Abstract
Data regarding immunogenicity of SARS-CoV-2 BNT162b2 vaccine in cystic fibrosis (CF) patients are limited. We prospectively measured total (TAbs-RBD; U/ml) and neutralizing (NAbs-RBD; %) antibodies of SARS-CoV-2 spike-receptor binding domain (RBD) protein in 33 CF patients and 66 healthy controls with median age (IQR): 19.6 (17.6–24.3) years and 31 (29–36) years, respectively and investigated possible associations with epidemiological and clinical parameters. Compared to healthy controls, CF patients had higher levels of TAbs-RBD and NAbs-RBD after both doses (P-value < 0.001). One month after the second dose, CF patients and controls had TAbs-RBD: median (IQR): 3396 (2443) and 1452 (1231) U/ml, respectively. Similarly, the NAbs-RBD (%) were: 97.30 (1.00) and 95.70 (3.71) %, respectively. CF patients also had fewer local and systemic adverse events (AEs) (P-value < 0.001). Among CF patients, no significant differences in immunogenicity were detected regarding the phenotype, genotype, medications, or severity of the disease. BNT162b2 vaccine was immunogenic with limited reactogenicity in CF patients regardless of the phenotype or severity of disease.
Keywords: Cystic fibrosis, SARS-CoV-2, COVID-19, BNT162b2, Vaccine, Antibody, Neutralizing
1. Introduction
Since the emergence of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) pandemic, comorbidities such as chronic lung diseases have been associated with severe clinical presentation and increased mortality [1,2].
Although individuals with cystic fibrosis (CF) generally experience acute exacerbations of lung disease from infectious agents, the incidence estimates indicate that they are not more likely to be affected by SARS-CoV-2 than the general population [3]. However, there are reports of subsets of CF, such as those who had organ transplants, that may experience a more severe COVID-19 course [2].
Data regarding immunogenicity of SARS-CoV-2 BNT162b2 vaccine in CF patients are limited. The purpose of this study was to investigate the immunogenicity of SARS-CoV-2 BNT162b2 vaccine and its association with epidemiological and clinical parameters in a cohort of CF patients and to compare it with a cohort of healthy individuals.
2. Study design and participants
This is a prospective cohort study with a control group involving genetically confirmed CF patients and healthy controls. CF participants were patients who were clinically followed at the Center for Cystic Fibrosis of ‘Aghia Sophia’ Children's Hospital and were willing to be vaccinated and participate in the study. As healthy controls were used healthcare workers (HCWs) without CF or underlying diseases. Both groups were vaccinated with the BNT162b2 COVID-19 vaccine (Comirnaty™, Pfizer-BioNTech) in April and May of 2021. Among the population with CF, there was no transplant recipient. The age range of the CF group was 16–35 years and of healthy HCWs control group was 24–40 years. During the study period there was no license for COVID-19 immunization for people <18 years, except for the high-risk groups and that was the reason why there was not an age-matched control group available.
Antibody tests were performed before the vaccination (to investigate the possibility of a previous COVID-19 infection), 1 day before the second dose of vaccine and one month after the second dose.
Each participant completed a form containing demographic, clinical data and adverse events (AEs) after each dose of COVID-19 vaccine. Data included sex, age, blood type and rhesus, body mass index (BMI, pancreatic status, CFTR genotype, CFRD (cystic fibrosis-related diabetes), CFTR modulators, history of chronic Pseudomonas aeruginosa (PA) infection, history of any other underlying diseases (cardiac, pulmonary, hyperlipidemia, Type 1 diabetes mellitus, Hashimoto's thyroiditis, rheumatoid arthritis, etc.) and allergies to medications. Local AEs including pain and edema or systemic AEs such as fatigue, fever, headache, lymphadenopathy, myalgias, arthralgias etc., were also recorded after each vaccine dose.
