Abstract
The ongoing Coronavirus Disease (COVID-19) pandemic has so far affected more than 500 million people. Lingering fatigue and cognitive difficulties are key concerns because they impede productivity and quality of life. However, the prevalence and duration of neurocognitive sequelae and association with functional outcomes after COVID-19 are unclear. This longitudinal study explored the frequency, severity and pattern of cognitive impairment and functional implications 1 year after hospitalisation with COVID-19 and its trajectory from 3 months after hospitalisation. Patients who had been hospitalised with COVID-19 from our previously published 3-months study at the Copenhagen University Hospital were re-invited for a 1-year follow-up assessment of cognitive function, functioning and depression symptoms. Twenty-five of the 29 previously assessed patients (86%) were re-assessed after 1 year (11±2 months). Clinically significant cognitive impairments were identified in 48-56 % of patients depending on the cut-off, with verbal learning and executive function being most severely affected. This was comparable to the frequency of impairments observed after 3 months. Objectively measured cognitive impairments scaled with subjective cognitive difficulties, reduced work capacity and poorer quality of life. Further, cognitive impairments after 3 months were associated with the severity of subsequent depressive symptoms after 1 year. In conclusion, the stable cognitive impairments in approximately half of patients hospitalized with COVID-19 and negative implications for work functioning, quality of life and mood symptoms underline the importance of screening for and addressing cognitive sequelae after severe COVID-19.
Keywords: COVID-19, Cognitive impairment, Depression, quaLity of life
Introduction
The ongoing pandemic caused by the SARS-CoV-2 virus (COVID-19) has so far affected more than 500 million people worldwide. A key concern is the frequent lingering physical, cognitive, neurological or psychiatric symptoms that persist for 12 or more weeks after the recovery from acute illness and are collectively referred to as ‘long-COVID’, or ‘post-COVID syndrome’ (NICE., 2021). While studies have shown that even asymptomatic or mild infection may lead to persistent symptoms (Bliddal et al., 2021; de Graaf et al., 2021), the risk appears to be greater amongst those with severe COVID-19 illness (Nakamura et al., 2021; Schou et al., 2021; Taquet et al., 2021a), older age and greater comorbidity (Ceban et al., 2022). Accordingly, studies indicate that the frequency of long-COVID symptoms is 20-40 % in general (Bliddal et al., 2021; Llach and Vieta, 2021; Logue et al., 2021), but up to 85% for patients who have been hospitalised with COVID-19 (Vejen et al., 2022). Given this high prevalence of lingering symptoms, inter-disciplinary long-COVID clinics have been established to address the needs of these patients.
Long-term cognitive sequalae of COVID-19, also coined cognitive COVID, involve fatigue, ‘brain fog’, memory and concentration difficulties. Meta-analytic evidence indicates that cognitive COVID occurs in 20-30% of people with COVID-19 in general (Ceban et al., 2022) and in around 30% of hospitalized COVID-19 patients (Nakamura et al., 2021; Schou et al., 2021), with executive function, memory, and attention being most affected (Nakamura et al., 2021; Schou et al., 2021). Further, a large-scale internet-based study with > 80,000 patients with or without COVID-19, suggested executive dysfunction of substantial effect size, 6-months after infection in both hospitalized and non-hospitalized patients (Hampshire et al., 2021). Notably, estimates of cognitive impairments varied across the included original studies, likely due to differences in the sensitivity of the implemented cognitive test batteries (including MoCA, MMSE, SCIP, WAIS-IV) (Schou et al., 2021). We found, using a sensitive cognitive screener devoid of ceiling effects in populations with work-related stress (Jensen et al., 2022) or psychiatric conditions (Ott et al., 2021), that 59-65 % of hospitalized patients displayed clinically significant verbal learning and memory, executive function and working memory impairments 3 months after hospital discharge (Miskowiak et al., 2021). The biological mechanisms underlying these cognitive symptoms are likely multifactorial; Acute and ongoing systemic inflammation due to autoimmunity complications, direct viral invasion of the central nervous system due to leakiness of the blood-brain-barrier (Pajo et al., 2021; Proal and VanElzakker, 2021) and cerebral microhaemorrhages, as identified by white matter hyperintensities, may all play a key role (Baldini et al., 2021; Mazza et al., 2021; Moonis et al., 2020; Scardua Silva et al., 2021). From a biopsychosocial perspective, additional contributing factors to cognitive COVID may be the social aspect of having a serious illness, stigma, and isolation and inactivity related to the pandemic (Grover et al., 2021; Sykes et al., 2021).
The duration of cognitive COVID and long-term impact on functioning and quality of life are unclear. One study of hospitalized patients (n = 312) indicated little to no cognitive improvement within the first 6 months, with observations of cognitive impairments in 79% and 75% of patients after 3 months and 6 months, respectively (Poletti et al., 2021). Another longitudinal study found that overall, 47 % of patients had cognitive impairments. Specifically, 21% had memory impairments, 12% had executive dysfunction 1 year after hospitalisation (Méndez et al., 2022). A third study identified concentration difficulties in 31 % of patients 1 year after hospital discharge (Becker et al., 2021). Notably, 45 % of patients in the latter study, who had no prior history of psychiatric illness, reported psychiatric morbidity and poorer quality of life following COVID-19 (Méndez et al., 2022). This is comparable to a large-scale study (n = 236 379), which demonstrated that within 6 months after COVID-19, 38 % of hospitalised patients received a neuropsychiatric diagnosis (14 % with first onset), most commonly depression or anxiety (Taquet et al., 2021b). This co-occurrence of cognitive and psychiatric sequelae of COVID-19 is noteworthy given pre-existing evidence for close links between cognitive and depressive symptoms (Nys et al., 2006; Weiland-Fiedler et al., 2004; Weisenbach et al., 2012). This raises the possibility that these symptoms are interlinked. Indeed, cognitive impairment is a key contributor to functional impairment and mood symptoms across several neuropsychiatric illnesses (Cambridge et al., 2018; Mitchell et al., 2014; Woo et al., 2016), whereas depressive symptoms may also exacerbate cognitive impairments (McDermott and Ebmeier, 2009).
The present longitudinal study of patients hospitalized with COVID-19 aimed to explore (I) the frequency, pattern, and severity of cognitive impairments 1 year after hospitalisation with COVID-19, (II) the trajectory of cognitive impairments from 3 months to 1 year after hospitalisation, (III) the association between cognitive impairments, functioning and quality of life, and (IV) whether cognitive impairments 3 months after hospitalisation are associated with subsequent depression symptoms after 1 year.
Methods
Participants and recruitment
Participants were recruited to take part in a prospective follow-up study ‘IMPACT-COVID’ examining all adult patients (≥ 18 years) admitted with COVID-19 to Bispebjerg Hospital in Denmark from March 2020 until the end of the first wave of COVID-19 cases in July 2020 (Johnsen et al., 2021). COVID-19 diagnosis was confirmed upon hospitalisation based on a positive polymerase chain reaction (PCR) test for SARS-CoV-2 from the upper respiratory tract or a positive IgG titer for COVID-19. Participants were recruited as part of their 3-months post discharge assessment of physical and cognitive functions (see Johnsen et al 2021; Miskowiak et al 2021). Participants were excluded from the study if they presented insufficient Danish language abilities or pre-existing neurological comorbidities. Prior to data collection, regional ethics committee in the Capital Region of Denmark approval was obtained for all study procedures (protocol no. H-20035553). All participants provided informed written consent prior to study enrolment. For the present follow-up study, patients who underwent cognitive screening at their 3-months assessment, were re-invited by telephone call for a re-assessment approximately 1 year after hospital discharge.
