Abstract
Background
The late recognition of virologic failure (VF) places persons with HIV in Sub-Saharan Africa at risk for HIV transmission, disease progression, and death. We conducted a systematic review and meta-analysis to determine if the recognition and response to VF in the region has improved.
Methods
We searched for studies reporting CD4 count at confirmed VF or at switch to second-line antiretroviral therapy (ART). Using a random-effects metaregression model, we analyzed temporal trends in CD4 count at VF—or at second-line ART switch—over time. We also explored temporal trends in delay between VF and switch to second-line ART.
Results
We identified 26 studies enrolling patients with VF and 10 enrolling patients at second-line ART switch. For studies that enrolled patients at VF, pooled mean CD4 cell count at failure was 187 cells/mm3 (95% CI, 111 to 263). There was no significant change in CD4 count at confirmed failure over time (+4 cells/year; 95% CI, –7 to 15). Among studies that enrolled patients at second-line switch, the pooled mean CD4 count was 108 cells/mm3 (95% CI, 63 to 154). CD4 count at switch increased slightly over time (+10 CD4 cells/year; 95% CI, 2 to 19). During the same period, the mean delay between confirmation of VF and switch was 530 days, with no significant decline over time (–14 days/year; 95% CI, –58 to 52).
Conclusions
VF in Africa remains an event recognized late in HIV infection, a problem compounded by ongoing delays between VF and second-line switch.
Keywords: advanced HIV, AIDS, resource-limited settings, second-line antiretroviral therapy, Sub-Saharan Africa, virologic failure
Delayed recognition and response to antiretroviral therapy (ART) virologic failure in Sub-Saharan Africa places persons with HIV (PWH) in the region at increased risk for onward HIV transmission, disease progression, and death [1–5]. The causes of late detection are multiple but include suboptimal use of viral load monitoring in routine follow-up care and delays in the confirmation of HIV viremia; most national algorithms require 2 consecutive values >1000 copies/mm3 to establish virologic failure. An additional factor is that patients develop virologic failure as a result of challenges in adhering to treatment and care these challenges can also undermine prompt detection and response [6–8].
We conducted a systematic review and meta-analysis to determine the extent to which virologic failure remains a diagnosis made late in HIV disease in Sub-Saharan Africa, with the associated increased risk of poor outcomes that late recognition of ART failure entails. We investigated this question by assessing temporal trends in CD4 cell count at the time of virologic failure and temporal trends in CD4 cell count at the time of switch to second-line ART. Further, we sought to determine if the time delay between virologic failure and switch to second-line ART in Sub-Saharan Africa has improved.
METHODS
Data Sources and Search Strategy
We performed this systematic review in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [9]. We searched PubMed (Supplementary Figures 1 and 2) to identify articles published between January 2002 and December 2020 that reported CD4 cell count at virologic failure during first-line ART or at second-line switch among PWH in Sub-Saharan Africa. An initial search was conducted in January 2018 and updated in January 2021. In addition, relevant reviews were screened for the identification of additional individual studies. Based on the United Nations geoscheme, we included studies performed in countries from the region of Sub-Saharan Africa [10]. There were no language restrictions. All citations were imported into Covidence, online software designed to support meta-analysis abstract management [11].
Study Selection and Data Extraction
We included observational studies that met all the following criteria: (1) included >30 participants, (2) included PWH receiving ART in any country in Sub-Saharan Africa, (3) and reported a summary measure of CD4 cell count at the time of virologic treatment failure or at second-line ART switch following virologic failure. The primary search focused on patients experiencing first-line ART virologic failure (Supplementary Figure 1), and the secondary search focused on patients identified at the time of second-line ART switch (Supplementary Figure 2).
Because our aim was to estimate CD4 cell count at the time of these events in programmatic settings, we excluded randomized clinical trials. We also excluded case reports, case–control studies, and cross-sectional studies (ie, studies of patients who were tested for virologic failure at a single point in time). We excluded studies of both adults and children that did not report disaggregated estimates by age, studies that limited inclusion to participants on the basis of a specified CD4 cell count threshold (eg, CD4 cell count <200 cells/mm3), studies limited to participants with evidence of ART drug resistance (and excluding those without resistance) or participants experiencing a life-threatening event such as an infection, and studies that defined treatment failure without providing data on viral load. For studies that included data overlapping with publications in our search, we included the report with the larger sample.
