Abstract
Background
Concerns regarding the autoimmune safety of COVID-19 vaccines may negatively impact vaccine uptake. We aimed to describe the incidence of autoimmune conditions following BNT162b2 and CoronaVac vaccination and compare these with age-standardized incidence rates in non-vaccinated individuals.
Methods
This is a descriptive cohort study conducted in public healthcare service settings. Territory-wide longitudinal electronic medical records of Hong Kong Hospital Authority users (≥16 years) were linked with COVID-19 vaccination records between February 23, 2021 and June 30, 2021. We classified participants into first/second dose BNT162b2 groups, first/second dose CoronaVac groups and non-vaccinated individuals for incidence comparison. The study outcomes include hospitalized autoimmune diseases (16 types of immune-mediated diseases across six body systems) within 28 days after first and second dose of vaccination. Age-standardized incidence rate ratios (IRRs) with exact 95% confidence intervals (CIs) were estimated using Poisson distribution.
Results
This study included around 3.9 million Hong Kong residents, of which 1,122,793 received at least one dose of vaccine (BNT162b2: 579,998; CoronaVac: 542,795), and 721,588 completed two doses (BNT162b2: 388,881; CoronaVac: 332,707). Within 28 days following vaccination, cumulative incidences for all autoimmune conditions were below 9 per 100,000 persons, for both vaccines and both doses. None of the age-standardized incidence rates were significantly higher than the non-vaccinated individuals, except for an observed increased incidence of hypersomnia following the first dose of BNT162b2 (standardized IRR: 1.47; 95% CI: 1.10–1.94).
Conclusions
Autoimmune conditions requiring hospital care are rare following mRNA and inactivated COVID-19 vaccination with similar incidence to non-vaccinated individuals. The association between first dose BNT162b2 vaccination and immune-related sleeping disorders requires further research. Population-based robust safety surveillance is essential to detect rare and unexpected vaccine safety events.
Keywords: COVID-19 vaccines, mRNA vaccine, Inactivated virus vaccine, Autoimmune diseases, Pharmacovigilance
1. Introduction
There has been a long-standing debate about vaccination and its potential to trigger autoimmune diseases (AID). For example, the influenza vaccine and Guillain-Barré syndrome [1], the Measles-Mumps-Rubella vaccine and thrombocytopenic purpura [2]. Although causality is not fully established, the fear of new onset or recurrence of AID may contribute to vaccine hesitancy and negatively impact vaccine uptake [3]. Currently, several vaccines using different technology platforms (mRNA, inactivated virus, protein subunit and viral vector) have been developed and authorized for emergency use against COVID-19 worldwide [4]. The characteristics of these vaccine platforms could result in different levels of neutralizing antibodies, T-cell response, and avoidance/occurrence of immune-mediated diseases.
Autoimmune safety events reported in Phase II/III trials, such as Bell's Palsy in mRNA vaccine (Moderna) [5] and transverse myelitis in viral vector vaccine (AstraZeneca (ChAdOx1)) [6], were uncommon and have been labeled in the prescribing information. However, since the launch of global vaccination against COVID-19, several case reports and analyses have described autoimmune conditions following vaccination. In a prospective cohort analysis, Simpson et al. implied an association between viral vector vaccine (AstraZeneca (ChAdOx1)) and the development of immune thrombocytopenia [7]. Wan et al. suggested the safety signal of Bell's palsy with inactivated vaccine (CoronaVac) in a nested case-control and case series study [8]. Case reports or case series of Guillain-Barré syndrome [[9], [10], [11], [12]], cutaneous vasculitis [13], reactive arthritis [14] and immune-medicated disease flare [14] from both mRNA and inactivated virus platforms are continuously emerging, and the list is not exclusive. Given the ongoing COVID-19 pandemic and the likelihood of routine vaccination for infection control, it is of timely importance to assess the autoimmune safety of COVID-19 vaccines in real-world settings.
Hong Kong (HK) is among the few jurisdictions in the world that have approved the emergency use of COVID-19 vaccines from two different technology platforms: inactivated virus vaccine, CoronaVac, from Sinovac Biotech (HK) Limited and mRNA vaccine, BNT162b2, from Fosun-BioNTech (equivalent to Pfizer-BioNTech). The rollout of the public-funded mass vaccination program began with CoronaVac on February 23, 2021 and was closely followed by BNT162b2 on March 12, 2021. By June 30, 2021, more than 3.73 million doses of COVID-19 vaccine had been administered alongside the implementation of population-based surveillance program for safety monitoring. In this study, we analyzed the territory-wide electronic medical records (EMRs) database and summarized the incidence of AID across the predefined disease spectrum among CoronaVac and BNT162b2 recipients in HK. We assessed the population-level risk of AID following mRNA and inactivated virus COVID-19 vaccination to inform vaccination decisions.
2. Methods
2.1. Data sources
We obtained population-based EMRs from the Hospital Authority (HA) and vaccination records from the Department of Health (DH) of HK Government to conduct this study. The HA manages all public hospitals and clinics in HK and provides publicly funded health services to all eligible HK residents (>7 million). The EMRs database managed by HA is centralized from the territory-wide clinical management system of 42 public hospitals with high population coverage, representativeness, and coding accuracy [[15], [16], [17], [18]]. DH, the government health adviser and agency that executes health-related policies and statutory functions, manages COVID-19 vaccination records of all HK residents. EMRs from the HA were linked with the DH vaccination records using de-identified non-reversible pseudo-ID to protect patient privacy. The database has been used for several COVID-19 vaccine safety assessment in HK [8,[19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29]].
2.2. Study design and population
This was a population-based descriptive cohort study. The study population consisted of all Hong Kong residents ≥16 years who had ever used the HA service (emergency admission, general or specialist outpatient clinic visit, or hospital admission) between January 1, 2018 and June 30, 2021. DH vaccination records between February 23 and June 30, 2021 were linked to the HA database to confirm vaccination status. Following the vaccination record linkage, we generated pseudo index dates for the non-vaccinated individuals to facilitate cohort follow-up. Within each age and sex group, we matched each vaccine recipient with non-vaccinated individuals at maximum ratio (the number of vaccine recipients divided by the number of non-vaccinated individuals in each age-sex strata) and assigned the vaccination date as the pseudo index date for the non-vaccinated individuals. We treated first dose and second dose as independent episodes and conducted the non-vaccinated group matching separately.
We followed up these individuals from the index date (date of first dose vaccination, date of second dose vaccination, or age-sex matched pseudo index date for non-vaccinated individuals) to the date of event of interest, date of registered death, 28 days after index date, or the end date of data availability (June 30, 2021), whichever was earlier. To differentiate whether the event was completely related to the first dose, in the first dose analysis, we also censored the follow-up one day before the second dose, if any. The record linkage, matching procedure and cohort follow-up is illustrated in Supplementary Fig. 1.
