Abstract
Introduction
Macrolide antibiotics have immunomodulatory properties which may be beneficial in viral infections. However, the precise effects of macrolides on T cell responses to COVID, differences between different macrolides, and synergistic effects with other antibiotics have not been explored.
Methods
We investigated the effect of antibiotics (amoxicillin, azithromycin, clarithromycin, and combined amoxicillin with clarithromycin) on lymphocyte intracellular cytokine levels and monocyte phagocytosis in healthy volunteer PBMCs stimulated ex vivo with SARS-CoV-2 S1+2 spike protein. A retrospective cohort study was performed on intensive care COVID-19 patients.
Results
Co-incubation of clarithromycin with spike protein-stimulated healthy volunteer PBMCs ex vivo resulted in an increase in CD8+ (p = 0.004) and CD4+ (p = 0.007) IL-2, with a decrease in CD8+ (p = 0.032) and CD4+ (p = 0.007) IL-10. The addition of amoxicillin to clarithromycin resulted in an increase in CD8+ IL-6 (p = 0.010), decrease in CD8+ (p = 0.014) and CD4+ (p = 0.022) TNF-alpha, and decrease in CD8+ IFN-alpha (p = 0.038). Amoxicillin alone had no effect on CD4+ or CD8+ cytokines. Co-incubation of azithromycin resulted in increased CD8+ (p = 0.007) and CD4+ (p = 0.011) IL-2. There were no effects on monocyte phagocytosis. 102 COVID-19 ICU patients received antibiotics on hospital admission; 62 (61%) received clarithromycin. Clarithromycin use was associated with reduction in mortality on univariate analysis (p = 0.023), but not following adjustment for confounders (HR = 0.540; p = 0.076).
Conclusions
Clarithromycin has immunomodulatory properties over and above azithromycin. Amoxicillin in addition to clarithromycin is associated with synergistic ex vivo immunomodulatory properties. The potential benefit of clarithromycin in critically ill patients with COVID-19 and other viral pneumonitis merits further exploration.
Keywords: Anti-bacterial agents, COVID-19, Critical care, Immunomodulation
1. Introduction
Patients with COVID-19 have complex impairments in their immune system, and several immunomodulatory therapies have been proposed (e.g. monoclonal antibodies or stem cells) [1]. Such therapies are both costly and not without risk. The use of a low-cost and well-tolerated therapeutic agent, such a macrolide antibiotic, is an attractive option.
Macrolide antibiotics have immunomodulatory effects [2] which have theoretical benefits in the management of non-bacterial inflammatory diseases including viral severe acute respiratory illness (SARI) [3]. Whilst azithromycin lacks and clinical benefit in the management of COVID-19 [4], a within-class effect may exist with clarithromycin having different immunomodulatory potential. The precise effects of macrolides on T cell responses to COVID-19, dissimilarities between different macrolides, and synergistic effects with other antibiotics have not been explored.
Our primary objective was to test the hypothesis that different macrolides may have different immunomodulatory effects in COVID-19 ex vivo. We evaluated the effect of antibiotics on lymphocyte anti-viral responses on healthy volunteer isolated peripheral blood mononuclear cells (PBMCs) following exposure to SARS-CoV-2 spike protein S1 + S2. As critically ill patients with COVID-19 are at risk of bacterial co-infections, we assessed the effect of antibiotics on monocyte phagocytosis and antigen presentation (quantified by HLA-DR expression) ex vivo. Our secondary objective was to ascertain mortality associated with macrolide use compared to other antibiotics on hospital presentation among critically ill patients with COVID-19.
2. Methods and materials
2.1. Healthy volunteer PBMC stimulation
Methods on healthy volunteer PBMC isolation, stimulation, and flow cytometry are described in detail in the Appendix. Ethics was obtained from University College London Research Ethics Committee (REC ref 19181/001). Demographic data on healthy volunteers are detailed in Table A1. Briefly, healthy volunteer blood (10 mL) was obtained by venepuncture and Ficoll gradient separation used to isolate PBMCs. Recombinant SARS-CoV-2 S protein S1 + S2 at a concentration of 15 μg/ml, and/or antibiotics (Amoxicillin, Azithromycin, or Clarithromycin) at a concentration of 10 μg/ml were added to appropriate wells with controls and incubated with gentle agitation for 6 h at 37 °C.
Table 1.
Baseline patient characteristics.
