Abstract
Objectives
(1) To highlight the important causes of chronic and recurrent cough in children. (2) To discuss multidisciplinary approach to management of chronic/recurrent pediatric cough.
Methods
Review of scholarly articles, guidelines, expert panels via PubMed and Google Scholar.
Conclusion
Chronic cough (CC) in children is mainly attributed to persistent bacterial bronchitis, asthma, nonspecific cough, and gastroesophageal reflux disease (GERD) symptoms. A multi‐disciplinary approach is cost‐effective and aids with earlier diagnosis and appropriate treatment. Congenital or acquired narrowing of the subglottis is the leading ENT cause for recurrent croup (RC) in children. Laryngeal cleft‐type 1 is commonly seen in children with recurrent aspiration and CC. Children are usually referred to pulmonologists for wet cough not responding to treatment. Eosinophilic esophagitis (EoE) and GERD should be considered in the differential diagnosis of CC in children with both respiratory symptoms and failure to thrive.
Level of Evidence: 2a
Keywords: chronic cough, gastroenterology, multidisciplinary, otolaryngology, pediatric, pulmonary
1. INTRODUCTION
Chronic cough (CC) is the most common reason for presentation to pediatric and urgent care clinics. 1 , 2 CC in children is associated with impaired quality of life, missed school days, multiple physician visits, 3 and inappropriate use of antibiotics. 4 , 5 Studies 1 , 5 , 6 and systematic reviews 7 , 8 have shown that use of an “algorithmic approach” to cough in children may lead to earlier diagnosis and fewer use of antibiotics. This manuscript outlines the common causes of pediatric CC and highlights those causes that require a multidisciplinary approach among otolaryngologist (ENT), pulmonologist, and the Gastroenterologist (GI). The article is not intended to be a review article on CC, rather it provides guidelines for interdisciplinary referral and management of CC in children.
2. ETIO‐PATHOPHYSIOLOGY OF CC IN CHILDREN
In contrast to adults, cough in children is considered chronic if it lasts >4 weeks. 9 This is because, in children, most acute respiratory infections usually resolve within a 2–4 week‐period. There are major physiological differences in cough response between adults and children. This includes maturation differences of the airway and chest wall musculature as well as age related maturations of the respiratory center and respiratory control of the cough reflex. 10 , 11 , 12 Cough sensitivity in children is influenced by airway caliber and age. 13 The leading causes for CC in children are persistent bacterial bronchitis (PBB), Asthma and gastroesophageal reflux disease (GERD). Table 1 shows the most common causes of CC in children.
TABLE 1.
Important causes of chronic cough in children
| Children younger than 5 years of age | Children older than 5 years of age |
|---|---|
| Infections (viral URI with cough) | Asthma |
| Congenital airway abnormalities such as subglottic stenosis | Infections (viral URI with Cough), sinusitis |
| Protracted bacterial bronchitis | Protracted bacterial bronchitis |
| Foreign body inhalation | Psychogenic cough |
| Asthma | GERD |
| GERD | Post‐nasal drip |
Abbreviation: GERD, gastroesophageal reflux disease.
The American College of Chest Physicians have proposed guidelines and an algorithm 1 to assist clinicians in the management of pediatric CC. Figure 1 outlines a modified algorithm applicable to otolaryngology practices. The evaluation of a child with CC should include a detailed history, physical examination, and testing when appropriate.
FIGURE 1.
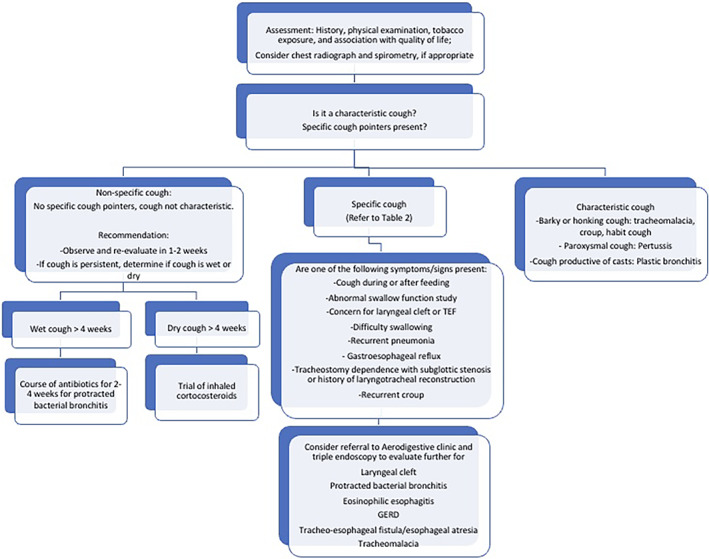
Algorithm for management of chronic cough in children. Modified from 2006 American College of Chest Physicians Algorithm
Patients are divided into patients with a characteristic cough, with specific cough pointers, and without specific cough pointers. Cough pointers essentially signify an identifiable cause or more severe illness. 9 , 14 Cough is considered “specific” when specific cough pointer symptoms and signs are present, and the etiology can be attributed to an underlying abnormality or disease. Cough pointers (Table 2) include nature of the cough (wet or dry), classically recognizable cough sounds (barky croup cough), auscultatory findings such as wheeze, and associated conditions such as digital clubbing, failure to thrive (FTT), feeding difficulties, recurrent pneumonia and abnormalities on chest radiograph or spirometry. Environmental factors such as tobacco smoke exposure, indoor and outdoor allergen exposure should also be evaluated.
