Abstract
Objectives
To examine the degree of agreement between MRI and histologically generated volumetric measurements of residual injection laryngoplasty material.
Methods
Following left recurrent laryngeal nerve transection, rabbit vocal cords were injected with jellyfish collagen, Cymetra®, or Restylane®. Laryngeal tissue was harvested 4 or 12 weeks post injection followed by MRI imaging and histologic cross‐sectioning. Two raters estimated the volume of remaining injection material in specimens within MRI and histologic axial cross sections. Wilcoxon signed rank tests were employed to detect gross differences between inter‐rater measurements and between imaging modalities across time. Agreement between rater measurements and imaging (histology and MRI) was assessed using intra‐class correlation coefficients.
Results
Data was available from 16 rabbits sacrificed at 4 weeks (n = 8) and 12 weeks (n = 8). Inter‐rater testing of MRI imaging revealed no significant differences (p > .05) between rater measurements across time points, and excellent agreement (0.93; 95% confidence interval 0.80–0.98) while histologically estimated volumes demonstrated a significant difference at 4 weeks (p < .05) and overall good agreement (0.89; 95% confidence interval 0.59–0.97). Comparison of MRI and histologically estimated volume measurements revealed significant differences at the 4‐week time point (p < .05) but not at 12 weeks (p > .05). Overall, there is only moderate agreement between MRI and histology estimates (0.72; 95% confidence interval 0.22–0.90).
Conclusions
MRI imaging demonstrates good reliability and similar estimates of volume to histologically estimated measurements of residual injection laryngoplasty material at time points clinically relevant for future injection laryngoplasty experiments.
Level of Evidence
NA.
Keywords: Cymetra®, histology, injection laryngoplasty, Jellagen®, jellyfish collagen, micronized acellular dermis, MRI, Restylane®
MRI and histologically generated volumetric measurements of residual injection laryngoplasty material were compared over several time points in a rabbit model of injection laryngoplasty using an assortment of injection laryngoplasty materials including jellyfish collagen, Cymetra®, or Restylane®. MRI demonstrated good reliability and similar estimates of volume to histologically estimated measurements of residual injection laryngoplasty material at time points clinically relevant for future injection laryngoplasty experiments.
1. INTRODUCTION
Injection laryngoplasty (IL) is a common otolaryngologic procedure for unilateral focal fold (VF) paralysis that aims to restore upper glottal competence caused by VF denervation secondary to idiopathic, iatrogenic, or traumatic events. VF paralysis causes the upper larynx to remain open during deglutition resulting in food aspiration into the airways. Historically, clinicians have utilized a variety of biomaterials for IL, ranging from the pioneering work of Bruening using paraffin injections to the recently FDA‐approved silk microparticles, currently in Phase I clinical trial. 1 To date, micronized acellular dermis (MACD; Cymetra®), cross‐linked hyaluronic acid (X‐HA; Restlyane®), and calcium hydroxyapatite (Prolaryn Plus®) are dominating the clinical space. Of the three, MACD is the only biomaterial that contains collagen, among other dermal‐derived proteins. Since collagen plays a critical role in cell growth, renewal and tissue regeneration, our group and others are investigating the use of collagen‐based biomaterials in IL. These investigations are initially performed in animal models, 2 , 3 , 4 but there is currently no consensus or established method of analyzing the residual volume of injectable material following the procedure. In previous experiments, we estimated residual volume through the calculation of the volume of an ellipsoid (an assumption made of the shape of the injected material within the paraglottic space) using measurements obtained through cross‐sectional analysis of histologic specimens from sacrificed animal larynges. 5 , 6
More recently we reported on our investigation of a novel jellyfish collagen substance (JC; Jellagen®) for IL, using MRI for volume estimation. 7 While MRI was not previously used for this purpose, there is experience with it for examining the relationship between foreign injected material and the native laryngeal parenchyma. 8 , 9 , 10 MRI has two potential advantages over histological measurements, namely digital visual access to the entire residual volume, eliminating the need for any assumptions about the shape of remaining injectate. Therefore, the purpose of this study was to perform a comparative analysis of the residual volumes obtained from MRI and histology using a clinically relevant and well‐documented rabbit model of IL.