Titles, abstracts, and full-text articles were reviewed by 2 authors (K.J.B., R.M.) using a standardized tool. The following data were extracted by a single author into Microsoft Excel (version 16.50 for Mac, Microsoft Corporation): country, year of publication, year(s) of the study, median age, proportion female, setting (urban, rural, or both), clinic type, sample size, median CD4 cell count at virologic failure or at second-line ART switch after failure, basis of treatment failure and frequency of viral load monitoring, and time to switch (in days).
Data Synthesis and Analysis
The main summary measures extracted from each included study were median and interquartile range (IQR) of the CD4 cell count at failure (or at second-line ART initiation). In order to pool CD4 cell counts across studies, we first estimated standard errors from the IQR [12]. Means and standard errors were pooled using random-effects models in Stata (version 16, College Station, TX, USA). We reported pooled mean CD4 cell counts with standard errors and 95% confidence intervals. We also used a random-effects metaregression model to estimate trends in CD4 cell count at virologic failure and at second-line switch using the meta set and meta regress commands in Stata; we reported the change in CD4 cell count over time with standard errors (Figure 2 and 3) and a 95% CI. Sensitivity analyses of CD4 cell count at virological failure were performed using a random-effects metaregression model; the subgroups evaluated were (1) studies that accrued patients over >36-month periods vs shorter accrual periods, (2) studies from South Africa vs other countries, and (3) studies from dedicated ART clinics vs other clinic types. CD4 cell count and 95% CI for all studies and for subgroups are shown in a forest plot; the forest plot was created using GraphPad Prism, version 9, for Windows (GraphPad Software, La Jolla, CA, USA) (Figure 4). For the 8 studies that reported time (in days) from treatment failure to switch, we used a random-effects model to report mean time (+/-SE) in days from virologic failure to switch (Figure 5). Data on publication and study characteristics were analyzed, and mean CD4 cell counts were plotted using R, version 4.0.4. For pooled mean CD4 cell counts in Table 1, means were pooled and 95% confidence intervals were generated using the meta set and meta forestplot commands in Stata.
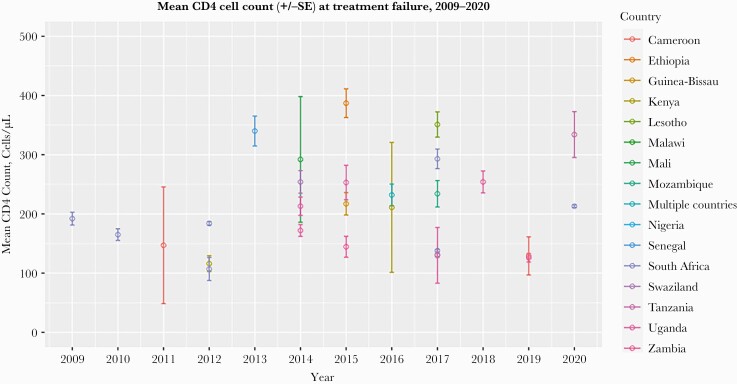
Figure 2.
Mean CD4 cell count (cells/µL) among HIV patients at virologic failure, by country, for studies during the period of 2009–2020. There were no studies that reported CD4 cell counts at the time of switch to second-line ART between 2018 and 2020. One study from 2014 only reported mean and no standard deviation, represented by a circle. Abbreviation: ART, antiretroviral therapy.
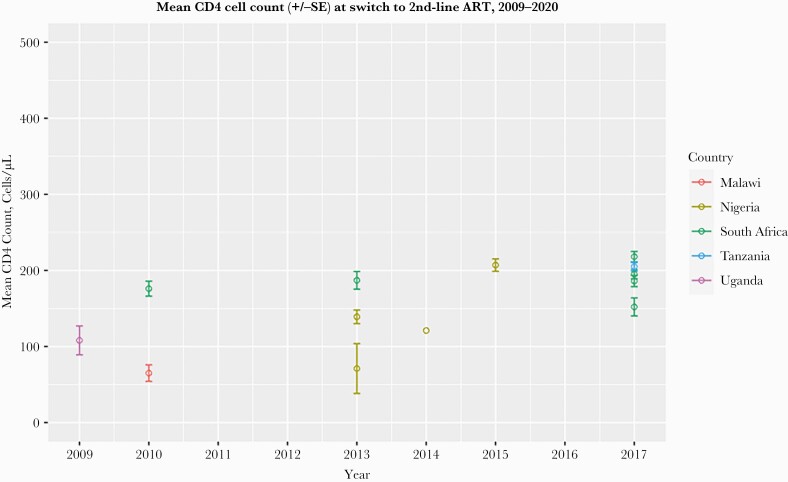
Figure 3.