| Table 1 Baseline characteristics of patient cohort | ||||
|---|---|---|---|---|
| Total cohort (n = 102) | Macrolide (n = 62) | Non-macrolide (n = 40) | p-value | |
| Demographics | ||||
| Age (years) | 67 (54–74) | 65 (55–73) | 67 (54–74) | 0.615 |
| Male | 74 (73%) | 51 (82%) | 23 (59%) | 0.008 |
| Body mass index (BMI) (kg. m−2) | 28 (25–32) | 27 (24–32) | 30 (26–32) | 0.117 |
| Symptom onset to hospital admission (days) | 8 (5–10) | 8 (5–10) | 7 (4–12) | 0.497 |
| Co-morbidities | ||||
| Hypertension | 57 (55%) | 33 (53%) | 24 (59%) | 0.596 |
| Diabetes | 28 (27%) | 17 (27%) | 11 (27%) | 0.947 |
| Smoker | 4 (4%) | 2 (3%) | 2 (5%) | 0.671 |
| Chronic obstructive pulmonary disease (COPD) | 7 (7%) | 4 (7%) | 3 (7%) | 0.864 |
| Ethnicity | ||||
| White | 50 (49%) | 32 (52%) | 18 (45%) | 0.731 |
| Other/Unknown | 23 (23%) | 13 (21%) | 10 (25%) | |
| Asian | 15 (15%) | 10 (16%) | 5 (12%) | |
| Black | 14 (14%) | 7(11%) | 7 (18%) | |
| Admission variables | ||||
| C-reactive protein (mg/dl) | 188 (106–285) | 203 (110–298) | 169 (89–275) | 0.325 |
| Haemoglobin | 133 (119–144) | 135 (123–146) | 131 (117–141) | 0.189 |
| Bilirubin | 11 (7–14) | 10 (8–14) | 11 (7–14) | 0.674 |
| Albumin | 37 (35–40) | 37 (36–40) | 37 (35–41) | 0.765 |
| Creatinine | 91 (73–112) | 92 (79–110) | 90 (73–113) | 0.816 |
| Lymphocytes (106. mL−1) | 0.75 (0.53–1.10) | 0.76 (0.59–1.00) | 0.74 (0.51–1.21) | 0.914 |
| Neutrophils (106. mL−1) | 7.56 (4.97–10.36) | 7.59 (4.78–10.35) | 7.08 (5.24–10.64) | 0.613 |
| Ratio | 9.57 (5.63–14.19) | 9.55 (5.22–13.35) | 9.57 (5.66–17.06) | 0.657 |
| Platelets (109 mL−1) | 231 (173–308) | 229 (178–303) | 231 (158–315) | 0.962 |
| Temperature (oC) | 38.0 (37.1–38.7) | 38.0 (37.2–38.6) | 38.0 (37.1–38.8) | 0.606 |
| SpO2:FiO2 ratio | 139 (95–362) | 142 (100–313) | 138 (93–375) | 0.975 |
| Treatments used | ||||
| Duration of antibiotics (days) | 3 (2–4) | 3 (2–4) | 3 (2–4) | 0.530 |
| Continuous positive airway pressure (CPAP) | 92 (89%) | 56 (90%) | 36 (88%) | 0.686 |
| Invasive mechanical ventilation | 34 (33%) | 21 (34%) | 13 (32%) | 0.819 |
| Renal replacement therapy | 11 (11%) | 7 (11%) | 4 (10%) | 0.805 |
| Noradrenaline use | 36 (35%) | 22 (36%) | 14 (34%) | 0.889 |
| Steroid use | 43 (41%) | 27 (44%) | 16 (39%) | 0.649 |
Table 1Baseline characteristics of patient cohort. Data expressed as median (inter-quartile range) if continuous, or number (n) (%).
Cell surface markers and intracellular cytokines were labelled with fluorophore-labelled antibodies. Products and concentrations used are detailed in Table A.2 and gating strategies in Fig. 1 . Whilst a number of inflammatory markers are elevated with COVID-19, we chose to investigate cytokines either with direct antiviral activity or cytokines associated with mortality in COVID-19. IFN-γ and TNF-α are responsible for tissue damage and mortality in COVID-19 [[5], [6], [7]], IL-6 and IL-10 are associated with mortality in COVID-19 [8], and type 1 interferons (IFN- α) are key to antiviral responses [9]. Monocyte phagocytosis was assessed using pHRodo red Staphylococcus aureus bioparticles (ThermoFischer Scientific, Waltham, MA) as per manufacturer recommendations.
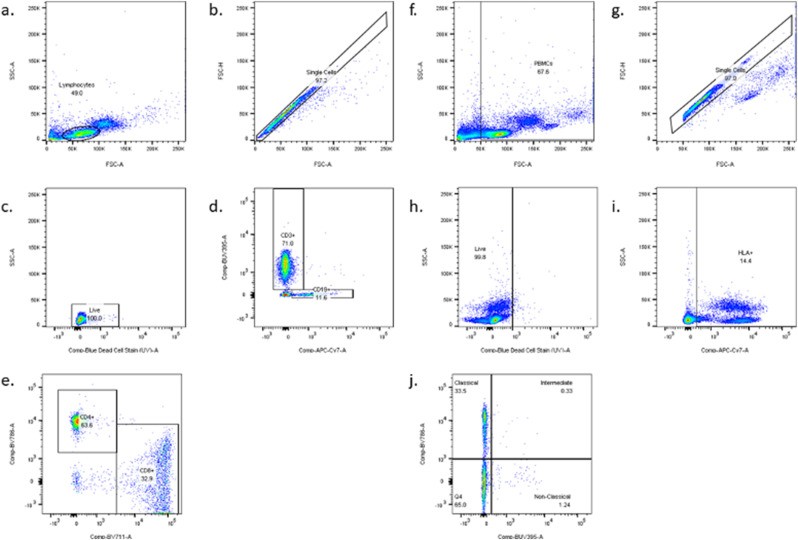
Fig. 1.
