Abstract
Liposomal formulations of rifabutin were developed, and the effects of some parameters on the incorporation efficiency were studied. The antimycobacterial activity of rifabutin incorporated into liposomes prepared with phosphatidylcholine and phosphatidylserine (molar ratio, 7:3) was evaluated in a murine model of infection with a virulent Mycobacterium avium strain (strain P1581) and was compared with that of free rifabutin. The influences of the size of the liposomal rifabutin formulation, the administered doses, and the treatment schedules on the evolution of infection were studied. Two types of treatment schedules were assayed: therapeutic and prophylactic. The therapeutic treatment started 2 weeks after infection, while the prophylactic treatment began 1 day before the experimental infection with mycobacteria. Incorporation of rifabutin in liposomes resulted in a significant enhancement of activity against M. avium infection compared to that of rifabutin in the free form in both schedules. These results demonstrate that liposomal formulations of antibiotics such as rifabutin may be effective for the treatment or prophylaxis of infectious diseases.
Mycobacterium avium complex (MAC) is made up of a group of intracellular bacteria that are able to survive and multiply inside macrophages. Before the 1980s, infections with these bacteria were uncommon in humans and were recognized as a slowly progressing pneumonitis in elderly patients with chronic pulmonary disorders, particularly in patients with silicosis (34) and, occasionally, immunocompromised leukemic patients (42). Since 1981, however, numerous medical institutions began to report cases of MAC bacteremia in AIDS patients (18) which were responsible for significant morbidity and mortality in human immunodeficiency virus (HIV)-positive patients (43). Disseminated infections with MAC organisms are diagnosed in only 3% of HIV-positive patients at the time of AIDS diagnosis (20). However, these infections are found in about half of the autopsied patients with AIDS (41). Although numerous antimycobacterial agents have been used to treat disseminated MAC infections, an efficient therapy for this disease is unknown, despite the availability of new and potent antibiotics. MAC isolates are intracellular pathogens resistant to many of the standard antituberculosis drugs. In many cases this resistance is due to the low levels of drug permeation into macrophages, as many antibiotics are unable to traverse the cell membranes, making it difficult to achieve sufficient concentrations at the infection sites (13, 14). In addition, degradation of drugs may occur before they reach target tissues (13, 14). On the other hand, when administered at high doses, in order to overcome their low levels of cell permeation, they may be severely toxic (19, 40). Thus, it is necessary either to find new antibiotics or to explore ways of enhancing the therapeutic activities of the currently available drugs. Our approach involves the use of drug carrier systems instead of the drugs in their free conventional form. Liposomes are ideal vehicles for directing antibiotics to infection sites. Following intravenous administration, liposomes are taken up by cells of the mononuclear phagocytic system (MPS), namely, the Kupffer cells in the liver and fixed macrophages in the spleen. These represent the major reservoir of the M. avium infection in the body (4). Other investigators have already demonstrated the efficacies of some liposome-encapsulated antibiotics against MAC infections. Increased therapeutic activity in animal models of MAC infection was achieved by encapsulating in liposomes drugs such as amikacin (32), gentamicin (23), streptomycin (10, 15), or clofazimine (27).
Rifabutin (RFB) is an antimycobacterial agent that has been demonstrated to have activity against MAC in both in vitro and in vivo models (35). More recently, the U.S. Public Health Service recommended the use of RFB as a prophylactic agent against infections due to MAC in patients with AIDS (29). Several European clinical trials with RFB for the treatment of MAC infections in AIDS patients have been completed or are in progress (8).
In the present work, our aim was to investigate the enhancement of the antimycobacterial activity of RFB by incorporating this antibiotic into liposomes. Liposomal RFB formulations were developed, and the therapeutic activities of the formulations were evaluated in an animal model of infection with M. avium. Different treatment schedules, doses, and liposome sizes were tested. Furthermore, the influences of these parameters on the reduction of the infection level were also studied.
MATERIALS AND METHODS
Drugs, lipids, and reagents.
Pharmacy Biotech AB (Uppsala, Sweden) kindly provided RFB. The following pure phospholipids were obtained from Sigma Chemical Co. (St. Louis, Mo.): phosphatidylcholine (PC), phosphatidylserine (PS), phosphatidylglycerol (PG), cholesterol (Chol), dimyristoylphosphatidylcholine (DMPC), dioleoylphosphatidylcholine (DOPC), dipalmitoylphosphatidylcholine (DPPC), dipalmitoylphosphatidylglycerol (DPPG), and phosphatidylinositol (PI). Middlebrook 7H9 broth and 7H10 agar, and BACTO Middlebrook oleic acid-albumin-dextrose-catalase and albumin-dextrose-catalase enrichments were obtained from Difco Laboratories (Detroit, Mich.). Polycarbonate membranes were purchased from Nuclepore filtration products (Cambridge, Mass.). All other reagents were of analytical grade. Male BALB/c mice (age, 5 to 7 weeks; weight, 25 to 30 g) were obtained from the Gulbenkian Institute of Science (Oeiras, Portugal). The animals were kept under standard hygiene conditions, fed commercial chow, and given acidified drinking water ad libitum. All of the experimental procedures were carried out with the permission of the local laboratory animal committee.
M. avium strain.
Inocula were prepared as described previously (38). Briefly, the transparent colonies of M. avium strain P1581 were subcultured in Middlebrook 7H9 broth with albumin-dextrose-catalase supplement and 0.04% Tween 80 (Sigma) and were allowed to grow at 37°C on an orbital shaker for 2 weeks. The bacteria were harvested by centrifugation (2,000 × g, 10 min) in a GPR Beckman centrifuge (Beckman Instruments, Inc., Palo Alto, Calif.), suspended in a small volume of saline with 0.04% Tween 80, sonicated at low energy for 90 s in a bath-type sonicator (Bandelin Sonorex RK156, Berlin, Germany) to disrupt bacterial clumps, diluted in the same medium to an optical density at 600 nm of 0.48, and stored frozen at −70°C until use. When needed, aliquots were thawed at 37°C, diluted to the desired concentration, and inoculated.
Infection of animals.
