Abstract
A recently identified SARS-CoV-2 variant, Lambda, has spread to many countries around the world. Here, we measured and evaluated the reduced sensitivity of Lambda variant to the neutralization by plasma polyclonal antibodies elicited by the natural SARS-CoV-2 infection and inactivated vaccine. The combination of two substitutions appearing in the RBD of spike protein (L452Q and F490S) resulted in noticeably reduced neutralization against Lambda variant. F490S contributed more than L452Q in affecting the neutralization. In addition, the neutralization test with 12 published nAbs binding to RBD of SARS-CoV-2 with defined structures suggested that Lambda variant resisted the neutralization by some antibodies from Class 2 and Class 3. Overall, these results suggest that pre-existing antibody neutralization established by natural infection from non-Lambda variants or immunization could be significantly decreased, re-emphasizing the importance of ongoing viral mutation monitoring.
Keywords: SARS-CoV-2, Lambda variant, Convalescent plasma, Inactivated vaccine, Monoclonal antibody, Neutralization
1. Introduction
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is still spreading across the world [1]. As a result, evolution of SARS-CoV-2 is facilitated, leading to the emergence of diverse variants. SARS-CoV-2 variants have been classified by the World Health Organization (WHO) into three main types: variants of concern (VOCs) including Alpha, Beta, Gamma, Delta, and Omicron, variants of interest (VOIs) including Lambda and Mu, and variants under monitoring (VUMs) including B.1.1.318, C.1.2, and B.1.640 [2]. The receptor binding domain (RBD) of the spike protein is responsible for binding to the cell receptor (angiotensin-converting enzyme 2, ACE2). Therefore, the mutations located in the RBD may affect the binding of virus to ACE2 and cause immune escape of SARS-CoV-2 from neutralizing antibodies (nAbs).
SARS-CoV-2 Lambda variant was first identified in Peru and has rapidly spread to other countries [3]. In the light of the high transmissibility it may has, Lambda variant was designated as a kind of VOI [2]. Meanwhile, Lambda variant carrying various mutations in the spike might lead to immune escape from nAbs [4]. Several preprint studies had shown that Lambda variant was resistant to the neutralization by mRNA vaccine-elicited antibodies [4, 5]. However, the neutralization of Lambda variant by antibodies elicited by the inactivated vaccine has yet to be reported, and little is known about the impact of spike mutations on monoclonal neutralizing antibodies (nAbs) targeting diverse epitopes.
In this study, we prepared SARS-CoV-2 pseudoviruses carrying distinct spike proteins of the Wuhan reference strain with D614G mutation (wild-type, D614G-WT) and Lambda variant, as well as L452Q/F490S, L452Q, or F490S mutations. We measured the susceptibility of these mutant viruses to the neutralization by plasma from convalescent patients and inactivated vaccine recipients and by nAbs from Class 1, 2, 3, and 4. These results showed that plasma neutralization against Lambda variant is compromised by L452Q and F490S mutations, which destroyed or weakened the binding and neutralizing activities of some antibodies from Class 2 and 3.
