Abstract
Background:
Neurodevelopmental impairment is common in children with congenital heart disease (CHD), yet postnatal variables explain only 30% of the variance in outcomes. To explore whether the antecedents for neurodevelopmental disabilities might begin in utero, we analyzed whether fetal brain volume predicted subsequent neurodevelopmental outcome in children with CHD.
Methods:
Fetuses with isolated CHD and sociodemographically comparable healthy control fetuses underwent fetal brain MRI and 2-year neurodevelopmental evaluation with the Bayley Scales of Infant and Toddler Development (Bayley-III) and the Adaptive Behavior Assessment System (ABAS-3). Hierarchical regression evaluated potential predictors of Bayley-III and ABAS-3 outcomes in the CHD group, including fetal total brain volume adjusted for gestational age and sex, sociodemographic characteristics, birth parameters, and medical history.
Results:
The CHD group (n=52) had lower Bayley-III cognitive, language, and motor scores than the control group (n=26), but fetal brain volumes were similar. Within the CHD group, larger fetal total brain volume correlated with higher Bayley-III cognitive, language, and motor scores, and ABAS-3 adaptive functioning scores (r=0.32–0.47; all P<0.05), but not in the control group. Fetal brain volume predicted 10–21% of the variance in neurodevelopmental outcome measures in univariate analyses. Multivariable models that also included social class and postnatal factors explained 18–45% of the variance in outcome, depending on developmental domain. Moreover, in final multivariable models, fetal brain volume was the most consistent predictor of neurodevelopmental outcome across domains.
Conclusions:
Small fetal brain volume is a strong independent predictor of 2-year neurodevelopmental outcomes and may be an important imaging biomarker of future neurodevelopmental risk in CHD. Future studies are needed to support this hypothesis. Our findings support inclusion of fetal brain volume in risk stratification models and as a possible outcome in fetal neuroprotective intervention studies.
Keywords: Fetal, Brain, Imaging, Magnetic Resonance Imaging (MRI), Congenital Heart Disease, Neurodevelopment
INTRODUCTION
Neurodevelopmental impairment is common in children with congenital heart disease (CHD). Young children with CHD are at greater risk for impaired cognitive, language, or motor development, whereas school-aged children often present with deficits in executive functioning, attention, visual-spatial skills, and social cognition.1–5 Given the high prevalence and impact of developmental disabilities, in 2012 the American Heart Association issued a statement recommending neurodevelopmental evaluation of all children who have undergone open heart surgery in infancy.6
Early identification of children with CHD at highest risk of neurodevelopmental impairment may improve outcome.7–9 However, predictive models from large prospective cohorts with comprehensive data collection, including the Boston Circulatory Arrest Trial and Single Ventricle Reconstruction Trial, have shown that sociodemographic, genetic, and medical factors measured after birth predict only 27–30% of the variance in outcome.1, 2, 10 Moreover, intervention studies concentrating on changing surgical methods have led to only modest improvements in clinical outcome.1
Magnetic resonance imaging (MRI) studies of neonates and fetuses with CHD indicate altered brain development before cardiac surgery11, 12 and small brain volumes in utero.13–16 Since postnatal neuroimaging abnormalities have been associated with adverse neurodevelopmental outcome,17, 18 it is possible that abnormal prenatal brain MRI findings reflecting disrupted fetal brain development may also relate to subsequent neurodevelopment. Establishing an association between fetal brain development and neurodevelopmental outcome would provide initial evidence for fetal brain disturbances as a mechanism of neurodevelopmental impairment.
We performed the first longitudinal study investigating the relationship between fetal brain MRI and postnatal neurodevelopment in CHD. In this hypothesis-generating study, we prospectively enrolled fetuses with and without CHD to evaluate the association between fetal brain volumes and 2-year neurodevelopment. We hypothesized that fetal brain volumes would explain a significant portion of the variance in neurodevelopmental outcome in the CHD group.
METHODS
Study Design and Participants
We analyzed data from a prospective, longitudinal fetal brain MRI and neurodevelopment study. Eligibility criteria and methods for the fetal MRI portion were previously published.15 Briefly, fetuses with isolated CHD and healthy control fetuses with a family history of CHD participated. Inclusion criteria for enrollment were maternal age during pregnancy of 18–45 years and fetal gestational age of 18–30 weeks. Exclusion criteria encompassed both maternal and fetal factors. Maternal exclusion criteria were multiple gestation pregnancy, maternal CHD, MRI contraindication, or clinician deemed inappropriate (e.g., considering termination). Fetal exclusion criteria were trivial/mild CHD as defined by Hoffman and colleagues,19 extracardiac anomaly, brain malformation, known genetic abnormality, or clinically detected congenital infection. To be eligible for the present neurodevelopment study, subjects also had to have available data from at least one fetal brain MRI (i.e., a fetus with only motion corrupted scan(s) was not eligible), and the child must have undergone either in-person or questionnaire-based neurodevelopmental assessment. Participants were excluded from the present analysis if they were diagnosed with a genetic abnormality after birth prior to their neurodevelopmental evaluation. Enrollment in the present study ended in March 2020, due to safety concerns and the unknown impact of personal protective equipment on test interpretation in the setting of COVID-19. The Boston Children’s Hospital Institutional Review Board approved the study, and parents provided written informed consent. The data, analytic methods, and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure from the corresponding author upon request.
MRI Acquisition and Processing
While the longitudinal study included multiple fetal brain MRI, for the present study, we analyzed the first fetal brain MRI from which usable data were obtained. We acquired multi-planar repeated T2-weighted single shot fast spin echo sequences on a 3-Tesla Siemens MRI scanner and processed images with our in-house built software for super-resolution volume reconstruction and semi-automated atlas-based segmentation to calculate regional and total brain volumes; details are previously described (Figure 1).15, 20, 21 Total brain volume included all parenchymal brain tissue and excluded cerebrospinal fluid.15 MRIs were reviewed for incidental findings.
Figure 1.