2.3. Outcome measures
We assessed the incidence of hospital admission related to a spectrum of 16 pre-specified AID, grouped by body systems including: 1) immune-mediated cardiovascular diseases (Kawasaki disease, single organ cutaneous vasculitis); 2) organ specific immune-mediated endocrine disorder (subacute thyroiditis); 3) immune-mediated hematological diseases (anti-phospholipid antibody syndrome, idiopathic thrombocytopenia); 4) immune-mediated multisystem diseases (Sjogren syndrome, systemic lupus erythematosus); 5) immune-mediated musculoskeletal diseases (acute aseptic arthritis, reactive arthritis, rheumatoid arthritis, psoriatic arthritis, spondyloarthritis); and 6) immune-mediated neurological disorders (acute disseminated encephalomyelitis, Guillain-Barré syndrome, narcolepsy and related disorders, and transverse myelitis). We used the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes for outcomes identification (Supplementary Table 1). The selection of autoimmune conditions was based on 1) the list of adverse events of special interests (AESI) according to the World Health Organization's Global Advisory Committee on Vaccine Safety (GACVS) [30]; 2) Brighton Collaboration Safety Platform for Emergency vACcines (SPEAC) [31]; 3) consultation with a local immunologist about the other immune-related AESI that might be of concern. All these diseases were pre-defined before analysis without the influence of the availability of ICD-9-CM diagnostic codes. We defined each individual outcome as an incident case if this was the patient's first relevant diagnosis (primary diagnosis) in the inpatient setting during the 28 days of follow-up. Patients with a diagnosis history within 365 days before the index date were excluded from the study cohort. The same procedure was conducted for first dose, second dose and non-vaccinated groups.
2.4. Main analysis
We reported two age-standardized health situation measurements as the main outcome of this study. First, we described the 28-day cumulative incidence (per 100,000 persons) of each condition, using 5-year age band weightings (16–19, 20–24, 25–29, 30–34, 35–39, 40–44, 45–49, 50–54, 55–59,60–64, 65–69, 70–74, 75–79, 80–84 and ≥ 85 years) based on the HK 2021 mid-year population census. Second, to account for the follow-up time differences among groups, we reported the age-standardized incidence rate (per 100,000 person-years) using the indirect standardization method. We treated the non-vaccinated group as an internal reference group and calculated the proportion of follow-up time (in person-years) in each age strata (16–19, 20–24, 25–29, 30–34, 35–39, 40–44, 45–49, 50–54, 55–59, 60–64, 65–69, 70–74, 75–79, 80–84 and ≥ 85 years) for incidence standardization. For each condition, we used observed number of cases, the follow-up time and proportion of person-years in each age strata to calculate age-specific crude incidence rate and aggregated the age-standardized incidence rate. We further estimated the expected cases in each group and calculated the standardized incidence rate ratios (IRRs) with exact 95% confidence intervals (CIs) using Poisson distribution [32,33].
2.5. Additional analyses
We conducted several additional analyses. First, in order to exclude the possibility that the autoimmune conditions were related to SARS-CoV-2 infection, we removed individuals who had positive SARS-CoV-2 PCR tests prior to June 30, 2021 and replicated the main analysis. Until Omicron variant began to spread in HK, PCR test was the gold standard for official diagnosis of COVID-19 and all suspected cases were required to undergo PCR test to be a confirmed case. Second, considering that some of the autoimmune manifestations, e.g., Guillain-Barré syndrome, may take longer than 28 days to develop, we removed the 28-day follow-up requirement in the second dose analysis and extended the observation period to the end date of data availability. Third, for the estimation of standardized IRRs, we further extended the case definition using both primary and secondary diagnoses at discharge, in consideration of potential different coding practices by clinicians. Fourth, given autoimmune diseases are more prevalent in females, we conducted the sex-stratified analysis to investigate the potential disease incidence differences between males and females. In addition, we conducted an ecological study to complement the main analysis. We assumed that the COVID-19 vaccination program (implemented from March to June 2021) will not impact the natural occurrence of AID at the population level and the incidence would be stable over the years within the study cohort. Hence, we illustrated a four-month (March–June) cumulative incidence trend for each AID between 2018 and 2021. The observed number of incident cases were divided by the total number of HA active patients (≥16 years) in the corresponding year to calculate the cumulative incidence. Age-standardization was also conducted based on the HK 2021 mid-year population census. All analyses were conducted using R 4.0.3 and cross-checked by two authors (LG and XT).
2.6. Ethics
Ethical approval for this study was granted by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (UW 21–149 and UW 21–138); and the Department of Health Ethics Committee (LM 21/2021). Patient identification was anonymized from HA and DH databases and patient consent was not required.
3. Results
We obtained the EMRs of 3,946,550 HA active patients with affirmed vaccination status. After age-sex matching with the non-vaccinated group, 1,122,793 vaccine recipients (BNT162b2: 579,998; CoronaVac: 542,795) were included in the first dose analysis, and 721,588 vaccine recipients (BNT162b2: 388,881; CoronaVac: 332,707) were included in the second dose analysis with follow-up until censoring (Supplementary Fig. 1). 63.0% in the first dose analysis and 71.9% in the second dose analysis had the injection on or before June 3, 2021 with the full 28 days observation from vaccination date to the study end date (June 30, 2021). Compared with non-vaccinated individuals, vaccine recipients were younger, more likely to be males, and less likely to have pre-existing chronic diseases. Detailed baseline characteristics of the study cohorts are summarized in Table 1 .
Table 1.
Baseline characteristics and pre-existing comorbidities of study cohorts.
