Abstract
The interaction of amphotericin B (AmB) and azole antifungal agents in the treatment of fungal infections is still a controversial issue. A checkerboard titration broth microdilution-based method that adhered to the recommendations of the National Committee for Clinical Laboratory Standards was applied to study the in vitro interactions of AmB with fluconazole (FLC), itraconazole (ITC), and the new investigational triazole SCH 56592 (SCH) against 15 clinical isolates of Cryptococcus neoformans. Synergy, defined as a fractional inhibitory concentration (FIC) index of ≤0.50, was observed for 7% of the isolates in studies of the interactions of both FLC-AmB and ITC-AmB and for 33% of the isolates in studies of the SCH-AmB interactions; additivism (FICs, >0.50 to 1.0) was observed for 67, 73, and 53% of the isolates in studies of the FLC-AmB, ITC-AmB, and SCH-AmB interactions, respectively; indifference (FICs, >1.0 to ≤2.0) was observed for 26, 20, and 14% of the isolates in studies of the FLC-AmB, ITC-AmB, and SCH-AmB interactions, respectively. Antagonism (FIC >2.0) was not observed. When synergy was not achieved, there was still a decrease, although not as dramatic, in the MIC of one or both drugs when they were used in combination. To investigate the effects of FLC-AmB combination therapy in vivo, we established an experimental model of systemic cryptococcosis in BALB/c mice by intravenous injection of cells of C. neoformans 2337, a clinical isolate belonging to serotype D against which the combination of FLC and AmB yielded an additive interaction in vitro. Both survival and tissue burden studies showed that combination therapy was more effective than FLC alone and that combination therapy was at least as effective as AmB given as a single drug. On the other hand, when cells of C. neoformans 2337 were grown in FLC-containing medium, a pronounced increase in resistance to subsequent exposures to AmB was observed. In particular, killing experiments conducted with nonreplicating cells showed that preexposure to FLC abolished the fungicidal activity of the polyene. However, this apparent antagonism was not observed in vivo. Rather, when the two drugs were used sequentially for the treatment of systemic murine cryptococcosis, a reciprocal potentiation was often observed. Our study shows that (i) the combination of triazoles and AmB is significantly more active than either drug alone against C. neoformans in vitro and (ii) the concomitant or sequential use of FLC and AmB for the treatment of systemic murine cryptococcosis results in a positive interaction.
The interaction of amphotericin B (AmB) and azole antifungal drugs in the treatment of fungal infections is still a controversial issue (1, 9, 11, 16–21, 23–27). AmB is believed to act primarily by damaging the fungal cell membrane after binding to fungal sterols, mainly to ergosterol. This binding alters membrane permeability, causing leakage of cations and hydrogen ions and eventually leading to cell death (24). Azoles appear to act by preventing fungal ergosterol biosynthesis via specific and selective inhibition of fungal lanosterol 14-demethylase, an enzyme of the cytochrome P450 superfamily (5). Thus, in theory, azoles could antagonize the effects of AmB. However, experimental data have demonstrated that the effects of this type of interaction can range from antagonistic to frankly synergistic (1, 9, 11, 16–21, 23–27). This broad variation of the results seems to be due to specific characteristics of the azole drug. It has been postulated that a lipophilic azole, such as itraconazole (ITC), by adsorbing to the cell membrane surface, could block the interaction of AmB at the cell membrane (21). On the other hand, a water-soluble azole, such as fluconazole (FLC), by penetrating the fungal cell, does not accumulate on the cell membrane, thereby allowing AmB to bind to the cell membrane ergosterol (21).
Cryptococcus neoformans is an important cause of morbidity and mortality in immunocompromised patients (4, 12, 30). The “gold standard” therapy for cryptococcosis remains AmB with or without flucytosine (4, 30). For suppression therapy a triazole, such as FLC or ITC, is the agent of choice (4, 5). Recently, the new investigational triazole SCH 56592 (SCH) was shown to have potent activity against isolates of C. neoformans (2, 8). Since in clinical practice AmB and triazoles are concomitantly or sequentially used for the treatment of cryptococcal infections, data that provide some insight into the effects of this interaction are needed.
Thus, in the present study we investigated the interactions of FLC, ITC, and SCH with AmB against C. neoformans.
MATERIALS AND METHODS
Isolates.
