Abstract
Background:
Thalamocortical white matter connectivity is disrupted in psychosis and is hypothesized to play a role in its etiology and associated cognitive impairment. Attenuated cognitive symptoms often begin in adolescence, during a critical phase of white matter and cognitive development. However, little is known about the development of thalamocortical white matter connectivity and its association with cognition.
Methods:
The present study characterized effects of age, sex, psychosis symptomatology, and cognition in thalamocortical networks in a large sample of youth (n = 1144, aged 8-22 years, 46% male) from the Philadelphia Neurodevelopmental Cohort (PNC), which included 316 typically-developing youth, 330 psychosis-spectrum youth, and 498 youth with other psychopathology. Probabilistic tractography was used to quantify percent total connectivity between the thalamus and six cortical regions, and assess microstructural properties (i.e. fractional anisotropy-FA) of thalamocortical white matter tracts.
Results:
Overall, percent total connectivity of the thalamus was weakly associated with age and was not associated with psychopathology or cognition. In contrast, FA of all thalamocortical tracts increased significantly with age, was generally higher in males than females, and was lowest in psychosis-spectrum youth. FA of tracts linking the thalamus to prefrontal and posterior parietal cortex was related to better cognitive function across subjects.
Conclusions:
By characterizing the pattern of typical development and alterations in those at risk for psychotic disorders, this study provides a foundation for further conceptualization of thalamocortical white matter microstructure as a marker of neurodevelopment supporting cognition and an important risk marker for psychosis.
Keywords: thalamus, cortex, neurodevelopment, DTI, white matter
INTRODUCTION
The thalamus is involved in the pathophysiology of psychosis. The most consistent findings include smaller thalamic volume (1–3), abnormal function during cognitive tasks (4–6), and lower neuronal markers of integrity (7) in patients with psychotic disorders. Deficits in thalamic function are positioned to cause far-reaching impacts on brain network function. The thalamus is involved in multiple brain networks, acting as a hub for cognitive processes and interface between sensory and motor systems. As a result, the thalamus has been proposed as part of a fundamental neural circuit contributing to the etiology of psychosis and cognitive impairment (8–11).
Thalamocortical dysconnectivity models are supported by functional and structural neuroimaging findings in psychosis patients. Abnormal functional connectivity between thalamus and cortex is among the most consistent findings in schizophrenia (12–14), with similar abnormalities observed in psychotic bipolar disorder (15). Diffusion imaging studies have begun to reveal accompanying deficits in white matter connections (16–20). Deficits in overall white matter structure are strongly linked to cognitive impairment in both healthy and neuropsychiatric samples (21–27), and thalamocortical white matter structure has been linked to cognitive impairment in psychosis (19; 20; 28), although less consistently (18). Intriguingly, recent findings indicate thalamocortical white matter structural connectivity deficits are similar across early and chronic stages of psychosis (18), suggesting white matter deficits may be a relatively stable neuropathological feature that precedes illness onset.
Evidence of abnormal thalamocortical structural connectivity early in the course of psychotic illness is consistent with the neurodevelopmental hypothesis of psychosis, which posits that psychosis originates from early-life brain abnormalities that influence normal maturation patterns (29; 30). Neuronal migration is a critical part of brain development, allowing neurons to make terminal connections and successfully interact (31). Because thalamic structural connections are central to multiple brain networks, disruptions during these early developmental processes have been hypothesized to contribute to the widespread dysconnectivity and cognitive deficits observed in psychosis (32–34). Moreover, the emergence of cognitive deficits precedes the onset of psychosis and can often be detected from early childhood. However, it remains unclear whether structural connectivity deficits in psychosis stem from early-life developmental abnormalities or are stable throughout adolescence. The onset of psychotic disorders typically begins in late adolescence, coinciding with a period of dynamic change in white matter maturation and brain connectivity (35–38), along with considerable cognitive development (39). Adolescence is characterized by important pubertal changes that are associated with overall faster white matter maturation and cognitive development (40) in females compared to males. Females also have lower risk for psychosis and later typical age of onset than males (41). Importantly, pubertal changes may exert spatially-localized effects on white matter maturation (38) that influence risk. Preclinical data support the hypothesis that thalamic dysfunction, in concert with the hippocampus, contributes to dopamine dysregulation and the emergence of positive symptoms (42), suggesting the period surrounding illness onset may mark a shift in thalamocortical function, precipitating the abnormalities observed later in illness.
Mapping thalamocortical structural connectivity across development will provide important insights into the timing and nature of white matter deficits and impacts on cognitive function in psychosis (43; 44). Early detection of brain network abnormalities has the potential to allow for both early identification of illness and better targeted treatments. Diffusion imaging has been shown to be sensitive to the detection of regional differences in thalamocortical pathways that begin during early development and persist postnatally (45). However, the delineation of a useful structural connectivity-based marker is significantly hampered by key remaining questions: what is the normal development of thalamocortical structural connections, when do deficits emerge in those at risk for psychosis, and is thalamocortical structural connectivity associated with variation in cognitive function during development?
To address these questions, we examined a large developmental sample consisting of 1144 participants (aged 8-22 years) from the Philadelphia Neurodevelopmental Cohort (PNC) (46). The PNC is a community-based sample that includes youth experiencing subclinical psychosis-spectrum symptoms, consistent with the notion that transitory psychotic-like experiences are reported with relative frequency in the population (47; 48). Subclinical psychosis-spectrum symptoms share etiological risk, cognitive correlates, and symptom profiles with psychotic illness (49), and are associated with increased risk for conversion to a psychotic disorder (50–52). The present study sought to: 1) chart age effects of thalamocortical structural connectivity across development; 2) characterize sex differences and sex by age interactions(53) in thalamocortical structural connectivity during development; 3) test whether youth displaying psychosis-spectrum symptoms show abnormalities in thalamocortical structural connectivity compared to typically-developing youth and youth with other psychopathologies; and 4) determine if thalamocortical structural connectivity is associated with cognitive function.
METHODS AND MATERIALS
Participants
The PNC dataset contains approximately 9500 individuals (aged 8-22 years), including 1396 participants with a diffusion-weighted imaging series. Of these, 53 were excluded for serious medical conditions, 63 for insufficient clinical data to reach a diagnosis, and 136 for data quality (see supplement for details). This yielded a final sample of 1144 participants. Using similar procedures as those described by Calkins et al. (46), participants were classified as typically-developing youth (n=316), psychosis-spectrum youth (n=330), or youth with other psychopathology (n=498). Demographic information are reported in Table 1.
Table 1.
Sample Characteristics
| Demographics | Typically Developing N = 316 |
Psychosis Spectrum N = 330 |
Other Psychopathology N = 498 |
Statistic | df | p | Post-Hoc |
|---|---|---|---|---|---|---|---|
| Age, years ± SD | 14.5 ± 3.7 | 16.1 ± 3.0 | 15.2 ± 3.4 | F = 16.96 | 2,1143 | <.001 | PS>OP>TD |
| Sex, % male | 50 | 45 | 44 | χ2 = 2.65 | 2 | .27 | -- |
| Race, % W:AA:O | 58:32:10 | 32:56:12 | 50:41:9 | χ2 = 47.47 | 4a | <.001 | -- |
| Education, years ± SD | 7.4 ± 3.6 | 8.4 ± 2.7 | 8.2 ± 3.3 | F = 7.59 | 2,1143 | <.001 | PS,OP>TD |
| Parental education, years ± SD | 14.5 ± 2.5 | 13.5 ± 2.2 | 14.2 ± 2.3 | F = 16.33 | 2,1136b | <.001 | TD,OP>PS |
| Cognition | |||||||
| CNB composite cognition, z-score | 0.10 ±.6 | 0.06 ± .5 | 0.12 ±.5 | F = 1.50 | 2,1144 | .23 | – |
| WRAT, standard score | 105.8 ± 15.9 | 99.1 ± 16.9 | 103.1 ± 16.1 | F = 14.13 | 2,1143d | <.001 | TD,O>PS |
Abbreviations: W=White, AA=African American, O=Other; WRAT=Wide Range Assessment Test; TD = Typically Developing; PS = Psychosis Spectrum; OP = Other Psychopathology.
