin-resistant TB are limited.
Methods
In preparation for and to inform the feasibility of an interventional trial, household contacts (HHC) of adults with pulmonary rifampin-resistant TB from high TB-burden countries were evaluated in a cross-sectional study. Using interferon gamma release assay (IGRA) and study-specific and 2015 international consensus definitions of intrathoracic TB in children, we evaluated the prevalence and predictors of TB infection and disease in child (<15 years) HHCs.
Results
Of 303 child HHCs, median age (range) 7 years (0–14), 57% (95% confidence interval (CI): 50%–64%) had a positive IGRA result (“TB infected”). TB infection was associated with the index case smoking (p=0.034), being the parent and/or sleeping in the same room (p=0.002), and the child HHC being age ≥5 years and having attended school (p=0.013). Four had study-defined confirmed TB and 9 had probable TB, a prevalence of 4.3% (95% CI: 2.6%–7.1%). Using the international consensus definitions, 4 had confirmed TB and 49 had unconfirmed TB, a prevalence of 17.2% (95% CI: 12.9%–22.4%). Twenty (7%) children had received TPT.
Conclusions
The prevalence of TB infection and disease was high in child HHC exposed to rifampin-resistant TB. Few children had routinely received TPT. High quality evidence is needed to inform strong recommendations for and access to TPT in children exposed to RR-TB.
Keywords: tuberculosis, rifampin resistance, interferon gamma release assay, household contacts, children
Introduction
The World Health Organization (WHO) estimated that in 2019 10 million people developed tuberculosis (TB) disease. Of these, 465,000 individuals had TB resistant to rifampin (RR-TB), 78% of which was also resistant to isoniazid, defined as multidrug-resistant (MDR)-TB. Children accounted for 8% of all TB cases globally.(1) Drug-resistant TB in children remains largely underdiagnosed and underreported and is complex to treat.(2)
The implementation of TB contact investigation in resource-limited settings with high TB burden, even when locally recommended, remains challenging.(3) Given the lack of robust evidence from randomized clinical trials (RCT), the WHO recommends that in selected high-risk HHCs exposed to RR-TB, individualized TPT be implemented “after a careful risk assessment, including the intensity of exposure, certainty of the source of disease, reliable information on the drug resistance pattern of the source and potential adverse drug reaction”(4). High-risk HHCs include children.(5) Observational cohort studies suggest that TPT in RR-TB child contacts may be effective and safe.(6, 7)
The A5300/I2003/PHOENIx Feasibility Study (8, 9) was conducted jointly between the AIDS Clinical Trials Group (ACTG) and the International Maternal, Pediatric, Adolescent AIDS Clinical Trials Network (IMPAACT) to inform the development, feasibility, and implementation of a multi-country interventional trial of TPT using delamanid versus isoniazid in high-risk HHCs of persons with infectious MDR-TB. Children under 15 years of age represented 30% of HHCs enrolled.(8) We characterized TB infection and TB disease in these children reported to have been exposed to RR-TB in the household.
MATERIALS AND METHODS
Study Design and Population
The study design has been reported elsewhere.(8) Briefly, using a cross-sectional design, 16 sites in eight high TB-burden countries enrolled adults with pulmonary RR-TB (hereafter termed “index participants”) and their HHCs. Index participants were adults with bacteriologically-confirmed RR-TB (including MDR-, pre-XDR- and XDR-TB), who had initiated routine RR-TB treatment in the local program in the preceding 6 months. HHCs lived in the same dwelling unit and shared housekeeping arrangements with the index participant and reported exposure within six months prior to the index participant starting RR-TB treatment. Index participants provided written informed consent for their participation and for study personnel to visit their households. Eligible child HHCs and their guardians provided consent and, if age-appropriate, assent, according to local guidelines. Child HHCs, defined here as those under 15 years of age at enrollment, were recruited in six countries (14 sites): Botswana (2), Haiti (2), India (2), Peru (2), South Africa (7), and Thailand (1); sites in Kenya (1) and Brazil (1) did not enroll children.
Study Evaluations
For index participants, we collected data on medical history, chest radiograph (CXR), HIV status, smoking history, and TB microbiological results from the TB program. A sputum sample was also tested for TB microbiology(10). Their household members were enumerated and a household questionnaire completed, which included questions describing the physical characteristics of the dwelling.
For HHCs, demographic information and medical history, including history of previous TB treatment and TPT, AIDS-defining conditions, diabetes, asthma, smoking, alcohol and substance use, relationship and exposure to the index participant, and exposure to individuals with TB outside of the household were collected. All HHCs were evaluated for TB infection and disease. Child HHCs with unknown TB status at study entry were evaluated using symptom screening and CXR. The protocol required collection of a respiratory sample for TB diagnostic testing in all HHC ≤5 years of age. For HHCs >5 years old, respiratory sampling was required if TB was suspected based on the presence of symptoms or CXR findings. HHCs with unknown HIV status were offered HIV testing.
Outcomes
The absence of a gold standard for TB infection presents challenges. Results from a 2018 latent class analysis that overcomes some of these challenges, in more than 10,000 US-born and non-US-born individuals at high risk for TB infection, including participants <5 and ≥5 years of age, suggested that IGRA is preferred over TST due to its higher specificity and similar sensitivity(11). Focusing on children <15 years of age, and including 54 children <2 years of age, Ahmed et al also concluded that IGRA was preferred to the TST in non-US born children (12). For this analysis, TB infection was defined exclusively based on IGRA. The study used locally available QuantiFERON TB Gold or Gold In-Tube (both Qiagen)(13), interpreted per manufacturer’s instructions. While TST was required per protocol, it was not done at all sites, largely due to a global reagent shortage when the study was conducted.
