Abstract
Introduction
Hearing loss is a major health problem, impacting education, communication, interpersonal relationships, and mental health. Drugs that prevent or restore hearing are lacking and hence novel drug targets are sought. There is the possibility of targeting the α9α10 nicotinic acetylcholine receptor (nAChR) in the prevention of noise-induced, hidden hearing loss and presbycusis. This receptor mediates synaptic transmission between medial olivocochlear efferent fibers and cochlear outer hair cells. This target is key since enhanced olivocochlear activity prevents noise-induced hearing loss and delays presbycusis.
Areas covered
The work examines the α9α10 nicotinic acetylcholine receptor (nAChR), it’s role in noise-induced, hidden hearing loss and presbycusis and the possibility of targeting. Data has been searched in Pubmed, the World Report on Hearing from the World Health Organization and the Global Burden of Disease Study 2019.
Expert opinion
The design of positive allosteric modulators of α9α10 nAChRs is proposed because of the advantage of reinforcing the MOC-hair cell endogenous neurotransmission without directly stimulating the target receptors, therefore avoiding receptor desensitization and reduced efficacy. The time is right for the discovery and development of α9α10 nAChRs targeting agents and high throughput screening assays will support this.
Keywords: α9α10 nicotinic receptors, hearing loss, positive allosteric modulators, presbycusis, tinnitus
1. Introduction
Hearing loss is one of the most prevalent sensory disabilities. It is a major public health problem, given that its impact on human communication and quality of life is devastating. Depending upon the age of onset, it impairs language development, education, communication, employment, mental health, interpersonal relationships and/or psychosocial well-being1, 2. Moreover, population-based observational studies have shown that hearing impairment is strongly related to accelerated cognitive decline and dementia risk in older adults3–5. Indeed, hearing loss is now known to be the largest modifiable risk factor for developing dementia, exceeding that of smoking, high blood pressure, lack of exercise, and social isolation6. According to the World Report on Hearing from the World Health Organization7 and the Global Burden of Disease Study 20198, more than 1.5 billion people worldwide experience some decline in their hearing capacity during the course of their life and at least 430 million will require care. If unaddressed hearing loss results in an annual global cost of more than $ 980 billion. An increase of more than 1.5-fold in hearing loss is projected for the coming decades and 2.45 billion people for 2050. Most dramatic, compared with other disease categories in the Global Burden of Disease Study8, age-related and other hearing loss was the third largest cause of global years lived with disability in 2019, after low back pain and migraine. Moreover, it ranked first among sensory disorders and was the leading cause for individuals older than 70 years. Hearing loss is sometimes accompanied with phantom sound perception, also known as tinnitus, which can be a debilitating condition on its own. Thus, tinnitus is perceived by approximately 20% and debilitating in 2–3% of the world population9. These striking facts indicate that hearing loss is a growing public health issue and burden, which requires an urgent call for global attention and action. In the present expert opinion I discuss the pros and cons of targeting inner ear hair cell α9α10 nicotinic acetylcholine receptors (nAChRs) for the prevention and/or treatment of hearing loss. The rational for targeting this receptor is based on the fact that it mediates synaptic transmission at the medial olivocochlear efferent fiber-outer hair cells synapse and that enhanced olivocochlear activity prevents noise-induced hearing loss and delays presbycusis10–13.
2. Noise-induced hearing loss and related disorders
2.1. Noise-induced hearing loss
Congenital hearing loss is the most common sensory disability and affects approximately 1–2 out of 1000 newborns. In more than 50% of cases the cause is genetic, being 70–80% non-syndromic14, 15. To date a total of 124 non-syndromic hearing loss genes have been identified16 and this number is constantly increasing, highlighting the wide variety of proteins involved in auditory physiology and development. Hearing loss increases with age, and age-related hearing loss or presbycusis is a multifactorial condition with contributions from, and interactions among, numerous variables including genetic factors that determine the rate and extent of hair cells and neural degeneration, pre-existing ear conditions, chronic illnesses, use of ototoxic medicines and lifestyles. Several environmental, lifestyle, or other modifiable factors contribute to the etiology of hearing impairment across the lifespan. This implies that hearing impairment in adults may be prevented or delayed17.
Within the environmental or lifestyle preventable factors that contribute the most to hearing loss is the exposure to elevated noise, which can be of occupational, recreational or environmental origin18–20. High levels of workplace noise remain a problem in all regions of the world7. A high risk of hearing loss is also faced due to loud levels of sound in recreational settings21–23. These include the prolonged listening to personal audio devices at high levels, attending to concerts or the use of firearms. The WHO estimates that over 50% of people aged 12–35 are at risk of having hearing problems due to the use of portable audio devices7.
Exposure to loud sounds is also the main known factor leading to tinnitus or phantom sound perception, the conscious awareness of a tonal or composite noise for which there is no identifiable corresponding external acoustic source24, 25. It is perceived by approximately 20–25 % of the word population9. Although the vast majority of people can live with their tinnitus, for 2–3 % of the world population, the auditory component is accompanied with suffering and so it is considered as tinnitus disorder26. In the latter case, the perceived sound is associated with emotional distress, cognitive dysfunction, and/or autonomic arousal, leading to behavioral changes and functional disability. Growing evidence indicates that the pathophysiology of tinnitus disorder involves changes in neuronal activity not only in different parts of the auditory pathway, but in different brain non-auditory areas as well24, 27, 28. At present there is not a single Food and Drug Administration (FDA) nor European Medicines Agency (EMA) approved drug on the market25, 29–31. Thus, there is still a significant unmet clinical need for a safe and effective drug targeting tinnitus relief. Even a drug that produces a small but significant effect would have a huge therapeutic impact. Since the exposure to overly loud sounds is the main known factor leading to tinnitus23, 32, drugs that prevent or restore noise-induced hearing loss, would be beneficial for tinnitus.
2.2. Hidden hearing loss
Hair cell damage was classically considered as the main contributor to the hearing loss produced by exposure to loud sounds, whereas neural degeneration was regarded as a secondary event to the loss of hair cells, due to loss of neurotrophic support33. Moreover, the consensus indicated that cochlear neural loss occurred only after hair cell death. Thus, hair cells swell within minutes and disappear hours after an exposure to a very loud sound, leading to permanent threshold elevations34. In contrast, the time course of neuronal degeneration was reported to be slower, since myelinated axons of cochlear nerve fibers begin to disappear 1–2 weeks postexposure and loss of their cell bodies in the spiral ganglion is evidenced after 1 month35. However, it has recently been demonstrated that synaptic connections between hair cells and cochlear neurons can be damaged in the absence of hair cell loss36. In fact, noise exposures causing only reversible hearing threshold shifts (and no hair cell loss) cause a permanent loss of >50% of the synaptic connections between hair cells and the auditory nerve, without hair cells loss33. This synaptic loss or synaptopathy silences large numbers of cochlear neurons and does not affect the test of threshold detection performed in the normal audiogram. Therefore, it has been named “hidden hearing loss”. Cochlear synaptopathy compromises understanding speech in a noisy environment37, which is a classic complaint of those who have been exposed to loud sounds or in aged people.
2.3. Presbycusis
Auditory function declines with age and includes the reduction in threshold sensitivity and poor speech discrimination and auditory processing, especially in noisy environments38. Genetic and ambient factors are probably involved in presbycusis. The decline in threshold sensitivity is most likely due to loss of hair cells38. However, even when auditory thresholds are preserved, degraded temporal resolution and the difficulty in understanding speech in background noise have been classically attributed to central and cognitive factors38. However, recent work in rodents has shown that synaptic aging is a key contributor to the hearing performance declines of aging listeners39. Thus, inner hair cell (IHC)-afferent synaptic loss progresses from youth to aged mice throughout the cochlea, long before changes in thresholds or hair cell counts are observed. Type I afferent fiber loss follows the synaptic loss, with a delay of several months39. This same synaptic aging most likely occurs in humans, as evidenced in a recent work performed in post-mortem human cochlea40. Although presbycusis occurs in the absence of exposure to loud sounds39, 40, this ambient factor adds further insult to aging.
3. Treatment
Prevention remains the best option for limiting the effects of acoustic trauma produced by the exposure to loud sounds. This requires education, regulations, legislation and workplace noise policy enforcement. A prospective, randomized controlled assessment of the short- and long-term efficacy of a hearing conservation education program in Canadian elementary school children, has shown that a community-based health promotion project around hearing loss aids students to develop their knowledge and skills in health advocacy, highlighting the importance of hearing protection education41. Moreover, a Cochrane systematic review has shown that enforcement of legislation and better implementation of occupational hearing loss prevention programs can reduce noise levels in workplaces42. However, not all countries have and/or enforce hearing protection regulation programs43.
In many situations prevention from exposure to loud sounds is not feasible to the extent necessary to protect hearing capabilities. A clear example is that observed in the military, where acute noise damage may result from a blast exposure, such as a discharge from a weapon or detonation of an explosive44, 45. In fact, hearing loss is the most common service-connected disability. Therefore, alternative strategies to merely prevention are needed. In this regard, the search for pharmacological treatments to prevent and/or treat noise-induced hearing loss is an active niche of research.
A plethora of drugs have been investigated and/or used in the treatment of noise-induced hearing loss, with different levels of outcomes and, in general, with poor solid evidence to support their use. In this regard, local or systemic steroids are commonly used to address the noise post-exposure inner ear inflammatory process46. A systematic review and meta-analysis on the use of steroids has recommended future additional studies with the inclusion of control groups, precise definition of acoustic trauma intensity and duration, and genetic polymorphisms46. Since oxidative stress and the release of free radicals in the form of reactive oxygen and nitrogen species take place during noise-induced hearing loss47, 48, antioxidants have been evaluated as a treatment option both in animal models and humans, with contradictory results. These include N-acetylcysteine, ginseng, co-enzyme, vitamin A, vitamin C, vitamin E, and vitamin B12, glutathione, D-methionine, ebselen and resveratrol49. On the other hand, noise exposure leads to hair cell death displaying features of both apoptosis and necrosis as well as necroptosis, a necrotic-like process49. Therefore, agents that prevent hair cell apoptosis by disrupting mitogen-activated protein kinase (MAPK) cell death signaling through peptide inhibition of c-Jun N-terminal Kinase have been tested50. Other compounds such as calcium antagonists, vasolidators, NMDA receptor antagonists and neurothrophins also have been tested in animal models49.