Race was not collected for 2 Typically Developing participant, 1 Psychosis Spectrum participants, and 4 Other Psychopathology participants.
Parental education was not collected for 1 Typically Developing participant, 2 Psychosis Spectrum participants, and 4 Other Psychopathology participants.
WRAT was not collected for 1 Other Psychopathology participant.
Clinical assessment.
Psychosis symptomatology was assessed using the PRIME Screen-Revised (PS-R), Schedule for Affective Disorders and Schizophrenia for School-Age Children (K-SADS) psychosis screen, and selected items from the Scale for Prodromal Syndromes (SOPS) assessing negative and disorganized symptoms. Psychosis-spectrum youth were identified based on: 1) having an age-deviant total PRIME score ≥ 2 SD above age-matched peers, or rating one item at > 6, or three items > 5; 2) endorsing definite or possible hallucinations or delusions on the K-SADS; and 3) having an age-deviant total SOPS score ≥ 2 SD above age-matched peers. Youth with other psychopathology were identified by endorsement of suprathreshold psychopathology symptoms and duration, consistent with SCID-IV diagnosis on the K-SADS, and endorsement of significant distress, but not meeting psychosis-spectrum criteria.
Neurocognitive Testing
Participants completed the Penn Computerized Neurocognitive Battery (CNB) (54). The CNB consists of 14 tests to evaluate multiple cognitive domains, including complex cognition (language reasoning, nonverbal reasoning, spatial ability), executive control (attention, working memory, mental flexibility), episodic memory (verbal, face, spatial), social cognition (emotion identification, emotion differentiation, age differentiation), and sensorimotor speed (finger tapping, sensorimotor processing speed). Domain-specific and overall composite CNB performance scores were transformed to standard equivalents (z-scores) as previously described (55).
Neuroimaging Data
Acquisition, preprocessing, and data quality.
High resolution T1-weighted structural (.93x.93x1mm voxels) and 64-direction diffusion-weighted data (1.875x1.875x2mm voxels) were acquired on a Siemens Tim Trio 3T scanner. Diffusion-weighted images were corrected for eddy current distortions and motion, coregistered to the mean b=0 image, and skull-stripped using the FMRIB Software Library (FSL, version 5.0.11) (56). Fractional anisotropy (FA) and mean diffusivity (MD) images were calculated using DTIFIT. Data quality metrics of mean relative displacement (motion) and contrast-to-noise ratio (CNR) were calculated and participants that did not meet quality thresholds were excluded. Full details of the acquisition, preprocessing, and data quality procedures are presented in the supplement.
Regions of interest.
Thalamus (seed) and cortical (target) regions of interest (ROIs) were defined in T1-weighted data using FreeSurfer developmental version 6 (57). The 6 bilateral cortical ROIs, created by combining selected cortical structures, included the: prefrontal (PFC); premotor/motor/supplementary motor; somatosensory; posterior parietal; temporal; and occipital cortex (see Supplementary Figure 1 for an example segmentation).
Thalamocortical connectivity analysis.
To quantify structural connectivity between the thalamus seed and each of the 6 cortical targets, seed-to-target probabilistic tractography analyses were run using probtrackx2 (see supplement for details). Connectivity values, expressed as “percent total connectivity”, were calculated as the total number of thalamus seed streamlines reaching the cortical target, divided by the total number of thalamus streamlines reaching any cortical target. In a planned secondary analysis, raw streamline counts were examined without converting to percent total connectivity.
Thalamocortical white matter microstructure analysis.
In addition to examining total white matter connections between cortex and thalamus, we also quantified microstructural properties of the white matter tracts linking cortex to thalamus. Cohort-specific tract overlap masks (>75% overlap) were used to extract individual mean FA and MD values for the 6 thalamocortical tracts. Group-level tract masks are presented in Supplementary Figure 2.
Statistical analysis
All statistical analyses were conducted in Statistical Analysis System (SAS Studio version 3.8). Percent total connectivity, white matter microstructure (FA), and cognition (CNB composite score) served as dependent variables in the primary analyses described below. Left and right hemisphere values were averaged as we did not have any a priori hypotheses regarding laterality, and to minimize the number of dependent variables. This resulted in six dependent variables per participant for each analysis, one for each cortical ROI. Results of age, sex, group, and cognition analyses were considered significant at a Bonferroni-corrected p-value for multiple comparisons (.05/6=.0083). All participants were included in analyses unless otherwise noted. Linear effects of age were modeled using linear regression, with age as the independent variable; sex and motion were included as covariates of no interest. Quadratic effects of age were modeled by including quadratic age as an independent variable in the regression model. Sex effects were investigated using one-way univariate ANCOVA, with sex as the independent variable; age, age2, and motion were included as covariates of no interest. Interactions of age by sex were investigated using the models described above. Group effects were investigated using one-way univariate ANCOVA, with group as the independent variable; sex, age, age2, and motion were included as covariates of no interest. Significant effects of group were further explored for interactions. In planned secondary analyses of connectivity, streamline counts served as the dependent variable, and the age, sex, and group models described above were repeated with an additional covariate of total streamline count to control for overall thalamic connectivity. Secondary analyses of age, sex, and group by hemisphere were investigated using the previously described models. Associations with cognition were examined using linear models, with percent total connectivity or FA for the six predefined thalamocortical connections serving as the predictor; sex, group, and motion were entered as covariates of no interest. Interactions between tract measures and group were investigated, with sex and motion entered as covariates of no interest. For significant associations, follow-up analyses were conducted to examine associations between five CNB cognitive domains and percent total connectivity or white matter microstructure, and were considered significant at p=.05. Mean MD was examined in follow-up analyses for any models that showed significant FA effects.
RESULTS
Thalamocortical Percent Total Connectivity
Age effects.
Percent total connectivity increased linearly with age for two regions, the motor and somatosensory cortex (Figure 1). Percent total connectivity also decreased linearly with age for two regions, the temporal and occipital cortex. All linear age effects were small and there were no quadratic effects of percent total connectivity (ps≥.32). Complete statistical results and secondary analyses by hemisphere and streamline counts are presented in the supplement.
Figure 1.
