Abstract
Malaria-associated morbidity and mortality are increasing because of widespread resistance to one of the safest and least expensive antimalarials, chloroquine. The availability of an inexpensive agent that is capable of reversing chloroquine resistance would have a major impact on malaria treatment worldwide. The interaction of nonylphenolethoxylates (NPEs, commercially available synthetic surfactants) with drug-resistant Plasmodium falciparum was examined to determine if NPEs inhibited the growth of the parasites and if NPEs could sensitize resistant parasites to chloroquine. NPEs inhibited the development of the parasite when present in the low- to mid-micromolar range (5 to 90 μM), indicating that they possess antimalarial activity. Further, the presence of <10 μM concentrations of NPEs caused the 50% inhibitory concentrations for chloroquine-resistant lines to drop to levels (≤12 nM) observed for sensitive lines and generally considered to be achievable with treatment courses of chloroquine. Long-chain (>30 ethoxylate units) NPEs were found to be most active in P. falciparum, which contrasts with previously observed maximal activity of short-chain (∼9 ethoxylate units) NPEs in multidrug-resistant mammalian cell lines. NPEs may be attractive chloroquine resistance reversal agents since they are inexpensive and may be selectively directed against P. falciparum without inhibiting mammalian tissue P glycoproteins. Antimalarial preparations that include these agents may prolong the effective life span of chloroquine and other antimalarials.
Chloroquine-resistant Plasmodium falciparum malaria was first recognized over 40 years ago and has since spread to almost all areas where malaria is endemic (20). When chloroquine-resistant malaria extended into the high-transmission areas of Africa, it raised fears of an impending public health crisis, since switching to alternative antimalarials, such as mefloquine, artemisinin derivatives, halofantrine, or quinine, is unaffordable for many countries in sub-Saharan Africa (24). Recent reports indicate that escalating mortality due to widespread malaria resurgence is now taking place (15). To meet this challenge, novel and inexpensive antimalarials must be developed, or a way to prolong the efficacy of chloroquine, such as by combining it with a safe and inexpensive sensitizing agent, must be found.
The mechanism of chloroquine resistance in P. falciparum is controversial, although it is frequently compared to multidrug resistance in mammalian cells, which is often mediated by P glycoproteins (4). Mammalian P glycoproteins are intrinsic membrane protein drug transporters that actively pump a wide variety of drugs and other xenobiotic compounds out of cells. Although P glycoproteins can pump many types of chemotherapeutic agents, like vinblastine and doxorubicin (Adriamycin), out of cancer cells, the multidrug resistance phenotype of such cells can be modified by a variety of compounds, including the immunosuppressant cyclosporine (7) and calcium channel blockers like verapamil. These chemosensitizers interact competitively with drug-binding sites on P glycoprotein, thereby interfering with the transport of chemotherapeutic agents out of cells.
The initial observation that drug resistance in P. falciparum could be modulated by verapamil (16) has led to reports that antipsychotics (e.g., chlorpromazine [1]), histamine (H-1) receptor antagonists (e.g., promethazine [17] and chlorpheniramine [2]), and other agents can reverse chloroquine resistance in vitro and in animal models (3). While these observations suggest that chloroquine combined with a second agent can be used to treat malaria, these agents have the disadvantage of being pharmacologically active compounds with multisystemic effects that may result in a variety of side effects. Furthermore, these compounds are often more expensive than chloroquine itself, and the concentrations required to reverse clinical drug resistance can be toxic.
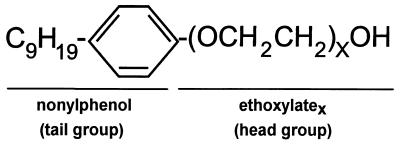
Nonylphenolethoxylates (6) (NPEs) (Fig. 1) are synthetic surfactants that are inexpensive enough to be used in a variety of household products. They have been used as wetting agents and as intestinal permeability enhancers to improve oral drug delivery (21). Their toxicology has been investigated (13), as have their absorption, distribution, and excretion in rodents and humans (12, 21). NPEs are rapidly absorbed orally and topically and are actively excreted into the urine of healthy control subjects by kidney P glycoprotein (6), an observation that led us to evaluate their use as P. falciparum chloroquine resistance reversal agents. We have determined that the nonylphenol (NP) series of ethoxylate (EO)-containing surfactants have antimalarial properties and reverse chloroquine resistance in both established laboratory lines of P. falciparum and patient isolates. Further, P. falciparum cultures and mammalian cell lines interact with NPEs with different EO contents. These findings suggest that NPEs alone or in combination with chloroquine could be used to treat P. falciparum malaria and that they can be directed to interact preferentially with the parasite simply by altering the number of EO units in the surfactant's structure.
