Abstract
Charcot–Marie–Tooth disease type 2A (CMT2A) is a rare inherited axonal neuropathy caused by mutations in MFN2 gene, which encodes Mitofusin 2, a transmembrane protein of the outer mitochondrial membrane. We performed a cross-sectional analysis on thirteen patients carrying mutations in MFN2, from ten families, describing their clinical and genetic characteristics. Evaluated patients presented a variable age of onset and a wide phenotypic spectrum, with most patients presenting a severe phenotype. A novel heterozygous missense variant was detected, p.K357E. It is located at a highly conserved position and predicted as pathogenic by in silico tools. At a clinical level, the p.K357E carrier shows a severe sensorimotor axonal neuropathy. In conclusion, our work expands the genetic spectrum of CMT2A, disclosing a novel mutation and its related clinical effect, and provides a detailed description of the clinical features of a cohort of patients with MFN2 mutations. Obtaining a precise genetic diagnosis in affected families is crucial both for family planning and prenatal diagnosis, and in a therapeutic perspective, as we are entering the era of personalized therapy for genetic diseases.
Subject terms: Neurological disorders, Genetics research
Introduction
Charcot–Marie–Tooth disease (CMT) includes a wide spectrum of primary inherited sensory-motor neuropathies associated with more than 100 different genetic culprits1. With an overall prevalence of 1/1200-2500, it represents the most common genetically inherited neuromuscular disorder2. CMTs are classified according to their neurophysiological properties and inheritance pattern. Demyelinating CMT type 1 is characterized by reduced motor nerve conduction velocity (MNCV), while axonal CMT type 2 shows preserved MNCV1. Among CMT2, CMT2A is the most frequent form, accounting for approximately 10–40% of axonal CMT cases and 4–7% of all CMTs with a genetic diagnosis2–6.
CMT2A is associated with mutations in the nuclear-encoded mitochondrial gene mitofusin 2 (MFN2), which is translated into the 757-amino acid long protein Mitofusin2 (MFN2). MFN2 is a highly conserved GTPase, anchored to the outer mitochondrial membrane via two adjacent transmembrane regions (Fig. 1A). Together with its homolog Mitofusin 1 (MFN1), it is implicated in the regulation of the balance between mitochondrial fusion and fission, two processes that are deemed critical in mitochondrial quality control, cellular stress response and apoptosis7.
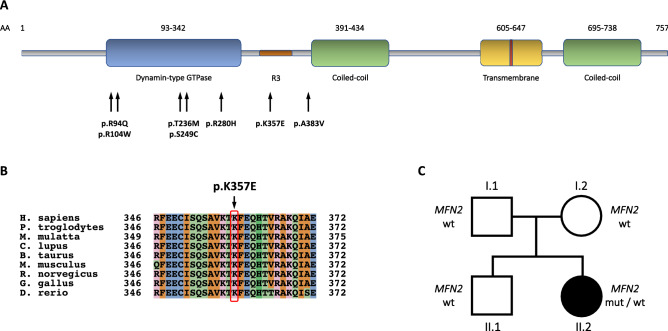
Figure 1.
Genetic and bioinformatic analysis. (A) Schematic representation of MFN2 protein structure, showing the localization of all the mutations detected in the current cohort (B) Conservation among orthologous genes of the K357 amino acid (mutation site), with the colours used by the Clustal Omega multiple sequence alignment program (red = hydrophobic, light blue = positively charged, pink = negatively charged, light green = polar, aquamarine = aromatic, dark green = glycine, orange = proline)49 (C) Pedigree of the patient carrying the p.K357E MFN2 variant. Circles correspond to females, squares to males. The black symbol indicates the proband affected by CMT2A, whereas the white ones are used for healthy relatives.
More than 100 MFN2 mutations have been detected in CMT2A patients; most of them are missense, whereas a limited number are nonsense variants or deletions. Although few recessive and semidominant forms have been described8–11, CMT2A is generally associated with autosomal dominant inheritance5,12–16. As described in other case series, most of mutations locate within the highly conserved GTPase or coiled-coil domains (Fig. 1A)16.
CMT2A has been associated to an early-onset, severe motor-predominant neuropathy, accompanied in some cases by significant proprioception loss15,16. In the largest cohort study of CMT2A natural history, childhood onset was shown to be the most predictive marker of disease severity16. Additional symptoms may be present and include optic nerve atrophy, scoliosis, hearing loss, vocal cord paralysis and upper motor neuron involvement15–19. White matter lesions can be detected on magnetic resonance imaging (MRI)20,21. Moreover, specific variants (e.g. those involving the arginine at the amino acid residue 104) have been associated with a neuropsychiatric syndrome characterized by developmental delay and cognitive impairment22. Overall, a wide inter-individual variability has been observed. Genotype–phenotype studies have been attempted, showing significant heterogeneity, also among members of the same family15–18,23.