2. Results
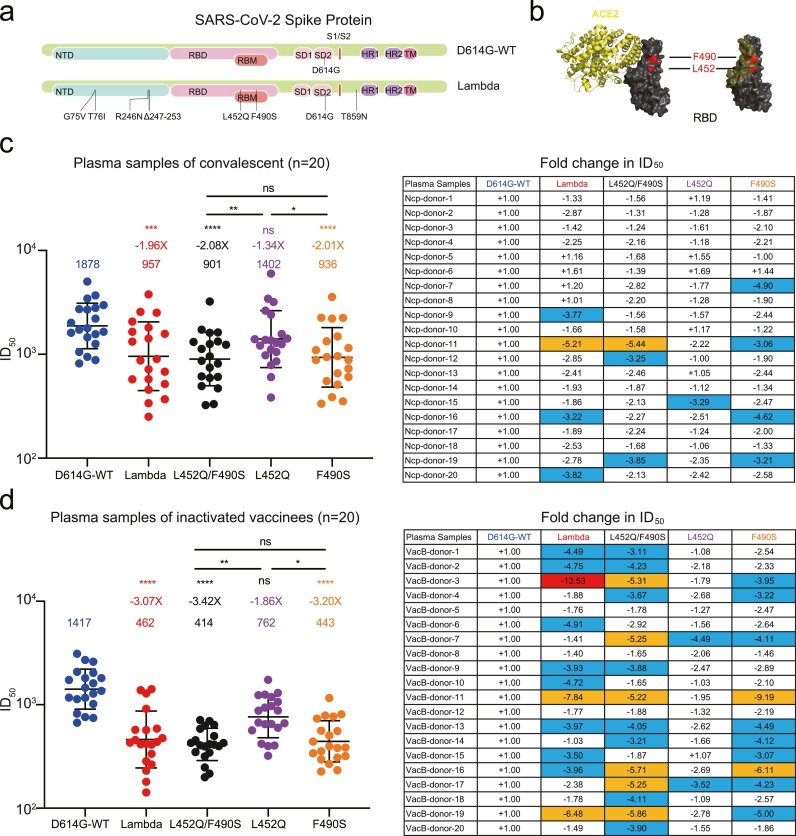
SARS-CoV-2 Lambda variant harbored G75V, T76I, R246N, and Δ246–252 mutations in the N terminal domain, L452Q and F490S in the RBD, D614G and T859N in other regions of spike (Fig. 1 a). The L452Q and F490S substitutions appeared in or near the binding interface between RBD and ACE2, indicating that Lambda variant may escape neutralization by nAbs in some degrees (Fig. 1b). We first established the pseudovirus-based neutralization assay. The ID50 of plasma from all 10 non-vaccinated healthy donors with no prior SARS-CoV-2 infection were < 1:20 against the D614G-WT and each variant virus (Figure S1). We measured the neutralization titers of plasma collected from 20 convalescent individuals (Figure S2a, Table S1). Compared with the WT strain (D614G-WT), both Lambda variant and L452Q/F490S mutated virus reduced the susceptibility by approximately 50% (Fig. 1c). The F490S played a more important role in reducing the neutralization than L452Q, with geometric mean titers (GMTs) being 936 and 1402, respectively. In addition, the neutralization of mutated virus strongly correlated to that of the WT strain by the same sample (Figure S2b).
Fig. 1.
Neutralization of SARS-CoV-2 Lambda variant by convalescent and inactivated vaccine-elicited plasma. (a) Mutations located in the spike protein were identified in Lambda variant compared with the wild-type (WT). (b) The location of L452 and F490 residuals (red) in the ACE2 (yellow) and SARS-CoV-2 RBD (black) complex (PDB: 7DMU). (c, d) Changes in neutralizing titers of convalescent (c) and inactivated vaccine-elicited plasma (d) against Lambda variant, L452Q/F490S, L452Q, and F490S mutated viruses compared with those against the D614G-WT. The data shown here are means of two independent experiments. Geometric mean titers (GMTs) in ID50 values and fold-change were calculated and shown above each variant in the left panel. The fold change in ID50 of each plasma against mutated viruses were represented in the right panel. Statistical analysis was performed with two-side Friedman test with Dunn's multiple comparison using GraphPad Prism 9 software. ns: P > 0.05; ***: P < 0.001, ****: P < 0.0001. The changes between 3-fold and 5-fold are marked in blue. The changes between 5-fold and 10-fold are marked in orange, and those above 10-fold are marked in red. The symbol ‘+’ indicates increased neutralization, and ‘-’ indicates decreased neutralization.
We next evaluated the neutralizing activity induced by SARS-CoV-2 inactivated vaccine against Lambda variant. Twenty plasma were collected after second immunization with BBIBP-CorV (Table S2). The levels of nAbs against Lambda variant, L452Q/F490S, and F490S mutated viruses were significantly lower than those against the WT virus, displaying a 3.07-fold, 3.42-fold, and 3.20-fold reduction, respectively (Fig. 1d, Figure S3a). The mutation L452Q contributed a 1.86-fold decline in neutralizing activity, which was weaker than the impact of F490S. There were strong correlations between the neutralization of the mutated and WT viruses (Figure S3b).