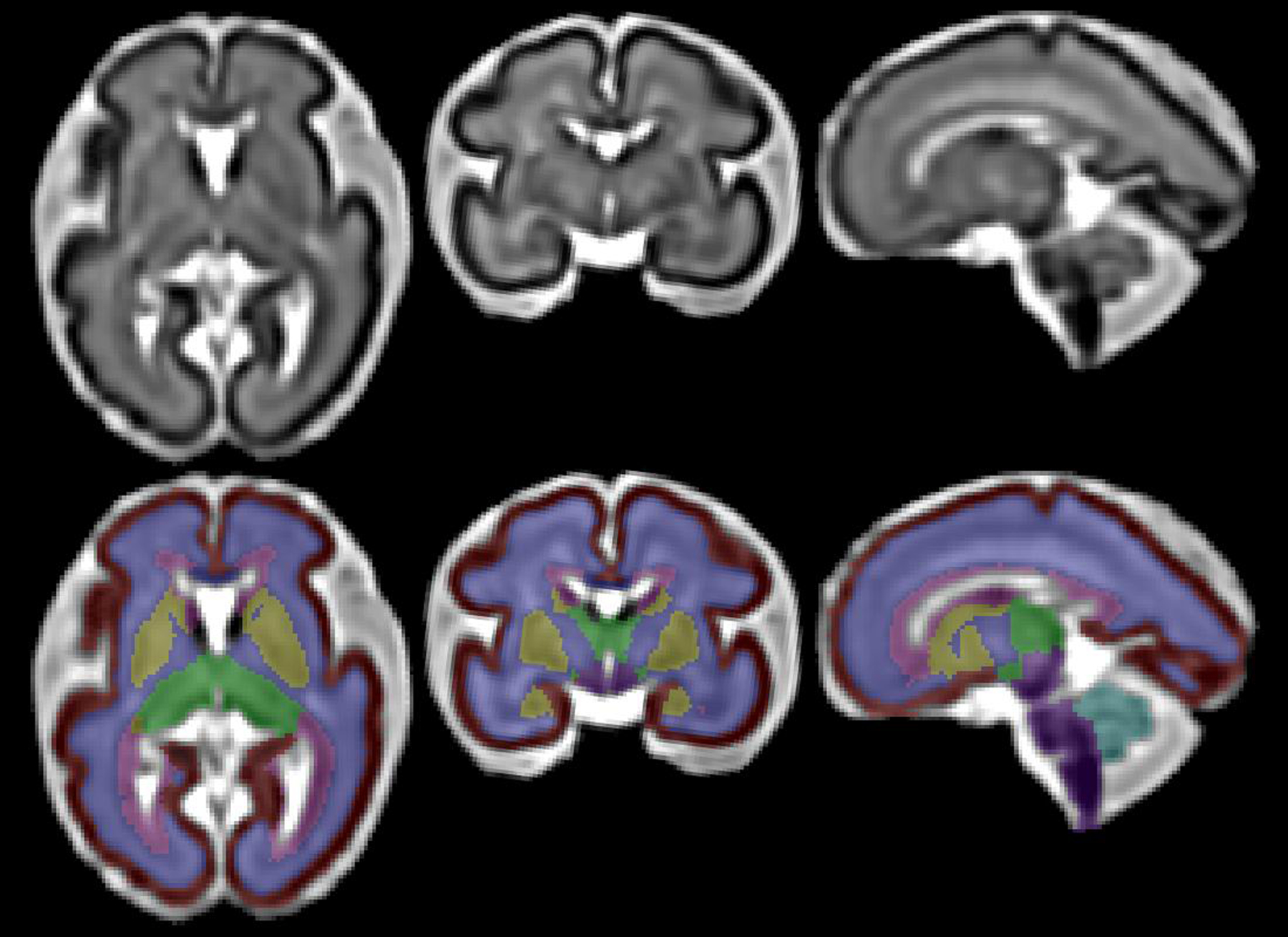
Reconstructed fetal brain MRI at 28 weeks in axial (left), coronal (middle), and sagittal (right) planes with brain structures segmented in bottom row. Red = Fetal cortex; Indigo = White matter; Yellow = Subcortical gray matter; Green = Diencephalon; Purple = Brainstem; Turqoise = Cerebellum.
Two-Year ND Evaluation
Children underwent neurodevelopmental assessment at 18–24 months of age. An intake questionnaire ascertained patient and family demographic characteristics. The Bayley Scales of Infant and Toddler Development, third edition (Bayley-III),22 a standardized measure of development, was administered by a licensed clinical psychologist (AS). When possible, the psychologist was blind to the child’s health status. However, in some cases the parents disclosed their child’s heart condition, or it was revealed as part of clinical care (33% of the CHD group). Composite scores (mean±SD: 100±15) and subscale scores (10±3) for the cognitive, language, and motor domains were calculated. Parents completed the Adaptive Behavior Assessment System, Third Edition (ABAS-3),23 a standardized questionnaire assessing observed adaptive functioning at home, from which we calculated the composite score for overall adaptive functioning (GAC: General Adaptive Composite). To evaluate clinical factors that may be associated with neurodevelopmental outcome, birth parameters (e.g., birth weight), cardiac anatomy, and neurological (e.g., stroke/seizure) data were extracted via chart review.
Statistical Analyses
To account for dramatic growth in the fetal brain that occurs over gestation15 and varying gestational age at MRI, as well as sex differences in brain structure that emerge in utero,24 total brain volumes were adjusted for sex and linear and quadratic gestational age at MRI. The residual total brain volume reflects the difference between the individual’s total brain volume and the expected value based on the linear regression equation. A subject whose brain size was smaller than predicted by linear regression for a given gestational age and sex would have a negative residual total brain volume, whereas a fetus whose brain size was larger than expected based on the regression equation would have a positive residual. These residual total brain volumes were used throughout all analyses and are subsequently referred to as “total brain volume” for simplicity.15 Correlations and interaction models assessed potential effect modification of total brain volume on neurodevelopmental outcomes by group (CHD versus control).
For our regression models, we considered several potential clinical predictors of neurodevelopmental outcome based on prior research: primary caregiver education, white race, male sex, single ventricle anatomy, diagnosis of hypoplastic left heart syndrome (HLHS) or transposition of the great arteries (TGA), cardiac class based on cardiac anatomy (two-ventricle vs single ventricle surgery, with/without arch obstruction),25 birth weight, gestational age at birth, length of stay at first hospitalization, stroke, seizure, total support duration during cardiopulmonary bypass, extracorporeal membrane oxygenation (ECMO), and age at first surgery greater than (vs less than/equal to) 30 days. When predictors demonstrated a high degree of collinearity, one factor was chosen to consider in regression modeling. For example, birth weight was chosen for inclusion rather than gestational age due to limited variability in gestational age in this cohort, the likelihood that birth weight provided additional information reflective of maternal environment, and data from the Single Ventricle Reconstruction Trial suggesting birth weight may have a greater association with neurodevelopmental outcome.26 Similarly, cardiac class was chosen as the single variable to classify cardiac anatomy due to its capture of both ventricular status and systemic outflow tract obstruction and known association with neurodevelopmental outcomes.1
For the primary hierarchical regression models for the CHD group, we started with fetal total brain volume, as this was the variable of major interest, then considered additional candidate variables separated into stages to reflect the order in which data become available. Stage 1 included fetal total brain volume, Stage 2 included demographic characteristics (primary caregiver education, race, and fetal sex), Stage 3 included cardiac class, Stage 4 included birth weight, and Stage 5 included postnatal medical variables (age of first surgery, total support time, stroke or seizure, ECMO, and length of stay). To enter the model, candidate variables at each stage were required to improve the Akaike Information Criterion (AIC) and have a positive partial R2. Variables that entered the model for a given stage were retained in later stages. To graphically display the hierarchical regression results, we calculated the relative contribution of each variable to the total adjusted R2 by dividing the adjusted partial R2 by the sum of the positive adjusted partial R2s, then scaling by the total adjusted R2 of the model.
We also employed two alternative regression analysis approaches to address the possibility that the primary analysis approach could amplify the predictive value of fetal brain MRI due to its early introduction to the model and retention by design in subsequent stages. For the first alternative approach, “alternative hierarchical regression,” we based the introduction of all variables on the order in which data would become clinically available: Stage 1: demographic characteristics; Stage 2: cardiac class; Stage 3: fetal total brain volume; Stage 4: birth weight; and Stage 5: postnatal medical variables. For the second alternative approach, “stepwise forward selection,” we applied stepwise forward selection regression models based on significant improvement in the AIC with no restriction on the ordering of which variables to include.
RESULTS
Demographic and Medical Characteristics
Of 90 subjects eligible for neurodevelopmental follow-up, 78 subjects (CHD=52, Control=26) completed the assessment (retention rate 87%; Figure 2). In the CHD group, subjects who returned for follow-up had higher gestational age at birth compared with those who did not (38.6±1.6 versus 37.1±2.4 weeks, P = 0.04). In the control group, subjects who returned had older mothers (32.5±4.1 versus 26.5±3.0 years, P = 0.01) who were more likely to be college educated (69% versus 0%, P = 0.01) than those who did not. Other fetal/maternal characteristics and brain volumes were comparable between those who did and did not return.
Figure 2. Recruitment and retention by group.