A murine model of M. avium infection described previously (31) was used. The animals were infected by intravenous injection in a lateral tail vein of 200 μl of an M. avium inoculum at a concentration of 106 CFU per ml.
Treatment schedule.
Two treatment schedules were studied: a therapeutic treatment schedule and a prophylactic treatment schedule. The therapeutic treatment was started 2 weeks after the infection. Each week treated animals received two or three intravenous injections of RFB in the free or liposomal form in a lateral tail vein. Two different control groups were used: mice injected with unloaded liposomes and nontreated mice. The therapeutic effects of the RFB formulations were evaluated after a treatment duration of 3 or 8 weeks. For the 8-week treatments, RFB formulations of 10 mg/kg of body weight were administered twice a week. For the 3-week treatments, two different doses (10 and 20 mg/kg of body weight) were administered three times a week. The dosing volume administered was 200 μl for both doses tested. The therapeutic effects of the RFB formulations were also evaluated following the administration of 20 mg/kg two and three times a week for 3 weeks.
The prophylactic treatment was undertaken before the infection became established and consisted of the daily administration of 5 or 10 mg of free or liposomal RFB per kg for up to a total of 7 injections starting 1 day before the experimental infection with mycobacteria.
Evaluation of M. avium growth in mice.
At selected times the mice were killed by cervical dislocation, and their spleens and livers were aseptically removed, homogenized, serially diluted in 0.04% Tween 80, and plated onto Middlebrook 7H10 agar medium for counting of the number of CFU. Colonies were counted and characterized after incubation at 37°C for 10 to 15 days. From these counts the growth index was calculated by using the difference between the log10 CFU at the end of treatment and the log10 CFU at the beginning of treatment.
Statistical analysis.
All results correspond to a mean value and standard deviation for at least six animals for each group studied. Statistical analysis was performed by the unpaired two-tailed Student's t test, and differences with P values of <0.05 were considered significant.
Liposomal RFB formulations.
Multilamellar vesicles composed of the selected lipids were prepared as described previously (5). The lipids and the RFB in a molar ratio of 1:10 previously solubilized in an organic solution were dried under a nitrogen stream. The film was solubilized with tert-butanol, and the solution obtained was frozen and lyophilized overnight. Rehydration of the lyophilized powder was performed in two steps: first, rehydration was done in a volume of 1/10 of the final volume with saline, and then, 30 min after that, rehydration was completed with the same solution. The nonincorporated RFB was separated from the nonsized liposomal suspension by gel filtration in a column of coarse Sephadex G50. The eluted liposomes were then centrifuged for concentration (38,000 × g, 30 min, 20°C), and the pellet of the nonsized RFB liposomes was finally resuspended in saline. In order to reduce and homogenize the diameters of the liposomal formulations, after the two hydration steps liposomes were diluted fivefold with saline and then sequentially filtered through polycarbonate membranes of different porosities under a nitrogen pressure of 100 to 500 lb/in2 with an Extruder device (Lipex; Biomembranes Inc., Vancouver, British Columbia, Canada). The liposomes collected after the last extrusion step were concentrated by ultracentrifugation (250,000 × g, 90 min, 20°C) in a Beckman ultracentrifuge (Beckman Instruments, Inc.).
Characterization of liposomal RFB formulations.
RFB was quantified spectrophotometrically at 500 nm after disruption of the liposomes with ethanol. Lipid determinations were performed by the method of Fiske and Subbarow (12) as modified by King (22). The incorporation efficiency (IE) was defined as percent (RFB/L)f/(RFB/L)i, where (RFB/L)f and (RFB/L)i are the ratios of the final and the initial rifabutin and lipid concentrations, respectively. The vesicle size determinations were carried out by dynamic laser light scattering in a ZetaSizer 3 (Malvern Instruments Ltd.), which can measure the mean diameters and the population distribution of particles in the range of 5 to 5,000 nm.
RESULTS
Characterization of liposomal RFB formulations. (i) Nonsized liposomes: effect of charge, cholesterol, and fluidity.
In order to optimize the liposome formulations, the effects of factors such as the fluidity of the bilayer membrane, the percentage of negatively charged lipids, the presence or absence of Chol on the incorporation parameters IE and (RFB/L)f were studied.
RFB, a lipid-soluble molecule, was incorporated into the hydrophobic core of the phospholipid bilayer. The fluidity of the liposome membrane is of crucial importance to the incorporation parameters. Therefore, the fluidity of the liposome membrane was modulated either by the inclusion of Chol in the lipid composition or by use of phospholipids with different transition temperatures (Tcs).
Table 1 shows the characteristics of nonsized RFB liposomes with mean diameters that ranged from 0.6 to 0.8 μm. The liposomes were prepared with neutral or charged phospholipids with or without Chol and lipid-charged mixtures with different phase Tcs.
TABLE 1.
Effect of lipid composition on (RFB/L)f and IE
| Liposome type, effect, and lipid composition | (RFB/L)f (nmol/μmol)a | IE (%)a | Lipid molar ratio |
|---|---|---|---|
| Nonsized liposomesb | |||
| Effect of Chol | |||
| PG (−6°C)c | 99 ± 1 | 98 ± 1 | |
| PG-Chol | 87 ± 2 | 79 ± 2 | 7:1 |
| DMPC (+20°C) | 60 ± 3 | 48 ± 2 | |
| DMPC-Chol | 40 ± 2 | 35 ± 3 | 7:1 |
| PC (−6°C) | 45 ± 4 | 51 ± 3 | |
| PC-Chol | 31 ± 2 | 33 ± 2 | 7:1 |
| Fluidity of membrane | |||
| PC-PG (−6 and −6°C) | 97 ± 3 | 99 ± 1 | 9:1 |
| DOPC-DPPG (+20 and +42°C) | 77 ± 4 | 54 ± 3 | 9:1 |
| DPPC-DPPG (+42 and +42°C) | 57 ± 3 | 42 ± 2 | 9:1 |
| Sized liposomesd | |||
| PC-PI | 49 ± 7 | 61 ± 2 | 7:3 |
| 40 ± 2 | 43 ± 3 | 9:1 | |
| PC-PG | 53 ± 6 | 53 ± 2 | 7:3 |
| 39 ± 6 | 45 ± 5 | 9:1 | |
| PC-PS | 45 ± 1 | 53 ± 2 | 7:3 |
| 42 ± 1 | 50 ± 7 | 9:1 |
Values are means ± standard deviations for at least three independent preparations.