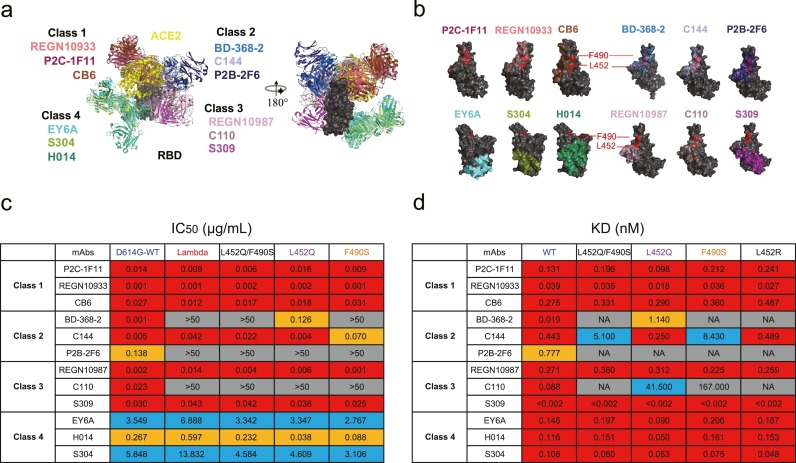
It is widely known that naturally occurring plasma antibodies induced by infection or vaccines are a group of polyclonal antibodies consisting of numerous monoclonal antibodies (mAbs), each recognizing a single epitope [6]. To explore what kind of mAbs lose their neutralizing activities, we summarized 12 published mAbs with defined structural information and divided them into 4 classes according to their competition with ACE2 and binding models to the RBD (Fig. 2 a) [7], [8], [9]. The mutations at L452 and F490 were both located in the RBD-binding sites of Class 2 antibodies and C110, a Class 3 antibody (Fig. 2b), suggesting that Lambda variant might escape from the recognition and neutralization by these nAbs in different degrees. All tested nAbs from Class 1 and Class 4 maintained their neutralizing activities against Lambda variant and site-mutated pseudoviruses (Fig. 2c, Figure S4). In contrast, Lambda variant was fully resistant to the neutralization by BD-368-2, P2B-2F6, and C110. The results of binding affinity showed that BD-368-2, P2B-2F6, and C110 hardly bound to the L452Q/F490S mutated RBD, revealing the escape mechanism of Lambda variant from the neutralization by nAbs (Fig. 2d, Table S3). Although L452Q and F490S mutations were both located in the binding epitope of C144 (a Class 2 antibody), F490S contributed more than L452Q to the reduction of neutralization and affinity. Analysis of two other Class 3 antibodies, REGN10987 and S309, showed that neither recognized the L452 and F490 residuals directly but each still neutralized the Lambda variant with similar potencies as those against D614G-WT. Overall, some nAbs from Class 2 and Class 3 lost or had decreased their binding and neutralizing activities against Lambda variant, resulting in a significant decline in the plasma neutralization in convalescent individuals and vaccine recipients.
Fig. 2.
Neutralization of SARS-CoV-2 Lambda variant by monoclonal nAbs. (a) Structural depiction of ACE2 and representative nAbs from each class binding to the RBD. Class 1: P2C-1F11 (PDB: 7CDI), REGN10933 (PDB: 6XDG), CB6 (PDB: 7C01); Class 2: BD-368–2 (PDB: 7CHH), C144 (PDB: 7K90), P2B-2F6 (PDB: 7BWJ); Class 3: REGN10987 (PDB: 6XDG), C110 (PDB: 7K8V), S309 (PDB: 6WPS); Class 4: EY6A (PDB: 6ZCZ), H014 (PDB: 7CAI), S304 (PDB: 7JW0)[7–9]. (b) Footprints of four classes of representative nAbs on the RBD. L452 and F490 residuals are shown in red. (c) The IC50 values of nAbs against SARS-CoV-2 pseudoviruses. The data shown here are means of two independent experiments. The IC50 values below 0.05 μg/mL are marked in red. The IC50 values between 0.05 and 1 μg/mL are marked in orange. The IC50 values between 1 and 50 μg/mL are marked in blue. The IC50 values above 50 μg/mL are highlighted in gray. (d) The affinity values in binding activities of nAbs to mutated and WT RBD. The data shown here are means of two independent experiments. The binding affinities of nAbs below 0.5 nM are marked in red. The binding affinities of nAbs between 0.5 and 5 nM are marked in orange. The binding affinities of nAbs between 5 and 50 nM are marked in blue. The binding affinity above 50 nM, or not available (NA), in gray, indicates that the affinity of nAbs to the mutated RBD was very weak.