For the study, the diagnosis of TB disease was adjudicated centrally and retrospectively by at least two clinicians provided with available clinical, microbiological, radiological, IGRA, and TST results. Study-specific TB disease definitions used a HHC management (active surveillance) strategy. The study protocol was finalized in July 2015 before publication of the updated international consensus clinical case definitions for classification of intrathoracic TB for diagnostic studies in children in October 2015 (hereafter “NIH 2015 definitions”)(14). TB disease was classified as confirmed (compatible symptoms or CXR and either culture or molecular tests positive for Mycobacterium tuberculosis, M.tb), probable (compatible symptoms and either AFB smear-positive or suggestive CXR), or possible TB (either compatible symptoms, AFB smear-positive, or suggestive CXR).(8) IGRA and TST results were not considered in the study-specific TB diagnosis. Our primary TB disease definition for this analysis included confirmed or probable TB only, with possible TB combined with meeting none of the criteria. We also classified children according to the NIH 2015 definitions(14). We provide results on confirmed, probable, or possible TB in the Supplemental Digital Content 1-8.
Statistical Analysis
Our goal was to estimate population-average responses, therefore we used logistic regression models using generalized estimating equations (GEE) with an exchangeable working correlation to account for clustering within households to estimate the prevalence of TB infection and disease and to evaluate covariates(15). When the estimated working correlation was negative (which only occurred when data were sparse), we substituted an independent working correlation. Robust standard errors were used to estimate 95% confidence intervals (CI). We report nominal two-sided score test p-values as overall tests for each covariate and Wald p-values for pairwise comparisons.
An exploratory approach was taken to create composite covariates based on the correlation between covariates and/or types of variables, (e.g., relationship and sleeping proximity of the index participant to HHC). Multivariable models included HHC age (or a composite variable including age); other candidate covariates were those significant at the 0.25 level in univariable models. Each omitted covariate was separately evaluated by addition to the multivariable models.
RESULTS
Participants and Characteristics
Three hundred five index participants enumerated 1,324 household members, of whom 1,016 HHCs were enrolled and evaluated. One hundred fifty-three households had 304 children <15 years of age (the mean number of children per household was 1, or 2.1 in households that had children). One child was currently on TB treatment at study entry and per protocol requirement had limited evaluations and was excluded from further analysis. Supplementary Digital Content 1 and Supplementary Digital Content 2 (figures) show the number of all HHCs enumerated and the number of child HHCs <15 years old with TB infection and TB disease using study-defined TB disease and NIH 2015 definitions of TB disease, respectively.
Table 1 displays key characteristics of the 303 child HHCs without TB disease at study entry, and their corresponding 152 index participants and households. The median age (range) of child HHCs was 7 years (0–14). Of 114 index participants with sputum smear results, 67% were positive; of 106 with CXR performed, 43% had cavitating disease. Thirty-two index participants had unknown resistance to isoniazid, primarily because it was not tested by the TB program. Additional details on resistance testing can be found elsewhere.(10)
Table 1.
Characteristics of Child Household Contacts (n=303) of RR-TB and their Adult Index Participants and Households (N=152)*
| Characteristics | Index Participants/ Households N=152 |
Household Contacts N=303 |
|---|---|---|
| Individual Characteristics | ||
| Country (Number of Sites): N (%) | ||
| Botswana (1) | 7 (5%) | 18 (6%) |
| Haiti (1) | 6 (4%) | 15 (5%) |
| India (2) | 23 (15%) | 36 (12%) |
| Peru (2) | 39 (26%) | 73 (24%) |
| South Africa (7) | 73 (48%) | 156 (51%) |
| Thailand (1) | 4 (3%) | 5 (2%) |
| Sex: N (%) | ||
| Male | 72 (47%) | 155 (51%) |
| Female | 80 (53%) | 148 (49%) |
| Age (years): median (range), IQR, N (%) | ||
| median (range) | 34 (18, 67) | 7 (0, 14) |
| interquartile range | (24, 41) | (3, 10) |
| <5 | 102 (34%) | |
| 5-9 | 112 (37%) | |
| 10-14 | 89 (29%) | |
| 18-24 | 39 (26%) | |
| 25-34 | 41 (27%) | |
| 35-49 | 51 (34%) | |
| ≥50 | 21 (14%) | |
| Age (years)/Currently or Ever in School: N (%) | N/A | |
| Age <5 | 102 (34%) | |
| Age 5-14, Currently or Ever in School | 187 (62%) | |
| Age 5-14, Never in School | 14 (5%) | |
| Smoking Status: N (%) | ||
| Current | 31 (20%) | 1 (<1%) |
| Previous/Never | 121 (80%) | 302 (>99%) |
| HIV Status: N (%) | ||