Initial lipid concentration, 16 μmol/ml; initial RFB concentration, 1.6 μmol/ml; mean liposome diameter, 0.6 to 0.8 μm;
Values in parentheses are Tcs and were obtained from another publication (17).
Effect of lipid charge. Initial RFB concentration, 1.6 μmol/ml; initial lipid concentration, 16 μmol/ml; liposome mean diameter, 0.3 μm.
The IEs of liposomes prepared with PG, DMPC, and PC decreased with the inclusion of Chol in the lipid composition from 98, 48, and 51% to 79, 35, and 33%, respectively.
The incorporation parameters for RFB in liposomes made with PC-PG, DOPC-DPPG and DPPC-DPPG at a molar ratio of 9:1 were evaluated. Liposomes prepared with mixtures of fluid phospholipids (PG and PC), which have a Tc of −6°C, showed the highest levels of incorporation: 97 nmol/μmol for (RFB/L)f and an IE of 99%. Liposomes made with more rigid lipids (DPPC and DPPG), which have Tc of 42°C, showed the lowest levels of incorporation: 57 nmol/μmol for (RFB/L)f and an IE of 42%. Intermediate values were observed for a lipid mixture of a fluid (DOPC) and a rigid (DPPG) phospholipid. These data indicate that the higher the rigidity of the liposome membrane the lower the level of incorporation of RFB.
For nonsized liposomes better incorporation parameters were achieved for lipid compositions with a negatively charged unsaturated phospholipid (PG) and with a mixture of a neutral phospholipid (PC with PG).
(ii) Sized liposomes: effects of charged lipids.
In order to obtain a smaller and more homogeneous liposome population, liposomal RFB formulations were prepared by using an extrusion procedure which resulted in a mean diameter of 0.3 μm. On the basis of previous results, several negatively charged lipid compositions were selected for the subsequent studies: mixtures of PC and negative lipids (PI, PG, or PS) in molar ratios of 7:3 and 9:1. As shown in Table 1, the highest IE and (RFB/L)f were achieved for liposomes prepared with a larger fraction of the negatively charged lipids (7:3). Regardless of the type of negatively charged phospholipid, the level of incorporation was dependent on the presence of the negatively charged phospholipids and increased concomitantly with the increase in the charge.
Therapeutic effects of liposomal RFB formulations.
According to the literature, liposomes that contain PS or PG administered intravenously are rapidly cleared from the circulation, mainly due to their uptake by the resident macrophages of the liver and spleen (7, 9). On the other hand, MPS uptake of liposomes that contain PI is inhibited and the circulation of the liposomes in the bloodstream is prolonged (2, 16). On the basis of these data and by taking into consideration the fact that in the animal model used in the present work the infection is predominantly localized in the liver and spleen, the lipid composition selected was PC-PS in a molar ratio of 7:3. The analysis of the therapeutic effect of this liposomal RFB formulation selected was carried out by considering the influence of the liposome diameters, treatment schedules, numbers of injections per week, durations of treatment, and administered doses.
Influences of liposome diameter and dose.
In order to evaluate the influence of the liposomal RFB formulation sizes on therapeutic efficacy, liposomes obtained by extrusion techniques (VET) with diameters of 0.1 μm (VET100) or 0.4 μm (VET400) were tested. Infected mice received intravenous injections of RFB formulations at a dose of 10 mg/kg twice a week for 8 weeks. The therapeutic effects of these formulations were estimated by comparing the bacterial counts in the infected organs with those in the organs of untreated mice or mice injected with RFB in the free form. As shown in Table 2, lower bacterial counts in the liver and spleen were found in mice treated with liposomal RFB formulations than in the groups treated with RFB in the free form or the control group. Statistically significant differences in the log10 CFU after treatment with VET400 versus that after treatment with free RFB were found in livers (P < 0.05) but not the spleens (P > 0.09).
TABLE 2.
Effects of RFB formulations on log10 CFU per organ for BALB/c mice infected with M. aviuma
| Treatment group | Log10 CFU/organ
|
|
|---|---|---|
| Liver | Spleen | |
| Control (beginning of treatment) | 6.0 ± 0.2 | 5.5 ± 0.2 |
| Control | 7.0 ± 0.1 | 7.7 ± 0.1 |
| Free RFB | 5.7 ± 0.2 | 6.6 ± 0.4 |
| VET400 | 5.3 ± 0.1 | 6.0 ± 0.3 |
| VET100 | 5.3 ± 0.3 | 6.2 ± 0.5 |
Mice were intravenously infected with 200 μl of an M. avium inoculum at a concentration of 106 CFU per ml. Treatment started 2 weeks after infection. Mice received intravenous injections of RFB formulations two times a week for 8 weeks at a dose of 10 mg/kg. Values are means ± standard deviations for at least six animals for each group studied. The lipid composition of the liposomes was PC-PS at a molar ratio 7:3. Mean liposome diameters were 0.4 μm (VET400) and 0.1 μm (VET100). The control group corresponds to untreated animals.
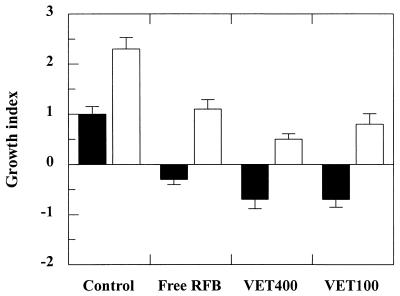
Figure 1 shows the effects of the RFB formulations administered twice a week for 8 weeks on the mycobacterial growth index. The growth indices for the livers of mice treated with liposomal RFB were lower than those for the livers of mice treated with free RFB: −0.7 ± 0.2 for VET400 and VET100 and −0.3 ± 0.2 for free RFB. The growth index for the livers of control mice was +1.0 ± 0.2. The differences in the growth indices for the livers of mice treated with VET400 and VET100 compared with those for the livers of mice treated with free RFB were found to be statistically significant (P < 0.025), as were the differences in the growth indices for the livers of mice treated with liposomal formulations compared with those for untreated animals, but to a greater extent (P < 0.001).
