Abstract
Objective
Chronic rhinosinusitis (CRS) is a highly prevalent and burdensome disease. The pathophysiology is not fully elucidated, but environmental pollutants have been suggested to impact the inflammatory component of the disease process. This review aims to summarize the role of environmental pollution in CRS onset and disease severity.
Methods
A systematic review was performed following Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines. PubMed, EMBASE, Cochrane Library, Web of Science, and Scopus databases were queried in August 2021. Original articles reporting on air pollution exposure in CRS were included. Other forms of sinonasal disease were excluded.
Results
Literature search produced 11,983 articles, of which 10 met inclusion criteria. Outcomes evaluated included incidence/prevalence, disease severity, quality of life, and histopathologic/microbial changes. Air pollutant exposure was associated with higher odds of CRS, particularly with particulate matter (PM) exposure. Increasing air pollution exposure was also associated with worsened disease severity and detectable histopathologic changes. Impact on quality of life was less clear.
Conclusion
Air pollution (particularly PM) is correlated with CRS incidence/prevalence and disease severity, with evidence of histopathologic changes in CRS tissue samples. Further research is warranted to better understand the mechanisms by which air pollution components may cause CRS and type 2 inflammation.
Level of Evidence
3a
Keywords: air pollutants, environmental pollution, quality of life, sinusitis
Short abstract
Recent evidence suggests a role for air pollution in the onset and severity of CRS, most notably with relation to PM2.5 exposure. This systematic review supports previous in vitro and in vivo models of pollution in CRS. This study further adds to the existing body of literature demonstrating the many negative health impacts of exposure to air pollution, including impacts on upper airway disease, lower airway disease, cardiac disease, and overall morbidity and mortality.
1. INTRODUCTION
Chronic rhinosinusitis (CRS) is a highly prevalent and burdensome disease, with large impacts on both the individual and population scales. 1 , 2 , 3 , 4 , 5 , 6 , 7 Although the pathogenesis of CRS is not well elucidated, multiple factors have been hypothesized to play a role, including chronic mucosal inflammation secondary to mucociliary clearance dysfunction, epithelial barrier abnormalities, or dysregulated immune response. 8 , 9 , 10 , 11 , 12 , 13
Notably, environmental exposures have been hypothesized to play a key role in disease onset and severity, as nasal mucosa is among the first lines of defense against said exposures. The Environmental Protection Agency (EPA) has defined six criteria air pollutants—ozone (O3), particulate matter (PM), carbon monoxide (CO), lead (Pb), sulfur dioxide (SO2), and nitrogen dioxide (NO2)—for which there are set air quality standards in the United States. 14 PM is often reported as PM10 or PM2.5, which corresponds to PM 10 μm or less in diameter or 2.5 μm or less in diameter, respectively. Elevated levels of these air pollutants have been repeatedly linked to multiple health conditions (e.g., cardiac disease, asthma, and chronic obstructive pulmonary disease) 15 , 16 , 17 as well as overall morbidity and mortality. 18 , 19 , 20 , 21
Recently, there has been increasing evidence for the role of air pollution in upper airway disease. Many air pollutants (including PM and O3) have been shown to upregulate reactive oxygen species, leading to DNA damage and increased oxidative stress and inflammation. 22 , 23 , 24 , 25 Cellular models and animal studies have supported this link between air pollution and oxidative stress in the pathogenesis, progression, and severity of CRS. One study found that human nasal epithelial cells exposure to PM demonstrated lower cell line viability and increased cytotoxicity. 26 Another group discovered that human sinonasal epithelial cell lines from patients undergoing surgery for CRS that were exposed to PM10 displayed disrupted epithelial barrier function. 27 Furthermore, murine models have demonstrated that chronic exposure to PM2.5 is associated with increased inflammatory changes 28 and tissue remodeling. 29
Over the past couple of decades, these hypotheses have been replicated in observational studies in humans. A previous systematic review was performed to evaluate the impact of air quality on CRS in humans, 30 but most of the evaluated studies were related to occupational exposures, and few conclusions could be drawn. Of the studies that considered environmental exposure, none evaluated the impact of the EPA criteria air pollutants. Although occupational exposures undoubtedly have a large impact on upper airway diseases, air pollution encountered in everyday life has the potential to have a significantly broader impact at the population level. Therefore, this systematic review aimed to evaluate the impact of air pollution on CRS pathogenesis, severity, and progression without considering the confounding effects of occupational exposures.
2. MATERIALS AND METHODS
2.1. Literature source and search
A literature search was performed following Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines utilizing PubMed, Cochrane Library, EMBASE, Web of Science, and SCOPUS. 31 The search was completed on August 5, 2021 by a qualified data informationist. No restrictions were placed on date of publication. Search terms including “environmental pollution,” “air pollution,” “particulate matter,” “ozone,” “carbon monoxide,” “sulfur dioxide,” and “nitrogen dioxide” were combined with “chronic rhinosinusitis,” “rhinitis,” “sinusitis,” “sinonasal disease,” and “CRS.” Full search summary details can be found online in Supporting Information S1. No additional records were obtained from outside sources. Studies were managed in Covidence (Veritas Health Innovation, Melbourne, Australia), and articles were reviewed for inclusion based on title/abstract by two independent reviewers (Evelyn M. Leland and Varun Vohra). Any disputes between reviewers were resolved by a third independent reviewer (Murugappan Ramanathan).
2.2. Inclusion and exclusion criteria
Studies focusing on the relationship between air pollution and CRS in adults (age ≥ 18) were included. The articles had to evaluate at least one of the six EPA criteria air pollutants (O3, PM2.5, PM10, CO, Pb, SO2, or NO2). 14 The diagnosis of CRS required documentation of provider diagnosis or use of professionally accepted criteria, such as the European Position Paper on Rhinosinusitis and Nasal Polyps. 32 Studies that included patients based on exclusively subjective symptoms (e.g., “Have you ever had a runny or blocked nose?”) were excluded. Additionally, studies without evidence of chronicity (e.g., >3 months) were also excluded. Other exclusion criteria included pediatric population, other rhinitis diagnoses (e.g., allergic rhinitis, vasomotor rhinitis), review articles, or occupational exposures. Although no restriction was placed on language during the initial search, an article was excluded if no English versions of the article were available.