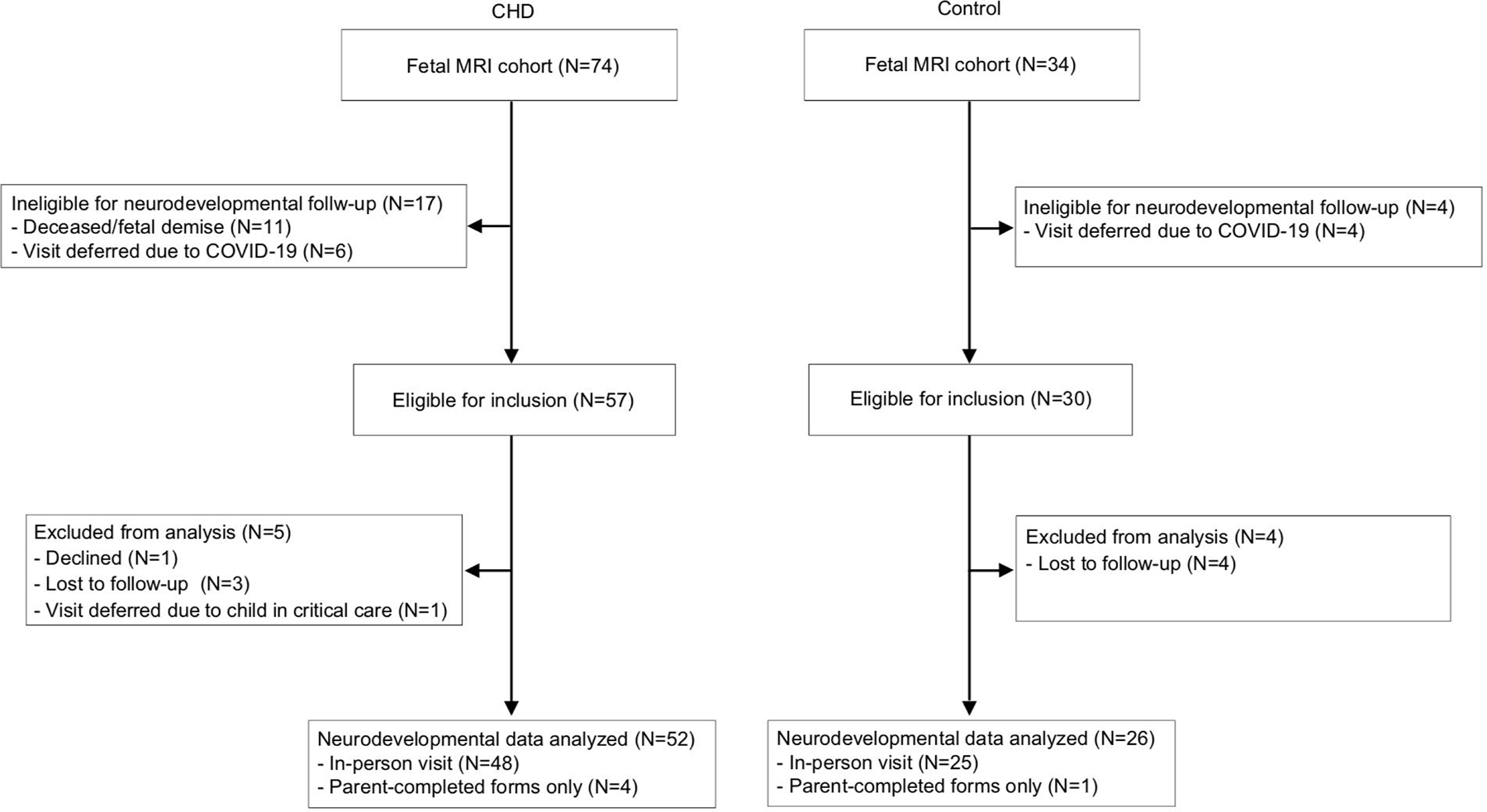
The diagram depicts subject recruitment, exclusions, and neurodevelopmental assessment by group.
Table 1 shows demographic and medical characteristics. Maternal hyperoxygenation was not administered to any subject. Within the CHD group, 32% had single ventricle heart disease, 8% had fetal cardiac intervention, and 63% underwent neonatal surgery. Table S1 reports the diagnosis and cardiac intervention for each participant. Median length of first hospital stay was 21 days. The CHD group had slightly lower birth weight than the control group, but otherwise the two groups had comparable maternal, fetal, and postnatal characteristics. The gestational age range at fetal brain MRI was 20–37 weeks. There were no group differences in total brain volume, and no fetus had parenchymal brain injury on MRI.
Table 1.
Demographic and Medical Characteristics
| Characteristic | CHD (N = 52) |
Control (N = 26) |
|---|---|---|
| Maternal characteristics | ||
| Maternal age (years) | 31.9 ± 4.7 | 32.5 ± 4.1 |
| Race | ||
| White | 42 (81) | 22 (85) |
| Black/African-American | 3 (6) | 2 (8) |
| Other/Unknown | 7 (13) | 2 (8) |
| Hispanic ethnicity | 7 (13) | 4 (15) |
| Education, college or greater | 35 (67) | 18 (69) |
| Fetal characteristics | ||
| Male sex | 33 (63) | 15 (58) |
| Gestational age at MRI (wks) | 28.1 ± 3.8 | 29.0 ± 4.4 |
| Total brain volume (per ml)† | −0.9 ± 12.6 | 1.7 ± 10.8 |
| Postnatal characteristics | ||
| Gestational age at birth (wks) | 38.6 ± 1.6 | 39.2 ± 1.4 |
| Head circumference (cm) | 33.8 ± 1.9 | 34.5 ± 1.4 |
| Cardiac class | – | |
| I | 22 (42) | |
| II | 13 (25) | |
| III | 8 (15) | |
| IV | 9 (17) | |
| Birth weight (kg) | 3.2 ± 0.6 | 3.5 ± 0.6 |
| Age at first surgery (days) | 9 [3, 44] | – |
| Total support time (min) | 133 [75, 203] | – |
| Stroke or seizure | 6 (12) | 0 |
| ECMO | 2 (4) | – |
Values are mean ± standard deviation, n (%), or median [IQR]
standard error
Total brain volume refers to residual after adjustment of fetal total brain volume for sex and gestational age (linear and quadratic terms)
ECMO = extracorporeal membrane oxygenation
Neurodevelopmental Assessment
Neurodevelopmental assessment occurred between June 2016 and March 2020, and all questionnaires were returned by May 2020. Mean age at assessment was comparable between groups (CHD, 22.9±4.3 months; Control, 24.1±4.2; P=0.24). On the Bayley-III, the CHD group had lower cognitive, language, and motor composite scores compared with the control group (d=−6.8, −12.9, −9.7 points; all P<0.05, Table 2). A greater proportion of the CHD group scored ≥1 SD below the normative mean than the control group in both the language and motor composites (CHD=17%, 21%; Control=4%, 0%). Across all subscales (i.e., expressive language, receptive language, gross motor, fine motor), the CHD group had significantly lower scores than the control group. The CHD group trended towards worse adaptive functioning (measured by the GAC), than the control group, though this difference did not reach statistical significance (P=0.06).
Table 2.