TABLE 2.
Specific cough pointers
| Abnormality | Examples of etiology |
|---|---|
| Digital clubbing | Suppurative lung disease |
| Growth failure | Cystic fibrosis |
| Hemoptysis | Suppurative lung disease, vascular abnormalities |
| Hypoxia/cyanosis | Airway or parenchymal disease, cardiac disease |
| Neurodevelopmental abnormality | Aspiration lung disease |
| Recurrent pneumonia | Immunodeficiency, congenital lung abnormalities, TE fistulas, suppurative lung disease, atypical infections |
| Facial pain/purulent nasal discharge | Chronic sinusitis, primary ciliary dyskinesia |
| Recurrent infections | Immunodeficiency |
| Hoarse voice/stridor | Laryngeal cleft/problems, airway abnormalities |
| Choking symptom | Foreign body inhalation |
| Cardiac abnormalities | Associated airway abnormalities, cardiac failure, arrhythmia |
| Chest pain | Arrhythmia, asthma |
| Monophonic wheeze | Large airway obstruction (e.g., foreign body aspiration, malacia and/or stenosis, vascular rings, lymphadenopathy, and mediastinal tumors) |
| Polyphonic wheeze | Asthma, bronchiolitis obliterans, bronchiolitis |
| Daily wet/productive cough | Protracted bacterial bronchitis, suppurative lung disease, recurrent aspiration, atypical infections, TB, diffuse pan‐bronchiolitis |
| Dyspnea or tachypnea or exertional dyspnea | Any airway or parenchymal disease |
| Chest wall deformity | Any pulmonary airway or parenchymal disease |
| Feeding difficulties | Any serious systemic including pulmonary illnesses, aspiration |
| Previous history of chronic lung or esophageal disease (e.g., neonatal lung disease, esophageal atresia) | Multiple causes (e.g., second H‐type fistula, bronchiectasis, aspiration, asthma) |
Abbreviations: TE, trachea‐esophageal fistula; TB, tuberculosis.
3. ROLE OF THE OTOLARYNGOLOGIST IN MANAGEMENT OF CC
Conditions that cause CC seen by a pediatric otolaryngologist differ from those seen by the pediatrician. 15 Otolaryngologists play a unique role in the multidisciplinary management of CC in children by aiding earlier identification of those cases of CC that require diagnostic work up and surgical intervention. They are also an integral part of an Aerodigestive Program. The role of the otolaryngologist in diagnosis and management of specific causes of CC are discussed below (after section on triple endoscopy).
4. ROLE OF THE PULMONOLOGIST IN MANAGEMENT OF CC
As mentioned earlier the common causes of CC in children are asthma, PBB, GERD, and nonspecific cough. Many of these can be initially managed by a non‐pulmonologist. However, if there is failure for the cough to resolve, recurrence, or concern for an underlying disease due to specific cough pointers, referral to a pulmonologist is recommended where they can undergo further evaluation and testing. Radiography (x‐ray or CT), pulmonary function testing, and flexible bronchoscopy (FB) with broncho‐alveolar lavage are diagnostic tests that can be coordinated with a pulmonologist, as needed.
5. ROLE OF GASTROENTEROLOGIST IN MANAGEMENT OF CC
GERD has been postulated as a cause for CC in children, although it appears to be less commonly identified as the etiology in comparison to adults with CC. 16 The mechanism for GERD‐induced cough is not completely clear but is generally hypothesized to be related to micro‐aspiration of refluxed gastric contents into the proximal airway versus a neuronal esophagobronchial reflex triggered by distal esophageal reflux and mediated via the vagus nerve. 5
Furthermore, proving a cause and effect relationship between GERD and CC is difficult, due in part to the lack of a gold standard diagnostic test for GERD in infants and children. 17 Thus given the tenuous relationship between GERD and CC, it is not recommended to use GERD treatments for CC in the absence of other clinical features of GERD, such as recurrent regurgitation, heartburn or chest/epigastric pain. 7 , 17 If the diagnosis of GERD is not clear, objective testing such as esophagogastroduodenoscopy (EGD) with biopsies or combined esophageal multichannel intraluminal impedance with pH monitoring (pH‐MII) may be considered. Endoscopy may reveal evidence of reflux esophagitis or other conditions such as eosinophilic esophagitis (EoE), while pH‐MII monitoring may be especially helpful for assessing the temporal correlation between cough and reflux events. When signs and symptoms or tests consistent with pathological gastroesophageal reflux are present, treatments such as thickened feeds in infants or pharmacological acid suppression with proton‐pump inhibitors (PPIs) or H2 receptor antagonists in older children may be considered on a short‐term trial basis. 17 Indeed, the potential for efficacy in treating CC should be balanced with the emerging concern for possible adverse events related to long‐term PPI use, such as increased risk of infections and bone fractures. 18
6. ROLE OF TRIPLE ENDOSCOPY IN CC