| HIV-positive | 57 (38%) | 6 (2%) |
| HIV-negative | 87 (57%) | 180 (59%) |
| Unknown | 8 (5%) | 117 (39%) |
| History of Previous TB before Current Diagnosis (Index Participant) or Previous TB Treatment (HHC): N (%) | ||
| Yes | 77 (51%) | 9 (3%) |
| No/unknown | 75 (49%) | 294 (97%) |
| Duration of Index Participant TB Treatment at Enrollment of Index Participant or HHC (days): median (range), IQR | ||
| median (range) | 56 (1, 190) | 68 (2, 239) |
| interquartile range | (20, 119) | (21, 116) |
| TB Preventive Therapy (HHC): N (%) | ||
| Current | 20 (7%) | |
| Previous | 29 (10%) | |
| Never | 254 (84%) | |
| Any Signs or Symptoms Consistent with TB (HHC): N (%) | N/A | |
| Yes | 69 (23%) | |
| No | 234 (77%) | |
| Cavitation on Chest X-raya: N (%) | ||
| Yes | 46 (30%) | 1 (<1%) |
| No | 60 (39%) | 54 (18%) |
| Unknown | 46 (30%) | 248 (82%) |
| Smear Status (Diagnosis for Index Participant and Current for HHC)a: N (%) | ||
| Positive | 76 (50%) | 2 (1%) |
| Negative | 38 (25%) | 91 (30%) |
| Unknown | 38 (25%) | 210 (69%) |
| Bacteriological Confirmation (Culture or Molecular Test)a: N (%) | ||
| Yes | 152 (100%) | 4 (1%) |
| No | 0 (0%) | 89 (29%) |
| Not Done/No Result | 0 (0%) | 210 (69%) |
| IGRA Result: N (%) | NA | |
| Positive | 160 (53%) | |
| Negative | 119 (39%) | |
| Indeterminate/Borderline | 4 (1%) | |
| Unknown | 20 (7%) | |
| Household Characteristics | ||
| Number of Household Members: median (range), IQR | ||
| median (range) | 6 (2, 22) | |
| interquartile range | (5, 8) | |
| Housing Type: N (%) | ||
| House | 102 (67%) | |
| Apartment/Unit off Open Hall or Courtyard | 10 (7%) | |
| Other | 40 (26%) | |
| Flooring Material: N (%) | ||
| Hardwood/Vinyl or Ceramic Tile/Carpet | 62 (41%) | |
| Planks/Cement/Dirt/Other | 89 (59%) | |
| Unknown | 1 (1%) | |
| Exterior Wall Material: N (%) | ||
| Brick or Cinderblock/Stone with Mortar | 115 (76%) | |
| Other | 36 (24%) | |
| Unknown | 1 (<1%) | |
| Any Smoking in Household: N (%) | ||
| Yes | 58 (38%) | |
| No | 92 (61%) | |
| Unknown | 2 (1%) | |
| Relationship of Index Participant to Child HHC and TB Exposure Characteristics | ||
| Relationship of IP to HHC: N (%) | ||
| Mother | 58 (19%) | |
| Father | 39 (13%) | |
| Other | 206 (68%) | |
| Sleeping Proximity of IP to HHC: N (%) | ||
| Same Room, Same Bed | 41 (14%) | |
| Same Room, Different Bed | 82 (27%) | |
| Different Room | 180 (59%) | |
| Relationship/Sleeping Proximity of IP with HHC: N (%) | ||
| Mother/Father, Same Room | 64 (21%) | |
| Mother/Father, Different Room | 33 (11%) | |
| Other Relationship, Same Room | 59 (19%) | |
| Other Relationship, Different Room | 147 (49%) | |
Percentages do not always total to 100% due to rounding.
Abbreviations and definitions: IP, index participant; HHC, household contact; N, number; IQR, interquartile range (25th and 75th percentiles); range (minimum and maximum); NA, not available.
For index participants, the AFB sputum smear and TB diagnostic testing was done at the time of TB diagnosis by routine the TB program prior to study entry. For HHCs without a current diagnosis of TB prior to study entry the protocol requirement for specimen collection for smear microscopy and TB diagnostic testing depended on their age: for those age > 5 years, a specimen was required if the HHC had symptoms consistent with TB or an abnormal chest X-ray, and for HHCs age ≤ 5 years, a specimen was required if disease status was unknown. Despite this, 74 (60%) of children age ≤ 5 years did not have a specimen collected.
The index participant was the parent for 32% of child HHCs. When the index participant was the child HHC’s parent, 66% shared the same room; whereas when the index participant was not the parent, 29% shared the same room. The median duration (range) the index participant had received MDR-TB treatment at the time of HHC enrollment was 68 days (2–239). Two percent of child HHCs were known to be living with HIV. A respiratory sample was collected for TB microbiology for 32% of child HHCs, 40% of children ≤5 years of age and 26% of children >5 years of age. The proportion of expectorated sputum samples increased by age. Gastric aspirates and induced sputum were 73% and 2% of samples, respectively, in children < 5 years of age; 8% and 14% of samples respectively, in children 5-9 years of age; and 0% and 4% in children 10-14 years of age. All other specimens were expectorated sputum.
Participant characteristics varied by country and site. Index participants’ HIV status varied from under 10% positive in Peruvian and Indian sites to over 50% positive from most sites in southern Africa. Timing of enrollment of index participants relative to their initiation of RR-TB treatment at sites varied from a median of 1 to over 17 weeks; HHC characteristics also varied by country and site (see Tables, Supplementary Digital Content 3 and Supplementary Digital Content 4).