2. METHODS
2.1. Animals
The animal model used in this investigation and the methodology employed for completing these experiments are described in a recently completed investigation. 7 It will be briefly highlighted below. After Institutional Animal Care and Use Committee (IACUC) approval (IACUC A4201), 17 three‐week‐old female New Zealand White rabbits underwent recurrent laryngeal nerve sectioning under anesthesia. Following a 2‐week recovery, animals were re‐anesthetized and received left IL with 100 μl of JC (225 mg/ml), MACD (275 mg/ml), or X‐HA (20 mg/ml). Injections of 100 μl were placed lateral to the vocal process as described before. 7 One animal in the Restylane group received 110 μl. Animals were euthanized 4 weeks or 12 weeks after injection. Laryngeal specimens were analyzed by MRI imaging and then sectioned for histologic measurements.
2.2. MRI and volumetric measurements
Prepared laryngeal specimens underwent MRI analysis using an Avance III 300 MHz (7 Tesla) wide bore NMR spectrometer equipped with micro‐imaging accessories (Bruker, BioSpin, Billerica, MA) and a 20 mm diameter volume coil. Samples were prepared and imaged as described previously. 7 A multi‐slice image collection of 26 separate 0.5 mm thick digital cross sections of the larynx starting from the cricothyroid cartilage moving superiorly to the end of the specimen was generated for each animal. A short scan (3–5 slices) was initially performed to ensure that medialized material was effectively captured within the multi‐slice collection. After generation of the multi‐slice collection, two independent raters reviewed the 26‐slide collection, identifying medialized material within each digital cross section. A freeform tool in Analyze imaging software (Mayo Clinic) was used to outline the edge of the medialized material (Figure 1); the software calculated the area within the outline (mm2). The volume in μl (1 mm3 = 1 μl) of each slice was calculated by multiplying the area by the slice thickness of 0.5 mm. All slice measurements for a single larynx were added to determine the volume of remaining material at 4‐ and 12 weeks post IL from MRI images.
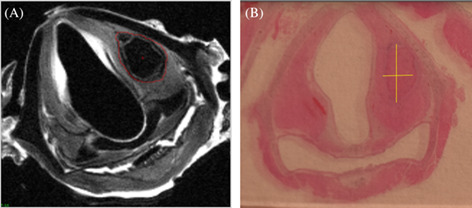
FIGURE 1.
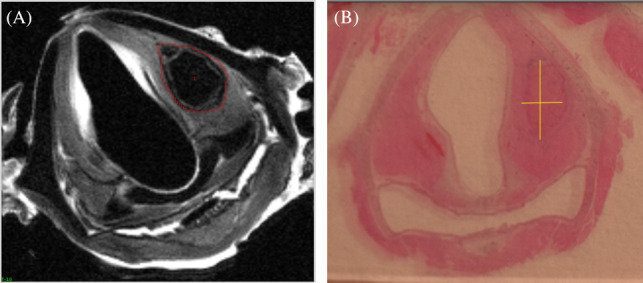
Axial MRI and histologic cross sections. The above images were obtained from laryngeal tissue from a rabbit harvested 12 weeks post injection laryngoplasty with Jellagen®. (A) represents a 0.5‐mm thick MRI axial cross section, with a free form line clearly demarcating residual jellyfish collagen material within the left vocal cord. (B) represents a 5‐μm thick histology axial cross section where the jellyfish collagen is visualized within the left vocal cord, with straight lines marking the short and long axis of the material. This was the same manner in which volume calculations were initiated
2.3. Histologic preparation and volume estimation
Once MRI imaging was completed, fixed tissues were embedded in paraffin, cut and stained with hematoxylin and eosin as previously described. 5 , 6 Briefly, each paraffin‐embedded larynx was divided into three blocks of equal length to generate top, middle, and bottom portions of the specimen and then 5 μm thickness sections from each block were layered onto slides for a total of 32 slides per larynx. This resulted in 96 separate 5 μm sections across the entire length of the larynges. Because of cost and convenience considerations we did not have access to all slices within the three paraffin blocks (i.e., 26 × 100 = 2600) for each larynx so we inferred laryngeal ellipsoid volumes from the available data. ImageJ software (https://imagej.nih.gov/ij/) was used to estimate the volume of remaining injected material within histology axial cross sections (μl), which was assumed to take the shape of an ellipsoid, using the formula:
where V is the ellipsoid volume (μl), L and W are the largest axial lengths and widths visualized within the 5 μm sections (Figure 1). H represents the height of the specimen which was estimated from the 7 T MRI imaging. As with MRI imaging, two independent evaluators generated an estimate of the size of injection remaining.