Mean CD4 count (cells/µL) +/-SE among HIV patients at the time of second-line ART switch, by country, for studies during the period of 2009–2020. Abbreviation: ART, antiretroviral therapy.
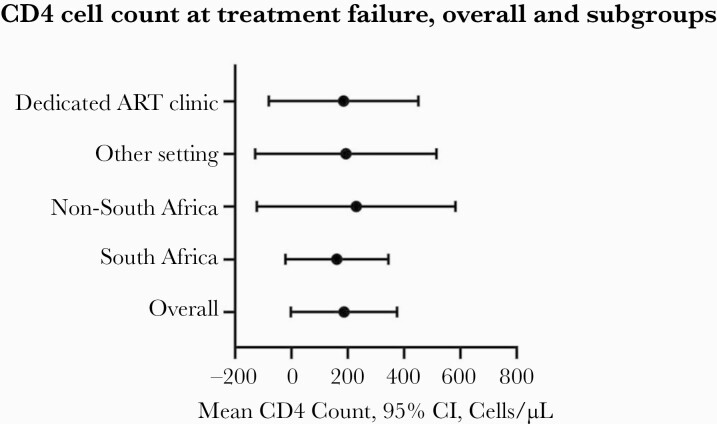
Figure 4.
Sensitivity analysis of mean CD4 count (95% CI) at virologic failure by country of origin (South Africa vs other) and clinic type (dedicated ART clinic vs other types). Abbreviation: ART, antiretroviral therapy.
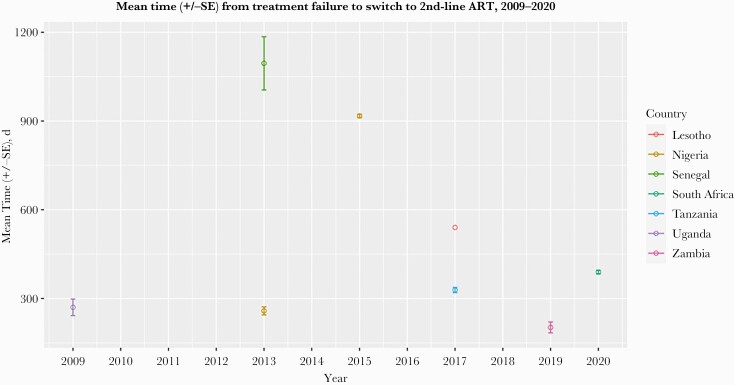
Figure 5.
Mean time (+/-SE) from virologic failure to switch to second-line ART, in days, among HIV patients by country. Abbreviation: ART, antiretroviral therapy.
Table 1.
