Abstract
Objective
To evaluate the effectiveness of nicergoline to prevent temporary threshold shift (TTS) in military personnel.
Study Design
A randomized control trial.
Methods
Two hundred and twenty‐four participants were enrolled. Nicergoline 30 mg twice daily intake was prescribed to the study group (n = 119) for 3 weeks. The placebo was prescribed to the control group (n = 105) for 3 weeks, as well. Audiometric thresholds were measured at baseline and within 24 h after the participants attended a 1‐day weapons firing practice. During the firing practice, all participants had to wear foam earplugs. The TTS was assessed by using a variety of published significant threshold shift (STS) definitions. Additionally, the effects of the treatment group on the magnitude of pre‐ to postexposure threshold shifts were estimated. Tinnitus and other adverse effects of the medication were recorded.
Results
The incidence of STS was 65.4% from the study group and 75% from the control group. The negative STS (thresholds improved) was 68.6% from the study group and 44.7% from the control group. The positive STS (thresholds worsened) from the study group and the control group was 31.4% and 55.3%, respectively. The effect of treatment in participants receiving nicergoline demonstrated significant coefficients (change in dB) in both ears (p = .001). The mean different threshold of participants receiving nicergoline showed negative STS in all tested frequencies without statistical significance. However, the mean different threshold of participants receiving a placebo showed positive STS with statistical significance. Additionally, there were 16 ears detecting a warning sign of permanent hearing loss. These participants from the control group presented a longer duration of tinnitus (p = .042). Moreover, the serious adverse effects of nicergoline were considerably low.
Conclusion
The study results suggest that nicergoline may attenuate noise‐related TTS and tinnitus, and justify further investigation on the effectiveness of this drug as an otoprotectant.
Level of Evidence
2
Keywords: nicergoline, significant threshold shift, temporary threshold shift, tinnitus
The temporary threshold shift (TTS) was found in the control group with statistically significant differences. The study results suggest that nicergoline may attenuate TTS and self‐reported tinnitus duration following noise exposure. Additionally, few serious adverse effects of nicergoline were shown. Further investigation on the effectiveness of nicergoline as an otoprotectant is warranted.
1. INTRODUCTION
A tenth of the world population is exposed to sound pressure level (SPL) which could potentially cause noise induced hearing loss (NIHL), and about one‐third of these people exposed to intense noise. 1 , 2 According to the Fiscal Year 2020 Annual Benefits Report, tinnitus has long been, and continues to be, the most prevalent disability (60.48%) connected to military service in the United States, and hearing loss continues to be the second most prevalent service‐connected disability (34.90%). 3 Military personnel suffer from tinnitus and hearing loss as a consequence of deployment but also of weapons firing practice or other military training programs. NIHL is divided into temporary threshold shift (TTS) and permanent threshold shift (PTS). Furthermore, TTS typically occurs and resolves within 16 to 48 h of the noise exposure, whereas PTS is confirmed with an audiometric retest at least 3 weeks after the noise exposure. 4 , 5 Additionally, tinnitus can be classified into primary tinnitus which is idiopathic and may be associated with sensorineural hearing loss. Whereas, secondary tinnitus is associated with a specific underlying cause (other than sensorineural hearing loss). Moreover, tinnitus duration can be divided into recent onset, lasting less than 6 months, and persistent tinnitus, lasting 6 months and longer. 6
The noise causes damage to the inner ears by two mechanisms. The first mechanism, the intense noise can damage the cochlea mechanically by decoupling the organ of Corti from the basilar membrane. 7 The second one is an intense metabolic activity, due to overstimulation. Consequently, the increased levels of reactive oxygen species (ROS), superoxide anion, hydroxyl radical, and reactive nitrogen species were demonstrated. Outer hair cells at the base of the cochlear seem to be most susceptible to free radical damage while supporting cells have considerably more survival capacity than hair cells. 8 , 9 , 10 Moreover, many studies have reported the use of antioxidants such as N‐acetyl‐cysteine (NAC), D‐methionine, salicylate, or Vitamin E to scavenge and eliminate the damaging ROS. 8 , 11 In 1994, Attia et al. 12 demonstrated that magnesium had a protective effect on NIHL among military personnel. Furthermore, Wu et al. 13 demonstrated the protective effectiveness of NAC, antioxidant against NIHL in male workers. Additionally, Kopke et al. conducted a randomized control trial (RCT) in the military personnel, and suggested that NAC treatment was superior to the placebo in preventing NIHL. 14 In animal models, glutathione, D‐methionine and ebselen also showed NIHL attenuation. 1 However, Campbell 15 conducted a clinical trial demonstrating D‐methionine attenuated the PTS associated with multi‐day weapon training activities with a statistically significant difference. Many studies reported the effectiveness of medications in preventing TTS, 16 but it is interesting to investigate whether other medications could attenuate the NIHL.