Neurodevelopmental Outcomes of Study Participants
| Outcome | CHD | Control | Difference [95% CI] |
|---|---|---|---|
| Bayley–III | N = 48 | N = 25 | |
| Cognitive composite | 99.4 ± 9.9 | 106.2 ± 14.6 | −6.8 [−12.6, −1.1] |
| ≤ 85 | 3 (6) | 0 | |
| Language composite | 96.1 ± 13.2 | 109.0 ± 18.2 | −12.9 [−20.3, −5.5] |
| ≤ 85 | 8 (17) | 1 (4) | |
| Receptive communication | 9.8 ± 2.6 | 12.2 ± 3.5 | −2.4 [−3.8, −0.9] |
| Expressive communication | 8.8 ± 2.4 | 10.8 ± 3.1 | −2.0 [−3.3, −0.7] |
| Motor composite | 93.6 ± 11.3 | 103.2 ± 10.5 | −9.7 [−15.1, −4.3] |
| ≤ 85 | 10 (21) | 0 | |
| Fine motor | 9.8 ± 2.1 | 11.0 ± 2.1 | −1.2 [−2.3, −0.2] |
| Gross motor | 8.1 ± 2.2 | 10.0 ± 2.1 | −2.0 [−3.0, −0.9] |
| ABAS-3 | N = 45 | N = 25 | |
| General adaptive composite | 103.1 ± 13.0 | 108.8 ± 10.1 | −5.7 [−11.5, 0.2] |
| ≤ 85 | 6 (13) | 0 |
Bayley-III = Bayley Scales of Infant Development, Third Edition
ABAS-3 = Adaptive Behavior Assessment System, Third Edition
Relationship between Fetal Brain MRI and Neurodevelopmental Outcome
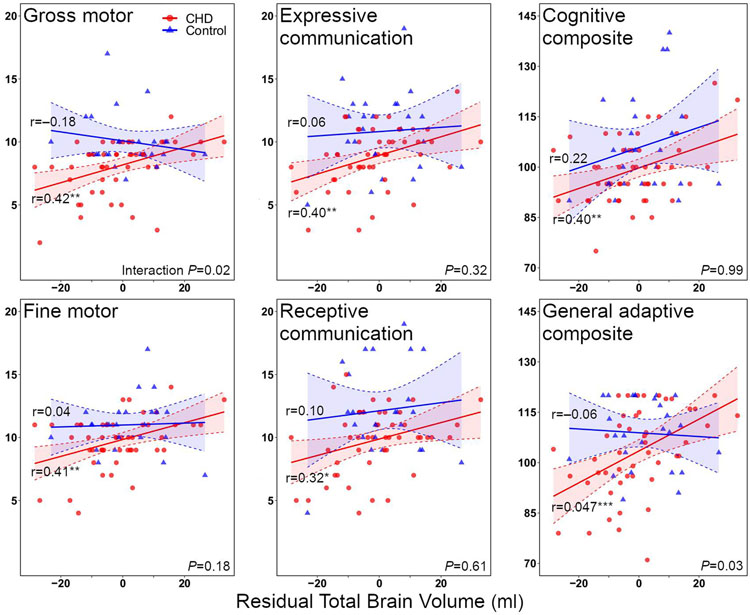
We examined relationships between total brain volume and neurodevelopmental outcomes separately in the CHD and control groups, then evaluated group interactions. Within the CHD group, larger total brain volume correlated with higher Bayley-III and ABAS-3 GAC scores (r=0.32–0.47; P<0.05; Figure 3, Table 3). In contrast, for the control group, total brain volume did not correlate with any outcome measure. Stronger associations of total brain volume with neurodevelopmental outcomes in the CHD group compared with the control group were especially evident for the Bayley-III gross motor and ABAS-3 GAC scores.
Figure 3. Associations between fetal total brain volume residuals and neurodevelopmental outcomes.
Congenital heart disease (CHD) group is shown in red, control group in blue. Total brain volume refers to residual after adjustment of fetal total brain volume for sex and gestational age (linear and quadratic terms). Within-group correlations are shown. Interaction term significance shown in bottom right corner of each pane. *P < 0.05; **P < 0.01; ***P < 0.001.
Table 3.
Correlations Between Regional Brain Volume Residuals and Neurodevelopmental Outcomes in the CHD Group
| Bayley-III | ABAS-3 | |||||
|---|---|---|---|---|---|---|
| Cognitive Composite | Receptive Communication | Expressive Communication | Fine Motor | Gross Motor | General Adaptive Composite | |
| Total brain volume (per ml)*† | 0.40** | 0.32* | 0.40** | 0.41** | 0.42** | 0.47*** |
| Fetal cortex | 0.36* | 0.29* | 0.23 | 0.43** | 0.34* | 0.43** |
| All white matter | 0.33* | 0.26 | 0.41** | 0.31* | 0.36* | 0.39** |
| Proliferative compartments | 0.30* | 0.21 | −0.02 | 0.44** | 0.27 | 0.31* |
| Subcortical gray matter | 0.30* | 0.24 | 0.42** | 0.13 | 0.30* | 0.16 |
| Diencephalon | 0.11 | 0.17 | 0.33* | 0.11 | 0.17 | 0.13 |
| Brain stem | 0.16 | 0.16 | 0.17 | 0.18 | 0.28 | 0.31* |
| Cerebellum | 0.22 | 0.25 | 0.41** | 0.22 | 0.31* | 0.25 |
Total brain volume refers to residual after adjustment of fetal total brain volume for sex and gestational age (linear and quadratic terms)
P<0.05
P<0.01
P<0.001
Bayley-III = Bayley Scales of Infant Development, Third Edition
ABAS-3 = Adaptive Behavior Assessment System, Third Edition
Hierarchical Regression of Fetal Brain Volume and Neurodevelopment
Hierarchical regression models identified risk factors associated with lower Bayley-III and ABAS-3 scores in the CHD group. Total brain volume at the first (univariable) stage explained 10–21% of the variance across developmental domains, dropping slightly to 6–21% after including all other risk factors (Table 4, Figure 4). Final hierarchical models including total brain volume and other significant risk factors explained 18–45% of the variance in neurodevelopmental scores. The highest percentage of explained variance was in the gross motor domain (45%), with total brain volume accounting for 15% of the variance. For adaptive functioning, total brain volume was the only significant predictor, accounting for 21% of the variance. Different clinical factors contributed to the models for each developmental domain. For example, primary caregiver education accounted for 5% and 9% of the variance in expressive and receptive language scores respectively, while cardiac class accounted for 25% of the variance in gross motor scores. The percent variance contributed by the fetal brain did not appreciably change with either the clinically-based alternative hierarchical regression approach or the stepwise forward selection approach based on the AIC. Specifically, total brain volume predicted 6–21% of the variance in neurodevelopmental scores for both of these final models (Tables S2 and S3).
Table 4.