2.3. Data extraction
Data extraction was performed by two independent reviewers (Evelyn M. Leland and Varun Vohra) with disagreements reviewed by the senior author (Murugappan Ramanathan). Data collected included study period, patient population demographics (diagnosis, diagnostic criteria, age, and sex), pollutant exposure, outcome data, and conclusions. Data and study conclusions were summarized in table format. Meta‐analysis was considered but deferred due to small sample sizes and heterogenous nature of available data.
2.4. Risk of bias assessment
Risk of bias was assessed by two independent reviewers with the methodological index for nonrandomized studies (MINORS), used to assess the quality of nonrandomized surgical studies. 33 Noncomparative studies are scored on either criteria (score 0–16), and comparative studies are scored on 12 criteria (0–24).
3. RESULTS
3.1. Study characteristics
The literature search resulted in 11,983 studies (Figure 1). After removal of 228 duplicates, 11,755 studies were screened for inclusion by title and abstract. A total of 115 articles underwent full‐text review, resulting in 10 studies meeting criteria for final data extraction and analysis (Table 1). Of the included studies, three were case–control, 34 , 36 , 42 and seven were ecological or cross‐sectional. Four studies evaluated incidence or prevalence of CRS. Six studies evaluated disease severity or quality of life. Two studies collected tissues for histopathologic or microbiological analysis. MINORS scores were 18 for comparative studies and 10 for noncomparative studies, indicating at least a moderate risk of bias for the included studies.
FIGURE 1.
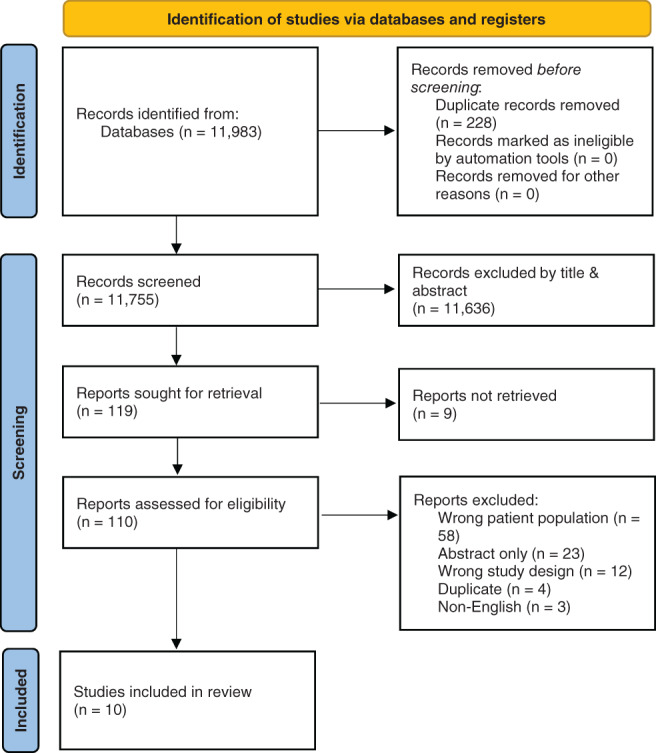
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) 2020 flow diagram. Source: Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. https://doi.org/10.1136/bmj.n71. For more information, visit: http://www.prisma‐statement.org/
TABLE 1.
Summary of included studies
| Author | Data period and location | Patient population | Sample size | Male % | Environmental pollution/exposure | Confounders | Conclusions |
|---|---|---|---|---|---|---|---|
| Diagnostic criteria | Age ± SD | MINORS score | |||||
| Padhye et al. 34 |
Jan 2015–July 2016 Chicago, Illinois |
CRS Nasal swab taken from middle nasal meatus under endoscopic guidance 2–3 weeks before tissue collection during surgery |
CRS 111 (71 underwent surgery) Controls 21 |
NR |
PM2.5 Exposure based on patient's home address. PM2.5 levels from US EPA 2011 Environmental Justice Screen dataset |
insurance, race, socioeconomic status, asthma, atopy, age, CRS duration |
CRS was associated with higher neighborhood PM2.5 levels than controls (p <.001) PM2.5 was associated with decreased relative abundance of Corynebacterium in CRS (p = .02) and controls (p = .04) PM2.5 levels were associated with eosinophilic aggregation in CRS patients (p <.01). Not associated with other evaluated markers |
| Patients diagnosed in tertiary rhinology clinic | NR | 18 | |||||
| Patel et al. 35 |
Jan 2015–May 2019 Chicago, Illinois |
Patients with CRS who underwent FESS Age ≥ 18 |
CRS 291 (CRSsNP 152 CRSwNP 131) |
46% |
O3, PM2.5 Exposure based on patient's home address Pollutant levels from US EPA 2018 Environmental Justice Screen Dataset |
Race, smoking history, inhalant allergy |
CRS: In multivariate models, increasing ozone exposure was associated with more severe inflammation (p = .031) and Charcot–Leyden crystals (p = .039). No trends were seen based on PM2.5 exposure Subgroups: There was no difference in exposure to O3 (p = .306) or PM2.5 (p = .815) based in CRSsNP versus CRSwNP subgroups In CRSwNP, increased O3 exposure was associated with increased inflammation (p = .004), presence of eosinophilic aggregates (p = .018), and presence of Charcot–Leyden crystals (p = .036). No associations were noted between PM2.5 exposure and CRSwNP No associations were noted with CRSsNP and O3 or PM2.5 exposure Not associated with other evaluated markers |
| Diagnosed based on 12 weeks of continuous symptoms supported by positive endoscopy and CT scan, and requirement of ESS after failure of appropriate medical therapy | 49.3 ± 16.1 | 10 | |||||
| Zhang et al. 36 |
NR Northeast United States |
CRS Age ≥ 18 |
CRS 2034 Controls 4068 |
CRS: 41.3% Control: 43.5% |
PM2.5 and O3 Exposure based on patient's home address at 12, 24, 36, and 60 months before diagnosis date Machine learning approaches were used to predict daily PM2.5 concentrations. Daily O3 exposure estimated from the National Air Monitoring Stations/State and Local Air Monitoring Stations |
Age, gender, race, BMI, alcohol consumption status, smoking status, hypertension, diabetes, COPD, asthma | At all time‐periods measured before diagnosis, PM2.5 exposure was associated with higher odds of CRS diagnosis. This was also noted across all sinusitis locations, most notably in cases of ethmoidal and severe (e.g., four sinus) sinusitis cases |
|
CRS ICD9/10 code by board‐certified otolaryngologist using nasal endoscopy and CT scans Excluded those with environmental allergies |
CRS: 51.5 ± 16.0 Controls: 51.9 ± 17.4 |
18 | |||||
| Lu et al. 37 |
Jan 1, 2015–Dec 31, 2018 Xinxiang, China |
Chronic sinusitis | 183,943 cases | 49.6% |
NO2, SO2, CO, PM10, PM2.5, and O3 Data from China Environmental Monitoring Centre website, gathered from four fixed site monitoring stations |
Co‐pollutants |