2.4. Statistical analysis
Given limited sample size among the different injectable groups, JC, MACD, X‐HA data were analyzed together across with data only stratified at 4‐week and 12‐week time points. To detect differences between rater measurements for MRI and histologically estimated volumes, Wilcoxon‐rank sum tests were performed at both 4‐week and 12‐week time points. This testing was followed by an assessment of intra‐class correlation coefficients to quantify the degree of agreement between rater measurements. 11 Due to sample size limitations, 4‐week and 12‐week data were combined when calculating the intra‐class correlation coefficients between the raters' MRI and histology volume estimates.
We used the same approach from our inter‐rater analysis for comparing MRI and histologically generated volumes. First, the mean MRI and histologic volumes for each laryngeal specimen were generated. A Wilcoxon ranked sum test was performed to assess differences between MRI and histologically generated measurements across 4‐week and 12‐week time points. The measure of agreement between MRI and histology measurement was assessed using the intra‐class correlation coefficient with 4‐week and 12‐week data again combined secondary to sample size limitations.
Mean residual injection volumes are presented as means ± standard deviation. For Wilcoxon rank‐sum testing, a p value of <.05 was used for significance. Intra‐class correlation coefficients are presented with a 95% confidence interval and with interpretating of their values using the following guidelines: coefficients <0.50 indicating poor agreement, coefficients between 0.50 and 0.75 indicating moderate agreement, coefficients between 0.75 and 0.90 indicating good agreement, and >0.90 indicating excellent agreement. 11 Statistical analysis was performed using either JMP (JMP Pro, 2014) and SAS version 9.4 (SAS Institute, Cary, NC).
3. RESULTS
Seventeen rabbits underwent IL with JC (N = 6), MACD (N = 5), or X‐HA (N = 6). Larynges from 8 rabbits (JC, N = 3; MACD, N = 3; X‐HA, N = 2) were collected 4 weeks after injection while the tissue from the remaining animals were collected at 12 weeks post injection. Residual injectate was not identifiable in one histologic sample (X‐HA, 12 weeks), therefore that sample was not included in subsequent analyses. In all samples, histologically determined volumes were below the known injection volume. In one sample (JC, 4 weeks), raters calculated the remaining volume to be greater than the injected volume (140 and 137 μl) by MRI. This was still included in the analysis.
3.1. Inter‐rater analysis of MRI volumes
MRI measurements calculated by each rater are depicted in Figure 2. No statistically significant inter‐rater differences were found between mean rater volumes for MRI values at 4 weeks (56.6 and 69.1 μl, respectively, mean difference 12.5 ± 23.5 μl, p > .05, n = 8) or 12 weeks (17.5 and 17.1 μl, respectively, mean difference 0.35 ± 0.98 μl, p > .05, n = 8). Furthermore, comparison of all MRI volumes estimated between the two raters generated an intra‐class correlation coefficient of 0.93 (95% CI 0.80–0.98), indicating excellent agreement.