Procedure
Patients attended the long-COVID clinic at the Bispebjerg Hospital or the Psychiatric Centre Copenhagen, Rigshospitalet, for a 1-year follow-up assessment of objective (performance-based) and subjective (self-reported) cognitive functions, mood symptoms, quality of life and work function (duration of approximately 1 hour).
Materials
Cognitive function was assessed with the Screen for Cognitive Impairment in Psychiatry Danish Version (SCIP-D) which consists of five subtests: (1) verbal learning (VLT-I), (2) working memory (WMT), (3) verbal fluency (VFT), (4) delayed memory (VLT-D) and (5) processing speed (PST) (Jensen et al., 2015; Purdon, 2005) and Trail Making Test- Part B (TMT-B) (TMT-B; Army Battery, 1944). The SCIP-D exists in 3 parallel forms for repeated testing, and 2 alternate versions were used at the 3-month and 1-year assessments. In addition, subjective cognitive functions were assessed with the Cognitive Failures Questionnaire (CFQ), a self-report inventory comprised of 25 items divided in 3 dimensions perception, memory, and motor function. Items are scored on a 5-point Likert scale (i.e., 0= “never” to 4= “very often”) (Broadbent et al, 1982).
Work functioning was assessed for patients who were currently employed with the Work Productivity and Activity Impairment Questionnaire (WPAI; Reilly et al, 1993), a 6-item self-report questionnaire assessing absenteeism, presenteeism, and activity impairments due to health problems in the past 7 days (e.g., “how many hours did you miss from work because of your health problems”) (WPAI; Reilly et al, 1993). Quality of Life was assessed with the EQ-5D-5L quality of life questionnaire which is comprised of 5 dimensions: self-care, usual activities, pain/discomfort, mobility, and anxiety/depression. (EQ5D; Lloyd and Pickard, 2019). Additionally, the EQ-5D-5L scale “EQ VAS” was included to assess self-rated health. Items were scored on a 5-point Likert scale (EQ5D; Lloyd and Pickard, 2019). Depressive symptoms were rated with the Hamilton Depression Rating scale 17-items (HDRS-17) (Hamilton, 1960).
Statistical analysis
All statistical tests were conducted using IBM SPSS statistics 25 for windows (IBM Corporation, Armonk, New York) with a significance level of α = 0.05 (two-tailed). Prior to statistical analyses relevant assumptions were tested. Regarding normality, appropriate analyses (parametric and non-parametric) tests were used accordingly for normally and non-normally distributed data.
Question (I): What is the frequency, pattern, and severity of cognitive impairments 1 year after hospital discharge?
This research question was examined with two complementary approaches: by comparing patients’ cognitive performance at their 1-year assessment (A) to their individual expected performance calculated with the regression based formulas based on their age, sex, years of education and the normative (expected) cognitive change with repeated testing (i.e., expected performance considering learning effects with repeated testing, as they had been tested previously, 3 months after hospital discharge) (Supplementary Table 1) and (B) to the cognitive performance of an age-, gender- and education-matched sample of 55 health controls (HC) from a pre-established normative data set following repeated testing (Ott et al., 2021).
In approach (A), regression-based formulas were applied for prediction of each patients expected SCIP test scores based on their age, sex, and years of education following repeated testing (Ott et al., 2021). The regression-based formula allows calculation of demographically corrected normative scores, applicable at an individual level (Duff, 2012). This enables precise estimation for each patient of whether their cognition scores deviate or align with the expected scores of a person with matched demographic characteristics. Reliable change indexes (RCI) provide standardized scores for the deviation of the observed scores from the predicted scores, calculated as (observed score – predicted score)/SEE, with SEE reflecting the standard error of the estimate for the regression equation (Attix et al., 2009). These regression-based models have previously been used to determine demographically adjusted norms and normative cognitive change over 1 year (Ott et al., 2021).
The frequency of clinically relevant ‘global cognitive impairment’ was based on a cut-off score defined as performance ≥1 standard deviations (SD) under the expected SCIP-D total score, whereas ‘selective cognitive impairment’ was defined as performance ≥ 1SD under the expected scores on ≥ 2 individual tests in the SCIP-D or TMT-B (Ott et al., 2021) (approach A). The same cut-offs for global and selective impairments were applied when estimating the frequency of clinically relevant cognitive impairments relative to the matched HC sample (approach B). These cut-offs for clinically relevant cognitive impairments were determined based on previous recommendations (Ott et al., 2021; Miskowiak et al., 2018).
Accordingly, the pattern of cognitive impairments was explored through comparisons between: (A) patients’ actual scores on SCIP-D and TMT-B at the 1-year assessment and their expected scores, using independent samples t-tests. For the TMT-B, normative change scores are not available. We therefore compared patients TMT-B scores with the normative cross-sectional TMT-B norms, based on our observation that TMT-B scores show no signs of learning effects with repeated testing in a large sample of HC (n = 141) from our ongoing cohort study (Kessing et al., 2017) over a period of 16 months (change, mean ± SD: 2.9 ± 13.4). Regarding (B), comparisons were made between patients’ SCIP-D scores after 1 year and those of the demographically matched HC group (TMT-B data not available for the HC group).
For normally and non-normally distributed data, independent samples t-tests and Mann-Whitney U tests were conducted, respectively. Severity of cognitive impairments (relative to expected performance [approach (A)] and cognitive performance in HC [approach (B)], respectively) was determined based on effect sizes for significant differences. For normally distributed data, Cohen's d effect sizes were estimated. For non-normally distributed data, effect sizes r were estimated (r = Z-score / √N) (Fritz et al., 2012). Due to unequal sample sizes between patient- and the HC group, severity of cognitive impairments relative to cognitive performance in the HC group, was estimated with Hedges’ g.
Question (II): Does cognition improve from 3 months to 1 year after hospitalisation?
This question was explored using the expected change norms based on participants’ age and baseline scores (Ott et al., 2021). To explore whether proportions of patients with global or selective cognitive impairment at the 1-year assessment were significantly different from 3 months post-discharge, we used non-parametric McNemar's tests for paired nominal data.
In addition, we analysed differences between patients’ actual change and expected change SCIP-D scores between the two assessments using paired sample t-tests, in line with approach (A). Further, we compared patients’ SCIP-D change scores between 3 months and 1 year with change scores in the HC group using independent sample t-tests (consistent with approach [B]). Since TMT-B data was not available for HC but is stable over time (Kessing et al., 2017), we analysed patients’ change in TMT-B through paired sample t-tests
Question (III): Are cognitive impairments associated with functioning and quality of life?
This was investigated with Pearson's correlations or Spearman's rho for normally and non-normally distributed data, respectively. The deviation from expected scores using the RCI for patients’ SCIP-D total scores was applied as a measure in the analyses of associations between objective cognitive function and subjective cognitive complaints, quality of life, mood symptoms and work function.
Question (IV): Are cognitive impairments 3 months after hospitalisation related to mood symptoms after 1 year?