Moreover, nicergoline, an anti‐oxidant, derivative of ergoline, provides neuroprotective effects and increases inner ear circulation. 17 , 18 In the other experimental study, nicergoline has been suggested an antioxidant inhibiting not only lipid peroxidation, but also free radical generation from neutrophils. 19 The clinical use of nicergoline such as in treating dementia, vascular, and balance disorders especially in vertigo and tinnitus, is widely recommended. 17 , 18 , 20 Nowadays, the studies of nicergoline on preventing NIHL are limited. Consequently, the primary objective of this study is to investigate the effectiveness of nicergoline in preventing TTS in Thai military servicemen undergoing annual firearms training. Additionally, the secondary objective is to investigate the impact of nicergoline on the duration of tinnitus among the military personnel attending the annual firing practice.
2. MATERIALS AND METHODS
An RCT was conducted, using a protocol that was approved by the Institutional Review Board of the Royal Thai Army Medical Department. The study adhered to the tenets of the Declaration of Helsinki and was performed according to the principles of Good Clinical Practice and the Consolidated Standards of Reporting Trials statement. The trial was registered at the Thai Clinical Trials Registry (TCTR20200519002).
Two hundred and thirty‐eight male conscripts from The Royal Thai Navy, aged 20 to 25 years, were informed about the aims and methods of the study, and then signed the informed consent prior to participate in the study. The participants consisting of 224 conscripts were divided into two groups depending on their two battalions by using the cluster randomization method. This method was able to be used to evaluate a new treatment affecting patient outcomes. Moreover, it was preferred when there was a significant potential for contamination in the study. All participants underwent a physical examination including an otoscopic examination of the ear canal, visual inspection of the tympanic membrane and baseline hearing threshold testing (Pure‐tone air‐conduction and bone‐conduction audiometry) before participating in the firing practice. Laboratory tests including a complete blood count, blood urea nitrogen, creatinine, uric acid, and liver function test were required, as well. Participants were excluded if they were diagnosed as having evidence of ear disease (such as a perforated tympanic membrane, an infected or inflamed ear), abnormal audiometry (such as baseline hearing threshold averaged across 2000, 3000, and 4000 Hz > 25 decibel [dB] hearing level [HL], baseline audiogram presenting air‐bone gap in either ear), abnormal blood chemistry and hypersensitivity or allergy to nicergoline. Additionally, the participants whose hearing thresholds were unable to be measured within 24 h would be excluded from the study. The participants in the study group and the control group were 119 and 105 respectively. After the physical examination was performed, 4 participants from the study group, and 10 participants from the other group were excluded from the study (Figure 1).
FIGURE 1.
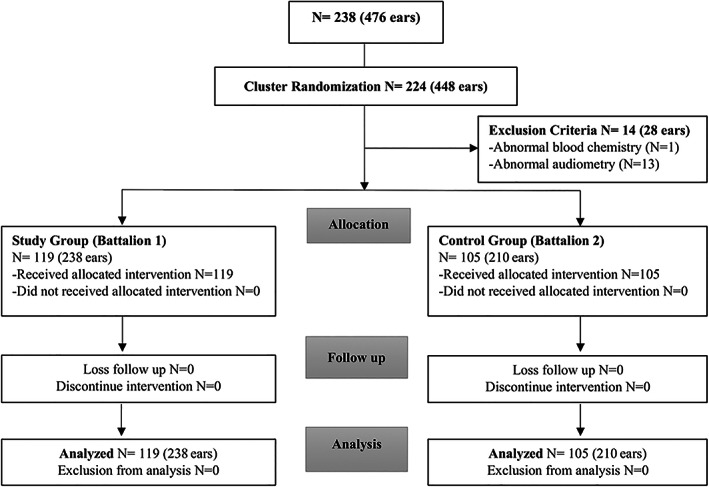
Flow diagram according to the CONSORT 2010 statement shows participants flow in this study
2.1. Noise exposure
From April 2019 to July 2019, all subjects participated in basic military training in the Royal Thai Marine Corps, Chonburi Province, Thailand. This included attending the annual firing practice conducted on August 31, 2019. All participants used M16 rifles and each person was to fire 30 shots in a row. All participants were assigned to trigger M16 rifles with their right hands. They were reminded to use foam earplugs (3M™ E‐A‐R™ Classic™) as the hearing protection device (HPD) during firing practice. The approximated noise intensity level of an M16 rifle is 157 dB SPL, 21 while the earplugs attenuated the noise level by 29 dB SPL. 22
2.2. Nicergoline administration and dosage
The blinded labels of nicergoline (30 mg) and placebo were provided in aluminum blister packets. The appropriate oral dosage of nicergoline was 30 mg administered twice daily (60 mg per day). This is the dosage that is generally prescribed for clinical efficacy in the treatment of dementia. 17 Furthermore, these packages were allocated to both groups. The participants were to take one tablet after meals twice daily for 3 weeks, and then they were observed for side effects such as nausea, drowsiness, diarrhea, fainting, headache, and vertigo 23 , 24 on their own. At the end of the second week of taking medication, they had to attend the firing practice.