4. The α9α10 nicotinic acetylcholine receptor: a possible pharmacological target?
4.1. α9α10 nicotinic acetylcholine receptors
Nicotinic acetylcholine receptors are a subfamily of the pentameric ligand-gated ion channels involved in many physiological and pathological processes51. Each receptor subtype is composed of different subunits, encoded by paralogous genes. They show a similar fivefold symmetrical arrangement of subunits around a central pore, and are composed of extracellular and transmembrane (TM) domains (Figure 1). The extracellular domain contains the orthosteric ligand binding sites and folds into a highly conserved immunoglobulin-like β-sandwich. The TM domain consists of four α-helices, with TM2 lining the channel pore, surrounded by a ring made of TM1 and TM3 α-helices51, 52. According to their main tissue of expression, in vertebrates, they are divided into three subgroups: neuronal, muscle and hair cell nAChRs53. Thus, neuronal nAChRs are formed by as yet not fully characterised combinatorial arrangements of α2-α7 (α8 in non-mammals) and β2–4 subunits54–56. In addition, receptors formed by the same subunits, but with alternate stoichiometry57–62, further extend the complexity of neuronal nAChRs. On the other hand, muscle receptors are formed by α12 β1γ, and δ, or ε subunits63, 64. Finally, and in contrast to neuronal receptors, the nAChR subunits expressed in cochlear hair cells have a very strict co-assembly pattern, encompassing only α9 and α10 subunits65–67.
Figure 1.
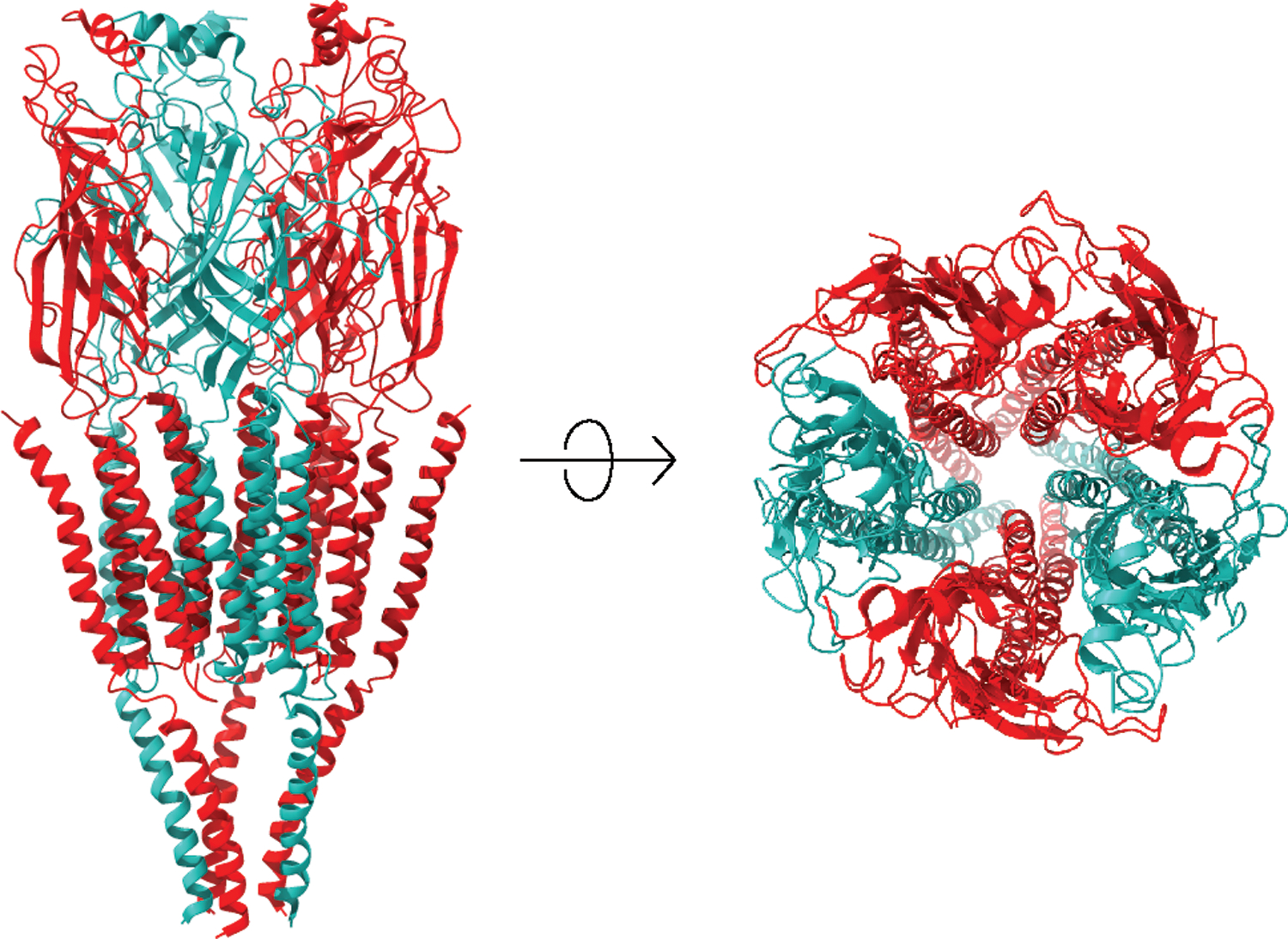
Ribbon structure of a heteromeric pentameric nicotinic acetylcholine receptor, showing the arrangement of subunits around the channel pore. Front and upper views are provided. The extracellular domain contains the orthosteric ligand binding sites and folds into a highly conserved immunoglobulin-like β-sandwich. The transmembrane domain consists of four α-helices, with TM2 lining the channel pore, surrounded by a ring made of TM1 and TM3 α-helice. Reproduced from Lipovsek et al (2021)79 under Creative Commons Attribution (CC BY) license.
The α9α10 heteromeric receptor is composed only of α subunits65, 67, 68. Alternate stoichiometries have been reported for this receptor. Thus, at equimolar expression of both subunits, an (α9)2(α10)3 stoichiometry has been determined69, whereas expression of a 10-fold excess of α9 compared with α10 in Xenopus oocytes can lead to an additional receptor isoform with a (α9)3(α10)2 stoichiometry70. The number of binding sites at the receptor in the different stoichiometries is unknown. However, the contribution of α9 and α10 subunits to the binding site is non-equivalent71, and the different binding sites in alternate stoichiometries can have differential pharmacological properties70. Recent crystal structures of the homomeric extracellular domain of the α9 subunit bound to antagonists, is beginning to shed light into the molecular interactions between binding site residues and ligands72. Moreover, they serve as a template for molecular dynamics simulations of the extracellular domains of the α9α10 nAChR in pentameric assemblies73.
α9α10 nAChRs are the most divergent within all nicotinic receptors, showing striking differences in their degree of sequence conservation compared to other nAChR subunits and to their orthologues in different species, their restricted expression pattern, their subunit co-assembly rules and their functional properties53, 67, 68, 74–78. In fact, whereas all nAChRs are activated by nicotine, the agonist that gives rise to the name of the family, α9α10 are blocked by this compound65, 67, 68, 76. Moreover, α9α10 nAChRs are potently blocked by antagonists of glycine, GABAA and serotonin type 3 receptors, thus sharing pharmacological properties with other members of the Cys loop family67, 75, 77. Therefore, α9α10 has been described as an odd cousin within the old family of α9α10 nAChRs79.
4.2. The efferent medial olivocochlear system
The best described function of the α9α10 nAChR is at the organ of Corti of the inner ear, where it mediates synaptic transmission between cholinergic medial olivocochlear fibers and outer hair cells (OHCs)53, 66–69, 74, 76–78, 80–86. This nicotinic synapse is inhibitory, since the activation of the α9α10 hair cell nAChR leads to an increase in intracellular Ca2+ and the subsequent opening of small conductance Ca2+-activated K+ (SK2) channels, thus leading to hyperpolarization of hair cells (Figure 2)87–93. Outer hair cells are responsible for amplification of incoming sounds and fine tuning of the basilar membrane through a mechanism known as somatic electromotility based on the motor protein prestin94, 95. The MOC neurons constitute a sound-evoked negative feedback loop. As sound pressure level increases, the firing rate of MOC fibers increases96. This results in the suppression of the contribution of OHCs to amplification of sound-induced motion in the sensory epithelium97. Thus, the MOC system reduces the gain of the cochlea through a direct inhibition of OHC function. Moreover, the strength of cochlear inhibition is proportional to the rate of MOC activity97–99. Thus, the MOC efferent system is part of a cochlea-brainstem-cochlea reflex pathway, which enables the central nervous system to modulate hearing at the periphery through the activation of α9α10 nAChRs, providing a stimulus-related control of the cochlea100.
Figure 2.
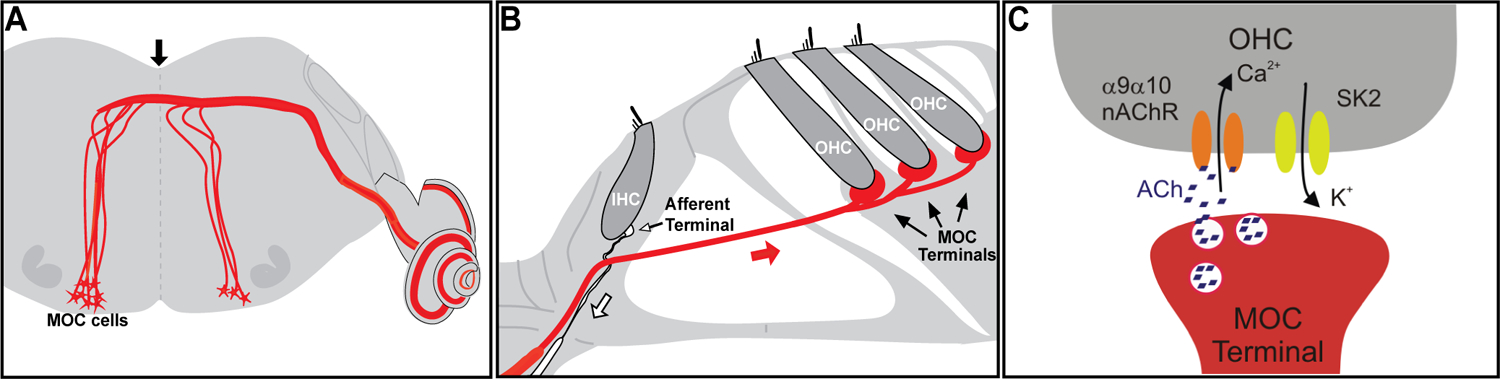
Schematics of the MOC System. (A) MOC efferent neurons are located in the superior olivary complex of the brainstem and project to the cochlea; (B) MOC fibers make direct synaptic contacts at the base of the OHCs; (C) At the MOC-OHC synapse ACh is released. It binds to α9α10 nAChRs present at the OHCs, leading to Ca2+- influx and the subsequent activation of Ca2+-dependent K+ (SK2) channels and hair cell hyperpolarization. The white arrow in (B) indicates the afferent fibers that bring the information from the IHCs to the central nervous system, and the red arrow indicates the MOC fibers. Reproduced from Taranda et al (2009)12 under Creative Commons Attribution (CC BY) license.