Triple endoscopy involves simultaneous evaluation of the upper airway, lungs and GI tract by an otolaryngologist, pulmonologist, and GI physicians respectively. It is usually performed by physicians as part of multidisciplinary care (aerodigestive team) for a child with recalcitrant CC and dysphagia. Studies 19 , 20 have shown a 20%–40% cost savings for children who are managed comprehensively in a multidisciplinary setting compared to a single specialty evaluation. Therefore, appropriate referral and evaluation of persistent/recalcitrant cough by aerodigestive team (multidisciplinary team) may lead to earlier accurate diagnosis with likely cost savings. In a study by Fracchia et al 21 in children undergoing triple endoscopy for CC, it was noted that 83.5% of the children with CC had at least one abnormal finding. When the abnormal findings in this group were further analyzed by subspecialty, it was found that about 42% had abnormalities in more than one subspecialty area: ENT, GI, and/or pulmonary. PBB was noted to be the leading cause of CC across all subspecialties. 6.4% of patients had otolaryngology abnormalities notably laryngeal clefts (LC). EoE and GERD were major findings noted on GI endoscopy. A longitudinal observational study 22 of 55 patients at a tertiary care aerodigestive program noted that CC was present in 44% of children as the presenting symptom. After undergoing triple endoscopy, 85% of children had a either a new diagnosis or confirmation of a working diagnosis. Similar, to the study by Fracchia et al, 21 LC was the most common abnormal ENT finding, followed by adenoid hypertrophy and vocal cord dysfunction. Pulmonary infection with positive cultures was seen in 73% of children after bronchoscopy and GERD and EoE were among the leading GI causes (Table 3). Both studies underscore the importance of comprehensive inter‐disciplinary management of children with CC and or concurrent other symptoms such as feeding/weight problems. Triple endoscopy in these children not only leads to correct diagnosis with excellent outcome after targeted treatment but also reduces costs and risks associated with repeated anesthesia.
TABLE 3.
Common triple endoscopy findings in children with chronic cough
| Laryngeal clefts |
| Tracheo‐esophageal fistula: congenital or acquired |
| Eosinophic esophagitis |
| Gastro‐esophageal reflux disease |
| Chronic aspiration: Children with neuromuscular disorders |
| Congenital syndromes or genetic abnormalities: Trisomy 21, cystic fibrosis, immunodeficiencies |
6.1. Recurrent croup
Croup is said to be recurrent when a child has 2–3 or more episodes of croup like symptoms within a single season. 23 Croup symptoms primarily occur due to infection with parainfluenza viruses. In contrast, recurrent croup (RC) is considered a symptom entity that can have various underlying causes. 23 , 24 , 25 Allergy, asthma, bronchial hyper‐responsiveness have all been implicated. 26 , 27 Approximately 5%–6% of children will have RC in the first 4 years of their life. 23 , 24 Even though RC does not meet criteria for “CC,” pediatric otolaryngologists are often involved in its management and therefore this entity will be discussed here.
6.1.1. Indications for Direct Laryngoscopy and Bronchoscopy
Children who have either a congenital or an acquired narrowed sub‐glottis are more prone to developing RC symptoms. There is increasing evidence in literature supporting the importance of performing Direct Laryngoscopy and Bronchoscopy (DLB) in all children with RC to identify any underlying airway pathology. 23 , 28 Studies by Chun et al 24 and Tan et al 29 have shown that age (<3 years) and need for hospitalization for RC symptoms should prompt DLB evaluation in these children. Ideally, DLB should be performed at least 3–4 weeks after the most recent croup episode and parents and caregivers should be counseled regarding implication of airway findings and their management.
6.1.2. Airway findings
The rate of positive airway findings in children undergoing DLB for RC symptoms ranges between 33%–55% in different studies 23 , 24 , 30 Among these, various grades of subglottic narrowing/stenosis and clinical findings suggestive of GERD are the leading abnormal findings. Sub‐glottic stenosis is generally Grade I or II (Myer‐Cotton grading system based on endotracheal tube size). Rankin et al in a study of 90 patients 23 identified findings suggestive of GERD in 28% of children. Other airway abnormalities include subglottic edema, tracheobronchomalacia, subglottic cysts, subglottic hemangioma and vascular compression of distal trachea. One should also have a high degree of suspicion for inhaled foreign bodies that may occasionally present with RC symptoms in this age group. It is important to note that >50% of children with RC will have normal airway findings. Presence or absence of airway abnormalities may help guide overall prognosis of patients with RC as discussed below in outcomes (e).