Currently, no curative treatment is available for CMT2A, although MFN2 agonists and histone-deacetylase inhibitors have shown promising results in experimental models24,25. Furthermore, gene therapy-based approaches are currently under investigation and pledge to revolutionize the therapeutic options of rare diseases26,27. In this perspective, unraveling the genetic and phenotypic spectrum of CMT2A is becoming of utmost importance.
Our study analyzes thirteen patients from ten families, describing their clinical and genetic characteristics.
Patients and methods
Patients’ clinical evaluation
Between January 2018 and December 2020, we recruited 13 patients belonging to 10 CMT2A families with confirmed heterozygous MFN2 mutations. Three patients from the same pedigree were already described by our group in a previously published work22; we report new clinical data. Neurological evaluation and chart reviews was performed by neurologists experienced in neuromuscular diseases of the IRCCS Fondazione Ca’ Granda Ospedale Maggiore Policlinico. CMT2A was defined as inherited axonal neuropathy with nerve conduction velocities in the upper extremity > 38 m/s associated with MFN2 mutations. Personal and family history was investigated and common acquired causes of axonal neuropathy were excluded. Standard clinical information was obtained, including previous neurophysiologic studies, MRI and sural nerve or muscle biopsies. Muscle strength was assessed manually using the standard medical research council (MRC) scale. Sensory involvement was evaluated in terms of level and severity of pain, temperature, vibration and position sense impairment. Charcot–Marie–Tooth Examination Score version 2 (CMTESv2), a subscore of Charcot–Marie–Tooth Neuropathy Score version 2 (CMTNSv2), was used to estimate disease severity and clinical disability in adult patients28,29. We selected CMTESv2 rather than CMTNSv2 as recent neurophysiological testing was not available for all patients. Rasch analysis-weighted CMTESv2 (CMTESv2-R) was also calculated and was used to establish disease severity as mild (0–9), moderate (10–18) and severe (≥ 19)30,31. We used CMT pediatric scale (CMTPeds) in children aged 3–1832. Disability was classified according to CMTPedS scores as mild (0–16), moderate (16–29) or severe (> 29). Intellective functions were assessed with the Wechsler Adult Intelligence Scale for adults and Wechsler Intelligence Scale for Children–Revised for children. Neurophysiological testing was performed with standard techniques. Optic atrophy was assessed by direct fundoscopy and ophthalmologic review and, when deemed necessary, Optical Coherence Tomography (OCT).
Genetic analysis
Genomic DNA was extracted from peripheral venous blood samples. Sanger sequencing of MFN2 was performed. If potential pathogenic MFN2 variants previously undescribed were reported, to exclude other potential genetic causes of the phenotype, patient's DNA was included in a library for the Next-Generation Sequencing (NGS) of a panel of genes causing hereditary neuropathies. NGS was run on an Illumina MiSeq platform, according to the manufacturer instruction. Then, in silico pathogenicity prediction tools were employed, including Combined Annotation Dependent Depletion (CADD), Mutation Taster, Sorting Intolerant From Tolerant (SIFT), Polymorphism Phenotyping version 2 (PolyPhen2), Functional Analysis Through Hidden Markov Models (FATHMM), Mutation Assessor and MutPred2. Candidate variants were screened in Genome Aggregation Database (gnomAD v2.1.1), Exome Aggregation Consortium (ExAC) and 1000 Genomes Project (1000G). Conservation among orthologous genes of the mutated amino acids was analyzed through the National Center for Biotechnology Information (NCBI) HomoloGene. The American College of Medical Genetics and Genomics (ACMG) criteria for interpretation of sequence variants were also used to classify novel variants33.
Protocol approval and informed consent
All patients or their legal representatives signed an informed consent form prior to enrollment. The study was approved by the local Institutional Review Board (Comitato Etico di Milano Area 2 Protocol Number 898_2020bis). The study was performed in accordance with relevant guidelines and regulations.
Results
Characterization of patient cohort and genotype–phenotype correlation
We evaluated thirteen patients belonging to ten different families, who showed a clinical and neurophysiological phenotype consistent with CMT2A and carried a pathogenic MFN2 variant. Demographic and clinical characteristics of patients enrolled are shown in Tables 1 and 2.
Table 1.