Serum samples were tested using Elecsys® Anti-SARS-CoV-2 S reagent (Roche Diagnostics, Basel, Switzerland) for detection of total antibodies (TAbs-RBD) of the receptor binding domain (RBD) of the S1 subunit of SARS-CoV-2 spike protein. Anti-RBD neutralization titers were measured with the ELISA cPassTM SARS-CoV-2 neutralization antibody detection kit (GenScript Biotech Corporation, Piscataway, New Jersey, USA).
The study protocol was approved by the Scientific and Bioethics Committee of the Children's Hospital ‘Aghia Sophia’ (No. 6292) and was carried out according to the Declaration of Helsinki. Written informed consent was obtained from all participants.
2.1. Statistical methods
Qualitative variables were described via absolute and relative frequencies (%), while median, and interquartile range (IQR) were used for quantitative data. Differences were evaluated using the Mann-Whitney U test, Kruskal-Wallis H test or Fisher's exact test. Statistical analysis was performed with SPSS version 26.0 (IBM Corp. Armonk, NY: IBM Corp).
3. Results
A total of 33 patients with CF and 66 healthy controls, who were fully vaccinated with 2 doses of the BNT162b2 vaccine according to the recommended schedule (21 days apart), were included in the study. The median age (IQR) of the CF group was 19.6 (17.6–24.3) years and 18 (54.4%) were females. In the control group, the median age (IQR) was 31 (29–36) years, and 51 (77.3%) were women.
In the CF group, severe CF phenotype and pancreatic insufficiency were present in 28 (84.8%) participants. Fifteen CF participants (45.5%) were homozygotes for the F508del mutation in the CFTR gene. Regarding current medication around the time of vaccine administration, 32 (97%) were treated with Pulmozyme (dornase alfa), 31 (93.9%) received azithromycin as anti-inflammatory therapy, 25 (75.8%) also received inhaled antibiotics and in 14 (42.4%) CFTR modulator therapy was administrated; lumacaftor/ivacaftor in 11/14 (78.6%), ivacaftor in 1/14 (7.1%) and a combination of tezacaftor/ivacaftor and ivacaftor in 2/14 (14.3%). Only 4 (12.1%) participants in the CF group have CFRD and another 4 (12.1%) have allergies to medications (ceftazidime, clindamycin, azithromycin, and minocycline).
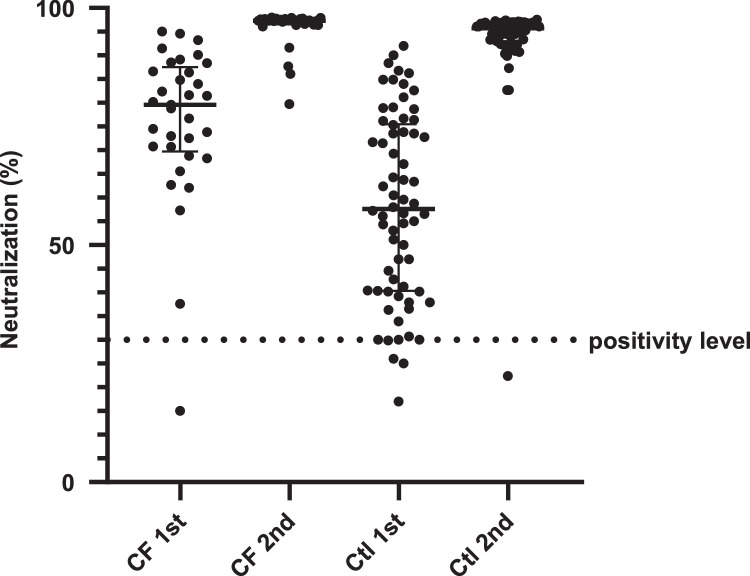
The comparison of TAbs-RBD (U/ml) and NAbs-RBD (%) between CF patients and healthy controls after the first and second doses of the vaccine is presented in Table 1 and Fig. 1 . There was no positive participant for past COVID-19 infection.
Table 1.