6.1.3. Role of GERD
Coughran et al 30 carried out a meta‐analysis of 15 studies to ascertain association between GERD and RC. They concluded that the prevalence of GERD in patients with RC is about 40%, though there is limited evidence of a causal link between the two. The meta‐analysis also noted that anti‐reflux medications helped decrease RC symptoms. An analysis to determine causal link between GERD and RC is difficult due to the heterogeneous, retrospective nature of the studies that are prone to moderate‐high bias risk. Only two studies in the meta‐analysis 31 , 32 performed pharyngeal and esophageal pH monitoring which provide an objective measurement of GERD episodes and overall severity. In many studies, the “diagnosis” of GERD was mostly based on a history of GERD symptoms or DLB findings. DLB findings which have been associated with GERD include erythema and edema of the arytenoids or posterior glottis or narrowing or strictures of the esophagus. 33 , 34 It is proposed that aspiration of refluxed acid into the trachea and upper airway causes mucosal sloughing and edema that makes the patient more susceptible to RC symptoms. 35 , 36 On the other hand, in a prospective study of 77 children presenting with CC who underwent DLB, EGD, and multichannel intraluminal impedance‐pH (MII‐pH) testing, there was no correlation between airway inflammation seen on DLB with pathologic reflux measured objectively by MII‐pH probe. 37 Furthermore, both pediatric and adult studies have demonstrated significant disagreement between otolaryngologists on airway findings seen on DLB suggesting poor inter‐rater reliability. 37 , 38 There should be a thoughtful discussion with the caregiver regarding the risks and benefits of anti‐reflux therapy when GERD is suspected and when in doubt GI referral should be considered.
6.1.4. RC and asthma
Asthma is often seen in conjunction with RC. In a sub‐study of a large longitudinal study of respiratory illnesses during childhood, Castro‐Rodriguez et al 39 concluded that children who present with croup in early life along with a positive history of wheezing, had a significant risk of persistent wheezing during the school years and warrant further evaluation by pediatric pulmonologist. They found that RSV was the most frequent cause of episodes of croup with wheezing while parainfluenza was the most frequent virus isolated in children with croup without wheezing. Therefore, it is likely that in the former, the upper airway symptoms are the manifestation of an extension of a disease process that affects mainly the lower airways.
6.1.5. Outcomes after treatment for airway abnormalities
About half of the children undergoing DLB for RC will have airway abnormalities. 23 , 24 A study done by Rankin et al 23 showed that subglottic narrowing was mild in majority of the children undergoing DLB and responded to observation or balloon dilation. Their study also showed that treatment with anti‐reflux medications significantly improved symptoms in 91% of their patients and they strongly advocate treatment of reflux when laryngotracheobronchial findings suggest reflux. The potential benefits of daily acid‐suppression medication must be carefully weighed along with the known risks with prolonged use of these medications. The long‐term prognosis for patients with airway abnormalities and RC are excellent once the underlying airway pathology is treated.
6.2. CC with/FTT
Table 4 shows the important causes of CC that are associated with poor feeding in children. The review of each of these causes is beyond the scope of this manuscript. LCs and tracheo‐esophageal fistulas are usually managed by a multidisciplinary approach involving ENT, GI, pediatric general surgeon, pulmonologist, and intensive care physician. A child with neurological/neuromuscular abnormality, altered swallow, weak cough reflex may have both CC and FTT and should be evaluated in an aerodigestive clinic along with speech therapists, radiologists and Physical Medicine and Rehabilitation physicians.
TABLE 4.
Important causes of chronic cough associated with feeding difficulties
| Upper airway and laryngoscopy findings | Enlarged adenoids, subglottic narrowing, subglottic cysts, type 1 laryngeal cleft, laryngomalacia, vocal fold dysfunction |
| Bronchoscopy findings | Protracted bacterial bronchitis with positive BAL, trachea‐broncho malacia |
| Esophagoscopy findings | Eosinophilic esophagitis and gastro‐esophageal reflux disease |
Abbreviation: BAL, bronchoalveolar lavage.
6.2.1. Laryngeal clefts
LC are congenital, relatively rare abnormalities characterized by abnormal communication between the airway and esophagus. The most widely used system to classify LC is the Benjamin‐Inglis classification system. 40 According to this classification, there are four types of LC, Type1 laryngeal cleft (LC1) is defined as defect in the inter‐arytenoid mucosa or musculature that does not extend below the vocal cords. LC1 usually presents with respiratory and aspiration symptoms such as CC or recurrent pneumonias. Feeding problems, dysphagia and difficulty with weight gain have also been reported. 21 Two recent systematic reviews 21 , 41 noted that the presenting symptoms of LC1 can be nonspecific and a high index of suspicion is required to diagnose this condition especially in a child with recurrent upper and lower airway symptoms with or without feeding problems. LC1 may also be associated with co‐morbidities such as laryngomalacia, trachea‐esophageal fistula, GERD, and syndromes such as Trisomy 21. 41
6.2.2. Management of LC1
Diagnosis of LC1 may be challenging due to varied nonspecific symptom presentation. Specific diagnosis may be delayed as symptoms may be attributed to other conditions such as asthma, allergies or GERD. Direct Laryngoscopy with palpation of the inter‐arytenoid area is considered the “gold standard” to diagnose LC1 (see figure‐picture demonstrating type 1 laryngeal cleft). LC1 can be managed conservatively or surgically. Conservative management includes thickened feeds, feeding and swallow therapy, and close monitoring for persistent symptoms. 22 , 42 , 43 Surgical treatment involves injection of the inter‐arytenoid area with various substances or endoscopic suture repair of the cleft. Most of the materials used in inter‐arytenoid injection are temporary, meaning some children will go on to require definitive repair after resorption. A recently published systematic review on management of LC1 43 showed that endoscopic repair of the cleft may be a better surgical option in patients who fail conservative treatment. This review also noted that conservative treatment alone improves symptoms in 50% of the children and injection of the cleft results in long term symptom improvement without the need for definite repair in a significant proportion of children. Management options should be tailored to the severity of patient's symptoms. Risks and benefits of conservative versus surgical treatment for long term symptom control should be discussed with the caregivers.