CD4 Cell Count at Virologic Failure or at Switch to Second-Line ART, Overall and by Country, in Sub-Saharan Africa
| Characteristic | Participants Identified at Time of Virologic Failure | Participants Identified at 2nd-Line ART Switch | ||||
|---|---|---|---|---|---|---|
| No. of Articles (%) | Sample Size, No. (%) |
a
CD4 Cell Count, Cells/µL, Mean (95% CI) |
No. of Articles (%) | Sample Size, No. (%) |
a CD4 Cell Count, Cells/µL, Mean (95% CI) | |
| Total sample | 26 (100) | 9883 (100) | 213 (181 to 246) | 10 (100) | 4306 (100) | 158 (128 to 188) |
| Country of origin | ||||||
| South Africa | 7 (27) | 8465 (86) | 178 (138 to 219) |
3 (30) | 1529 (36) |
187 (170 to 204) |
| Cameroon | 2 (7.7) | 175 (2) |
131 (71 to 191) | - | - | - |
| Ethiopia | 1 (3.8) | 15 (0.2) | 387 (299 to 426) | - | - | - |
| Guinea-Bissau | 1 (3.8) | 36 (0.4) | 217 (157 to 310) | - | - | - |
| Kenya | 2 (7.7) | 89 (0.9) |
117 (91 to 144) | - | - | - |
| Lesotho | 1 (3.8) | 138 (1) | 351 (182 to 520) | - | - | - |
| Mali | 1 (3.8) | 84 (0.8) | 292 (6 to 1319) | - | - | - |
| Malawi | - | - | - | 1 (10) | 106 (2) | 65 (22 to 173) |
| Mozambique | 1 (3.8) | 48 (0.5) | 234 (113 to 322) | - | - | - |
| Nigeria | - | - | - | 4 (40) | 871 (20) |
144 (70 to 218) |
| Senegal | 1 (3.8) | 79 (0.8) | 340 (178 to 481) | - | - | - |
| Swaziland | 1 (3.8) | 78 (0.8) | 254 (167 to 394) | - | - | - |
| Tanzania | 1 (3.8) | 63 (0.6) | 334 (134 to 549) | 1 (10) | 1760 (41) | 205b |
| Uganda | 4 (15) | 317 (3) |
223 (154 to 292) | 1 (10) | 40 (1) | 108 (43 to 205) |
| Zambia | 2 (7.7) | 233 (2) | 129 (116 to 142) |
- | - | - |
| Study included multiple countriesa | 1 (3.8) | 63 (0.6) | 232 (128 to 324) | - | - | - |
Abbreviations: ART, antiretroviral therapy; IQR, interquartile range.
For countries with >1 study, mean CD4 cell count and 95% CI were reported; for countries with 1 study, median CD4 cell count and IQR are shown as reported in the publication.
This study reported mean CD4 and standard deviation only.
To assess how programmatic responses to treatment failure have changed over calendar time, we conducted an exploratory analysis in the subset of studies that reported the time between detection of virologic failure and switch to second-line ART. We fit a random-effects metaregression model in Stata specifying the duration of time as the dependent variable and calendar year as the explanatory variable, and each study contributed a weighted estimate based on the mean and standard deviation of days in the cohort between failure and second-line ART switch. For studies where only medians were reported, means and standard deviations were estimated [13, 14].
The protocol for this meta-analysis was not registered in advance, and ethics approval was not sought.
RESULTS
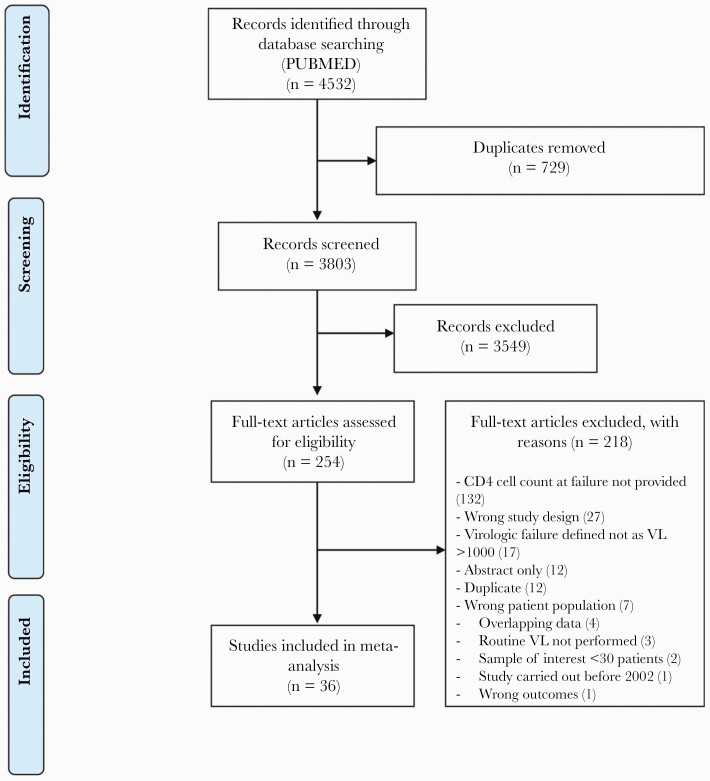
Among 3803 initial abstracts screened, 254 articles were reviewed for full-text eligibility. Among studies that underwent full-text review, 216 of 254 studies were excluded. Thirty-six studies met the eligibility criteria and were included for review and spanned the period from 2009 to 2020 (Figure 1).