This question was analysed with Pearson correlation analysis. SCIP Total score at 3-months was used as a measure investigating the association with depressive symptoms at 1-year as measured by Hamilton Depression Rating Scale (HDRS-17).
Results
Participants
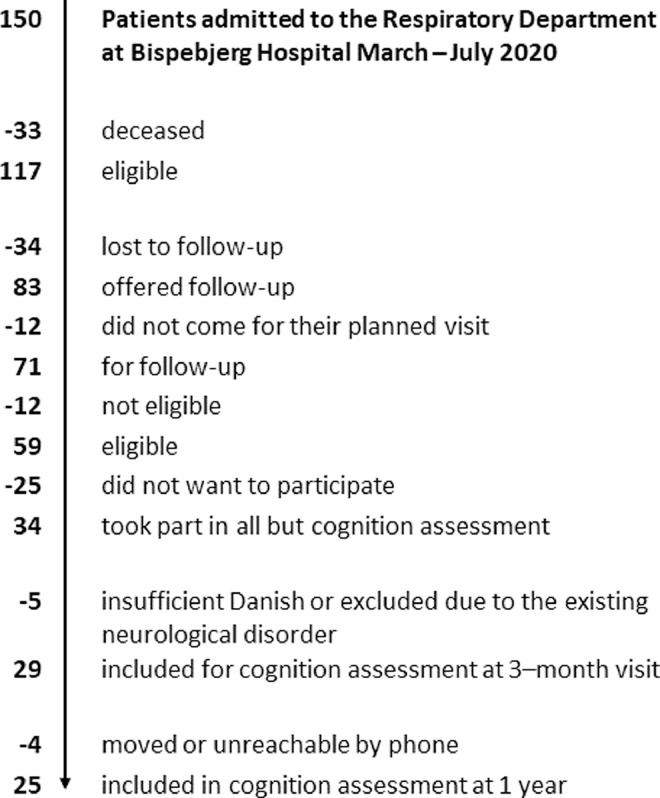
Following hospitalisation for COVID-19 at Bispebjerg Hospital, 70 % of patients (n = 83) were offered follow-up at two-time points; after 3 months and 1 year. Of those offered participation in the study, 86 % accepted (n = 71) (See Miskowiak et al., 2021). Fig. 1 illustrates the recruitment process (See Appendix A). For the 3 months follow-up, 29 patients took part (n = 29). The results of the 3 months cognition assessments have previously been published (Miskowiak et al., 2021). For the 1-year (mean ± SD: 11 ± 2 months) follow-up, we reassessed 25 (86%) of these patients. Of the 4 patients lost to follow-up, 1 had moved abroad while the other 3 were unreachable by telephone. Importantly, these 4 patients did not differ significantly from the remaining patients on the demographic variables age and gender (p-levels > .09). Clinical and demographical characteristics of the resulting sample (n = 25) are displayed in Table 1 .
Fig. 1.
Flow Chart for recruitment of patients in post-COVID cognition assessments.
Table 1.
Demographic and clinical characteristic variables of patients at the 11-months follow-up assessment after hospitalisation with COVID-19 and demographic characteristics of the matched healthy controls (HC).
| Patients (n = 25) | Healthy controls (n = 55) | P-value | |
|---|---|---|---|
| Demographics | |||
| Age (years), mean (SD) | 56 (10.7) | 56.7 (5.2) | .91 |
| Sex, no. Females (%) | 12 (48) | 25 (46) | .83 |
| Years of education, mean (SD) | 14.84 (3.8) | 14 (2.7) | .27 |
| Work status, no. employed (%)a | 12 (48) | ||
| Ethnicity, no. Caucasian (%) | 19 (75) | ||
| Clinical Characteristics | |||
| EQ-5D-5L Quality of Life Questionnaire | |||
| Quality of Life Questionnaire totalb | 9.1 (2.6) | ||
| Movementc | 1.6 (0.9) | ||
| Personal cared | 1.1 (0.3) | ||
| Usual activitye | 1.8 (0.6) | ||
| Painf | 2.6 (0.9) | ||
| Anxiety/Depressiong | 1.7 (1.1) | ||
| EQ-VAS healthh | 70 (17.1) | ||
| CFQ total, mean (SD)i | 67 (14.8) | ||
| Hamilton Depression Rating Scalej | 3.0 (4.2) | ||
| Work productivity and activity impairment | |||
| Percent overall work impairment due to healthk | 20.0 [0.0, 100.0] | ||
| Percent work time missed due to health (absenteeism) | 0.0 [0.0, 100.0] | ||
| Percent impairment while working due to health (presenteeism) | 15.0 [0.0, 100.0] | ||
| Percent activity impairment due to healthl | 20.0 [0.0, 90.0] |
Data is presented as mean (SD) or number (percentage) for demographics, clinical characteristics, and quality of life data. Work Productivity and Activity Impairment data is reported as median [minimum, maximum].
Missing data for n = 7a,b,h,j,l, Missing data for n = 9 c,d,e,f,g, Missing data for n = 2i; Missing data for n = 1k
Question (I): What is the frequency, pattern, and severity of cognitive impairments 1 year after hospital discharge?
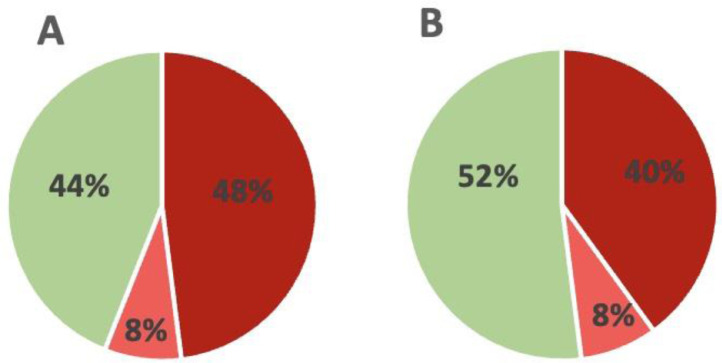
In total, 14 (56%) patients fulfilled the criterion for clinically relevant cognitive impairments compared with their expected performance based on their individual age, education level and gender; 12 (48 %) patients fulfilled the criterion for global impairment, and 2 (8%) patients as selectively impaired, while 11 (44%) were cognitively normal. In comparison with the HC sample, 12 (48%) patients were identified as cognitively impaired; 10 (40%) with global impairments and 2 (8%) with selective impairment, while 13 (52%) were cognitively normal. Cognition data from patients, expected scores and HC is displayed in Table 2 and Fig. 2 .
Table 2.
Objective and subjective cognition data from patients and a matched control group as well as the expected scores based on patients age, sex, and education.
| Patients (n = 25) | Expected scores based on age, sex, and education | Healthy controls (n = 55) | P-value all patients actual vs. Expected | P-value all patients vs. Healthy controls | |
|---|---|---|---|---|---|
| SCIP total score, mean (SD) | 69.0 (14.5) | 78.3 (4) | 77.3 (8.4) | .002 | .012 |
| VLT-L, mean (SD) | 21.2 (4.4) | 23.7 (0.9) | 23.4 (2.4) | .008 | .032 |
| WMT, mean (SD) | 18.2 (2.8) | 20.1 (0.6) | 19.8 (2.9) | .002 | .007 |
| VFT, mean (SD) | 14 (5.6) | 16.8 (0.9) | 16.2 (4.5) | .015 | .065 |
| VLT-D, mean (SD) | 6.5 (2.8) | 7.7 (0.4) | 7.8 (1.6) | .052 | .044 |
| PST, mean (SD) | 9.0 (3.0) | 10.4 (1.1) | 10.2 (1.8) | .020 | .076 |
| TMT-B, mean (SD) | 94.3 (42.1) | 78.0 (16.2) | .090 | ||
| CFQ, total mean (SD) | 67 (14.8) |
Data is presented as mean (SD) or number (percentage). CFQ data was only available for 23 of the 25 patients; SCIP, Screen for Cognitive Impairment in Psychiatry; SD, standard deviation; VLT-L, verbal learning test-learning; WMT, working memory test; VFT, verbal fluency test; VLT-D, verbal learning test-delayed recall; PST, psychomotor speed test; TMT-B, Trail Making Test B; CFQ, Cognitive Failures Questionnaire.