Prevalence and Predictors of TB Infection
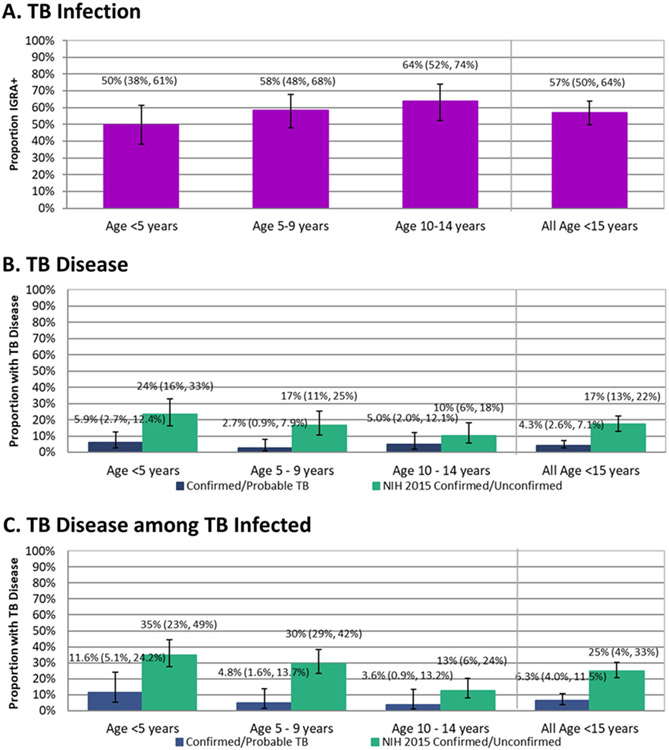
One hundred sixty [57% (95% CI: 50%–64%)] child HHCs had TB infection as defined by IGRA. TST results at the 11 sites that performed both tests indicated that lack of TST as part of the definition of TB infection may have potentially underestimated TB infection prevalence by approximately 12% (see table, Supplementary Digital Content 5). The proportion TB infected in 5-year age increments was 50%, 58%, and 64% for those under 5, 5 to 9, and 10 to 14 years, respectively (any difference p=0.10, Figure 1A).
Figure 1.
TB Infection and TB Disease Prevalence in Child HHCs Overall and by 5 Year Age Groupings*
Abbreviations: HHC, household contact; IGRA, interferon gamma release assay; TB, tuberculosis
*TB infection is based on interferon gamma release assay using QuantiFERON TB Gold or QuantiFERON Gold In-Tube. TB disease was adjudicated by a study outcomes review group. Panel A TB infection includes N=279 HHCs age <15 years with determinate IGRA results, Panel B N=303 HHCs age <15 years, and Panel C N=160 HHCs age <15 years with IGRA-positive results. Proportions were estimated by logistic regression using generalized estimating equations and confidence intervals are based on robust standard error. The vertical bars are 95% confidence intervals.
Table 2 shows results of selected univariable models and a multivariable model for TB infection. Univariable models displayed include HHC age and sex and covariates included in the multivariable models. Multivariable modeling results showed that the prevalence of TB infection varied (non-monotonely) by the age of the adult index participant (p=0.049) and was highest when the index participant was under 25 years old. The composite variable of HHC age and school attendance was significantly associated with TB infection (p=0.013); HHCs who were school age (age≥5 year) and having ever attended school were more likely to be TB infected than those <5 years of age, (aOR=2.4, 95% CI: 1.4–4.2, p=0.003 for the pairwise comparison) and the aOR comparing those ever-attended school versus never attended school among school age was 2.6 (95% CI: 0.9–7.2) (p=0.07). The prevalence of TB infection varied by the relationship of the index participant to the child HHC and their sleeping proximity (p=0.002); when the index participant was a parent and slept in the same room the TB infection prevalence was highest and lowest when the index participant was not the parent and slept in a different room. If the index participant currently smoked, the child HHC was more likely to have TB infection (aOR=2.3 (95% CI: 1.02–5.0)) compared to when the index participant never or previously smoked (p=0.034). We also fit a multivariable model substituting a composite smoking variable that delineated smoking in the index participant from other household members (not shown); only smoking in the index participant was associated with higher prevalence of TB infection compared to when there were no smokers in the household. HIV infection in the index participant was not associated with prevalence of TB infection in child HHCs (p=0.63).
Table 2.
Logistic Regression Models for TB Infection (n/N=160/279) in Child Household Contacts of Adults with Pulmonary RR-TB
| n/N (%) 160/279 (57%) |
Univariable |
Multivariable |
||||
|---|---|---|---|---|---|---|
| Characteristic | Odds Ratio (95% CI) |
P | Odds Ratio (95% CI) |
P | ||
| Household Contact Characteristics | ||||||
| HHC Age | 0.10 | |||||
| < 5 years | 43/88 (49%) | 0.5 (0.3, 0.9) | 0.03 | |||
| 5 - 9 years | 62/104 (60%) | 0.9 (0.6, 1.5) | 0.77 | |||
| 10 - 14 years | 55/87 (63%) | 1.0 ref | ||||
| HHC Sex | 0.35 | |||||
| Male | 88/145 (61%) | 1.3 (0.8, 2.0) | ||||
| Female | 72/134 (54%) | 1.0 ref | ||||
| HHC Age and School Attendance | 0.033 | 0.013 | ||||
| Age <5 years | 43/88 (49%) | 1.0 ref | 1.0 ref | |||
| Age 5 - 14 years, Never in School | 6/14 (43%) | 0.9 (0.3, 2.3) | 0.78 | 0.9 (0.3, 2.6) | 0.89 | |
| Age 5 - 14 years, Currently or Ever in School | 111/177 (63%) | 2.0 (1.2, 3.5) | 0.010 | 2.4 (1.4, 4.2) | 0.003 | |
| Previous TB Treatment | 0.77 | |||||
| Yes | 5/9 (56%) | 1.2 (0.3, 5.0) | ||||
| No/Unknown | 155/270 (57%) | 1.0 ref | ||||
| Index Participant Characteristics a | ||||||
| Index Participant Age | 0.09 | 0.049 | ||||
| <25 years | 46/78 (59%) | 1.0 ref | 1.0 ref | |||
| 25 - 34 years | 33/74 (45%) | 0.5 (0.2, 1.0) | 0.06 | 0.3 ( 0.1, 0.8) | 0.010 | |
| 35 - 49 years | 63/95 (66%) | 1.2 (0.6, 2.7) | 0.58 | 0.8 ( 0.3, 1.9) | 0.62 | |