3. Discussion
Currently, a series of significant achievements have been made in the development of vaccines and nAbs for preventing and treating COVID-19 [10]. However, with the rapid emergence of SARS-CoV-2 variants, concerns have arisen about antibody escape capabilities of the variants. Current vaccines or nAbs were designed or isolated based on the original strain in the early phase of the COVID-19 pandemic [11]. Although, according to the latest report from the WHO, Lambda is not a dominant VOI anymore, some of its feature mutations are possible to appear in future variants. Thus, evaluating the antibody escape of Lambda is still formative for designing next-generation vaccines and screening broadly therapeutic nAbs.
Although the number of samples was limited and all donors were Chinese in this study, we found that the neutralizing activities of convalescent and inactivated vaccine-elicited plasma against SARS-CoV-2 Lambda variant were significantly decreased compared with those against D614G-WT. The results of site-mutated neutralization assay showed that the mutations in RBD, especially F490S, played an important role in the reduced sensitivity of Lambda variant. Previous studies have also shown that both the L452 and F490 residuals are the key recognition sites of multiple nAbs. The more common mutation at L452 in the RBD was the L452R substitution, which has been found in Epsilon, Kappa, and Delta variants [12], [13], [14]. Thus, we performed a head-to-head comparison of the binding affinity of nAbs to the RBD-L452R and RBD-L452Q. BD-368-2 and C110 totally lost their binding activities to the RBD-L452R, but still maintained some degree of affinity to the RBD-L452Q, suggesting that an arginine at L452 residual might have a greater impact on antibody recognition than the glutamine. The F490S substitution introducing a potential O-linked glycosylation site once appeared in some variants sporadically before it became the characteristic mutation of Lambda [15], [16], [17]. The protective efficacies of different types of vaccines against Lambda variant need to be further studied in the real world.
Finally, it is worth noticing that a monoclonal nAb we previously identified, P2C-1F11 [18], [19], [20], could potently neutralize Lambda variant. As a combination therapy, the Fc-modified version (Brii-196) has been submitted to the Food and Drug Administration for the emergency use authorization. These results further confirmed the broadly neutralizing activity of Brii-196 and showed its effectiveness in fighting against SARS-CoV-2 variants [20], [21], [22].
Author contributions
Z.Z. is the principal investigator of this study. Z.Z. and B.J. conceived and designed the study. H.G., Q.F., and S.Song performed all experiments together with assistance from B.Z., H.W., L.C., and X.G. Senlin S. summarized the structural information of published nAbs and analyzed their binding modes and footprints on the RBD. Z.Z., B.J., and H.G. wrote the manuscript and all authors read and approved this version of manuscript.
Declaration of Competing Interests
The authors have no conflict of interest.
Acknowledgments
We thank all participants who recovered from COVID-19 and who received inactivated vaccine and all of the healthy providers from Shenzhen Third People's Hospital for the work they have done. This study was supported by the National Science Fund for Distinguished Young Scholars (82025022), the National Natural Science Foundation of China (82002140, 82171752, 82101861), the Guangdong Basic and Applied Basic Research Foundation (2021B1515020034, 2019A1515011197, 2021A1515011009, 2020A1515110656), and the Shenzhen Science and Technology Program (RCYX20200714114700046, JSGG20200207155251653, JSGG20200807171401008, KQTD20200909113758004, JCYJ20190809115617365).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jcv.2022.105162.