FIG. 1.
General structure of NPEs. NPEs consist of a hydrophobic tail group with a polymeric hydrophilic head portion consisting of repeating units of EO. NPEs are synthesized by copolymerization of ethylene oxide with NP, thereby producing a polydisperse mixture of head group lengths (X values).
MATERIALS AND METHODS
Parasite strains and culture.
P. falciparum cultures were grown in A+ blood obtained by venipuncture of volunteers. Cultures of the laboratory lines ItG and 3D7 (8) and the patient isolates were maintained by the method of Trager and Jensen (22), using RPMI 1640 supplemented with 10% human serum and 50 μM hypoxanthine. Patient isolates were obtained from pretreatment blood samples of patients enrolled in ongoing and ethically approved studies at the Tropical Disease Unit, University of Toronto (11, 26). A molecular characterization of their resistance phenotypes (A.-C. Labbé, [University of Toronto], personal communication) showed that isolate 1 has a wild-type Pfmdr locus and type RII clinical resistance, whereas isolate 2 has a mutant Pfmdr locus and type RIII clinical resistance. In vitro drug susceptibility testing was performed using the World Health Organization in vitro microtest (Mark III) (25). The 50% inhibitory concentrations (IC50) were determined using a nonlinear regression analysis of the dose-response curve.
CHO cells were grown in RPMI 1640 supplemented with 10% fetal calf serum, HEPES, and gentamicin. CHO cell viability was determined using an MTT assay (5). NPEs were gifts from Union Carbide and were extensively dried by lyophilization before being made up as 1% (wt/vol) stock solutions in water.
RESULTS
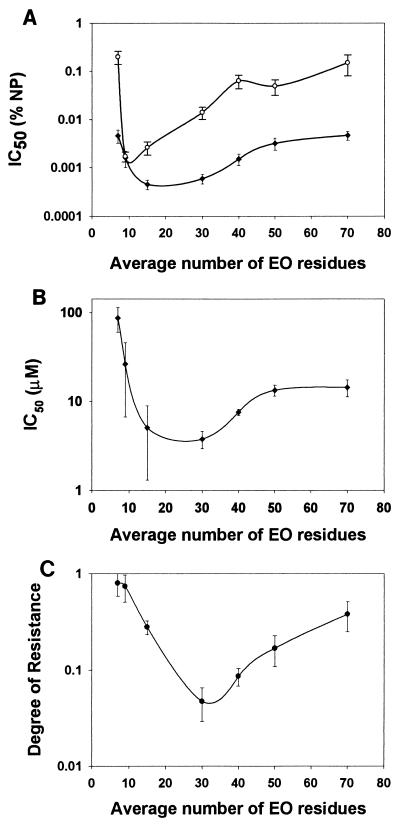
Initial experiments were undertaken to determine the level of chloroquine resistance present (e.g., IC50) in the laboratory lines 3D7 and ItG and in patient isolates 1 and 2 (Table 1). We then proceeded to determine what effect increasing concentrations of surfactant alone had on P. falciparum cultures. NPE preparations with a common hydrophobic tail group but with hydrophilic head groups having various average EO chain lengths (Fig. 1) were assayed for their direct activity against P. falciparum and CHO cell cultures. On a per-weight basis, NPEs with an average EO head length of >10 but <40 had the greatest anti-P. falciparum activity, while NP9 has maximum activity against CHO cells (Fig. 2A). When the results were corrected for the average molecular weights of the preparations, it was observed that NPEs with average EO head lengths of >10 and <50 had maximum activity in P. falciparum cultures (Fig. 2B). The IC50 of the surfactants in P. falciparum were significantly lower than the concentrations at which micelles form (>100 μM); therefore, the mechanism of action of NPEs is unlikely to be due to gross disruption of membrane integrity, which suggests that the NPEs interact with a cellular component(s) of the parasite. To determine if NPEs and chloroquine, two compounds that have been implicated as substrates for intercellular membrane drug pumps, were capable of acting synergistically, NPE-chloroquine combination experiments were performed in both P. falciparum and CHO cell cultures. CHO cultures were unaffected by chloroquine-NP30 combinations, even when concentrations of up to 25 μM chloroquine were used in the presence of NP30 concentrations of up to 0.01% (data not shown). Initial experiments with P. falciparum cultures indicated that 8 μM NP15 was able to reverse chloroquine resistance as effectively as 1 μM verapamil in the chloroquine-resistant ItG line and two drug-resistant patient isolates, one from India and one from Africa (Table 1).