Lymphocyte and monocyte gating strategies. Lymphocytes were initially gated using forward and side scatter and 10,000 lymphocyte gate events were acquired for each sample. The following cell surface markers were used to identify subpopulations of interest: CD3 (BUV395, BD Biosciences, San Jose, CA), CD4 (BV785, Biolegend, San Diego, CA), CD8 (BV711, BD Biosciences, San Jose, CA), and CD19 (APC-Cy7, BD Biosciences, San Jose, CA). Monocytes were identified using HLA-DR (APC-Cy7, BD Biosciences, San Jose, CA) positive PBMCs and 10,000 monocyte gate events were acquired for each sample. CD14 (BV785, Biolegend, San Diego, CA) and CD16 (BUV395, BD Biosciences, San Jose, CA) cell surface markers were used to identify specific subpopulations.
(a.
-e). Lymphocyte gating strategy: a. Lymphocytes; b. Single cells; c. Live cells; d. CD3 and CD19 cells; e. CD4 and CD8 cells. (f.-j). Monocyte gating strategy: f. Peripheral blood mononuclear cells (PBMCs); g. Single cells; h. Live cells; i. HLA-DR positive cells j. CD14 and CD16 differentiation into classical (CD14++CD16lo), Intermediate (CD14+CD16+) and non-classical (CD14loCD16+) monocytes.
Cells were acquired on an LSR II flow cytometer (BD Biosciences, San Jose, CA) running BD FACSDiva version 9 (BD Biosciences, San Jose, CA). Flow cytometry data was analysed using FlowJo (version 10.7.1, BD Biosciences, San Jose, CA).
2.2. Clinical data
We performed a retrospective cohort study of consecutive patients admitted to the respiratory high dependency unit (HDU) or intensive care unit (ICU) at University College London Hospital between 1st March and 30th June 2020 with PCR-confirmed or clinically suspected COVID-19. Ethics approval was granted by the London-Westminster Research Ethics Committee with approval by the Health Research Authority and Health and Care Research Wales (REC ref 20/HRA/2505 and IRAS ID 284088).
We included patients who were commenced on antibiotics within 24 h of admission. Patients receiving immunomodulatory therapies (e.g., monoclonal antibodies) including early steroids were excluded. Patients transferred in from other hospitals were excluded as admission blood tests and detailed clinical data on antimicrobial use were unavailable. Patients with haemato-oncological diagnoses were also excluded as recent chemotherapy may have impacted immune function and outcome. Moribund patients who died within 24hrs were excluded.
Patient demographics, clinical data (including date of symptom onset, admission tissue oxygen saturation (SpO2), respiratory rate, temperature), treatments and outcome were recorded from electronic healthcare records on a standardized data collection form. Patients were followed up to death or hospital discharge.
2.3. Statistics
Analysis of clinical data was performed using anonymized data. Continuous and categorical variables are reported as median (interquartile range) and n (%), respectively. Mann Whitney U tests was used for comparison of continuous variables between groups. Categorical data were compared using the chi-square test. Changes in continuous variables over time between groups was assessed using two-way ANOVA. Unadjusted survival differences were assessed using log-rank test and displayed using a Kaplan-Meier curve. Adjusted hazards ratios of factors associated with mortality was analysed using Cox regression. Graphs were constructed, and statistical analysis performed using SPSS version 26.0 (IBM Corp, Armonk, NY, USA) and Prism version 9 (GraphPad, San Diego, CA).
Flow cytometry data was analysed using FlowJo (version 10.7.1, BD Biosciences, San Jose, CA) and data presented as dots signifying median fluorescence intensity (MFI; arbitrary units) of individual replicate values, column height representing median of the combined replicates and bars showing inter-quartile ranges. Heatmaps were constructed to visualise summary of lymphocyte intracellular cytokine data. Differences between groups were compared using a non-parametric Kruskal- Wallis test without Dunnett's correction. A p-value <0.05 was deemed significant. Graphs were constructed, and statistical analysis performed using Prism version 9 (GraphPad, San Diego, CA).