FIGURE 2.
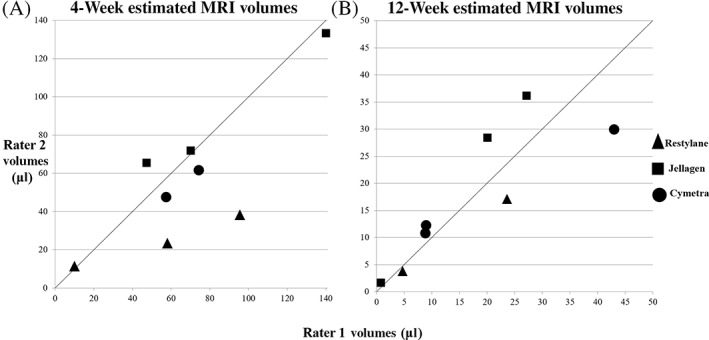
Estimated MRI volumes between two separate raters. (A) Four‐week MRI volumes determined by rater 2 are graphed against values reported by rater 1. (B) Twelve‐week MRI volumes are shown as in (A), n = 8. The line indicates where matching volumes from each rater would appear if estimates were identical
3.2. Inter‐rater analysis of histology volumes
Individual histologic volume measurements calculated by each rater for each laryngeal specimen is depicted in Figure 3. Values between mean histologic volumes between the two raters were significantly different at 4 weeks (32.5 and 44.8 μl respectively, mean difference of 12.4 ± 8.3 μl, p < .05) but were similar at 12 weeks (11.4 and 14.8 μl, respectively, mean difference of 3.4 ± 12.8 μl, p > .05). Comparison of all histology volumes between the two raters generated an intra‐class correlation coefficient of 0.89 (95% CI 0.59–0.97), indicating good agreement.
FIGURE 3.
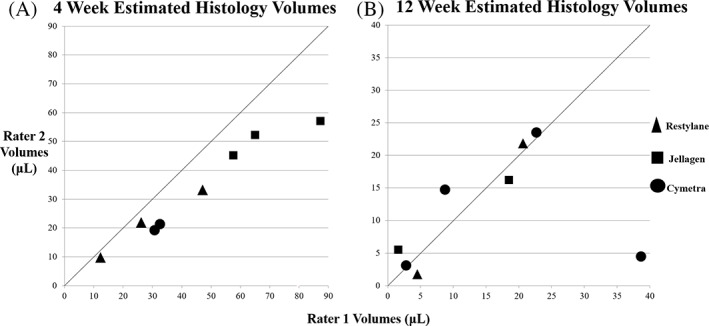
Estimated histology volumes between two separate raters. (A) Four‐week histology volumes determined by rater 2 are graphed against values reported by rater 1. (B) Twelve‐week histology volumes are shown as in (A), n = 8. The line indicates where matching volumes from each rater would appear if estimates were identical
3.3. Comparison of MRI and histologically estimated volumes
Figure 4 shows histology volumes for each larynx plotted against their corresponding MRI volumes. MRI and histologic volumes were significantly different at 4 weeks (62.9 and 38.7 μl, respectively, mean difference of 24.2 ± 20.9 μl, p < .05) from each other but were similar at 12 weeks (17.3 and 13.1 μl respectively, mean difference of 4.2 ± 5.8 μl, p > .05). Comparison of the agreement between all MRI volumes and histologically estimated volumes generated an intra‐class correlation coefficient of 0.72 (95% CI 0.22–0.90), indicating moderate agreement between MRI and histology volumes.
FIGURE 4.