Final Hierarchical Regression Models to Predict Neurodevelopmental Outcomes in the CHD Group
| Outcome | Variable | Beta Coefficient [95% CI] | Partial Adjusted R2 | Total Adjusted R2 |
|---|---|---|---|---|
| Bayley–III | ||||
| Cognitive composite | Total brain volume (per ml)* | 0.28 [0.08, 0.48] | 0.14 | 0.32 |
|
|
||||
| White race | 1.85 [−5.80, 9.50] | −0.02 | ||
|
|
||||
| Stroke or seizure | 7.20 [−3.05, 17.45] | 0.02 | ||
| Length of stay (per day) | −0.10 [−0.19, −0.02] | 0.17 | ||
|
| ||||
| Language composite | Total brain volume (per ml)* | 0.32 [0.03, 0.60] | 0.08 | 0.22 |
|
|
||||
| Education, college or greater† | 8.95 [1.48, 16.42] | 0.09 | ||
|
| ||||
| Motor composite | Total brain volume (per ml)* | 0.32 [0.09, 0.55] | 0.16 | 0.39 |
|
|
||||
| Male sex | −3.79 [−9.93, 2.35] | 0.01 | ||
|
|
||||
| Cardiac class II | 0.34 [−7.02, 7.69] | 0.03 | ||
| Cardiac class III | −7.88 [−16.37, 0.61] | – | ||
| Cardiac class IV | −4.45 [−13.15, 4.24] | – | ||
|
|
||||
| Length of stay (per day) | −0.10 [−0.19, −0.01] | 0.10 | ||
|
|
||||
| ECMO | −9.92 [−23.82, 3.98] | 0.03 | ||
|
| ||||
| Receptive communication | Total brain volume (per ml)* | 0.05 [−0.002, 0.11] | 0.06 | 0.18 |
|
|
||||
| Education, college or greater† | 1.75 [0.25, 3.25] | 0.09 | ||
|
| ||||
| Expressive communication | Total brain volume (per ml)* | 0.06 [0.014, 0.12] | 0.11 | 0.21 |
|
|
||||
| Education, college or greater† | 1.19 [−0.17, 2.54] | 0.05 | ||
|
|
||||
| ECMO | −2.61 [−5.71, 0.49] | 0.04 | ||
|
| ||||
| Fine motor | Total brain volume (per ml)* | 0.05 [0.003, 0.10] | 0.08 | 0.26 |
|
|
||||
| Male sex | 0.49 [−0.53, 1.51] | −0.001 | ||
|
|
||||
| Length of stay (per day) | −0.02 [−0.04, −0.003] | 0.10 | ||
|
| ||||
| Gross motor | Total brain volume (per ml)* | 0.06 [0.02, 0.10] | 0.15 | 0.45 |
|
|
||||
| White race | 1.39 [0.09, 2.70] | 0.08 | ||
|
|
||||
| Cardiac class II | −0.44 [−1.62, 0.75] | 0.25 | ||
| Cardiac class III | −2.53 [−3.99, −1.06] | – | ||
| Cardiac class IV | −1.98 [−3.32, −0.64] | – | ||
|
|
||||
| ECMO | −3.12 [−5.54, −0.71] | 0.12 | ||
|
| ||||
| ABAS-3 | ||||
| General adaptive composite | Total brain volume (per ml)* | 0.49 [0.22, 0.76] | 0.21 | 0.21 |
Total brain volume refers to residual after adjustment of fetal total brain volume for sex and gestational age (linear and quadratic terms)
Education refers to primary caregiver education
ECMO = extracorporeal membrane oxygenation
Bayley-III = Bayley Scales of Infant Development, Third Edition
ABAS-3 = Adaptive Behavior Assessment System, Third Edition
Figure 4. Bar plots depicting the contribution to the variance explained by each set of predictors in hierarchical regression models for each developmental domain.
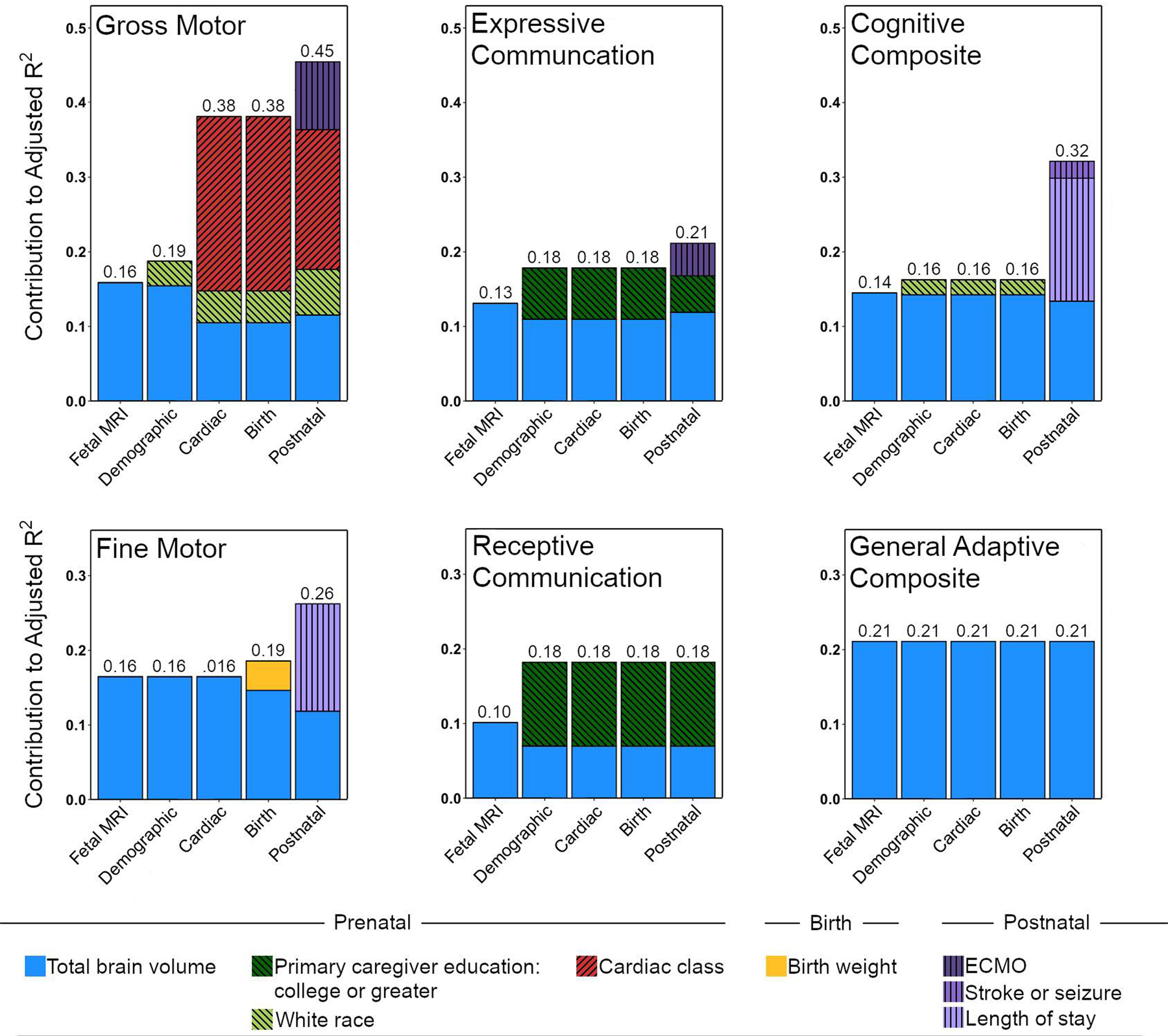
Stage 1 included fetal total brain volume residual, Stage 2 could add demographic information (primary caregiver education, race, or fetal sex), Stage 3 could add cardiac class, Stage 4 could add birth weight, and Stage 5 could add postnatal medical variables (total support time, stroke or seizure, ECMO, or length of stay).
ECMO = Extracorporeal membrane oxygenation
DISCUSSION
Recent reports have shown brain dysmaturity in fetuses with CHD, yet the extent to which fetal brain development impacts later neurodevelopment has not been studied. This hypothesis-generating study sought to evaluate whether fetal brain volume predicts 2-year neurodevelopment using a cohort of fetuses with isolated CHD and a sociodemographically comparable control group. Fetal total brain volume correlated with scores in all neurodevelopmental domains assessed in the CHD group, but not the control group. A predictive model including total brain volume along with sociodemographic and medical data accounted for up to 45% of the variance in neurodevelopmental outcome within the CHD group. These findings suggest that small fetal brain volume in CHD may be an important imaging biomarker of future neurodevelopmental risk. The findings highlight the need for further studies to assess the role of fetal brain volume as an imaging biomarker, its mechanisms, and its validity as an outcome marker for fetal neuroprotective intervention trials.