Median values and interquartile range (IQR) of TAbs-RBD (U/ml) and NAbs-RBD (%) SARS-CoV-2 spike antibodies after the first and second dose of BNT162b2 vaccine in the CF group (n = 33) and in the control group (n = 66).
| After 1st dose |
After 2nd dose |
||||
|---|---|---|---|---|---|
| TAbs-RBD (U/ml) | NAbs-RBD (%) | TAbs-RBD (U/ml) | NAbs-RBD (%) | ||
| Group | Cystic fibrosis n=33 |
222.45 (1162.05) |
79.60 (15.90) |
3396.00 (2443.00) |
97.30 (1.00) |
| Controls n=66 |
35.41 (105.75) |
57.63 (34.95) |
1452.00 (1231.00) |
95.70 (3.71) |
|
| P-value | <.001 | <.001 | <.001 | <.001 | |
Abbreviations: TAbs-RBD; Total antibodies against Receptor Binding Domain of SARS-CoV-2 spike protein, NAbs-RBD; Neutralizing antibodies (%). Values refer to median (interquartile range) and P-value of Mann-Whitney U test. Statistically significant values are marked in bold.
Fig. 1.
SARS-CoV-2 neutralizing – receptor binding domain antibodies (%) in cystic fibrosis (CF) patients (n = 33) and in healthy controls (Ctl) (n = 66), 20 days after vaccination with the first dose and one month after the second dose of the BNT162b2 vaccine. Black lines represent median (IQR) values. CF: cystic fibrosis, Ctl: control.
Statistically significant higher values for TAbs-RBD and NAbs-RBD were detected in the CF group after both vaccine doses (P-value < 0.001). One month after the second dose the TAbs-RBD (median, IQR) for CF group and control group were 3396 U/ml (2443) and 1452 U/ml (1231), respectively (P-value < 0.001). Likewise, the NAbs-RBD (median, IQR) for the CF group and control group were 97.3% (1) and 95.7% (3.71), respectively (P-value < 0.001).
The associations of antibody responses after both vaccine doses with clinical and demographic characteristics in CF patients are presented in Table 2 . Among CF patients no statistically significant differences were detected for TAbs-RBD or NAbs-RBD regarding gender, local or systemic AEs, pancreatic status, CFRD, CFTR genotype of CF, use of CFTR modulators and chronic PA infection (Table 2).
Table 2.
Differences in median values of TAbs-RBD (U/ml) and NAbs-RBD (%) SARS-CoV-2 spike antibodies after the first and second dose of BNT162b2 vaccine regarding demographic and clinical characteristics in cystic fibrosis patients (n = 33).
| After 1st dose |
After 2nd dose |
||||
|---|---|---|---|---|---|
| n (%) | TAbs-RBD (U/ml) | NAbs-RBD (%) | TAbs-RBD (U/ml) | NAbs-RBD (%) | |
|
Gender |
Male 15 (45.5) |
280.70 (1736.70) |
80.20 (20.80) |
3381.00 (2776.00) |
97.30 (1.60) |
| Female 18 (54.5) |
214.70 (164.10) |
79.20 (15.60) |
3642.50 (2322.00) |
97.35 (0.40) |
|
| P-value | .526 | .845 | .532 | .421 | |
|
Local Adverse Events |
No 1st dose: 25 (75.8) 2nd dose: 24 (72.7) |
231.00 (1401.45) |
81.60 (15.40) |
3785.00 (3271.00) |
97.30 (1.00) |
| Yes 1st dose: 8 (24.2) 2nd dose: 9 (27.3) |
166.55 (855.74) |
69.80 (11.50) |
2881.00 (1544.00) |
97.30 (0.60) |
|
| P-value | .380 | .089 | .486 | .677 | |
|
Systemic Adverse Events |
No 1st dose: 29 (87.9) 2nd dose: 24 (72.7) |
223.25 (1253.95) |
79.60 (15.60) |
3388.50 (2497.00) |
97.30 (0.95) |
| Yes 1st dose: 4 (12.1) 2nd dose: 9 (27.3) |
140.91 (218.75) |
75.30 (29.60) |
4445.00 (5319.00) |
97.30 (0.90) |
|