4.3. α9α10 nicotinic acetylcholine receptors and protection from noise-induced hearing loss
The MOC system has been implicated in several functions of the auditory process; important for this Expert Opinion is the protection from damage produced by the exposure to loud sounds11, 12, 101–105. The protective effect of the MOC system from acute and chronic noise-induced hearing loss has been described in different animal models103, 106–109. In addition, the strength of the MOC system is inversely correlated with the degree of noise-induced hearing loss102. Moreover, activation of the MOC system also reduces neuropathy produced during hidden hearing loss10. Short-term plasticity of the MOC-OHC synapse is responsible for shaping MOC inhibition and encodes the transfer function from efferent firing frequency to the gain of the cochlear amplifier110. In this regard, the activity of the α9α10 nAChR is key in the protective effect of the MOC system. Thus, overexpression of the α9 nAChR subunits in OHCs reduces acoustic injury from exposures causing either temporary or permanent damage11. In addition, a Chrna9Ĺ9T “gain-of-function” knockin mice with a threonine for leucine change at position 9´ in the second transmembrane domain of the α9 nAChR subunit, leading to an enhanced strength of MOC-mediated cochlear inhibition12, 111, shows less permanent hearing loss following exposure to intense noise12. The introduction of this threonine for leucine change, results in an α9α10 nAChR with a decrease in the desensitization rate, an increase in the potency of ACh and spontaneous receptor openings when expressed in an heterologous expression system112 and in prolonged synaptic currents with slower desensitization kinetics when assessed in an ex-vivo organ of Corti preparation (Figure 3)12, 110, 111. Chrna9Ĺ9T knockin, with enhanced efferent activity, have also proven that the extent of hidden hearing loss produced by noise exposure is dependent upon the level of MOC cholinergic activity113. Thus, strengthening MOC feedback by enhancement of α9α10 nAChR activity can reduce noise-induced hearing loss.
Figure 3.
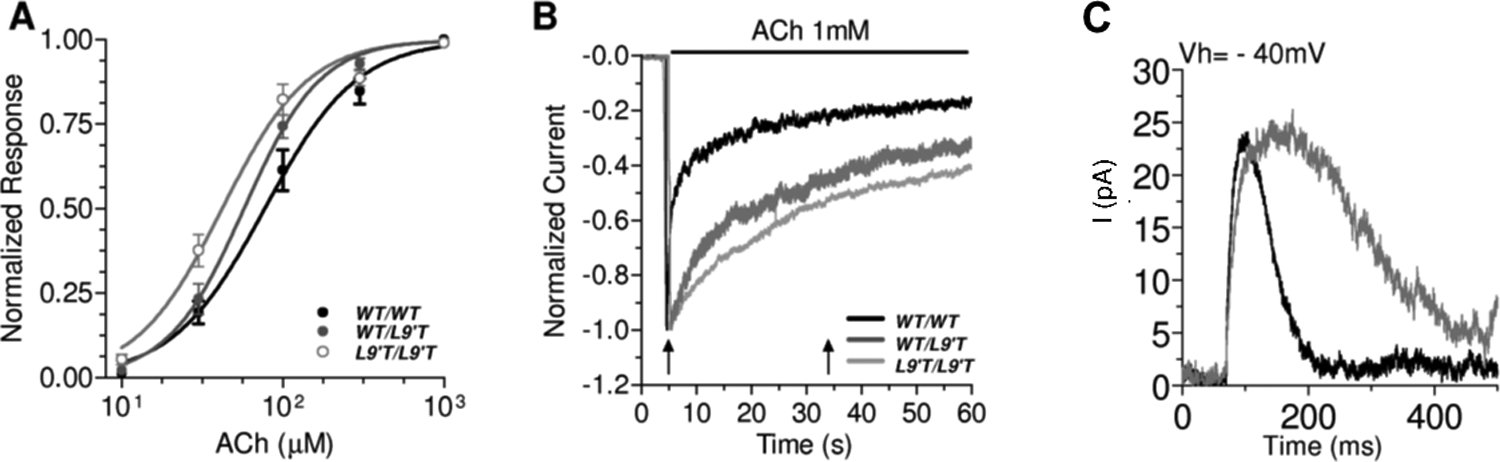
Hypersensitive and Slowly Desensitizing responses in Chrna9Ĺ9T knockin mice. (A) Acetylcholine is more potent in α9α10 nAChRs of mutant mice; (B) Hair cell responses to 1 mM ACh during 1 min show slower desensitization kinetics in mutant mice; (C) Spontaneous synaptic MOC-hair cell currents are prolonged in mutant mice. Adapted from Taranda et al (2009)12 under Creative Commons Attribution (CC BY) license.
The mechanism/s underlying the protective effect of the MOC system are poorly understood. One can propose that they could be the consequence of either a mechanical or a metabolic OHC effect. The former would derive from the reduction of amplification of cochlear vibrations produced by OHC electromotility and the latter from a direct protection from damage of the very acoustic vulnerable OHCs11. A mechanical effect is less favored, since the magnitude of the reduction of cochlear vibrations by MOC activation is higher at low-mid, but probably not at high levels of acoustic input114. This suggests that the MOC protective effects are likely independent of SK2 activation that leads to the OHC K+ efflux, cell hyperpolarization and inhibition of electromotility. This is further supported by the finding that, contrary to that observed when overexpressing the α9 nAChR subunit11, a mouse model that overexpresses SK2 and shows enhanced MOC-evoked cochlear suppression, does not exhibit resistance to acoustic injury115.
If an alternative metabolic effect of MOC activation leads to protection of OHCs damage, a downstream effect of Ca2+ signaling needs to be considered, due to the high calcium permeability of mammalian α9α10 nAChR74, 76, 116. An increase in intracellular Ca2+ might activate protein phosphorylation of the motor protein prestin or of cytoskeletal components, leading to changes in OHC axial stiffness117, 118. On the other hand, a traumatic insult leads to the activation of multiple cellular signaling pathways that affect gene expression and are a balancing act between those involved in cell survival to restore homeostasis and in cell death via apoptotic or necrotic pathways. Although in general one considers that calcium overload leads to cell death, the controlled Ca2+ entry through ion channels is also involved in pro-survival or anti-apoptotic pathways, through the activation of protein kinase C119. One accepted mechanism of hair cell damage is the accumulation of reactive oxygen and reactive nitrogen species120, 121, which are mainly produced in the mitochondria and increased by the accumulation of extracellular calcium120. On the other hand, mitogen-activated protein kinases (MAPKs) are important mediators of both damage and survival signals120. Thus, stress-activated MAPKs include c-Jun-N-terminal kinase (JNK) isoforms and p38 MAPK can lead to apoptosis and necrosis, whereas the extracellular regulated kinase (ERK) is associated with cell survival and proliferation120. The ERK protective pathway is dependent upon Ca2+ activation122. In addition, the phosphoinositide-3 kinase/protein kinase B (PI3K/Akt) pathway is implicated in hair cell survival123. One can propose that Ca2+ entry at the base of OHC through α9α10 nAChRs could participate in hair cell survival mechanisms. In this regard, Ca2+ entry and distribution at the base of OHCs is tightly controlled93. Electron micrographs have shown postsynaptic cisterns within OHCs, closely aligned in apposition with presynaptic efferent synaptic contacts (Engström, 1958; Fuchs et al., 2014; Saito, 1980; Smith & Sjöstrand, 1961). These might serve as a barrier for free calcium diffusion in the cytoplasm. Moreover, they can serve as a Ca2+ store, modulating efferent synaptic responses through both Ca2+ ATPases (of the sarcoplasmic type, SERCA) and ryanodine receptors (RyR) (Evans et al., 2000; Grant et al., 2006; Lioudyno et al., 2004; Sridhar et al., 1997).
4.4. α9α10 nicotinic acetylcholine receptors and presbycusis
Recent experiments in mice have shown that MOC efferents are important for the long-term maintenance of cochlear function during aging, even in the absence of acoustic overexposure124. Thus, MOC de-efferentation accelerates age-related amplitude reduction in cochlear neural responses and increases the loss of afferent synapses, a characteristic of hidden hearing loss124. Moreover, C57 mice, which are used as a model for presbycusis, show decline in the neural population of the trapezoid body nuclei and efferent inhibition125 and in MOC-OHC synaptic terminals, independent of OHC loss126. In addition, experiments in mice have shown that MOC decline precedes age-related hearing loss125. Recent work has shown that, compared to rodents, MOC efferent innervation in humans is less abundant and also decreases with aging127. Functional studies have further indicated that the contralaterally evoked MOC reflex is weakened for frequencies <1500 Hz (where medial efferent effects are largest) in middle age human subjects128. Taken together, the presented evidence suggests that loss of MOC function may play a role in the development of presbycusis in both humans and animal models127. Therefore, the MOC-OHC synapse plays a key function in age-related hearing loss. In the absence of high levels of sounds as a confounding factor, aged Chrna9Ĺ9T knockin mice with enhanced MOC strength, are protected from the loss of acoustic sensitivity, cochlear synaptopathy and hair cell loss, compared to aged wild-type mice which exhibit elevated acoustic thresholds together with loss of afferent synapses of IHCs throughout the cochlea and some OHC loss13. The mechanisms underlying the protective effect of the MOC system on inner ear aging are mainly unknown, but could derive from the activation of pro-survival or anti-apoptotic pathways as suggested above for noise-induced hearing loss. Taking together, these results suggest that strengthening MOC feedback by enhancement of α9α10 nAChR activity can slow cochlear aging.