All pollutants except for O3 had some impact on hospital outpatient cases of chronic sinusitis. Notably, these trends were seen in patients age < 65 (most significantly in pediatric population), but not significant in a subgroup of adults age ≥ 65 After adjusting for co‐pollutants, the relationship between PM2.5 and NO2 and chronic sinusitis cases remained |
| Chronic sinusitis as coded with the ICD‐10 J32 code | 70.1% between 15–65 years old | 12 | |||||
| Velasquez et al. 38 |
2013–2015 Pittsburgh, Pennsylvania |
CRSsNP CRSwNP ≥18 years old Same residence and occupation for past 5 years Imaging studies within 3 years of study protocol |
CRSsNP 96 CRSwNP 113 |
CRSsNP: 39.6% CRSwNP: 61.1% |
PM2.5, BC Utilized home address to estimate exposure based on a spatial model from Pittsburgh air pollution |
NR |
Air pollutant exposure did not vary between groups. LMS, FESS, and steroid usage were not associated with air pollutant exposure in CRSsNP or CRSwNP. Authors thought due to small subgroup analyses |
|
CRS diagnosed based on International Consensus Statement on Allergy and Rhinology All patients seen by one of the two rhinologists at institution |
CRSsNP: 51.2 CRSwNP: 51 |
11.5 | |||||
| Park et al. 39 |
2009 South Korea |
Age ≥ 19 Data from KNHANES |
NR | NR |
PM10, NO2, O3, SO2, CO Data from the Korean National Institute of Environmental Research (2009) |
Age, sex, region |
No correlation between CRS incidence and air pollution levels For each 1 μg/m3 unit increase in PM10 level, prevalence of CRS increased significantly, with an OR of 1.22 (95% CI 1.02–1.46; p = .031) |
| CRS diagnosed by trained residents in those with visible nasal polyps endoscopically or with at least two of the following symptoms: anterior/posterior nasal drip, nasal obstruction, facial pain/tenderness, and olfactory dysfunction more than 3 months in duration (either an anterior/posterior nasal drip or nasal obstruction was required as a presenting symptom) | NR | 10 | |||||
| Mady et al. 40 |
2013–2015 Pittsburgh, Pennsylvania |
CRSsNP CRSwNP ≥18 years old Described rhinitis symptoms and had subsequent allergy testing Same residence and occupation for past 5 years Imaging studies within 3 years of study protocol |
CRSsNP 58 (22 allergy‐negative) CRSwNP 67 (23 allergy‐negative) |
CRSsNP: 41.4% CRSwNP: 53.7% |
PM2.5, BC Utilized home address to estimate exposure based on a spatial model from Pittsburgh air pollution |
Age, sex |
Allergy‐negative patients had higher exposure levels to both PM2.5 (p = .030) and BC (p = .044) than their allergy‐positive counterparts. This trend was carried by CRSwNP patients, in which allergy‐negative patients had higher PM2.5 (p = .032) and BC (p = .017) exposure than allergy‐positive patients. Allergy‐negative CRSwNP patients also had higher PM2.5 exposure than allergy‐positive CRSsNP patients (p = .023) There were no differences in exposure to PM2.5/BC noted between allergy‐positive and allergy‐negative CRSsNP patients. There was no difference in SNOT‐22, LMS, or steroid usage between allergy‐positive and allergy‐negative patients In CRSsNP, BC correlated with SNOT‐22 (r = .55, p = .042, log‐transformed r = .56, p = .039). No correlation with other measured severity metrics In CRSwNP, there was no relationship between PM2.5/BC exposure and any evaluated severity metric |
| All patients seen by one of the two rhinologists at institution |
CRSsNP: 47 ± 15.7 CRSwNP: 49 ± 15.5 |
10 | |||||
| Mady et al. 41 |
2013–2015 Pittsburgh, Pennsylvania |
CRSsNP CRSwNP ≥18 years old Same residence and occupation for past 5 years Imaging studies within 3 years of study protocol |
CRSsNP 96 CRSwNP 138 |
CRSsNP: 39.6% CRSwNP: 59.4% |
PM2.5, BC Utilized home address to estimate exposure based on a spatial model from Pittsburgh air pollution |
Age, sex |
Mean PM2.5 levels were higher in Pittsburgh (11.28 vs. 10.98 μg/m3, p = .002). CRSwNP patients living in Pittsburgh had higher PM2.5 exposure versus patients living elsewhere (11.28 vs. 10.95 μg/m3, p = .008), but this was not seen in CRSsNP patients (11.27 vs. 11.03 μg/m3, p = .097). No significant difference based on BC levels Air pollutant exposure was not different between CRSsNP and CRSwNP groups for PM2.5 or BC CRSsNP: PM2.5 exposure was associated with FESS in CRSsNP patents (p = .015), with each unit increased in PM2.5 exposure corresponding to a 1.89‐fold increased risk in proportion of CRSsNP patients needing more surgery (p = .015). BC exposure was associated with SNOT‐22 scores in CRSsNP patients (p = .015), with each 0.1‐unit increase in BC corresponding to a 7.97‐unit increase in SNOT‐22 scores in CRSsNP patients (p = .008). No relationship in other evaluated metrics and PM2.5 /BC exposure in CRSsNP patients CRSwNP: No relationship between PM2.5 /BC exposure and any evaluated metric in CRSwNP patients |
| All patients seen by one of the two rhinologists at institution |
CRSsNP: 51.2 ± 16.5 CRSwNP: 51.4 ± 15.4 |
10 | |||||
| Sommar et al. 42 |
2008–2010 Swedish cities: Umeå, Uppsala, Stockholm, and Gothenburg |
CRS Based on GA2LEN survey from 19 European countries |
CRS 110 Control 226 |
CRS: 54% Control: 49% |
NO2, NOx Exposure based on patient's home address Pollutant levels estimated based on modeling and local emission data |
Exposure to nitric oxides did not differ between controls and CRS European quality of life‐5D was not associated with measures of NO2 and NOx in controls or CRS |
|
| EPOS 2012 criteria |
CRS: 45.4 (95% CI: 42.5–48.3) Control: 47.5 (95% CI: 45.5–49.5) |
19 | |||||
| Wolf 43 |
1990–1999 Cologne, Germany |
Patients with CRS who underwent FESS and | 1435 patients, evaluated based on districts | NR |