TABLE 1.
Chloroquine sensitivity and activity of reversal agents on chloroquine-resistant laboratory lines and wild P. falciparum isolatesa
| Isolateb | Source | Resistance presentc | Chloroquine IC50 (nM) | Chloroquine IC50 (nM) with agent | Reversal agent |
|---|---|---|---|---|---|
| 3D7 | Laboratory | No | 2.4 | NDd | |
| ItG | Laboratory | Yes | 60 ± 20 | 12 ± 3 | 7 μM NP15 |
| ItG | Laboratory | Yes | 60 ± 20 | 21 ± 7 | 7 μM NP40 |
| ItG | Laboratory | Yes | 60 ± 20 | 13 ± 6 | 7 μM NP70 |
| ItG | Laboratory | Yes | 60 ± 20 | 21 ± 7 | 1 μM verapamil |
| ItG | Laboratory | Yes | 60 ± 20 | 54 ± 22 | 7 μM PEG |
| Isolate 1 | India | Yes | >150 | 9 ± 2 | 7 μM NP15 |
| Isolate 2 | Africa | Yes | >300 | 7 ± 1 | 7 μM NP15 |
| Isolate 2 | Africa | Yes | >300 | 28 ± 7 | 1 μM verapamil |
The chloroquine IC50 were determined in the presence and absence of resistance reversal agents using the WHO Mark III microtest (see Materials and Methods).
The laboratory line 3D7 is a chloroquine-sensitive line of presumed African origin. ItG is a chloroquine-resistant line of Southeast Asian origin. Isolates 1 and 2 were obtained from patients who had traveled to India and Africa, respectively.
Clinical resistance is frequently defined as an IC50 of >100 nM (1).
ND, not determined.
FIG. 2.
Effect of EO content on viability in the absence and presence of chloroquine. NPE solutions with increasing average EO contents were tested for CHO cell (○) and P. falciparum (isolate 2 [●]) toxicity. The IC50 of these materials were determined using a nonlinear regression analysis. IC50 results are expressed both on a per-weight basis (A) and on a molar-concentration basis (B). The ability of a 5 μM concentration of NPEs to sensitize P. falciparum in vitro to chloroquine was determined (C). The degree of chloroquine resistance is calculated as the IC50 observed in the presence of various NPEs divided by the control (no NPE added) chloroquine IC50 (240 ± 60 nM). Values greater than 1 indicate that the NPE rendered the P. falciparum parasites less sensitive to chloroquine, while values less than 1 indicate that the NPE sensitized P. falciparum to chloroquine. Anti-P. falciparum activity and sensitization are representative results obtained from multiple determinations. Results are plotted as means, with the standard errors (as calculated by Sigma plot) indicated with bars.
Previous results (Fig. 2B) suggested that the EO content of the NPE influenced the surfactant-parasite interaction. Therefore, the reversal potentials of a series of NPEs with increasing EO content were determined using 5 μM concentrations of each surfactant. An NPE preparation with an average EO head length of 30 was found to be the most effective chloroquine resistance reversal agent (Fig. 2C). To determine if both the tail and head groups (Fig. 1) were required for activity, the effect of the EO polymer polyethylene glycol (PEG, n ∼ 75), which has no tail group, was assayed. PEG was ineffective as a chloroquine-sensitizing agent (Table 1). This result, in combination with the low activity of short-head NPEs (e.g., NP7), indicates that both the head and tail portions of NPEs are required for drug reversal activity.
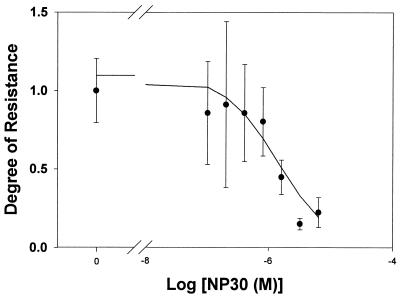
The effect of adding increasing amounts of NP30 on the degree of chloroquine resistance of P. falciparum was determined (Fig. 3). The degree of chloroquine resistance is calculated as the IC50 observed in the presence of various concentrations of NP30 divided by the control (no NPE added) chloroquine IC50 (274 ± 56 nM). Values greater than 1 indicate that the particular NPE rendered the P. falciparum parasites less sensitive to chloroquine, while values less than 1 indicate that the particular NPE sensitized P. falciparum to chloroquine (17). Nonlinear analysis of the chloroquine IC50 at known NP30 concentrations indicated that a concentration of approximately 1 μM (0.0002% on a weight/volume basis) resulted in a 50% decrease in the degree of chloroquine resistance of the parasites. We have examined six other isolates and have found that NP30 is an effective antimalarial, either killing parasites on its own or sensitizing them to chloroquine (data not shown). Separate experiments also indicate that NPEs can sensitize P. falciparum to quinine (degree of resistance, <0.25 at 0.005%) and quinidine (degree of resistance, ∼0.25 at 0.005%), but not to artemisinin.