Fig. 2.
Frequency of clinically significant cognitive impairments. (A) Proportion of patients with clinically relevant global or selective cognitive impairments using a cut-off for global impairment (dark red) defined as SCIP Total scores ≥1 below demographically adjusted norms and – for selective impairments (light red) – performance ≥1 SD below the demographically adjusted norms, on ≥2 individual tests. (B) Finally, proportion of patients with clinically relevant global or selective cognitive impairments using a cut-off for global impairment defined as SCIP Total scores ≥1 below 55 age- and education matched healthy controls (HC) following repeated testing and – for selective impairments – performance ≥1 SD below HC ≥2 individual tests. Dark red = global cognitive impairment; light red= selective cognitive impairment; green = cognitively normal.
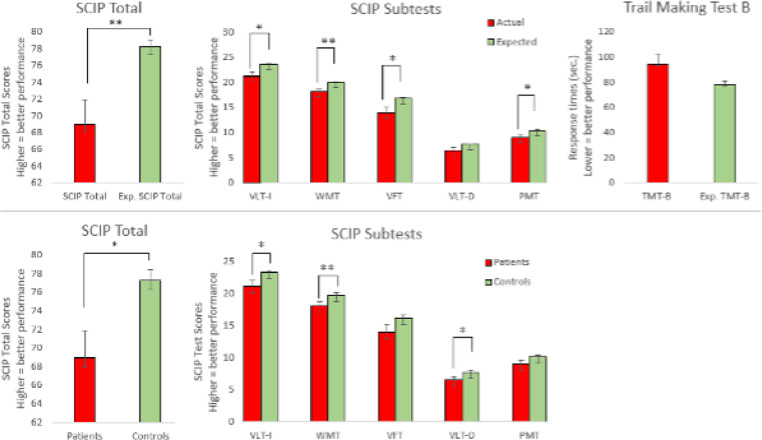
Comparison between patients’ actual and expected scores based on the age, gender and education revealed that on average, patients displayed pronounced global cognitive impairment on the SCIP with a large effect size (SCIP Total: t= -3.09, df= 27.42, p = 0.002, Cohen's d=-0.87). Large effect size was observed on the working memory test (t= -3.37, df= 25.94, p = 0.002, Cohen's d= -0.91). Moderate to large impairments were observed in verbal learning test -immediate (t= -2.67, df= 25.93, p = 0.008, Cohen's d= -0.75), verbal fluency test (t= -2.48, df= 25.25, p = 0.015, Cohen's d= -0.70), and psychomotor speed test (t= -2.15, df= 30.73, p = 0.020, Cohen's d= -0.61). In contrast, a non-significant trend was observed on the trail making test B (t= 1.73, df= 28.40, p = 0.09, Cohen's d= 0.51) and verbal learning test -delayed (t= -2.04, df= 25.02, p = 0.052, Cohen's d= -0.58) (see Fig. 3 ).
Fig. 3.
Pattern and severity of cognitive impairments in patients 1 year after COVID-19 hospitalisation. Top: cognitive impairments in patients (red) in comparison with expected normative scores adjusted for age, sex and education (green). Bottom: cognitive impairments in patients (red) in comparison with scores of 55 healthy demographically matched controls following repeated testing. Graphs represent the mean whereas error bars represent the standard error of the mean. *= p < 0.05 (two-tailed); **= p < 0.01 (two-tailed); *** = p < 0.001 (two-tailed).
Comparisons of patients with the matched HC group revealed similar results. Patients exhibited global cognitive impairments with a large effect size (SCIP total: t= 2.67, df= 31.4, p = 0.012, Hedges’ g= 0.78). Moderate to large effect sizes were observed in verbal learning test -immediate (t= 2.25, df = 30.73, p = 0.032, Hedges’ g= 0.67), WMT (U= 814,00 p = 0.007, r= -0.30) and -delayed (t= 2.10, df= 31.30, p = 0.044, Hedges’ g= 0.62). Non-significant trends toward group differences were found for psychomotor speed and verbal fluency (PST: t= 1.84, df= 32.24, p = 0.076, Hedges’ g= 0.53; VFT: t= 1.87, df = 78, p = 0.065, Hedges’ g= 0.45). The pattern of cognitive deficits across groups is displayed in Fig. 3.
Question (II): Does cognition improve from 3 months to 1 year after hospitalisation?
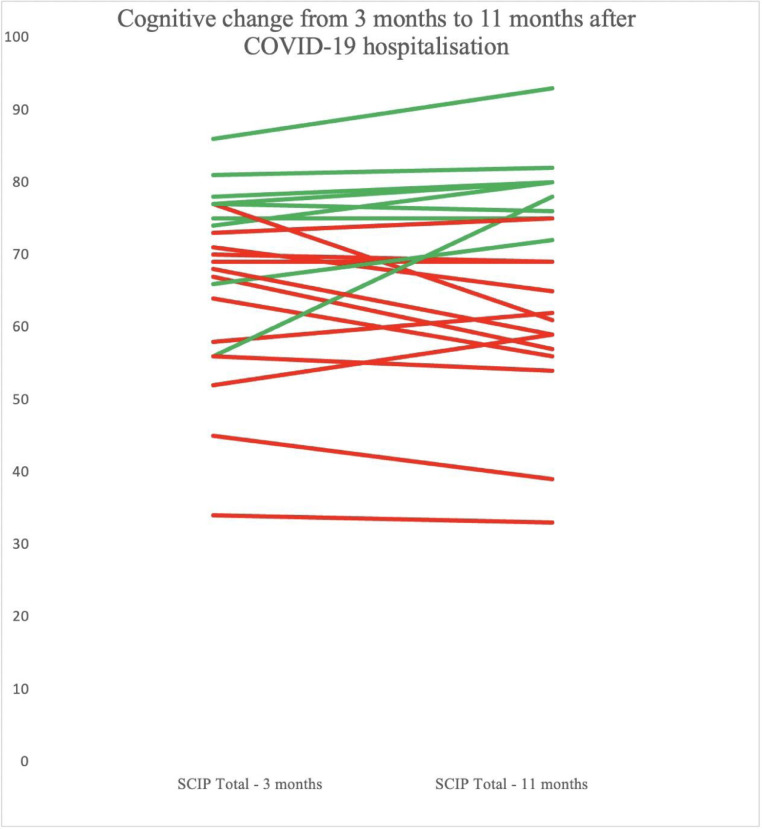
There was no significant difference in the proportion of patients with global or selective cognitive impairment between the 3-months assessment (32% and 24 %, respectively) and the 1 year assessment (48% and 8%, respectively) (p-values ≥ 0.22 and ≥ 0.13. respectively). Cognitive change in individual patients from the 3-months to 11-months assessments is illustrated in Fig. 4 .