Phenotype and clinical severity of patients with MFN2 mutations.
| Mutation | Patient # | Age at onset | Age at review | Sex | CMTESv2 | CMTESv2-R | Ambulation | Motor symptoms | Sensory symptoms |
|---|---|---|---|---|---|---|---|---|---|
| R94Q | 01–1 | 3 y | 22 y | M | 13 | 18 | Ambulatory (bilateral support, AFOs) | + | + |
| R104W | 02–1 | 1 y | 5 y | M | 26 (CMTPeds) | / | Ambulatory (AFOs) | + | − |
| 03–1 | 14 y | 55 y | M | 18 | 22 | Ambulatory (bilateral support) | + | + | |
| 03–2 | 2 y | 23 | M | 16 | 20 | Non-ambulatory | + | − | |
| 03–3 | 4 y | 20 | M | 18 | 24 | Non-ambulatory | + | + | |
| T236M | 04–1 | 5 y | 8 y | M | 12 (CMTPeds) | / | Ambulatory (plantars) | + | − |
| S249C | 05–1 | 17 y | 39 y | F | 7 | 8 | Ambulatory (autonomous) | + | − |
| R280H | 06–1 | 69 y | 77 y | F | 9 | 12 | Ambulatory (unilateral support) | + | − |
| 07–1 | 48 y | 76 y | F | 14 | 19 | Ambulatory (bilateral support) | + | + | |
| 08–1 | 58 y | 71 y | M | 3 | 3 | Ambulatory (autonomous) | + | − | |
| K357E | 09–1 | 1 y | 23 y | F | 19 | 26 | Non-ambulatory | + | + |
| A383V | 10–1 | 7 y | 48 y | F | 12 | 13 | Ambulatory (AFOs) | + | − |
| 10–2 | 12 y | 58 y | F | 11 | 16 | Ambulatory (walking aids) | + | + |
AFO ankle–foot orthosis, CMTES Charcot–Marie–Tooth examination scale, CMTES-R Rasch analysis-weighted CMTES, CMTPeds CMT Pediatric Scale.
Table 2.
Clinical features of patients with MFN2 mutations.
| Mutation | Patient # | Proximal weakness UL * | Distal weakness UL * | Proximal weakness LL * | Distal weakness LL * | Cutaneous sensation UL/LL§ | Pallesthesia UL/LL§ | Proprioception UL/LL§ | Optic atrophy | Scoliosis | Intellectual disability | Restrictive lung disease/Non-invasive respiratory support | Additional symptoms |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R94Q | 01–1 | − | +++ | + | +++ | +/+ | −/− | −/− | − | − | − | − | − |
| R104W | 02–1 | − | +++ | + | +++ | −/− | −/− | −/− | + | − | + | − | Bilateral cataracts, epilepsia partialis continua |
| 03–1 | + | + | ++ | ++ | −/− | −/+ | −/− | + | − | + | − | Lower limbs myoclonus, spastic paraparesis, dysphagia, sensorineural hearing defect | |
| 03–2 | + | +++ | +++ | +++ | −/− | −/− | −/− | − | − | + | + | Dysarthria, ataxic gait features | |
| 03–3 | ++ | +++ | ++ | +++ | −/+ | −/− | −/− | + | − | + | − | Dysarthria, lower limbs myoclonus | |
| T236M | 04–1 | − | − | + | ++ | −/− | −/− | −/− | − | − | − | − | − |
| S249C | 05–1 | − | + | + | +++ | −/− | −/− | −/− | − | − | − | − | − |
| R280H | 06–1 | − | + | + | ++ | −/− | −/− | −/− | + | − | − | − | Sensorineural hearing defect |
| 07–1 | − | ++ | − | +++ | −/− | −/− | −/− | − | − | − | − | Dysphagia, ptosis | |
| 08–1 | − | − | − | ++ | −/− | −/− | −/− | − | − | − | + | MEPs/SSEPs alteration | |
| K357E | 09–1 | + | +++ | +++ | +++ | +/+ | +/+ | +/+ | + | + | − | + | Vocal cord paresis |
| A383V | 10–1 | − | + | + | +++ | −/− | −/− | −/− | − | − | − | − | − |
| 10–2 | − | +++ | − | +++ | −/− | +/+ | −/− | − | − | − | − | − |
LL lower limbs, LMN lower motor neuron, MEP motor evoked potentials, SSEP somato-sensory evoked potentials, UL upper limbs.
*Motor weakness assessed by Medical Research Council scale (MRC): UL proximal weakness assessed by deltoids, biceps brachii and triceps, UL distal weakness assessed by first dorsal interosseus, abductor pollicis brevis and adductor digiti minimi muscles, LL proximal weakness assessed by iliopsoas, quadriceps and hamstring muscles, LL distal weakness assessed by anterior tibialis, gastrocnemius and extensor hallucis longus muscles. −: no weakness; +: slight weakness (> / = 4);++: moderate weakness (3 to 4);+++: severe weakness (< / = 3).