FIG. 3.
Effect of adding increasing amounts of NP30 on degree of chloroquine resistance of P. falciparum. The degree of chloroquine resistance in isolate 2 is calculated as the IC50 observed in the presence of various concentrations of NP30 divided by the control (no NPE added) chloroquine IC50 (274 ± 56 nM). Values greater than 1 indicate that the NPE rendered the P. falciparum parasites less sensitive to chloroquine, while values less than 1 indicate that the NPE sensitized P. falciparum to chloroquine. Nonlinear analysis of the data points indicates that an NP30 concentration of approximately 1 μM (0.0002% on a weight/volume basis) results in a 50% decrease in the degree of chloroquine resistance of the parasites.
DISCUSSION
Our studies indicate that P. falciparum cultures are far more sensitive to the presence of synthetic surfactants (NPEs) than CHO cells are and that these surfactants interact with cellular components that may include elements involved in chloroquine resistance. The treatment of malaria with a chloroquine-NPE combination could provide at least two benefits.
The first benefit is that NPEs, even in the absence of chloroquine, have antimalarial activity. This finding is not unexpected since NP9 is used as a spermicidal agent, and therefore some of the surfactants can be selectively toxic to some cell types. NPEs are uncharged molecules that should cross membranes easily, and therefore their site(s) of interaction could be located anywhere in the cell. The anti-Plasmodium activity of NPEs may result from the interaction of the surfactant with a specific cellular component, or it may result from an alteration of the permeability of the membranes present in the parasitized erythrocyte (9). It is of interest that P. falciparum cultures are affected by surfactant structures that are larger and more hydrophilic than those that optimally interact with mammalian P glycoproteins (Fig. 2) (14). This suggests that NPEs can be preferentially targeted to the parasite by using longer-head NPEs.
The second benefit of treatment with NPEs is that while NPEs and chloroquine inhibit development of the parasite when used separately, in combination they have synergistic effects that make them potent antimalarials. The maximum synergistic effect between an NPE and chloroquine was observed with an NPE having an average EO content of 30 EO units (Fig. 2C). The similar EO optima and IC50 for the antimalarial activity of the NPE alone and its synergistic interaction with chloroquine suggest that these properties may be related. While it is tempting to compare the effect of NPEs on the drug resistance mechanisms of mammalian cells and Plasmodium, the basis of chloroquine resistance in P. falciparum is still poorly defined and may be multifactorial. Preliminary observations that the activities of some of the other quinoline antimalarials are modulated by NPEs (data not shown) are consistent with the observation that alterations in the Pfmdr protein can affect several quinoline sensitivities (18, 19). However, our data do not directly support the conclusion that NPEs interact with a protein such as Pfmdr, and it is unknown if NPEs directly compete for a chloroquine-binding site on drug transporters involved in malarial drug resistance.
Long-chain NPEs are relatively well tolerated by CHO cells and interact poorly with mammalian P glycoproteins (14). NP9 is the optimal substrate for renal P glycoprotein (its primary route of excretion [6]). This implies that NP30 may be excreted much more slowly than NP9, and the time it spends in circulation is predicted to be longer in the absence of another renal clearance mechanism. Whether NP30 can be maintained at sufficient levels to provide effective chemosensitization to chloroquine is an important issue that will require animal studies. Chloroquine causes irreversible damage to malaria parasites (10), and therefore even transient impaired chloroquine efflux by NPEs may contribute to effective therapy.