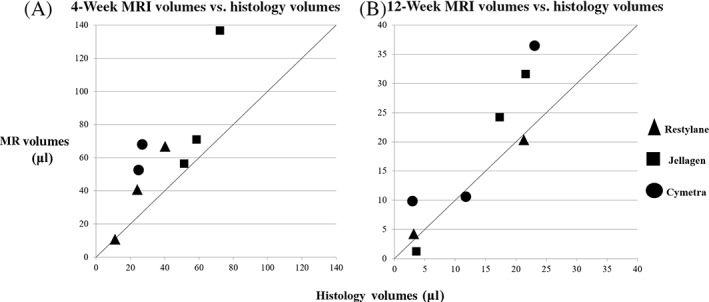
Estimated MRI and histologic estimates of the residual volume. (A) Four‐week MRI volumes are graphed against histology volumes. (B) Twelve‐week MRI and histology volumes are shown as in (A), n = 8. The line indicates where matching volumes from each method would appear if estimates were identical
4. DISCUSSION
There is no consensus on the method to estimate the residual volume of material following IL in animal models. Our investigation compared MRI to histologically estimated volume measurements using a two‐rater system that was previously shown to yield a useful analysis of data reliability. 5 , 6 Our results suggest that MRI measurements demonstrates excellent agreement among raters across 4‐week and 12‐week time points and provides results that are comparable to histological estimated volumes at 12 weeks.
IL is a common procedure employed by otolaryngologists to treat a variety of patients with laryngeal pathology including vocal cord paralysis and paresis. In the past, substances such as Cymetra® and Restylane® have dominated this clinical space over the last decade. Recently however, Cymetra® has been discontinued due to new FDA regulations regarding ease of use for IL injectables (senior author email correspondence). This has spurred intense interest in identifying new materials that are safe and provide a lasting effect as their predecessors. 2 , 10
We previously evaluated the longevity of MACD with and without augmentation with adipose derived stem cells using a rabbit model at 4‐ and 12‐week time points. 6 At that time, we developed a histological method to measure residual injectate that was based on a previous study evaluating subdermal injections of MACD in a canine model. 12 The dimensions of this presumed ellipsoid were estimated by identifying the laryngeal cross section with the greatest area of material. However as is required with histology‐based analysis of fixed paraffin‐embedded tissue, where slices of sectioned tissue must be micrometers in thickness, we were limited in our ability to perform cross sectioning of our entire laryngeal specimens, given that attempting such an endeavor would require the generation of thousands of slides. Additionally, with tissue fixation, there is known contraction of specimens, 13 , 14 thus making measuring the absolute true volume impossible. These limitations were addressed by assuming the final shape of the injection material resembles an ellipsoid and inferring the volume of the material remaining from a small collection of tissue sections. While histologic analysis served us well in our prior experiments, there was a conscious effort to identify a different methodology more amenable for performing whole organ volumetry.
MRI imaging is now more readily available and economically feasible for lab experimentation at our institution, as is occurring at most major institutions in the United States. For this reason, we proceeded to use this imaging modality to estimate the residual volume of JC in a rabbit model. 7 Our desire to use this imaging modality was partially motivated by animal experiments using this modality to assess the intrinsic behavior of injection laryngoplasty material with the native laryngeal parenchyma. 8 , 9 , 10 Additionally, MRI imaging in theory provides further benefits that histologic estimation does not, including allowing for a larger portion of the material to be included in the estimation of residual volume. Finally, as this modality is non‐invasive and can be done in vivo, there is the potential for future injection laryngoplasty experiments to obtain repeated estimates of residual volume size within the same animal, increasing the confidence in conclusions drawn from studies. Additionally, MRI imaging provides certain advantages over histologic estimation, such as incorporating nearly the whole residual specimen for estimation of residual volume, as well as the potential for repeated in vivo measurements without sacrifice of the animal. Finally, MRI volume estimation does not require the assumption of the final shape of the injected material in specimen, further increasing the confidence of conclusions drawn from these studies.