§Cutaneous sensation is based on pinprick examination: normal is no definite decrease compared to a normal reference point. Pallesthesia is assessed with Rydel-Seiffer tuning fork: normal is ≥ 5. Proprioception is based on joint position sensation. In the Table, cutaneous sensation, pallesthesia and proprioception are defined as normal (+) or impaired (−) in upper limbs/lower limbs.
Age of disease onset varied widely, ranging from early childhood to late adulthood (1–69 years, mean 18.5, SD + /− 23.6). Walking difficulties were the most common initial symptoms. Clinical severity also ranged from mild to severe, with most patients presenting a severe phenotype, as already observed by previous studies (Table 1, Supplementary Table 1) 15,16. CMTESv2 in adult patients ranged from 3 to 19 points (mean 12.7, SD + /− 5), while Rasch-modified CMTESv2 ranged from 3 to 26 points (mean 16.5, SD + /− 6,9). Overall, patients with childhood-onset CMT2A (1–20 years of age) presented a more severe phenotype comparing with patients with adult-onset CMT2A (> 20 years of age). Interestingly, all the three adult-onset CMT2A patients carried the p.R280H mutation.
Clinical sensory involvement was reported only in 6/13 (46%) patients and did not seem to be associated to specific mutations nor to age of onset. Additional symptoms were described in a total of 8/13 (61%) patients. Among them, optic atrophy and reduced visual acuity occurred in 5/13 (38%) patients, in line with previous reports18.Furthermore, one p.R280H carrier (08-1) showed concomitant alterations in motor and somatosensory evoked potentials (MEP/SSEP). Two patients, one carrying the p.R104W mutation (03-1) and one carrying the p.R280H mutation (06-1), presented a sensorineural hearing defect. Another carrier of the p.R280H mutation (07–1) displayed dysphagia and ptosis.
As already reported, all subjects carrying the p.R104W variant presented central nervous system involvement with intellectual disability and developmental delay, in some cases with dysarthric speech (patients 03-2 and 03-3), spastic paraparesis (patient 03-1) and ataxic gait (patient 03-2). In addition to that, three patients among them had epileptic manifestations, namely lower limbs myoclonus in two patients from the same family (03-2, 03-3) and epilepsia partialis continua in a third, unrelated patient (02-1). Two patients (02-1, 03-1) displayed punctate white matter alterations at MRI investigation.
Characterization of MFN2 genetic spectrum and identification of one novel mutation
Except for the proband (09-1), all patients carried a known heterozygous missense MFN2 mutation already associated to CMT2A phenotype (p.R94Q5,14,15,18,23,34,35, p.R104W16,22, p.T236M34, p.S249C16, p.R280H5,14,16–18,23, p.A383V16,36,37). Most of them (p.R94Q, p.R104W, p.T236M, p.S249C and p.R280H) are located within the highly conserved GTPase domain (Fig. 1A). The affected members of three separate kindreds carried the p.R280H mutation. The p.R104W mutation was observed in four patients from two families. The other mutations (p.R94Q, p.T236M, p.S249C, p.A383V) were reported in one family each.
We detected one novel, heterozygous pathogenic variant, namely the c.1069A > G, p.K357E (NM_014874). The mutation, located in MFN2 exon 11, is absent from public databases and is predicted as pathogenic by in silico tools (Supplementary Table 2). MFN2 sequencing in the parents excluded the presence of the variant, thus confirming its de novo occurrence (Fig. 1C). The p.K357E replaces a highly conserved lysine (Fig. 1B) in the R3 region (Fig. 1A), which was found to be necessary for mitochondrial fragmentation and fusion in vitro38. A different amino acid substitution at the same site (c.1071G > C, p.K357N) was previously described by Kijima et al. in 200534. The variant fulfils the ACMG criteria for a likely pathogenic variant33.
The proband carrying the p.K357E mutation presented a severe, early-onset phenotype characterized by severe weakness and muscle atrophy in both proximal and distal segments of lower limbs and in the distal segments of the upper limbs, and mild weakness and atrophy in the proximal segments of the upper limbs. The patient is not able to walk autonomously, uses the wheelchair and needs assistance for daily activities and self-care. She had scoliosis and foot deformities (pes cavus), and a severe restrictive lung disease with respiratory insufficiency requiring nocturnal non-invasive ventilatory support (NIV). She also displayed complete loss of tactile, vibratory and proprioceptive sensation, optic atrophy and bilateral vocal cord paresis determining dysphonia.