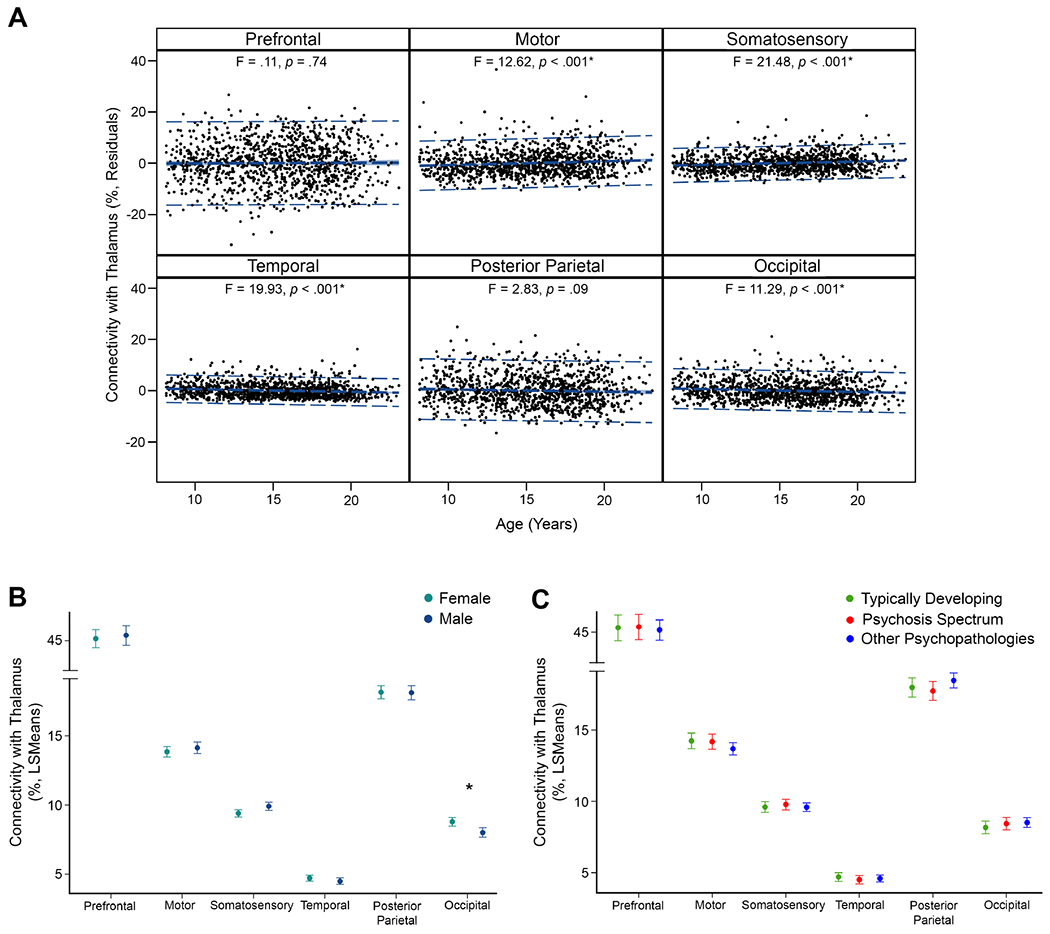
Thalamocortical percent total connectivity effects of age, sex, and group. Error bars for sex and group effects are 95% confidence intervals. Asterisks denote significant effects (p ≤ .008). (A) Percent total thalamic connectivity with the motor and somatosensory cortex was positively associated with age, while percent total connectivity with the temporal and occipital cortex was negatively associated with age. (B) Females had higher total thalamic connectivity with the occipital cortex than males. (C) Typically-developing youth, psychosis-spectrum youth, and youth with other psychopathologies had similar total thalamic connectivity across cortical regions.
Sex effects.
Females showed a small effect of greater thalamic-occipital cortex percent total connectivity compared to males (Figure 1). No other connections showed significant sex effects.
Group differences.
There were no between-group differences in percent total connectivity with any region (ps≥.20; Figure 1). Secondary analysis of streamline counts by group also showed no between-group differences (Supplementary Table 9).
Interactions.
There was a small interaction between linear age and sex in the occipital cortex. Older females had greater thalamic-occipital percent total connectivity than older males (Supplementary Figure 6). No other region showed an interaction between linear age and sex (ps≥.02).
Cognitive correlates.
Cognitive function, measured by CNB composite score, was not associated with percent total connectivity for any cortical ROI (ps≥.60; Supplementary Table 11).
Thalamocortical White Matter Microstructure
Age effects.
White matter microstructure, measured as mean FA within thalamocortical tracts, increased linearly with age for all tracts, with effect sizes ranging from small to large (Figure 2). The largest effects were observed in PFC and posterior parietal tracts. White matter microstructure in tracts linking the thalamus with the posterior parietal and occipital cortex also showed quadratic associations with age, although effects were small. These tracts demonstrated an inverted U-shaped association, with higher FA values observed in late adolescence and lower FA values observed in early childhood/early adulthood. Follow-up analysis showed that MD values decreased linearly with age across regions. For each region, older participants had lower MD values than younger participants. Follow-up analyses also showed quadratic associations between MD and age across regions (trend effect in thalamic-somatosensory tract). Each tract showed a U-shaped association, with lower MD values observed in late adolescence and higher MD values observed in early childhood/early adulthood. Specificity analyses confirmed that age effects were not associated with generalized, whole-brain differences in FA. Full statistical results and secondary analyses by hemisphere are presented in the supplement.
Figure 2.
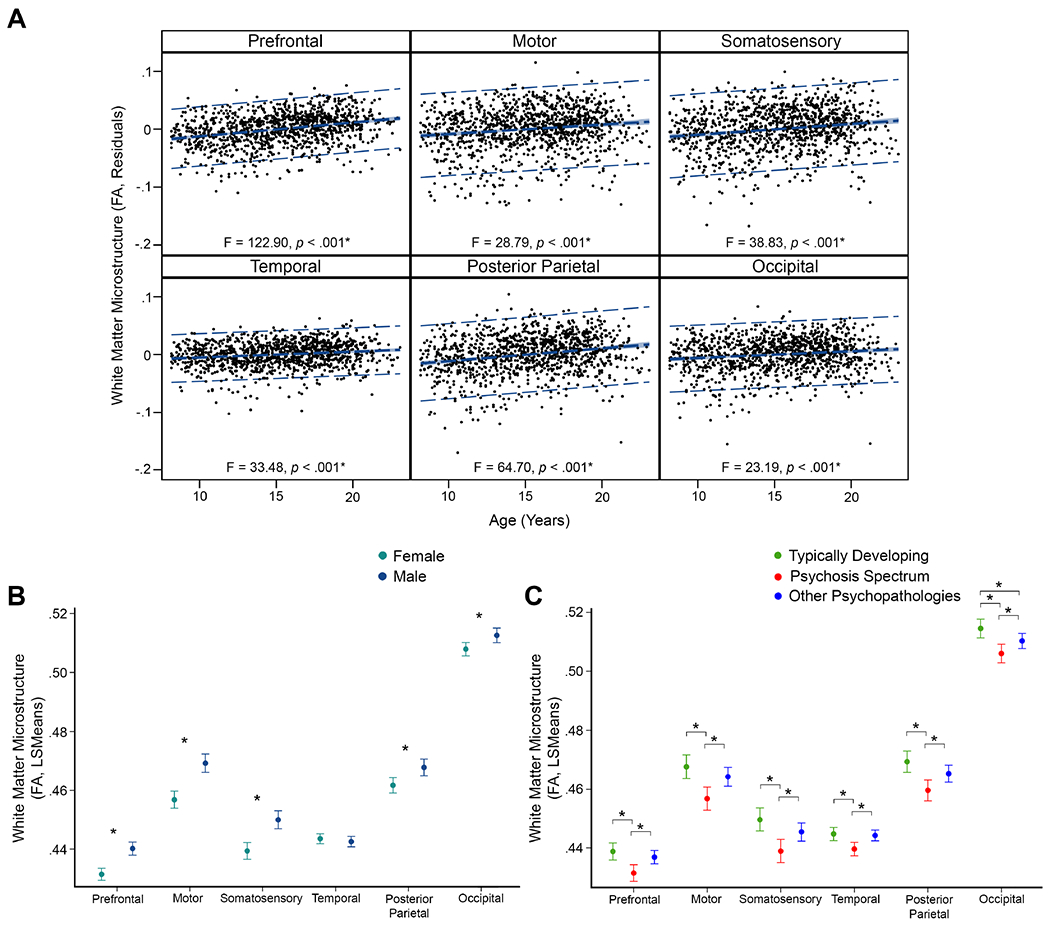
White matter microstructure (FA) effects of age, sex, and group for thalamocortical tracts. Error bars for sex and group effects are 95% confidence intervals. Asterisks denote significant effects (p ≤ .008). (A) FA was positively associated with age across thalamocortical tracts, with the largest effects in prefrontal and posterior parietal cortex tracts. (B) Males had higher FA values than females in tracts linking the thalamus with the prefrontal, motor, somatosensory, posterior parietal, and occipital cortex. (C) Psychosis-spectrum youth had the lowest FA values of all three groups across all thalamocortical tracts. Typically-developing youth had FA values similar to youth with other psychopathologies for most tracts, although FA values were lower in youth with other psychopathologies for the thalamic-occipital tract.