3. Results
3.1. Lymphocyte intracellular cytokines
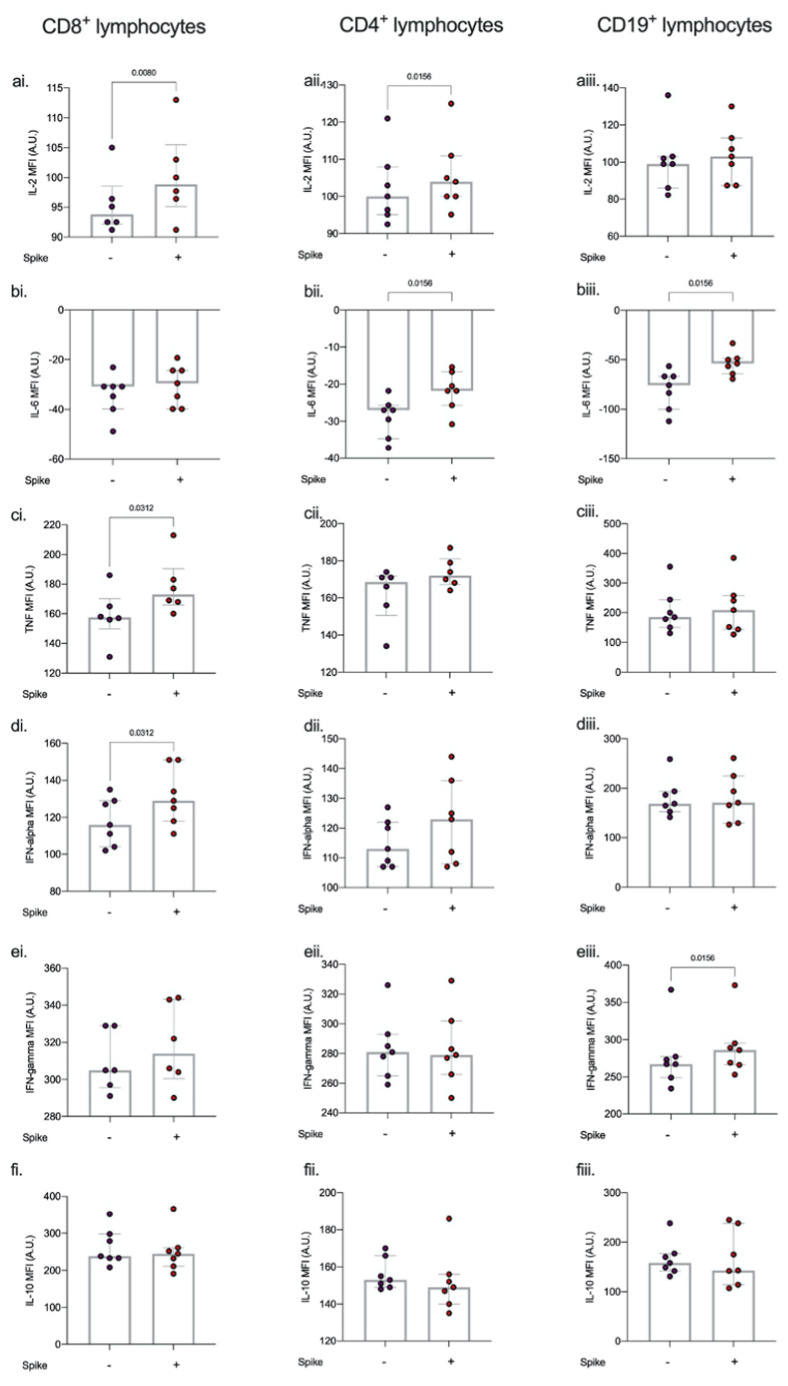
Median healthy volunteer PBMC viability was 95% (93%–97%) with no differences on stimulation with spike protein or co-incubation with antibiotics. Stimulation of PBMCs with spike protein resulted in an increase in CD8+ IL-2 (p = 0.008), TNF-α (p = 0.031) and IFN-α (p = 0.031); CD4+ IL-2 (p = 0.016), and IL-6 (p = 0.016); and CD19+ IL-6 (p = 0.016) and IFN-γ (p = 0.016). (Fig. 2 , Figure A1).
Fig. 2.
Effect of spike protein on lymphocyte intracellular cytokine production.
Intracellular cytokines a) IL-2, (b). IL-6, (c). TNF-alpha, (d). interferon-alpha, (e). interferon gamma, and (f). IL-10 in CD4, CD8, and CD19 cells following 6 h spike protein stimulation ex vivo. Stimulation of PBMCs with spike protein resulted in an increase in CD8+ IL-2 (p = 0.008), TNF-α (p = 0.031) and IFN-α (p = 0.031); CD4+ IL-2 (p = 0.016), and IL-6 (p = 0.016); and CD19+ IL-6 (p = 0.016) and IFN-γ (p = 0.016). Intracellular cytokine concentration is expressed as median fluorescent intensity (MFI) measured in arbitrary units (A.U.). Dots represent individual replicate values (n = 7), column heights represent the median of the replicates, bars show inter-quartile range Differences between groups were compared using Mann- Whitney U test.