The NPEs used in this study are a subset of the commercially available head and tail group combinations of surfactants. They represent a new class of P. falciparum-sensitizing agents, since they are uncharged molecules that do not have the requisite nitrogen atom in their structure (4). Commercial preparations of NPEs are synthesized by the copolymerization of ethylene oxide with NP (23). Such preparations are polydisperse mixtures of surfactants consisting of molecules with a common tail hydrophobe (NP) and a range of EO hydrophile head lengths. Further separation of polydisperse NPE preparations into fractions with uniform head group lengths will allow us to further define the optimal head group length (EO number) and antimalarial activity. An examination of other types of surfactants will allow us to determine if ones with other head and/or tail groups are also active.
White and colleagues (24) have recently argued that the loss of cheap and effective antimalarials to resistance “may represent the single most important threat to the health of people in tropical countries.” It is possible that the life of antimalarials such as chloroquine could be significantly extended by combining them with resistance-reversing or sensitizing agents, like NPEs. NPEs have several desirable features that make them well suited as sensitizing agents: (i) they are inexpensive enough to be used in developing countries, (ii) they are as stable as chloroquine itself and require no special storage conditions, (iii) they may promote the uptake of chloroquine and inhibit its excretion, and (iv) they do not require the introduction of pharmacological agents with undesirable side effects. With further development, the combination of chloroquine and synthetic surfactants to treat drug-resistant P. falciparum may be an effective solution to the current malaria crisis in Africa and elsewhere.
REFERENCES
- 1.Basco L K, Le Bras J. In vitro activities of chloroquine in combination with chlorpromazine or prochlorperazine against isolates of Plasmodium falciparum. Antimicrob Agents Chemother. 1992;36:209–213. doi: 10.1128/aac.36.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basco L K, Le Bras J. In vitro reversal of chloroquine resistance with chlorpheniramine against African isolates of Plasmodium falciparum. Jpn J Med Sci Biol. 1994;47:59–63. doi: 10.7883/yoken1952.47.59. [DOI] [PubMed] [Google Scholar]
- 3.Bray P G, Mungthin M, Ridley R G, Ward S A. Access to hematin: the basis of chloroquine resistance. Mol Pharmacol. 1998;54:170–179. doi: 10.1124/mol.54.1.170. [DOI] [PubMed] [Google Scholar]
- 4.Bray P G, Ward S A. A comparison of the phenomenology and genetics of multidrug resistance in cancer cells and quinoline resistance in Plasmodium falciparum. Pharmacol Ther. 1998;77:1–28. doi: 10.1016/s0163-7258(97)00083-1. [DOI] [PubMed] [Google Scholar]
- 5.Campling B G, Pym J, Galbraith P R, Cole S P. Use of the MTT assay for rapid determination of chemosensitivity of human leukemic blast cells. Leuk Res. 1988;12:823–831. doi: 10.1016/0145-2126(88)90036-7. [DOI] [PubMed] [Google Scholar]
- 6.Charuk J H, Grey A A, Reithmeier R A. Identification of the synthetic surfactant nonylphenol ethoxylate: a P-glycoprotein substrate in human urine. Am J Physiol. 1998;274:F1127–F1139. doi: 10.1152/ajprenal.1998.274.6.F1127. [DOI] [PubMed] [Google Scholar]
- 7.Charuk J H, Wong P Y, Reithmeier R A. Differential interaction of human renal P-glycoprotein with various metabolites and analogues of cyclosporin A. Am J Physiol. 1995;269:F31–F39. doi: 10.1152/ajprenal.1995.269.1.F31. [DOI] [PubMed] [Google Scholar]
- 8.Dolan S A, Herrfeldt J A, Wellems T E. Restriction polymorphisms and fingerprint patterns from an interspersed repetitive element of Plasmodium falciparum DNA. Mol Biochem Parasitol. 1993;61:137–142. doi: 10.1016/0166-6851(93)90166-u. [DOI] [PubMed] [Google Scholar]
- 9.Drori S, Eytan G D, Assaraf Y G. Potentiation of anticancer-drug cytotoxicity by multidrug-resistance chemosensitizers involves alterations in membrane fluidity leading to increased membrane permeability. Eur J Biochem. 1995;228:1020–1029. doi: 10.1111/j.1432-1033.1995.tb20352.x. [DOI] [PubMed] [Google Scholar]