Fifteen isolates of C. neoformans were included in this study. They comprised 14 clinical strains isolated from blood, cerebrospinal fluid, or skin biopsy specimens of AIDS patients and one strain from the American Type Culture Collection: C. neoformans ATCC 90112. All the strains were maintained on Sabouraud dextrose agar (SDA; Difco Laboratories, Detroit, Mich.) slants at 4°C.
Antifungal agents.
Stock solution of AmB (Sigma Chemical, Milan, Italy) was prepared in dimethyl sulfoxide (Sigma). Stock solutions of FLC (Pfizer Inc., New York, N.Y.) were prepared in sterile distilled water. Stock solutions of ITC (Janssen, Beerse, Belgium) and SCH (Shering-Plough Research Institute, Kenilworth, N.J.) were prepared in polyethylene glycol 400 (Janssen Chimica, Geel, Belgium). Further dilutions of all drugs were prepared in the test medium (13). For in vivo studies AmB (Fungizone) was purchased from Brystol-Myers, Squibb S.p.A., Sermoneta, Italy, while FLC (Diflucan) was purchased from Pfizer, Roerig S.p.A., Latina, Italy.
In vitro experiments. (i) Combination therapy.
Drug interactions were assessed by a checkerboard titration broth microdilution-based method that adhered to the recommendations of the National Committee for Clinical Laboratory Standards (NCCLS) (13). Testing was performed in RPMI 1640 medium (Sigma) buffered to pH 7.0 with 0.165 M morpholinepropanesulfonic acid (MOPS) buffer (Gibco Laboratories, Milan, Italy). AmB was tested at concentrations that ranged from 0.03 to 2.0 μg/ml. FLC was tested at concentrations that ranged from 0.06 to 32 μg/ml, both ITC and SCH were tested at concentrations that ranged from 0.0078 to 4.0 μg/ml. The trays were incubated at 35°C and were read at 72 h. Readings were performed spectrophotometrically with an automatic plate reader (model MR 700; Dynatech) set at 490 nm (3). MIC endpoints were determined as the first concentration of the antifungal agent tested alone and in combination at which the turbidity in the well was >80% less than that in the control well. Drug interactions were classified as synergistic, additive, indifferent, or antagonistic on the basis of the fractional inhibitory concentration (FIC) index (7). The FIC index is the sum of the FICs of each drug; the FIC is defined as the MIC of each drug when used in combination divided by the MIC of the drug when used alone. The interaction was defined as synergistic if the FIC index was 0.50, additive if the FIC index was >0.50 to 1.0, indifferent if the FIC index was >1.0 to 2.0, and antagonistic if the FIC index was >2.0 (7).
(ii) Sequential therapy.
Sequential therapy experiments were performed only with FLC. The effect of preexposure to FLC on the anticryptococcal activity of AmB was investigated against C. neoformans 2337. Serotyping, performed by M. A. Viviani at the Istituto di Igiene e Medicina Preventiva Università degli Studi di Milano, showed that this strain belongs to serotype D. Briefly, cells of C. neoformans were grown overnight in FLC-free medium (CN) or in medium with FLC at 50 μg/ml (CN-50). Cells were harvested by low-speed centrifugation, washed twice with phosphate-buffered saline (PBS), adjusted to a final inoculum of 1.0 × 105 to 5.0 × 105 CFU/ml, and suspended in 10 ml of medium (replicating cells) or PBS (nonreplicating cells) containing AmB at variable concentrations. At time points of 0, 1, 2, 4, 6, and 24 h following the introduction of the isolate into the system, 100-μl aliquots were removed from each test solution. After 10-fold serial dilution, a 50-μl aliquot from each dilution was streaked in duplicate onto SDA plates for colony count determination. The plates were incubated for from 48 to 72 h at 35°C, and then the number of CFU was counted. Fungicidal activity was considered to be achieved when the number of CFU per milliliter was <99.9% compared with the initial inoculum size. Each experiment was performed three times.
Animal studies.