FIG. 1.
Effect of RFB formulations on the growth indices for the livers (black bars) and spleens (white bars) of BALB/c mice infected with M. avium. Mice were intravenously infected with 200 μl of an M. avium inoculum at a concentration of 106 CFU per ml. Treatment started 2 weeks after infection. Mice received intravenous injections of RFB formulations two times a week for 8 weeks at a dose of 10 mg/kg. Values are means ± standard deviations for at least six animals for each group studied. The lipid composition of the liposomes was PC-PS at a molar ratio of 7:3. Mean liposome diameters were 0.4 μm (VET400) and 0.1 μm (VET100). The control group corresponds to untreated animals.
Treatment with the RFB formulations was not as effective for the spleens as for the livers. Despite the positive growth indices observed for this organ for all groups, a decrease in the progression of infection was observed for animals injected with liposomal RFB formulations compared to that observed for control mice. Statistically significant differences were obtained for treatment with VET400 and VET100 compared to no treatment (P < 0.0009). However, the therapeutic effects of the liposomal RFB formulations versus those of free RFB were not statistically significant (P > 0.09) for this organ. The results presented in Table 2 and Fig. 1 show that the therapeutic effects of the two liposomal formulations that differed in particle size were not statistically different (P > 0.05) for both organs studied.
In order to achieve a higher level of preferential targeting of the liposomes to the spleen and to further increase the therapeutic effect of RFB, liposomal formulations with larger mean liposome diameters, different doses, and different treatment schedules were studied.
Liposomes with diameters of 0.3 μm (VET300) and 0.6 μm (VET600) were tested at doses of 10 and 20 mg/kg. As shown in Table 3, a strong reduction in the bacterial loads in the livers and spleens was observed for all groups treated with liposomal RFB formulations (groups D, E, G, and H). For the higher dose, a stronger reduction in the number of viable bacteria in both organs was observed for all groups treated with liposomal formulations. The therapeutic effect of liposomal RFB formulations at the dose of 10 mg/kg versus that at a dose of 20 mg/kg for either VET600 or VET300 was statistically significant for the livers (P < 0.03) and to a greater extent for the spleens (P < 0.001). The use of different liposome sizes for the same dose did not result in statistically significant differences in the therapeutic effect (P > 0.05). For free RFB, statistically significant differences for both organs under study were not observed with an increase in the administered dose from 10 to 20 mg/kg (P > 0.05).
TABLE 3.
Effects of RFB formulations on log10 CFU/organ after 3 weeks of treatment of BALB/c mice infected with M. aviuma
| Treatment group (dose [mg/kg]) | Log10 CFU/organ
|
|
|---|---|---|
| Liver | Spleen | |
| Control (beginning of treatment) | 6.6 ± 0.4 | 5.6 ± 0.2 |
| A. Control | 7.4 ± 0.1 | 7.1 ± 0.1 |
| B. Unloaded liposomes | 7.5 ± 0.3 | 6.9 ± 0.3 |
| C. Free RFB (10) | 6.5 ± 0.2 | 6.1 ± 0.3 |
| D. VET600 (10) | 6.2 ± 0.3 | 5.7 ± 0.3 |
| E. VET300 (10) | 6.0 ± 0.2 | 5.8 ± 0.2 |
| F. Free RFB (20) | 6.4 ± 0.2 | 5.9 ± 0.4 |
| G. VET600 (20) | 5.7 ± 0.1 | 5.1 ± 0.2 |
| H. VET300 (20) | 5.7 ± 0.1 | 5.1 ± 0.2 |
Mice were intravenously infected with 200 μl of an M. avium inoculum at a concentration of 106 CFU per ml. Treatment started 2 weeks after infection. Mice received intravenous injections of RFB formulations three times a week for 3 weeks. Two doses were tested: 10 and 20 mg/kg. Values are means ± standard deviations for at least six animals for each group studied. The lipid composition of the liposomes was PC-PS at a molar ratio of 7:3. Mean liposome diameters were 0.6 μm (VET600) and 0.3 μm (VET300).
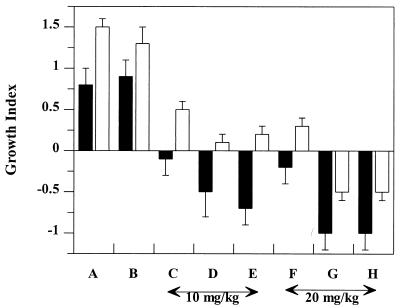
The therapeutic effect was also analyzed by comparing the growth indices for all animal groups. As shown in Fig. 2, negative values were obtained for both infected organs only for animals treated with either VET300 or VET600 at a dose of 20 mg/kg. In the case of the spleen, statistically significant differences in the growth indices were obtained for animals treated with liposomal RFB formulations at a dose of 10 mg/kg compared with those for animals treated with liposomal RFB formulations at a dose of 20 mg/kg (P < 0.0009). In order to determine the effect of phospholipids on the course of M. avium infection, a group of animals was treated with unloaded liposomes at a dose that corresponded to the amount of lipid used in formulations with 10 mg of liposomal RFB per kg. No statistically significant differences were found between untreated mice (group A) and mice injected with unloaded liposomes (group B) (P > 0.05).
FIG. 2.
Effect of treatment with different RFB formulations on the growth indices for the livers (black bars) and spleens (white bars) of BALB/c mice infected with M. avium. Mice were intravenously infected with 200 μl of an M. avium inoculum at a concentration of 106 CFU per ml. Treatment started 2 weeks after infection. Mice received intravenous injections of RFB formulations three times a week for 3 weeks. Untreated animals (A) and animals injected with empty liposomes (B), free RFB (10 mg/kg) (C), VET600 (10 mg/kg) (D), VET300 (10 mg/kg) (E), free RFB (20 mg/kg) (F), VET600 (20 mg/kg) (G), or VET300 (20 mg/kg) (H) were studied. Values are means ± standard deviations for at least six animals for each group studied. The lipid composition of the liposomes was PC-PS at a molar ratio of 7:3. Mean liposome diameters were 0.6 μm (VET600) and 0.3 μm (VET300).