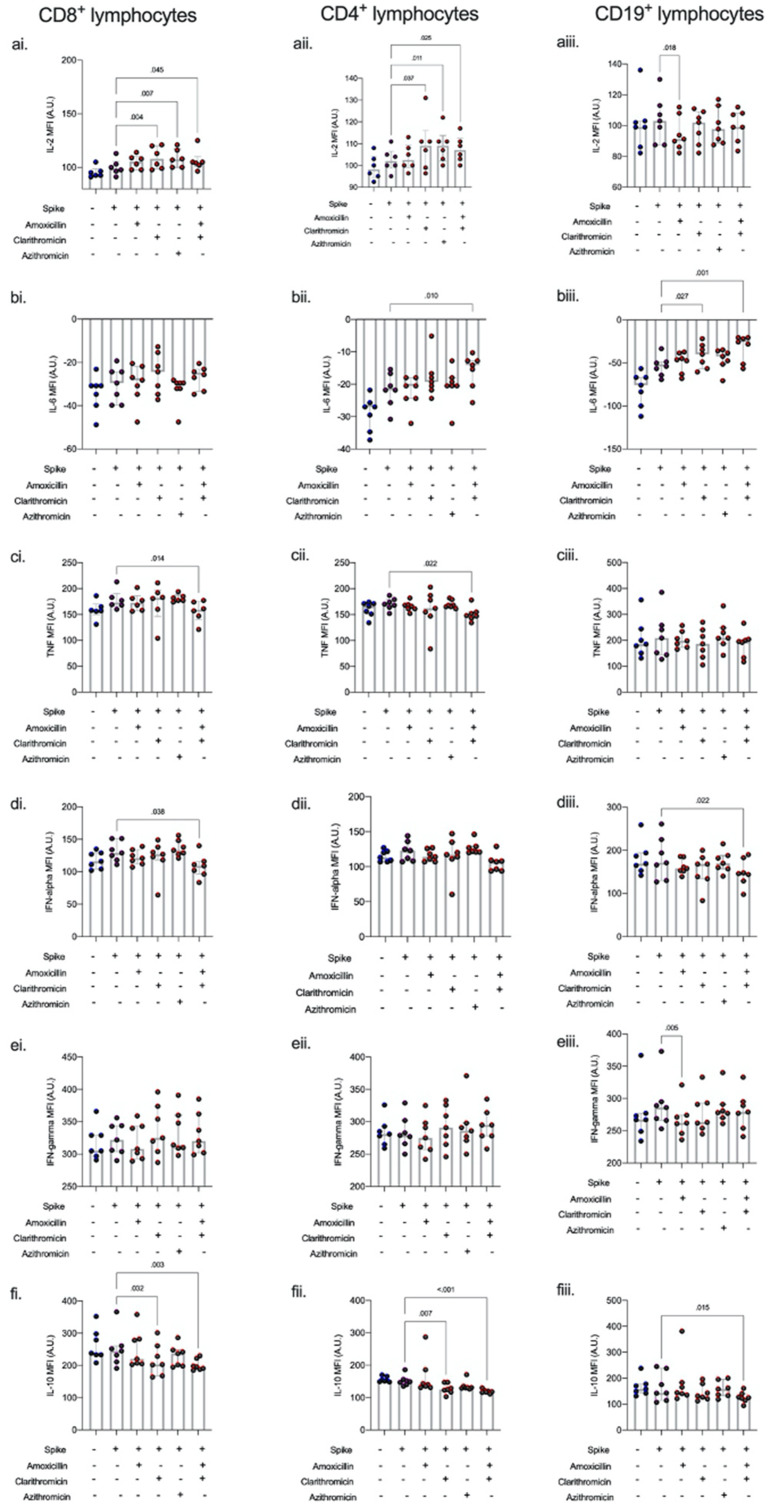
Co-incubation with azithromycin resulted in an increase in CD8+ (p = 0.007) and CD4+ (p = 0.011) IL-2. Similarly, co-incubation with clarithromycin increased CD8+ (0.004) and CD4+ (p = 0.007) IL-2. In addition, co-incubation with clarithromycin resulted in an increase in CD19+ IL-6 (p = 0.027) and decrease in CD4+ (p = 0.007) and CD8+ (p = 0.032) IL-10. Co-incubation of spike protein-stimulated cells with amoxicillin resulted in a decrease in CD19+ IFN-γ (p = 0.005). The combination of amoxicillin and clarithromycin had synergistic effects on spike-protein stimulated lymphocytes. A significant decrease in IL-10 was seen in CD4+(p < 0.001), CD8+(p = 0.003), and CD19+ (p = 0.015) lymphocytes. Additionally, TNF-α was reduced in both CD4+ (p = 0.022) and CD8+ (p = 0.014) lymphocytes and IFN-α was decreased in CD8+ (p = 0.038) and CD19+ (p = 0.022) lymphocytes (Fig. 3 , Figure A1).
Fig. 3.
Intracellular cytokines on stimulation with spike protein S1 + S2 with antibiotic co-incubation.
Intracellular cytokines a) IL-2, (b). IL-6, (c). TNF-alpha, (d). interferon-alpha, (e). interferon gamma, and (f). IL-10 in CD4, CD8, and CD19 cells following 6 h spike protein stimulation ± antibiotic co-incubation ex vivo. Co-incubation with azithromycin resulted in an increase in CD8+ (p = 0.007) and CD4+ (p = 0.011) IL-2. Co-incubation with clarithromycin increased CD8+ (0.004) and CD4+ (p = 0.007) IL-2. In addition, co-incubation with clarithromycin resulted in an increase in CD19+ IL-6 (p = 0.027) and decrease in CD4+ (p = 0.007) and CD8+ (p = 0.032) IL-10. Co-incubation of spike protein-stimulated cells with amoxicillin resulted in a decrease in CD19+ IFN-γ (p = 0.005). The combination of amoxicillin and clarithromycin had synergistic effects on spike-protein stimulated lymphocytes; a significant decrease in IL-10 was seen in CD4+(p < 0.001), CD8+(p = 0.003), and CD19+ (p = 0.015) lymphocytes. Additionally, TNF-α was reduced in both CD4+ (p = 0.022) and CD8+ (p = 0.014) lymphocytes and IFN-α was decreased in CD8+ (p = 0.038) and CD19+ (p = 0.022) lymphocytes. Intracellular cytokine concentration is expressed as median fluorescent intensity (MFI) measured in arbitrary units (A.U.). Dots represent individual replicate values (n = 7), column heights represent the median of the replicates, and bars show inter-quartile range. Differences between groups were compared using a non-parametric Kruskal- Wallis test without Dunnett's correction.