2.3. Pure‐tone air‐conduction audiometry
This auditory sensitivity test, performed by certified audiologists and technicians, was used to evaluate the hearing threshold. The procedure was usually performed in both ears. All participants, audiologists, study coordinators were blinded as to who was allocated in the study group or the control one. The mobile van service containing two four double‐wall sound attenuated booths which was compatible with American National Standards Institute (ANSI 2014) was used. The GSI Pello™, a product of Grason–Stadler company, was manufactured to meet the ANSI S3.6, and international standards. This device was used to measure the pure‐tone air‐conduction and bone‐conduction thresholds as the baseline before the participants attended the firing practice. Additionally, the post‐noise exposure audiometry would be measured as soon as possible within 24 h, and the audiometry was measured at the frequencies of noise at 250, 500, 1 K, 2 K, 3 K, 4 K, 6 K, and 8 K Hz. Furthermore, the bone‐conduction testing was conducted at 500, 1 K, 2 K, 3 K, and 4 K Hz. Thresholds that show ≤25 dB HL are considered normal, whereas thresholds above 25 dB HL represent various levels of hearing loss. 25
2.4. Threshold shift
Only TTS were considered for this study, and that TTS was defined in four different ways following different published criteria. The four TTS definitions are: (1) significant threshold shift (STS) defined as either a 10 dB INCREASE or DECREASE in hearing threshold averaged across 2, 3, and 4 kHz in the same ear from an individual's baseline, confirmed by a retest, and shifting to a pure‐tone average threshold that exceeds 25 dB HL; (2) significant negative threshold shift (Negative STS), defined as a decrease (improvement) of 10 dB or greater for the average of 2, 3, and 4 kHz in either ear; (3) significant positive threshold shift (Positive STS), defined as an increase (worsening) of 10 dB or greater for the average of 2, 3, and 4 kHz in either ear; and (4) early warning sign of permanent hearing loss, defined as a post‐noise exposure threshold shift at a single frequency of 15 dB at 1, 2, 3, or 4 kHz and that results in a hearing loss as defined as a threshold in excess of 25 dB HL. 5 , 26 , 27
2.5. Measurement of tinnitus
A portable self‐recording form with the necessary details was given to each qualified participant in order to record the side effects of the medication on their own during 3 weeks of the protocol. Additionally, at the end of the firing practice, each participant would record the exact time when tinnitus began until the symptoms were resolved.
2.6. Statistical analysis
The statistical analysis was performed by a certificated statistician by using STATA Software. Descriptive statistics such as number, percentage, mean, standard deviation, minimum, and maximum were reported. The analysis of the effect of the treatment between the treatment and the control groups (nicergoline versus placebo) was performed separately for left and right ears. Within the same ears, baseline hearing thresholds (dB) at eight‐tested frequencies (Hz) were measured between the treatment and the control groups defined as pre‐noise exposure hearing thresholds. Post‐noise exposure hearing thresholds were later measured according to the study protocol. Generalized estimating equations (GEE) modeling was used to compare hearing threshold difference (post − pre) at the eight tested frequencies. GEE modeling was also used to estimate the average hearing threshold difference between the two groups. Coefficients obtained from the GEE modeling were interpreted as change in dB. The one‐way ANOVA testing was used to compare the mean difference thresholds within group. The Independent Samples t Test was used to compare the mean difference of the two independent groups. Categorical data was analyzed by using χ 2 test while Mann–Whitney U test was used to analyze the average of two independent groups. The p value <.05 was considered to be statistically significant.