We recently completed an investigation of novel jellyfish collagen, assessing for residual injectate volumes at 4 weeks and 12 weeks post injection. 7 For this investigation we sought to use the MRI modality for assessment of residual volume, unlike in our previous experiments. 5 , 6 Given our intention to perform further experiments, preferably using MRI as the primary measuring tool, the following experiment sought to determine whether MRI estimates provide volumes that are vastly different from estimates generated by our former measuring modality. We also used this study to assess for inter‐rater reliability for MRI and histology modalities. Through our study we found that MRI measurements were not statistically different between raters both at 4‐week and 12‐week time points. Additionally, intra‐class coefficient analysis indicated excellent agreement between two separate raters using MRI imaging to estimate volume. In contrast, histological estimations were significantly different at 4‐weeks between the raters and intra‐class coefficient analysis indicated only good agreement, suggesting that MRI provides more consistent results between raters.
When MRI measurements were compared to histologically calculated volumes, we found that MRI images consistently provided statistically larger estimates of the residual volume at the 4‐week time point. MRI estimates were also larger on average than histologically generated volumes at 12 weeks, however, the difference was not statistically significant. Intra‐class correlation analysis indicated the two methods showed only moderate agreement; likely this is due to the differences in measurement at the 4‐week time point. However, the 4‐week time point is of less clinical interest than 12 weeks, which future studies would likely choose as a time point or further post injection. Given the strong agreement between mean MRI and histology volumes at 12 weeks, a more clinically relevant time point, we are confident that MRI is reliable across raters and provides accurate estimates of the size of material remaining post injection.
Our study has several limitations. First, there was an animal that was excluded from the experiment when residual material was detectable by MRI, but not on histological examination. This indicates the potential for false detection of material by MRI imaging. Furthermore, at the 4‐week time point, one animal injected with X‐HA was estimated by MRI to have a residual volume larger than the volume reported during IL. As both specimens were collected 4 weeks after the injection, when inflammation may not have fully resolved, distinguishing between residual material and native laryngeal parenchyma can lead to the overestimation of residual volume. This issue with MRI imaging, is not unique to our experiment, given that similar findings were recently reported in an investigation comparing MRI imaging of pancreatic intraductal masses to other imaging modalities. 15 Fortunately, these issues were uncommon in our experiment, and additionally occurred at the 4‐week time point, a time point of less clinical significance compared to the 12‐week time point.
Our study was also limited in the ability to perform a stratified analysis of the level of agreement between MRI and histology based on time point and injection type. We attempted to partially address this issue by performing Wilcoxon ranked sum testing to detect gross differences between residual volumes between the two modalities. Together, we feel that our analyses demonstrate that MRI imaging will be a reliable, sound method to estimate volume in future studies on novel injectable materials.
5. CONCLUSIONS
MRI imaging is an imaging modality that provides repeatable measurements of residual injection volume following injection laryngoplasty in animal model that complements prior estimates from histologic examination. Future investigations of injectable materials can use this modality to follow the results of novel biomaterials, with histologic analysis serving as a failsafe should there be discrepancies in reported volumes.
CONFLICT OF INTEREST
The study authors declare no competing financial interests. SSM is a member of the Scientific Advisory Board for Jellagen® Pty Ltd.
ACKNOWLEDGMENTS
There are no acknowledgements.
Bowen AJ, San‐Marina S, Hunter D, et al. MRI imaging versus histologic volumetric estimation of residual injection laryngoplasty material. Laryngoscope Investigative Otolaryngology. 2022;7(2):454‐459. doi: 10.1002/lio2.744
Meeting Information: This research was presented at the American Laryngological Association 142nd Year Annual Meeting from April 7 to 8, part of the 2021 Combined Otolaryngology Spring Meetings.
Financial Disclosure:Funds for the project were provided in part by Jellagen® Pty Ltd. under a work agreement with the Mayo Clinic, with SSM and DCE as Principal IACUC Investigators.