Fig. 4.
The trajectory of cognitive change from 3 months to 1 year in patients hospitalised with COVID-19. SCIP-D Total scores measured at the 3-months (M= 69.2, SD= 13.2) and 11-months (M= 69.0, SD= 14.5) assessments, respectively. Dark red= cognitive impairment; green = cognitively normal.
There were also no significant differences between patients’ test scores at 3 months and 1 year on the SCIP-D or TMT-B (p-vales ≥ 0.19). However, when adjusting the analyses for the normative change due to learning effects with repeated testing, patients showed a lack of the expected increase over time in SCIP-D total scores (mean ± SD, actual change: -0.2 ± 7.4; expected change: 9.1 ± 11.8; t(24)= -3.49, p = 0.002, Cohen's d = 0.94) and on the five SCIP-D subtests (p-values ≤ 0.05) but not for TMT-B (p = 0.06). Comparison with in the HC group (approach [B]) also revealed less improvement in patients in SCIP-D total scores (mean ± SD, patients: -0.20 ± 7.4; HC: 3.0 ± 6.0; t = 2.02, df = 39.1, p = 0.046, Hedges’ g = 0.49) but not on the individual SCIP-D subtests (p-values ≥ 0.09).
Question (III): Are cognitive impairments associated with functioning and quality of life?
Global cognitive impairments correlated moderately with subjective cognitive complaints (Pearson's correlation: r = -0.61 p < .01). Regarding quality-of-life measurements, global cognitive impairments revealed a moderate correlation with EQ-5D-5L Quality of Life Questionnaire Total (Pearson's correlation: r = -0.56, p = 0.02). Additionally, more global cognitive impairment correlated moderately with more anxiety and depression (EQ-5D-5L subscale ‘anxiety and depression’, Pearson's correlation: r = -0.66, p = 0.01), poorer health (Pearson's correlation: r = -0.54, p = 0.02), and more pain and discomfort (Pearson's correlation: r = -0.50, p = 0.05) measured with the EQ-5D-5L. In addition, global cognitive impairment correlated with greater subsyndromal depression symptoms, measured with HDRS-17 (Pearson's correlation; r = -0.54, p = 0.02). Global cognitive impairment also correlated strongly with impairments in several aspects of work function measured with the WPAI, including work productivity loss’ (i.e., overall work impairment; Spearman's Rho; r = -0.75, p = 0.01), ‘presenteeism’ (i.e., impairment at work; Spearman's Rho’; r = -0.70, p = 0.01), and ‘activity impairment’ (Spearman's Rho; r = -0.69, p < 0.01). In contrast, no relation was found between global cognitive impairment and the WPAI ‘absenteeism’ (i.e., work time missed) (p≥.46).
Question (IV): Are cognitive impairments 3 months after hospitalisation related to mood symptoms after 1 year?
A moderate correlation was found between cognitive impairments 3 months after hospital discharge and the severity of depressive symptoms after 1 year (Pearson's correlation; r = -0.49, p = 0.04).
Discussion
In this longitudinal study of patients hospitalised with COVID-19, we examined the frequency and pattern of cognitive impairments and associations with quality of life and work functioning 1 year after hospital discharge (mean ± SD; 11 ± 2 months) and the cognitive trajectory from patients’ prior 3-months assessments (Miskowiak et al., 2021). The percentages of patients with clinically relevant cognitive impairments after 1 year were 48 % and 56 % when compared to a matched healthy control group and demographically adjusted norms, respectively. This did not differ significantly from the frequency of impairments observed 3 months after hospitalisation. Regarding the pattern of deficits, verbal learning and executive function were most affected, with moderate to large effect sizes for deficits in these domains. These objective cognitive impairments correlated strongly with overall work impairment, presenteeism, and activity impairment. In addition, objective cognitive impairments correlated moderately with subjective cognitive difficulties and with poorer quality of life, including poor health, pain, anxiety and depression. Finally, cognitive impairments 3 months after hospitalisation correlated moderately with depressive symptoms 1 year after hospitalisation.
The observed trajectory of cognitive functions from 3 months to 1 year after hospitalisation with COVID-19 indicates that patients with impaired cognition 3 months after hospitalisation do not improve after 1 year, while patients with no impairments after 3 months remain cognitively normal. This is consistent with meta-analytic evidence suggesting patients’ cognitive impairments after COVID-19 persist over time (Ceban et al., 2022). Our findings corroborate with 3 longitudinal studies that identified cognitive impairments both 6 (Poletti et al., 2021) and 12 months (Méndez et al., 2022; Ferrucci et al., 2022) after COVID-19 hospitalisation, with most pronounced impairments within verbal learning, memory and executive function (Méndez et al., 2022). While Ferrucci and colleagues observed improvement in verbal memory, attention, and processing speed, cognitive functions were still affected after 1 year, with processing speed, visuospatial and verbal memory being most affected (Ferrucci et al., 2022). No study has yet investigated whether cognitive COVID persists beyond 1 year. However, longer-term follow-up assessment of patients with other respiratory illnesses, including SARS-CoV-1 and MERS, indicates that neurocognitive and functional impairments may last up to 5 years after hospitalisation with these diseases, particularly in severely ill patients with acute respiratory distress syndrome (ARDS) (O'Sullivan, 2021; Sasannejad et al., 2019). Nevertheless, studies with longer follow-up times are needed to determine the long-term trajectory of cognitive deficits after COVID-19.
The moderate to strong associations between cognitive impairments and daily functioning, quality of life and low mood 1 year after hospitalisation with COVID-19 is noteworthy. This finding is in keeping with the observation by Mendez and colleagues in a larger sample of patients (n = 171) that cognitive impairments, subjective cognitive complaints, psychiatric morbidity, and poorer quality of life are prevalent 1 year after hospitalisation with COVID-19 (Méndez et al., 2022). The lack of cognitive improvements from 3 months to 1 year is also consistent with the observations that patients often do not return to previous levels of work capacity even 1 year after COVID-19 hospitalisation (Davis et al., 2021; Frontera et al., 2021). Importantly, we showed here for the first time that cognitive impairments are strongly associated with reduced work capacity, and moderately associated with self-reported cognitive difficulties in daily life, poorer quality of life, and elevated mood symptoms 1 year after hospital discharge. In particular, the moderate correlation between objectively measured and subjective self-reported cognitive impairment suggests that patients’ insight into their cognitive status was adequate. This contrasts with the generally poor correlation between subjective and objective cognition in patients with mood disorders (e.g., Petersen et al., 2019). Cognitive screening with easy-to-administer self-report measures may thus be adequate for patients in long-COVID clinics.
Interestingly, a longitudinal study of 1,276 hospitalised patients in China revealed that while most patients had returned to work within 12 months after hospitalisation with COVID-19 (88%), a substantial subset (26 %) was battling with unchanged levels of mood symptoms from 6 to 12 months after their hospital discharge (L. Huang et al., 2021). It is likely that patients’ return to work despite lingering symptoms will aid their recovery and help them regain their cognitive abilities and daily functioning after COVID-19. Indeed, environmental stimulation seems crucial for enhancing and preserving cognitive function (Shaffer, 2016), possibly through a positive impact on patients’ neuroplasticity. However, work demands should not exceed people's cognitive capacity, because this would likely result in stress and poor mental health. In this regard, cognitive screening after COVID-19 hospitalisation and implementation of appropriate support for patients with identified cognitive impairments may be clinically useful.