6.2.3. Important non‐ENT causes of CC with feeding/weight issues: EoE
EoE should also be considered in children who present with poor feeding in the context of respiratory symptoms such as CC. EoE is a chronic inflammatory condition of the esophagus characterized by symptoms related to esophageal dysfunction and eosinophilic inflammation, which is typically defined as >15 eosinophils per high powered field of the esophageal mucosa. 44 Younger patients tend to present with more nonspecific symptoms, including feeding difficulties, dysphagia to liquids and/or solids, vomiting, regurgitation, and poor weight gain, while older adolescents and adults present with dysphagia to solids and food impactions. 45 , 46 Although EoE is generally considered a condition of the gastrointestinal tract, EoE can also present with extraesophageal manifestations, including airway and respiratory symptoms such as CC, as well as RC, hoarseness, and dysphonia. 47 There is a strong association between EoE and other atopic conditions, such as food allergies, environmental allergies, asthma, and atopic dermatitis. EGD with visual examination and biopsies of the upper GI tract is considered the diagnostic test of choice for EoE. Gross endoscopic findings include mucosal edema, linear furrows, white specks of the esophageal mucosa, or may be relatively normal in appearance, which is why multiple mucosal biopsies must be obtained from both the distal and proximal levels of the esophagus for histologic evaluation and ultimately diagnosis of EoE. 44 Potential treatment options for EoE currently include PPIs, swallowed corticosteroid preparations such as fluticasone propionate and budesonide, and dietary therapy. Within dietary therapy, there are three types of diet approaches: (1) Elemental diet where all foods are replaced with an elemental, amino acid based formula; (2) targeted elimination diet, in which foods are eliminated based on allergy testing; or (3) empiric elimination diet, where the six most common food allergens (dairy, soy, wheat, egg, tree nuts/peanuts and fish/shellfish) are eliminated from the diet without the need for allergy testing. 48 A multidisciplinary approach to children who present with feeding difficulties and CC not only improves ongoing gastrointestinal and otolaryngologic symptoms, but can also reduce the risk of future complications due to uncontrolled inflammation in the esophagus such as fibrosis and stricture. 48
6.3. Protracted bacterial bronchitis
PBB is one of the most common causes of CC in pre‐school children (<5 years of age). It is characterized by a history of chronic wet cough, absence of specific cough pointers, positive bronchoalveolar lavage (BAL) culture and cough resolving after two‐week course of an appropriate oral antibiotic. PBB is caused by typical respiratory pathogens, such as Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Children with PBB are eight times more likely to attend daycare, therefore are more likely to have recurrent viral infections that cause airway injury and inflammation making it easier for bacteria to grow and cause infection. 49
FB is indicated when the chronic wet cough does not resolve after repeated antibiotic treatment. BAL also helps with choice of appropriate, culture directed antibiotics in these cases. Bronchoscopy findings in these children include secretions and/or mucus commonly found in the bronchi and sometimes in the trachea; bronchitis with airway inflammation and edema, and occasionally airway malacia. 49 Treatment for PBB includes prolonged course of antibiotics, with a minimum course of 2 weeks. The most widely used antibiotic is oral amoxicillin‐clavulanate. Alternatives include most oral second or third generation cephalosporins, trimethoprim‐sulfamethoxazole, or a macrolide. Recurrent PBB or ineffective treatment of PBB can lead to bronchiectasis. 50
6.4. Asthma
Asthma is a common chronic disorder of the airways that is complex and characterized by variable and recurring symptoms, airflow obstruction, bronchial hyperresponsiveness, and an underlying inflammation. 51 Cough is a frequent symptom in patients with asthma, particularly persistent nocturnal cough. Cough that is associated with asthma is typically dry, but a wet cough does not exclude asthma. Other symptoms of asthma include wheezing, shortness‐of‐breath, or chest tightness. Asthma is often associated with exertional dyspnea, allergies, and/or atopy. Asthma symptoms are usually episodic and triggered by respiratory viral infections, allergens, weather changes, exercise, strong emotions, or irritants such as environmental tobacco smoke. 52 Clinical response to asthma medications, including short acting bronchodilators and systemic steroids, is also consistent with asthma. There are many different phenotypes under the broad diagnosis of asthma. Given the complexities of establishing a definitive diagnosis, when asthma is suspected, further evaluation by pulmonologists, often with chest radiographs, spirometry (when age appropriate), and response to bronchodilators, is indicated. 53
7. ROLE OF DYNAMIC AIRWAY CT IN MANAGEMENT OF PERSISTENT RESPIRATORY SYMPTOMS
Rigid (or Flexible) direct visualization of the airways and bronchi (DLB) is considered the gold standard for diagnosis of airway and bronchial abnormalities in children with persistent respiratory symptoms. In a 2017 study by Ullmann et al, 54 the authors evaluated 34 children with persistent respiratory symptoms (commonest being difficulty breathing/respiratory distress) by performing a non‐sedated dynamic airway CT scan and showed that CT was 100% sensitive and 82% specific in diagnosing tracheobronchomalacia in these patients. The authors also found good correlation between CT and DLB and suggested that non‐sedated dynamic airway CT should be considered in children who are medically fragile to undergo DLB or triple endoscopy under general anesthesia. Disadvantages of airway CT (authors did not discuss in their article) may potentially include inability to palpate the airway for LC, inability to simultaneously obtain BAL cultures or perform EGD with biopsies for recalcitrant CC. Another study by Ahmed et al 55 suggested that use of low dose non‐contrast airway CT for evaluation of children with “intermediate risk” for airway foreign bodies (determined by an institution protocol) significantly decreased the rate of negative bronchoscopies in these children. A 2011 study published in Chest 56 evaluated children with productive CC for 6 weeks with high‐resolution computer tomography (HRCT) and correlated findings on HRCT with FB/BAL. The authors found good correlation between HRCT and FB for increasing severity and symptom duration as well as with severity of neutrophilic inflammation on BAL. However, HRCT was found to be less sensitive in detecting airway lesions as compared to FB/BAL. Though the role and indications of dynamic airway CT are still evolving, this technique may be considered as a non‐invasive option for evaluation of airway abnormalities in higher risk children and the two diagnostic modalities can be considered complementary for evaluation of recalcitrant cough in children.