This study is the first to show a direct relationship between fetal brain MRI measures and neurodevelopmental outcome in CHD. Fetal brain volume alone predicted 10–21% of the variance in neurodevelopment scores in the hierarchical regression models. Notably, it remained a significant predictor in all domains after accounting for other known prenatal and postnatal risk factors. Although these associations were observed across all domains of development, age two years is still early in the developmental trajectory; thus, definitive conclusions cannot be made about associations between fetal brain volume and long-term functioning. Further study of this cohort is underway to determine whether these associations persist at school age. Of note, fetal brain volume was the only variable that predicted adaptive functioning, for which it explained 21% of the variance. This finding is of particular interest given that deficits in functional outcomes (e.g., self-care, communication, and social skills) often persist throughout life.27
Our results build upon existing literature suggesting a relationship between fetal brain health and subsequent outcomes. Williams and colleagues found that in utero cerebrovascular resistance was associated with Bayley-III neurodevelopment scores in CHD, though the relationship varied depending on cardiac class.28, 29 An exploratory study of at-risk fetuses, including 12 with CHD, found an association between ultrasound-based fetal brain measures and questionnaire-based neurodevelopment.30 Finally, in other populations, such as preterm populations and older children with CHD, brain MRI measures have been associated with long-term brain structure and neurodevelopment.18, 31–34
We employed a hierarchical regression approach for primary analysis in this study, first assessing the predictive value of fetal brain volume, the variable of interest, then sequentially adding other prenatal, birth, and postnatal data. We adopted this approach because we sought to identify biomarkers of neurodevelopmental risk that could be deployed for prenatal counseling or antenatal medical care, when birth and postnatal medical data would not be available. However, the results appear robust to analysis approach, as two alternative approaches, a clinically-based alternative hierarchical regression model and a stepwise forward selection model yielded similar results.
While this study was not designed to determine the underlying mechanisms contributing to impaired fetal brain growth and neurodevelopmental impairment, existing literature suggests several possibilities. The developing fetal brain is highly dependent on cerebral oxygen/nutrient delivery and may be particularly vulnerable to physiologic disturbances.35, 36 Abnormal cardiac anatomy can directly reduce oxygen/nutrient delivery to fetal brain; indeed lower oxygen consumption has been associated with smaller brain size in CHD.16 The importance of fetal cerebral hemodynamics is supported by variation in fetal brain growth among congenital heart lesions with differing physiologies. A prior analysis of the present cohort found that fetuses with HLHS and TGA, diagnoses with the greatest disruption in prenatal brain oxygen/nutrient delivery, displayed the most significant reductions in regional brain volumes.15 Single ventricle status was also associated with smaller total brain volume. While the current study was not powered to investigate differences by diagnosis, those within class III and IV (single ventricle anatomy) had the lowest gross motor scores. Of note, in the present study, fetal brain volume accounted for a greater percentage of variance in neurodevelopmental outcome than cardiac class.
Placental abnormalities may also impact the developing brain,37 with maternal factors that are more common in CHD pregnancies (e.g., pre-eclampsia, diabetes) influencing placental development and function that may enhance physiologic disturbances.38–40 Additionally, socioeconomic factors may contribute to an adverse maternal-fetal environment via maternal comorbidities like smoking or diabetes that alter placental physiology or through social determinants of health (e.g., access to care, home environment).41–43 Regardless of mechanism, our data suggest that fetal brain volume may be a useful biomarker for future fetal neuroprotection trials addressing these different physiologic pathways.
Beyond the physiologic environment, genetic factors are a potential contributor to fetal brain dysmaturation in CHD. Shared genetic pathways may alter both heart and brain development, for example through single gene disorders (e.g., CHARGE), copy number variants (e.g., DiGeorge), or gene variants with milder phenotypes.44 The present study excluded children with known extracardiac or genetic abnormalities, lowering the likelihood that major genetic abnormalities would account for the associations in our data. Moreover, total brain volumes in fetuses with and without CHD were similar. However, universal genetic testing was not a part of this study. Damaging de novo or rare variants affecting heart and brain development may still be present in our cohort and contribute to associations between brain volume and neurodevelopment.
The control group provides additional insight into the association between fetal brain volume and neurodevelopment. This group was comprised of healthy fetuses with a family history of CHD (usually a father or sibling15) who shared similar sociodemographic characteristics to the CHD group and had similar brain volumes. Within the control group, brain volume did not correlate with neurodevelopment, while in the CHD group, it did. Because sociodemographic variables were comparable, and a family history of CHD may provide some overlap in genetic background and in utero/maternal factors between the groups, our findings are consistent with the hypothesis that fetal circulatory derangements directly or indirectly account for the association between fetal brain volume and neurodevelopmental outcome. This hypothesis is further supported by our previous findings that fetuses expected to have the lowest fetal cerebral oxygen/nutrient delivery have the most marked disturbances in fetal brain growth, particularly in regions known to be sensitive to hypoxia.15
Finally, the present study underscores the well-known multifactorial etiology of neurodevelopmental impairment in CHD. Multivariable models explained up to 45% of the variance in neurodevelopmental outcomes, a significant advance beyond postnatal studies, which explained 30% of the variance at most.1, 2 In accordance with existing literature, cardiac anatomy, maternal education, and length of stay all contributed to models predicting neurodevelopmental outcomes. As expected, cardiac anatomy was associated with worse motor outcomes while lower maternal education was associated with poorer language skills.45, 46 In this series, overt brain injury, such as seizure or stroke, was not associated with significant worsening in neurodevelopmental outcome; however patients did not undergo routine postoperative electroencephalography or neuroimaging in the absence of clinical concern. Given the high rate of occult brain injury in the CHD population,6 our observed rates likely represent a lower bound, and improved detection might have yielded greater predictive value. Nonetheless, across all domains in this study, fetal brain volume was the most consistent predictor of neurodevelopmental outcome.
Limitations
Our study has limitations. The moderate sized sample for this study was from a single center and not racially/ethnically diverse. This did not allow for a subgroup analysis of different CHD diagnoses to establish whether the strength of the association between fetal brain volume and neurodevelopment differs between cardiac subtypes. Future multisite studies with a larger, more diverse sample may shed light on this question. We also did not conduct whole exome or whole genome sequencing on these patients, and cannot exclude an underlying genetic disorder that isn’t clinically apparent. While our study was not designed to determine mechanisms, the lack of correlation between fetal brain volume and neurodevelopment in the control group of a similar sociodemographic distribution would be consistent with a cardiac physiologic etiology. It is however possible that a third factor, such as genetics or the maternal environment, may account for this association. Future studies should integrate fetal brain MRI, sociodemographic measures, cardiac physiologic measures, and placental functioning with next generation sequencing of all fetuses. Third, the sample was evaluated at two years of age, yet for many children with CHD, higher-order deficits in executive functioning, attention, and social cognition do not become apparent until school-age. Long-term follow-up is needed to determine the persistence of associations found in the present cohort.
Conclusions
We present the first data showing an association between fetal brain development and neurodevelopmental outcome in CHD. Smaller fetal brain volume correlated with worse outcome at age 2 years across all domains of development and adaptive functioning in the CHD group. Fetal brain volume was the most consistent predictor of neurodevelopment compared with other prenatal and postnatal variables examined. In an era where most children with CHD are diagnosed prenatally in economically developed countries, small fetal brain volume may be an important biomarker of future neurodevelopmental risk. Trials are underway examining whether pharmacologic approaches (e.g., progesterone, allopurinol), or behavioral interventions (e.g., maternal stress reduction) could improve fetal brain development in CHD. While future studies are needed to confirm the validity of fetal brain volume as a predictor of neurodevelopment in children with CHD, our findings suggest that this imaging biomarker may inform risk stratification and be used as an outcome in trials of fetal neuroprotective interventions.
Supplementary Material
Clinical Perspectives.
What is new?
In children with congenital heart disease (CHD), smaller total brain volume on fetal MRI correlated with worse neurodevelopmental outcome at two years of age across all domains of development and adaptive functioning.