Depressive symptoms were found in a previous study to be the main predictor of impaired cognitive performance 6 months after hospitalisation with COVID-19 (Poletti et al., 2021). Our finding that cognitive impairments 3 months after hospitalisation were moderately associated with subsequent depressive symptomatology after 1 year provides preliminary evidence that the opposite may also be the case. While the sample size was too small for multiple regression analyses with covariation for other potential contributing factors, this association provides preliminary evidence for a potential role of cognitive COVID in the development of depressive symptoms. Cognitive impairments and mood symptoms are thus likely to have a bi-directional relation; In a mutually reinforcing cycle, cognitive impairments may exacerbate depression and anxiety symptoms due to difficulties overcoming cognitive challenges in daily life and work functioning and, thereby, result in poorer quality of life, stress, and mood symptoms. On the other hand, mood and anxiety symptoms may also exacerbate cognitive impairment and functional disability. This highlights a need to screen for and target both cognitive impairment and mood symptoms after severe COVID-19 illness. Regarding cognitive screening, the finding that objectively measured cognitive impairment scaled with subjectively self-reported cognitive difficulties indicates that patients may have accurate insight into their cognitive status. This supports the use of the CFQ or similar easy-to-administer self-report measures to screen for cognitive difficulties after COVID-19.
While the functional implications of cognitive impairments after COVID-19 are evident, the neurobiological origins of these long-term impairments remain unclear and are likely multifactorial (Baldini et al., 2021; Mazza et al., 2021; Moonis et al., 2020; Scardua Silva et al., 2021). In our 3-months assessment study of these patients, we had observed that the degree of cognitive impairment was related to higher d-dimer levels during acute illness and residual pulmonary dysfunction. This suggests that reduced oxygen delivery to the brain and possible thrombosis or coagulation may play a role in patients’ cognitive impairments (Miskowiak et al., 2021). In particular, the hippocampus -an essential brain structure for memory function- is highly susceptible to hypoxia-related injury, suggesting that cerebral oxygen starvation during acute illness may contribute to these patients’ long-term verbal learning and memory difficulties.
The careful longitudinal evaluations over 1 year of cognition, functioning and quality of life and the well-defined sample of patients hospitalized with COVID-19 were strengths of the study (Miskowiak et al., 2021). A key limitation, however, was the small sample size (n = 25) and consequent low statistical power, which limited our ability to conduct extensive and thorough statistical analyses of the associations between variables. Nevertheless, we were able to observe robust group differences in cognitive functions with moderate to large effect sizes, similar to a prior larger study (Méndez et al., 2022) as well as moderate to strong associations between cognitive impairments and work difficulties, mood symptoms and quality of life. It is possible that they experienced no cognitive-COVID, indicating that our findings may only be generalised to patients with lingering symptoms. However, the reasons for declining were often exhaustion and lack of energy for cognitive testing, which would speak against a sampling bias. Further, the lack of control group of patients with a different respiratory illness prevented insight into whether the observed cognitive impairments were specific to COVID-19 illness. Finally, SCIP-D is a brief cognition screener and may not replace comprehensive neuropsychological evaluation. Nevertheless, the SCIP-D is short, sensitive and feasible, rendering complete longitudinal datasets for all included participants.
Conclusion
In conclusion, our prospective follow-up study revealed that 48-56 % of patients suffer from clinically relevant cognitive impairments 1 year after hospitalisation with COVID-19, which were of a moderate to large effect size for the global cognition measure, verbal learning and executive function. Longitudinal analyses showed stability of these impairments from 3 months to 1 year after hospitalisation. Further, the cognitive impairments after 1 year were moderately to strongly associated with poorer quality of life, more mood symptoms and greater work impairments. The observed association between cognitive impairments after 3 months and severity of depressive symptoms 1 year after hospitalisation provides preliminary evidence for a possible role of cognitive status in mental health outcomes after hospitalisation with COVID-19. Based on these findings, we suggest that there is a need to assess and address cognitive difficulties, mood symptoms and functional impairments after severe COVID-19 illness.
Role of Funding Source
Department of Pulmonology Medicine and Respiratory Research Unit, Bispebjerg University Hospital, provided financial support for the study. The funder had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Contributors
KWM and SJ defined the aim and hypotheses of this report. KWM, AEJ, SMS, DP and SJ were involved in conducting the study and assessing the patients under supervision of CMP. LF and AEJ conducted the statistical analyses under supervision of KWM. KWM and LF wrote the first draft. All authors contributed to and approved the final manuscript.
Conflict of interest
The authors report no conflicts of interest in relation to the current manuscript. Outside of the present work, KWM reports having received consultancy fees from Janssen-Cilag and Lundbeck; JR reports having received consultancy fees from Novo Nordisk, Boehringer-Ingelheim and Astra-Zeneca; CMP reports having received consultancy honararia and unrestricted grants from Astra Zeneca, Novartis, Sanofi, GSK, TEVA, ALK, Chiesi and Pharmaxis in the past three years; SJ, DP and SMS report no conflicts of interest outside of the present work.
Acknowledgements
The authors thank the Department of Pulmonology Medicine and Respiratory Research Unit, Bispebjerg University Hospital, for the financial support for the study. KWM would like to thank the Lundbeck Foundation for her five-year Lundbeck Foundation Fellowship (grant no. R215-2015-4121). JR thanks the Jascha Foundation and Skibsreder Per Henriksen, R. og Hustrus foundation for support.