8. CONCLUSION
CC in children is mainly attributed to PBB, asthma, nonspecific cough, and GERD symptoms. A multi‐disciplinary approach is cost‐effective and aids with earlier diagnosis and appropriate treatment. Congenital or acquired narrowing of the subglottis is the leading ENT cause for RC in children. LC1 is commonly seen in children with recurrent aspiration and CC. Children are usually referred to pulmonologists for wet cough not responding to treatment. EoE and GERD should be considered in the differential diagnosis of CC in children with both respiratory symptoms and FTT.
CONFLICT OF INTEREST
None.
ACKNOWLEDGMENTS
None.
Mukerji SS, Yenduri NJS, Chiou E, Moonnumakal SP, Bedwell JR. A multi‐disciplinary approach to chronic cough in children. Laryngoscope Investigative Otolaryngology. 2022;7(2):409‐416. doi: 10.1002/lio2.778
Meeting presented at the COSM/ASPO spring meeting, April 2021.
REFERENCES
- 1. Chang AB, Robertson CF, van Asperen PP, et al. A cough algorithm for chronic cough in children: a multicenter, randomized controlled study. Pediatrics. 2013;131(5):e1576‐e1583. [DOI] [PubMed] [Google Scholar]
- 2. Irwin RS, Baumann MH, Bolser DC, et al. Diagnosis and management of cough executive summary: ACCP evidence‐based clinical practice guidelines. Chest. 2006;129(1 Suppl):1S‐23S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marchant JM, Newcombe PA, Juniper EF, Sheffield JK, Stathis SL, Chang AB. What is the burden of chronic cough for families? Chest. 2008;134(2):303‐309. [DOI] [PubMed] [Google Scholar]
- 4. Thomson F, Masters IB, Chang AB. Persistent cough in children and the overuse of medications. J Paediatr Child Health. 2002;38(6):578‐581. [DOI] [PubMed] [Google Scholar]
- 5. Chang AB, Oppenheimer JJ, Weinberger M, et al. Etiologies of chronic cough in pediatric cohorts: CHEST guideline and expert panel report. Chest. 2017;152(3):607‐617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Karabel M, Kelekçi S, Karabel D, Gürkan MF. The evaluation of children with prolonged cough accompanied by American College of Chest Physicians guidelines. Clin Respir J. 2014;8(2):152‐159. [DOI] [PubMed] [Google Scholar]
- 7. Chang AB, Oppenheimer JJ, Weinberger MM, et al. Use of management pathways or algorithms in children with chronic cough: CHEST Guideline and Expert Panel Report. Chest. 2017;151(4):875‐883. [DOI] [PubMed] [Google Scholar]
- 8. McCallum GB, Bailey EJ, Morris PS, Chang AB. Clinical pathways for chronic cough in children. Cochrane Database Syst Rev. 2014;9:CD006595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chang AB, Oppenheimer JJ, Irwin RS, CHEST Expert Cough Panel . Managing chronic cough as a symptom in children and management algorithms: CHEST Guideline and Expert Panel Report. Chest. 2020;158(1):303‐329. [DOI] [PubMed] [Google Scholar]
- 10. Nunn JF. Nunn's Applied Respiratory Physiology. Butterworths; 1993. [Google Scholar]
- 11. Polgar G, Weng TR. The functional development of the respiratory system from the period of gestation to adulthood. Am Rev Respir Dis. 1979;120(3):625‐695. [DOI] [PubMed] [Google Scholar]
- 12. Thach BT. Maturation and transformation of reflexes that protect the laryngeal airway from liquid aspiration from fetal to adult life. Am J Med. 2001;3(111 Suppl 8A):69S‐77S. [DOI] [PubMed] [Google Scholar]
- 13. Chang AB, Phelan PD, Sawyer SM, Del Brocco S. Robertson CCough sensitivity in children with asthma, recurrent cough, and cystic fibrosis. Arch Dis Child. 1997;77(4):331‐334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Merchant JM, Masters B, Taylor SM, Chang AB. Utility of signs and symptoms of chronic cough in predicting specific cause in children. Thorax. 2006;61(8):694‐698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cash H, Trosman S, Abelson T, Yellon R, Anne S. Chronic cough in children. JAMA Otolaryngol Head Neck Surg. 2015;141(5):417‐423. [DOI] [PubMed] [Google Scholar]
- 16. Lee AS, Lee JS, He Z, Ryu JH. Reflux‐aspiration in chronic lung disease. Ann Am Thorac Soc. 2020;17(2):155‐164. [DOI] [PubMed] [Google Scholar]