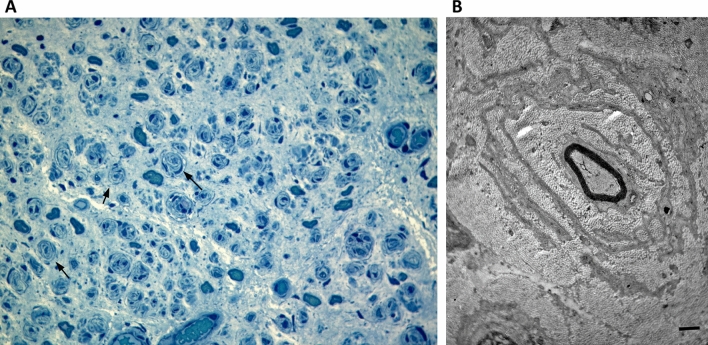
The biopsy of the sural nerve was consistent with the clinical severity of the disease showing an almost complete absence of fibers, important connective substitution and several onion bulb formations (Fig. 2), as reported in severe CMT2A cases 22,39,40.
Figure 2.
Nerve biopsy of patient carrying the p.K357E variant. (A) Sural nerve, semithin section, toluidine blue (400× magnification): several onion bulbs (arrows). (B) Electron micrograph; transverse section. One myelinated axon is surrounded by concentric proliferation of Schwann cells. Bar = 1 µm.
Discussion
We identified seven pathogenic MFN2 mutations (p.R94Q, p.R104W, p.T236M, p.S249C, p.R280H, p.A383V, p.K357E), which co-segregate with CMT2A phenotype in thirteen patients of ten separate families, including the novel heterozygous p.K357E. We observed the prevalence of moderate or severe phenotypes (38% respectively) among our cohort. The mean CMTESv2 score at review was 12.7, similarly to what has been observed by Pipis and colleagues in a large natural history study16, and a great proportion of patients (11, 85%) was not able to walk independently but required aids or wheelchair. Despite the limited number of patients in our cohort and the variable age at review do not allow to draw definite conclusions, these data demonstrate the presence of moderate-to-severe burden of disease in daily life, as opposed to other forms of CMT41–43.
As regards genotype–phenotype correlation, the small number of patients does not allow to make precise inferences. It is interesting to note that the p.R280H mutation caused a late-onset phenotype in all the three carriers. This mutation has been previously associated with a highly variable age at onset16–18,44. Some of our patients had pure motor neuropathies whereas others showed symptoms and signs of large sensory fibers involvement. These discrepancies were not mutation specific as some variants (p.R104W, p.R280H, p.A383V) were associated with either motor or sensorimotor phenotypes in different individuals. Proximal weakness of lower limbs appeared quite frequently in our patients, suggesting that CMT2A impairment might be less length dependent than other forms of CMT, as already hypothesized by other authors15,23. However, proximal involvement in some of our patients may be as well due to the presence of a severe length dependent axonal neuropathy, typical of CMT2A. Therefore, the hypothesis of a non-length dependent proximal involvement remains purely speculative. Notably, three probands carrying the p.R104W mutation suffered from epileptic manifestations, namely myoclonus in two probands from the same kindred and epilepsia partialis continua in a third, unrelated proband. Even though this variant is known to cause an encephalopathic syndrome with developmental delay16, epileptic phenomena have been reported only sporadically, in the form of temporo-parietal slow waves45 or complex partial seizures with abdominal aura46. Thus, the epileptic symptoms observed in our patients further expand the spectrum of manifestations known to occur in the carriers of the R104W variant. Proband 09-1, carrier of the p.K357E mutation, displays a severe phenotype, with severe motor and sensory involvement in both upper and lower limbs, respiratory insufficiency, skeletal deformities, optic atrophy and bilateral vocal cord paresis.
Most of the mutations detected in our series are located in the MFN2 dynamin-GTPase domain, as reported in other studies done so far15,16. Nevertheless, the muted amino acid of the novel p.K357E mutation is located outside this functional domain, in a helix bundle formed by helices from the N-terminal and the C-terminal parts of the MFN2 protein (highly conserved R3 region). This helix bundle is required for the expression of the GTPase domain. Furthermore, its structural integrity allows close contact between membranes from adjacent mitochondria, thus resulting in correct mitochondrial fusion38. Notably, the impairment in mitochondrial fusion has been proposed as one of the pathogenic mechanisms linking MFN2 dysfunction to neuronal die-back and degeneration47. The recent finding that MFN2 agonists reverse mitochondrial defects in CMT2A preclinical models, by mimicking the peptide-peptide interface of MFN2 and consequently activating MFN2 and promoting mitochondrial fusion, further confirms this observation25,48. Intriguingly, a report by Kijima and colleagues described a patient with a different amino acid substitution at the same site (p.K357N), presenting with mild-to-moderate symptoms and onset in early childhood. The phenotype severity differed markedly from that of our proband, who harbors the p.K357E mutation. Although we cannot be sure of the reason of such differences in genotype–phenotype correlation, it may be that the substitution of a lysine (K), which is positively charged, with an asparagine (N), a polar amino acid, does not alter the structure and the ionic charge of the helix bundle as the substitution with a glutamic acid (E), which is negatively charged. The specific amino acid might influence the interaction between mitochondrial membranes and, therefore, have a different impact on the regulation of mitochondrial fusion.