A predictive model including total brain volume along with sociodemographic and medical data accounted for up to 45% of the variance in neurodevelopmental outcome within the CHD group.
Fetal brain volume was the most consistent predictor of outcomes across neurodevelopmental domains compared with other sociodemographic and medical/surgical variables examined.
What are the clinical implications?
These findings suggest that small fetal brain volume may be an important imaging biomarker of future neurodevelopmental risk in CHD.
While our results are hypothesis generating, they support testing the efficacy of fetal interventions to protect the brain and improve the developmental trajectory for children with CHD.
Acknowledgments:
The authors acknowledge contributions from Judy Estroff, Ed Yang, Reem Chamseddine, Sarah Perelman, and Jeanette Beaute.
Sources of Funding:
Research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke K23NS101120 (C.K.R.), the National Heart, Lung, and Blood Institute K23HL141602 (C.M.O.), the National Institute of Biomedical Imaging and Bioengineering R01EB013248 (S.K.W.), R01EB018988 (A.G.), R01NS106030 (A.G.) and R01EB031849 (A.G), and a National Heart, Lung, and Blood Institute Pediatric Heart Network Scholar Award (C.K.R.); the American Academy of Neurology Clinical Research Training Fellowship (C.K.R.); the Brain and Behavior Research Foundation NARSAD Young Investigator (C.K.R.) and Distinguished Investigator (S.K.W.) Awards; the McKnight Foundation Technological Innovations in Neuroscience Award (A.G.); the Office of Faculty Development at Boston Children’s Hospital Career Development Awards (A.G., C.K.R.); the Mend A Heart Foundation (C.M.O.); and the Farb Family Fund (J.W.N). The content does not necessarily represent the official views of the NIH or other funding agencies.
Non-standard Abbreviations and Acronyms.
- ABAS-3
Adaptive Behavior Assessment System
- AIC
Akaike Information Criterion
- Bayley-III
Bayley Scales of Infant and Toddler Development
- CHD
congenital heart disease
- ECMO
extracorporeal membrane oxygenation
- GAC
General Adaptive Composite
- HLHS
hypoplastic left heart syndrome
- MRI
Magnetic resonance imaging
- TGA
transposition of the great arteries
Footnotes
Disclosures: None
Supplemental Materials
REFERENCES
- 1.The International Cardiac Collaborative on Neurodevelopment (ICCON) Investigators. Impact of operative and postoperative factors on neurodevelopmental outcomes after cardiac operations. Ann Thorac Surg. 2016;102:843–849. [DOI] [PubMed] [Google Scholar]
- 2.Gaynor JW, Stopp C, Wypij D, Andropoulos DB, Atallah J, Atz AM, Beca J, Donofrio MT, Duncan K, Ghanayem NS, et al. Neurodevelopmental outcomes after cardiac surgery in infancy. Pediatrics. 2015;135:816–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schaefer C, von Rhein M, Knirsch W, Huber R, Natalucci G, Caflisch J, Landolt MA and Latal B. Neurodevelopmental outcome, psychological adjustment, and quality of life in adolescents with congenital heart disease. Dev Med Child Neurol. 2013;55:1143–1149. [DOI] [PubMed] [Google Scholar]
- 4.Newburger JW, Sleeper LA, Bellinger DC, Goldberg CS, Tabbutt S, Lu M, Mussatto KA, Williams IA, Gustafson KE, Mital S, et al. Early developmental outcome in children with hypoplastic left heart syndrome and related anomalies: the single ventricle reconstruction trial. Circulation. 2012;125:2081–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mussatto KA, Hoffmann RG, Hoffman GM, Tweddell JS, Bear L, Cao Y and Brosig C. Risk and prevalence of developmental delay in young children with congenital heart disease. Pediatrics. 2014;133:e570–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marino BS, Lipkin PH, Newburger JW, Peacock G, Gerdes M, Gaynor JW, Mussatto KA, Uzark K, Goldberg CS, Johnson WH, et al. American Heart Association Congenital Heart Defects Committee, Council on Cardiovascular Disease in the Young, Council on Cardiovascular Nursing, Council on Cardiovascular Disease in the Young, Council on Cardiovascular Nursing and Stroke Council. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American Heart Association. Circulation. 2012;126:1143–1172. [DOI] [PubMed] [Google Scholar]
- 7.Dawson G, Rogers S, Munson J, Smith M, Winter J, Greenson J, Donaldson A and Varley J. Randomized, controlled trial of an intervention for toddlers with autism: the Early Start Denver Model. Pediatrics. 2010;125:e17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lipkin PH, Macias MM and Council on Children with Disabilities SoDaBP. Promoting Optimal Development: Identifying Infants and Young Children With Developmental Disorders Through Developmental Surveillance and Screening. Pediatrics. 2020;145:e20193449. [DOI] [PubMed] [Google Scholar]
- 9.Council on Children With D, Section on Developmental Behavioral P, Bright Futures Steering C and Medical Home Initiatives for Children With Special Needs Project Advisory C. Identifying infants and young children with developmental disorders in the medical home: an algorithm for developmental surveillance and screening. Pediatrics. 2006;118:405–420. [DOI] [PubMed] [Google Scholar]
- 10.Bellinger DC, Wypij D, duPlessis AJ, Rappaport LA, Jonas RA, Wernovsky G and Newburger JW. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg. 2003;126:1385–1396. [DOI] [PubMed] [Google Scholar]
- 11.Licht DJ, Shera DM, Clancy RR, Wernovsky G, Montenegro LM, Nicolson SC, Zimmerman RA, Spray TL, Gaynor JW and Vossough A. Brain maturation is delayed in infants with complex congenital heart defects. J Thorac Cardiovasc Surg. 2009;137:529–36; discussion 536–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller SP, McQuillen PS, Hamrick S, Xu D, Glidden DV, Charlton N, Karl T, Azakie A, Ferriero DM, Barkovich AJ and Vigneron DB. Abnormal brain development in newborns with congenital heart disease. N Engl J Med. 2007;357:1928–1938. [DOI] [PubMed] [Google Scholar]
- 13.Clouchoux C, du Plessis AJ, Bouyssi-Kobar M, Tworetzky W, McElhinney DB, Brown DW, Gholipour A, Kudelski D, Warfield SK, McCarter RJ, et al. Delayed cortical development in fetuses with complex congenital heart disease. Cereb Cortex. 2013;23:2932–2943. [DOI] [PubMed] [Google Scholar]
- 14.Limperopoulos C, Tworetzky W, McElhinney DB, Newburger JW, Brown DW, Robertson RL Jr., Guizard N, McGrath E, Geva J, Annese D, et al. Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation. 2010;121:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rollins CK, Ortinau CM, Stopp C, Friedman KG, Tworetzky W, Gagoski B, Velasco-Annis C, Afacan O, Vasung L, Beaute JI, et al. Regional brain growth trajectories in fetuses with congenital heart disease. Ann Neurol. 2021;89:143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun L, Macgowan CK, Sled JG, Yoo SJ, Manlhiot C, Porayette P, Grosse-Wortmann L, Jaeggi E, McCrindle BW, Kingdom J, et al. Reduced fetal cerebral oxygen consumption is associated with smaller brain size in fetuses with congenital heart disease. Circulation. 2015;131:1313–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beca J, Gunn JK, Coleman L, Hope A, Reed PW, Hunt RW, Finucane K, Brizard C, Dance B and Shekerdemian LS. New white matter brain injury after infant heart surgery is associated with diagnostic group and the use of circulatory arrest. Circulation. 2013;127:971–979. [DOI] [PubMed] [Google Scholar]