- 17. Rosen R, Vandenplas Y, Singendonk M, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2018;66(3):516‐554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. DeBruyne P, Ito S. Toxicity of long‐term use of proton pump inhibitors in children. Arch Dis Child. 2018;103(1):78‐82. [DOI] [PubMed] [Google Scholar]
- 19. Garcia JA, Mistry B, Hardy S, et al. Time‐driven activity‐based costing to estimate cost of care at multidisciplinary aerodigestive centers. Laryngoscope. 2017. Sep;127(9):2152‐2158. [DOI] [PubMed] [Google Scholar]
- 20. Collaco JM, Aherrera AD, Au Yeung KJ, Lefton‐Greif MA, Hoch J, Skinner ML. Interdisciplinary pediatric aerodigestive care and reduction in health care costs and burden. JAMA Otolaryngol Head Neck Surg. 2015. Feb;141(2):101‐105. [DOI] [PubMed] [Google Scholar]
- 21. Fracchia MS, Diercks G, Cook A, et al. The diagnostic role of triple endoscopy in pediatric patients with chronic cough. Int J Pediatr Otorhinolaryngol. 2019;116:58‐61. [DOI] [PubMed] [Google Scholar]
- 22. Rotsides JM, Krakovsky GM, Pillai DK, et al. Is a multidisciplinary aerodigestive clinic more effective at treating recalcitrant aerodigestive complaints than a single specialist? Ann Otol Rhinol Laryngol. 2017;126(7):537‐543. [DOI] [PubMed] [Google Scholar]
- 23. Rankin I, Wang SM, Waters A, Clement WA, Kubba H. The management of recurrent croup in children. J Laryngol Otol. 2013;127(5):494‐500. [DOI] [PubMed] [Google Scholar]
- 24. Chun R, Preciado DA, Zalzal GH, Shah RK. Utility of bronchoscopy for recurrent croup. Ann Otol Rhinol Laryngol. 2009;118(7):495‐499. [DOI] [PubMed] [Google Scholar]
- 25. Farmer TL. Wohl DL diagnosis of recurrent intermittent airway obstruction ("recurrent croup") in children. Ann Otol Rhinol Laryngol. 2001;110(7 Pt 1):600‐605. [DOI] [PubMed] [Google Scholar]
- 26. Konig P. The relationship between croup and asthma. Ann Allergy. 1978;41:227‐231. [PubMed] [Google Scholar]
- 27. Zach M, Erben A, Olinsky A. Corup, recurrent group, allergy and airways hyper‐reactivity. Arch Dis Child. 1981;56:336‐341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cressman WR, Myer CM III. Diagnosis and management of croup and epiglottitis. Pediatr Clin North Am. 1994;41:265‐276. [DOI] [PubMed] [Google Scholar]
- 29. Tan AK, Manoukian JJ. Hospitalized croup (bacterial and viral): the role of rigid endoscopy. J Otolaryngol. 1992;21:48‐53. [PubMed] [Google Scholar]
- 30. Coughran A, Balakrishnan K, Ma Y, et al. The relationship between croup and gastroesophageal reflux: a Sustematic review and meta‐analysis. Laryngoscope. 2021;131:209‐217. [DOI] [PubMed] [Google Scholar]
- 31. Contencin P, Narcy P. Gastresophageal reflux in infants and children: a pharyngeal pH monitoring study. Arch Otolaryngol Head Neck Surg. 1992;118:1028‐1030. [DOI] [PubMed] [Google Scholar]
- 32. Arslan Z, Cipe FE, Ozmen S, Kondolot M, Piskin IE, Yoney A. Evaluation of allergic sensitization and gastroesophageal reflux disease in children with recurrent croup. Pediatr Int. 2009;51:661‐665. [DOI] [PubMed] [Google Scholar]
- 33. Duval M, Tarasidis G, Grimmer JF, et al. Role of operative airway evaluation in children with recurrent croup: a retrospective cohort study. Clin Otolaryngol. 2015;40:227‐233. [DOI] [PubMed] [Google Scholar]
- 34. Jabbour N, Parker NP, Finkelstein M, Lander TA, Sidman JD. Incidence of operative endoscopy findings in recurrent coup. Otolaryngol Head Neck Surg. 2011;144:596‐601. [DOI] [PubMed] [Google Scholar]
- 35. Hon M, Kingsley KL, Cottichia JM. Correlating the clinical course of recurrent croup with endoscopic findings: a retrospective observational study. Ann Otol Rhinol Laryngol. 2008;117:464‐469. [DOI] [PubMed] [Google Scholar]
- 36. Kwong R, Hon M, Coticchia J. Recurrent croup presentation, diagnosis, and management. Am J Otolaryngol. 2007;28:401‐407. [DOI] [PubMed] [Google Scholar]