| 50+ years | 18/32 (56%) | 0.7 (0.3, 1.8) | 0.69 | 0.6 ( 0.2, 1.8) | 0.35 | |
| Index Participant Sex | 0.74 | |||||
| Male | 70/125 (56%) | 0.9 (0.5, 1.6) | ||||
| Female | 90/154 (58%) | 1.0 ref | ||||
| Index Participant Smoking Status | 0.07 | 0.034 | ||||
| Current | 41/58 (71%) | 1.9 (0.95, 3.9) | 2.3 (1.02, 5.0) | |||
| Previous/Never | 119/221 (54%) | 1.0 ref | 1.0 ref | |||
| Exposure Characteristics | ||||||
| Index Participant-HHC Relationship and Sleeping Arrangement | 0.024 | 0.002 | ||||
| Mother/Father, Slept in Same Room | 41/59 (69%) | 2.8 (1.5, 5.5) | 0.002 | 5.1 (2.4, 11) | <0.001 | |
| Mother/Father, Slept in Different Room | 20/32 (63%) | 2.2 (1.02, 4.9) | 0.045 | 2.4 (1.1, 5.6) | 0.037 | |
| Other Relationship, Slept in Same Room | 35/57 (61%) | 2.1 (1.0, 4.3) | 0.05 | 2.6 (1.1, 5.7) | 0.023 | |
| Other Relationship, Slept in Different Room | 64/131 (49%) | 1.0 ref | 1.0 ref | |||
| Index Participant-HHC Relationship | 0.009 | |||||
| Mother/Father | 61/91 (67%) | 2.0 (1.2, 3.4) | ||||
| Other | 99/188 (53%) | 1.0 ref | ||||
Abbreviations: HHC, household contact; CI, confidence interval; P, overall p-value from score test, and for categorical variables with more than 2 categories Wald tests for pairwise comparisons to the reference category; n/N (%), number of HHCs positive on outcome/total with results (crude percentages that do not account for clustering within household); OR, odds ratio; ref, reference group.
Odds ratios, univariable or adjusted from multivariable models, are based on logistic regression using a generalized estimating equation approach to account for correlation among HHCs within a household.
Characteristics of index participants according to TB infection status of their HHCs. An index participant with more than one HHC will contribute more than once to the sample counts.
Prevalence and Predictors of TB Disease
Of the 303 child HHCs who did not have TB at study entry, 4 (1.3%) were adjudicated to have confirmed TB and 9 (3.0%) to have probable TB according to study definitions. All HHCs with study-defined confirmed and probable TB were classified by the NIH 2015 definitions as having confirmed and unconfirmed TB, respectively; an additional 40 HHCs with study-defined possible TB were classified as unconfirmed TB (see Table, Supplementary Digital Content 6). Using study definitions, we estimated that 4.3% (95% CI: 2.6%–7.1%) of child HHCs had prevalent TB disease. Two child HHCs with TB disease had negative IGRA; one of whom had bacteriologically confirmed TB; 1.7% (95% CI: 0.5%–6.0%) had TB disease among child HHCs with negative IGRA test results and 6.3% (95% CI: 3.6%–10.8%) had TB disease among those with positive IGRA results (p=0.028). TB disease prevalence among the 303 child HHCs without a diagnosis of TB at study entry is shown in 5-year age increments in Figure 1B. Using the more inclusive NIH 2015 definitions, the prevalence of TB disease was 17.2% (95% CI: 12.9%–22.4%).
The prevalence of study-defined TB disease varied non-monotonely with the age of child HHC but was not statistically significant (p=0.51). Using the NIH 2015 definitions, the prevalence of TB disease varied significantly by HHC age (p=0.040 for any difference). We also estimated the proportion with TB disease by HHC age among those with a positive IGRA result (see Figure 1C), which showed a non-statistically significant increase with younger age (p=0.12). Among children with a positive IGRA result, the proportion with TB using the NIH 2015 definitions increased with younger age (p=0.007). Supplementary Digital Content 7 (figure) shows TB Disease prevalence for confirmed, probable, or possible TB.
Table 3 shows results of selected univariable logistic regression models and a multivariable model for the NIH 2015 TB outcome. Because of the limited number of child HHCs with confirmed or probable TB disease, only univariable models of study-defined TB disease are shown. TB disease was less likely in child HHCs if the index participant was male (OR=0.2 (95% CI: 0.04–0.9), p=0.012; Table 3). Smoking in the index participant was not associated with the child HHC having TB disease (p=0.86). Using the NIH 2015 definitions, HHC age, previous TB treatment of the HHC, and male HHC sex, were associated with child HHC having confirmed or unconfirmed TB. HHC age and sex were significant in the multivariable model using the NIH 2015 definitions. Supplementary Digital Content 8 (table) is a model for the study-defined outcome of confirmed, probable, or possible TB.
Table 3.