4.5. α9α10 nicotinic acetylcholine receptors in pharmacoterapeutics
In general, nicotinic receptor ligands can be classified into agonists, allosteric agonists, antagonists and allosteric modulators129. Orthosteric agonists and antagonists contact highly conserved amino acids in the ACh-binding site at the interface of two adjacent subunits129. An additional unorthodox orthosteric ACh-binding site has been recently discovered at some α/α and β/α subunit interfaces, in nAChRs with alternative stoichiometries bearing 3 α and 2 β subunits. Unorthodox sites synergize with orthodox sites to promote activation130–134. Allosteric agonists induce nAChR activation but do not bind to the orthosteric binding site. On the other hand, allosteric modulators may stimulate (PAM) or inhibit (NAM) nAChR function elicited by the agonist by binding to regulatory sites other than ACh binding sites129, 135–137. In addition, silent allosteric modulators (SAMs) have also been reported for nAChRs. These compounds can block the effect of other allosteric modulators (PAMs, NAMs or allosteric agonists) by binding competitively at an allosteric binding site. Three types of effects from PAMs have been identified for nAChRs. Type I PAMs increase the agonist peak responses in the absence of changes in desensitization kinetics138–140; type II PAMs increase agonist peak responses, slow desensitization kinetics and reactivate desensitized receptors138, 139, 141; type III PAMs are allosteric agonists, they increase agonist activation and can also activate nAChRs directly in the absence of agonists142, 143. Nicotinic receptor agonists (both full and partial) have beneficial effects in clinical and/or preclinical studies for CNS disorders. This include addiction, obsessive–compulsive disorder, pain, schizophrenia, autism, attention deficit/hyperactivity disorder and Parkinson’s and Alzheimer’s disease (for reviews see129, 144, 145). However, chronic treatment with agonists may provide suboptimal benefit because sustained receptor activation leads to desensitization. Moreover, the fact that neuronal subunits assemble into different combinatorial assemblies giving rise to a plethora of nAChRs that play a role in a number of different neural functions, leads to agonists with considerable off target side effects. On the other hand, PAMs which can reinforce the endogenous cholinergic neurotransmission without directly stimulating the target receptors, do not lead to desensitization, have less side effects and have recently appeared as attractive pharmacotherapeutic compounds for CNS disorders129.
The observation that the α9α10 nAChR has a restricted expression pattern, are not expressed in the brain66, 84, 146–148 and have a different pharmacological profile when compared to other nAChRs, makes this receptor a suitable pharmacotherapeutic target for the design of drugs with less side effects, specially of central origin. The pharmacotherapeutic activity of α9 and/or α9α10 nAChR antagonists have been investigated in several types of pain in animal models, probably due to the expression of α9α10 nAChR in different immune cells involved in inflammatory processes149–152. Moreover, α9 and/or α10 nAChR subunit blockers have been also suggested to be of use in animal models of immune diseases such as experimental autoimmune encephalomyelitis153, 154. In addition, the NMDA and α9α10 nAChR antagonist neramexane155, 156, has been tested in clinical trials for the treatment of tinnitus without success (https://clinicaltrials.gov/). The allosteric α9α10 nAChR antagonist alphaO-Conotoxin GeXIVA, has resulted in antitumor effects157, 158. However, the potential use of α9α10 nAChR PAMs in therapeutics has not been investigated. The observation that in Chrna9Ĺ9T knockin mice, which are resistant to permanent noise-induced and hidden hearing loss and have a delayed presbycusis12, 111, efferent synaptic responses are prolonged and ACh responses are potentiated and have a slower desensitization kinetics (Figure 3), poses putative α9α10 nAChR type II PAMs as potential inner ear pharmacotheraputic drugs. The serendipitous discovery that the store active compound ryanodine159 and the serotonin type 3 receptor agonist 1-(m-chlorophenyl)-biguanid, potentiate α9α10-mediated ACh-responses77, with no intrinsic activity per se, opens a possible avenue for the design of positive allosteric modulators for this nAChR subtype. So far the search for α9α10 PAMS has been hampered by the lack of high throughput screening assays of small molecule libraries. The recent successful expression of these receptors in HEK cells coupled to FLIPR calcium assay160 opens a new area in the search for α9α10 nAChR selective lead compounds as otoprotectants. Moreover, the availability of the crystal structure of the α9 subunit extracellular domain72, and the feasibility of radioligand binding assays of compounds to purified extracellular α9 protein fragments, will further aid to decipher the orthosteric or allosteric interaction of novel α9α10 nAChR ligands161.
5. Conclusions
Hearing loss is a major health problem that affects the quality of life if left unattended. The exposure to loud sounds is the most prevalent and modifiable environmental risk factor leading to hearing loss. Thus, prevention from exposure to damaging sound levels is the most efficient and safe way to proceed. This requires education and regulation enforcement. However, protection from and/or avoiding the exposure to loud sounds is not always possible. Therefore, a plethora of drugs have been used to prevent from or treat noise-induced hearing loss, with very limited efficacy. Medial olivocochlear fibres that contact OHCs, protect the inner ear from the damage produced by the exposure to overly loud sounds. Thus, compounds that enhance MOC activity appear as a near physiological way to approach the problem. In this regard, the properties of the α9α10 nAChR, which mediates synaptic transmission between MOC fibres and OHCs, has been extensively studied in recent years and appears as a possible pharmacotherapeutic target. Positive allosteric modulators of nicotinic receptors, compounds which can reinforce the endogenous cholinergic neurotransmission without directly stimulating the target receptors, do not lead to desensitization, have less side effects and have recently appeared as attractive pharmacotherapeutics for CNS disorders. Similar compounds that target α9α10 nAChRs would be an alternative way to tackle the debilitating condition resulting from noise-induced hearing loss.
8. Expert opinion
The market for a drug indicated for prevention of noise-induced, hidden hearing loss and presbycusis is huge and will grow further. In spite of the existence of such a huge market, compounds in pharma pipelines are scarce. Although a wide range of compounds with different cellular targets have been tested in animal models and some used in the clinics, their effectiveness is limited, and serendipitous discoveries of effective pharma treatments are lacking. In this regard, the α9α10 nAChR emerges as a new target to be investigated. The rationale behind this new avenue of research is that activation of the α9α10 nAChRs present in OHCs prevents noise-induced, hidden hearing loss and presbycusis. Two alternative compounds could be developed: agonists that bind to the orthostheric ligand bind site or positive allosteric modulators that enhance agonist activity. Compared to other nAChRs, very few agonists of α9α10 nAChRs have been described so far and most classical nAChR agonists act as antagonists of this receptor67. The crystal structure of the α9 extracellular domain, together with molecular docking simulations and mutagenesis experiments, are beginning to decipher residues that impair agonist binding in α9α10 compared to other nAChRs72, 162, 163. In this regard, very recent discoveries shed light into novel α9α10 nAChR agonists164.
In the absence of α9α10 nAChRs agonists, positive allosteric modulators, appear as the best option. In fact, PAMS of nAChRs in general are considered as a better pharmacotherapeutic tool, since in vivo efficacy of ligands binding to the orthosteric site is limited due to desensitization. This is circumvented by using PAMs, which lack any intrinsic activity, but can enhance the effect of the orthosteric endogenous agonists138, 139, 141. The best example of PAMs used in clinical settings are the benzodiazepines which enhance GABAA receptor activity, another member of the pentameric ligand-gated ion superfamily165. Although no nAChR PAMS are approved for therapeutics yet, a wide range of them have been developed for neuronal receptors with promising results. The proof of concept that allosteric potentiation of α9α10 nAChRs responses is feasible is provided by the results with ryanodine159 and the serotonin type 3 receptor agonist 1-(m-chlorophenyl)-biguanid77. However, the first next step in order to prove that α9α10 nAChRs PAMS are effective in hearing disorders, is to test them in animal models of noise-induced hearing loss. Moreover, additional α9α10 nAChRs PAMS need to be discovered and tested. This is now facilitated by the recent success in the expression of these receptors in cells coupled to calcium imaging, which allows high throughput small molecule screening160. Moreover, the known crystal structure of the α9 subunit, coupled to molecular modelling, will aid to decipher the binding site of PAMS and facilitated further virtual screening for compounds166.
Targeting α9α10 nAChRs as a pharmacotherapeutic approach leads to several open questions that will need to be addressed in the future. First, although it is well established that enhancement of the MOC system prevents noise-induced and hearing hidden loss, will this approach be efficacious as otoprotectants immediately after the damage is produced? This would be ideal in order not only to prevent, but to treat these disorders. Second, the pharmacokinetics of these compounds and the passage through the blood-cochlea barrier need to be taken into consideration for a systemic versus a local drug delivery. Finally, auditory perceptual side effects due to the activation of the MOC system and the reduction of the gain of the cochlea need to be investigated. Despite these caveats, much progress has been made in understanding cochlear physiology and the biophysical and pharmacological properties α9α10 nAChRs, an odd member of the nicotinic cholinergic family of receptors. The time is right for the search of α9α10 nAChRs targeting compounds.
Article highlights.
Hearing loss is one of the most prevalent sensory disabilities. It is a major public health problem, given that its impact on human communication and quality of life is devastating.
Within the environmental or lifestyle preventable factors that contribute the most to hearing loss is the exposure to elevated noise, which can be of occupational, recreational, or environmental origin.
Several drugs have been investigated and/or used in the treatment of noise-induced hearing loss; a spectrum of outcomes has been observed and there is poor evidence to support their use.
The protective effect of the medial olivocochlear system from acute and chronic noise-induced hearing loss has been described in animal models. Thus, enhancing the strength of the MOC system appears is a means to protect from noise-induced hearing loss.
Since the α9α10 nAChR mediates synaptic transmission between MOC fibers and cochlear hair cells, it appears to be a novel target for the development of drugs that prevent noise-induced hearing loss.
The development of positive allosteric modulators of α9α10 nAChRs is proposed as a novel approach in the prevention from hearing disorders produced by exposure to loud sounds.
Funding
This work was supported by Agencia Nacional de Promoción Científica y Técnica and Scientific Grand Prize from the Fondation Pour L’Audition, NIH grant R01 DC001508.