| P-value | .254 | .811 | .437 | .706 | |
|
Pancreatic Status |
Pancreatic insufficiency 28 (84.8) |
214.70 (1350.50) |
79.20 (16.75) |
3388.50 (2430.50) |
97.30 (0.95) |
| Pancreatic sufficiency 5 (15.2) |
244.00 (324.80) |
80.20 (15.20) |
3681.00 (2018.00) |
97.60 (0.50) |
|
| P-value | >.999 | .903 | .643 | .478 | |
|
CFRD |
No 29 (87.9) |
212.65 (1269.05) |
78.80 (16.00) |
3396.00 (2311.00) |
97.30 (1.20) |
| Yes 4 (12.1) |
231.00 (106.86) |
88.80 (9.80) |
4987.00 (6813.50) |
97.20 (0.40) |
|
| P-value | .763 | .133 | .730 | .690 | |
|
CFTR Modulators |
No 19 (57.6) |
231.80 (1679.30) |
76.70 (19.60) |
3917.00 (2443.00) |
97.40 (1.20) |
| Yes 14 (42.4) |
197.60 (201.94) |
81.95 (14.10) |
3127.00 (2629.00) |
97.30 (0.80) |
|
| P-value | .195 | .529 | .627 | .900 | |
|
CFTR genotype§ |
F508del/F508del 15 (45.5) |
184.30 (214.14) |
79.60 (18.30) |
3268.00 (2224.00) |
97.30 (1.00) |
| F508del/other 13 (39.4) |
230.20 (1678.90) |
76.70 (17.70) |
4708.00 (4825.00) |
97.50 (0.60) |
|
| Other 5 (15.2) |
244.00 (76.70) |
81.60 (5.20) |
3381.00 (1246.00) |
97.20 (0.30) |
|
| P-value | .324 | .955 | .718 | .412 | |
|
Chronic PA |
No 19 (57.6) |
268.45 (1129.20) |
81.50 (17.60) |
3889.00 (3817.00) |
97.30 (1.30) |
| Yes 14 (42.4) |
153.25 (201.94) |
75.25 (16.80) |
3324.50 (2486.00) |
97.30 (0.60) |
|
| P-value | .111 | .274 | .362 | .971 | |
Abbreviations: TAbs-RBD; Total antibodies against Receptor Binding Domain of SARS-CoV-2 spike protein, NAbs-RBD; Neutralizing antibodies (%), CFRD; Cystic fibrosis-related diabetes, PA; Pseudomonas aeruginosa. Values refer to median (interquartile range) and P-value of Mann-Whitney U test or §Kruskal-Wallis H test. Statistically significant values are marked in bold.
Local AEs after both vaccine doses were reported less frequently in the CF group than in the control group (P-value < 0.001). Regarding systemic AEs, no significant differences were detected between the two groups after the first dose (P-value = 0.408), with higher reporting in the control group after the second dose (P-value < 0.001). In the CF group, the most common local self-limited AEs after the first and second doses were injection site pain (24.2% and 27.3%, respectively). The most frequently reported systemic AEs after both doses were fatigue (6.1% and 12.1%, respectively) and fever (6.1% and 15.2%, respectively).
4. Discussion
In our study population, the BNT162b2 vaccine induced higher antibody responses in the CF group compared to the older healthy control group. Τhis difference can be attributed to the younger age of the CF group compared to the control. In a previous study, we have detected an inverted association of age with antibody responses after immunization with the BNT162b2 vaccine, which has also been found in other studies [4,5]. CF patients mounted good antibody responses after immunization irrelevant of CFTR genotype, related comorbidities, or treatment type. However, it remains questionable whether CF itself predisposes to enhanced adaptive immune responses after COVID-19 immunization, as no relevant data have been published. Thus, it is reasonable that further studies are required to elucidate the pathophysiologic basis for these findings with comparison with an age-matched control group.