Effects of number of administrations per week.
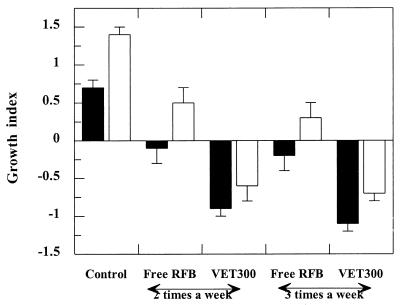
The influence of the number of administrations per week on the reduction in the level of infection was evaluated for the dose of 20 mg/kg. Mice were injected two or three times a week for 3 weeks. As shown in Fig. 3, administration of liposomal RFB always resulted in a stronger reduction in the level of infection compared to that achieved after administration of free RFB.
FIG. 3.
Effect of the number of administrations of RFB formulations per week on the growth indices for the livers (black bars) and spleens (white bars) of BALB/c mice infected with M. avium. Mice were intravenously infected with 200 μl of an M. avium inoculum at a concentration of 106 CFU per ml. Treatment started 2 weeks after infection. Mice were treated with a dose of 20 mg/kg, with the RFB formulations intravenously administered two or three times a week for 3 weeks. Values are means ± standard deviations for at least six animals for each group studied. The lipid composition of the liposomes was PC-PS at a molar ratio of 7:3. The mean liposome diameter was 0.3 μm (VET300).
For mice treated with liposomal RFB, significant differences in the level of reduction of the infection in the livers were achieved between animals that received two or three administrations per week (P < 0.009). However, no statistically significant differences were observed for the spleens of mice that received either two or three injections of liposomal RFB per week (P > 0.05). Administration of RFB in the free form two or three times per week did not result in statistically significant differences of RFB in the therapeutic effect for either organ (P > 0.05).
Prophylactic effects of RFB formulations.
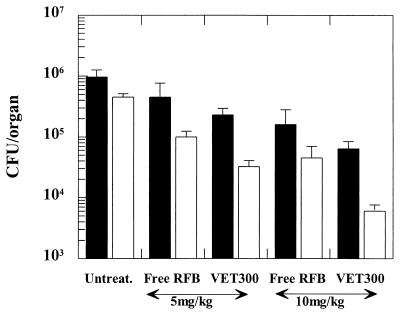
The prophylactic effects of free and liposomal RFB administered before experimental infection with M. avium were analyzed. Two doses were studied: 5 and 10 mg/kg. According to the data presented in Fig. 4, both the livers and the spleens of animals treated with liposomal RFB had smaller bacterial loads than the livers and spleens of mice treated with free RFB or untreated animals irrespective of the dose tested (5 or 10 mg/kg). Significant differences in the level of reduction of infection in the spleen or the liver were achieved for mice treated with RFB in the free or liposomal form for both doses (P < 0.05). Similar CFU counts were achieved either by treating mice with 10 mg of free RFB per kg or by injecting animals with 5 mg of RFB in the liposomal form per kg. This indicates that when liposomes are used in a prophylactic scheme smaller doses of the antibiotic can be administered.
FIG. 4.
Prophylactic effects of free and liposomal RFB on CFU counts for the livers (black bars) and spleens (white bars) of BALB/c mice. Mice received daily intravenous injections, which started 1 day before the injection of mycobacteria, for a total of 7 injections. Two doses were tested: 5 and 10 mg/kg. Mice were intravenously infected with 200 μl of an M. avium inoculum at a concentration of 106 CFU per ml. Values are means ± standard deviations for at least six animals for each group studied. The lipid composition of the liposomes was PC-PS at a molar ratio of 7:3. The mean liposome diameter was 0.3 μm (VET300).
DISCUSSION
Among the battery of antibiotics available for the treatment of MAC infections, RFB has been demonstrated to have activity both in vitro and in vivo (35). Clinical trials of combination therapies have demonstrated that the activities of agents such as ethambutol and clarithromycin are markedly enhanced when these drugs are used in association with RFB (39). In the present work the strategy used to enhance the antimycobacterial activity of RFB was the incorporation of this antibiotic in liposomes.
The first aim of our work was optimization of the liposomal RFB formulations under the galenic point of view. First, the effects of some parameters on RFB incorporation efficiency were evaluated. As RFB is partially soluble in lipids, the effect of Chol was investigated since it has an important modulatory effect on the liposomal membrane due to a strong interaction with the phospholipids. Chol acts by reducing the fluidity of membranes above the phase Tc and increasing it in the case of rigid membranes (28). Data obtained in this study are in accordance with those reported in the literature, as a decrease in the level of incorporation of hydrophobic drugs after Chol inclusion had been reported (5). This effect is due to a competition between the lipophilic drugs and Chol for similar locations in the hydrophobic membrane (3). In experiments in which RFB was incorporated into liposomes, by using lipids with negative- and positive-phase Tc, we found that RFB incorporation is affected by the rigidity of the liposome membrane. The greater the order of the membrane structure the more difficult RFB incorporation within the lipid bilayer will be. A similar behavior concerning the incorporation of another hydrophobic ansamycin, rifampin, has been described previously (5).