Logistic Regression Models for TB Disease in Child Household Contacts of Adults with Pulmonary RR-TB
| Characteristic | Confirmed/Probable TB Disease |
NIH 2015 Confirmed/Unconfirmed TB Disease |
||||||
|---|---|---|---|---|---|---|---|---|
| n/N (%) 13/303 (4%) |
Univariable |
n/N (%) 53/303 (17%) |
Univariable |
Multivariable |
||||
| Odds Ratio (95% CI) |
P | Odds Ratio (95% CI) |
P | Odds Ratio (95% CI) |
P | |||
| Household Contact Characteristics | ||||||||
| HHC Age | 0.51 | 0.040 | 0.041 | |||||
| < 5 years | 6/102 (6%) | 1.3 (0.4, 4.8) | 0.67 | 24/102 (24%) | 2.6 (1.2, 5.7) | 0.014 | 2.7 (1.2, 5.9) | 0.012 |
| 5 - 9 years | 3/112 (3%) | 0.6 (0.1, 2.7) | 0.50 | 20/112 (18%) | 1.9 (0.8, 4.2) | 0.13 | 1.9 (0.8, 4.3) | 0.14 |
| 10 - 14 years | 4/89 (4%) | 1.0 ref | 9/89 (10%) | 1.0 ref | – | 1.0 ref | – | |
| HHC Sex | 0.12 | 0.02 | 0.021 | |||||
| Male | 9/155 (6%) | 2.5 (0.7, 8.5) | 35/155 (23%) | 2.0 (1.1, 3.7) | 2.1 (1.1, 3.9) | |||
| Female | 4/148 (3%) | 1.0 ref | 18/148 (12%) | 1.0 ref | 1.0 ref | |||
| HHC Age and School Attendance | 0.52 | 0.14 | ||||||
| Age <5 years | 6/102 (6%) | 1.0 ref | 24/102 (24%) | 1.0 ref | – | |||
| Age 5 - 14 years, Never in School | 1/14 (7%) | 1.5 (0.2, 10) | 0.71 | 1/14 (7%) | 0.3 (0.3, 2.5) | 0.24 | ||
| Age 5 - 14 years, Currently or Ever in School | 6/187 (3%) | 0.5 (0.2, 1.7) | 0.29 | 28/187 (15%) | 0.6 (0.3, 1.1) | 0.09 | ||
| Previous TB Treatment | ND | 0.05 | ||||||
| Yes | 0/9 (0%) | 5/9 (56%) | 6.6 (1.9, 23.8) | |||||
| No/Unknown | 13/294 (4%) | 48/294 (16%) | 1.0 ref | |||||
| IGRA Status a | 0.028 | <0.001 | ||||||
| Positive | 10/160 (6%) | 3.6 (0.7, 18) | 41/160 (26%) | 3.8 (1.8, 7.7) | 0.25 | |||
| Indeterminate/Borderline | 0/4 (0%) | – | 1/4 (25%) | 3.6 (0.4, 32.4) | ||||
| Negative | 2/119 (2%) | 1.0 ref | 10/119 (8%) | 1.0 ref | ||||
| Index Participant Characteristics b | ||||||||
| Index Participant Age | 0.91 | 0.41 | ||||||
| <25 years | 3/86 (3%) | 1.0 ref | 10/86 (12%) | 1.0 ref | – | |||
| 25 - 34 years | 4/81 (5%) | 1.4 (0.3, 6.0) | 0.66 | 17/81 (21%) | 1.9 (0.8, 4.9) | 0.16 | ||
| 35 - 49 years | 4/104 (4%) | 1.1 (0.3, 4.8) | 0.85 | 20/104 (19%) | 1.8 (0.8, 4.1) | 0.15 | ||
| 50+ years | 2/32 (6%) | 1.9 (0.4, 10) | 0.44 | 6/32 (19%) | 1.6 (0.5, 5.4) | 0.46 | ||
| Index Participant Sex | 0.012 | 0.41 | ||||||
| Male | 2/137 (1%) | 0.2 (0.04, 0.9) | 20/137 (15%) | 0.7 (0.3, 1.4) | ||||
| Female | 11/166 (7%) | 1.0 ref | 33/166 (20%) | 1.0 ref | ||||
| Index Participant Smoking Status | 0.86 | 0.26 | ||||||
| Current | 3/65 (5%) | 1.1 (0.3, 3.8) | 15/65 (23%) | 1.5 (0.8, 3.1) | ||||
| Previous/Never | 10/238 (4%) | 1.0 ref | 38/238 (16%) | 1.0 ref | ||||
| Exposure Characteristics | ||||||||
| IP-HHC Relationship and Sleeping Arrangement | ND | 0.37 | ||||||
| Mother/Father, Slept in Same Room | 7/64 (11%) | 18/64 (28%) | 2.1 (0.9, 4.5) | 0.07 | ||||
| Mother/Father, Slept in Different Room | 0/33 (0%) | 6/33 (18%) | 1.1 (0.4, 3.5) | 0.81 | ||||
| Other Relationship, Slept in Same Room | 0/59 (0%) | 8/59 (14%) | 0.9 (0.3, 2.6) | 0.83 | ||||
| Other Relationship, Slept in Different Room | 6/147 (4%) | 21/147 (14%) | 1.0 ref | |||||
| Index Participant-HHC Relationship | 0.12 | 0.13 | 0.12 | |||||
| Mother/Father | 7/97 (7%) | 2.6 (0.9, 7.5) | 24/97 (25%) | 1.8 (0.9, 3.4) | 1.8 (0.9, 3.5) | |||
| Other | 6/206 (3%) | 1.0 ref | 29/206 (14%) | 1.0 ref | 1.0 ref | |||
Abbreviations: IP, index participant; HHC, household contact; CI, confidence interval; P, overall p-value from score test, and for categorical variables with more than 2 categories Wald tests for pairwise comparisons to the reference category; ND, not done; n/N (%), number of HHCs positive on outcome/total with results (crude percentages that do not account for clustering within household); OR, odds ratio; ref, reference group.