References
- Army Individual Test Battery . War Department, Adjutant General's Office; Washington, DC: 1944. Manual of Directions and Scoring. [Google Scholar]
- Attix D.K., Story T.J., Chelune G.J., Ball J.D., Stutts M.L., Hart R.P., Barth J.T. The Clinical Neuropsychologist The prediction of Change: Normative neuropsychological trajectories. Clin. Neuropsychol. 2009;23(1):21–38. doi: 10.1080/13854040801945078. [DOI] [PubMed] [Google Scholar]
- Baldini T., Asioli G.M., Romoli M., Carvalho Dias M., Schulte E.C., Hauer L., Aguiar De Sousa D., Sellner J., Zini A. Cerebral venous thrombosis and severe acute respiratory syndrome coronavirus-2 infection: A systematic review and meta-analysis. Eur. J. Neurol. 2021;28(10):3478–3490. doi: 10.1111/ene.14727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker C., Beck K., Zumbrunn S., Memma V., Herzog N., Bissmann B., Gross S., Loretz N., Mueller J., Amacher S.A., Bohren C., Schaefert R., Bassetti S., Fux C., Mueller B., Schuetz P., Hunziker S. Long COVID 1 year after hospitalisation for COVID-19: a prospective bicentric cohort study. Swiss Med. Weekly. 2021;2021(41):41. doi: 10.4414/smw.2021.w30091. [DOI] [PubMed] [Google Scholar]
- Bliddal S., Banasik K., Pedersen O.B., Nissen J., Cantwell L., Schwinn M., Tulstrup M., Westergaard D., Ullum H., Brunak S., Tommerup N., Feenstra B., Geller F., Ostrowski S.R., Grønbæk K., Nielsen C.H., Nielsen S.D., Feldt-Rasmussen U. Acute and persistent symptoms in non-hospitalized PCR-confirmed COVID-19 patients. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-92045-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent D.E., Cooper P.F., FitzGerald P., Parkes K.R. The cognitive failures questionnaire (CFQ) and its correlates. Br. J. Clin. Psychol. 1982;21(1):1–16. doi: 10.1111/j.2044-8260.1982.tb01421.x. [DOI] [PubMed] [Google Scholar]
- Cambridge O.R., Knight M.J., Mills N., Baune B.T. The clinical relationship between cognitive impairment and psychosocial functioning in major depressive disorder: a systematic review. Psychiatry Res. 2018;269:157–171. doi: 10.1016/j.psychres.2018.08.033. [DOI] [PubMed] [Google Scholar]
- Ceban F., Ling S., Lui L.M.W., Lee Y., Gill H., Teopiz K.M., Rodrigues N.B., Subramaniapillai M., di Vincenzo J.D., Cao B., Lin K., Mansur R.B., Ho R.C., Rosenblat J.D., Miskowiak K.W., Vinberg M., Maletic V., McIntyre R.S. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: a systematic review and meta-analysis. Brain Behav. Immun. 2022;101:93–135. doi: 10.1016/j.bbi.2021.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis H.E., Assaf G.S., McCorkell L., Wei H., Low R.J., Re'em Y., Redfield S., Austin J.P., Akrami A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38 doi: 10.1016/j.eclinm.2021.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf M.A., Antoni M.L., ter Kuile M.M., Arbous M.S., Duinisveld A.J.F., Feltkamp M.C.W., Groeneveld G.H., Hinnen S.C.H., Janssen V.R., Lijfering W.M., Omara S., Postmus P.E., Ramai S.R.S., Rius-Ottenheim N., Schalij M.J., Schiemanck S.K., Smid L., Stöger J.L., Visser L.G., Roukens A.H.E. Short-term outpatient follow-up of COVID-19 patients: a multidisciplinary approach. EClinicalMedicine. 2021;32 doi: 10.1016/j.eclinm.2021.100731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff K. Current topics in science and practice evidence-based indicators of neuropsychological change in the individual patient: Relevant concepts and methods. Arch. Clin. Neuropsychol. 2012;27(3):248–261. doi: 10.1093/arclin/acr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz C.O., Morris P.E., Richler J.J. Effect size estimates: current use, calculations, and interpretation. J. Exp. Psycholo. 2012;141(1):2–18. doi: 10.1037/a0024338. [DOI] [PubMed] [Google Scholar]
- Frontera J.A., Yang D., Lewis A., Patel P., Medicherla C., Arena V., Fang T., Andino A., Snyder T., Madhavan M., Gratch D., Fuchs B., Dessy A., Canizares M., Jauregui R., Thomas B., Bauman K., Olivera A., Bhagat D., Galetta S. A prospective study of long-term outcomes among hospitalized COVID-19 patients with and without neurological complications. J. Neurol. Sci. 2021;426 doi: 10.1016/j.jns.2021.117486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover S., Sahoo S., Mishra E., Gill K.S., Mehra A., Nehra R., Suman A., Bhalla A., Puri G.D. Fatigue, perceived stigma, self-reported cognitive deficits and psychological morbidity in patients recovered from COVID-19 infection. Asian J. Psychiatry. 2021;64 doi: 10.1016/j.ajp.2021.102815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. Psychiat. 1960:56. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A., Trender W., Chamberlain S.R., Jolly A.E., Grant J.E., Patrick F., Mazibuko N., Williams S.C., Barnby J.M., Hellyer P., Mehta M.A. Cognitive deficits in people who have recovered from COVID-19. EClinicalMedicine. 2021 doi: 10.1016/j.eclinm.2021.101044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Yao Q., Gu X., Wang Q., Ren L., Wang Y., Hu P., Guo L., Liu M., Xu J., Zhang X., Qu Y., Fan Y., Li X., Li C., Yu T., Xia J., Wei M., Chen L., Cao B. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet North Am. Ed. 2021;398(10302):747–758. doi: 10.1016/S0140-6736(21)01755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J.H., Miskowiak K.W., Purdon S.E., Thomsen J.F., Eller N.H. Screening for cognitive impairment among patients with work-related stress complaints in Denmark: validation and evaluation of objective and self-report tools. Scand. J. Work Environ. Health. 2022;48(1):71–80. doi: 10.5271/sjweh.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsen S., Sattler S.M., Miskowiak K.W., Kunalan K., Victor A., Pedersen L., Andreassen H.F., Jørgensen B.J., Heebøll H., Andersen M.B., Marner L., Hædersdal C., Hansen H., Ditlev S.B., Porsbjerg C., Lapperre T.S. Descriptive analysis of long COVID sequelae identified in a multidisciplinary clinic serving hospitalised and non-hospitalised patients. ERJ Open Res. 2021;7(3) doi: 10.1183/23120541.00205-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessing L.V., Munkholm K., Faurholt-Jepsen M., Miskowiak K.W., Nielsen L.B., Frikke-Schmidt R., Vinberg M. The Bipolar Illness Onset study: research protocol for the BIO cohort study. BMJ open. 2017;7(6) doi: 10.1136/bmjopen-2016-015462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llach C.D., Vieta E. Mind long COVID: psychiatric sequelae of SARS-CoV-2 infection. Eur. Neuropsychopharmacol. 2021;49:119–121. doi: 10.1016/j.euroneuro.2021.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd A., Pickard A.S. The EQ-5D and the EuroQol group. Value Health. 2019;22(1):21–22. doi: 10.1016/j.jval.2018.12.002. [DOI] [PubMed] [Google Scholar]
- Logue J.K., Franko N.M., McCulloch D.J., McConald D., Magedson A., Wolf C.R., Chu H.Y. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw. Open. 2021;4(2) doi: 10.1001/jamanetworkopen.2021.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza M.G., Palladini M., de Lorenzo R., Magnaghi C., Poletti S., Furlan R., Ciceri F., Rovere-Querini P., Benedetti F. Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: effect of inflammatory biomarkers at three-month follow-up. Brain Behav. Immun. 2021;94:138–147. doi: 10.1016/j.bbi.2021.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott L.M., Ebmeier K.P. A meta-analysis of depression severity and cognitive function. J. Affect. Disord. 2009;119(1–3):1–8. doi: 10.1016/j.jad.2009.04.022. [DOI] [PubMed] [Google Scholar]