The lipid compositions of liposomes selected for therapeutic purposes cannot be based strictly on the incorporation parameters. The in vivo behaviors of liposomal formulations are dependent on the mean diameter, the presence of Chol, the fluidity of the membrane, and the charge, among other things. Besides its effect on the incorporation parameters, Chol also influences the in vivo behaviors of liposomes, stabilizing the bilayers and inhibiting their clearance from the circulation, presumably by inhibiting the binding of liposomes to serum opsonins and, consequently, their phagocytosis (26). As reported by Semple and coworkers (37), the highest degree of binding of blood serum proteins to liposomes, and, consequently, their most rapid clearance, was obtained when liposomes without Chol were injected intravenously. Taking into consideration that our aim was to target liposomal RFB formulations to infections in cells of the MPS, the exclusion of Chol from the lipid composition selected seemed to be a good choice (26, 37). The dependence of RFB incorporation on negatively charged phospholipids such as PS, PG, and PI was tested, as it is known that negatively charged phospholipids should be included in liposomal formulations to target MPS cells (10, 25, 26, 44). While higher levels of incorporation were observed for liposomes prepared with larger fractions of the negatively charged lipids, no differences in (RFB/L)f among the lipids mentioned above were found when they were used at the same molar ratio. However, different in vivo behaviors have been reported. PI inhibits uptake by the MPS and prolongs the circulation times of liposomes (2, 16, 30), while liposomes with PS or PG are rapidly cleared from the circulation (7, 9). These data, together with the good values for the incorporation parameters obtained in the present work, led to the selection of a lipid mixture that contained PC and PS (7:3).
The in vivo tests showed that liposomal RFB formulations reduced the number of bacteria in the spleen and reduced the number of bacteria to an even greater extent in the liver. Negative growth indices for the spleen were seen only when higher doses were used. The administration of the higher dose of liposomal RFB formulations (20 mg/kg) results in the injection of twice as many lipids compared to the number administered as part of the lower dose. The superior efficacy of higher doses, particularly for the spleen, could be explained by the temporary saturation of the liver with liposomes, and consequently, the numbers of particles that remain in the circulation and that could be conceivably taken up by the spleen may increase (33).
Comparison of the therapeutic activities of liposomal RFB formulations reported in the present work with data on other antimycobacterial agents reported in the literature is complex. This is due to differences in experimental conditions, namely, the timing of treatment after the induction of infection, the treatment schedule, the mean liposome diameters, the lipid composition, the treatment duration, the M. avium strain virulence, and the infection dose (10, 15, 23, 27, 32). In most of the studies with other antimycobacterial drugs described above, negatively charged lipids were used, but the lipid composition selected for our work has not been used previously. Nevertheless, we demonstrated in the present study the advantage of using liposomes to deliver RFB to M. avium infection sites, which is in agreement with previously published work with other antibiotics (10, 15, 23, 27, 32). We have also investigated the possible therapeutic effects of the phospholipids on M. avium infection level by injecting a group of animals with unloaded liposomes. No statistically significant differences were observed between untreated mice and mice treated with empty liposomes. According to data in the literature (6), statistically significant differences in M. avium loads between untreated mice and mice injected with unloaded liposomes have been observed only when high lipid doses were used. In the present study, the amount of lipid injected into animals treated with 20 mg of liposomal RFB per kg ranged from 6 to 7 μmol of lipid, which is between three and four times less than the amount used in the work reported by Cynamon et al. (6). Therefore, we can infer that no significant effect on bacterial proliferation would be induced by the small amount of lipid in unloaded liposomes used in the present work.
We have also studied the influences of liposome size, dose, and treatment schedule on the level of reduction of the number of bacteria in BALB/c mice infected with a virulent M. avium strain. The liposome sizes tested ranged from 0.1 to 0.6 μm, but no statistically significant differences in the effect of size on the therapeutic efficiencies of RFB formulations were found. Several researchers have investigated the influence of liposomal size on the therapeutic activity but have used a much broader range of liposomal sizes: 0.2 to 3 μm. Duzgunes et al. (11) studied the therapeutic effects of liposomes that incorporate amikacin on an M. avium infection in the beige mouse model. They found that larger liposomes (diameters, 2 to 3 μm) were slightly more effective in the livers and spleens than unilamellar vesicles with diameters of 0.2 μm (11). Other investigators did not find an effect of size on therapeutic efficacy for liposomes with streptomycin that ranged from 0.2 μm to 1.7 to 2.2 μm in diameter. Other factors besides liposome size may influence the therapeutic activities of the liposomal antibiotic formulations. The effect of the dose and the interval of dosing were also investigated, as they appear to be important factors in reducing the level of infection. Regarding the liposomal RFB dose, the greatest therapeutic effect was observed when the dose increased from 10 to 20 mg/kg. Similar conclusions were reported by Kansal and coworkers (21) when different doses of another liposomal antimycobacterial agent were tested.
The treatment schedule is of great importance, as it is known that the development of drug resistance may occur when low doses of antimicrobial agents are administered over a long dosing interval. Duzgunes et al. (11) found that the efficacy of administration of six doses of streptomycin was not substantially greater than the efficacy of administration of four doses once a week at a relatively low dose (10). In the present study, we have evaluated the effect of the number of administrations of RFB formulations per week. Our results show that the reduction of the level of infection was more dependent on the administered dose than on the number of doses administered per week. These data are in accordance with the observations of Cynamon et al. (6) obtained with liposomal amikacin.
Liposomal RFB formulations could be administered as prophylactic treatment against MAC infections in AIDS patients. Therefore, we evaluated the prophylactic effect of the liposomal form of RFB, and this scheme was found to be advantageous since the administration of 5 mg of RFB in the liposomal form per kg was as effective as the administration of 10 mg of antibiotic in the free form per kg. This observation corroborates the results obtained with other liposomal antibiotics in prophylactic schemes (23, 24).
In the animal model used in the present study, the liver has a higher bacterial load than the spleen. Twenty-four hours after infection by the intravenous route, the liver contains approximately 90% of the injected mycobacteria (data not shown). However, after the establishment of the infection, both organs remain infected. The greater reduction in the level of infection observed in livers may be due to the fact that liposomes that contain PS are preferentially targeted to the liver (7). On the other hand, the prophylactic administration of liposomes resulted in a greater reductions in the bacterial loads in the spleen. This was probably because the drug reached the spleen before the infection was established.
In conclusion, the incorporation of RFB into liposomes seems to be a very promising therapeutic system for the treatment or prophylaxis of infectious diseases. The intravenous administration of liposomal RFB to mice infected with M. avium resulted in a greater reductions in the level of infection compared to the administration of the RFB in the free form either before or after the establishment of infection.