Logistic regression use a generalized estimating equations for estimation. Univariable models are not adjusted for other covariates. Multivariable models are adjusted for covariates shown.
Two hundred eighty-three child HHCs had IGRA test results. Of the 20 without a result, 15 did not have specimen obtained for IGRA testing (7 declined the blood draw, 8 attempted without success) and 5 had specimen collection but no result due to insufficient sample or sample rejection. Because no child HHC with indeterminate/borderline results had confirmed/probable TB, the univariable model evaluating IGRA status for the confirmed/probable TB outcome used 279 HHCs with determinate IGRA results (i.e., positive or negative) only. IGRA status was not a candidate for the multivariable models.
Characteristics of index participants according to TB disease status of their HHCs. An index case with more than one HHC will contribute more than once to the sample counts.
Tuberculosis Preventive Therapy
Few (20/303, 7%) child HHCs were receiving TPT at the time of enrollment. Use of TPT was heterogeneous by country and was more likely if the HHC was enrolled at South African or Indian sites. The TPT regimen was INH alone for 8, and INH, ethambutol, and a fluoroquinolone for 12 children. TPT was more likely if the HHC was under 5 years of age (17 of 102 vs. 3 of 201 5–14 years of age; p<0.001). One child receiving TPT had probable TB and two additional had unconfirmed TB (NIH 2015) (three total). All eligible children were referred for TPT based on local guidelines.
DISCUSSION
Across diverse sites in Africa, Asia, South America, and Haiti using immunological, radiological, clinical, and microbiological evaluations across all sites, we studied both TB infection and TB disease in child HHCs of adults with pulmonary RR-TB. We aimed to characterize TB infection and TB disease in children living with individuals with infectious RR-TB who have been shown to be at high risk of disease progression once infected(16).
The prevalence of TB infection and TB disease in our study was high in child HHCs exposed to RR-TB, with 57% having TB infection, and 4.3% having TB disease using study definitions and 17% using the NIH 2015 classification of intrathoracic TB in children.
We identified factors associated with TB infection in child HHCs, including smoking by the index participant with RR-TB, the child being ≥5 years of age and having attended school, and having greater degree of exposure to the index participant. Smoking in the index participant could have led to more frequent or stronger coughing or greater extent of disease in the index participant and therefore greater exposure of HHCs to infectious M.tb aerosols.
School attendance was independently associated with TB infection. This result is consistent with schools being a potential additional point of TB transmission outside the home for children or with accumulating M.tb exposure with age.
Our finding that shared sleeping space and a parent-child relationship between the index participant and child was associated with TB infection is consistent with other studies for (presumed) drug-susceptible TB(17-21). The fact that our results align with the findings from HHC studies of drug-susceptible TB is reassuring considering emerging evidence supporting differences in virulence of drug-resistant versus drug-susceptible mycobacterial strains(22).
Children represented 30% of contacts exposed to RR-TB in the household. A third of these children were highly exposed, because the index participant was a parent. In the context of DS-TB, the risk of TB progression is high in young children and may occur acutely, with TPT providing excellent protection against developing disease(23). TPT may also be useful for preventing RR-TB, and WHO currently conditionally recommends (based on very low quality of evidence) that preventive treatment for high-risk RR-TB contacts may be considered and recommends “[s]trict clinical observation and close monitoring for the development of active TB disease for at least 2 years, regardless of the provision of preventive treatment”(5). The high prevalence of TB infection and disease and high level of exposure support the potential value of these recommendations.
Limitations
Limitations of our study include its cross-sectional design. TB infection and disease may be a result of exposure to TB beyond the household. Because IGRA had imperfect sensitivity and specificity and pediatric TB is challenging to diagnose, our estimates of prevalence of TB infection and TB disease are likely underestimated. The findings of higher TB infection prevalence with age and having attended school are consistent with accumulated exposure outside of the household and therefore our TB infection outcome might represent a combination of primary infection and older (remote) infection.
We found substantial variation by site and country. This may reflect differences in risk factors, including HIV prevalence, and delays in household contact investigation of TB cases.
The definitions used for TB disease substantially affected the prevalence estimate of disease and its predictors and our ability to detect differences. The latter underscores the need for improved TB diagnostic strategies for children and suggests caution in interpreting results.
Summary
The high prevalence of TB infection and TB disease in child HHCs highlights the importance of rapid and careful investigation of children exposed to RR-TB to identify those at risk of TB disease and provide prompt treatment for those with disease. High quality evidence is needed to inform strong, unreserved recommendations for and access to TPT in children exposed to RR-TB across the age spectrum.
Supplementary Material
Acknowledgements
The authors thank the study participants, site community advisory boards, local TB program staff; Rohan Hazra, clinical representative; Roxana Rustomjee, clinical representative; Richard E Chaisson, investigator; Kimberly Scarsi, investigator; Sharon Nachman, investigator; Mark Harrington, investigator; Savita Kanade, field representative; Janet Nicotera, field representative; Barbara Heckman, data manager; Lynne Jones, data manager; Patricia Anthony, laboratory technologist; Christopher Lane, laboratory technologist; Ujwala A Kadam, community scientific subcommittee representative; Ronald Ssenyonga, community scientific subcommittee representative; Lara Hosey, clinical trials specialist; Akbar Shahkolahi, international site specialist; and Laura Hovind, laboratory data manager, for their contributions.