| First dose |
Second dose |
|||||||
|---|---|---|---|---|---|---|---|---|
| None |
BNT162b2 |
CoronaVac |
SMD |
None |
BNT162b2 |
CoronaVac |
SMD |
|
| N | 2816133 | 579998 | 542795 | – | 1892783 | 388881 | 332707 | – |
| Male (%) | 1183236 (42.0) | 275965 (47.6) | 266681 (49.1) | 0.095 | 831868 (43.9) | 190562 (49.0) | 175487 (52.7) | 0.118 |
| Age (mean (SD)) | 55.67 (19.17) | 46.72 (15.06) | 54.01 (13.85) | 0.374 | 56.81 (19.27) | 47.49 (14.88) | 54.67 (13.97) | 0.389 |
| Charlson Comorbidity Index (mean (SD)) | 0.35 (0.91) | 0.10 (0.41) | 0.15 (0.49) | 0.250 | 0.37 (0.93) | 0.10 (0.41) | 0.15 (0.49) | 0.263 |
| Myocardial infarction (%) | 16935 (0.6) | 617 (0.1) | 891 (0.2) | 0.057 | 12229 (0.6) | 418 (0.1) | 548 (0.2) | 0.060 |
| Congestive Heart Failure (%) | 32695 (1.2) | 425 (0.1) | 912 (0.2) | 0.096 | 24414 (1.3) | 302 (0.1) | 581 (0.2) | 0.102 |
| Peripheral vascular disease (%) | 7525 (0.3) | 249 (0.0) | 362 (0.1) | 0.039 | 5631 (0.3) | 175 (0.0) | 240 (0.1) | 0.042 |
| Cerebrovascular disease (%) | 111165 (3.9) | 4309 (0.7) | 7350 (1.4) | 0.145 | 80569 (4.3) | 2964 (0.8) | 4611 (1.4) | 0.153 |
| Stroke or systemic embolism (%) | 37828 (1.3) | 1226 (0.2) | 2197 (0.4) | 0.088 | 27494 (1.5) | 852 (0.2) | 1360 (0.4) | 0.093 |
| Asthma (%) | 27317 (1.0) | 3665 (0.6) | 3309 (0.6) | 0.027 | 18798 (1.0) | 2468 (0.6) | 2043 (0.6) | 0.028 |
| Chronic obstructive pulmonary disease (%) | 58132 (2.1) | 4778 (0.8) | 5343 (1.0) | 0.070 | 41664 (2.2) | 3244 (0.8) | 3365 (1.0) | 0.075 |
| Dementia (%) | 11122 (0.4) | 103 (0.0) | 149 (0.0) | 0.057 | 8477 (0.4) | 69 (0.0) | 105 (0.0) | 0.061 |
| Diabetes without chronic complication (%) | 351241 (12.5) | 27787 (4.8) | 41633 (7.7) | 0.185 | 245849 (13.0) | 18561 (4.8) | 25251 (7.6) | 0.196 |
| Diabetes with chronic complication (%) | 20940 (0.7) | 871 (0.2) | 1302 (0.2) | 0.060 | 14766 (0.8) | 594 (0.2) | 795 (0.2) | 0.063 |
| Chronic renal failure (%) | 38702 (1.4) | 1155 (0.2) | 2048 (0.4) | 0.091 | 28288 (1.5) | 778 (0.2) | 1297 (0.4) | 0.097 |
| Mild liver disease (%) | 4056 (0.1) | 231 (0.0) | 324 (0.1) | 0.023 | 2846 (0.2) | 158 (0.0) | 188 (0.1) | 0.024 |
| Moderate-severe liver disease (%) | 2871 (0.1) | 141 (0.0) | 188 (0.0) | 0.021 | 1980 (0.1) | 95 (0.0) | 108 (0.0) | 0.021 |
| Rheumatoid arthritis and other inflammatory polyarthropathies (%) | 10584 (0.4) | 813 (0.1) | 873 (0.2) | 0.031 | 7010 (0.4) | 479 (0.1) | 456 (0.1) | 0.033 |
| Malignancy (%) | 75657 (2.7) | 4682 (0.8) | 5220 (1.0) | 0.096 | 52132 (2.8) | 3054 (0.8) | 3107 (0.9) | 0.100 |
| Metastatic solid tumor (%) | 14757 (0.5) | 348 (0.1) | 418 (0.1) | 0.058 | 10256 (0.5) | 226 (0.1) | 234 (0.1) | 0.060 |
Note: P-values for vaccinated and non-vaccinated groups comparison all <0.001. Abbreviation: SMD, standard mean difference (defined as the average of SMD among unvaccinated, CoronaVac and BNT162b2).
3.1. Main analysis
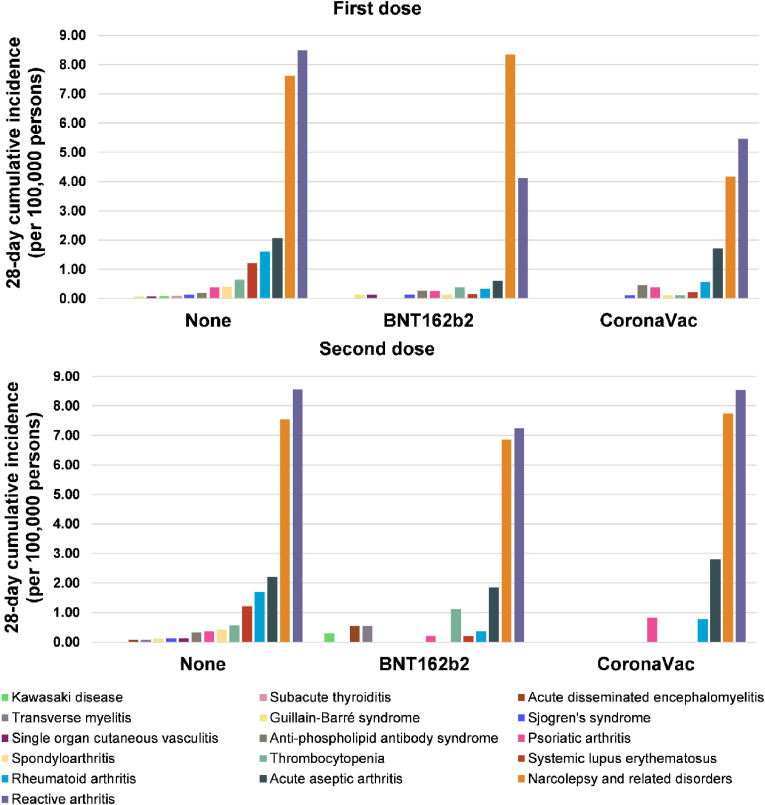
Within 28 days after the index date, autoimmune conditions requiring hospitalization were generally rare (cumulative incidence all below 9 per 100,000 persons) among vaccine recipients and non-vaccinated individuals (Fig. 1 ). After the first dose, most of the analyzed immune-mediated cardiovascular, endocrine, hematological, nervous, and multisystem diseases had a cumulative incidence lower than 1 per 100,000 persons for both BNT162b2 and CoronaVac recipients, translating to fewer than 10 cases per 1 million of first dose administration (Table 2 ). Several autoimmune conditions following immunization are extremely rare and zero cases were recorded in our database for acute disseminated encephalomyelitis, Kawasaki disease, subacute thyroiditis, and transverse myelitis for BNT162b2 and CoronaVac and also Guillain-Barré syndrome, single organ cutaneous vasculitis for CoronaVac. The incidence of reactive arthritis and narcolepsy-related disorders were relatively higher compared to other AIDs, with cumulative incidence ranging between 4.12 and 8.35 per 100,000 persons among first dose vaccine recipients (Table 2). Similarly, the majority of the autoimmune conditions following the second dose also had a 28-day cumulative incidence of less than 1 per 100,000 persons (Fig. 1). Reactive arthritis, narcolepsy-related disorders and thrombocytopenia appeared to have a numerically higher incidence than the corresponding incidence reported after the first dose.
Fig. 1.
Cumulative incidence of hospitalized autoimmune diseases among vaccine recipients and non-vaccinated individuals.
Table 2.