SO2, TSP, NOx Pollutant levels estimated from modeling programs and simulation study considering meteorological parameters Data then aggregated to level of city “administrative districts.” Exposure status determined based on which administrative district the patient lived in. |
Demographic and socioeconomic composition of the city districts—social status, family status Social status, residential stability of city district, distance between university hospital and city district |
Evaluated disease prevalence through age‐standardized rates of patients per 100,000 inhabitants per year who underwent surgery in the 1990s. Broken into quintiles from low to high rates. In the overall case, there was no relationship between patient rates and air pollution (r = −.025). When breaking data down into city districts above and below the average air pollution level and controlling for confounders, city districts with an above average air pollution level were associated with patient rates (r = .382, p <.05). In districts with air pollution below average, city rates were not associated with air pollution (r = −.135, p >.10). When broken up by time period, this relationship was maintained in 1990–1994 (r = .427, p <.05), but not in 1995–1999 (p = .265), which authors note was during a period of decreasing air pollution |
| Diagnosed at the Otorhinolaryngology Department at the University of Cologne | NR | 8.5 |
Abbreviations: BC, black carbon; BMI, body mass index; CO, carbon monoxide; COPD, chronic obstructive pulmonary disease; CRS, chronic rhinosinusitis; CRSsNP, chronic rhinosinusitis without nasal polyps; CRSwNP, chronic rhinosinusitis with nasal polyps; EPA, Environmental Protection Agency; EPOS, European position paper on rhinosinusitis and nasal polyps; FESS, functional endoscopic sinus surgery; GA2LEN, Global Allergy and Asthma European Network; KNHANES, Korea National Health and Nutrition Examination Survey; LMS, Lund‐Mackay Score; MINORS, methodological index for nonrandomized studies; NO2, nitrogen dioxide; NOx, nitrogen oxides; NR, not reported; O3, ozone; PM2.5, particulate matter ≤2.5 μm in aerodynamic diameter; PM10, particular matter ≤10 μm in aerodynamic diameter; SNOT‐22, sinonasal outcomes test‐22; SO2, sulfur dioxide; TSP, total suspended particulate.
3.2. Patient population characteristics
There were 4215 patients included in the studies (excluding Lu et al., 2020). Three studies were completed by the same author group, and it was assumed that the patient population was similar between the group. 38 , 40 , 41 Therefore, only one of the patient groups was included in the population summary calculations. Average age was 51.0 ± 15.7 (n = 2669) for CRS cases and 51.7 ± 17.0 (n = 4294) for controls. The CRS group was 43.2% male (n = 2669); the control group was 43.8% male (n = 4294). Only two studies reported the distinction between CRSsNP and CRSwNP. In those studies (n = 517), there were 48% CRSsNP patients and 52% CRSwNP patients.
3.3. CRS incidence and prevalence
Four studies investigated whether air pollution was associated with CRS incidence or prevalence, and all noted a significant relationship. Each study was from a different geographical region, and all studies utilized patients' home addresses to estimate air pollutant exposure.
One study performed in Cologne, Germany evaluated the impact of a model‐estimated air pollution level (based on the calculated levels of SO2, total suspended particulate, and NOx) on rates of CRS patients within predefined city districts. 43 Authors noted that city districts with above average air pollution levels were associated with higher patient rates (r = .382, p <.05). This was primarily driven by earlier years (1990–1994) versus later years (1995–1999), which authors note may be due to decreasing air pollution over the years.
Another study based on the Korea National Health and Nutrition Examination Survey evaluated the impact of PM10, NO2, O3, SO2, and CO. 39 There was a significant correlation between PM10 exposure and CRS prevalence, with each 1 μg/m3 increase in PM10 level correlating with a 1.2‐fold higher increase in developing CRS (OR: 1.22 [95% CI: 1.02–1.46], p = .031). No significant correlation was found between any air pollutant and CRS incidence.
A third study utilized ICD‐9/ICD‐10 codes from hospitals in Xinxiang, China to identify patients with chronic sinusitis. 37 Authors evaluated the impact of PM2.5, PM10, NO2, O3, SO2, and CO on hospital outpatient cases of chronic sinusitis. All pollutants except for O3 were associated with an increase in hospital outpatient cases. After adjusting for exposure to co‐pollutants, only PM2.5 and NO2 relationships remained significant. A 10 μg/m3 increase in PM2.5 or NO2 was associated with a 0.48% (95% CI: 0.22–0.74%) and 1.98% (95% CI: 1.31–2.64%) increase in hospital outpatients, respectively. Authors analyzed cases based on patient age, and these relationships were seen in patients age <65, but not in the subgroup of adults ≥65.
Most recently, a large case–control study was performed which evaluated the impact of long‐term PM2.5 exposure on the development of CRS. 36 The authors determined PM2.5 exposure at 12, 24, 36, and 60 months prior to CRS diagnosis, and location of involved sinuses was recorded. At all measured timepoints, a 5 μg/m3 increase in PM2.5 was associated with higher odds of CRS diagnosis, most significantly in cases of ethmoidal sinusitis (e.g., 60‐month OR: 3.27 [95% CI: 2.03–5.25]) and severe (e.g., involving maxillary, ethmoid, sphenoid, and frontal sinuses) sinusitis (e.g., 36‐month OR: 7.91 [95% CI: 3.06–20.42]).