Appendix. Supplementary materials
Data availability statements: We are happy to share reagents and information in this study upon request.
References
- 1.Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G., et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798) doi: 10.1038/s41586-020-2008-3. 265-9Epub 2020/02/06. doi: 10.1038/s41586-020-2008-3. PubMed PMID: 32015508; PubMed Central PMCID: PMCPMC7094943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/2022
- 3.Wink P.L., Volpato F.C.Z., Monteiro F.L., Willig J.B., Zavascki A.P., Barth A.L., et al. First identification of SARS-CoV-2 Lambda (C.37) variant in Southern Brazil. Infect. Control Hosp. Epidemiol. 2021:1–7. doi: 10.1017/ice.2021.390. Epub 2021/09/03. doi: 10.1017/ice.2021.390. PubMed PMID: 34470685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kimura I., Kosugi Y., Wu J., Yamasoba D., Butlertanaka E.P., Tanaka Y.L., et al. SARS-CoV-2 Lambda variant exhibits higher infectivity and immune resistance. bioRxiv. 2021 doi: 10.1101/2021.07.28.454085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu H., Wei P., Zhang Q., Aviszus K., Linderberger J., Yang J., et al. The Lambda variant of SARS-CoV-2 has a better chance than the Delta variant to escape vaccines. bioRxiv. 2021 doi: 10.1101/2021.08.25.457692. PubMed PMID: 34462744; PubMed Central PMCID: PMCPMC8404886. [DOI] [Google Scholar]
- 6.Barnes C.O., West A.P., Huey-Tubman K.E., Hoffmann M.A.G., Sharaf N.G., Hoffman P.R., et al. Structures of human antibodies bound to SARS-CoV-2 spike reveal common epitopes and recurrent features of antibodies. Cell. 2020 doi: 10.1016/j.cell.2020.06.025. Jr. PubMed PMID: 32645326; PubMed Central PMCID: PMCPMC7311918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnes C.O., Jette C.A., Abernathy M.E., Dam K.A., Esswein S.R., Gristick H.B., et al. SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. Nature. 2020 doi: 10.1038/s41586-020-2852-1. PubMed PMID: 33045718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan R., Wang R., Ju B., Yu J., Zhang Y., Liu N., et al. Structural basis for bivalent binding and inhibition of SARS-CoV-2 infection by human potent neutralizing antibodies. Cell Res. 2021;31(5):517–525. doi: 10.1038/s41422-021-00487-9. PubMed PMID: 33731853; PubMed Central PMCID: PMCPMC7966918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng L., Song S., Fan Q., Shen S., Wang H., Zhou B., et al. Cross-neutralization of SARS-CoV-2 Kappa and Delta variants by inactivated vaccine-elicited serum and monoclonal antibodies. Cell Discov. 2021;7(1):112. doi: 10.1038/s41421-021-00347-1. PubMed PMID: 34811350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Checcucci E., Piramide F., Pecoraro A., Amparore D., Campi R., Fiori C., et al. The vaccine journey for COVID-19: a comprehensive systematic review of current clinical trials in humans. Panminerva Med. 2020 doi: 10.23736/S0031-0808.20.03958-0. Epub 2020/05/28. doi: 10.23736/s0031-0808.20.03958-0. PubMed PMID: 32456404. [DOI] [PubMed] [Google Scholar]
- 11.Chen R.E., Zhang X., Case J.B., Winkler E.S., Liu Y., VanBlargan L.A., et al. Resistance of SARS-CoV-2 variants to neutralization by monoclonal and serum-derived polyclonal antibodies. Nat. Med. 2021;27(4):717–726. doi: 10.1038/s41591-021-01294-w. Epub 2021/03/06. doi: 10.1038/s41591-021-01294-w. PubMed PMID: 33664494; PubMed Central PMCID: PMCPMC8058618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Starr T.N., Greaney A.J., Dingens A.S., Bloom J.D. Complete map of SARS-CoV-2 RBD mutations that escape the monoclonal antibody LY-CoV555 and its cocktail with LY-CoV016. Cell Rep. Med. 2021;2(4) doi: 10.1016/j.xcrm.2021.100255. Epub 2021/04/13. doi: 10.1016/j.xcrm.2021.100255. PubMed PMID: 33842902; PubMed Central PMCID: PMCPMC8020059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu C., Ginn H.M., Dejnirattisai W., Supasa P., Wang B., Tuekprakhon A., et al. Reduced neutralization of SARS-CoV-2 B.1.617 by vaccine and convalescent serum. Cell. 2021;184(16) doi: 10.1016/j.cell.2021.06.020. 