28-day cumulative incidence (per 100,000 persons) of autoimmune conditions among vaccine recipients and non-vaccinated individuals.
| Body system | Disease | First dose |
Second dose |
||||
|---|---|---|---|---|---|---|---|
| None | BNT162b2 | CoronaVac | None | BNT162b2 | CoronaVac | ||
| Cardiovascular system | Kawasaki disease | 0.080 | 0.00 | 0.00 | 0.00 | 0.29 | 0.00 |
| Single organ cutaneous vasculitis | 0.066 | 0.13 | 0.00 | 0.12 | 0.00 | 0.00 | |
| Endocrine system | Subacute thyroiditis | 0.090 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Hematological system | Anti-phospholipid antibody syndrome | 0.19 | 0.27 | 0.46 | 0.32 | 0.00 | 0.00 |
| Thrombocytopenia | 0.64 | 0.39 | 0.11 | 0.57 | 1.11 | 0.00 | |
| Multisystem | Sjogren's syndrome | 0.13 | 0.14 | 0.11 | 0.12 | 0.00 | 0.00 |
| Systemic lupus erythematosus | 1.21 | 0.15 | 0.22 | 1.21 | 0.20 | 0.00 | |
| Musculoskeletal system | Acute aseptic arthritis | 2.07 | 0.61 | 1.71 | 2.21 | 1.85 | 2.80 |
| Reactive arthritis | 8.49 | 4.12 | 5.46 | 8.56 | 7.24 | 8.53 | |
| Rheumatoid arthritis | 1.60 | 0.34 | 0.57 | 1.69 | 0.36 | 0.77 | |
| Psoriatic arthritis | 0.38 | 0.26 | 0.39 | 0.37 | 0.20 | 0.82 | |
| Spondyloarthritis | 0.40 | 0.14 | 0.12 | 0.42 | 0.00 | 0.00 | |
| Nervous system | Acute disseminated encephalomyelitis | 0.00 | 0.00 | 0.00 | 0.071 | 0.55 | 0.00 |
| Guillain-Barré syndrome | 0.062 | 0.14 | 0.00 | 0.10 | 0.00 | 0.00 | |
| Narcolepsy and related disorders | 7.62 | 8.35 | 4.17 | 7.55 | 6.86 | 7.74 | |
| Transverse myelitis | 0.00 | 0.00 | 0.00 | 0.07 | 0.55 | 0.00 | |
| All-cause death | Death | 60.47 | 3.29 | 4.54 | 63.18 | 6.83 | 3.98 |
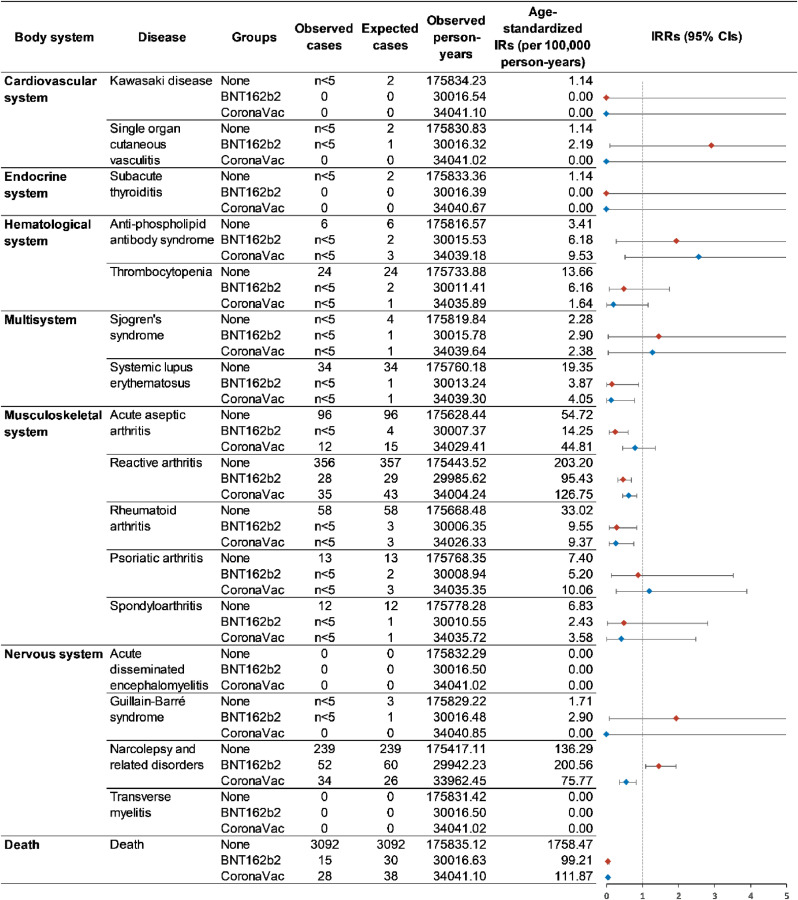
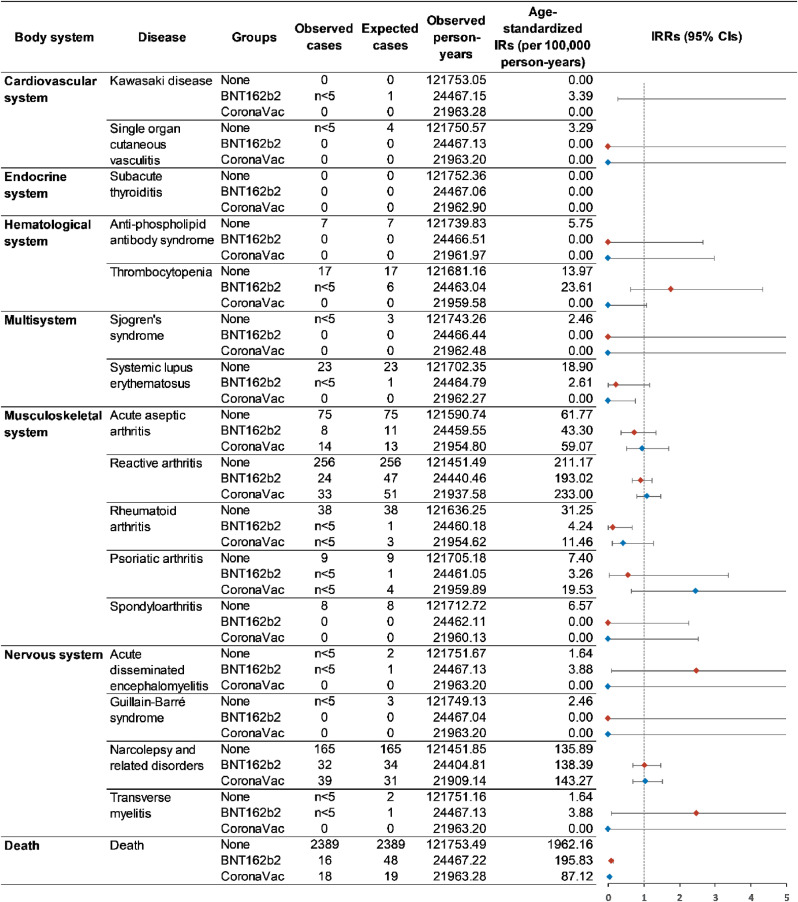
When considering age and follow-up time into the measurement of disease occurrence using standardized incidence rate, we did not detect a significantly increased incidence rate of all the interested autoimmune conditions among the vaccine recipients (Fig. 2 first dose analysis; Fig. 3 second dose analysis), except narcolepsy and related disorders among first dose BNT162b2 recipients (standardized incidence: 220.56 versus 136.29 per 100,000 person-years; standardized IRR: 1.47; 95% CI: 1.10–1.94). The age-standardized all-cause mortality was considerably lower in the vaccine groups, suggesting that individuals who received the vaccine might be healthier than the non-vaccinated.