Figure 1.
Flowchart for search and selection strategy for studies in the meta-analysis of CD4 cell count at virologic treatment failure and at time of switch to second-line ART in Sub-Saharan Africa. Abbreviations: ART, antiretroviral therapy; VL, viral load.
Study Characteristics
Studies from South Africa, Uganda, and Nigeria comprised the majority (53%; n = 19) of those included (Table 1). The median sample size of studies that identified patients at the time of virologic failure and at second-line ART switch (IQR) was 74 (55–124) and 186 (106–269), respectively. Sixty-one percent of studies took place in urban settings (n = 22), 22% took place in rural settings (n = 8), 8% included patients from both (n = 3), and 8% did not report (n = 3). Fifty-eight percent of studies enrolled participants over a period of <36 months (n = 21). Fifty-three percent of studies took place in dedicated ART clinics (n = 19), 36% in hospital-based clinics (n = 13), 8% did not report or involved multiple clinic types (n = 3), and 3% took place in a primary health clinic (n = 1). In 44% of studies, virologic failure was defined as 2 viral loads >1000 copies/mL (n = 16); however, 28% of studies used a single viral load >1000 copies/mL (n = 10). Fifty percent of programs monitored viral loads every 6 months (n = 18), and 50% used a strategy involving less frequent testing (n = 18).
Pooled CD4 Cell Counts
For studies that identified patients at the time of confirmed virologic failure (n = 26), the pooled mean CD4 cell count at failure was 187 cells/mm3 +/- 39 (95% CI, 111 to 263), and during the period between 2009 and 2020, the CD4 cell count at the time of virologic failure did not increase significantly over time (+4 cells/mm3 +/- 6 per year; 95% CI, –7 to 15) (Figure 2). Among the studies (n = 10) that enrolled patients at the time of second-line ART switch after virologic failure, the pooled mean CD4 cell count was 108 cells/mm3 +/- 23 (95% CI, 63 to 154). During the period from 2009 to 2020, we found that CD4 cell count at second-line switch increased slightly (+10 CD4 cells/mm3 per year; 95% CI, 2 to 19; P < .05) (Figure 3).
Sensitivity Analyses
We sought to assess if a temporal trend in CD4 cell count at virologic treatment failure could have been obscured by pooling studies that enrolled participants over longer time periods. We found that studies with a >36-month inclusion period and studies with a <36-month inclusion period had a pooled mean CD4 count of 200 cells/mm3 (95% CI, 122 to 278) and 144 cells/mm3 (95% CI, –20 to 307), respectively. For studies with a <36-month inclusion period, we found that CD4 cell count at the time of virologic failure did not increase significantly over time (+12 cells/mm3 +/- 11 per year; 95% CI, –10 to 35) during the period from 2009 to 2020. For studies with a >36-month inclusion period, we also found that CD4 cell count at the time of virologic failure did not increase significantly over time (–1 cells/mm3 +/- 6 per year; 95% CI, –13 to 10) during the period from 2009 to 2020. We also examined CD4 cell count at virologic failure by study origin and clinic type. For the first analysis, we compared South African with non–South African studies. Studies from South Africa had a pooled CD4 cell count of 162 cells/mm3 (95% CI, 88 to 235); studies from other countries had a pooled CD4 cell count of 230 cells/mm3 (95% CI, 88 to 372). In the second analysis, we compared studies reporting CD4 cell count at virologic failure from dedicated ART clinics vs other clinic types. Studies from dedicated ART clinics had a pooled CD4 cell count of 186 cells/mm3 (95% CI, 79 to 293); studies from other clinic types had a pooled CD4 cell count of 193 cells/mm3 (95% CI, 64 to 323) (Figure 4). Further, we found no evidence of temporal improvement in CD4 cell count at treatment failure by study origin or by clinic type, as defined above.