3. RESULTS
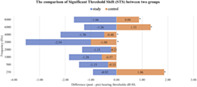
Fourteen participants with abnormal blood chemistry and abnormal pre‐exposure audiometry were excluded from the study because they did not meet the inclusion criteria. The information of 224 participants consisting of 448 ears was analyzed. The average age of the participants in the study group and the control group was 22.31 ± 1.44 and 22.36 ± 1.40, respectively. In Table 1, the incidence of STS was demonstrated at 65.4% from the study group and 75% from the control group. Furthermore, the negative STS was 68.6% from the study group and 44.7% from the control group. The positive STS from the study group and the control group was 31.4% and 55.3%, respectively. In addition, the early warning sign of permanent hearing loss was demonstrated in 8 (out of 153) ears from the study group and in 8 (out of 150) ears from the control group. Additionally, the effect of the treatment group (nicergoline treatment versus control) on post‐noise exposure hearing thresholds (dB) was assessed for the right and the left ears, after the frequency and the corresponding baseline pre‐noise exposure hearing thresholds were adjusted (Table 2). The comparison of STS between the two groups is shown in Figure 2. Mean difference (post − pre) hearing thresholds that were negative (improvement in hearing threshold) were referred to as “negative STS,” and the positive mean difference thresholds were referred to as “positive STS.” The mean threshold difference of participants receiving nicergoline showed negative STS in all tested frequencies without statistical significance (p = .108). However, the mean threshold difference of participants receiving placebo showed positive STS (hearing worsened) with statistical significance at the frequencies of 250 Hz, 3 KHz, 4 KHz, 6 KHz, and 8 KHz (p < .001).
TABLE 1.
Comparison of incidence rates for STS between the two groups
| Study group (N = 119) | Control group (N = 105) | |
|---|---|---|
| Age; years (mean ± SD) | 22.31 ± 1.44 | 22.36 ± 1.40 |
| Number of ears | 238 | 210 |
| Pre‐noise exposure | ||
| Normal hearing thresholds | 234 (98.3%) | 200 (95.2%) |
| Post‐noise exposure | ||
| STS | 153 (65.4%) | 150 (75.0%) |
| Positive STS (increase in hearing threshold) | 48 (31.4%) | 83 (55.3%) |
| Right ears | 23 (47.9%) | 43 (51.8%) |
| Left ears | 25 (52.1%) | 40 (48.2%) |
| Negative STS (improvement in hearing threshold) | 105 (68.6%) | 67 (44.7%) |
| Right ears | 55 (52.4%) | 30 (44.8%) |
| Left ears | 50 (40.6%) | 37 (55.2%) |
| Early warning sign of permanent hearing loss | 8 (5.2%) | 8 (5.3%) |
| Right ears | 5 (62.5%) | 5 (62.5%) |
| Left ears | 3 (37.5%) | 3 (37.5%) |
Note: STS—either a 10 dB INCREASE or DECREASE in hearing threshold averaged across 2, 3, and 4 kHz in the same ear from an individual's baseline, confirmed by a retest, and shifting to a pure‐tone average threshold that exceeds 25 dB HL. Positive STS—an increase (worsening) of 10 dB or greater for the average of 2, 3, and 4 kHz in either ear. Negative STS—a decrease (improvement) of 10 dB or greater for the average of 2, 3, and 4 kHz in either ear. Early warning sign of permanent hearing loss – a post‐noise exposure threshold shift at a single frequency of 15 dB at 1, 2, 3, or 4 kHz and that results in a hearing loss as defined as a threshold in excess of 25 dB HL.
Abbreviation: STS, significant threshold shift.
TABLE 2.
Effect of the treatment group (nicergoline treatment versus control) in the right and the left ears on post‐noise exposure thresholds
| Effect of nicergoline versus control | Coefficients (as change in dB) | p‐value | 95% CI |
|---|---|---|---|
| Right ear | −1.60 | .001 | −2.57, −0.63 |
| Left ear | −1.21 | .001 | −2.17, −0.26 |
Note: The analyzed effect of the treatment between the two groups adjusted for frequency and baseline hearing threshold was conducted by using generalized estimating equations modeling. The effect of the treatment in the treatment group (receiving nicergoline) shown in the right and the left ears, results in estimated threshold shift accounting for −1.60 and −1.21 dB, respectively. The post‐noise exposure thresholds in the group treated with nicergoline were better as compared with the control group.
FIGURE 2.
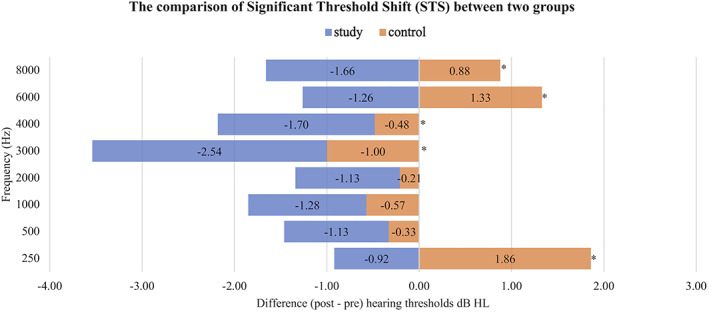
The comparison of significant threshold shift (STS) between two groups. Negative STS represents an improvement in hearing threshold from the reference audiogram. Positive STS represents an increase in hearing threshold from the reference audiogram. The comparison of the mean threshold difference within group by using one‐way ANOVA testing shows the significant difference in the control panel (p < .001), whereas the study group does not show the significant difference (p = .108). The mean different hearing thresholds between two groups demonstrate the significant difference at 250 Hz, 3 KHz, 4 KHz, 6 KHz, and 8 KHz. *Independent Samples t Test
Subgroup analysis of STS between two groups is shown in Table 3. In the negative STS results, the negative mean different hearing threshold of the study group showed the better results of improvement in hearing threshold than the control group without significance. Additionally, in the positive STS results, the positive mean different hearing threshold of the study group demonstrated the lower results of an increase in hearing threshold than the control group at frequencies of 250 Hz, 3 KHz, 4 KHz, 6 KHz, and 8 KHz without significance.