Sex effects.
Males had higher FA values than females in tracts linking the thalamus with the prefrontal, motor, somatosensory, posterior parietal, and occipital cortex (Figure 2). All sex effects were small. Follow-up analysis showed that males also had lower MD values compared to females in most thalamic-cortical tracts. Specificity analyses confirmed that sex differences were not associated with generalized, whole-brain differences in FA.
Group differences.
Typically-developing youth had higher FA values than psychosis-spectrum youth in all white matter tracts linking the thalamus and cortex (Figure 2). Youth with other psychopathologies also had larger FA values than psychosis-spectrum youth and were similar to typically-developing youth for all tracts except for the thalamic-posterior parietal tract, which showed lower FA values than typically-developing youth. All effect sizes were small. Follow-up analysis showed that MD values were lower in typically-developing youth compared to psychosis-spectrum youth in the thalamic-somatosensory and thalamic-posterior parietal tracts. In both of these tracts, youth with other psychopathologies had lower MD values than typically-developing youth, but higher MD values than psychosis-spectrum youth. Sensitivity analyses confirmed that between-group differences in white matter microstructure were not associated with whole-brain FA, race, parental education, or CNR differences by group.
Interactions.
There was an interaction between linear age and sex in tracts connecting the thalamus with the prefrontal, motor, somatosensory, and posterior parietal cortex. Older males had higher FA values than older females (Supplementary Figure 11). All effects were small. There were no significant interactions between age and group, or sex and group (ps≥.10).
Cognitive correlates.
Associations between cognitive function and thalamocortical white matter microstructure are presented in Table 2. Better cognitive function, as measured by composite CNB score, was associated with higher FA values in thalamic-prefrontal and thalamic-posterior parietal cortex tracts. Both effects were small. There were no interactions between FA and group (ps≥.17). Follow-up analyses of cognitive domains showed that higher FA values in thalamic-prefrontal and thalamic-posterior parietal tracts were correlated with higher complex cognition, higher executive function, higher social cognition, and lower sensorimotor scores. Higher FA values in thalamic-posterior parietal cortex tracts were also associated with better episodic memory.
Table 2.
Associations between cognition and thalamocortical white matter microstructure
| Composite Score | ||||
|---|---|---|---|---|
|
| ||||
| Brain Region | F^ | β | p | Partial η2 |
| Prefrontal | 13.91 | 3.53 | <.001* | .012 |
| Motor | 6.54 | −2.37 | .01 | .006 |
| Somatosensory | 1.35 | 1.07 | .25 | .001 |
| Temporal | 0.23 | 0.48 | .63 | .000 |
| Posterior parietal | 11.43 | 3.46 | .001* | .010 |
| Occipital | 5.92 | −2.36 | .02 | .005 |
| Complex Cognition | ||||
|
| ||||
| Brain Region | F^^ | β | p | Partial η2 |
|
| ||||
| Prefrontal | 61.12 | 6.59 | <.001◊ | .051 |
| Posterior Parietal | 63.84 | 5.47 | <.001◊ | .053 |
| Executive Function | ||||
|
| ||||
| Brain Region | F^^ | β | p | Partial η2 |
|
| ||||
| Prefrontal | 35.88 | 4.45 | <.001◊ | .031 |
| Posterior Parietal | 33.17 | 3.45 | <.001◊ | .028 |
| Episodic Memory | ||||
|
| ||||
| Brain Region | F^^ | β | p | Partial η2 |
|
| ||||
| Prefrontal | 1.31 | 0.74 | .25 | .001 |
| Posterior Parietal | 1.84 | 0.72 | .018◊ | .002 |
| Social Cognition | ||||
|
| ||||
| Brain Region | F^^ | β | p | Partial η2 |
|
| ||||
| Prefrontal | 20.53 | 3.57 | <.001◊ | .018 |
| Posterior Parietal | 14.89 | 2.46 | <.001◊ | .013 |
| Sensorimotor Speed | ||||
|
| ||||
| Brain Region | F^^ | β | p | Partial η2 |
|
| ||||
| Prefrontal | 9.37 | −1.75 | .002◊ | .008 |
| Posterior Parietal | 6.02 | −1.13 | .01◊ | .005 |
Composite Score Degrees of freedom = 10,1133. For each region tested, all other regions, along with age, sex, group and motion are included as effects of no interest.
Domain Score Degrees of freedom = 5,1135. Age, sex, group, and motion are included as effects of no interest.
Composite Score effects are considered significant at Bonferroni-corrected p = .008.
Follow-up analyses of domain scores are considered significant at p = .05.
DISCUSSION
Abnormal thalamocortical structural connectivity is associated with neurodevelopmental disorders such as psychosis. Typical development of thalamocortical circuits, and whether structural connectivity deficits emerge during development and contribute to cognitive disruption prior to the onset of illness, remains unclear. Here, we characterized the effects of age, sex, and clinical symptomatology on thalamocortical structural connectivity in a large youth sample (aged 8-22 years). We found that percent total connectivity was weakly associated with age and showed a mixed pattern of increases and decreases across thalamocortical tracts, was generally similar in males and females, and was associated with neither clinical symptomatology nor cognition. In contrast, thalamocortical white matter microstructure showed robust age, sex, and group effects, characterized by moderate to large increases with age, generally higher values in males than females, and lower values in psychosis-spectrum youth. Furthermore, white matter microstructure in tracts linking the thalamus to the PFC and the posterior parietal cortex was associated with better cognitive function across participants. Together, these findings suggest that abnormal development of thalamocortical white matter microstructure may contribute to cognitive impairment and risk for psychosis.
A key motivation of our investigation was to chart the typical developmental trajectory of thalamocortical connections by age and sex. Our results showed that white matter microstructure increased linearly with age, was generally higher in males, and increased more rapidly with age in males. Quadratic age effects were smaller than linear effects across all tracts but suggested that, in tracts linking the thalamus with posterior parietal and occipital cortex, FA values may be higher during adolescence and decline slightly in early adulthood (although sampling of the early adulthood age range in the PNC is sparse). These findings are consistent with the linear increases in FA and white matter volume typically observed from childhood through adolescence (36; 37; 58), thought to reflect increases in axon organization and myelination (35). Similarly, sex effects were consistent with developmental findings of overall larger white matter volumes and more rapid white matter volume increases in males (37), which is predicted by larger brain volume (59). The majority of evidence suggests broad sex differences in white matter microstructure persist into adulthood and may be related to hormonal expression (60). Whether males continue to show higher thalamocortical white matter microstructure into adulthood requires further study.
Our second goal was to determine whether thalamocortical connections differ in youth experiencing subclinical psychosis symptoms. Our findings provide compelling evidence that thalamocortical white matter microstructure is compromised in psychosis-spectrum youth. White matter microstructure in psychosis-spectrum youth was consistently lower across tracts than in typically-developing youth. In contrast, youth with other psychopathologies were largely similar to typically-developing youth, suggesting white matter pathology may be a specific marker of psychosis risk. Thalamocortical deficits were not explained by whole brain microstructural deficits, suggesting the key importance of thalamocortical connections. Schizophrenia has long been considered a disorder of brain dysconnectivity (30; 61). Recent work has highlighted a role for brain dysconnectivity in the pathophysiology of psychotic bipolar disorder (62; 63), suggesting dysconnectivity may be a transdiagnostic psychosis marker. The establishment of structural markers reflecting aberrant brain development has significant potential to improve detection of those at risk for developing psychosis (64). White matter microstructure deficits are widespread in psychosis (65; 66), associated with genetic alterations that increase risk for psychotic disorders (67), and can be detected in early psychosis (34) and clinical-high risk individuals (68). However, the contribution of thalamocortical white matter microstructure to the onset of a frank psychotic disorder remains unclear. Studies in early psychosis and clinical-high risk cohorts show a mixed spatial pattern of white matter microstructural changes (34; 65), which may reflect interactions between normal development and illness (69–72). Myelination begins postnatally but completes in young adulthood near the typical onset of psychotic illness. During the period surrounding illness onset, reduced expression of genes associated with oligodendrocyte function are thought to contribute to psychosis through hypomyelination and loss of axonal metabolic support (73–76). However, longitudinal studies are essential to understand how thalamocortical white matter microstructure is affected in the period surrounding illness onset.