3.2. Monocyte phagocytosis
Co-incubation of PBMCs with spike protein alone resulted in an increase in phagocytic capacity among 4 of 6 individuals, although not statistically significant. Addition of antibiotics to PBMCs treated with spike protein did not affect monocyte phagocytosis (Figure A2). In the absence of spike protein, phagocytosis of pHRodo red S. aureus bioparticles resulted in a decrease in monocyte surface HLA-DR expression (p = 0.009). Co-incubation with both clarithromycin and amoxicillin resulted in a small but statistically significant reduction in surface HLA-DR expression (Figure A2)
3.3. Clinical data
192 patients were identified of whom 90 were excluded (56 were inter-hospital transfers, 14 had active haematological malignancy, 11 received no antibiotics on hospital admission, 8 died within 24 h of admission), and one patient received azithromycin on day 4, leaving 102 patients for the final analysis. The baseline demographics of patients included are summarized in Table 1 . At the time the study was conducted, patients were infected with the wild type virus. No variants of concern were identified in the UK at the time.
Of these patients, 62 received clarithromycin, and 40 received other antibiotic combinations (Table A3). Half the patients received a combination of a macrolide and penicillin. Patients receiving immunomodulatory therapies (e.g., monoclonal antibodies) including early steroids were excluded. Two patients receiving clarithromycin and one patient receiving non-macrolide antibiotics were administered Remdesivir. The dose of clarithromycin administered was 500 mg twice daily. None of the patients received clarithromycin alone.
None of the patients were diagnosed with co-existing atypical pneumonia nor had positive blood cultures within 2 days of admission. Only eight patients had sputum cultures taken on admission as many were unable to expectorate. Fourteen (12%) patients were intubated in the first 48 h, limiting the number of tracheal or deeper aspirates. A total of 14 sputum/tracheal aspirate samples taken within the first 48 h, of which four were positive on microbial culture.
Baseline patient characteristics were well matched except for gender, with a greater proportion of males compared to females receiving clarithromycin (82% vs. 29%; p = 0.008) (Table 1). The median duration of macrolide antibiotics and non-macrolide antibiotics were similar (3(2–4) vs. 3(2–4) days; p = 0.530). None of the patients received immunomodulatory therapies [10], as the cohort were admitted prior to publication of the RECOVERY trial results [11,12], although 43 patients received steroids late (>10 days) after initial presentation as rescue therapy for non-resolving ARDS; with no difference between patients who received macrolide and non-macrolide antibiotics (44% vs. 39%; p = 0.649).
Three days following antibiotic initiation, the change in CRP (p = 0.644), temperature (p = 0.459), neutrophil (p = 0.430) and lymphocyte count (p = 0.602) were similar between patients receiving macrolide and non-macrolide antibiotics. Unadjusted hospital survival was better among patients receiving macrolide compared to non-macrolide antibiotics (30% vs. 70%; p = 0.023 on log-rank), but similar between patients receiving amoxicillin compared to non-amoxicillin antibiotics (32% vs. 68%; p = 0.362 on log-rank).
Following adjustment for gender, age, CRP, macrolide use, penicillin use, and duration of antibiotic exposure, advancing age (HR = 1.072 (1.040–1.105); p < 0.001), and higher CRP (HR = 1.003 (1.001–1.006); p = 0.013) were associated with increased mortality risk. Longer duration of antibiotics (HR = 0.839 (0.705–0.997); p = 0.047) and macrolide use (HR = 0.540 (0.275–1.079); p = 0.076) were associated with a decreased mortality risk, although the latter did not statistical significance. Neither male gender (HR = 1.48 (0.755–2.754); p = 0.228) nor amoxicillin use (HR = 1.092 (0.548–2.244); p = 0.804) was associated with mortality.
4. Discussion
We showed that on stimulation with SARS-CoV-2 spike protein, the effect of azithromycin and clarithromycin on lymphocytes differed. Azithromycin had relatively limited immunomodulatory properties in comparison to clarithromycin. Whilst amoxicillin alone had minimal immunomodulatory properties, the combination of amoxicillin and clarithromycin had synergistic effects. Immunomodulatory properties vary between macrolides. As examples, suppressed T-cell activation with azithromycin only occurs at high concentrations of clarithromycin [13], and suppressed monocyte cytokine release occurs with azithromycin but not clarithromycin [14].
Whilst a number of non-immune cells are able to secrete cytokines, we chose to measure lymphocyte – associated cytokines as lymphopenia and lymphocyte dysfunction is common in COVID-19 [15], suggesting immunomodulation of lymphocytes is important. The increase in lymphocyte IL-2 associated with macrolide use may facilitate resolution of lymphopenia, and improves survival in severe viral illness. Whilst raised IL-6 is associated with mortality in COVID-19 [16], the elevated intracellular levels of IL-6 associated with clarithromycin (ex vivo) may facilitate viral clearance. The suppression of TNF-α in COVID-19 may seem beneficial, given the degree of systemic inflammation. INF-α is important for viral clearance [9]. It is unclear if reduction in CD8+ and CD19+ lymphocyte intracellular IFN-α associated with clarithromycin/amoxicillin results in any functional impairment.