TABLE 3.
Subgroup analysis of STS between two groups
| Frequency (Hz) | Ears | Mean difference (post − pre) hearing threshold dB HL | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Negative STS (improvement in hearing threshold) | Positive STS (increase in hearing threshold) | ||||||||
| Study | Control | Study | Control | ||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| 250 | Right | −3.182 | 6.692 | −1.833 | 6.086 | 6.087 | 6.208 | 6.512 | 5.725 |
| Left | −3.100 | 6.218 | −2.568 | 5.965 | 1.400 | 5.686 | 5.875 | 6.969 | |
| 500 | Right | −2.727 | 5.678 | −2.833 | 5.522 | 3.043 | 8.082 | 2.674 | 6.487 |
| Left | −2.900 | 5.722 | −3.649 | 4.662 | 2.800 | 5.788 | 2.375 | 6.503 | |
| 1000 | Right | −2.727 | 5.166 | −3.333 | 5.142 | 1.304 | 5.481 | 1.860 | 5.003 |
| Left | −3.300 | 6.195 | −3.514 | 4.694 | 1.000 | 5.000 | 2.500 | 5.189 | |
| 2000 | Right | −3.273 | 6.102 | −3.167 | 4.251 | 3.696 | 5.049 | 1.744 | 3.761 |
| Left | −2.900 | 5.258 | −1.892 | 4.142 | 0.800 | 4.933 | 1.625 | 5.357 | |
| 3000 | Right | −5.545 | 5.906 | −3.833 | 5.363 | 1.304 | 4.322 | 1.279 | 5.128 |
| Left | −3.600 | 5.349 | −3.514 | 4.227 | 0.600 | 4.163 | 1.625 | 6.736 | |
| 4000 | Right | −3.273 | 6.471 | −4.167 | 6.029 | 0.435 | 5.623 | 2.558 | 5.913 |
| Left | −2.800 | 4.861 | −4.189 | 6.402 | 2.600 | 7.089 | 3.750 | 7.824 | |
| 6000 | Right | −5.000 | 7.071 | −2.500 | 5.835 | 4.130 | 7.783 | 5.581 | 9.711 |
| Left | −2.700 | 7.299 | −2.568 | 6.414 | 3.400 | 8.505 | 4.375 | 7.778 | |
| 8000 | Right | −4.000 | 6.625 | −4.667 | 6.288 | 3.261 | 7.008 | 5.465 | 10.846 |
| Left | −3.200 | 6.606 | −4.324 | 7.743 | 0.800 | 6.238 | 5.375 | 10.401 | |
Note: In the negative STS results, the negative mean different hearing threshold of the study group showed the better results of improvement in hearing threshold than the control group without significance. Additionally, in the positive STS results, the positive mean different hearing threshold of the study group demonstrated the lower results of increase in hearing threshold than the control group at frequencies of 250 Hz, 3 KHz, 4 KHz, 6 KHz, and 8 KHz without significance. Independent Samples t Test.
Abbreviation: STS, significant threshold shift.
There were 16 ears detecting a warning sign of permanent hearing loss from both groups but the comparison did not show the statistical significance (Table 1). However, the participants having a risk of permanent hearing loss from the control group were affected by longer duration of tinnitus with statistical significance (p = .042) (Table 4). Moreover, the participants in the study group and the control group reported the side effects of the medication during the 3‐week protocol such as dizziness, fainting, nausea, diarrhea, vertigo, and headache (Table 5). More participants in the study group experienced side effects of the medication but without significance. Although nicergoline might increase the uric acid levels in blood serum, the study demonstrated increased uric acid levels in both groups without significance. The comparison of uric acid levels in blood serum was shown in Table 6.
TABLE 4.