REFERENCES
- 1.Allen T M, Hansen C, Rutledge J. Liposomes with prolonged circulation times: factors affecting uptake by reticuloendothelial and other tissues. Biochim Biophys Acta. 1989;981:27–35. doi: 10.1016/0005-2736(89)90078-3. [DOI] [PubMed] [Google Scholar]
- 2.Allen T M, Austin G A, Chonn A, Lin L, Lee K C. Uptake of liposomes by cultured mouse bone marrow macrophages: influence of liposome composition and size. Biochim Biophys Acta. 1991;1061:56–64. doi: 10.1016/0005-2736(91)90268-d. [DOI] [PubMed] [Google Scholar]
- 3.Blume G, Cevc G. Molecular mechanisms of the lipid vesicle longevity in vivo. Biochim Biophys Acta. 1993;1146:157–168. doi: 10.1016/0005-2736(93)90351-y. [DOI] [PubMed] [Google Scholar]
- 4.Chester A C, Winn W C. Unusual and newly recognized patterns of nontuberculous mycobacterial infection with emphasis on immunocompromised host. Pathol Annu. 1986;21:251–270. [PubMed] [Google Scholar]
- 5.Constantino L, Cruz M E M, Mehta R, Lopez-Berestein G. Formulation and toxicity of liposomes containing rifampicin. J Liposome Res. 1993;2:275–301. [Google Scholar]
- 6.Cynamon M H, Swenson C E, Palmer G S, Ginsberg R S. Liposome-encapsulated-amikacin therapy of Mycobacterium avium complex infection in beige mice. Antimicrob Agents Chemother. 1989;33:1179–1183. doi: 10.1128/aac.33.8.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daemen T, Velinova M, Regts J, Jager M, Kalicharan R, Donga J, van der Want J J L, Scherphof G L. Different intrahepatic distribution of phosphatidylglycerol and phosphatidylserine liposomes in the rat. Hepatology. 1997;26:416–423. doi: 10.1002/hep.510260223. [DOI] [PubMed] [Google Scholar]
- 8.Dautzenberg B. Rifabutin in the treatment of Mycobacterium avium complex infection: experience in Europe. Clin Infect Dis. 1996;22(Suppl.1):S33–S36. doi: 10.1093/clinids/22.supplement_1.s33. [DOI] [PubMed] [Google Scholar]
- 9.Dijkstra J, Van Galen M, Scherphof G. Influence of liposome charge on the association of liposomes with Kupffer cells in vitro. Effects of divalent cations and competition with latex particles. Biochim Biophys Acta. 1985;813:287–297. doi: 10.1016/0005-2736(85)90244-5. [DOI] [PubMed] [Google Scholar]
- 10.Duzgunes N, Ashtekar D R, Flasher D L, Ghori N, Debs R J, Friend D S, Gangadharam P R J. Treatment of Mycobacterium avium-intracellulare complex infection in beige mice with free and liposome-encapsulated streptomycin: role of liposome type and duration of treatment. J Infect Dis. 1991;164:143–151. doi: 10.1093/infdis/164.1.143. [DOI] [PubMed] [Google Scholar]
- 11.Duzgunes N, Perumal V K, Kesavalu L, Goldstein J A, Debs R J, Gangadharam P R J. Enhanced effect of liposome-encapsulated amikacin on Mycobacterium avium-M. intracellulare complex infection in beige mice. Antimicrob Agents Chemother. 1988;9:1404–1411. doi: 10.1128/aac.32.9.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fiske C H, Subbarow Y. The colorimetric determination of phosphorus. J Biol Chem. 1925;66:375–400. [Google Scholar]
- 13.Gangadharam P R J, Iseman M D. Antimycobacterial drugs. In: Peterson P K, Verhoel J, editors. Antimicrobial agents annual 2. Amsterdam, The Netherlands: Elsevier Science Publishers; 1987. pp. 14–35. [Google Scholar]
- 14.Gangadharam P R J, Iseman M D. Antimycobacterial drugs. In: Peterson P K, Verhoel J, editors. Antimicrobial agents annual 1. Amsterdam, The Netherlands: Elsevier Science Publishers; 1986. pp. 17–39. [Google Scholar]
- 15.Gangadharam P R J, Ashtekar D R, Flasher D L, Düzgünes N. Therapy of Mycobacterium avium complex infections in beige mice with streptomycin encapsulated in sterically stabilized liposomes. Antimicrob Agents Chemother. 1995;39:725–730. doi: 10.1128/AAC.39.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaspar M M, Perez-Soler R, Cruz M E M. Biological characterization of l-asparaginase liposomal formulations. Cancer Chemother Pharmacol. 1996;38:373–377. doi: 10.1007/s002800050497. [DOI] [PubMed] [Google Scholar]
- 17.Gunstone F D, Harwood J L, Padley F B, editors. The lipid handbook. London, United Kingdom: Chapman & Hall; 1986. [Google Scholar]
- 18.Havlik J A, Jr, Horsburgh C R, Jr, Metchock B, Williams P P, Fann S A, Thompson S E. Disseminated Mycobacterium avium complex infection: clinical identification and epidemiologic trends. J Infect Dis. 1992;165:577–580. doi: 10.1093/infdis/165.3.577. [DOI] [PubMed] [Google Scholar]
- 19.Higgins K. Potential toxicity of ciprofloxacin. Ophthalmology. 1991;98:120–121. [PubMed] [Google Scholar]
- 20.Horsbrugh C R, Selik R M. The epidemiology of disseminated nontuberculous mycobacterial infection in the acquired immunodeficiency syndrome (AIDS) Am Rev Respir Dis. 1989;139:4–7. doi: 10.1164/ajrccm/139.1.4. [DOI] [PubMed] [Google Scholar]