Studies with animals were performed only with FLC. A murine model of systemic cryptococcosis was established in male BALB/c mice (weight, 30 g; Charles River Laboratories, Calco, Italy) by intravenous injection of viable cells of C. neoformans 2337. Both FLC and AmB were administered intraperitoneally. FLC was given at concentrations that ranged from 3 to 30 mg/kg of body weight/day, and AmB was given at concentrations that ranged from 0.5 to 1.5 mg/kg/day. Either combination or sequential therapies were evaluated (see below). In survival studies, treatment was begun 24 h after infection and was continued for 10 days. The mice were observed through day 30, and deaths were recorded daily. In tissue burden studies, therapy was given for from 7 to 13 consecutive days, depending on the experiment (see below). Twenty-four hours after the end of therapy, the mice were euthanized by CO2-induced asphyxia, and the number of viable CFU per gram of brain, lungs, spleen, liver, and kidneys of each animal was determined by quantitative plating of organ homogenates on SDA plates. There were 10 mice per group in the survival studies and 7 mice per group in the tissue burden studies.
Serum FLC levels.
In some experiments serum FLC concentrations were determined by reverse-phase high-pressure liquid chromatography (HPLC) (14, 29). Briefly, samples were deproteinated and spiked with an extraction mixture of acetonitrile containing 0.5 μg of internal standard (UK-54,373) per ml by vortex mixing for 30 s, followed by centrifugation at 10,000 × g for 1 min at room temperature and then a repeat of the mixing. After centrifugation the organic layer was removed and evaporated with nitrogen to near dryness. The residue was reconstituted in 100 μl of mobile phase and filtered, and an aliquot was injected into the HPLC column. Separation was achieved with a reversed-phase analytical column (C18; 3.9 by 150 or 300 mm) at room temperature and a wavelength of 205 nm with 10 mM acetonitrile–ammonium acetate (30:70; vol/vol) with 0.5% diethylamine as the mobile phase. The pump was set at 1 ml/min. Working serum standards with FLC concentrations of 0.25 to 50 μg/ml were prepared. Controls with FLC concentrations of 0.5, 2.0, 7.5, and 37.5 μg/ml were similarly prepared. Best-fit standard curves were obtained by linear regression analysis with a correlation coefficient not less than 0.99. Intra- and interassay precisions were determined, and the results were considered acceptable when both intra- and interassay differences were less than 10%.
Statistical analysis.
The MIC data were transformed logarithmically to approximate a normal distribution before statistical analysis. Continuous variables were compared by Student's t test or the Mann-Whitney test. Survival was plotted as Kaplan-Meier curves, and groups were compared by log rank analysis. The results of the fungal burden studies were analyzed by the Mann-Whitney test. Significance was defined as a P value of <0.05.
RESULTS
In vitro studies.
To investigate the interactions between triazoles and AmB in vitro, we performed experiments in which drugs were tested either in combination or through a sequential scheme.
(i) Combination therapy.