3.4. CRS disease severity and quality of life
Other commonly evaluated outcomes were metrics of disease severity and quality of life. Four studies evaluated these metrics, but notably, three of the studies were performed by the same author group. Common disease severity indicators evaluated included sinonasal outcome test (SNOT‐22), Lund‐Mackay score (LMS), systemic steroid usage, and functional endoscopic sinus surgery (FESS) requirement.
The first report studied disease severity in CRSsNP and CRSwNP patients through SNOT‐22 scores, LMS, steroid usage, and FESS requirement. 41 PM2.5 and black carbon (BC, a major component of PM) exposures were calculated. In CRSsNP patients, PM22.5 exposure was associated with FESS requirement (p = .015), with each unit increase in PM2.5 exposure corresponding to a 1.89‐fold increased risk in proportion of CRSsNP patients requiring revision FESS surgery (p = .015). Additionally, BC exposure was associated with SNOT‐22 scores in CRSsNP patients (p = .015), with each 0.1‐unit increase in BC corresponding to a 7.97‐unit increase in SNOT‐22 scores (p = .008). There was no relationship with LMS scores or steroid usage in CRSsNP patients, and there was no relationship between air pollution and any disease severity metric in CRSwNP patients. This was not explained by differences in air pollutant exposure, as exposure was similar between CRSsNP and CRSwNP groups.
A second paper by the same author group was performed similarly but considered allergy status of patients. 40 In this report, similar to above data, in CRSsNP patients, BC exposure was associated with higher SNOT‐22 scores. PM2.5 exposure was not correlated with disease severity metrics in CRSsNP patients, and neither air pollutant was associated with outcomes in CRSwNP patients. Allergy status was not associated with severity metrics in either CRSsNP or CRSwNP patients. Interestingly, allergy‐negative patients were associated with higher exposure levels to both PM2.5 (p = .030) and BC (p = .044) than allergy‐positive counterparts. This trend was carried by CRSwNP patients—in which allergy‐negative patients had higher PM2.5 (p = .032) and BC (p = .017) exposure than allergy‐positive patients—but not seen in CRSsNP patients.
Finally, the third paper from the group evaluated the impact of both air pollutants and occupational exposures on CRSsNP and CRSwNP disease severity. 38 This review will focus solely on the air pollutant exposure. In this study, air pollutant exposure did not vary between subgroups, and disease severity metrics were not correlated with air pollutant exposure in either CRSsNP or CRSwNP groups. Although this is presumably the same study population as the previous papers, 40 , 41 a subgroup analysis on aspirin‐exacerbated respiratory disease was also performed by Velasquez et al. Authors hypothesized the loss of significance was due to small subgroup analyses. 38
The last paper evaluated the quality of life in CRSsNP and CRSwNP patients based on NO2 and NOx exposure. 42 Authors utilized the Swedish Global Allergy and Asthma European Network to collect data on patients with CRS and/or asthma. There was no difference in air pollutant exposure between CRSsNP and CRSwNP patients. The Euro Quality of Life questionnaire was utilized, and air pollution was not associated with the quality of life in CRS patients.
3.5. CRS histopathology and microbiology
Two studies evaluated the impact of air pollution exposure on histopathologic features of CRS tissue samples. Notably, as tissue samples were obtained from patients undergoing FESS, this suggests cases of severe disease that have failed conservative/medical management. 44
One study assessed O3 and PM2.5 exposure in patients with CRSsNP and CRSwNP. 35 In the overall CRS population, O3 exposure was associated with increased inflammatory changes (p = .031) and Charcot–Leyden crystals (p = .039), but no associations were seen based on PM2.5 exposure. Subgroup analysis revealed that this trend was carried by CRSwNP. In patients with CRSwNP, increased O3 exposure was associated with increased inflammation (p = .004), presence of eosinophilic aggregates (p = .018), and presence of Charcot–Leyden crystals (p = .036). Again, no associations were seen between PM2.5 exposure and CRSwNP, and there were no significant associations between CRSsNP and either evaluated pollutant. Notably, these changes were not due to varying exposure levels, as exposure to O3 and PM2.5 did not vary between CRSsNP and CRSwNP patients.
On the other hand, another study looked at the impact of only PM2.5 in both cases and controls. 34 Cases of CRS were associated with higher neighborhood PM2.5 levels than controls (p <.001). PM2.5 levels were also associated with eosinophilic markers in CRS patients (p <.01), but no other relationship was noted with other histopathologic markers evaluated. Additionally, Padhye et al. (2021) assessed for microbial changes associated with air pollution. Interestingly, higher PM2.5 exposure was associated with relatively lower amounts of Corynebacterium in both CRS and control cases (r = −.197, p = .02). Authors note that decreased Corynebacterium has previously been associated with sinonasal inflammation, supporting a link between air pollution exposure and CRS. 45
4. DISCUSSION
The pathophysiology of CRS is complex and multifactorial, with chronic inflammation likely playing a large role. Given nasal and sinus mucosa are among our first lines of defense against air pollution, previous studies have investigated and demonstrated that air pollution may be involved in CRS pathogenesis or progression. This systematic review identified and analyzed 10 human studies investigating the impact of EPA criteria air pollutants on CRS disease onset and severity. Overall, most studies indicated evidence of a correlation between air pollution and CRS. All studies investigating the impact of air pollution on CRS incidence/prevalence found a correlation, notably with PM (PM2.5 and PM10) and NOx pollutants. Additionally, disease severity was worse in CRS patients with higher PM or BC exposure, but only in CRSsNP patients. The biological plausibility of a relationship between environmental exposures and CRS was supported by evidence of objective histopathologic and microbial changes with air pollutant exposure.