Higher white matter microstructure in two tracts—the thalamic-PFC and thalamic-posterior parietal cortex tracts—was selectively associated with better cognitive function across a number of domains, including complex cognition, executive function, social cognition, and sensorimotor speed. Cognitive deficits in these domains have been noted in psychotic disorders (77) and associated with white matter deficits in tracts that overlap those investigated in our study (78–81). White matter microstructure, particularly in PFC tracts, is well-established as a neural correlate of cognitive function in healthy populations (82–84) and patients with neurocognitive deficits (85), is linked to cognitive development during adolescence (86; 87), and has been proposed as a marker for cognitive deficits in psychosis (88). Our findings provide further support that the development of thalamocortical white matter microstructure in PFC and posterior parietal tracts is critical for normal cognitive function across a range of cognitive domains.
In contrast to our white matter microstructure findings, thalamocortical percent total connectivity showed only small associations with age, including subtle increases in thalamic-somatosensory and motor structural connectivity and subtle decreases in thalamic-temporal and occipital connections. These results are broadly consistent with thalamocortical functional connectivity findings over a similar developmental age range (89). However, the deficits in thalamic-PFC connectivity and increases in thalamic-somatosensory and thalamic-motor connectivity consistently found in psychotic disorders (18; 19), and to a lesser extent in clinical-high risk (16), were not evident in psychosis-spectrum youth. Psychosis-spectrum youth in PNC were younger than other clinical-high-risk samples (90) and may not show age-related changes observed in older groups. It’s also possible that psychosis-related alterations stem from abnormal refinement of connections surrounding illness onset. While substantial axon elimination and synaptic pruning occurs during the first postnatal year, a protracted period of selective refinement continues through the second decade of life (91–94). Disruptions to these processes are known to result in long-lasting effects on neural communication and cognition, and disruptions occurring in late adolescence, in the period surrounding typical illness onset, have been long-hypothesized to play a role in psychosis (32; 95). Specifically, evidence that genetic variants associated with illness (96; 97) and hormonal shifts during adolescence (98) result in excess synaptic pruning have led some researchers to hypothesize that dysregulations in synaptic remodeling during adolescence could trigger the onset of disease (43; 99). Neuropathological studies in cortex have broadly supported this hypothesis, showing reductions in neuropil (100), expression of synaptic markers (101), and dendritic spine density (102) in schizophrenia. Likewise, gray matter volume declines in many cortical regions during adolescence (103), and this decline appears more pronounced in schizophrenia (104), particularly in those with childhood onset (105). Further, recent imaging findings in the thalamus suggest neuropil reductions are present in the early stage of schizophrenia (106). Reduced synaptic neuropil in the thalamus and cortex may contribute to abnormalities in neuronal synchronization, and ultimately, changes in structural connectivity. Alternatively, psychosis-related alterations may result from prenatal disruptions. Thalamocortical axons migrate during the second and third trimester of human gestation (107), laying the fundamental structure for efficient communication across the brain. Defective migration and altered programmed cell death during this window of vulnerability, perhaps from second-trimester maternal infection (108), have also been theorized to underlie risk for schizophrenia (32). As most at-risk individuals do not ultimately transition to psychosis (109), structural alterations in the current sample may be obscured. Ultimately, prospective longitudinal studies in clinical-high risk individuals are necessary to determine the timing of occurrence of structural connectivity changes.
It is important to consider the limitations of the study when interpreting the findings. The PNC dataset is comprised of cross-sectional data spanning a developmental window. Our findings expand the small existing literature of how structural connectivity deficits during development may contribute to cognitive deficits in psychosis, although longitudinal studies will be essential to determine white matter biomarkers that predict subsequent clinical or cognitive outcomes.
While diffusion-based methods can detect differences in structural connectivity, the nature of identified differences should be interpreted with caution. Microstructural differences may result from differences in myelination, fiber bundle cohesion, or water content. Probabilistic tracking can be influenced by crossing fibers and regions where anatomical boundaries between tracts are close. Advancements in acquisition of higher-resolution diffusion images and comparisons with other measures of tissue microstructure, such as myelin-based (110) or free-water imaging (111), may further refine our understanding of structural connectivity across development. CNR was lower in the psychosis-spectrum group despite similar motion measures across groups. Although motion was included as a covariate, accounting for non-linear motion effects may improve tractography accuracy (112).
In conclusion, we find thalamocortical white matter microstructure increases with age, is higher in males, is lower in psychosis-spectrum youth across development, and is correlated with cognitive function. These findings suggest disturbances in maturation of thalamocortical white matter microstructure may be an early marker for psychosis and contribute to cognitive deficits. The characterization of thalamocortical network structural connectivity by age, sex, and clinical symptomatology in a population-based, developmental sample provides a foundation for further neurodevelopmental studies of thalamocortical network dysconnectivity in psychotic disorders. Future studies should use longitudinal samples to determine the predictive validity of thalamocortical white matter microstructure in conversion to a psychotic disorder and clinical interventions that strengthen structural connectivity and may ameliorate risk.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by R01 MH115000 (awarded to NDW and AA), R01 MH102266 (awarded to NDW), R01 MH123563 (awarded to SNV), and the Vanderbilt Institute for Clinical and Translational Research (through grant 1-UL-1-TR000445 from the National Center for Research Resources/NIH). This work was conducted in part using the resources of the Advanced Computing Center for Research and Education and the Center for Computational Imaging at Vanderbilt University, Nashville, TN.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
All authors report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Dorph-Petersen KA, Lewis DA (2017): Postmortem structural studies of the thalamus in schizophrenia. Schizophr Res. 180: 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Erp TGMM, Hibar DP, Rasmussen JM, Glahn DC, Pearlson GD, Andreassen OA, et al. (2016): Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry. 21: 547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang AS, Rogers BP, Sheffield JM, Jalbrzikowski ME, Anticevic A, Blackford JU, et al. (2020): Thalamic nuclei volumes in psychotic disorders and in youths with psychosis spectrum symptoms. Am J Psychiatry. 177: 1159–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC (2009): Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 66: 811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ragland JD, Laird AR, Ranganath C, Blumenfeld RS, Gonzales SM, Glahn DC (2009): Prefrontal Activation Deficits During Episodic Memory in Schizophrenia. Am J Psychiatry. 166: 863–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cerullo MA, Adler CM, Delbello MP, Strakowski SM (2009): The functional neuroanatomy of bipolar disorder. Int Rev Psychiatry. 21: 314–322. [DOI] [PubMed] [Google Scholar]