In conclusion, our work expands the genetic spectrum of CMT2A by providing a detailed description of clinical features of CMT2A patients who underwent genetic analysis and were confirmed to carry either a known or a novel pathogenic mutation of MFN2.
Supplementary Information
Acknowledgements
We thank “Associazione Amici del Centro Dino Ferrari” and “Progetto Mitofusina 2 onlus” for their support.
Abbreviations
- CMT
Charcot–Marie–Tooth
- CMTESv2
Charcot–Marie–Tooth Examination Score version 2
- CMTESv2-R
Rasch analysis-weighted CMTESv2
- CMTNSv2
Charcot–Marie–Tooth Neuropathy Score version 2
- CMTPeds
CMT pediatric scale
- MFN1/2
Mitofusin1/2
- MNCV
Motor nerve conduction velocity
- NIV
Non-invasive ventilatory support
Author contributions
E.A. and S.C. conceived and designed the work. E.A. gave major contributions in the acquisition, analysis, and interpretation of data; and drafted the work. A.M. gave major contributions in the analysis and interpretation of data and drafted the work. D.V., R.D.B., L.N., F.R., M.M., N.B., E.B., M.T.B., M.G.D., G.P.C. and S.C. gave substantial contributions in the acquisition, analysis and interpretation of data and substantively revised the draft. All authors have approved the submitted version and have agreed both to be personally accountable for each author's own contributions.
Funding
This study was funded by the Italian Ministry of Health grant GR-2018-12365358 to FR (2018–2021) and RC 2020 and RC 2021 to MTB and MGD, by the Telethon Foundation GGP19002 to SC, by the grant CP 20/2018 (Care4NeuroRare) from Fondazione Regionale per la Ricerca Biomedica (FRRB) to SC and MTB, and by the Foundation IRCCS Cà Granda Ospedale Maggiore Policlinico Ricerca Corrente 2022 to NB and GC. GC and SC are members of the European Reference Network for Rare Neuromuscular Diseases (ERN EURO-NMD).
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Elena Abati and Arianna Manini.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-10220-0.
References
- 1.Laurá M, Pipis M, Rossor AM, Reilly MM. Charcot-marie-Tooth disease and related disorders: An evolving landscape. Curr. Opin. Neurol. 2019;32:641–650. doi: 10.1097/WCO.0000000000000735. [DOI] [PubMed] [Google Scholar]
- 2.Braathen GJ. Genetic epidemiology of charcot-marie-tooth disease. Acta Neurol. Scand. Suppl. 2012 doi: 10.1111/ane.12013. [DOI] [PubMed] [Google Scholar]
- 3.Murphy SM, et al. Charcot-Marie-Tooth disease: Frequency of genetic subtypes and guidelines for genetic testing. J. Neurol. Neurosurg. Psychiatry. 2012;83:706–710. doi: 10.1136/jnnp-2012-302451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fridman V, et al. CMT subtypes and disease burden in patients enrolled in the inherited neuropathies consortium natural history study: A cross-sectional analysis. J. Neurol. Neurosurg. Psychiatry. 2015;86:873–878. doi: 10.1136/jnnp-2014-308826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Züchner S, et al. Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nat. Genet. 2004;36:449–451. doi: 10.1038/ng1341. [DOI] [PubMed] [Google Scholar]
- 6.Engelfried K, et al. Charcot-marie-tooth neuropathy type 2A: novel mutations in the mitofusin 2 gene (MFN2) BMC Med. Genet. 2006 doi: 10.1186/1471-2350-7-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chandhok G, Lazarou M, Neumann B. Structure, function, and regulation of mitofusin-2 in health and disease. Biol. Rev. Camb. Philos. Soc. 2018;93:933–949. doi: 10.1111/brv.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomaselli PJ, et al. Semi-dominant mutations in MFN2-related neuropathy and implications for genetic counselling. J. Peripher. Nerv. Syst. 2016;21:52–54. doi: 10.1111/jns.12155. [DOI] [PubMed] [Google Scholar]
- 9.Polke JM, et al. Recessive axonal charcot-marie-tooth disease due to compound heterozygous mitofusin 2 mutations. Neurology. 2011;77:168–173. doi: 10.1212/WNL.0b013e3182242d4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piscosquito G, et al. Mutational mechanisms in MFN2-related neuropathy: Compound heterozygosity for recessive and semidominant mutations. J. Peripher. Nerv. Syst. 2015;20:380–386. doi: 10.1111/jns.12145. [DOI] [PubMed] [Google Scholar]