The comparison of the duration of tinnitus
| Study group | Control group | p‐value | |
|---|---|---|---|
| STS | |||
| Number of ears (%) | 5 (3.268%) | 8 (5.333%) | |
| Duration of tinnitus (h) | 0.113 ± 0.126 | 9.138 ± 12.308 | .135 |
| Negative STS | |||
| Number of ears (%) | 1 (0.952%) | 0 | |
| Duration of tinnitus (h) | 0.1 | 0 | |
| Positive STS | |||
| Number of ears (%) | 4 (8.333%) | 8 (9.369%) | |
| Duration of tinnitus (h) | 0.117 ± 0.145 | 9.138 ± 12.308 | .077 |
| Early warning sign of permanent hearing loss | |||
| Number of ears (%) | 8 (5.2%) | 8 (5.3%) | |
| Duration of tinnitus (h) | 0.583 ± 0.114 | 6.104 ± 11.026 | .042 a |
Abbreviations: STS, significant threshold shift.
Mann–Whitney U test.
TABLE 5.
The adverse effects of the medications
| Study N (%) | Control N (%) | p‐value | |
|---|---|---|---|
| Dizziness | 11 (9.24%) | 8 (7.61%) | .672 |
| Fainting | 8 (6.72%) | 3 (2.86%) | .183 |
| Nausea | 1 (0.84%) | 2 (1.9%) | .490 |
| Diarrhea | 1 (0.84%) | 2 (1.9%) | .490 |
| Vertigo | 3 (2.52%) | 8 (7.61%) | .082 |
| Headache | 11 (9.24%) | 10 (9.52%) | .942 |
Note: χ 2 test; Mann–Whitney U test.
TABLE 6.
The comparison of the serum uric acid levels
| Uric acid a (3.4–7.0 mg/100 ml) | Study group | Control group | p‐value b | p‐value c within study group | p‐value c within control group |
|---|---|---|---|---|---|
| Baseline | 6.17 ± 1.16 | 6.19 ± 1.29 | .843 | ||
| 2 weeks | 6.26 ± 1.31 | 6.29 ± 1.26 | .806 | .378 | .345 |
| Change | 0.09 ± 1.04 | 0.12 ± 1.04 | .747 |
Uric acid levels of Thai population adapted from Pongpaew P, Saovakontha S, Schelp F P, Serum uric acid level of Thai individuals in comparison with the nutritional status and some other physical and biochemical parameters, Am J Clin Nutr. 1977 Dec;30(12):2122–5. doi: 10.1093/ajcn/30.12.2122.
Independent t‐test.
Paired t‐test.
4. DISCUSSION
According to many previous studies demonstrating the effectiveness of medications such as Magnesium, 12 NAC, 14 or d‐methionine 15 to attenuate NIHL, these clinical trials assessed the prevention of PTS associated with multi‐day weapon training activities. Additionally, this study is the pilot to determine whether nicergoline attenuates NIHL (TTS) among the participants attending the annual firing practice. As the results demonstrated in Tables 1, 2, and Figure 2, these findings suggested that the participants from the study group were less suffered from TTS. These results were compatible with the previous studies. 12 , 14 , 15 , 25 The effect of the nicergoline treatment, coefficients, shown for the right ear was an estimated −1.6 dB, which showed thresholds improved. These results demonstrated better average threshold shift compared with that of the control one. A smaller but significant group effect was demonstrated for the left ear (Table 2). The authors distinguished the “early warning sign of permanent hearing loss” from the TTS since the subjects with the early warning sign of permanent hearing loss demonstrated the post‐noise exposure hearing thresholds >25 dB HL, and the tinnitus lasted longer in the control group with statistical significance (Table 4). In Table 3, the subgroup analysis demonstrated that the study group revealed lower mean different hearing thresholds, especially in the right ears, than the control group at a number of frequencies. According to Kopke et al. 14 firing an M‐16 rifle increased thresholds by about 6 dB (SPL) for the trigger hand ear for right‐handed shooters. The participants often showed a shift at a single frequency, but not at adjacent frequencies. 14 Consequently, it was relatively difficult to diagnose positive STS among the participants. In the present study, the results indicated that the mean hearing thresholds in the study group significantly improved after participants attended the firing practice, maybe associated with the cognitive or arousal enhancement properties of nicergoline. 10 , 17 , 18 These results could imply that participants in the study group might benefit from the indirect effects of nicergoline. This represents a possible confound that could be investigated and controlled in a future study.
Based on the results from animal experimentation, McArthur et al. 10 indicated that intake of nicergoline for 11 weeks was an effective cognitive enhancer in a learning model of age‐related deficits. It remains unclear whether nicergoline effects cognitive function in young adults. A future study using the standard firing practice protocol for military personnel in Thailand could confirm whether nicergoline reduces TTS even after controlling for cognitive effects. In addition, it could begin to address limitations of the research regarding the effect of nicergoline on cognitive function in young adults.