- 7.Kraguljac NV, Reid M, White D, Jones R, den Hollander J, Lowman D, Lahti AC (2012): Neurometabolites in schizophrenia and bipolar disorder - a systematic review and meta-analysis. Psychiatry Res. 203: 111–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andreasen N, Arndt S, Swayze V, Cizadlo T, Flaum M, O’Leary D, et al. (1994): Thalamic abnormalities in schizophrenia visualized through magnetic resonance image averaging. Science (80-). 266: 294–298. [DOI] [PubMed] [Google Scholar]
- 9.Andreasen NC (1997): The Role of the Thalamus in Schizophrenia. Can J Psychiatry. 42: 27–33. [DOI] [PubMed] [Google Scholar]
- 10.Scheibel AB (1997): The thalamus and neuropsychiatric illness. J Neuropsychiatry Clin Neurosci. 9: 342–53. [DOI] [PubMed] [Google Scholar]
- 11.Swerdlow NR (2010): Integrative circuit models and their implications for the pathophysiologies and treatments of the schizophrenias. Curr Top Behav Neurosci. 4: 555–83. [DOI] [PubMed] [Google Scholar]
- 12.Giraldo-Chica M, Woodward ND (2017): Review of thalamocortical resting-state fMRI studies in schizophrenia. Schizophr Res. 180: 58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramsay IS (2019): An Activation Likelihood Estimate Meta-analysis of Thalamocortical Dysconnectivity in Psychosis. Biol Psychiatry Cogn Neurosci Neuroimaging. 4: 859–869. [DOI] [PubMed] [Google Scholar]
- 14.Woodward ND, Karbasforoushan H, Heckers S (2012): Thalamocortical dysconnectivity in schizophrenia. Am J Psychiatry. 169: 1092–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anticevic A, Cole MW, Repovs G, Murray JD, Brumbaugh MS, Winkler AM, et al. (2014): Characterizing thalamo-cortical disturbances in schizophrenia and bipolar illness. Cereb Cortex. 24: 3116–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho KIK, Shenton ME, Kubicki M, Jung WH, Lee TY, Yun JY, et al. (2016): Altered thalamo-cortical white matter connectivity: Probabilistic tractography study in clinical-high risk for psychosis and first-episode psychosis. Schizophr Bull. 42: 723–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kubota M, Miyata J, Sasamoto A, Sugihara G, Yoshida H, Kawada R, et al. (2013): Thalamocortical disconnection in the orbitofrontal region associated with cortical thinning in schizophrenia. Arch Gen Psychiatry. 70: 12–21. [DOI] [PubMed] [Google Scholar]
- 18.Sheffield JM, Huang AS, Rogers BP, Giraldo-Chica M, Landman BA, Blackford JU, et al. (2020): Thalamocortical anatomical connectivity in schizophrenia and psychotic bipolar disorder. Schizophr Bull. 46: 1062–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giraldo-Chica M, Rogers BP, Damon SM, Landman BA, Woodward ND (2018): Prefrontal-Thalamic Anatomical Connectivity and Executive Cognitive Function in Schizophrenia. Biol Psychiatry. 83: 509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marenco S, Stein JL, Savostyanova AA, Sambataro F, Tan HY, Goldman AL, et al. (2012): Investigation of anatomical thalamo-cortical connectivity and fMRI activation in schizophrenia. Neuropsychopharmacology. 37: 499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szeszko PR, Robinson DG, Ashtari M, Vogel J, Betensky J, Sevy S, et al. (2008): Clinical and Neuropsychological Correlates of White Matter Abnormalities in Recent Onset Schizophrenia. Neuropsychopharmacology. 33: 976–984. [DOI] [PubMed] [Google Scholar]
- 22.Levin HS, Wilde EA, Chu Z, Yallampalli R, Hanten GR, Li X, et al. (2008): Diffusion tensor imaging in relation to cognitive and functional outcome of traumatic brain injury in children. J Head Trauma Rehabil. 23: 197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell LE, Daly E, Toal F, Stevens A, Azuma R, Karmiloff-Smith A, et al. (2009): Brain structural differences associated with the behavioural phenotype in children with Williams syndrome. Brain Res. 1258: 96–107. [DOI] [PubMed] [Google Scholar]
- 24.Cornblath EJ, Ashourvan A, Kim JZ, Betzel RF, Ciric R, Adebimpe A, et al. (2020): Temporal sequences of brain activity at rest are constrained by white matter structure and modulated by cognitive demands. Commun Biol. 3: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aoki Y, Yoncheva YN, Chen B, Nath T, Sharp D, Lazar M, et al. (2017): Association of White Matter Structure With Autism Spectrum Disorder and Attention-Deficit/Hyperactivity Disorder. JAMA psychiatry. 74: 1120–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Unger A, Alm KH, Collins JA, O’Leary JM, Olson IR (2016): Variation in white matter connectivity predicts the ability to remember faces and discriminate their emotions. J Int Neuropsychol Soc. 22: 180–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kochunov P, Zavaliangos-Petropulu A, Jahanshad N, Thompson PM, Ryan MC, Chiappelli J, et al. (2021): A White Matter Connection of Schizophrenia and Alzheimer’s Disease. Schizophr Bull. 47: 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qiu A, Zhong J, Graham S, Chia MY, Sim K (2009): Combined analyses of thalamic volume, shape and white matter integrity in first-episode schizophrenia. Neuroimage. 47: 1163–1171. [DOI] [PubMed] [Google Scholar]
- 29.Weinberger DR (1987): Implications of Normal Brain Development for the Pathogenesis of Schizophrenia. Arch Gen Psychiatry. 44: 660–669. [DOI] [PubMed] [Google Scholar]
- 30.McGlashan TH, Hoffman RE (2000): Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Arch Gen Psychiatry. 57. [DOI] [PubMed] [Google Scholar]
- 31.Sidman RL, Rakic P (1973): Neuronal migration, with special reference to developing human brain: a review. Brain Res. 62: 1–35. [DOI] [PubMed] [Google Scholar]
- 32.Jones EQ (1997): Cortical development and thalamic pathology in schizophrenia. Schizophr Bull. 23: 483–501. [DOI] [PubMed] [Google Scholar]
- 33.Stephan KE, Friston KJ, Frith CD (2009): Dysconnection in Schizophrenia: From abnormal synaptic plasticity to failures of self-monitoring. Schizophr Bull. 35: 509–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samartzis L, Dima D, Fusar-Poli P, Kyriakopoulos M (2014): White matter alterations in early stages of schizophrenia: A systematic review of diffusion tensor imaging studies. J Neuroimaging. 24: 101–110. [DOI] [PubMed] [Google Scholar]
- 35.Tamnes CK, Østby Y, Fjell AM, Westlye LT, Due-Tønnessen P, Walhovd KB (2010): Brain maturation in adolescence and young adulthood: Regional age-related changes in cortical thickness and white matter volume and microstructure. Cereb Cortex. 20: 534–548. [DOI] [PubMed] [Google Scholar]
- 36.Giedd JN, Lalonde FM, Celano MJ, White SL, Wallace GL, Lee NR, Lenroot RK (2009): Anatomical brain magnetic resonance imaging of typically developing children and adolescents. J Am Acad Child Adolesc Psychiatry. 48: 465–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, et al. (2007): Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 36: 1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asato MR, Terwilliger R, Woo J, Luna B (2010): White matter development in adolescence: A DTI study. Cereb Cortex. 20: 2122–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blakemore SJ, Choudhury S (2006): Development of the adolescent brain: Implications for executive function and social cognition. J Child Psychol Psychiatry Allied Discip. 47: 296–312. [DOI] [PubMed] [Google Scholar]
- 40.Lenroot RK, Giedd JN (2010): Sex differences in the adolescent brain. Brain Cogn. 72: 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aleman A, Kahn RS, Selten J-P (2003): Sex differences in the risk of schizophrenia: evidence from meta-analysis. Arch Gen Psychiatry. 60: 565–71. [DOI] [PubMed] [Google Scholar]