Funding
This work was supported by the National Institute of Allergy and Infectious Diseases with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health of the National Institutes of Health [UM1AI068634, UM1AI068636, UM1AI106701, UM1AI068616, UM1AI068632, and UM1AI106716]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Global Tuberculosis Report 2020. World Health Organization; 2020. [Google Scholar]
- 2.Dodd PJ, Sismanidis C, Seddon JA. Global burden of drug-resistant tuberculosis in children: a mathematical modelling study. Lancet Infect Dis. 2016;16:1193–1201. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Recommendations for Investigating Contacts of Persons with Infectious Tuberculosis in Low- and Middle-Income Countries. World Health Organization; 2012. [PubMed] [Google Scholar]
- 4.WHO consolidated guidelines on tuberculosis: tuberculosis preventive treatment: Module 1: prevention. Geneva: 2020. [PubMed] [Google Scholar]
- 5.World Health Organization. Latent tuberculosis infection: Updated and consoliated guidelines for programmatic management. 2018. [PubMed] [Google Scholar]
- 6.Seddon JA, Hesseling AC, Finlayson H, et al. Preventive therapy for child contacts of multidrug-resistant tuberculosis: a prospective cohort study. Clin Infect Dis. 2013;57:1676–1684. [DOI] [PubMed] [Google Scholar]
- 7.Schaaf HS, Gie RP, Kennedy M, Beyers N, Hesseling PB, Donald PR. Evaluation of young children in contact with adult multidrug-resistant pulmonary tuberculosis: a 30-month follow-up. Pediatrics. 2002;109:765–771. [DOI] [PubMed] [Google Scholar]
- 8.Gupta A, Swindells S, Kim S, et al. Feasibility of Identifying Household Contacts of Rifampin-and Multidrug-resistant Tuberculosis Cases at High Risk of Progression to Tuberculosis Disease. Clin Infect Dis. 2020;70:425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swindells S, Gupta A, Kim S, et al. Resource utilization for multidrug-resistant tuberculosis household contact investigations (A5300/I2003). Int J Tuberc Lung Dis. 2018;22:1016–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demers AM, Kim S, McCallum S, et al. Drug susceptibility patterns of Mycobacterium tuberculosis from adults with multidrug-resistant tuberculosis and implications for a household contact preventive therapy trial. BMC Infect Dis. 2021;21:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stout JE, Wu Y, Ho CS, et al. Evaluating latent tuberculosis infection diagnostics using latent class analysis. Thorax. 2018;73:1062–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmed A, Feng PI, Gaensbauer JT, et al. Interferon-gamma Release Assays in Children <15 Years of Age. Pediatrics. 2020;145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.QuantiFERON-TB Gold Plus (QFT®) [package insert]. Germantown M, USA: Qiagen. https://www.quantiferon.com/us/products/quantiferon-tb-gold/package-inserts/. [Google Scholar]
- 14.Graham SM, Cuevas LE, Jean-Philippe P, et al. Clinical Case Definitions for Classification of Intrathoracic Tuberculosis in Children: An Update. Clin Infect Dis. 2015;61 Suppl 3:S179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis. Hoboken, N.J.: Wiley-Interscience; 2004. [Google Scholar]
- 16.Martinez L, Cords O, Horsburgh CR, Andrews JR, Pediatric TBCSC. The risk of tuberculosis in children after close exposure: a systematic review and individual-participant meta-analysis. Lancet. 2020;395:973–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mandalakas AM, Kirchner HL, Lombard C, et al. Well-quantified tuberculosis exposure is a reliable surrogate measure of tuberculosis infection. Int J Tuberc Lung Dis. 2012;16:1033–1039. [DOI] [PubMed] [Google Scholar]
- 18.Rathi SK, Akhtar S, Rahbar MH, Azam SI. Prevalence and risk factors associated with tuberculin skin test positivity among household contacts of smear-positive pulmonary tuberculosis cases in Umerkot, Pakistan. Int J Tuberc Lung Dis. 2002;6:851–857. [PubMed] [Google Scholar]
- 19.Acuña-Villaorduña C, Jones-López EC, Fregona G, et al. Intensity of exposure to pulmonary tuberculosis determines risk of tuberculosis infection and disease. Eur Respir J. 2018;51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bates MN, Khalakdina A, Pai M, Chang L, Lessa F, Smith KR. Risk of tuberculosis from exposure to tobacco smoke: a systematic review and meta-analysis. Arch Intern Med. 2007;167:335–342. [DOI] [PubMed] [Google Scholar]
- 21.Lienhardt C, Fielding K, Sillah J, et al. Risk factors for tuberculosis infection in sub-Saharan Africa: a contact study in The Gambia. Am J Respir Crit Care Med. 2003;168:448–455. [DOI] [PubMed] [Google Scholar]
- 22.Golla V, Snow K, Mandalakas AM, et al. The impact of drug resistance on the risk of tuberculosis infection and disease in child household contacts: a cross sectional study. BMC Infect Dis. 2017;17:593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marais BJ, Ayles H, Graham SM, Godfrey-Faussett P. Screening and preventive therapy for tuberculosis. Clin Chest Med. 2009;30:827–846, x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.