The pathophysiology of CRS is further complicated by the different disease classifications—CRSsNP (80%) and CRSwNP (20%). Although these phenotypes may present with significant overlap clinically, there are differences in histopathologic findings, inflammatory markers, and associated comorbidities. 7 , 46 , 47 , 48 Importantly, it has been suggested that there are distinct pathophysiologic mechanisms for CRSsNP and CRSwNP. Only three studies demonstrated a difference in outcomes based on polyp status of patients, while others did not classify patients as CRSsNP or CRSwNP. In two such papers (with the same patient population), worsened air pollution was associated with worsened disease severity in CRSsNP patients. 40 , 41 On the other hand, CRSwNP patients demonstrated histopathologic changes associated with air pollution that were not seen in CRSsNP patients, suggesting that the two subtypes may respond differently to air pollution. 35 Thus, studies that do not account for this distinction in subtypes may arrive at alternate conclusions.
Another confounding factor in the diagnosis and management of rhinosinusitis is the role of allergens. The one study considering the impact of allergens noted that pollutant exposure (PM2.5 and BC) was higher in allergy‐negative CRS patients than allergy‐positive patients. 40 Notably, this trend was driven by CRSwNP but not seen in CRSsNP patients. Additionally, allergy status played no role in disease severity outcomes. This study further highlighted the complex relationship between air pollution, CRS subtypes, and allergens but suggests that environmental pollutants may have more of an impact on nonallergic disease than allergic disease.
Multiple evaluated pollutants were associated with CRS, including PM, BC (a major component of PM), NOx, and O3. Of the evaluated pollutants, PM is the most well studied. Most studies (8/10) investigated the impact of PM (either PM2.5 or PM10), and even when controlling for additional air pollutant exposure, the independent impact of PM alone was apparent. PM is formed from chemical reactions between other pollutants produced from various sources, including industrial plants and cars. 14 Since 1990, PM2.5 and PM10 emissions have dropped 38% and 31%, respectively, and emissions of the other criteria air pollutants have fallen even more dramatically over the same time period. 49 Despite these improvements, almost 100 million Americans are living in a county with at least one air pollutant over the National Ambient Air Quality Standards. 49 Additionally, data from other parts of the world demonstrate challenges in controlling air pollutant exposure. 50 , 51 Given the demonstrated large impacts that air pollution has on the health of individuals, increased effort on regulation and control of this pollution has the potential to impact millions of lives.
This review benefits from many strengths, including being the first systematic review focused on EPA air pollutants and CRS disease markers. By including only studies utilizing objective diagnostic criteria, this review limits the ambiguity in diagnosis for CRS, although it is challenging to limit completely. Additionally, by including only papers that evaluated at least one EPA criteria air pollutant, this review studies the impact of large‐scale pollutants that affect populations worldwide. Therefore, this review provides evidence for the utility of a large‐scale population study evaluating the effects of criteria air pollutants on CRS, with emphasis on the distinction between CRSsNP and CRSwNP.
Despite the strengths of the review, there are inherent limitations in the studies included. Studies utilized home addresses to determine pollutant exposure criteria. Although this is a commonly employed technique that allows for estimated exposure levels to be calculated, it is imprecise and does not account for time spent away from the home or other encountered sources of exposure. Although the use of personal air pollution monitors worn by study participants would allow for better approximations of air pollutant exposure, this would be a challenging, costly study design, which may limit its application. Additionally, in the available studies, there is significant heterogeneity in the data, especially with regard to the type of environmental pollutants evaluated, the methodology for determining exposure, and the outcomes of interest. Therefore, this limits the ability to make definitive conclusions. Finally, most studies were retrospective in nature without defined healthy control groups. Future studies would benefit from the addition of control populations to determine the impact of air pollution on those without diagnosed upper airway disease.
5. CONCLUSION
Recent evidence suggests a role for air pollution in the onset and severity of CRS, most notably with relation to PM2.5 exposure. This supports previous in vitro and in vivo models of pollution in CRS. This further adds to the existing body of literature demonstrating the many negative health impacts of exposure to air pollution, including impacts on upper airway disease, lower airway disease, cardiac disease, and overall morbidity and mortality. Given this evidence, consideration should be given to further investigation and implications for air pollution exposure and supports the need for increased regulation toward measures to improve air quality.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Supporting information
Supplementary 1 Literature search summary
Leland EM, Vohra V, Seal SM, Zhang Z, Ramanathan M Jr. Environmental air pollution and chronic rhinosinusitis: A systematic review. Laryngoscope Investigative Otolaryngology. 2022;7(2):349‐360. 10.1002/lio2.774doi: 10.1002/lio2.774
Funding information National Institute of Allergy and Infectious Diseases, Grant/Award Number: R01AI143731
REFERENCES
- 1. Dietz de Loos D, Lourijsen ES, Wildeman MAM, et al. Prevalence of chronic rhinosinusitis in the general population based on sinus radiology and symptomatology. J Allergy Clin Immunol. 2019;143(3):1207‐1214. doi: 10.1016/j.jaci.2018.12.986 [DOI] [PubMed] [Google Scholar]