Fig. 2.
Standardized incidence rates of autoimmune conditions among first dose vaccine recipients and non-vaccinated individuals.
Fig. 3.
Standardized incidence rates of autoimmune conditions among second dose vaccine recipients and non-vaccinated individuals.
3.2. Additional analyses
In total, we removed 10,185 individuals who had previous SARS-CoV-2 infection before our study end date, and the results were consistent with the main analysis (Supplementary Figs. 2 and 3). After removing the 28-day follow-up time restriction in the second dose analysis, there were more autoimmune conditions with non-significant IRRs greater than one, including Sjogren's syndrome, acute disseminated encephalomyelitis, and transverse myelitis in both BNT162b2 and CoronaVac groups, psoriatic arthritis in CoronaVac group, and narcolepsy and related disorders in the BNT162b2 group (Supplementary Fig. 4). When using both primary and secondary diagnoses for case definition, the standardized IRRs for all disease groups yielded consistent results as the main analysis (Supplementary Figs. 5 and 6). The tendency for increased risk of narcolepsy and related disorder among first dose BNT162b2 recipients remained but became non-significant (standardized incidence: 242.26 versus 197.88 per 100,000 person-years standardized IRR: 1.23; 95% CI: 0.95–1.58). In general, we did not find significant differences between males and females (Supplementary Table 2 and Supplementary Table 3), but the observed tendency of increased incidence of thrombocytopenia after the second dose of BNT162b2 in the main analysis became prominent in females. In the ecological analysis of 3.9 million HA active patients, we observed a relatively stable trend of a four-month cumulative incidence between 2018 and 2021 for all autoimmune conditions analyzed (Supplementary Fig. 7).
4. Discussion
HK is among the ten jurisdictions in the world implementing both mRNA and inactivated virus vaccines for emergency use, which allows us to analyze the autoimmune safety performance of the two technology platforms in one population cohort. In this territory-wide descriptive cohort study following more than 1 million people with BNT162b2 and CoronaVac vaccination in HK, we found that autoimmune conditions that need inpatient management are rare, with a comparable incidence rate with non-vaccinated individuals. Under the circumstance of emerging case reports on autoimmune conditions following COVID-19 vaccination, this study provides population-level perspectives on autoimmune safety, with the potential to enhance vaccine confidence among the public.
Among the autoimmune conditions analyzed in this study, published case reports focused mainly on Guillain-Barré syndrome, immune thrombocytopenia, and cutaneous vasculitis [7,[9], [10], [11], [12], [13],34]. Because of the low incidence, the observed number of these three conditions are all less than 5 among our 1.1 million vaccine recipient records. Within 28 days after vaccination, most of the standardized incidence rates were not significantly higher than in non-vaccinated individuals, and we were unable to detect major safety signals for these conditions for both vaccines. In the additional analysis that removed the requirement of 28 days follow-up, we observed more diseases with an IRR greater than one although they did not reach significant levels. This might suggest that some autoimmune manifestations may take longer than 28 days to develop and the risk window for vaccine safety monitoring needs thoughtful adjustment. The natural rarity of autoimmune diseases with or without the vaccination proposes a huge challenge for signal detection and association evaluation. The World Health Organization has called for pharmacovigilance preparedness for COVID-19 vaccines in all countries [35]. Our study highlights that, for rare safety events monitoring, it is particularly imperative to establish a population-based, real-time surveillance system through multiple stakeholder collaboration and capacity building. In addition, in line with the existing literature [23,36], we observed a tendency of increased incidence of thrombocytopenia following the second dose of BNT162b2; and the safety signal prominent in females as shown in the age-stratified analysis. A similar observation was reported in the safety study on ChAdOx1-S (Oxford-AstraZeneca COVID-19 vaccine) [37]. The potential sex-specific effect of vaccine-associated thrombocytopenia warrants further investigation.
Similar to the observations from other COVID-19 vaccine safety studies, individuals who received the vaccines are generally healthier with fewer comorbidities than the general population [37,38]. In HK, the rollout of the COVID-19 vaccination program prioritized healthcare workers, personnel maintaining critical public services and care home residents at the initial stage [39]. Four months after the program commencement, 44% of the eligible HK population had already received the first vaccine dose while the uptake in care home residents was only 5%. This possibly explains the significantly lower incidence of systemic lupus erythematosus, reactive arthritis, and rheumatoid arthritis, and most pronouncedly, all-cause mortality, among vaccine recipients found in this study. In addition, Hong Kong maintained a very low mortality rate due to COVID-19 before the recent circulation and pandemic of Omicron. Up to June 30, 2021 (the study end date), the cumulative number of death due to COVID-19 is only 212 out of 11,937 confirmed cases [40], therefore, we anticipate the attribution of death from COVID-19 on the all-cause mortality is minimal. The current mortality analysis mainly indicated the ‘healthy-cohort’ effect from the vaccinated group.
Notably, this observation should not be misinterpreted as the vaccine lowers the risk of these diseases or prevents death. Due to the limited case numbers of each disease in this descriptive cohort study, we considered only age and follow-up time, not other confounding factors, when assessing autoimmune safety following COVID-19 vaccination. It is anticipated that more individuals with well-controlled chronic conditions, including the elderly, will be vaccinated given the government's commitment to achieve optimal vaccine uptake and herd immunity. The number of rare safety outcomes could possibly accumulate, and further analytical epidemiological studies are highly encouraged to evaluate the association with comprehensive covariate adjustment.
With great interest, we observed a potential safety signal of narcolepsy and related disorders following the first dose of BNT162b2. The initial motivation for including narcolepsy in the list of interested AIDs was due to the widely reported increased risk of narcolepsy among Pandemrix (AS03 adjuvanted pandemic A/H1N1 influenza vaccine) recipients in European countries and Canada during the 2009H1N1 pandemic [[41], [42], [43], [44], [45], [46]], which could contribute to vaccine hesitancy against COVID-19 [47,48]. Considering the potential delay and miscoded diagnosis of narcolepsy from inpatient settings [49],we used a series of ICD-9-CM codes (307.4x, 347.xx and 780.5x, Supplementary Table 1) to investigate a broader range of relevant sleeping disorders according to ACCESS - a project funded by the EMA for the safety monitoring of COVID-19 vaccines [50]. When breaking down the ICD-9-CM codes, we found that hypersomnia (ICD-9-CM: 780.53) was predominately coded but no case was coded as narcolepsy (ICD-9-CM: 347). Therefore, the increased incidence may not be attributed to narcolepsy, but mainly associated with hypersomnia, a likely manifestation of fatigue and increased sleep duration commonly reported after the COVID-19 vaccination [38,51,52]. To date, no narcolepsy-related disorder case report is related to COVID-19 vaccination. There is a recent proof-of-concept study using wearable devices found an increased sleeping duration up to four days post-BNT162b2 vaccination [53]. The sleeping signal detected in this study encourages further investigation on the immunogenicity, mechanism and association between mRNA vaccine and sleeping disorders.