- Méndez R., Balanzá Martínez V., Luperdi S.C., Estrada I., Latorre A., González-Jiménez P., Bouzas L., Yépez K., Ferrando A., Reyes S., Menéndez R. Long-term neuropsychiatric outcomes in COVID-19 survivors: A 1-year longitudinal study. J. Intern. Med. 2022;291(2):247–251. doi: 10.1111/joim.13389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskowiak K.W., Burdick K.E., Martinez-Aran A., Bonnin C.M., Bowie C.R., Carvalho A.F., Gallagher P., Lafer B., López-Jaramillo C., Sumiyoshi T., McIntyre R.S., Schaffer A., Porter R.J., Purdon S., Torres I.J., Yatham L.N., Young A.H., Kessing L.v., Vieta E. Assessing and addressing cognitive impairment in bipolar disorder: the International Society for Bipolar Disorders Targeting Cognition Task Force recommendations for clinicians. Bipol. Disord. 2018;20(3):184–194. doi: 10.1111/bdi.12595. [DOI] [PubMed] [Google Scholar]
- Miskowiak K.W., Johnsen S., Sattler S.M., Nielsen S., Kunalan K., Rungby J., Lapperre T., Porsberg C.M. Cognitive impairments four months after COVID-19 hospital discharge: pattern, severity and association with illness variables. Eur. Neuropsychopharmacol. 2021;46:39–48. doi: 10.1016/j.euroneuro.2021.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell A.J., Kemp S., Benito-León J., Reuber M. The influence of cognitive impairment on health-related quality of life in neurological disease. Acta Neuropsychiatrica. 2014;22(1):2–13. [Google Scholar]
- Moonis G., Filippi C.G., Kirsch C.F.E., Mohan S., Stein E.G., Hirsch J.A., Mahajan A. The Spectrum of Neuroimaging Findings on CT and MRI in Adults With COVID-19. 2020;217(4):959–974. doi: 10.2214/AJR.20.24839. [DOI] [PubMed] [Google Scholar]
- Nakamura Z.M., Nash R.P., Laughon S.L., Rosenstein D.L. Neuropsychiatric complications of COVID-19. Curr. Psychiatry Rep. 2021;23(5) doi: 10.1007/s11920-021-01237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nys G.M.S., van Zandvoort M.J.E., van der Worp H.B., de Haan E.H.F., de Kort P.L.M., Jansen B.P.W., Kappelle L.J. Early cognitive impairment predicts long-term depressive symptoms and quality of life after stroke. J. Neurol. Sci. 2006;247(2):149–156. doi: 10.1016/j.jns.2006.04.005. [DOI] [PubMed] [Google Scholar]
- O’Sullivan O. Long-term sequelae following previous coronavirus epidemics. Clinic. Med. 2021;21(1):e68. doi: 10.7861/clinmed.2020-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott C.V., Knorr U., Jespersen A., Obenhausen K., Røen I., Purdon S.E., Miskowiak K.W. Norms for the Screen for Cognitive Impairment in Psychiatry and cognitive trajectories in bipolar disorder. J. Affect. Disord. 2021;281:33–40. doi: 10.1016/j.jad.2020.11.119. [DOI] [PubMed] [Google Scholar]
- Petersen J.Z., Porter R.J., Miskowiak K.W. Clinical characteristics associated with the discrepancy between subjective and objective cognitive impairment in depression. J. Affect. Disord. 2019;246:763–774. doi: 10.1016/j.jad.2018.12.105. [DOI] [PubMed] [Google Scholar]
- Pajo A.T., Espiritu A.I., Apor A.D.A.O., Jamora R.D.G. Neuropathologic findings of patients with COVID-19: a systematic review. Neurolog. Sci. 2021;42(4):1255–1266. doi: 10.1007/s10072-021-05068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poletti S., Palladini M., Mazza M.G., de Lorenzo R., COVID-19 BioB Outpatient Clinic Study group, Furlan R., Ciceri F., Rovere-Querini P., Benedetti F. Long-term consequences of COVID-19 on cognitive functioning up to 6 months after discharge: role of depression and impact on quality of life. Eur. Arch. Psychiatry Clin. Neurosci. 2021;1:1–10. doi: 10.1007/s00406-021-01346-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proal A.D., VanElzakker M.B. Long COVID or post-acute sequelae of COVID-19 (PASC): an overview of biological factors that may contribute to persistent symptoms. Front. Microbiol. 2021;12:1494. doi: 10.3389/fmicb.2021.698169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdon S.E., Psych R. PNL; Edmonton, Alberta, Canada: 2005. The Screen for Cognitive Impairment in Psychiatry. Administration and Psychometric Properties. [Google Scholar]
- Reilly M.C., Zbrozek A.S., Dukes E.M. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4(5):353–365. doi: 10.2165/00019053-199304050-00006. [DOI] [PubMed] [Google Scholar]
- Sasannejad C., Ely E.W., Lahiri S. Long-term cognitive impairment after acute respiratory distress syndrome: A review of clinical impact and pathophysiological mechanisms. Critic. Care. 2019;23(1):1–12. doi: 10.1186/s13054-019-2626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scardua Silva L., Batista Joao R., Henrique Nogueira M., Karmann Aventurato I., Machado de Campos B., Rabelo de Brito M., Koutsodontis Machado Alvim M., Vieira Nunes Ludwig G., Rocha C., Kaue Alves Silva Souza T., Amorim da Costa B., Julia Mendes M., Waku T., de Oliveira Boldrini V., Silva Brunetti N., Nora Baptista S., da Silva Schmitt G., Gabriela Duarte de Sousa J., Aparecida Marchiori de Oliveira Cardoso T., Yasuda C.L. Functional and microstructural brain abnormalities, fatigue, and cognitive dysfunction after mild COVID-19. MedRxiv, 2021. 03.20.21253414. 2021 [Google Scholar]
- Schou T.M., Joca S., Wegener G., Bay-Richter C. Psychiatric and neuropsychiatric sequelae of COVID-19 – A systematic review. Brain Behav. Immun. 2021 doi: 10.1016/j.bbi.2021.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer J. Neuroplasticity and clinical practice: Building brain power for health. Front. Psychol. 2016;7(JUL):1118. doi: 10.3389/fpsyg.2016.01118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes D.L., Holdsworth L., Jawad N., Gunasekera P., Morice A.H., Crooks M.G. Post-COVID-19 symptom burden: what is long-COVID and how should we manage it? Lung. 2021;199(2):113–119. doi: 10.1007/s00408-021-00423-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taquet M., Dercon Q., Luciano S., Geddes J.R., Husain M., Harrison P.J. Incidence, co-occurrence, and evolution of long-COVID features: A 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 2021;18(9) doi: 10.1371/journal.pmed.1003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taquet M., Geddes J.R., Husain M., Luciano S., Harrison P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8(5):416–427. doi: 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vejen M., Frausing Hansen E., Nabil Ibrahim Al-Jarah B., Jensen C., Thaning P., Nielsen Jeschke K., Suppli Ulrik C., Suppli C. Hospital admission for COVID-19 pneumonitis – long-term impairment in quality of life and lung function. 2022;9(1) doi: 10.1080/20018525.2021.2024735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland-Fiedler P., Erickson K., Waldeck T., Luckenbaugh D.A., Pike D., Bonne O., Charney D.S., Neumeister A. Evidence for continuing neuropsychological impairments in depression. J. Affect. Disord. 2004;82(2):253–258. doi: 10.1016/j.jad.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Weisenbach S.L., Boore L.A., Kales H.C. Depression and Cognitive Impairment in Older Adults. Curr. Psychiatry Rep. 2012;14(4):280–288. doi: 10.1007/s11920-012-0278-7. [DOI] [PubMed] [Google Scholar]
- Woo Y.S., Rosenblat J.D., Kakar R., Bahk W.M., McIntyre R.S. Cognitive deficits as a mediator of poor occupational function in remitted major depressive disorder patients. Clinic. Psychopharmacol. Neurosci. 2016;14(1):1. doi: 10.9758/cpn.2016.14.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]