- 21.Kansal R G, Gomez-Flores R, Sinha I, Mehta R T. Therapeutic efficacy of liposomal clofazimine against Mycobacterium avium complex in mice depends on size of initial inoculum and duration of infection. Antimicrob Agents Chemother. 1997;41:17–23. doi: 10.1128/aac.41.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.King E J. The colorimetric determination of phosphurus. Biochem J. 1932;26:292–297. doi: 10.1042/bj0260292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klemens S P, Cynamon M H, Swenson C E, Ginsberg R S. Liposome-encapsulated-gentamicin therapy of Mycobacterium avium complex infection in beige mice. Antimicrob Agents Chemother. 1990;34:967–970. doi: 10.1128/aac.34.6.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Conte P, Le Gallou F, Potel G, Struillou L, Baron D, Drugeon H B. Pharmacokinetics, toxicity, and efficacy of liposomal capreomycin in disseminated Mycobacterium avium beige mouse model. Antimicrob Agents Chemother. 1994;38:2695–2701. doi: 10.1128/aac.38.12.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee K D, Hong K, Papahadjopoulos D. Recognition of liposomes by cell: in vitro binding and endocytosis mediated by specific lipid headgroups and surface charge density. Biochim Biophys Acta. 1992;1103:185–197. doi: 10.1016/0005-2736(92)90086-2. [DOI] [PubMed] [Google Scholar]
- 26.Lee K D, Hong K, Nir S, Papahadjopoulos D. Quantitative analysis of liposome-cell interactions in vitro: rate constants of binding and endocytosis with suspension and adherent J774 cells and human monocytes. Biochemistry. 1993;32:889–899. doi: 10.1021/bi00054a021. [DOI] [PubMed] [Google Scholar]
- 27.Mehta R T. Liposome encapsulation of clofazimine reduces toxicity in vitro and in vivo and improves therapeutic efficacy in the beige mouse model of disseminated Mycobacterium avium-M. intracellulare complex infection. Antimicrob Agents Chemother. 1996;40:1893–1902. doi: 10.1128/aac.40.8.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.New R R C. Influence of liposome characteristics on their properties and fate. In: Philippot J R, Schuber F, editors. Liposomes as tools in basic research and industry. Boca Raton, Fla: CRS Press, Inc.; 1995. pp. 3–20. [Google Scholar]
- 29.Nichols C W. Mycobacterium avium complex infection, rifabutin, and uveitis—is there a connection? Clin Infect Dis. 1996;22:S43–S49. doi: 10.1093/clinids/22.supplement_1.s43. [DOI] [PubMed] [Google Scholar]
- 30.Park Y S, Maruyama K, Huang L. Some negatively charged phospholipid derivatives prolong the liposome circulation in vivo. Biochim Biophys Acta. 1992;1108:257–260. doi: 10.1016/0005-2736(92)90034-j. [DOI] [PubMed] [Google Scholar]
- 31.Pedrosa J, Flórido M, Kunze Z M, Castro A G, Portaels F, McFadden J, Silva M T, Appelberg R. Characterization of the virulence of Mycobacterium avium complex (MAC) isolates in mice. Clin Exp Immunol. 1994;98:210–216. doi: 10.1111/j.1365-2249.1994.tb06127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petersen E A, Grayson J B, Hersh E M, Dorr R T, Chiang S M, Oka M, Proffitt R T. Liposomal amikacin: improved treatment of Mycobacterium avium complex infection in the beige mouse model. J Antimicrob Chemother. 1996;38:819–828. doi: 10.1093/jac/38.5.819. [DOI] [PubMed] [Google Scholar]
- 33.Poste G. Liposome targeting in vivo: problems and opportunities. Biol Cell. 1983;47:19–38. [Google Scholar]
- 34.Rosenzweig D Y. Pulmonary mycobacterial infections due to Mycobacterium intracellulare-avium complex. Clinical features and course in 100 consecutive cases. Chest. 1979;75:115–119. doi: 10.1378/chest.75.2.115. [DOI] [PubMed] [Google Scholar]
- 35.Saito H, Sato K, Tomioka H. Comparative in vitro and in vivo activity of rifabutin and rifampicin against Mycobacterium avium complex. Tubercle. 1988;69:187–192. doi: 10.1016/0041-3879(88)90020-7. [DOI] [PubMed] [Google Scholar]
- 36.Scherphof G L, Velinova M, Kamps J, Donga J, van der Want H, Kuipers F, Havekes L, Daemen T. Modulation of pharmacokinetic behaviour of liposomes. Adv Drug Delivery Rev. 1997;24:179–191. [Google Scholar]
- 37.Semple S C, Chonn A, Cullis P R. Influence of cholesterol on the association of plasma proteins with liposomes. Biochemistry. 1996;35:2521–2525. doi: 10.1021/bi950414i. [DOI] [PubMed] [Google Scholar]
- 38.Silva M T, Appelberg R, Silva M N T, Macedo P M. In vivo killing and degradation of Mycobacterium avium within mouse peritoneal macrophages. Infect Immun. 1987;55:2000–2016. doi: 10.1128/iai.55.9.2006-2016.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sullam P M. Rifabutin therapy for disseminated Mycobacterium avium complex infection. Clin Infect Dis. 1996;22(Suppl.1):S37–S49. doi: 10.1093/clinids/22.supplement_1.s37. [DOI] [PubMed] [Google Scholar]
- 40.Taneja J, Kaur D. Study on hepatotoxicity and other side effects on antituberculosis drugs. J Indian Med Assoc. 1990;88:278–280. [PubMed] [Google Scholar]
- 41.Walker J M, Hannah J B. Mycobacterium avium complex infection in patients with the acquired immunodeficiency syndrome. Chest. 1988;93:926–932. doi: 10.1378/chest.93.5.926. [DOI] [PubMed] [Google Scholar]
- 42.Wolinski E. Nontuberculosis mycobacteria and associated diseases. Am Rev Respir Dis. 1979;119:107–159. doi: 10.1164/arrd.1979.119.1.107. [DOI] [PubMed] [Google Scholar]
- 43.Young L S. Mycobacterium avium complex infection. J Infect Dis. 1988;157:863–867. doi: 10.1093/infdis/157.5.863. [DOI] [PubMed] [Google Scholar]
- 44.Yu-Kyoung O, Nix D E, Straubinger R M. Formulation and efficacy of liposome-encapsulated antibiotics for therapy of intracellular Mycobacterium avium infection. Antimicrob Agents Chemother. 1995;39:2104–2111. doi: 10.1128/aac.39.9.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]