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers
- 1.Olusanya BO, Neumann KJ, Saunders JE. The global burden of disabling hearing impairment: a call to action. Bulletin of the World Health Organization 2014. May 1;92(5):367–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nordvik Ø, Laugen Heggdal PO, Brännström J, Vassbotn F, Aarstad AK, Aarstad HJ. Generic quality of life in persons with hearing loss: a systematic literature review. BMC ear, nose, and throat disorders 2018;18:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deal JA, Goman AM, Albert MS, Arnold ML, Burgard S, Chisolm T, et al. Hearing treatment for reducing cognitive decline: Design and methods of the Aging and Cognitive Health Evaluation in Elders randomized controlled trial. Alzheimer’s & dementia (New York, N Y) 2018;4:499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loughrey DG, Kelly ME, Kelley GA, Brennan S, Lawlor BA. Association of Age-Related Hearing Loss With Cognitive Function, Cognitive Impairment, and Dementia: A Systematic Review and Meta-analysis. JAMA otolaryngology-- head & neck surgery 2018. Feb 1;144(2):115–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, et al. Dementia prevention, intervention, and care. Lancet (London, England) 2017. Dec 16;390(10113):2673–734. [DOI] [PubMed] [Google Scholar]
- 6.Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet (London, England) 2020. Aug 8;396(10248):413–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO. World report on hearing. https://wwwwhoint/publications/i/item/world-report-on-hearing 2021;
- 8.Haile LM, Collaborators GHL. Hearing loss prevalence and years lived with disability, 1990–2019: findings from the Global Burden of Disease Study 2019. Lancet (London, England) 2021. Mar 13;397(10278):996–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCormack A, Edmondson-Jones M, Somerset S, Hall D. A systematic review of the reporting of tinnitus prevalence and severity. Hear Res 2016. Jul;337:70–9. [DOI] [PubMed] [Google Scholar]
- 10.Maison SF, Usubuchi H, Liberman MC. Efferent feedback minimizes cochlear neuropathy from moderate noise exposure. J Neurosci 2013. Mar 27;33(13):5542–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maison SF, Luebke AE, Liberman MC, Zuo J. Efferent protection from acoustic injury is mediated via alpha9 nicotinic acetylcholine receptors on outer hair cells. J Neurosci 2002. Dec 15;22(24):10838–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taranda J, Maison SF, Ballestero JA, Katz E, Savino J, Vetter DE, et al. A point mutation in the hair cell nicotinic cholinergic receptor prolongs cochlear inhibition and enhances noise protection. PLoS Biol 2009. Jan 20;7(1):e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boero LE, Castagna VC, Terreros G, Moglie MJ, Silva S, Maass JC, et al. Preventing presbycusis in mice with enhanced medial olivocochlear feedback. Proc Natl Acad Sci U S A 2020. May 26;117(21):11811–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buonfiglio P, Bruque CD, Luce L, Giliberto F, Lotersztein V, Menazzi S, et al. GJB2 and GJB6 Genetic Variant Curation in an Argentinean Non-Syndromic Hearing-Impaired Cohort. Genes 2020. Oct 21;11(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korver AM, Smith RJ, Van Camp G, Schleiss MR, Bitner-Glindzicz MA, Lustig LR, et al. Congenital hearing loss. Nature reviews Disease primers 2017. Jan 12;3:16094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Camp G, Smith R. Hereditary Hearing Loss Homepage, https://hereditaryhearingloss.org.. 2021.
- 17.Zhan W, Cruickshanks KJ, Klein BE, Klein R, Huang GH, Pankow JS, et al. Modifiable determinants of hearing impairment in adults. Preventive medicine 2011. Oct;53(4–5):338–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Concha-Barrientos M, Campbell-Lendrum D, Steenland K. Occupational noise: assessing the burden of disease from work-related hearing impairment at national and local levels. Geneva, World Health Organization; (WHO Environmental Burden of Disease Series, No 9) 2004. [Google Scholar]
- 19.Lie A, Skogstad M, Johannessen HA, Tynes T, Mehlum IS, Nordby KC, et al. Occupational noise exposure and hearing: a systematic review. International archives of occupational and environmental health 2016. Apr;89(3):351–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Śliwińska-Kowalska M, Zaborowski K. WHO Environmental Noise Guidelines for the European Region: A Systematic Review on Environmental Noise and Permanent Hearing Loss and Tinnitus. International journal of environmental research and public health 2017. Sep 27;14(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ivory R, Kane R, Diaz RC. Noise-induced hearing loss: a recreational noise perspective. Curr Opin Otolaryngol Head Neck Surg 2014. Oct;22(5):394–8. [DOI] [PubMed] [Google Scholar]
- 22.Neitzel RL, Fligor BJ. Risk of noise-induced hearing loss due to recreational sound: Review and recommendations. J Acoust Soc Am 2019. Nov;146(5):3911. [DOI] [PubMed] [Google Scholar]
- 23.Pienkowski M. Loud Music and Leisure Noise Is a Common Cause of Chronic Hearing Loss, Tinnitus and Hyperacusis. International journal of environmental research and public health 2021. Apr 16;18(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elgoyhen AB, Langguth B, De Ridder D, Vanneste S. Tinnitus: perspectives from human neuroimaging. Nat Rev Neurosci 2015. Oct;16(10):632–42. [DOI] [PubMed] [Google Scholar]
- 25.Langguth B, Elgoyhen AB, Cederroth CR. Therapeutic Approaches to the Treatment of Tinnitus. Annu Rev Pharmacol Toxicol 2019. Jan 6;59:291–313. [DOI] [PubMed] [Google Scholar]
- 26.De Ridder D, Schlee W, Vanneste S, Londero A, Weisz N, Kleinjung T, et al. Tinnitus and tinnitus disorder: Theoretical and operational definitions (an international multidisciplinary proposal). Prog Brain Res 2021;260:1–25. [DOI] [PubMed] [Google Scholar]
- 27.De Ridder D, Elgoyhen AB, Romo R, Langguth B. Phantom percepts: tinnitus and pain as persisting aversive memory networks. Proc Natl Acad Sci U S A 2011. May 17;108(20):8075–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Ridder D, Vanneste S, Weisz N, Londero A, Schlee W, Elgoyhen AB, et al. An integrative model of auditory phantom perception: tinnitus as a unified percept of interacting separable subnetworks. Neuroscience and biobehavioral reviews 2014. Jul;44:16–32. [DOI] [PubMed] [Google Scholar]
- 29.Elgoyhen AB, Langguth B. Pharmacological approaches to the treatment of tinnitus. Drug discovery today 2010. Apr;15(7–8):300–5. [DOI] [PubMed] [Google Scholar]
- 30.Elgoyhen AB, Langguth B, Nowak W, Schecklmann M, De Ridder D, Vanneste S. Identifying tinnitus-related genes based on a side-effect network analysis. CPT: pharmacometrics & systems pharmacology 2014. Jan 29;3(1):e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Langguth B, Salvi R, Elgoyhen AB. Emerging pharmacotherapy of tinnitus. Expert opinion on emerging drugs 2009. Dec;14(4):687–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheppard A, Ralli M, Gilardi A, Salvi R. Occupational Noise: Auditory and Non-Auditory Consequences. International journal of environmental research and public health 2020. Dec 2;17(23). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bohne BA, Harding GW. Degeneration in the cochlea after noise damage: primary versus secondary events. The American journal of otology 2000. Jul;21(4):505–9. [PubMed] [Google Scholar]
- 34.Wang Y, Hirose K, Liberman MC. Dynamics of noise-induced cellular injury and repair in the mouse cochlea. J Assoc Res Otolaryngol 2002. Sep;3(3):248–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liberman MC, Kiang NY. Acoustic trauma in cats. Cochlear pathology and auditory-nerve activity. Acta Otolaryngol Suppl 1978;358:1–63. [PubMed] [Google Scholar]
- 36.Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci 2009. Nov 11;29(45):14077–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liberman MC, Epstein MJ, Cleveland SS, Wang H, Maison SF. Toward a Differential Diagnosis of Hidden Hearing Loss in Humans. PLoS One 2016;11(9):e0162726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gordon-Salant S. Hearing loss and aging: new research findings and clinical implications. Journal of rehabilitation research and development 2005. Jul-Aug;42(4 Suppl 2):9–24. [DOI] [PubMed] [Google Scholar]
- 39.Sergeyenko Y, Lall K, Liberman MC, Kujawa SG. Age-related cochlear synaptopathy: an early-onset contributor to auditory functional decline. J Neurosci 2013. Aug 21;33(34):13686–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Viana LM, O’Malley JT, Burgess BJ, Jones DD, Oliveira CA, Santos F, et al. Cochlear neuropathy in human presbycusis: Confocal analysis of hidden hearing loss in post-mortem tissue. Hear Res 2015. Sep;327:78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neufeld A, Westerberg BD, Nabi S, Bryce G, Bureau Y. Prospective, randomized controlled assessment of the short- and long-term efficacy of a hearing conservation education program in Canadian elementary school children. Laryngoscope 2011. Jan;121(1):176–81. [DOI] [PubMed] [Google Scholar]
- 42.Verbeek JH, Kateman E, Morata TC, Dreschler WA, Mischke C. Interventions to prevent occupational noise-induced hearing loss: a Cochrane systematic review. International journal of audiology 2014. Mar;53 Suppl 2(0 2):S84–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arenas JP, Suter AH. Comparison of occupational noise legislation in the Americas: an overview and analysis. Noise & health 2014. Sep-Oct;16(72):306–19. [DOI] [PubMed] [Google Scholar]