The results of combination therapy for 15 isolates of C. neoformans are reported in Table 1. AmB MICs ranged from 0.25 to 1.0 μg/ml, with an MIC at which 50% of isolates are inhibited (MIC50) and an MIC90 of 1.0 μg/ml each. FLC MICs ranged from 1.0 to 16 μg/ml, with an MIC50 and an MIC90 of 4.0 and 8.0 μg/ml, respectively. When AmB and FLC were given in combination, there were significant reductions in the geometric mean AmB MIC (from 0.73 to 0.07 μg/ml; P = 0.0001) and FLC MIC (from 4.1 to 1.8 μg/ml; P = 0.029). For 7% (1 of 15) of the isolates the interactions were synergistic, for 67% (10 of 15) they were additive, and for 26% (4 of 15) they were indifferent, while antagonism was not observed. For isolate 526 there was a fourfold reduction in the MIC of each drug upon use of the drugs in combination. When additivism was documented, the median reductions in MICs were 8-fold (range, 2- to 32-fold) for AmB and 2-fold (range, 2- to 128-fold) for FLC. For four isolates (isolates 486, 492, 1993, and 3123) the interactions were indifferent: the initial AmB MICs for the isolates were reduced 16- to 32-fold upon combination of AmB with FLC. ITC MICs ranged from 0.25 to 1.0 μg/ml, with an MIC50 and an MIC90 of 0.5 and 1.0 μg/ml, respectively. When AmB and ITC were given in combination, there were significant reductions in the geometric mean AmB MIC (from 0.83 to 0.10 μg/ml; P = 0.0001) and ITC MIC (from 0.41 to 0.17 μg/ml; P = 0.009). For 7% (1 of 15) of the isolates the interactions were synergistic, for 73% (11 of 15) they were additive, and for 20% (3 of 15) they were indifferent, while antagonism was not observed. For isolate 2881 there was a fourfold reduction in the MIC of each drug upon use of the drugs in combination. When additivism was documented, the median reductions in MICs were 4-fold (range, 2- to 32-fold) for AmB and 2-fold (range, 2- to 32-fold) for ITC. For three isolates (isolates 492, 1880, and 2337) the interactions were indifferent: the initial AmB MICs were reduced 8- to 32-fold upon combination of AmB with ITC. SCH MICs ranged from 0.125 to 1.0 μg/ml, with an MIC50 and an MIC90 of 0.5 and 1.0 μg/ml, respectively. When AmB and SCH were given in combination, there were significant reductions in the geometric mean AmB MIC (from 0.57 to 0.15 μg/ml; P = 0.0001) and SCH MIC (from 0.45 to 0.08 μg/ml; P = 0.0001). For 33% (5 of 15) of the isolates the interactions were synergistic, for 53% (8 of 15) they were additive, and for 14% (2 of 15) they were indifferent, while antagonism was not observed. When synergy was documented, the median reductions in MICs were 4-fold for AmB and 16-fold (range, 2- to 32-fold) for SCH. When additivism was documented, the median reductions in MICs were 2-fold (range, 2- to 4-fold) for AmB and 4-fold (range, 2- to 64-fold) for SCH. For two isolates (isolates 1880 and 2337) the interactions were indifferent: for both isolates the initial AmB MIC was reduced 16-fold upon combination of AmB with SCH.
TABLE 1.
Mode of interactions between triazoles and AmB in 15 isolates of C. neoformans
| Isolate | MIC (μg/ml)
|
AmB-FLC FIC | MIC (μg/ml)
|
AmB-ITC FIC | MIC (μg/ml)
|
AmB-SCH FIC | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| AmBa | FLC | AmB-FLC | ITC | AmB-ITC | SCH | AmB-SCH | ||||
| ATCC 90112 | 1.0 | 8.0 | 0.03/4.0 | 0.53 | 0.25 | 0.5/0.125 | 1.00 | 1.0 | 0.5/0.015 | 0.51 |
| 486 | 0.5/1.0/0.5 | 8.0 | 0.03/8.0 | 1.06 | 1.0 | 0.06/0.5 | 0.56 | 1.0 | 0.125/0.06 | 0.31 |
| 491 | 0.25/1.0/0.5 | 1.0 | 0.125/0.5 | 1.00 | 0.5 | 0.06/0.25 | 0.56 | 0.125 | 0.25/0.015 | 0.62 |
| 492 | 0.5/1.0/0.5 | 4.0 | 0.03/4.0 | 1.06 | 1.0 | 0.03/1.0 | 1.03 | 1.0 | 0.125/0.5 | 0.75 |
| 526 | 1.0/1.0/0.5 | 8.0 | 0.25/2.0 | 0.50 | 0.5 | 0.03/0.25 | 0.53 | 0.5 | 0.125/0.015 | 0.28 |
| 1094 | 0.5/1.0/0.5 | 8.0 | 0.25/0.06 | 0.51 | 0.5 | 0.25/0.25 | 0.75 | 0.5 | 0.125/0.25 | 0.75 |
| 1880 | 1.0/0.5/0.5 | 2.0 | 0.03/1.0 | 0.53 | 0.25 | 0.03/0.25 | 1.06 | 0.25 | 0.03/0.25 | 1.06 |
| 1993 | 0.5/1.0/0.5 | 2.0 | 0.03/2.0 | 1.06 | 0.25 | 0.5/0.007 | 0.53 | 0.5 | 0.125/0.03 | 0.31 |