As patients with COVID-19 may present with bacterial co-infection [17], we also explored the ex vivo effect of antibiotics on bacterial phagocytosis with and without the presence of COVID-19 spike protein 1 + 2. We found the immunomodulatory effects of macrolides were limited to lymphocytes, with no effect on monocyte phagocytosis or antigen presentation.
Compared to non-macrolide antibiotics, clarithromycin use in critically ill patients with COVID-19 was associated with a significant survival benefit on unadjusted analysis, albeit this significance was lost following adjustment for covariates. Longer antibiotic duration was associated with improved survival, suggesting that there might have been a survival benefit associated with greater exposure to clarithromycin. As expected, advancing age and elevated CRP were also associated with higher mortality risk.
Despite mechanistic and observational data supporting the use of macrolides in COVID-19, clinical trials have yet to show survival benefit. This may be related to patient selection and timing of treatment. Azithromycin has been the most commonly studied macrolide in COVID-19. However, our data supports a role for clarithromycin [18]. The dose of macrolide required to achieve adequate immunomodulatory effects in vivo is unknown. Many ex-vivo studies demonstrate immunomodulation occurs at higher doses than might be achievable clinically [19].
A significant proportion of critically ill patients with COVID-19 are treated with empirical antibiotics, as exclusion of bacterial co-infection is often vexatious. The median duration of antibiotics in our centre was 3 days, reflecting the Surviving Sepsis Guidelines recommendation that initial empirical antibiotic therapy is continued until further microbiology results become available [20]. Although the absolute difference in antibiotic duration may not appear significant (3(2–4) days), the relative difference in antibiotic duration between patients is not insignificant (e.g. twice as much antibiotic exposure with a 4-day compared to two-day course). The association between lower mortality and longer course of antibiotics may be explained by the treatment of an undiagnosed bacterial co-infection rather than the immunomodulatory effect of antibiotics.
Macrolides have demonstrated numerous potentially beneficial immunomodulatory properties in the context of non-COVID-19 viral infections, gram-negative sepsis and ventilator associated pneumonia (VAP) [2]. However, the ex-vivo effect of macrolides on immune function in SARS-CoV-2 is relatively unknown.
As with all retrospective analyses, we acknowledge the possibility of residual confounding, and that results are associative. Only a minority of patients had sputum cultures or tracheal aspirates taken on admission as many were unable to expectorate. We did not include patients who did not receive antibiotics on admission to hospital as their illness severity was milder and thus not comparable.
All data have been performed ex vivo on healthy volunteer PBMCs using a single concentration of both spike protein and antibiotics. We have not demonstrated if a similar effect occurs in vivo, or at different concentrations or timepoints. Assessment of intracellular protein (cytokine) synthesis ex vivo following cell stimulation requires use of monensin to prevent protein transport beyond the Golgi apparatus. However, beyond 6 h, monensin is cytotoxic, precluding longer incubation times.
Although we demonstrate alterations in intracellular cytokines, the underlying mechanisms have not been explored. Our ex-vivo model utilises the SARS-CoV-2 S1+2 domain of the spike protein; other parts of the SARS-CoV-2 virus may have different immunogenic properties. We were unable to model the effect of a prolonged viral infection prior to commencement of antibiotics in keeping with clinical COVID-19 infection. Additionally, the effect of antibiotics on SARS-CoV-2 S1+2 – stimulated non-immune cells is unknown. Our healthy volunteers were younger than the clinical cohort, although the sex distribution was similar.
5. Conclusion
Crucially, empirical antibiotic courses at our centre were for limited duration (<4 days) and we do not advocate clinicians take a carte blanche attitude to prescribing antibiotics for theoretical benefits. Clarithromycin has immunomodulatory properties over and above azithromycin. Amoxicillin in addition to clarithromycin is associated with synergistic ex vivo immunomodulatory properties. The potential benefit of clarithromycin in critically ill patients with COVID-19 and other viral pneumonitis merits further exploration.
Funding
European Society of Intensive Care Medicine (ESICM) New Investigator Award 2018 (NA), Belgium; University College London Precision AMR Grant 2020 (NA, TS, MS), United Kingdom.
Author contribution
Study design (NA), data collection (AL), ex vivo experiments (TS), flow cytometry (TS), statistics (NA and TS), drafting manuscript (TS and NA), finalising manuscript (all authors).
Declaration of competing interest
None.