From the previous studies, 14 , 16 there are limited data on the duration of tinnitus. In the control group participants in the present study, those diagnosed as having an “early warning sign of permanent threshold shift” also had the longest duration of tinnitus. However, the number of the participants affected by tinnitus was relatively small, and the duration of protocol was relatively short. Future studies could be conducted to determine whether nicergoline is effective in preventing PTS and/or cochlear synaptopathy 28 , 29 leading to tinnitus. According to the study conducted by Boismare and Lefrancois, 24 nicergoline affects the cardiovascular system. Intravenous nicergoline (5 mg) made the patients in that study sustain lowered blood pressure, bradycardia, and elevated cardiac output. This could explain why more participants from the study group reported side effects of nicergoline in the present study. Another side effect of nicergoline is asymptomatic hyperuricemia which is characterized by increased serum uric acid levels without clinical presentation. Additionally, the normal range of serum uric acid levels of the Thai population is referred to 3.4–7.0 mg/100 ml. 30 In the present study, we found that the serum uric acid levels were elevated in both groups but without significance compared with the reference values. Although, the incidence of STS was higher than previous studies, 12 , 14 , 15 the number of participants suffered from tinnitus was relatively small (Table 4). Additionally, the results demonstrate that using HPD with nicergoline may prevent the military personnel who attend the short period of weapon training activities from the TTS and tinnitus. Another advantage of nicergoline is that the tablets are contained in an aluminum blister which is portable and durable. Military personnel can take it instantly, as well.
There are some limitations of the study. Safety profiles of nicergoline dosage are limited for women with pregnancy or lactation. Therefore, female participants were not enrolled in the study. The duration of drug administration was relatively short (3 weeks). Moreover, there was the limitation of the audiometry. Auditory tests were not included that might reveal auditory nerve degeneration, which could occur after the participants were exposed to intense noise, but might not increase hearing thresholds. 28 , 29 Additionally, the schedule of Naval basic military training was strict and could cause the appointment for post‐noise exposure audiometry to be delayed, causing some participants to be excluded from this study. Finally, the participants moved to other areas every 3 weeks. Consequently, it was not possible to determine whether the participants were affected by PTS.
5. CONCLUSION
The study results suggest that nicergoline may attenuate TTS and self‐reported tinnitus duration following noise exposure. Additionally, few serious adverse effects of nicergoline were shown. Further investigation on the effectiveness of nicergoline as an otoprotectant is warranted.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
ACKNOWLEDGMENTS
The authors are grateful to Mr. Apichart Treeprapankit for data collection, Miss Supak Ukritchon for valuable statistical analysis, and Mrs. Kornkanok Supawaropas for English improvement. This study was supported by Siam Pharmaceutical Co., LTD.
Klamkam P, Pagcharoenpol R, Treesaranuwattana T, et al. A clinical trial of nicergoline to prevent temporary threshold shift. Laryngoscope Investigative Otolaryngology. 2022;7(2):515‐522. doi: 10.1002/lio2.746
REFERENCES
- 1. Oishi N, Schacht J. Emerging treatments for noise‐induced hearing loss. Expert Opin Emerg Drugs. 2011;16(2):235‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Le TN, Straatman LV, Lea J, Westerberg B. Current insights in noise‐induced hearing loss: a literature review of the underlying mechanism, pathophysiology, asymmetry, and management options. J Otolaryngol Head Neck Surg. 2017;46(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Affairs DoV. VBA annual benefits report fiscal year 2020. 2020.
- 4. Yong JS, Wang DY. Impact of noise on hearing in the military. Mil Med Res. 2015;2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Campbell K, Hammill T, Hoffer M, Kil J, Le Prell C. Guidelines for auditory threshold measurement for significant threshold shift. Otol Neurotol. 2016;37(8):e263‐e270. [DOI] [PubMed] [Google Scholar]
- 6. Tunkel DE, Bauer CA, Sun GH, et al. Clinical practice guideline: tinnitus. Otolaryngol Head Neck Surgery. 2014;151(2 Suppl):S1‐s40. [DOI] [PubMed] [Google Scholar]
- 7. Slepecky N. Overview of mechanical damage to the inner ear: noise as a tool to probe cochlear function. Hear Res. 1986;22:307‐321. [DOI] [PubMed] [Google Scholar]