- 44.Hecht QA, Hammill TL, Calamia PT, Smalt CJ, Brungart DS. Characterization of acute hearing changes in United States military populations. J Acoust Soc Am 2019. Nov;146(5):3839. [DOI] [PubMed] [Google Scholar]
- 45.Wells TS, Seelig AD, Ryan MA, Jones JM, Hooper TI, Jacobson IG, et al. Hearing loss associated with US military combat deployment. Noise & health 2015. Jan-Feb;17(74):34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahmed MM, Allard RJ, Esquivel CR. Noise-Induced Hearing Loss Treatment: Systematic Review and Meta-analysis. Mil Med 2021. Jan 11. [DOI] [PubMed] [Google Scholar]
- 47.Yamashita D, Jiang HY, Schacht J, Miller JM. Delayed production of free radicals following noise exposure. Brain Res 2004. Sep 3;1019(1–2):201–9. [DOI] [PubMed] [Google Scholar]
- 48.Ohlemiller KK, Wright JS, Dugan LL. Early elevation of cochlear reactive oxygen species following noise exposure. Audiol Neurootol 1999. Sep-Oct;4(5):229–36. [DOI] [PubMed] [Google Scholar]
- 49.Sha SH, Schacht J. Emerging therapeutic interventions against noise-induced hearing loss. Expert opinion on investigational drugs 2017. Jan;26(1):85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang J, Ruel J, Ladrech S, Bonny C, van de Water TR, Puel JL. Inhibition of the c-Jun N-terminal kinase-mediated mitochondrial cell death pathway restores auditory function in sound-exposed animals. Mol Pharmacol 2007. Mar;71(3):654–66. [DOI] [PubMed] [Google Scholar]
- 51.Karlin A. Ion channel structure: emerging structure of the nicotinic acetylcholine receptors. Nature Reviews Neurosc 2002;3:102–14. [DOI] [PubMed] [Google Scholar]
- 52.Corringer PJ, Poitevin F, Prevost MS, Sauguet L, Delarue M, Changeux JP. Structure and pharmacology of pentameric receptor channels: from bacteria to brain. Structure (London, England : 1993) 2012. Jun 6;20(6):941–56. [DOI] [PubMed] [Google Scholar]
- 53.Marcovich I, Moglie MJ, Carpaneto Freixas AE, Trigila AP, Franchini LF, Plazas PV, et al. Distinct Evolutionary Trajectories of Neuronal and Hair Cell Nicotinic Acetylcholine Receptors. Mol Biol Evol 2020. Apr 1;37(4):1070–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zoli M, Pistillo F, Gotti C. Diversity of native nicotinic receptor subtypes in mammalian brain. Neuropharmacology 2015. Sep;96(Pt B):302–11. [DOI] [PubMed] [Google Scholar]
- 55.Zoli M, Pucci S, Vilella A, Gotti C. Neuronal and Extraneuronal Nicotinic Acetylcholine Receptors. Current neuropharmacology 2018;16(4):338–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gotti C, Clementi F, Fornari A, Gaimarri A, Guiducci S, Manfredi I, et al. Structural and functional diversity of native brain neuronal nicotinic receptors. Biochem Pharmacol 2009. Oct 1;78(7):703–11. [DOI] [PubMed] [Google Scholar]
- 57.Nelson ME, Kuryatov A, Choi CH, Zhou Y, Lindstrom J. Alternate stoichiometries of alpha4beta2 nicotinic acetylcholine receptors. Mol Pharmacol 2003. Feb;63(2):332–41. [DOI] [PubMed] [Google Scholar]
- 58.Benallegue N, Mazzaferro S, Alcaino C, Bermudez I. The additional ACh binding site at the α4(+)/α4(−) interface of the (α4β2)2α4 nicotinic ACh receptor contributes to desensitization. Br J Pharmacol 2013. Sep;170(2):304–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mazzaferro S, Bermudez I, Sine SM. α4β2 Nicotinic Acetylcholine Receptors: RELATIONSHIPS BETWEEN SUBUNIT STOICHIOMETRY AND FUNCTION AT THE SINGLE CHANNEL LEVEL. J Biol Chem 2017. Feb 17;292(7):2729–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moroni M, Bermudez I. Stoichiometry and pharmacology of two human alpha4beta2 nicotinic receptor types. J Mol Neurosci 2006;30(1–2):95–6. [DOI] [PubMed] [Google Scholar]
- 61.Moroni M, Zwart R, Sher E, Cassels BK, Bermudez I. alpha4beta2 nicotinic receptors with high and low acetylcholine sensitivity: pharmacology, stoichiometry, and sensitivity to long-term exposure to nicotine. Mol Pharmacol 2006. Aug;70(2):755–68. [DOI] [PubMed] [Google Scholar]
- 62.Krashia P, Moroni M, Broadbent S, Hofmann G, Kracun S, Beato M, et al. Human α3β4 neuronal nicotinic receptors show different stoichiometry if they are expressed in Xenopus oocytes or mammalian HEK293 cells. PLoS One 2010. Oct 26;5(10):e13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mishina M, Takai T, Imoto K, Noda M, Takahashi T, Numa S, et al. Molecular distinction between fetal and adult forms of muscle acetylcholine receptor. Nature 1986. May 22–28;321(6068):406–11. [DOI] [PubMed] [Google Scholar]
- 64.Cetin H, Beeson D, Vincent A, Webster R. The Structure, Function, and Physiology of the Fetal and Adult Acetylcholine Receptor in Muscle. Frontiers in molecular neuroscience 2020;13:581097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sgard F, Charpentier E, Bertrand S, Walker N, Caput D, Graham D, et al. A novel human nicotinic receptor subunit, α10, that confers functionality to the α9-subunit. Molec Pharmacol 2002;61:150–59. [DOI] [PubMed] [Google Scholar]
- 66.Elgoyhen AB, Johnson DS, Boulter J, Vetter DE, Heinemann S. Alpha 9: an acetylcholine receptor with novel pharmacological properties expressed in rat cochlear hair cells. Cell 1994. Nov 18;79(4):705–15. [DOI] [PubMed] [Google Scholar]
- 67.Elgoyhen AB, Vetter DE, Katz E, Rothlin CV, Heinemann SF, Boulter J. alpha10: a determinant of nicotinic cholinergic receptor function in mammalian vestibular and cochlear mechanosensory hair cells. Proc Natl Acad Sci U S A 2001. Mar 13;98(6):3501–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Elgoyhen AB, Johnson DS, Boulter J, Vetter DE, Heinemann S. a9: an acetylcholine receptor with novel pharmacological properties expressed in rat cochlear hair cells. Cell 1994;79:705–15. [DOI] [PubMed] [Google Scholar]
- 69.Plazas PV, Katz E, Gomez-Casati ME, Bouzat C, Elgoyhen AB. Stoichiometry of the alpha9alpha10 nicotinic cholinergic receptor. J Neurosci 2005. Nov 23;25(47):10905–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Indurthi DC, Pera E, Kim HL, Chu C, McLeod MD, McIntosh JM, et al. Presence of multiple binding sites on α9α10 nAChR receptors alludes to stoichiometric-dependent action of the α-conotoxin, Vc1.1. Biochem Pharmacol 2014. May 1;89(1):131–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boffi JC, Marcovich I, Gill-Thind JK, Corradi J, Collins T, Lipovsek MM, et al. Differential Contribution of Subunit Interfaces to α9α10 Nicotinic Acetylcholine Receptor Function. Mol Pharmacol 2017. Mar;91(3):250–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zouridakis M, Giastas P, Zarkadas E, Chroni-Tzartou D, Bregestovski P, Tzartos SJ. Crystal structures of free and antagonist-bound states of human α9 nicotinic receptor extracellular domain. Nat Struct Mol Biol 2014. Nov;21(11):976–80. [DOI] [PubMed] [Google Scholar]
- 73.Zouridakis M, Papakyriakou A, Ivanov IA, Kasheverov IE, Tsetlin V, Tzartos S, et al. Crystal Structure of the Monomeric Extracellular Domain of α9 Nicotinic Receptor Subunit in Complex With α-Conotoxin RgIA: Molecular Dynamics Insights Into RgIA Binding to α9α10 Nicotinic Receptors. Frontiers in pharmacology 2019;10:474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lipovsek M, Im GJ, Franchini LF, Pisciottano F, Katz E, Fuchs PA, et al. Phylogenetic differences in calcium permeability of the auditory hair cell cholinergic nicotinic receptor. Proc Natl Acad Sci U S A 2012. Mar 13;109(11):4308–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rothlin CV, Katz E, Verbitsky M, Elgoyhen AB. The alpha9 nicotinic acetylcholine receptor shares pharmacological properties with type A gamma-aminobutyric acid, glycine, and type 3 serotonin receptors. Mol Pharmacol 1999. Feb;55(2):248–54. [DOI] [PubMed] [Google Scholar]
- 76.Gomez-Casati ME, Fuchs PA, Elgoyhen AB, Katz E. Biophysical and pharmacological characterization of nicotinic cholinergic receptors in rat cochlear inner hair cells. J Physiol 2005. Jul 1;566(Pt 1):103–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rothlin CV, Lioudyno MI, Silbering AF, Plazas PV, Casati ME, Katz E, et al. Direct interaction of serotonin type 3 receptor ligands with recombinant and native alpha 9 alpha 10-containing nicotinic cholinergic receptors. Mol Pharmacol 2003. May;63(5):1067–74. [DOI] [PubMed] [Google Scholar]
- 78.Verbitsky M, Rothlin C, Katz E, Elgoyhen AB. Mixed nicotinic-muscarinic properties of the a9 nicotinic cholinergic receptor. Neuropharmacology 2000;39:2515–24. [DOI] [PubMed] [Google Scholar]
- 79.Lipovsek M, Marcovich I, Elgoyhen A. The hair cell α9α10 nicotinic acetylcholine receptor: odd cousin in an old family. Frontiers in Cellular Neuroscience 2021;in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fuchs PA, Murrow BW. A novel cholinergic receptor mediates inhibition of chick cochlear hair cells. Proc Biol Sci 1992. Apr 22;248(1321):35–40. [DOI] [PubMed] [Google Scholar]
- 81.Franchini LF, Elgoyhen AB. Adaptive evolution in mammalian proteins involved in cochlear outer hair cell electromotility. Mol Phylogenet Evol 2006. Dec;41(3):622–35. [DOI] [PubMed] [Google Scholar]
- 82.Vetter DE, Katz E, Maison SF, Taranda J, Turcan S, Ballestero J, et al. The alpha10 nicotinic acetylcholine receptor subunit is required for normal synaptic function and integrity of the olivocochlear system. Proc Natl Acad Sci U S A 2007. Dec 18;104(51):20594–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Katz E, Elgoyhen AB, Gomez-Casati ME, Knipper M, Vetter DE, Fuchs PA, et al. Developmental regulation of nicotinic synapses on cochlear inner hair cells. J Neurosci 2004. Sep 8;24(36):7814–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morley B, Li H, Hiel H, Drescher D, Elgoyhen AB. Identification of the subunits of the nicotinic cholinergic receptors in the rat cochlea using RT-PCR and in situ hybridization. Molec Brain Res 1998;53:78–87. [DOI] [PubMed] [Google Scholar]