| 2337 | 1.0/0.5/0.5 | 4.0 | 0.5/0.5 | 0.62 | 0.25 | 0.06/0.25 | 1.12 | 0.25 | 0.03/0.25 | 1.06 |
| 2341 | 1.0 | 4.0 | 0.25/2.0 | 0.75 | 0.5 | 0.06/0.25 | 0.56 | 0.5 | 0.25/0.125 | 0.50 |
| 2715 | 1.0/0.5/1.0 | 16 | 0.03/8.0 | 0.53 | 0.5 | 0.125/0.25 | 0.75 | 1.0 | 0.25/0.25 | 0.50 |
| 2853 | 1.0/1.0/0.5 | 2.0 | 0.125/1.0 | 0.62 | 0.25 | 0.5/0.03 | 0.62 | 0.25 | 0.25/0.015 | 0.56 |
| 2881 | 1.0/1.0/0.5 | 8.0 | 0.03/4.0 | 0.53 | 1.0 | 0.25/0.25 | 0.50 | 1.0 | 0.25/0.25 | 0.75 |
| 3059 | 0.5 | 4.0 | 0.03/2.0 | 0.56 | 0.25 | 0.25/0.125 | 1.00 | 0.5 | 0.25/0.25 | 1.00 |
| 3123 | 1.0/1.0/0.5 | 2.0 | 0.03/2.0 | 1.03 | 0.25 | 0.03/0.125 | 0.53 | 0.125 | 0.25/0.03 | 0.74 |
When only one value (e.g., 1.0 μg/ml) is given, the AmB MIC was always, for example, 1.0 μg/ml for each drug combination; when three values (e.g., 1.0/1.0/0.5 μg/ml) are reported, it means that the AmB MICs were, for example, 1.0, 1.0, and 0.5 μg/ml in the experiments performed with FLC, ITC, and SCH, respectively.
(ii) Sequential therapy.
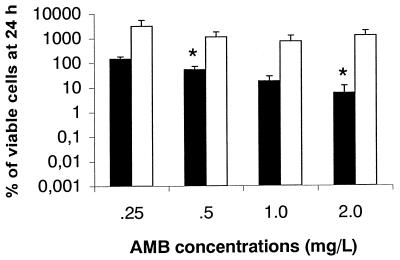
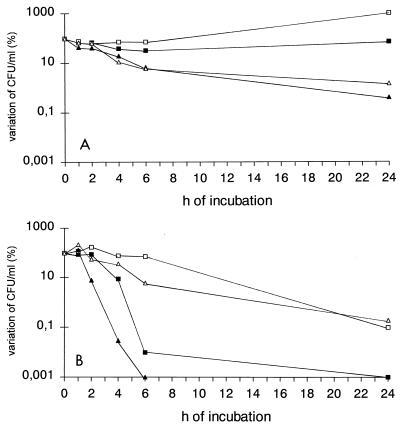
Sequential therapy experiments were performed only with FLC. Since additivism was the most common interaction seen among these isolates, C. neoformans 2337 was selected as a representative strain for further experiments. The isolate was grown overnight either in FLC-free medium (CN) or in medium containing FLC at 50 μg/ml (CN-50). Quantitative plating showed that all in vitro experiments were performed with an initial inoculum that ranged from 1.0 × 105 to 5.0 × 105 CFU/ml. Figure 1 shows the viability of CN or CN-50 cells incubated for 24 h with various concentrations of AmB. In this experiment the cells were incubated without agitation at 35°C. The anticryptococcal activity of AmB against CN cells was dose dependent, with 149, 53, 17, and 5% of the cells surviving incubation with AmB at concentrations of 0.25, 0.5, 1.0, and 2.0 μg/ml, respectively. On the other hand, the polyene was clearly ineffective against CN-50 cells, as shown by a dramatic increase in the numbers of CFU after 24 h of incubation with all AmB concentrations. In particular, the reduction in the number of viable CN cells was significantly greater than the reduction in the number of CN-50 viable cells with AmB at concentrations of 0.5 μg/ml (P = 0.048) and 2.0 μg/ml (P = 0.044). Figure 2A shows the anticryptococcal activity of AmB at concentrations of 0.5 and 1.0 μg/ml against replicating cells (incubation in RPMI 1640 medium). In these experiments the cells were gently shaken through the 24-h period. In this system, AmB at 0.5 μg/ml exerted fungistatic activity against both types of cells for up to 6 h of incubation. However, the regrowth at 24 h was more pronounced for CN-50 cells than it was for CN cells. When cells were incubated with AmB at 1.0 μg/ml, a progressive decrease in the number of CFU was noted through the 24-h period for both types of cells. The percent viability at the end of the experiment was 1.5 for CN-50 cells and 0.4 for CN cells. Figure 2B shows the anticryptococcal activity of AmB at concentrations of 0.5 and 1.0 μg/ml against nonreplicating cells (incubation in PBS). Both AmB concentrations exerted fungicidal activity against CN cells (>99.9% reduction in the number of CFU) after 6 h of incubation. On the other hand, AmB at 0.5 and 1.0 μg/ml did not exert fungicidal activity against CN-50 cells during the 24-h incubation period. The viabilities after 24 h of incubation were 0.1 and 0.2% for CN-50 cells with AmB at 0.5 and 1.0 μg/ml, respectively.