Time From Confirmed Virologic Failure to Second-Line ART Switch
Among the 8 studies that presented data describing time from confirmation of virologic failure to switch to second-line ART, we estimated a mean delay of 530 days between the 2 events, without evidence of an improvement with increasing calendar time (14 fewer days per each passing year since 2009; 95% CI, –81 to 52) (Figure 5).
DISCUSSION
Our systematic review provides evidence that in Sub-Saharan Africa the CD4 cell count at the time of identification of virologic failure—and at switch to second-line ART—has not meaningfully changed over more than a decade. We found, in exploratory analysis, that the number of days from virologic failure to second-line ART switch remains quite prolonged, at ~530 days, well beyond the interval recommended by international guidelines, with no evidence of a significant improvement since 2009 [15].
Delayed diagnosis of virologic failure in African settings could result from a variety of causes. First, while viral load monitoring is part of many public sector HIV programs, the implementation has proven challenging. Despite the existence of viral load monitoring in a growing number of countries, a substantial proportion of patients in longitudinal HIV care do not benefit from routine viral load testing [6]. Second, impeding prompt recognition is the requirement that virologic failure be defined—in most national treatment guidelines—as persistent viremia across 2 time points. This requirement, while reducing some unnecessary second-line ART switching, introduces an inherent delay in establishing treatment failure. Third, the health care worker responsible for responding to elevated viral load values has not, in some clinics, been clearly identified [16]. This responsibility is critical because an elevated viral load requires a deliberate set of interventions, including a return visit, an adherence intervention, and repeat viral load testing, which, our research demonstrates, currently plays out over not weeks—as was anticipated in treatment guidelines—but months. Once virologic failure is confirmed, clinician concerns about adherence to the next regimen and about exhausting second-line ART options when access to salvage regimens is limited can contribute to a delay in switching. Additionally, switch can be delayed because patients with treatment failure, compared with patients who remain undetectable, are more likely to miss clinic visits and cycle in and out of care, making rapid switch after persistently elevated viral load values difficult to quickly execute [17, 18].
Our findings have considerable implications for clinical practice and program implementation. For the individual patient receiving first-line ART in Sub-Saharan Africa, the findings suggest that, if virologic failure occurs, it is likely that it will be identified in the setting of relatively advanced HIV infection, that a change to second-line ART after confirmed virologic failure will follow a substantial time delay, and that a switch to second-line ART—if available—will take place at a time of even greater immune suppression. During this period, an individual patient will be at increased risk for opportunistic infection and death, given a concomitant depressed CD4 cell count and ongoing viral replication [2–5].
How can viral load testing be utilized more effectively? Although viral load testing clearly improves the quality of HIV care, health services research is needed to help direct how to deploy it better. For example, a marked improvement in viral load testing rates was observed in clinics in South Africa that deployed a nurse-led “viral load champion” program [16]. Using this approach, overall detection of virologic treatment failure improved rapidly in a programmatic setting without additional human resources. Another promising line of operational research in this area involves task sharing. When enrolled nurses were given the responsibility for ART prescribing, monitoring, and second-line ART switch—using point-of-care viral load in South Africa—viral suppression outcomes and retention in HIV care improved [19]. Additional investments in implementation research are needed to ensure that the systems are in place to fully realize the benefits of viral load monitoring.
Earlier identification of virologic failure and a timelier switch to second-line ART may also result from other important changes to the diagnostic and treatment landscape. Recent modeling suggests that for patients receiving an efavirenz-based ART, regimen switch after a single viral load >1000 copies/mL could avoid ~10 215 deaths annually in South Africa [20], and these findings support recent guidelines issued by the World Health Organization recommending a single viral load switch for patients on an non-nucleoside reverse transcriptase inhibitor-based regimen [21]. Where available, drug resistance testing could also help with more rapidly assigning patients to either an accelerated switch pathway in the presence of significant antiretroviral resistance or to continue an existing active regimen [22]. Finally, the rollout of dolutegravir-based ART may reduce the overall burden of treatment failure in the region [23].