- 85.Vetter DE, Liberman MC, Mann J, Barhanin J, Boulter J, Brown MC, et al. Role of alpha9 nicotinic ACh receptor subunits in the development and function of cochlear efferent innervation. Neuron 1999. May;23(1):93–103. [DOI] [PubMed] [Google Scholar]
- 86.Elgoyhen AB, Katz E, Fuchs PA. The nicotinic receptor of cochlear hair cells: a possible pharmacotherapeutic target? Biochem Pharmacol 2009. Oct 1;78(7):712–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fuchs PA, Murrow BW. Cholinergic inhibition of short (outer) hair cells of the chick’s cochlea. J Neurosci 1992. Mar;12(3):800–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Glowatzki E, Fuchs PA. Cholinergic synaptic inhibition of inner hair cells in the neonatal mammalian cochlea. Science 2000. Jun 30;288(5475):2366–8. [DOI] [PubMed] [Google Scholar]
- 89.Oliver D, Klocker N, Schuck J, Baukrowitz T, Ruppersberg JP, Fakler B. Gating of Ca2+-activated K+ channels controls fast inhibitory synaptic transmission at auditory outer hair cells. Neuron 2000;26:595–601. [DOI] [PubMed] [Google Scholar]
- 90.Dulon D, Lenoir M. Cholinergic responses in developing outer hair cells of the rat cochlea. European J Neurosci 1996;8:1945–52. [DOI] [PubMed] [Google Scholar]
- 91.Dulon D, Luo L, Zhang C, Ryan AF. Expression of small-conductance calcium-activated potassium channels (SK) in outer hair cells of the rat cochlea. Eur J Neurosci 1998;10:907–15. [DOI] [PubMed] [Google Scholar]
- 92.Moglie MJ, Fuchs PA, Elgoyhen AB, Goutman JD. Compartmentalization of antagonistic Ca(2+) signals in developing cochlear hair cells. Proc Natl Acad Sci U S A 2018. Feb 27;115(9):E2095–e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Moglie MJ, Wengier DL, Elgoyhen AB, Goutman JD. Synaptic contributions to cochlear outer hair cell Ca(2+) dynamics. J Neurosci 2021. Jul 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dallos P. Cochlear amplification, outer hair cells and prestin. Curr Opin Neurobiol 2008. Oct 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zheng J, Shen W, He DZ, Long KB, Madison LD, Dallos P. Prestin is the motor protein of cochlear outer hair cells. Nature 2000. May 11;405(6783):149–55. [DOI] [PubMed] [Google Scholar]
- 96.Liberman MC, Brown MC. Physiology and anatomy of single olivocochlear neurons in the cat. Hear Res 1986;24(1):17–36. [DOI] [PubMed] [Google Scholar]
- 97.Wiederhold ML, Kiang NY. Effects of electric stimulation of the crossed olivocochlear bundle on single auditory-nerve fibers in the cat. J Acoust Soc Am 1970. Oct;48(4):950–65. [DOI] [PubMed] [Google Scholar]
- 98.Gifford ML, Guinan JJ Jr., Effects of electrical stimulation of medial olivocochlear neurons on ipsilateral and contralateral cochlear responses. Hear Res 1987;29(2–3):179–94. [DOI] [PubMed] [Google Scholar]
- 99.Galambos R. Suppression of auditory nerve activity by stimulation of efferent fibers to cochlea. J Neurophysiol 1956. Sep;19(5):424–37. [DOI] [PubMed] [Google Scholar]
- 100.Guinan JJ. Physiology of the Medial and Lateral Olivocochlear Systems. In: Ryugo DK, Fay RR, Popper AN, eds. Auditory and Vestibular Efferents. New York: Springer; 2011:39–81. [Google Scholar]
- 101.Liberman MC. The olivocochlear efferent bundle and susceptibility of the inner ear to acoustic injury. J Neurophysiol 1991. Jan;65(1):123–32. [DOI] [PubMed] [Google Scholar]
- 102.Maison SF, Liberman MC. Predicting vulnerability to acoustic injury with a noninvasive assay of olivocochlear reflex strength. J Neurosci 2000. Jun 15;20(12):4701–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kujawa SG, Liberman MC. Conditioning-related protection from acoustic injury: effects of chronic deefferentation and sham surgery. J Neurophysiol 1997. Dec;78(6):3095–106. [DOI] [PubMed] [Google Scholar]
- 104.Handrock M, Zeisberg J. The influence of the effect system on adaptation, temporary and permanent threshold shift. Arch Otorhinolaryngol 1982;234(2):191–5. [DOI] [PubMed] [Google Scholar]
- 105.Liberman MC, Gao WY. Chronic cochlear de-efferentation and susceptibility to permanent acoustic injury. Hear Res 1995. Oct;90(1–2):158–68. [DOI] [PubMed] [Google Scholar]
- 106.Rajan R. Centrifugal pathways protect hearing sensitivity at the cochlea in noisy environments that exacerbate the damage induced by loud sound. J Neurosci 2000. Sep 01;20(17):6684–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rajan R. Effect of electrical stimulation of the crossed olivocochlear bundle on temporary threshold shifts in auditory sensitivity. I. Dependence on electrical stimulation parameters. J Neurophysiol 1988;60:549–68. [DOI] [PubMed] [Google Scholar]
- 108.Rajan R. Functions of the efferent pathways to the mammalian cochlea. Information Processing in Mammalian Auditory and Tactile Systems: Alan R. Liss, Inc. 1990:81–96. [Google Scholar]
- 109.Reiter ER, Liberman MC. Efferent-mediated protection from acoustic overexposure: relation to slow effects of olivocochlear stimulation. J Neurophysiol 1995. Feb;73(2):506–14. [DOI] [PubMed] [Google Scholar]
- 110.Ballestero J, Zorrilla de San Martin J, Goutman J, Elgoyhen AB, Fuchs PA, Katz E. Short-term synaptic plasticity regulates the level of olivocochlear inhibition to auditory hair cells. J Neurosci 2011. Oct 12;31(41):14763–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wedemeyer C, Vattino LG, Moglie MJ, Ballestero J, Maison SF, Di Guilmi MN, et al. A Gain-of-Function Mutation in the α9 Nicotinic Acetylcholine Receptor Alters Medial Olivocochlear Efferent Short-Term Synaptic Plasticity. J Neurosci 2018. Apr 18;38(16):3939–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Plazas PV, De Rosa MJ, Gomez-Casati ME, Verbitsky M, Weisstaub N, Katz E, et al. Key roles of hydrophobic rings of TM2 in gating of the alpha9alpha10 nicotinic cholinergic receptor. Br J Pharmacol 2005. Aug;145(7):963–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Boero LE, Castagna VC, Di Guilmi MN, Goutman JD, Elgoyhen AB, Gómez-Casati ME. Enhancement of the Medial Olivocochlear System Prevents Hidden Hearing Loss. J Neurosci 2018. Aug 22;38(34):7440–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Guinan JJ Jr., Stankovic KM. Medial efferent inhibition produces the largest equivalent attenuations at moderate to high sound levels in cat auditory-nerve fibers. J Acoust Soc Am 1996. Sep;100(3):1680–90. [DOI] [PubMed] [Google Scholar]
- 115.Maison SF, Parker LL, Young L, Adelman JP, Zuo J, Liberman MC. Overexpression of SK2 channels enhances efferent suppression of cochlear responses without enhancing noise resistance. J Neurophysiol 2007. Apr;97(4):2930–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Weisstaub N, Vetter DE, Elgoyhen AB, Katz E. The alpha9alpha10 nicotinic acetylcholine receptor is permeable to and is modulated by divalent cations. Hear Res 2002. May;167(1–2):122–35. [DOI] [PubMed] [Google Scholar]
- 117.Zhang M, Kalinec GM, Urrutia R, Billadeau DD, Kalinec F. ROCK-dependent and ROCK-independent control of cochlear outer hair cell electromotility. J Biol Chem 2003. Sep 12;278(37):35644–50. [DOI] [PubMed] [Google Scholar]
- 118.Sziklai I, Szõnyi M, Dallos P. Phosphorylation mediates the influence of acetylcholine upon outer hair cell electromotility. Acta Otolaryngol 2001. Jan;121(2):153–6. [DOI] [PubMed] [Google Scholar]
- 119.Cerella C, Diederich M, Ghibelli L. The dual role of calcium as messenger and stressor in cell damage, death, and survival. International journal of cell biology 2010;2010:546163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kurabi A, Keithley EM, Housley GD, Ryan AF, Wong AC. Cellular mechanisms of noise-induced hearing loss. Hear Res 2017. Jun;349:129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Henderson D, Bielefeld EC, Harris KC, Hu BH. The role of oxidative stress in noise-induced hearing loss. Ear Hear 2006. Feb;27(1):1–19. [DOI] [PubMed] [Google Scholar]
- 122.Miningou N, Blackwell KT. The road to ERK activation: Do neurons take alternate routes? Cell Signal 2020. Apr;68:109541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen J, Yuan H, Talaska AE, Hill K, Sha SH. Increased Sensitivity to Noise-Induced Hearing Loss by Blockade of Endogenous PI3K/Akt Signaling. J Assoc Res Otolaryngol 2015. Jun;16(3):347–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liberman MC, Liberman LD, Maison SF. Efferent feedback slows cochlear aging. J Neurosci 2014. Mar 26;34(13):4599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhu X, Vasilyeva ON, Kim S, Jacobson M, Romney J, Waterman MS, et al. Auditory efferent feedback system deficits precede age-related hearing loss: contralateral suppression of otoacoustic emissions in mice. J Comp Neurol 2007. Aug 10;503(5):593–604. [DOI] [PubMed] [Google Scholar]