- 2. Fu QL, Ma JX, Ou CQ, et al. Influence of self‐reported chronic rhinosinusitis on health‐related quality of life: a population‐based survey. PLoS One. 2015;10(5):e0126881. doi: 10.1371/journal.pone.0126881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Khan A, Huynh TMT, Vandeplas G, et al. The GALEN rhinosinusitis cohort: chronic rhinosinusitis with nasal polyps affects health‐related quality of life. Rhinology. 2019;57(5):343‐351. doi: 10.4193/Rhin19.158 [DOI] [PubMed] [Google Scholar]
- 4. Phillips KM, Hoehle LP, Bergmark RW, Caradonna DS, Gray ST, Sedaghat AR. Acute exacerbations mediate quality of life impairment in chronic rhinosinusitis. J Allergy Clin Immunol Pract. 2017;5(2):422‐426. doi: 10.1016/j.jaip.2016.09.015 [DOI] [PubMed] [Google Scholar]
- 5. Rudmik L. Economics of chronic rhinosinusitis. Curr Allergy Asthma Rep. 2017;17(4):20. doi: 10.1007/s11882-017-0690-5 [DOI] [PubMed] [Google Scholar]
- 6. Smith KA, Orlandi RR, Rudmik L. Cost of adult chronic rhinosinusitis: a systematic review. Laryngoscope. 2015;125(7):1547‐1556. doi: 10.1002/lary.25180 [DOI] [PubMed] [Google Scholar]
- 7. Talat R, Speth MM, Gengler I, et al. Chronic rhinosinusitis patients with and without polyps experience different symptom perception and quality of life burdens. Am J Rhinol Allergy. 2020;34(6):742‐750. doi: 10.1177/1945892420927244 [DOI] [PubMed] [Google Scholar]
- 8. Cao PP, Wang ZC, Schleimer RP, Liu Z. Pathophysiologic mechanisms of chronic rhinosinusitis and their roles in emerging disease endotypes. Ann Allergy Asthma Immunol. 2019;122(1):33‐40. doi: 10.1016/j.anai.2018.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gudis D, Zhao KQ, Cohen NA. Acquired cilia dysfunction in chronic rhinosinusitis. Am J Rhinol Allergy. 2012;26(1):1‐6. doi: 10.2500/ajra.2012.26.3716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khalmuratova R, Park JW, Shin HW. Immune cell responses and mucosal barrier disruptions in chronic rhinosinusitis. Immune Netw. 2017;17(1):60‐67. doi: 10.4110/in.2017.17.1.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Majima Y, Sakakura Y, Matsubara T, Miyoshi Y. Possible mechanisms of reduction of nasal mucociliary clearance in chronic sinusitis. Clin Otolaryngol Allied Sci. 1986;11(2):55‐60. doi: 10.1111/j.1365-2273.1986.tb00108.x [DOI] [PubMed] [Google Scholar]
- 12. Soyka MB, Wawrzyniak P, Eiwegger T, et al. Defective epithelial barrier in chronic rhinosinusitis: the regulation of tight junctions by IFN‐gamma and IL‐4. J Allergy Clin Immunol. 2012;130(5):1087‐1096.e10. doi: 10.1016/j.jaci.2012.05.052 [DOI] [PubMed] [Google Scholar]
- 13. Tieu DD, Kern RC, Schleimer RP. Alterations in epithelial barrier function and host defense responses in chronic rhinosinusitis. J Allergy Clin Immunol. 2009;124(1):37‐42. doi: 10.1016/j.jaci.2009.04.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Agency USEP Criteria air pollutants. March 22, 2021. https://www.epa.gov/criteria-air-pollutants. Accessed February 09, 2022.
- 15. Al‐Kindi SG, Brook RD, Biswal S, Rajagopalan S. Environmental determinants of cardiovascular disease: lessons learned from air pollution. Nat Rev Cardiol. 2020;17(10):656‐672. doi: 10.1038/s41569-020-0371-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kelly FJ, Fussell JC. Air pollution and airway disease. Clin Exp Allergy. 2011;41(8):1059‐1071. doi: 10.1111/j.1365-2222.2011.03776.x [DOI] [PubMed] [Google Scholar]
- 17. Thurston GD, Balmes JR, Garcia E, et al. Outdoor air pollution and new‐onset airway disease. An official American Thoracic Society workshop report. Ann Am Thorac Soc. 2020;17(4):387‐398. doi: 10.1513/AnnalsATS.202001-046ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim H, Kim J, Kim S, et al. Cardiovascular effects of long‐term exposure to air pollution: a population‐based study with 900 845 person‐years of follow‐up. J Am Heart Assoc. 2017;6(11). doi: 10.1161/JAHA.117.007170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Logue JM, Price PN, Sherman MH, Singer BC. A method to estimate the chronic health impact of air pollutants in U.S. residences. Environ Health Perspect. 2012;120(2):216‐222. doi: 10.1289/ehp.1104035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Manisalidis I, Stavropoulou E, Stavropoulos A, Bezirtzoglou E. Environmental and health impacts of air pollution: a review. Front Public Health. 2020;8:14. doi: 10.3389/fpubh.2020.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pope CA 3rd, Lefler JS, Ezzati M, et al. Mortality risk and fine particulate air pollution in a large, representative cohort of U.S. adults. Environ Health Perspect. 2019;127(7):77007. doi: 10.1289/EHP4438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Borm PJ, Kelly F, Kunzli N, Schins RP, Donaldson K. Oxidant generation by particulate matter: from biologically effective dose to a promising, novel metric. Occup Environ Med. 2007;64(2):73‐74. doi: 10.1136/oem.2006.029090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ghio AJ, Carraway MS, Madden MC. Composition of air pollution particles and oxidative stress in cells, tissues, and living systems. J Toxicol Environ Health B Crit Rev. 2012;15(1):1‐21. doi: 10.1080/10937404.2012.632359 [DOI] [PubMed] [Google Scholar]
- 24. Mumby S, Chung KF, Adcock IM. Transcriptional effects of ozone and impact on airway inflammation. Front Immunol. 2019;10:1610. doi: 10.3389/fimmu.2019.01610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ohyama M, Otake T, Adachi S, Kobayashi T, Morinaga K. A comparison of the production of reactive oxygen species by suspended particulate matter and diesel exhaust particles with macrophages. Inhal Toxicol. 2007;19(Suppl 1):157‐160. doi: 10.1080/08958370701496103 [DOI] [PubMed] [Google Scholar]
- 26. Shin CH, Byun J, Lee K, et al. Exosomal miRNA‐19a and miRNA‐614 induced by air pollutants promote proinflammatory M1 macrophage polarization via regulation of RORα expression in human respiratory mucosal microenvironment. J Immunol. 2020;205(11):3179‐3190. doi: 10.4049/jimmunol.2000456 [DOI] [PubMed] [Google Scholar]