FIG. 1.
Effects of AmB on the growth of C. neoformans 2337 grown overnight in FLC-free medium (CN) (■) or medium containing FLC at 50 μg/ml (CN-50) (□). Cells were incubated under the conditions described by NCCLS (13) without shaking. Data are the averages of three experiments, and error bars denote standard deviations. Experiments were performed with an initial inoculum that ranged from 1.0 × 105 to 5.0 × 105 CFU/ml. ∗, P < 0.05 for CN versus CN-50.
FIG. 2.
Anticryptococcal activities of AmB at concentrations of 0.5 μg/ml (squares) and 1.0 μg/ml (triangles) against replicating (A) and nonreplicating (B) cells of C. neoformans 2337 grown overnight in FLC-free medium (CN) (black symbols) or medium containing FLC at 50 μg/ml (CN-50) (white symbols). Cells were incubated under the conditions described by NCCLS (13) with gentle shaking. Each datum point represents the average of three different experiments. Experiments were performed with an initial inoculum that ranged from 1.0 × 105 to 5.0 × 105 CFU/ml.
In vivo studies.
To investigate the interactions between FLC and AmB in vivo, we established an experimental model of systemic cryptococcosis in BALB/c mice by intravenous injection of cells of C. neoformans 2337. Overall, four studies were performed.
(i) Combination therapy.
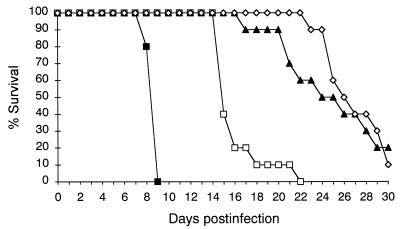
In study 1, the mice were challenged with 9.4 × 105 viable cells of C. neoformans, and 24 h after the challenge, the mice were randomized into one of the following treatment groups: (i) placebo, (ii) FLC at 10 mg/kg/day, (iii) AmB at 0.5 mg/kg/day, and (iv) FLC at 10 mg/kg/day plus AmB at 0.5 mg/kg/day. Therapy was given for 10 consecutive days, and deaths were recorded daily through day 30 postinfection (Fig. 3). All treatment regimens were effective in prolonging the survival compared with the length of survival for the controls (P < 0.0001). AmB was more effective than FLC (P < 0.0001). Combination therapy was more effective than FLC therapy (P < 0.0001), but it was not better than AmB therapy (P = 0.067). In study 2, the mice were challenged with 6.5 × 104 viable cells of C. neoformans, and 24 h after the challenge, the mice were randomized into one of the following treatment groups: (i) placebo, (ii) AmB at 0.5 mg/kg/day, (iii) FLC at 3 mg/kg/day, (iv) FLC at 10 mg/kg/day, (v) AmB at 0.5 mg/kg/day plus FLC at 3 mg/kg/day, and (vi) AmB at 0.5 mg/kg/day plus FLC at 10 mg/kg/day. Therapy was administered for 7 consecutive days, and the mice were killed on day 8 postinfection (Table 2). All treatment regimens were effective in reducing the fungal burdens compared with the burdens in the organs of the controls with the exception of AmB for the brain. The effectiveness of FLC was shown to be dose dependent, with FLC at 10 mg/kg/day being more effective than FLC at 3 mg/kg/day at reducing the fungal burdens in all organs with the exception of the liver. AmB was more effective than both FLC dosing regimens at reducing the fungal burdens in the lung and kidney, while the polyene was more effective than FLC at 3 mg/kg/day but not FLC at 10 mg/kg/day at reducing the fungal burden in the liver. Both FLC dosing regimens were more effective than AmB at reducing the fungal burdens in the brain and spleen. Both combination therapies were shown to be more effective than each single therapy at reducing the fungal burdens in the liver and spleen.