- 126.Fu B, Le Prell C, Simmons D, Lei D, Schrader A, Chen AB, et al. Age-related synaptic loss of the medial olivocochlear efferent innervation. Molecular neurodegeneration 2010. Nov 26;5:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liberman LD, Liberman MC. Cochlear Efferent Innervation Is Sparse in Humans and Decreases with Age. J Neurosci 2019. Nov 27;39(48):9560–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zettel ML, Zhu X, O’Neill WE, Frisina RD. Age-related decline in Kv3.1b expression in the mouse auditory brainstem correlates with functional deficits in the medial olivocochlear efferent system. J Assoc Res Otolaryngol 2007. Jun;8(2):280–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang J, Lindstrom J. Orthosteric and allosteric potentiation of heteromeric neuronal nicotinic acetylcholine receptors. Br J Pharmacol 2018. Jun;175(11):1805–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jain A, Kuryatov A, Wang J, Kamenecka TM, Lindstrom J. Unorthodox Acetylcholine Binding Sites Formed by α5 and β3 Accessory Subunits in α4β2* Nicotinic Acetylcholine Receptors. J Biol Chem 2016. Nov 4;291(45):23452–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mazzaferro S, Bermudez I, Sine SM. Potentiation of a neuronal nicotinic receptor via pseudo-agonist site. Cell Mol Life Sci 2019. Mar;76(6):1151–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mazzaferro S, Benallegue N, Carbone A, Gasparri F, Vijayan R, Biggin PC, et al. Additional acetylcholine (ACh) binding site at alpha4/alpha4 interface of (alpha4beta2)2alpha4 nicotinic receptor influences agonist sensitivity. J Biol Chem 2011. Sep 2;286(35):31043–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Harpsøe K, Ahring PK, Christensen JK, Jensen ML, Peters D, Balle T. Unraveling the high- and low-sensitivity agonist responses of nicotinic acetylcholine receptors. J Neurosci 2011. Jul 27;31(30):10759–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang J, Kuryatov A, Sriram A, Jin Z, Kamenecka TM, Kenny PJ, et al. An Accessory Agonist Binding Site Promotes Activation of α4β2* Nicotinic Acetylcholine Receptors. J Biol Chem 2015. May 29;290(22):13907–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Williams DK, Wang J, Papke RL. Positive allosteric modulators as an approach to nicotinic acetylcholine receptor-targeted therapeutics: advantages and limitations. Biochem Pharmacol 2011. Oct 15;82(8):915–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chatzidaki A, Millar NS. Allosteric modulation of nicotinic acetylcholine receptors. Biochem Pharmacol 2015. Oct 15;97(4):408–17. [DOI] [PubMed] [Google Scholar]
- 137.Grupe M, Grunnet M, Bastlund JF, Jensen AA. Targeting α4β2 nicotinic acetylcholine receptors in central nervous system disorders: perspectives on positive allosteric modulation as a therapeutic approach. Basic & clinical pharmacology & toxicology 2015. Mar;116(3):187–200. [DOI] [PubMed] [Google Scholar]
- 138.Grønlien JH, Håkerud M, Ween H, Thorin-Hagene K, Briggs CA, Gopalakrishnan M, et al. Distinct profiles of alpha7 nAChR positive allosteric modulation revealed by structurally diverse chemotypes. Mol Pharmacol 2007. Sep;72(3):715–24. [DOI] [PubMed] [Google Scholar]
- 139.Collins T, Young GT, Millar NS. Competitive binding at a nicotinic receptor transmembrane site of two α7-selective positive allosteric modulators with differing effects on agonist-evoked desensitization. Neuropharmacology 2011. Dec;61(8):1306–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nielsen BE, Stabile S, Vitale C, Bouzat C. Design, Synthesis, and Functional Evaluation of a Novel Series of Phosphonate-Functionalized 1,2,3-Triazoles as Positive Allosteric Modulators of α7 Nicotinic Acetylcholine Receptors. ACS Chem Neurosci 2020. Sep 2;11(17):2688–704. [DOI] [PubMed] [Google Scholar]
- 141.Wang J, Kuryatov A, Jin Z, Norleans J, Kamenecka TM, Kenny PJ, et al. A Novel α2/α4 Subtype-selective Positive Allosteric Modulator of Nicotinic Acetylcholine Receptors Acting from the C-tail of an α Subunit. J Biol Chem 2015. Nov 27;290(48):28834–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gill JK, Savolainen M, Young GT, Zwart R, Sher E, Millar NS. Agonist activation of alpha7 nicotinic acetylcholine receptors via an allosteric transmembrane site. Proc Natl Acad Sci U S A 2011. Apr 5;108(14):5867–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Horenstein NA, Papke RL, Kulkarni AR, Chaturbhuj GU, Stokes C, Manther K, et al. Critical Molecular Determinants of α7 Nicotinic Acetylcholine Receptor Allosteric Activation: SEPARATION OF DIRECT ALLOSTERIC ACTIVATION AND POSITIVE ALLOSTERIC MODULATION. J Biol Chem 2016. Mar 4;291(10):5049–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gotti C, Riganti L, Vailati S, Clementi F. Brain neuronal nicotinic receptors as new targets for drug discovery. Current pharmaceutical design 2006;12(4):407–28. [DOI] [PubMed] [Google Scholar]
- 145.Hurst R, Rollema H, Bertrand D. Nicotinic acetylcholine receptors: from basic science to therapeutics. Pharmacol Ther 2013. Jan;137(1):22–54. [DOI] [PubMed] [Google Scholar]
- 146.Morley BJ, Whiteaker P, Elgoyhen AB. Commentary: Nicotinic Acetylcholine Receptor α9 and α10 Subunits Are Expressed in the Brain of Mice. Front Cell Neurosci 2018;12:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zuo J, Treadaway J, Buckner TW, Fritzsch B. Visualization of alpha9 acetylcholine receptor expression in hair cells of transgenic mice containing a modified bacterial artificial chromosome. Proc Natl Acad Sci U S A 1999. Nov 23;96(24):14100–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Allen Institute for Brain Science. Allen Brain Atlas API. Available from: brain-map.org/api/index.htm. 2015.
- 149.Hone AJ, Servent D, McIntosh JM. α9-containing nicotinic acetylcholine receptors and the modulation of pain. Br J Pharmacol 2018. Jun;175(11):1915–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Romero HK, Christensen SB, Di Cesare Mannelli L, Gajewiak J, Ramachandra R, Elmslie KS, et al. Inhibition of α9α10 nicotinic acetylcholine receptors prevents chemotherapy-induced neuropathic pain. Proc Natl Acad Sci U S A 2017. Mar 7;114(10):E1825–e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hone AJ, McIntosh JM. Nicotinic acetylcholine receptors in neuropathic and inflammatory pain. FEBS Lett 2018. Apr;592(7):1045–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Christensen SB, Hone AJ, Roux I, Kniazeff J, Pin JP, Upert G, et al. RgIA4 Potently Blocks Mouse α9α10 nAChRs and Provides Long Lasting Protection against Oxaliplatin-Induced Cold Allodynia. Front Cell Neurosci 2017;11:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Liu Q, Li M, Whiteaker P, Shi FD, Morley BJ, Lukas RJ. Attenuation in Nicotinic Acetylcholine Receptor α9 and α10 Subunit Double Knock-Out Mice of Experimental Autoimmune Encephalomyelitis. Biomolecules 2019. Dec 4;9(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Simard AR, Gan Y, St-Pierre S, Kousari A, Patel V, Whiteaker P, et al. Differential modulation of EAE by α9*- and β2*-nicotinic acetylcholine receptors. Immunology and cell biology 2013. Mar;91(3):195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Plazas PV, Savino J, Kracun S, Gomez-Casati ME, Katz E, Parsons CG, et al. Inhibition of the alpha9alpha10 nicotinic cholinergic receptor by neramexane, an open channel blocker of N-methyl-D-aspartate receptors. Eur J Pharmacol 2007. Jul 2;566(1–3):11–9. [DOI] [PubMed] [Google Scholar]
- 156.Rammes G. Neramexane: a moderate-affinity NMDA receptor channel blocker: new prospects and indications. Expert review of clinical pharmacology 2009. May;2(3):231–8. [DOI] [PubMed] [Google Scholar]
- 157.Sun Z, Zhangsun M, Dong S, Liu Y, Qian J, Zhangsun D, et al. Differential Expression of Nicotine Acetylcholine Receptors Associates with Human Breast Cancer and Mediates Antitumor Activity of αO-Conotoxin GeXIVA. Marine drugs 2020. Jan 17;18(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Luo S, Zhangsun D, Harvey PJ, Kaas Q, Wu Y, Zhu X, et al. Cloning, synthesis, and characterization of αO-conotoxin GeXIVA, a potent α9α10 nicotinic acetylcholine receptor antagonist. Proc Natl Acad Sci U S A 2015. Jul 28;112(30):E4026–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Boffi JC, Wedemeyer C, Lipovsek M, Katz E, Calvo DJ, Elgoyhen AB. Positive modulation of the alpha9alpha10 nicotinic cholinergic receptor by ascorbic acid. Br J Pharmacol 2013. Feb;168(4):954–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gu S, Knowland D, Matta JA, O’Carroll ML, Davini WB, Dhara M, et al. Hair cell α9α10 nicotinic acetylcholine receptor functional expression regulated by ligand binding and deafness gene products. Proc Natl Acad Sci U S A 2020. Sep 29;117(39):24534–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kryukova EV, Ivanov IA, Lebedev DS, Spirova EN, Egorova NS, Zouridakis M, et al. Orthosteric and/or Allosteric Binding of α-Conotoxins to Nicotinic Acetylcholine Receptors and Their Models. Marine drugs 2018. Nov 22;16(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Giastas P, Zouridakis M, Tzartos SJ. Understanding structure-function relationships of the human neuronal acetylcholine receptor: insights from the first crystal structures of neuronal subunits. Br J Pharmacol 2018. Jun;175(11):1880–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Moglie MJ, Marcovich I, Corradi J, Carpaneto Freixas AE, Gallino S, Plazas PV, et al. Loss of Choline Agonism in the Inner Ear Hair Cell Nicotinic Acetylcholine Receptor Linked to the α10 Subunit. Frontiers in molecular neuroscience 2021;14:639720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Papke RL, Andleeb H, Stokes C, Quadri M, Horenstein NA. Selective Agonists and Antagonists of α9 Versus α7 Nicotinic Acetylcholine Receptors. ACS Chem Neurosci 2022. Feb 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sigel E, Ernst M. The Benzodiazepine Binding Sites of GABA(A) Receptors. Trends in pharmacological sciences 2018. Jul;39(7):659–71. [DOI] [PubMed] [Google Scholar]
- 166.Smelt CLC, Sanders VR, Newcombe J, Burt RP, Sheppard TD, Topf M, et al. Identification by virtual screening and functional characterisation of novel positive and negative allosteric modulators of the α7 nicotinic acetylcholine receptor. Neuropharmacology 2018. Sep 1;139:194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]