4220-36.e13. Epub 2021/07/10. doi: 10.1016/j.cell.2021.06.020. PubMed PMID: 34242578; PubMed Central PMCID: PMCPMC8218332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Planas D., Veyer D., Baidaliuk A., Staropoli I., Guivel-Benhassine F., Rajah M.M., et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021;596(7871):276–280. doi: 10.1038/s41586-021-03777-9. Epub 2021/07/09. doi: 10.1038/s41586-021-03777-9. PubMed PMID: 34237773. [DOI] [PubMed] [Google Scholar]
- 15.Yi C., Sun X., Lin Y., Gu C., Ding L., Lu X., et al. Comprehensive mapping of binding hot spots of SARS-CoV-2 RBD-specific neutralizing antibodies for tracking immune escape variants. Genome Med. 2021;13(1):164. doi: 10.1186/s13073-021-00985-w. Epub 2021/10/16. doi: 10.1186/s13073-021-00985-w. PubMed PMID: 34649620; PubMed Central PMCID: PMCPMC8515915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Z., VanBlargan L.A., Bloyet L.M., Rothlauf P.W., Chen R.E., Stumpf S., et al. Identification of SARS-CoV-2 spike mutations that attenuate monoclonal and serum antibody neutralization. Cell Host Microbe. 2021;29(3):477–488. doi: 10.1016/j.chom.2021.01.014. e4. Epub 2021/02/04. doi: 10.1016/j.chom.2021.01.014. PubMed PMID: 33535027; PubMed Central PMCID: PMCPMC7839837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Z., VanBlargan L.A., Bloyet L.M., Rothlauf P.W., Chen R.E., Stumpf S., et al. Identification of SARS-CoV-2 spike mutations that attenuate monoclonal and serum antibody neutralization. Cell Host Microbe. 2021 doi: 10.1016/j.chom.2021.01.014. PubMed PMID: 33535027; PubMed Central PMCID: PMCPMC7839837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ju B., Zhang Q., Ge J., Wang R., Sun J., Ge X., et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature. 2020 doi: 10.1038/s41586-020-2380-z. PubMed PMID: 32454513. [DOI] [PubMed] [Google Scholar]
- 19.Ge J., Wang R., Ju B., Zhang Q., Sun J., Chen P., et al. Antibody neutralization of SARS-CoV-2 through ACE2 receptor mimicry. Nat. Commun. 2021;12(1):250. doi: 10.1038/s41467-020-20501-9. PubMed PMID: 33431856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Q., Ju B., Ge J., Chan J.F., Cheng L., Wang R., et al. Potent and protective IGHV3-53/3-66 public antibodies and their shared escape mutant on the spike of SARS-CoV-2. Nat. Commun. 2021;12(1):4210. doi: 10.1038/s41467-021-24514-w. PubMed PMID: 34244522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang R., Zhang Q., Ge J., Ren W., Zhang R., Lan J., et al. Analysis of SARS-CoV-2 variant mutations reveals neutralization escape mechanisms and the ability to use ACE2 receptors from additional species. Immunity. 2021 doi: 10.1016/j.immuni.2021.06.003. PubMed PMID: 34166623; PubMed Central PMCID: PMCPMC8185182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang P., Nair M.S., Liu L., Iketani S., Luo Y., Guo Y., et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 2021 doi: 10.1038/s41586-021-03398-2. PubMed PMID: 33684923. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data availability statements: We are happy to share reagents and information in this study upon request.