- 37. Rosen R, Mitchell PD, Amirault J, Amin M, Watters K, Rahbar R. The edematous and erythematous airway does not denote pathologic gastroesophageal reflux. J Pediatr. 2017;183:127‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chang BA, MacNeil SD, Morrison MD, Lee PK. The reliability of the reflux finding score among general otolaryngologists. J Voice. 2015;29(5):572. [DOI] [PubMed] [Google Scholar]
- 39. Castro‐Rodríguez JA, Holberg CJ, Morgan WJ, et al. Relation of two different subtypes of croup before age three to wheezing, atopy, and pulmonary function during childhood: a prospective study. Pediatrics. 2001;107(3):512‐518. [DOI] [PubMed] [Google Scholar]
- 40. Wilmott RW, Boat TF, Bush A, Chernick V, Deterding RR, Ratjen F. Kendig and Chernick's Disorders of the Respiratory Tract in Children. Elseiver; 2019. [Google Scholar]
- 41. Reddy P, Byun YJ, Downs J, Nguyen SA, White DR. Presentation and management of type 1 laryngeal clefts: a systematic review and meta‐analysis. Int J Pediatr Otorhinolaryngol. 2020;138:110370. [DOI] [PubMed] [Google Scholar]
- 42. Parsons DS, Stivers FE, Giovanetto DR, Phillips SE. Type I posterior laryngeal clefts. Laryngoscope. 1998;108(3):403‐410. [DOI] [PubMed] [Google Scholar]
- 43. Van der Doef HP, Yntema JB, van den Hoogen FJ, Marres HA. Clinical aspects of type 1 posterior laryngeal clefts: literature review and a report of 31 patients. Laryngoscope. 2007;117(5):859‐863. [DOI] [PubMed] [Google Scholar]
- 44. Timashpolsky A, Schild SD, Ballard DP, Leventer SP, Rosenfeld RM, Plum AW. Management of type 1 laryngeal clefts: a systematic review and meta‐analysis. Otolaryngol Head Neck Surg. 2021;164(3):489‐500. [DOI] [PubMed] [Google Scholar]
- 45. Dellon ES, Liacouras CA, Molina‐Infante J, et al. Updated international consensus diagnostic criteria for eosinophilic esophagitis; proceedings of the AGREE conference. Gastroenterology. 2018;155(4):1022‐1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sun RW, Bonilla‐Velez J, Pesek RD, Johnson AB, Cleves MA, Richter GT. Eosinophilic esophagitis in children under the age of 5 years: clinical characteristics. Laryngoscope. 2018;128(4):798‐805. [DOI] [PubMed] [Google Scholar]
- 47. Noel RJ, Putnam PE, Rothenberg ME. Eosinophilic esophagitis. N Engl J Med. 2004;351(9):940‐941. [DOI] [PubMed] [Google Scholar]
- 48. Kubik M, Thottoam P, Shaffer A, Choi S. The role of the otolaryngologist in the evaluation and diagnosis of eosinophilic esophagitis. Laryngoscope. 2017;127(6):1459‐1464. [DOI] [PubMed] [Google Scholar]
- 49. Kumar S, Choi S, Gupta SK. Eosinophilic esophagitis – a primer for otolaryngologists. JAMA Otolaryngol Head Neck Surg. 2019;145(4):373‐380. [DOI] [PubMed] [Google Scholar]
- 50. Chang AB, Upham JW, Masters IB, et al. Protracted bacterial bronchitis: the last decade and the road ahead. Pediatr Pulmonol. 2016. Mar;51(3):225‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wurzel DF, Marchant JM, Yerkovich ST, et al. Protracted bacterial bronchitis in children: natural history and risk factors for bronchiectasis. Chest. 2016. Nov;150(5):1101‐1108. [DOI] [PubMed] [Google Scholar]
- 52. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma . National Asthma Education and Prevention Program, Third Expert Panel on the Diagnosis and Management of Asthma. National Heart, Lung, and Blood Institute (US); 2007. [Google Scholar]
- 53. Patel SJ, Teach SJ. Asthma. Pediatr Rev. 2019;40:549‐567. doi: 10.1542/pir.2018-0282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ullmann N, Secinaro A, Menchini L, et al. Dynamic expiratory CT: an effective non‐invasive diagnostic exam for fragile children with suspected trachea‐bronchomalacia. Pediatric Pulmonol. 2018;53(1):73‐80. [DOI] [PubMed] [Google Scholar]
- 55. Ahmed OG, Guillerman RP, Giannoni CM. Protocol incorporating airway CT decreases negative bronchoscopy rates for suspected foreign bodies in pediatric patients. Int J Pediatr Otorhinolaryngol. 2018;109:133‐137. [DOI] [PubMed] [Google Scholar]
- 56. Douros K, Alexopoulou E, Nicopoulou A, et al. Bronchoscopic and high resolution CT scan findings in children with chronic wet cough. Chest. 2011;140(2):317‐323. [DOI] [PubMed] [Google Scholar]