- 27. London NR Jr, Tharakan A, Rule AM, Lane AP, Biswal S, Ramanathan M Jr. Air pollutant‐mediated disruption of sinonasal epithelial cell barrier function is reversed by activation of the Nrf2 pathway. J Allergy Clin Immunol. 2016;138(6):1736‐1738.e4. doi: 10.1016/j.jaci.2016.06.027 [DOI] [PubMed] [Google Scholar]
- 28. Ramanathan M Jr, London NR Jr, Tharakan A, et al. Airborne particulate matter induces nonallergic eosinophilic sinonasal inflammation in mice. Am J Respir Cell Mol Biol. 2017;57(1):59‐65. doi: 10.1165/rcmb.2016-0351OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhao R, Guo Z, Dong W, et al. Effects of PM2.5 on mucus secretion and tissue remodeling in a rabbit model of chronic rhinosinusitis. Int Forum Allergy Rhinol. 2018;8(11):1349‐1355. doi: 10.1002/alr.22182 [DOI] [PubMed] [Google Scholar]
- 30. Sundaresan AS, Hirsch AG, Storm M, et al. Occupational and environmental risk factors for chronic rhinosinusitis: a systematic review. Int Forum Allergy Rhinol. 2015;5(11):996‐1003. doi: 10.1002/alr.21573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fokkens WJ, Lund VJ, Hopkins C, et al. Executive summary of EPOS 2020 including integrated care pathways. Rhinology. 2020;58(2):82‐111. doi: 10.4193/Rhin20.601 [DOI] [PubMed] [Google Scholar]
- 33. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non‐randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712‐716. doi: 10.1046/j.1445-2197.2003.02748.x [DOI] [PubMed] [Google Scholar]
- 34. Padhye LV, Kish JL, Batra PS, Miller GE, Mahdavinia M. The impact of levels of particulate matter with an aerodynamic diameter smaller than 2.5 μm on the nasal microbiota in chronic rhinosinusitis and healthy individuals. Ann Allergy Asthma Immunol. 2021;126(2):195‐197. doi: 10.1016/j.anai.2020.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Patel TR, Tajudeen BA, Brown H, et al. Association of air pollutant exposure and sinonasal histopathology findings in chronic rhinosinusitis. Am J Rhinol Allergy. 2021;35(6):761‐767. doi: 10.1177/1945892421993655 [DOI] [PubMed] [Google Scholar]
- 36. Zhang Z, Kamil RJ, London NR, et al. Long‐term exposure to particulate matter air pollution and chronic rhinosinusitis in non‐allergic patients. Am J Respir Crit Care Med. 2021;204(7):859‐862. doi: 10.1164/rccm.202102-0368LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lu M, Ding S, Wang J, et al. Acute effect of ambient air pollution on hospital outpatient cases of chronic sinusitis in Xinxiang, China. Ecotoxicol Environ Saf. 2020;202:110923. doi: 10.1016/j.ecoenv.2020.110923 [DOI] [PubMed] [Google Scholar]
- 38. Velasquez N, Moore JA, Boudreau RM, Mady LJ, Lee SE. Association of air pollutants, airborne occupational exposures, and chronic rhinosinusitis disease severity. Int Forum Allergy Rhinol. 2020;10(2):175‐182. doi: 10.1002/alr.22477 [DOI] [PubMed] [Google Scholar]
- 39. Park M, Lee JS, Park MK. The effects of air pollutants on the prevalence of common ear, nose, and throat diseases in South Korea: a national population‐based study. Clin Exp Otorhinolaryngol. 2019;12(3):294‐300. doi: 10.21053/ceo.2018.00612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mady LJ, Schwarzbach HL, Moore JA, et al. The association of air pollutants and allergic and nonallergic rhinitis in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2018;8(3):369‐376. doi: 10.1002/alr.22060 [DOI] [PubMed] [Google Scholar]
- 41. Mady LJ, Schwarzbach HL, Moore JA, et al. Air pollutants may be environmental risk factors in chronic rhinosinusitis disease progression. Int Forum Allergy Rhinol. 2018;8(3):377‐384. doi: 10.1002/alr.22052 [DOI] [PubMed] [Google Scholar]
- 42. Sommar JN, Ek A, Middelveld R, et al. Quality of life in relation to the traffic pollution indicators NO2 and NOx: results from the Swedish GA(2)LEN survey. BMJ Open Respir Res. 2014;1(1):e000039. doi: 10.1136/bmjresp-2014-000039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wolf C. Urban air pollution and health: an ecological study of chronic rhinosinusitis in Cologne, Germany. Health Place. 2002;8(2):129‐139. doi: 10.1016/s1353-8292(01)00040-5 [DOI] [PubMed] [Google Scholar]
- 44. Luong A, Marple BF. Sinus surgery: indications and techniques. Clin Rev Allergy Immunol. 2006;30(3):217‐222. doi: 10.1385/CRIAI:30:3:217 [DOI] [PubMed] [Google Scholar]
- 45. Mahdavinia M, Engen PA, LoSavio PS, et al. The nasal microbiome in patients with chronic rhinosinusitis: analyzing the effects of atopy and bacterial functional pathways in 111 patients. J Allergy Clin Immunol. 2018;142(1):287‐290.e4. doi: 10.1016/j.jaci.2018.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Benjamin MR, Stevens WW, Li N, et al. Clinical characteristics of patients with chronic rhinosinusitis without nasal polyps in an academic setting. J Allergy Clin Immunol Pract. 2019;7(3):1010‐1016. doi: 10.1016/j.jaip.2018.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kuhar HN, Tajudeen BA, Mahdavinia M, Gattuso P, Ghai R, Batra PS. Inflammatory infiltrate and mucosal remodeling in chronic rhinosinusitis with and without polyps: structured histopathologic analysis. Int Forum Allergy Rhinol. 2017;7(7):679‐689. doi: 10.1002/alr.21943 [DOI] [PubMed] [Google Scholar]
- 48. Staudacher AG, Peters AT, Kato A, Stevens WW. Use of endotypes, phenotypes, and inflammatory markers to guide treatment decisions in chronic rhinosinusitis. Ann Allergy Asthma Immunol. 2020;124(4):318‐325. doi: 10.1016/j.anai.2020.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. United States Environmental Protection Agency Air Quality—National Summary. 2021. https://www.epa.gov/air-trends/air-quality-national-summary. Accessed February 09, 2022.
- 50. Hammer MS, van Donkelaar A, Li C, et al. Global estimates and long‐term trends of fine particulate matter concentrations (1998–2018). Environ Sci Technol. 2020;54(13):7879‐7890. doi: 10.1021/acs.est.0c01764 [DOI] [PubMed] [Google Scholar]
- 51. Wang Y, Li W, Gao W, et al. Trends in particulate matter and its chemical compositions in China from 2013–2017. Sci China Earth Sci. 2019;62(12):1857‐1871. doi: 10.1007/s11430-018-9373-1 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary 1 Literature search summary