Funding information Jellagen Pty Ltd
REFERENCES
- 1. Gulka CP, Brown JE, Giordano JEM, et al. A novel silk‐based vocal fold augmentation material: 6‐month evaluation in a canine model. Laryngoscope. 2019;129(8):1856‐1862. [DOI] [PubMed] [Google Scholar]
- 2. Karajanagi SS, Lopez‐Guerra G, Park H, et al. Assessment of canine vocal fold function after injection of a new biomaterial designed to treat phonatory mucosal scarring. Ann Otol Rhinol Laryngol. 2011;120(3):175‐184. [DOI] [PubMed] [Google Scholar]
- 3. Moon IH, Park KN, Kim HK, Lee S. Utility and safety of commercially available injection laryngoplasty materials in a rabbit model. J Voice. 2015;29(1):125‐128. [DOI] [PubMed] [Google Scholar]
- 4. Staskowski PA, Ford CN, Inagi K. The histologic fate of autologous collagen injected into the canine vocal fold. Otolaryngol Head Neck Surg. 1998;118(2):187‐190. [DOI] [PubMed] [Google Scholar]
- 5. Oldenburg MS, Ekbom DC, San Marina S, et al. Preliminary results of tissue‐engineered injection laryngoplasty material in a rabbit model. Laryngoscope. 2018;128(1):160‐167. [DOI] [PubMed] [Google Scholar]
- 6. Oldenburg MS, Janus J, Voss S, et al. Histologic evaluation of micronized AlloDerm after injection laryngoplasty in a rabbit model. Laryngoscope. 2017;127(5):E166‐E169. [DOI] [PubMed] [Google Scholar]
- 7. San‐Marina S, Bowen AJ, Oldenburg MS, et al. mri study of jellyfish collagen, hyaluronic acid, and cadaveric dermis for injection laryngoplasty. Laryngoscope. 2021;131(8):E2452‐E2460. [DOI] [PubMed] [Google Scholar]
- 8. Kruschewsky L d S, de Mello‐Filho FV, dos Santos AC, Rosen CA. Autologous fat graft absorption in unilateral paralyzed canine vocal folds. Laryngoscope. 2007;117(1):96‐100. [DOI] [PubMed] [Google Scholar]
- 9. Herrera VLM, Viereck JC, Lopez‐Guerra G, et al. 11.7 tesla magnetic resonance microimaging of laryngeal tissue architecture. Laryngoscope. 2009;119(11):2187‐2194. [DOI] [PubMed] [Google Scholar]
- 10. Zeitels SM, Lombardo PJ, Chaves JL, et al. Vocal fold injection of absorbable materials: a histologic analysis with clinical ramifications. Ann Otol Rhinol Laryngol. 2019;128(3_suppl):71S‐81S. [DOI] [PubMed] [Google Scholar]
- 11. Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15(2):155‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sclafani AP, Romo T, Jacono AA, McCormick S, Cocker R, Parker A. Evaluation of acellular dermal graft in sheet (AlloDerm) and injectable (micronized AlloDerm) forms for soft tissue augmentation. Clinical observations and histological analysis. Arch Facial Plast Surg. 2000;2(2):130‐136. [DOI] [PubMed] [Google Scholar]
- 13. Tran H, Jan NJ, Hu D, et al. Formalin fixation and cryosectioning cause only minimal changes in shape or size of ocular tissues. Sci Rep. 2017;7(1):12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tran T, Sundaram CP, Bahler CD, et al. Correcting the shrinkage effects of formalin fixation and tissue processing for renal tumors: toward standardization of pathological reporting of tumor size. J Cancer. 2015;6(8):759‐766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huynh T, Ali K, Vyas S, et al. Comparison of imaging modalities for measuring the diameter of intraductal papillary mucinous neoplasms of the pancreas. Pancreatology. 2020;20(3):448‐453. [DOI] [PMC free article] [PubMed] [Google Scholar]