The administration of the BNT162b2 vaccine had a good safety profile in CF patients comparable or even better from the control group, with most local or systemic AEs being self-limited, lasting 2-3 days after vaccination and without preventing daily activities. No severe hypersensitivity reactions, including anaphylaxis, had been observed among vaccine recipients in both groups during the study period. This may be attributed to the younger age of CF participants comparing with the control and is a finding that is also detected in the initial clinical studies [6]. A recent study regarding the safety profile of mRNA SARS-CoV-2 vaccines in 424 patients with CF reported that were well-tolerated and safe without significant differences among different age groups. However, in this study both mRNA-1273 and BNT162b2 vaccine were used [7].
The present study has certain limitations, including the limited number of participants in the CF group, as well as the lack of age and gender matching between the two study groups, due to the restriction of the COVID-19 vaccine license during the study period. However, to the best of our knowledge, this is the first study reporting immunogenicity in patients with CF after BNT162b2 vaccination.
In conclusion, BNT162b2 vaccine appears to be immunogenic with limited adverse events in CF population. Long-term studies, with a higher number of participants regarding vaccine effectiveness, antibody kinetics after immunization, T cell responses, and rare AEs are important in order mass vaccination policies to be optimized.
CRediT authorship contribution statement
Athanasios Michos: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. Filippos Filippatos: Data curation, Formal analysis, Investigation, Project administration, Writing – original draft, Writing – review & editing. Elizabeth-Barbara Tatsi: Data curation, Formal analysis, Investigation, Project administration, Writing – original draft, Writing – review & editing. Charilaos Dellis: Data curation, Investigation. Vasiliki Efthymiou: Data curation, Formal analysis, Investigation. Ioanna Zarkada: Data curation, Investigation. Evgenia Troupi: Data curation, Investigation. Vasiliki Syriopoulou: Data curation, Investigation, Methodology. Ioanna Loukou: Conceptualization, Data curation, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
None.
Funding
This research did not receive specific grant from funding agencies in the public, commercial or not-for-profit sectors.
References
- 1.Harrison S.L., Fazio-Eynullayeva E., Lane D.A., Underhill P., Lip G.Y.H. Comorbidities associated with mortality in 31,461 adults with COVID-19 in the United States: a federated electronic medical record analysis. PLoS Med. 2020;17 doi: 10.1371/JOURNAL.PMED.1003321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathew H.R., Choi M.Y., Parkins M.D., Fritzler M.J. Systematic review: cystic fibrosis in the SARS-CoV-2/COVID-19 pandemic. BMC Pulm Med. 2021;21:1–11. doi: 10.1186/s12890-021-01528-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burgel P.R., Goss C. COVID-19 outcomes in people with cystic fibrosis. Curr Opin Pulm Med. 2021;27:538–543. doi: 10.1097/MCP.0000000000000823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Terpos E., Trougakos I.P., Apostolakou F., Charitaki I., Sklirou A.D., Mavrianou N., et al. Age-dependent and gender-dependent antibody responses against SARS-CoV-2 in health workers and octogenarians after vaccination with the BNT162b2 mRNA vaccine. Am J Hematol. 2021;96:E257–E259. doi: 10.1002/ajh.26185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michos A., Tatsi E.B., Filippatos F., Dellis C., Koukou D., Efthymiou V., et al. Association of total and neutralizing SARS-CoV-2 spike -receptor binding domain antibodies with epidemiological and clinical characteristics after immunization with the 1st and 2nd doses of the BNT162b2 vaccine. Vaccine. 2021;39:5963–5967. doi: 10.1016/j.vaccine.2021.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frenck R.W., Klein N.P., Kitchin N., Gurtman A., Absalon J., Lockhart S., et al. Safety, immunogenicity, and efficacy of the BNT162b2 COVID-19 vaccine in adolescents. N Engl J Med. 2021;385:239–250. doi: 10.1056/nejmoa2107456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alicandro G., Daccó V., Cariani L., Contarini M., Morlacchi L.C., Rosazza C., et al. Safety of mRNA-based vaccines against SARS-CoV-2 in people with cystic fibrosis aged 12 years and over. J Cyst Fibros. 2022 doi: 10.1016/j.jcf.2022.02.009. S1569-1993(22)00041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]