- 11.Nicholson GA, et al. Severe early-onset axonal neuropathy with homozygous and compound heterozygous MFN2 mutations. Neurology. 2008;70:1678–1681. doi: 10.1212/01.wnl.0000311275.89032.22. [DOI] [PubMed] [Google Scholar]
- 12.Cartoni R, Martinou J-C. Role of mitofusin 2 mutations in the physiopathology of Charcot–Marie–Tooth disease type 2A. Exp. Neurol. 2009;218:268–273. doi: 10.1016/j.expneurol.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Chen H, et al. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J. Cell Biol. 2003;160:189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Detmer SA, Chan DC. Complementation between mouse Mfn1 and Mfn2 protects mitochondrial fusion defects caused by CMT2A disease mutations. J. Cell Biol. 2007;176:405–414. doi: 10.1083/jcb.200611080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feely SME, et al. MFN2 mutations cause severe phenotypes in most patients with CMT2A. Neurology. 2011;76:1690–1696. doi: 10.1212/WNL.0b013e31821a441e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pipis M, et al. Natural history of charcot-marie-tooth disease type 2A: A large international multicentre study. Brain. 2021 doi: 10.1093/brain/awaa323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung KW, et al. Early onset severe and late-onset mild Charcot-Marie-Tooth disease with mitofusin 2 (MFN2) mutations. Brain. 2006;129:2103–2118. doi: 10.1093/brain/awl174. [DOI] [PubMed] [Google Scholar]
- 18.Verhoeven K, et al. MFN2 mutation distribution and genotype/phenotype correlation in Charcot-Marie-Tooth type 2. Brain. 2006;129:2093–2102. doi: 10.1093/brain/awl126. [DOI] [PubMed] [Google Scholar]
- 19.Pareyson D, Marchesi C. Diagnosis, natural history, and management of Charcot-Marie-Tooth disease. Lancet. Neurol. 2009;8:654–667. doi: 10.1016/S1474-4422(09)70110-3. [DOI] [PubMed] [Google Scholar]
- 20.Chung KW, et al. Early-onset charcot-marie-tooth patients with mitofusin 2 mutations and brain involvement. J. Neurol. Neurosurg. Psychiatry. 2010;81:1203–1206. doi: 10.1136/jnnp.2009.181669. [DOI] [PubMed] [Google Scholar]
- 21.Lee M, et al. Cerebral white matter abnormalities in patients with charcot-marie-tooth disease. Ann. Neurol. 2017;81:147–151. doi: 10.1002/ana.24824. [DOI] [PubMed] [Google Scholar]
- 22.Del Bo R, et al. Mutated mitofusin 2 presents with intrafamilial variability and brain mitochondrial dysfunction. Neurology. 2008;71:1959–1966. doi: 10.1212/01.wnl.0000327095.32005.a4. [DOI] [PubMed] [Google Scholar]
- 23.Bombelli F, et al. Charcot-Marie-Tooth disease type 2A: from typical to rare phenotypic and genotypic features. JAMA Neurol. 2014;71:1036–1042. doi: 10.1001/jamaneurol.2014.629. [DOI] [PubMed] [Google Scholar]
- 24.Picci C, et al. HDAC6 inhibition promotes α-tubulin acetylation and ameliorates CMT2A peripheral neuropathy in mice. Exp. Neurol. 2020;328:113281. doi: 10.1016/j.expneurol.2020.113281. [DOI] [PubMed] [Google Scholar]
- 25.Rocha AG, et al. MFN2 agonists reverse mitochondrial defects in preclinical models of Charcot-Marie-Tooth disease type 2A. Science. 2018;360:336–341. doi: 10.1126/science.aao1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schiza N, et al. Gene replacement therapy in a model of Charcot-Marie-Tooth 4C neuropathy. Brain. 2019;142:1227–1241. doi: 10.1093/brain/awz064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kagiava A, et al. Gene replacement therapy after neuropathy onset provides therapeutic benefit in a model of CMT1X. Hum. Mol. Genet. 2019;28:3528–3542. doi: 10.1093/hmg/ddz199. [DOI] [PubMed] [Google Scholar]
- 28.Murphy SM, et al. Reliability of the CMT neuropathy score (second version) in Charcot-Marie-Tooth disease. J. Peripher. Nerv. Syst. 2011;16:191–198. doi: 10.1111/j.1529-8027.2011.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shy ME, et al. Reliability and validity of the CMT neuropathy score as a measure of disability. Neurology. 2005;64:1209–1214. doi: 10.1212/01.WNL.0000156517.00615.A3. [DOI] [PubMed] [Google Scholar]