- 18.Rollins CK, Asaro LA, Akhondi-Asl A, Kussman BD, Rivkin MJ, Bellinger DC, Warfield SK, Wypij D, Newburger JW and Soul JS. White matter volume predicts language development in congenital heart disease. J Pediatr. 2017;181:42–48 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffman JIE and Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900. [DOI] [PubMed] [Google Scholar]
- 20.Kainz B, Steinberger M, Wein W, Kuklisova-Murgasova M, Malamateniou C, Keraudren K, Torsney-Weir T, Rutherford M, Aljabar P, Hajnal JV et al. Fast volume reconstruction from motion corrupted stacks of 2D slices. IEEE Trans Med Imaging. 2015;34:1901–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gholipour A, Rollins CK, Velasco-Annis C, Ouaalam A, Akhondi-Asl A, Afacan O, Ortinau CM, Clancy S, Limperopoulos C, Yang E, et al. A normative spatiotemporal MRI atlas of the fetal brain for automatic segmentation and analysis of early brain growth. Sci Rep. 2017;7:476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bayley N Bayley Scales of Infant and Toddler Development, third edition. 2006. Pearson Education,San Antonio, TX. [Google Scholar]
- 23.Harrison PL and Oakland T. ABAS-3: Adaptive Behavior Assessment System 3rd edition. 2015. Pearson Education, San Antonio,TX. [Google Scholar]
- 24.Studholme C, Kroenke CD and Dighe M. Motion corrected MRI differentiates male and female human brain growth trajectories from mid-gestation. Nat Commun. 2020;11:3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clancy RR, McGaurn SA, Wernovsky G, Spray TL, Norwood WI, Jacobs ML, Murphy JD, Gaynor JW and Goin JE. Preoperative risk-of-death prediction model in heart surgery with deep hypothermic circulatory arrest in the neonate. J Thorac Cardiovasc Surg. 2000;119:347–357. [DOI] [PubMed] [Google Scholar]
- 26.Miller TA, Ghanayem NS, Newburger JW, McCrindle BW, Hu C, DeWitt AG, Cnota JF, Tractenberg FL, Pemberton VL, Wolf MJ, et al. Pediatric Heart Network Investigators. Gestational age, birth weight, and outcomes six years after the Norwood procedure. Pediatrics. 2019;143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nattel SN, Adrianzen L, Kessler EC, Andelfinger G, Dehaes M, Côté-Corriveau G and Trelles MP. Congenital heart disease and neurodevelopment: clinical manifestations, genetics, mechanisms, and implications. Can J Cardiol. 2017;33:1543–1555. [DOI] [PubMed] [Google Scholar]
- 28.Williams IA, Fifer C, Jaeggi E, Levine JC, Michelfelder EC and Szwast AL. The association of fetal cerebrovascular resistance with early neurodevelopment in single ventricle congenital heart disease. Am Heart J. 2013;165:544–550 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams IA, Tarullo AR, Grieve PG, Wilpers A, Vignola EF, Myers MM and Fifer WP. Fetal cerebrovascular resistance and neonatal EEG predict 18-month neurodevelopmental outcome in infants with congenital heart disease. Ultrasound Obstet Gynecol. 2012;40:304–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Welling MS, Husen SC, Go A, Groenenberg IAL, Willemsen SP, Bijma HH and Steegers-Theunissen RPM. Growth trajectories of the human fetal brain in healthy and complicated pregnancies and associations with neurodevelopmental outcome in the early life course. Early Hum Dev. 2020;151:105224. [DOI] [PubMed] [Google Scholar]
- 31.Thompson DK, Matthews LG, Alexander B, Lee KJ, Kelly CE, Adamson CL, Hunt RW, Cheong JLY, Spencer-Smith M, Neil JJ, et al. Tracking regional brain growth up to age 13 in children born term and very preterm. Nat Commun. 2020;11:696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woodward LJ, Anderson PJ, Austin NC, Howard K and Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med. 2006;355:685–694. [DOI] [PubMed] [Google Scholar]
- 33.Rollins CK, Watson CG, Asaro LA, Wypij D, Vajapeyam S, Bellinger DC, DeMaso DR, Robertson RL Jr., Newburger JW and Rivkin MJ. White matter microstructure and cognition in adolescents with congenital heart disease. J Pediatr. 2014;165:936–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von Rhein M, Buchmann A, Hagmann C, Huber R, Klaver P, Knirsch W and Latal B. Brain volumes predict neurodevelopment in adolescents after surgery for congenital heart disease. Brain. 2014;137:268–276. [DOI] [PubMed] [Google Scholar]
- 35.Morton PD, Korotcova L, Lewis BK, Bhuvanendran S, Frank JA, Jonas RA, Gallo V and Ishibashi N. Abnormal neurogenesis and cortical growth in congenital heart disease. Sci Transl Med. 2017;9:eaah7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lawrence KM, McGovern PE, Mejaddam A, Rossidis AC, Baumgarten H, Kim A, Grinspan JB, Licht DJ, Didier RA, Vossough A, et al. Chronic intrauterine hypoxia alters neurodevelopment in fetal sheep. J Thorac Cardiovasc Surg. 2019;157:1982–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schlatterer SD, Murnick J, Jacobs M, White L, Donofrio MT and Limperopoulos C. Placental pathology and neuroimaging correlates in neonates with congenital heart disease. Sci Rep. 2019;9:4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ernst LM. Maternal vascular malperfusion of the placental bed. APMIS. 2018;126:551–560. [DOI] [PubMed] [Google Scholar]
- 39.Auger N, Fraser WD, Healy-Profitos J and Arbour L. Association between preeclampsia and congenital heart defects. JAMA. 2015;314:1588–1598. [DOI] [PubMed] [Google Scholar]
- 40.Oyen N, Diaz LJ, Leirgul E, Boyd HA, Priest J, Mathiesen ER, Quertermous T, Wohlfahrt J and Melbye M. Prepregnancy diabetes and offspring risk of congenital heart disease: a nationwide cohort study. Circulation. 2016;133:2243–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bonthrone AF, Chew A, Kelly CJ, Almedom L, Simpson J, Victor S, Edwards AD, Rutherford MA, Nosarti C and Counsell SJ. Cognitive function in toddlers with congenital heart disease: The impact of a stimulating home environment. Infancy. 2021;26:184–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu Y, Kapse K, Jacobs M, Limperopoulos C, Niforatos-Andescavage N, Donofrio MT, Vezina G, Wessel DL, duPlessis A and Limperopoulos C. Association of maternal psychological distress with in utero brain development in fetuses with congenital heart disease. JAMA Pediatr. 2020;174:e195316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu YC, Kapse K, Andersen N, Quistorff J, Lopez C, Fry A, Cheng J, Andescavage N, Wu Y, Espinosa K, Vezina G, du Plessis A and Limperopoulos C. Association between socioeconomic status and in utero fetal brain development. JAMA Netw Open. 2021;4:e213526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Homsy J, Zaidi S, Shen Y, Ware JS, Samocha KE, Karczewski KJ, DePalma SR, McKean D, Wakimoto H, Gorham J, et al. De novo mutations in congenital heart disease with neurodevelopmental and other congenital anomalies. Science. 2015;350:1262–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Snookes SH, Gunn JK, Eldridge BJ, Donath SM, Hunt RW, Galea MP and Shekerdemian L. A systematic review of motor and cognitive outcomes after early surgery for congenital heart disease. Pediatrics. 2010;125:e818–827. [DOI] [PubMed] [Google Scholar]
- 46.Dollaghan CA, Campbell TF, Paradise JL, Feldman HM, Janosky JE, Pitcairn DN and Kurs-Lasky M. Maternal education and measures of early speech and language. J Speech Lang Hear Res. 199;42:1432–1443. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.