Our findings should be interpreted with some limitations in mind. First, several studies included in our meta-analysis enrolled patients over a multiyear period, potentially obscuring trends in CD4 cell count at the time of virologic failure or at switch that may have been evident if the inclusion periods were narrower [24]. Following previous work, we addressed this potential aggregation bias by analyzing studies with shorter inclusion periods separately and found no evidence of differential trends when we plotted the 2 types of studies (short vs long inclusion periods) separately [25]. Second, since the initial ART rollout, public health authorities have increased the recommended CD4 cell count at which ART is initiated in treatment-naïve patients, with initiation irrespective of CD4 cell count now almost universally adopted. Although these changes could theoretically have had an impact on the CD4 cell count at which ART treatment failure is identified, summary data suggest that changes in these thresholds have had only a modest impact on the actual CD4 cell count at ART initiation and, as we show, on CD4 cell count at virologic failure [25, 26]. Third, since we utilized IQR to estimate standard errors for CD4 cell counts, greater precision in calculating standard errors could be achieved if minimum and maximum values were also reported in studies [12]. This is a limitation in the published literature because studies generally report the median and IQR, rather than the mean with minimum and maximum values. Fourth, we searched a single database, PubMed, and did not search the gray literature or unpublished abstracts. Finally, only a subgroup of studies provided estimates of time between virologic failure and second-line ART switch; thus, we were unable to estimate with high confidence trends over time in switch delay, and we therefore present these findings as exploratory.
CONCLUSIONS
Prolonged virologic failure poses a threat to patients with HIV infection in Africa largely because, under current conditions, it is unlikely to be recognized until late in HIV disease and is compounded by a substantial delay before second-line ART switch. Although some HIV programs have achieved a high level of viral load coverage among patients in active follow-up, this scale-up has less value to patients or the health system if it is not partnered with effective longitudinal care [27]. Clearly additional investment in health services research is needed to investigate novel strategies to improve the recognition and response to virologic failure. Addressing these challenges will be key to improving the quality of long-term HIV care and avoiding unnecessary on-treatment HIV transmission, morbidity, and mortality.
Supplementary Data
Supplementary materials are available at the Journal of The Pediatric Infectious Diseases Society online (http://jpids.oxfordjournals.org).
Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Acknowledgments
We would like to offer our deepest appreciation for the trust and generosity of those individuals living with HIV infection at clinical research sites throughout Africa.
Financial support. This research was supported by the UCLA-Clinical and Translational Research Institute (UCLA-CTSI) at Lundquist/Harbor-UCLA and from the Emory Center for AIDS Research (P30 AI050409).
Potential conflicts of interest. V.C.M. has received investigator-initiated research grants (to the institution) and consultation fees (both unrelated to the current work) from Eli Lilly, Bayer, Gilead Sciences, and ViiV. A.C.T. reports receiving a financial stipend from Elsevier, Inc., for his work as Co-Editor in Chief of the journal SSM-Mental Health. All other authors declare no conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. K.J.B.: executed literature search, screened and selected studies, extracted data, data analysis, drafting of manuscript. M.J.S.: manuscript conception, revision, data analysis, and drafting of manuscript. A.C.T.: manuscript conception, revision, and data analysis. V.C.M.: manuscript conception, revision. R.A.M.: manuscript conception, screened and selected studies, extracted data, revision.
Patient consent. Our study does not include factors necessitating patient consent.
References
- 1. Murphy RA, Court R, Maartens G, Sunpath H.. Second-line antiretroviral therapy in Sub-Saharan Africa: it is time to mind the gaps. AIDS Res Hum Retroviruses 2017; 33:1181–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bell Gorrod H, Court R, Schomaker M, Maartens G, Murphy RA.. Increased mortality with delayed and missed switch to second-line antiretroviral therapy in South Africa. J Acquir Immune Defic Syndr 2020; 84:107–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bell-Gorrod H, Fox MP, Boulle A, et al. The impact of delayed switch to second-line antiretroviral therapy on mortality, depending on failure time definition and CD4 count at failure. Am J Epidemiol 2020; 189:811–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Petersen ML, Tran L, Geng EH, et al. Delayed switch of antiretroviral therapy after virologic failure associated with elevated mortality among HIV-infected adults in Africa. AIDS 2014; 28:2097–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rohr JK, Ive P, Horsburgh CR, et al. Marginal structural models to assess delays in second-line HIV treatment initiation in South Africa. PLoS One 2016; 11:e0161469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sunpath H, Hatlen TJ, Naidu KK, et al. Targeting the third “90”: introducing the viral load champion. Public Heal Action 2018; 8:225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hermans LE, Carmona S, Nijhuis M, et al. Virological suppression and clinical management in response to viremia in South African HIV treatment program: a multicenter cohort study. PLoS Med 2020; 17:e1003037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moyo F, Chasela C, Brennan AT, et al. Treatment outcomes of HIV-positive patients on first-line antiretroviral therapy in private versus public HIV clinics in Johannesburg, South Africa. Clin Epidemiol 2016; 8:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015; 4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. United Nations Department of Economic and Social Affairs, Statistics Division UN Geoscheme. Available at: https://unstats.un.org/unsd/methodology/m49/. Accessed 23 August 2021.