With the availability of territory-wide longitudinal EMRs, this study systematically reported the incidence of autoimmune conditions among vaccine recipients and non-vaccinated controls in the same period and within the same health system. This would avoid reporting bias from enhanced surveillance when using the historical background incidence as the control. There are also limitations in this study. Due to limited data sources, our case definition was restricted to autoimmune conditions that were severe enough to warrant hospitalization and inpatient care. Mild, self-limiting autoimmune conditions not requiring inpatient treatment are beyond the scope of this study. Similarly, our EMRs database does not include health information from the private sector, and information on other vaccination which may cause such diseases. Finally, case identification largely depended on ICD-9-CM diagnosis codes, which could cast some uncertainty on coding practices and affirmative diagnosis. However, previous studies using the EMRs database from the HA showed high coding accuracy [[15], [16], [17], [18]] and our additional analyses using both primary and secondary diagnosis would minimize this risk.
5. Conclusions
Within 28 days after BNT162b2 and CoronaVac vaccination, autoimmune conditions requiring hospitalization are rare and similar to disease occurrence among the non-vaccinated population. The association between first dose BNT162b2 vaccination and immune-related sleeping disorders requires further research. Population-based active safety surveillance is essential to detect rare and unexpected adverse events and serves as a useful tool for future hypothesis testing epidemiology studies.
Author statements
Xue Li: Conceptualization, Methodology, Resources, Data Curation, Writing - Original Draft, Writing - Review & Editing, Visualization, Project administration. Le Gao: Conceptualization, Methodology, Software, Formal analysis, Writing - Original Draft, Writing - Review & Editing, Project administration. Xinning Tong: Methodology, Software, Formal analysis, Validation, Writing - Review & Editing. Vivien K.Y. Chan: Formal analysis, Writing - Review & Editing, Project administration. Celine S.L. Chui: Conceptualization, Resources, Data Curation, Writing - Review & Editing, Project administration. Francisco T.T. Lai: Conceptualization, Resources, Data Curation, Writing - Review & Editing, Project administration. Carlos K.H. Wong: Conceptualization, Resources, Data Curation, Writing - Review & Editing, Project administration. Eric Y.F. Wan: Conceptualization, Resources, Data Curation, Writing - Review & Editing, Project administration. Esther W.Y. Chan: Conceptualization, Resources, Data Curation, Writing - Review & Editing. Kui Kai Lau: Investigation, Writing - Review & Editing. Chak Sing Lau: Conceptualization, Methodology, Writing - Review & Editing. Ian C.K. Wong: Conceptualization, Methodology, Resources, Data Curation, Writing - Review & Editing, Supervision, Funding acquisition.
Declaration of competing interest
XL received research grants from Research Fund Secretariat of the Food and Health Bureau (HMRF, HKSAR), Research Grants Council Early Career Scheme RGC/ECS, HKSAR), Janssen and Pfizer; internal funding from the University of Hong Kong; consultancy fee from Merck Sharp & Dohme, unrelated to this work. CSLC has received grants from the Food and Health Bureau of the Hong Kong Government, Hong Kong Research Grant Council, Hong Kong Innovation and Technology Commission, Pfizer, IQVIA, and Amgen; personal fee from Primevigilance Ltd.; outside the submitted work. FTTL has been supported by the RGC Postdoctoral Fellowship under the Hong Kong Research Grants Council and has received research grants from Food and Health Bureau of the Government of the Hong Kong SAR, outside the submitted work. CKHW reports the receipt of Health and Medical Research Fund, Food and Health Bureau, Government of Hong Kong SAR; General Research Fund, Research Grant Council, Government of Hong Kong SAR; EuroQol Research Foundation, all outside the submitted work. EYFW has received research grants from the Food and Health Bureau of the Government of the Hong Kong SAR, and the Hong Kong Research Grant Council, outside the submitted work. EWYC reports honorarium from Hospital Authority, grants from Research Grants Council (RGC, Hong Kong), grants from Research Fund Secretariat of the Food and Health Bureau, grants from National Natural Science Fund of China, grants from Wellcome Trust, grants from Bayer, grants from Bristol-Myers Squibb, grants from Pfizer, grants from Janssen, grants from Amgen, grants from Takeda, grants from Narcotics Division of the Security Bureau of HKSAR, outside the submitted work. KKL reports research funding from the Food and Health Bureau of the Hong Kong Government, Hong Kong Research Grants Council, Hong Kong Innovation and Technology Commission, Boehringer Ingelheim, Eisai and Pfizer, all unrelated to the submitted work. ICKW reports research funding outside the submitted work from Amgen, Bristol-Myers Squibb, Pfizer, Janssen, Bayer, GSK, Novartis, the Hong Kong RGC, and the Hong Kong Health and Medical Research Fund, National Institute for Health Research in England, European Commission, National Health and Medical Research Council in Australia, and also received speaker fees from Janssen and Medice in the previous 3 years. He is also an independent non-executive director of Jacobson Medical in Hong Kong. LG, XT, VKYC, and CSL do not report any competing interests.
Funding Research Grant from the Food and Health Bureau, the Government of the Hong Kong Special Administrative Region (Ref. No. COVID19F01).
Acknowledgments
The project was funded Research Grant from the Food and Health Bureau, The Government of the Hong Kong Special Administrative Region (Ref. No.COVID19F01). LFTT and IW's post were partly funded by D24H; hence this work was partly supported by AIR@InnoHK administered by Innovation and Technology Commission. We thank members of the Expert Committee on Clinical Events Assessment Following COVID-19 Immunization for case assessment and colleagues from the Drug Office of the Department of Health and from the Hospital Authority for providing vaccination and clinical data. We also thank Mr. Kuan Peng for technical support and Ms. Lisa Lam for proofreading the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jaut.2022.102830.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
The authors do not have permission to share data.