Acknowledgements
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jiac.2022.04.001.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Snow T.A.C., Singer M., Arulkumaran N. Immunomodulators in COVID-19: two sides to every coin. Am J Respir Crit Care Med. Nov 15 2020;202(10):1460–1462. doi: 10.1164/rccm.202008-3148LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reijnders T.D.Y., Saris A., Schultz M.J., van der Poll T. Immunomodulation by macrolides: therapeutic potential for critical care. Lancet Respir Med. Jun 2020;8(6):619–630. doi: 10.1016/s2213-2600(20)30080-1. eng) [DOI] [PubMed] [Google Scholar]
- 3.Doan T., Hinterwirth A., Arzika A.M., Worden L., Chen C., Zhong L., et al. Reduction of Coronavirus burden with mass azithromycin distribution. Clin Infect Dis. Nov 19 2020;71(16):2282–2284. doi: 10.1093/cid/ciaa606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furtado R.H.M., Berwanger O., Fonseca H.A., Correa T.D., Ferraz L.R., Lapa M.G., et al. Azithromycin in addition to standard of care versus standard of care alone in the treatment of patients admitted to the hospital with severe COVID-19 in Brazil (COALITION II): a randomised clinical trial. Lancet. Oct 3 2020;396(10256):959–967. doi: 10.1016/S0140-6736(20)31862-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karki R., Sharma B.R., Tuladhar S., Williams E.P., Zalduondo L., Samir P., et al. Synergism of TNF-alpha and IFN-gamma triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes. Cell. Jan 7 2021;184(1):149–168. doi: 10.1016/j.cell.2020.11.025. e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu Z.-J., Xu J., Yin J.-M., Li L., Hou W., Zhang L.-L., et al. Lower circulating interferon-gamma is a risk factor for lung fibrosis in COVID-19 patients. Original Res. 2020;11(2348) doi: 10.3389/fimmu.2020.585647. (in English), Frontiers in Immunology. September-29 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feldmann M., Maini R.N., Woody J.N., Holgate S.T., Winter G., Rowland M., et al. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. Lancet. May 2 2020;395(10234):1407–1409. doi: 10.1016/S0140-6736(20)30858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laing A.G., Lorenc A., Del Molino Del Barrio I., Das A., Fish M., Monin L., et al. A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat Med. Oct 2020;26(10):1623–1635. doi: 10.1038/s41591-020-1038-6. [DOI] [PubMed] [Google Scholar]
- 9.Schreiber G. The role of type I interferons in the pathogenesis and treatment of COVID-19. Review. 2020-September;11(2599) doi: 10.3389/fimmu.2020.595739. (in English), Frontiers in Immunology. 30 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Longobardo A., Snow T.A.C., Montanari C., Shulman R., Singer M., Arulkumaran N. COVID-19 and non-COVID ARDS patients demonstrate a distinct response to low dose steroids- A retrospective observational study. J Crit Care. Nov 21 2020;62:46–48. doi: 10.1016/j.jcrc.2020.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. Feb 25 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Group R.C. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. May 1 2021;397(10285):1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ratzinger F., Haslacher H., Poeppl W., Hoermann G., Kovarik J.J., Jutz S., et al. Azithromycin suppresses CD4(+) T-cell activation by direct modulation of mTOR activity. Sci Rep. Dec 11 2014;4:7438. doi: 10.1038/srep07438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gualdoni G.A., Lingscheid T., Schmetterer K.G., Hennig A., Steinberger P., Zlabinger G.J. Azithromycin inhibits IL-1 secretion and non-canonical inflammasome activation. Sci Rep. Jul 8 2015;5 doi: 10.1038/srep12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laing A.G., Lorenc A., Del Molino Del Barrio I., Das A., Fish M., Monin L., et al. A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat Med. Oct 2020;26(10):1623–1635. doi: 10.1038/s41591-020-1038-6. [DOI] [PubMed] [Google Scholar]
- 16.McElvaney O.J., McEvoy N.L., McElvaney O.F., Carroll T.P., Murphy M.P., Dunlea D.M., et al. Characterization of the inflammatory response to severe COVID-19 illness. Am J Respir Crit Care Med. Sep 15 2020;202(6):812–821. doi: 10.1164/rccm.202005-1583OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu X., Ge Y., Wu T., Zhao K., Chen Y., Wu B., et al. Co-infection with respiratory pathogens among COVID-2019 cases. Virus Res. Aug 2020;285 doi: 10.1016/j.virusres.2020.198005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsiakos K., Tsakiris A., Tsibris G., Voutsinas P.M., Panagopoulos P., Kosmidou M., et al. Early start of oral clarithromycin is associated with better outcome in COVID-19 of moderate severity: the ACHIEVE open-label single-arm trial. Infect Dis Ther. Aug 6 2021 doi: 10.1007/s40121-021-00505-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyamoto D., Hasegawa S., Sriwilaijaroen N., Yingsakmongkon S., Hiramatsu H., Takahashi T., et al. Clarithromycin inhibits progeny virus production from human influenza virus-infected host cells. Biol Pharm Bull. Feb 2008;31(2):217–222. doi: 10.1248/bpb.31.217. [DOI] [PubMed] [Google Scholar]
- 20.Alhazzani W., Moller M.H., Arabi Y.M., Loeb M., Gong M.N., Fan E., et al. Surviving sepsis campaign: Guidelines on the management of critically ill adults with Coronavirus disease 2019 (COVID-19) Crit Care Med. Jun 2020;48(6):e440–e469. doi: 10.1097/CCM.0000000000004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.