- 8. Poirrier AL, Pincemail J, Van Den Ackerveken P, Lefebvre PP, Malgrange B. Oxidative stress in the cochlea: an update. Curr Med Chem. 2010;17(30):3591‐3604. [DOI] [PubMed] [Google Scholar]
- 9. Sha SH, Taylor R, Forge A, Schacht J. Differential vulnerability of basal and apical hair cells is based on intrinsic susceptibility to free radicals. Hear Res. 2001;155(1–2):1‐8. [DOI] [PubMed] [Google Scholar]
- 10. McArthur RA, Carfagna N, Banfi L, et al. Effects of nicergoline on age‐related decrements in radial maze performance and acetylcholine levels. Brain Res Bull. 1997;43(3):305‐311. [DOI] [PubMed] [Google Scholar]
- 11. Henderson D, Bielefeld EC, Harris KC, Hu BH. The role of oxidative stress in noise‐induced hearing loss. Ear Hear. 2006;27(1):1‐19. [DOI] [PubMed] [Google Scholar]
- 12. Attias J, Weisz G, Almog S, et al. Oral magnesium intake reduces permanent hearing loss induced by noise exposure. Am J Otolaryngol. 1994;15(1):26‐32. [DOI] [PubMed] [Google Scholar]
- 13. Lin CY, Wu JL, Shih TS, et al. N‐Acetyl‐cysteine against noise‐induced temporary threshold shift in male workers. Hear Res. 2010;269(1–2):42‐47. [DOI] [PubMed] [Google Scholar]
- 14. Kopke R, Slade MD, Jackson R, et al. Efficacy and safety of N‐acetylcysteine in prevention of noise induced hearing loss: a randomized clinical trial. Hear Res. 2015;323:40‐50. [DOI] [PubMed] [Google Scholar]
- 15. Campbell KCM. Phase 2 Clinical Trials: D‐Methionine to Reduce Noise‐induced Hearing Loss. Final report for award W81XWH‐11‐C‐0033. Southern Illinois University School of Medicine, Springfield, Illinois, 62794, U.S. Army Medical Research and Materiel Command Fort Detrick M; 2016.
- 16. Le Prell CG, Hammill TL, Murphy WJ. Noise‐induced hearing loss and its prevention: integration of data from animal models and human clinical trials. J Acoust Soc Am. 2019;146(5):4051‐4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Winblad B, Fioravanti M, Dolezal T, et al. Therapeutic use of nicergoline. Clin Drug Investig. 2008;28(9):533‐552. [DOI] [PubMed] [Google Scholar]
- 18. Saletu B, Garg A, Shoeb A. Safety of nicergoline as an agent for management of cognitive function disorders. Biomed Res Int. 2014;2014:610103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tanaka M, Yoshida T, Okamoto K, Hirai S. Antioxidant properties of nicergoline; inhibition of brain auto‐oxidation and superoxide production of neutrophils in rats. Neurosci Lett. 1998;248(1):68‐72. [DOI] [PubMed] [Google Scholar]
- 20. Sortino MA, Battaglia A, Pamparana F, Carfagna N, Post C, Canonico PL. Neuroprotective effects of nicergoline in immortalized neurons. Eur J Pharmacol. 1999;368(2–3):285‐290. [DOI] [PubMed] [Google Scholar]
- 21. Humes LJ, Joellenbeck LM, Durch J, et al. Noise and military service implications on hearing loss and tinnitus. In: Humes LJ, Joellenbeck LM, Durch JS, eds. Noise and Noise‐Induced Hearing Loss in Military. Institute of Medicine of The National Academes; 2005:81. [Google Scholar]
- 22. The noise attenuation of the 3M E‐A‐R Classic Ear plugs [Internet]. 2020. .
- 23. Fioravanti M, Nakashima T, Xu J, Garg A. A systematic review and meta‐analysis assessing adverse event profile and tolerability of nicergoline. BMJ Open. 2014;4(7):e005090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boismare F, Lefrançois J. Haemodynamic effects of nicergoline in man at rest and during exercise. Clin Exp Pharmacol Physiol. 1980;7(2):105‐112. [DOI] [PubMed] [Google Scholar]
- 25. (ASHA) AS‐L‐HA . Degree of hearing loss 1997–2021. https://www.asha.org/public/hearing/degree-of-hearing-loss/
- 26. (NIOSH) TNIfOSaH . Noise and hearing loss prevention. https://www.cdc.gov/niosh/topics/noise/preventhearingloss/hearlosspreventprograms.html
- 27. DoD Instruction 6055.12 Hearing Conservation Program (HCP) [Internet]. 2019. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/605512p.pdf%3Fver=2019-08-14-073309-537
- 28. Bramhall NF, McMillan GP, Gallun FJ, Konrad‐Martin D. Auditory brainstem response demonstrates that reduced peripheral auditory input is associated with self‐report of tinnitus. J Acoust Soc Am. 2019;146(5):3849‐3862. [DOI] [PubMed] [Google Scholar]
- 29. Liberman MC, Kujawa SG. Cochlear synaptopathy in acquired sensorineural hearing loss: manifestations and mechanisms. Hear Res. 2017;349:138‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pongpaew P, Saovakontha S, Schelp FP. Serum uric acid level of Thai individuals in comparison with the nutritional status and some other physical and biochemical parameters. Am J Clin Nutr. 1977;30(12):2122‐2125. [DOI] [PubMed] [Google Scholar]