References
- 1.Vellozzi C., Iqbal S., Broder K. Guillain-Barre syndrome, influenza, and influenza vaccination: the epidemiologic evidence. Clin. Infect. Dis. 2014;58:1149–1155. doi: 10.1093/cid/ciu005. [DOI] [PubMed] [Google Scholar]
- 2.Andrews N., Stowe J., Miller E., Svanström H., Johansen K., Bonhoeffer J., et al. A collaborative approach to investigating the risk of thrombocytopenic purpura after measles-mumps-rubella vaccination in England and Denmark. Vaccine. 2012;30:3042–3046. doi: 10.1016/j.vaccine.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 3.Dubé E., Vivion M., MacDonald N.E. Vaccine hesitancy, vaccine refusal and the anti-vaccine movement: influence, impact and implications. Expert Rev. Vaccines. 2015;14:99–117. doi: 10.1586/14760584.2015.964212. [DOI] [PubMed] [Google Scholar]
- 4.Creech C.B., Walker S.C., Samuels R.J. SARS-CoV-2 vaccines. JAMA. 2021;325:1318–1320. doi: 10.1001/jama.2021.3199. [DOI] [PubMed] [Google Scholar]
- 5.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simpson C.R., Shi T., Vasileiou E., Katikireddi S.V., Kerr S., Moore E., et al. First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat. Med. 2021;27:1290–1297. doi: 10.1038/s41591-021-01408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wan E.Y.F., Chui C.S.L., Lai F.T.T., Chan E.W.Y., Li X., Yan V.K.C., et al. Bell's palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study. Lancet Infect. Dis. 2022;22:64–72. doi: 10.1016/S1473-3099(21)00451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hasan T., Khan M., Khan F., Hamza G. Case of Guillain-Barré syndrome following COVID-19 vaccine. BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2021-243629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maramattom B.V., Krishnan P., Paul R., Padmanabhan S., Cherukudal Vishnu Nampoothiri S., Syed A.A., et al. Guillain-barre syndrome following ChAdOx1-S/nCoV-19 vaccine. Ann. Neurol. 2021;90:312–314. doi: 10.1002/ana.26143. [DOI] [PubMed] [Google Scholar]
- 11.Ogbebor O., Seth H., Min Z., Bhanot N. Guillain-Barré syndrome following the first dose of SARS-CoV-2 vaccine: a temporal occurrence, not a causal association. IDCases. 2021;24 doi: 10.1016/j.idcr.2021.e01143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finsterer J. Exacerbating guillain–barré syndrome eight days after vector-based COVID-19 vaccination. Case Rep Infect Dis. 2021;2021:3619131. doi: 10.1155/2021/3619131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McMahon D.E., Amerson E., Rosenbach M., Lipoff J.B., Moustafa D., Tyagi A., et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J. Am. Acad. Dermatol. 2021;85:46–55. doi: 10.1016/j.jaad.2021.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watad A., De Marco G., Mahajna H., Druyan A., Eltity M., Hijazi N., et al. Immune-mediated disease flares or new-onset disease in 27 subjects following mRNA/DNA SARS-CoV-2 vaccination. Vaccines. 2021;9:435. doi: 10.3390/vaccines9050435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan E.W., Lau W.C.Y., Leung W.K., Mok M.T.C., He Y., Tong T.S.M., et al. Prevention of dabigatran-related gastrointestinal bleeding with gastroprotective agents: a population-based study. Gastroenterology. 2015;149:586–595. doi: 10.1053/j.gastro.2015.05.002. e3. [DOI] [PubMed] [Google Scholar]
- 16.Lau W.C.Y., Chan E.W., Cheung C.-L., Sing C.W., Man K.K.C., Lip G.Y.H., et al. Association between dabigatran vs warfarin and risk of osteoporotic fractures among patients with nonvalvular atrial fibrillation. JAMA. 2017;317:1151–1158. doi: 10.1001/jama.2017.1363. [DOI] [PubMed] [Google Scholar]
- 17.Man K.K.C., Coghill D., Chan E.W., Lau W.C.Y., Hollis C., Liddle E., et al. Association of risk of suicide attempts with methylphenidate treatment. JAMA Psychiatry. 2017;74:1048–1055. doi: 10.1001/jamapsychiatry.2017.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong A.Y.S., Root A., Douglas I.J., Chui C.S.L., Chan E.W., Ghebremichael-Weldeselassie Y., et al. Cardiovascular outcomes associated with use of clarithromycin: population based study. BMJ. 2016;352:h6926. doi: 10.1136/bmj.h6926. [DOI] [PubMed] [Google Scholar]
- 19.Li X., Tong X., Wong I.C.K., Peng K., Chui C.S.L., Lai F.T.T., et al. Lack of inflammatory bowel disease flare-up following two-dose BNT162b2 vaccine: a population-based cohort study. Gut. 2022 doi: 10.1136/gutjnl-2021-326860. Published Online First: 08 February 2022:gutjnl-2021-326860. [DOI] [PubMed] [Google Scholar]
- 20.Lai F.T.T., Li X., Peng K., Huang L., Ip P., Tong X., et al. Carditis after COVID-19 vaccination with a messenger rna vaccine and an inactivated virus vaccine : a case-control study. Ann. Intern. Med. 2022;175(3):362–370. doi: 10.7326/M21-3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X., Tong X., Yeung W.W.Y., Kuan P., Yum S.H.H., Chui C.S.L., et al. Two-dose COVID-19 vaccination and possible arthritis flare among patients with rheumatoid arthritis in Hong Kong. Ann. Rheum. Dis. 2021;81(4):564–568. doi: 10.1136/annrheumdis-2021-221571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chua G.T., Kwan M.Y.W., Chui C.S.L., Smith R.D., Cheung E.C., Tian T., et al. Epidemiology of acute myocarditis/pericarditis in Hong Kong adolescents following comirnaty vaccination. Clin. Infect. Dis. 2021 doi: 10.1093/cid/ciab989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sing C.W., Tang C.T.L., Chui C.S.L., Fan M., Lai F.T.T., Li X., et al. COVID-19 vaccines and risks of hematological abnormalities: nested case-control and self-controlled case series study. Am. J. Hematol. 2022;97(4):470–480. doi: 10.1002/ajh.26478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai F.T.T., Huang L., Peng K., Li X., Chui C.S.L., Wan E.Y.F., et al. Post-Covid-19-vaccination adverse events and healthcare utilization among individuals with or without previous SARS-CoV-2 infection. J. Intern. Med. 2022 doi: 10.1111/joim.13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wan E.Y.F., Chui C.S.L., Wang Y., Ng V.W.S., Yan V.K.C., Lai F.T.T., et al. Herpes zoster related hospitalization after inactivated (CoronaVac) and mRNA (BNT162b2) SARS-CoV-2 vaccination: a self-controlled case series and nested case-control study. Lancet Reg Health West Pac. 2022;21:100393. doi: 10.1016/j.lanwpc.2022.100393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiong X., Wong C.K.H., Au I.C.H., Lai F.T.T., Li X., Wan E.Y.F., et al. Safety of inactivated and mRNA COVID-19 vaccination among patients treated for hypothyroidism: a population-based cohort study. Thyroid. 2022 doi: 10.1089/thy.2021.0684. [DOI] [PubMed] [Google Scholar]