FIG. 3.
Survival of mice infected intravenously with 9.4 × 105 viable cells of C. neoformans 2337 and treated for 10 days with FLC at 10 mg/kg/day (□), AmB at 0.5 mg/kg/day (▴), and FLC at 10 mg/kg/day plus AmB at 0.5 mg/kg/day (◊). ■, control.
TABLE 2.
Effects of combination therapy on fungal burdens of mice infected with C. neoformans 2337a
| Therapy (dose [mg/kg/day]) | Fungal burden (log10 CFU/g) in the following tissues:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lung
|
Brain
|
Kidney
|
Liver
|
Spleen
|
||||||
| Mean | Range | Mean | Range | Mean | Range | Mean | Range | Mean | Range | |
| None | 11.0 | 8.8–11.5 | 7.5 | 7.4–7.6 | 7.4 | 7.1–7.7 | 8.4 | 7.8–8.8 | 7.5 | 7.2–7.6 |
| AmB | 5.4bcd | 4.4–5.9 | 7.2 | 6.7–7.6 | 4.4bcd | 3.3–4.8 | 4.7bc | 3.3–5.4 | 6.3b | 4.1–7.0 |
| FLC (3) | 7.8b | 5.5–8.5 | 6.4be | 5.8–7.0 | 6.2b | 5.0–6.6 | 5.3b | 4.7–5.5 | 5.6b, e | 5.2–6.0 |
| FLC (10) | 6.7bf | 6.4–6.9 | 5.4bef | 4.8–6.0 | 5.2bf | 4.9–5.4 | 5.3b | 4.2–6.0 | 4.7bef | 4.4–4.9 |
| AmB + FLC (3) | 5.3b | 4.8–5.6 | 6.2b | 4.0–6.6 | 3.9b | 3.1–4.5 | 3.7bg | 2.0–3.9 | 4.7bg | 3.1–5.3 |
| AmB + FLC (10) | 5.3b | 4.9–5.5 | 5.2b | 4.0–5.8 | 3.5b | 2.9–3.9 | 3.2bg | 2.0–3.7 | 3.6bg | 2.9–4.1 |
See the text for details. There were seven mice in each treatment gorup.
P < 0.05, any treatment versus control.
P < 0.05, AmB versus FLC at 3 mg/kg/day.
P < 0.05, AmB versus FLC at 10 mg/kg/day.
P < 0.05, FLC (any dose) versus AmB.
P < 0.05, FLC at 10 mg/kg/day versus FLC at 3 mg/kg/day.
P < 0.05, combination therapy versus any monotherapy.
(ii) Sequential therapy.
In study 3, the mice were challenged with 5.0 × 105 viable cells of C. neoformans, and 24 h after the challenge they were randomized into one of the following treatment groups: (i) placebo; (ii) FLC at 15 mg/kg/day on days 1, 2, and 3 postinfection and FLC at 10 mg/kg/day on days 5 and 7 postinfection; (iii) AmB at 1.5 mg/kg/day from day 8 to day 13 postinfection; and (iv) FLC at 15 mg/kg/day on days 1, 2, and 3 postinfection and FLC at 10 mg/kg/day on days 5 and 7 postinfection followed by AmB at 1.5 mg/kg/day from day 8 to day 13 postinfection. Tissue burden studies were performed on day 14 postinfection (Table 3). FLC was effective in reducing the fungal burdens in all organs compared with the burdens in the organs of the controls. Although AmB therapy was initiated on day 8 postinfection, it yielded significant reductions in the fungal burdens in all organs compared to those in the organs of the controls with the exception of those in the lung. For reduction of the fungal burden in the lung, FLC was superior to AmB. No significant differences were seen between FLC and AmB in reducing the fungal burdens in the remaining four organs. Sequential therapy yielded significant reductions in the fungal burdens in all organs compared to the burdens in the organs of the controls. Additionally, it was better than both monotherapies in reducing the fungal burdens in the lung, brain, and spleen. Sequential therapy was better than FLC therapy but not AmB therapy in reducing the fungal burden in the kidney. Conversely, it