- 10.Foley M, Tilley L. Quinoline antimalarials: mechanisms of action and resistance and prospects for new agents. Pharmacol Ther. 1998;79:55–87. doi: 10.1016/s0163-7258(98)00012-6. [DOI] [PubMed] [Google Scholar]
- 11.Kain K C, Harrington M A, Tennyson S, Keystone J S. Imported malaria: prospective analysis of problems in diagnosis and management. Clin Infect Dis. 1998;27:142–149. doi: 10.1086/514616. [DOI] [PubMed] [Google Scholar]
- 12.Knaak J, Eldridge J, Sullivan L. Excretion of certain polyethylene glycol ether adducts of nonylphenol by the rat. Toxicol Appl Pharmacol. 1966;9:331–340. doi: 10.1016/0041-008x(66)90129-3. [DOI] [PubMed] [Google Scholar]
- 13.Larson P, Borzelleca J, Bowman E, Crawford E, Smith J, Hennigar G. Toxicological studies on a preparation of p-tertiary octylphenoxy-polyethyl ethanols (Triton X-405) Toxicol Appl Pharmacol. 1963;5:782–789. doi: 10.1016/0041-008x(63)90070-x. [DOI] [PubMed] [Google Scholar]
- 14.Loe D W, Sharom F J. Interaction of multidrug-resistant Chinese hamster ovary cells with amphiphiles. Br J Cancer. 1993;68:342–351. doi: 10.1038/bjc.1993.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marsh K. Malaria disaster in Africa. Lancet. 1998;352:924. doi: 10.1016/S0140-6736(05)61510-3. [DOI] [PubMed] [Google Scholar]
- 16.Martin S, Oduola A, Milhous W. Reversal of chloroquine resistance in Plasmodium falciparum by verapamil. Science. 1987;235:899–901. doi: 10.1126/science.3544220. [DOI] [PubMed] [Google Scholar]
- 17.Oduola A M, Sowunmi A, Milhous W K, Brewer T G, Kyle D E, Gerena L, Rossan R N, Salako L A, Schuster B G. In vitro and in vivo reversal of chloroquine resistance in Plasmodium falciparum with promethazine. Am J Trop Med Hyg. 1998;58:625–629. doi: 10.4269/ajtmh.1998.58.625. [DOI] [PubMed] [Google Scholar]
- 18.Price R, Robinson G, Brockman A, Cowman A, Krishna S. Assessment of pfmdr 1 gene copy number by tandem competitive polymerase chain reaction. Mol Biochem Parasitol. 1997;85:161–169. doi: 10.1016/s0166-6851(96)02822-8. [DOI] [PubMed] [Google Scholar]
- 19.Reed M B, Saliba K J, Caruana S R, Kirk K, Cowman A F. Pgh1 modulates sensitivity and resistance to multiple antimalarials in Plasmodium falciparum. Nature. 2000;403:906–909. doi: 10.1038/35002615. [DOI] [PubMed] [Google Scholar]
- 20.Su X, Kirkman L A, Fujioka H, Wellems T E. Complex polymorphisms in an approximately 330-kDa protein are linked to chloroquine-resistant P. falciparum in Southeast Asia and Africa. Cell. 1997;91:593–603. doi: 10.1016/s0092-8674(00)80447-x. [DOI] [PubMed] [Google Scholar]
- 21.Swenson E S, Milisen W B, Curatolo W. Intestinal permeability enhancement: structure-activity and structure-toxicity relationships for nonylphenoxypolyoxyethylene surfactant permeability enhancers. Pharm Res. 1994;11:1501–1504. doi: 10.1023/a:1018920728800. [DOI] [PubMed] [Google Scholar]
- 22.Trager W, Jensen J. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 23.Weinheimer R, Varineau P. Polyoxyethylene alkylphenols. In: Van Os N, editor. Nonionic surfactants: organic chemistry. New York, N.Y: Marcel Dekker, Inc.; 1998. pp. 39–85. [Google Scholar]
- 24.White N J, Nosten F, Looareesuwan S, Watkins W M, Marsh K, Snow R W, Kokwaro G, Ouma J, Hien T T, Molyneux M E, Taylor T E, Newbold C I, Ruebush II T K, Danis M, Greenwood B M, Anderson R M, Olliaro P. Averting a malaria disaster. Lancet. 1999;353:1965–1967. doi: 10.1016/s0140-6736(98)07367-x. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization. In vitro micro-test (mark III) for the assessment of the response of Plasmodium falciparum to chloroquine, mefloquine, quinine, amodiaquine, sulfadoxine/pyrimethamine and artemisinin. Geneva, Switzerland: World Health Organization; 1997. [Google Scholar]
- 26.Zhong K J Y, Kain K C. Evaluation of a colorimetric PCR-based assay to diagnose Plasmodium falciparum malaria in travelers. J Clin Microbiol. 1999;37:339–341. doi: 10.1128/jcm.37.2.339-341.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]