- 42.Lisman JE, Pi HJ, Zhang Y, Otmakhova NA (2010): A Thalamo-Hippocampal-Ventral Tegmental Area Loop May Produce the Positive Feedback that Underlies the Psychotic Break in Schizophrenia. Biol Psychiatry. 68: 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keshavan MS (1999): Development, disease and degeneration in schizophrenia: A unitary pathophysiological model. J Psychiatr Res. 33: 513–521. [DOI] [PubMed] [Google Scholar]
- 44.Xiao Y, Sun H, Shi S, Jiang D, Tao B, Zhao Y, et al. (2018): White matter abnormalities in never-treated patients with long-term schizophrenia. Am J Psychiatry. 175: 1129–1136. [DOI] [PubMed] [Google Scholar]
- 45.Wilkinson M, Kane T, Wang R, Takahashi E (2017): Migration pathways of thalamic neurons and development of thalamocortical connections in humans revealed by diffusion MR tractography. Cereb Cortex. 27: 5683–5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Calkins ME, Moore TM, Merikangas KR, Burstein M, Satterthwaite TD, Bilker WB, et al. (2014): The psychosis spectrum in a young U.S. community sample: Findings from the Philadelphia Neurodevelopmental Cohort. World Psychiatry. 13: 296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Os J, Linscott RJ, Myin-Germeys I, Delespaul P, Krabbendam L (2009): A systematic review and meta-analysis of the psychosis continuum: Evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol Med. 39: 179–195. [DOI] [PubMed] [Google Scholar]
- 48.Linscott RJ, Van Os J (2013): An updated and conservative systematic review and meta-analysis of epidemiological evidence on psychotic experiences in children and adults: On the pathway from proneness to persistence to dimensional expression across mental disorders. Psychol Med. 43: 1133–1149. [DOI] [PubMed] [Google Scholar]
- 49.DeRosse P, Karlsgodt KH (2015): Examining the Psychosis Continuum. Curr Behav Neurosci reports. 2: 80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poulton R, Caspi A, Moffitt TE, Cannon M, Murray R, Harrington H (2000): Children’s self-reported psychotic symptoms and adult schizophreniform disorder: a 15-year longitudinal study. Arch Gen Psychiatry. 57: 1053–8. [DOI] [PubMed] [Google Scholar]
- 51.David AS, Ajnakina O (2016): Psychosis as a continuous phenotype in the general population: the thin line between normality and pathology. World Psychiatry. 15: 129–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fusar-Poli P, Bonoldi I, Yung AR, Borgwardt S, Kempton MJ, Valmaggia L, et al. (2012): Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 69: 220–9. [DOI] [PubMed] [Google Scholar]
- 53.Kaczkurkin AN, Raznahan A, Satterthwaite TD (2019): Sex differences in the developing brain: insights from multimodal neuroimaging. Neuropsychopharmacology. 44: 71–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gur RC, Richard J, Hughett P, Calkins ME, Macy L, Bilker WB, et al. (2010): A cognitive neuroscience-based computerized battery for efficient measurement of individual differences: Standardization and initial construct validation. J Neurosci Methods. 187: 254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moore TM, Reise SP, Gur RE, Hakonarson H, Gur RC (2015): Psychometric properties of the penn computerized neurocognitive battery. Neuropsychology. 29: 235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, et al. (2004): Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 23: 208–219. [DOI] [PubMed] [Google Scholar]
- 57.Dale AM, Fischl B, Sereno MI (1999): Cortical Surface-Based Analysis. Neuroimage. 9: 179–194. [DOI] [PubMed] [Google Scholar]
- 58.Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C (2008): Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 40: 1044–1055. [DOI] [PubMed] [Google Scholar]
- 59.Lüders E, Steinmetz H, Jäncke L (2002): Brain size and grey matter volume in the healthy human brain. Neuroreport. 13: 2371–4. [DOI] [PubMed] [Google Scholar]
- 60.Van Hemmen J, Saris IMJ, Cohen-Kettenis PT, Veltman DJ, Pouwels PJW, Bakker J (2017): Sex Differences in White Matter Microstructure in the Human Brain Predominantly Reflect Differences in Sex Hormone Exposure. Cereb Cortex. 27: 2994–3001. [DOI] [PubMed] [Google Scholar]
- 61.Catani M, Ffytche DH (2005): The rises and falls of disconnection syndromes. Brain. 128: 2224–2239. [DOI] [PubMed] [Google Scholar]
- 62.Perry A, Roberts G, Mitchell PB, Breakspear M (2019): Connectomics of bipolar disorder: a critical review, and evidence for dynamic instabilities within interoceptive networks. Mol Psychiatry. 24: 1296–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fernandes HM, Cabral J, van Hartevelt TJ, Lord LD, Gleesborg C, Møller A, et al. (2019): Disrupted brain structural connectivity in Pediatric Bipolar Disorder with psychosis. Sci Rep. 9: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Steullet P (2020): Thalamus-related anomalies as candidate mechanism-based biomarkers for psychosis. Schizophr Res. 226: 147–157. [DOI] [PubMed] [Google Scholar]
- 65.Cetin-Karayumak S, Di Biase MA, Chunga N, Reid B, Somes N, Lyall AE, et al. (2019): White matter abnormalities across the lifespan of schizophrenia: a harmonized multi-site diffusion MRI study. Mol Psychiatry. 3208–3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kelly S, Jahanshad N, Zalesky A, Kochunov P, Agartz I, Alloza C, et al. (2018): Widespread white matter microstructural differences in schizophrenia across 4322 individuals: Results from the ENIGMA Schizophrenia DTI Working Group. Mol Psychiatry. 23: 1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bohlken MM, Brouwer RM, Mandl RCW, Van Den Heuvel MP, Hedman AM, De Hert M, et al. (2016): Structural brain connectivity as a genetic marker for schizophrenia. JAMA Psychiatry. 73: 11–19. [DOI] [PubMed] [Google Scholar]
- 68.Bernard JA, Orr JM, Mittal VA (2015): Abnormal hippocampal-thalamic white matter tract development and positive symptom course in individuals at ultra-high risk for psychosis. npj Schizophr. 1: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ho B-C, Andreasen NC, Nopoulos P, Arndt S, Magnotta V, Flaum M (2003): Progressive Structural Brain Abnormalities and Their Relationship to Clinical Outcome. Arch Gen Psychiatry. 60: 585. [DOI] [PubMed] [Google Scholar]
- 70.Karlsgodt KH (2016): Diffusion Imaging of White Matter In Schizophrenia: Progress and Future Directions. Biol psychiatry Cogn Neurosci neuroimaging. 1: 209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peters BD, Szeszko PR, Radua J, Ikuta T, Gruner P, Derosse P, et al. (2012): White matter development in adolescence: Diffusion tensor imaging and meta-analytic results. Schizophr Bull. 38: 1308–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peters BD, Karlsgodt KH (2015): White matter development in the early stages of psychosis. Schizophr Res. 161: 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goudriaan A, De Leeuw C, Ripke S, Hultman CM, Sklar P, Sullivan PF, et al. (2014): Specific glial functions contribute to Schizophrenia susceptibility. Schizophr Bull. 40: 925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nave KA, Ehrenreich H (2014): Myelination and oligodendrocyte functions in psychiatric diseases. JAMA Psychiatry. 71: 582–584. [DOI] [PubMed] [Google Scholar]