- 27.Li X., Lai F.T.T., Chua G.T., Kwan M.Y.W., Lau Y.L., Ip P., et al. Myocarditis following COVID-19 BNT162b2 vaccination among adolescents in Hong Kong. JAMA Pediatr. 2022 doi: 10.1001/jamapediatrics.2022.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lai F.T.T., Chua G.T., Chan E.W.W., Huang L., Kwan M.Y.W., Ma T., et al. Adverse events of special interest following the use of BNT162b2 in adolescents: a population-based retrospective cohort study. Emerg. Microb. Infect. 2022;11:885–893. doi: 10.1080/22221751.2022.2050952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong C.K.H., Xiong X., Lau K.T.K., Chui C.S.L., Lai F.T.T., Li X., et al. Impact of a delayed second dose of mRNA vaccine (BNT162b2) and inactivated SARS-CoV-2 vaccine (CoronaVac) on risks of all-cause mortality, emergency department visit, and unscheduled hospitalization. BMC Med. 2022;20:119. doi: 10.1186/s12916-022-02321-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization . World Health Organization; 2020. Background Paper on Covid-19 Disease and Vaccines: Prepared by the Strategic Advisory Group of Experts (SAGE) on Immunization Working Group on COVID-19 Vaccines, 22 December 2020. https://apps.who.int/iris/handle/10665/338095. [Google Scholar]
- 31.Law B. Safety Platform for Emergency vACcines (SPEAC); 2021. SO2-D2. 1.2 Priority List of COVID-19 Adverse Events of Special Interest: Quarterly Update December 2020.https://brightoncollaboration.us/wp-content/uploads/2020/12/SO2_D2.1.2_V1.2_COVID-19_AESI-update-23Dec2020.pdf [Google Scholar]
- 32.Cohen G.R., Yang S.Y. Mid-P confidence intervals for the Poisson expectation. Stat. Med. 1994;13:2189–2203. doi: 10.1002/sim.4780132102. [DOI] [PubMed] [Google Scholar]
- 33.Fay M.P. Two-sided exact tests and matching confidence intervals for discrete data. The R Journal. 2010;2(1):53–58. [Google Scholar]
- 34.Kharkar V., Vishwanath T., Mahajan S., Joshi R., Gole P. Asymmetric cutaneous vasculitis following COVID-19 vaccination with unusual eosinophil preponderance. Clin. Exp. Dermatol. 2021 doi: 10.1111/ced.14797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.The World Health Organization . 2020. Covid-19 Vaccines: Safety Surveillance Manual.https://www.who.int/publications/i/item/10665338400 [Google Scholar]
- 36.Hippisley-Cox J., Patone M., Mei X.W., Saatci D., Dixon S., Khunti K., et al. Risk of thrombocytopenia and thromboembolism after covid-19 vaccination and SARS-CoV-2 positive testing: self-controlled case series study. BMJ. 2021;374:n1931. doi: 10.1136/bmj.n1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pottegård A., Lund L.C., Karlstad Ø., Dahl J., Andersen M., Hallas J., et al. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: population based cohort study. BMJ. 2021;373:n1114. doi: 10.1136/bmj.n1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Menni C., Klaser K., May A., Polidori L., Capdevila J., Louca P., et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect. Dis. 2021;21:939–949. doi: 10.1016/S1473-3099(21)00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.The Government of the Hong Kong Special Administrative Region . 2021. Vaccination Priority Groups to Be Expanded to Cover People Aged 30 or above.https://www.info.gov.hk/gia/general/202103/15/P2021031500626.htm [Google Scholar]
- 40.Centre for Health Protection | Department of Health | The Government of the Hong Kong Special Administrative Region. Latest local situation of COVID-19 (Details of previous cases). https://www.chp.gov.hk/files/xls/previous_cases_covid19_en.xlsx. (accessed 28 March, 2022).
- 41.Gadroen K., Straus S., Pacurariu A., Weibel D., Kurz X., Sturkenboom M. Patterns of spontaneous reports on narcolepsy following administration of pandemic influenza vaccine; a case series of individual case safety reports in Eudravigilance. Vaccine. 2016;34:4892–4897. doi: 10.1016/j.vaccine.2016.08.062. [DOI] [PubMed] [Google Scholar]
- 42.Granath F., Gedeborg R., Smedje H., Feltelius N. Change in risk for narcolepsy over time and impact of definition of onset date following vaccination with AS03 adjuvanted pandemic A/H1N1 influenza vaccine (Pandemrix) during the 2009 H1N1 influenza pandemic. Pharmacoepidemiol. Drug Saf. 2019;28:1045–1053. doi: 10.1002/pds.4788. [DOI] [PubMed] [Google Scholar]
- 43.Montplaisir J., Petit D., Quinn M.J., Ouakki M., Deceuninck G., Desautels A., et al. Risk of narcolepsy associated with inactivated adjuvanted (AS03) A/H1N1 (2009) pandemic influenza vaccine in Quebec. PLoS One. 2014;9 doi: 10.1371/journal.pone.0108489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O'Flanagan D., Barret A.S., Foley M., Cotter S., Bonner C., Crowe C., et al. Investigation of an association between onset of narcolepsy and vaccination with pandemic influenza vaccine, Ireland April 2009-December 2010. Euro Surveill. 2014;19:15–25. [PubMed] [Google Scholar]
- 45.Stowe J., Andrews N., Kosky C., Dennis G., Eriksson S., Hall A., et al. Risk of narcolepsy after AS03 adjuvanted pandemic A/H1N1 2009 influenza vaccine in adults: a case-coverage study in England. Sleep. 2016;39:1051–1057. doi: 10.5665/sleep.5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Winstone A.M., Stellitano L., Verity C., Andrews N., Miller E., Stowe J., et al. Clinical features of narcolepsy in children vaccinated with AS03 adjuvanted pandemic A/H1N1 2009 influenza vaccine in England. Dev. Med. Child Neurol. 2014;56:1117–1123. doi: 10.1111/dmcn.12522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Narcolepsy fiasco spurs Covid vaccine fears in Sweden. 2020; https://medicalxpress.com/news/2020-11-narcolepsy-fiasco-spurs-covid-vaccine.html. (accessed 22 July, 2021).
- 48.Silberner J. Overcoming vaccine hesitancy: five minutes with Heidi Larson. BMJ. 2019;364:l1259. doi: 10.1136/bmj.l1259. [DOI] [PubMed] [Google Scholar]
- 49.Wei Y.T., Lee P.Y., Lin C.Y., Chen H.J., Lin C.C., Wu J.S., et al. Non-alcoholic fatty liver disease among patients with sleep disorders: a Nationwide study of Taiwan. BMC Gastroenterol. 2020;20:32. doi: 10.1186/s12876-020-1178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.VAC4EU COVID-19 vaccine monitoring. 2021; https://vac4eu.org/covid-19-vaccine-monitoring/. (accessed 28 July, 2021).
- 51.Lai F.T.T., Leung M.T.Y., Chan E.W.W., Huang L., Lau L.K.W., Peng K., et al. Self-reported reactogenicity of CoronaVac (Sinovac) compared with Comirnaty (Pfizer-BioNTech): a prospective cohort study with intensive monitoring. Vaccine. 2022;40:1390–1396. doi: 10.1016/j.vaccine.2022.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chan E.W.W., Leung M.T.Y., Lau L.K.W., Leung J., Lum D., Wong R.S., et al. Comparing self-reported reactogenicity between adolescents and adults following the use of BNT162b2 (Pfizer-BioNTech) messenger RNA COVID-19 vaccine: a prospective cohort study. Int. J. Infect. Dis. 2022;116:47–50. doi: 10.1016/j.ijid.2021.12.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hajduczok A.G., DiJoseph K.M., Bent B., Thorp A.K., Mullholand J.B., MacKay S.A., et al. Physiologic response to the pfizer-BioNTech COVID-19 vaccine measured using wearable devices: a prospective observational study. JMIR Form Res. 2021 doi: 10.2196/28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors do not have permission to share data.