- 30.Fridman V, et al. A longitudinal study of CMT1A using Rasch analysis based CMT neuropathy and examination scores. Neurology. 2020;94:e884–e896. doi: 10.1212/WNL.0000000000009035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sadjadi R, et al. Psychometrics evaluation of charcot-marie-tooth neuropathy score (CMTNSv2) second version, using Rasch analysis. J. Peripher. Nerv. Syst. 2014;19:192–196. doi: 10.1111/jns.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burns J, et al. Validation of the charcot-marie-tooth disease pediatric scale as an outcome measure of disability. Ann. Neurol. 2012;71:642–652. doi: 10.1002/ana.23572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richards S, et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kijima K, et al. Mitochondrial GTPase mitofusin 2 mutation in charcot-marie-tooth neuropathy type 2A. Hum. Genet. 2005;116:23–27. doi: 10.1007/s00439-004-1199-2. [DOI] [PubMed] [Google Scholar]
- 35.Baloh RH, Schmidt RE, Pestronk A, Milbrandt J. Altered axonal mitochondrial transport in the pathogenesis of charcot-marie-tooth disease from mitofusin 2 mutations. J. Neurosci. 2007;27:422–430. doi: 10.1523/JNEUROSCI.4798-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bergamin G, et al. Novel mutation of the mitofusin 2 gene in a family with Charcot-Marie-Tooth disease type 2. Muscle Nerve. 2014;49:145–146. doi: 10.1002/mus.23985. [DOI] [PubMed] [Google Scholar]
- 37.Muglia M, et al. Clinical and genetic study of a large Charcot-Marie-Tooth type 2A family from southern Italy. Neurology. 2001;56:100–103. doi: 10.1212/WNL.56.1.100. [DOI] [PubMed] [Google Scholar]
- 38.Honda S, Aihara T, Hontani M, Okubo K, Hirose S. Mutational analysis of action of mitochondrial fusion factor mitofusin-2. J. Cell Sci. 2005;118:3153–3161. doi: 10.1242/jcs.02449. [DOI] [PubMed] [Google Scholar]
- 39.Lv H, et al. A cohort study of Han Chinese MFN2-related charcot-marie-tooth 2A. J. Neurol. Sci. 2015;358:153–157. doi: 10.1016/j.jns.2015.08.1528. [DOI] [PubMed] [Google Scholar]
- 40.Luigetti M, et al. Clinical, electrophysiological and pathological findings of a patient with CMT2 due to the p.Ala738Val mitofusin 2 mutation. J. Neurol. Sci. 2011;307:168–170. doi: 10.1016/j.jns.2011.04.025. [DOI] [PubMed] [Google Scholar]
- 41.Rossor AM, et al. Pilot phenotype and natural history study of hereditary neuropathies caused by mutations in the HSPB1 gene. Neuromuscul. Disord. 2017;27:50–56. doi: 10.1016/j.nmd.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Panosyan FB, et al. Cross-sectional analysis of a large cohort with X-linked charcot-marie-tooth disease (CMTX1) Neurology. 2017;89:927–935. doi: 10.1212/WNL.0000000000004296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanmaneechai O, et al. Genotype-phenotype characteristics and baseline natural history of heritable neuropathies caused by mutations in the MPZ gene. Brain. 2015;138:3180–3192. doi: 10.1093/brain/awv241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakhro K, Park JM, Choi BO, Chung KW. Missense mutations of mitofusin 2 in axonal charcot-marie-tooth neuropathy: Polymorphic or incomplete penetration? Animal Cells Syst. 2013;17:228–236. doi: 10.1080/19768354.2013.814587. [DOI] [Google Scholar]
- 45.Tufano M, et al. Early onset charcot-marie-tooth neuropathy type 2A and severe developmental delay: Expanding the clinical phenotype of MFN2-related neuropathy. J. Peripher. Nerv. Syst. 2015;20:415–418. doi: 10.1111/jns.12148. [DOI] [PubMed] [Google Scholar]
- 46.Klein CJ, et al. Large kindred evaluation of mitofusin 2 novel mutation, extremes of neurologic presentations, and preserved nerve mitochondria. Arch. Neurol. 2011;68:1295. doi: 10.1001/archneurol.2011.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dorn GW. Mitofusin 2 dysfunction and disease in mice and men. Front. Physiol. 2020;11:1–9. doi: 10.3389/fphys.2020.00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Franco A, et al. Correcting mitochondrial fusion by manipulating mitofusin conformations. Nature. 2016;540:74–79. doi: 10.1038/nature20156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McWilliam H, et al. Analysis tool web services from the EMBL-EBI. Nucleic Acids Res. 2013;41:597–600. doi: 10.1093/nar/gkt376. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.