- 75.Roussos P, Haroutunian V (2014): Schizophrenia: susceptibility genes and oligodendroglial and myelin related abnormalities. Front Cell Neurosci. 8: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Takahashi N, Sakurai T, Davis KL, Buxbaum JD (2011): Linking oligodendrocyte and myelin dysfunction to neurocircuitry abnormalities in schizophrenia. Prog Neurobiol. 93: 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Irani F, Brensinger CM, Richard J, Calkins ME, Moberg PJ, Bilker W, et al. (2012): Computerized neurocognitive test performance in schizophrenia: a lifespan analysis. Am J Geriatr Psychiatry. 20: 41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Roalf DR, Ruparel K, Verma R, Elliott MA, Gur RE, Gur RC (2013): White matter organization and neurocognitive performance variability in schizophrenia. Schizophr Res. 143: 172–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang M, Gao S, Zhang X (2020): Cognitive deficits and white matter abnormalities in never-treated first-episode schizophrenia. Transl Psychiatry. 10. doi: 10.1038/s41398-020-01049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Subramaniam K, Gill J, Fisher M, Mukherjee P, Nagarajan S, Vinogradov S (2018): White matter microstructure predicts cognitive training-induced improvements in attention and executive functioning in schizophrenia. Schizophr Res. 193: 276–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kochunov P, Coyle TR, Rowland LM, Jahanshad N, Thompson PM, Kelly S, et al. (2017): Association of white matter with core cognitive deficits in patients with schizophrenia. JAMA Psychiatry. 74: 958–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nagy Z, Westerberg H, Klingberg T (2004): Maturation of white matter is associated with the development of cognitive functions during childhood. J Cogn Neurosci. 16: 1227–1233. [DOI] [PubMed] [Google Scholar]
- 83.Turken AU, Whitfield-Gabrieli S, Bammer R, Baldo JV., Dronkers NF, Gabrieli JDE (2008): Cognitive processing speed and the structure of white matter pathways: Convergent evidence from normal variation and lesion studies. Neuroimage. 42: 1032–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gold BT, Powell DK, Xuan L, Jiang Y, Hardy PA (2007): Speed of lexical decision correlates with diffusion anisotropy in left parietal and frontal white matter: Evidence from diffusion tensor imaging. Neuropsychologia. 45: 2439–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Boespflug EL, Storrs J, Sadat-Hossieny S, Eliassen J, Shidler M, Norris M, Krikorian R (2014): Full diffusion characterization implicates regionally disparate neuropathology in Mild Cognitive Impairment. Brain Struct Funct. 219: 367–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schmithorst VJ, Wilkes M, Dardzinski BJ, Holland SK (2005): Cognitive functions correlate with white matter architecture in a normal pediatric population: A diffusion tensor HRI study. Hum Brain Mapp. 26: 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Luna B (2009): Developmental Changes in Cognitive Control through Adolescence. Adv Child Dev Behav. (Vol. 37), Elsevier, doi: 10.1016/S0065-2407(09)03706-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thomas MB, Raghava JM, Pantelis C, Rostrup E, Nielsen MØ, Jensen MH, et al. (2021): Associations between cognition and white matter microstructure in first-episode antipsychotic-naive patients with schizophrenia and healthy controls: A multivariate pattern analysis. Cortex. 1–16. [DOI] [PubMed] [Google Scholar]
- 89.Fair (2010): Maturing thalamocortical functional connectivity across development. Front Syst Neurosci. 4: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Catalan A, Salazar De Pablo G, Aymerich C, Damiani S, Sordi V, Radua J, et al. (2021): Neurocognitive Functioning in Individuals at Clinical High Risk for Psychosis: A Systematic Review and Meta-analysis. JAMA Psychiatry. 78: 859–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huttenlocher PR (1979): Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 163: 195–205. [DOI] [PubMed] [Google Scholar]
- 92.Huttenlocher PR, de Courten C, Garey LJ, Van der Loos H (1982): Synaptogenesis in human visual cortex--evidence for synapse elimination during normal development. Neurosci Lett. 33: 247–52. [DOI] [PubMed] [Google Scholar]
- 93.Huttenlocher PR, Dabholkar AS (1997): Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 387: 167–178. [DOI] [PubMed] [Google Scholar]
- 94.Casey BJ, Tottenham N, Liston C, Durston S (2005): Imaging the developing brain: What have we learned about cognitive development? Trends Cogn Sci. 9: 104–110. [DOI] [PubMed] [Google Scholar]
- 95.Feinberg I (1982): Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res. 17: 319–34. [DOI] [PubMed] [Google Scholar]
- 96.Sellgren CM, Gracias J, Watmuff B, Biag JD, Thanos JM, Whittredge PB, et al. (2019): Increased synapse elimination by microglia in schizophrenia patient-derived models of synaptic pruning. Nat Neurosci. 22: 374–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sekar A, Bialas AR, De Rivera H, Davis A, Hammond TR, Kamitaki N, et al. (2016): Schizophrenia risk from complex variation of complement component 4. Nature. 530: 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Trotman HD, Holtzman CW, Ryan AT, Shapiro DI, MacDonald AN, Goulding SM, et al. (2013): The development of psychotic disorders in adolescence: A potential role for hormones. Horm Behav. 64: 411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sakai J (2020): Core Concept: How synaptic pruning shapes neural wiring during development and, possibly, in disease. Proc Natl Acad Sci U S A. 117: 16096–16099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Selemon LD, Rajkowska G, Goldman-Rakic PS (1995): Abnormally high neuronal density in the schizophrenic cortex. A morphometric analysis of prefrontal area 9 and occipital area 17. Arch Gen Psychiatry. 52: 805–18; discussion 819–20. [DOI] [PubMed] [Google Scholar]
- 101.Glantz LA, Lewis D a (1997): Reduction of synaptophysin immunoreactivity in the prefrontal cortex of subjects with schizophrenia. Regional and diagnostic specificity. Arch Gen Psychiatry. 54: 943–52. [DOI] [PubMed] [Google Scholar]
- 102.Garey LJ, Ong WY, Patel TS, Kanani M, Davis A, Mortimer AM, et al. (1998): Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J Neurol Neurosurg Psychiatry. 65: 446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. (1999): Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 2: 861–3. [DOI] [PubMed] [Google Scholar]
- 104.van Haren NEM, Pol HEH, Schnack HG, Cahn W, Brans R, Carati I, et al. (2008): Progressive Brain Volume Loss in Schizophrenia Over the Course of the Illness: Evidence of Maturational Abnormalities in Early Adulthood. Biol Psychiatry. 63: 106–113. [DOI] [PubMed] [Google Scholar]
- 105.Sporn AL, Greenstein DK, Gogtay N, Jeffries NO, Lenane M, Gochman P, et al. (2003): Progressive brain volume loss during adolescence in childhood-onset schizophrenia. Am J Psychiatry. 160: 2181–9. [DOI] [PubMed] [Google Scholar]
- 106.Prasad KM, Burgess AM, Keshavan MS, Nimgaonkar VL, Stanley JA (2016): Neuropil pruning in Early-Course Schizophrenia: Immunological, Clinical, and Neurocognitive Correlates. Biol psychiatry Cogn Neurosci neuroimaging. 1: 528–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wilson S, Pietsch M, Cordero-Grande L, Price AN, Hutter J, Xiao J, et al. (2021): Development of human white matter pathways in utero over the second and third trimester. Proc Natl Acad Sci. 118: e2023598118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fraile SC, Ruíz PC, Peinado AG (2015): Prenatal Infections and Schizophrenia. Eur Psychiatry. 30: 1700. [Google Scholar]
- 109.Salazar De Pablo G, Radua J, Pereira J, Bonoldi I, Arienti V, Besana F, et al. (2021): Probability of Transition to Psychosis in Individuals at Clinical High Risk: An Updated Meta-analysis. JAMA Psychiatry. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.van der Weijden CWJ, García DV, Borra RJH, Thurner P, Meilof JF, van Laar PJ, et al. (2021): Myelin quantification with MRI: A systematic review of accuracy and reproducibility. Neuroimage. 226. doi: 10.1016/j.neuroimage.2020.117561. [DOI] [PubMed] [Google Scholar]
- 111.Pasternak O, Kelly S, Sydnor VJ, Shenton ME (2018): Advances in microstructural diffusion neuroimaging for psychiatric disorders. Neuroimage. 182: 259–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Baum GL, Roalf DR, Cook PA, Ciric R, Rosen AFG, Xia C, et al. (2018): The impact of in-scanner head motion on structural connectivity derived from diffusion MRI. Neuroimage. 173: 275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