- 11. Veritas Health Innovation. Covidence systematic review software. Available at: www.covidence.org. Accessed 15 January 2022. [Google Scholar]
- 12. Hozo SP, Djulbegovic B, Hozo I.. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005; 5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wan X, Wang W, Liu J, Tong T.. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014; 14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Higgins JPT, Li T, Deeks JJ.. Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.3. Cochrane, 2022. Available at: www.training.cochrane.org/handbook. Accessed 26 March 2022. [Google Scholar]
- 15. World Health Organization. Consolidated Guidelines on HIV Prevention, Testing, Treatment, Service Delivery and Monitoring; Recommendations for a Public Health Approach. World Health Organization; 2021. [PubMed] [Google Scholar]
- 16. Sunpath H, Pillay S, Hatlen T, et al. A nurse-led intervention to improve management of virologic failure in public-sector HIV clinics in Durban, South Africa: a pre-post implementation evaluation. South African Med J 2021; 111:299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kaplan SR, Oosthuizen C, Stinson K, et al. Contemporary disengagement from antiretroviral therapy in Khayelitsha, South Africa: a cohort study. PLoS Med 2017; 14:e1002407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kyaw NTT, Harries AD, Kumar AMV, et al. High rate of virological failure and low rate of switching to second-line treatment among adolescents and adults living with HIV on first-line ART in Myanmar, 2005-2015. PLoS One 2017; 12:e0171780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Drain PK, Dorward J, Violette LR, et al. Point-of-care HIV viral load testing combined with task shifting to improve treatment outcomes (STREAM): findings from an open-label, non-inferiority, randomised controlled trial. Lancet HIV 2020; 7:e229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shroufi A, Van Cutsem G, Cambiano V, et al. Simplifying switch to second-line antiretroviral therapy in Sub Saharan Africa: predicted effect of using a single viral load to define efavirenz-based first-line failure. AIDS 2019; 33:1635–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Global HIV, Hepatitis and Sexually Transmitted Infections Programmes, Guidelines Review Committee. Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach. 2021. Available at: https://www.who.int/publications/i/item/9789240031593. Accessed 7 January 2022.
- 22. Siedner MJ, Bwana MB, Moosa M-YS, et al. The REVAMP trial to evaluate HIV resistance testing in sub-Saharan Africa: a case study in clinical trial design in resource limited settings to optimize effectiveness and cost effectiveness estimates. HIV Clin Trials 2017; 18:149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McCluskey SM, Siedner MJ.. Population effectiveness of Dolutegravir Implementation in Sub-Saharan Africa (DISCO). ClinicalTrials.gov NCT04066036. Available at: https://clinicaltrials.gov/ct2/show/NCT04066036. Accessed 12 September 2021. [Google Scholar]
- 24. Ford N, Mills EJ, Egger M.. Immunodeficiency at start of antiretroviral therapy: the persistent problem of late presentation to care. Clin Infect Dis 2015; 60:1128–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Siedner MJ, Ng CK, Bassett IV, Katz IT, Bangsberg DR, Tsai AC.. Trends in CD4 count at presentation to care and treatment initiation in Sub-Saharan Africa, 2002-2013: a meta-analysis. Clin Infect Dis 2015; 60:1120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. IeDEA and COHERE Cohort Collaborations. Global trends in CD4 Cell count at the start of antiretroviral therapy: collaborative study of treatment programs. Clin Infect Dis 2018; 66:893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lecher S, Ellenberger D, Kim AA, et al. Scale-up of HIV viral load monitoring—seven Sub-Saharan African countries. MMWR Morb Mortal Wkly Rep 2015; 64:1287–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.