was better than AmB but not FLC in reducing the fungal burden in the liver. In study 4, the mice were given FLC at 30 mg/kg/day prior to the infection. Azole prophylaxis commenced on day −5 and lasted until day 0, with the last dose given 2 h prior to the infection. The mice were challenged with 4.5 × 105 viable cells of C. neoformans. Starting at 24 h postinfection, both FLC-pretreated mice and naive mice were given AmB at 0.5 or 1.5 mg/kg/day for 9 consecutive days, and they were killed on day 10 postinfection (Table 3). Prophylaxis with FLC was not effective in reducing the fungal burden compared with the fungal burden in the controls. AMB at 0.5 mg/kg/day was effective in reducing the fungal burdens in all organs with the exception of the brain. AmB at 1.5 mg/kg/day was effective in reducing the fungal burdens in all organs. The effectiveness of AmB was shown to be dose dependent with AmB at 1.5 mg/kg/day being more effective than AmB at 0.5 mg/kg/day in reducing the fungal burdens in all organs with the exception of the brain. In general, AmB given after FLC prophylaxis was as effective as AmB given alone, irrespective of the doses used. However, the administration of AmB at 1.5 mg/kg/day after the administration of FLC was shown to be more effective than AmB alone in reducing the fungal burden in the brain. In this study, additional mice were used for determination of serum FLC levels. Blood was sampled at 2, 24, 48, 72, 96, and 120 h after administration of the last dose of FLC given as prophylaxis. Concentrations in serum were 33.4 and 1.1 μg/ml 2 and 24 h, respectively, after administration of the last dose of the azole, while they were undetectable by day 2.
TABLE 3.
Effects of sequential therapy on fungal burdens of mice infected with C. neoformans 2337a
| Study no. | Therapy (dose [mg/kg/day]) | Log10 CFU/g in the following tissues:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lung
|
Brain
|
Kidney
|
Liver
|
Spleen
|
|||||||
| Mean | Range | Mean | Range | Mean | Range | Mean | Range | Mean | Range | ||
| 3 | None | 10.2 | 8.7–10.5 | 7.5 | 6.9–7.9 | 7.3 | 6.1–7.6 | 7.4 | 6.8–7.6 | 7.3 | 7.0–7.5 |
| FLC | 8.2bc | 7.1–8.7 | 6.6b | 5.9–6.8 | 5.9b | 5.2–6.2 | 6.2b | 5.3–6.7 | 6.0b | 5.3–6.4 | |
| AmB | 10.6 | 7.8–11.2 | 6.6b | 5.7–7.1 | 5.4b | 4.5–5.7 | 6.3b | 5.7–6.7 | 6.5b | 4.8–6.9 | |
| FLC-AmB | 6.7bde | 5.7–5.9 | 5.2bde | 3.7–5.7 | 4.7bd | 3.2–5.3 | 5.3be | 3.2–5.7 | 4.2bde | 4.0–4.5 | |
| 4 | None | 8.0 | 7.7–8.3 | 7.4 | 6.7–7.8 | 6.8 | 5.7–7.4 | 6.9 | 6.1–7.4 | 7.0 | 6.8–7.3 |
| FLC | 7.7 | 7.0–8.0 | 7.3 | 6.6–7.7 | 6.6 | 5.8–7.1 | 6.8 | 5.9–7.2 | 6.9 | 5.7–7.2 | |
| AmB(0.5) | 4.8f | 4.3–5.1 | 6.9 | 5.6–7.5 | 3.8f | 3.3–4.3 | 3.6f | 2.9–4.0 | 4.3f | 3.2–4.9 | |
| AmB (1.5) | 3.0fg | 2.7–3.3 | 5.9f | 5.0–6.4 | 2.7fg | 2.4–2.9 | 2.7fg | 0.3–3.4 | 3.3fg | 3.0–3.5 | |
| FLC-AmB (0.5) | 5.0f | 4.1–5.3 | 7.1 | 3.6–7.4 | 4.0f | 3.2–4.5 | 4.0f | 2.6–4.5 | 4.6f | 4.1–5.0 | |
| FLC-AmB (1.5) | 3.3f | 1.4–3.8 | 2.9fh | 2.8–3.0 | 2.9f | 2.6–3.1 | 2.5f | 0.6–3.0 | 3.2f | 3.0–3.4 | |
See the text for details. There were seven mice in each treatment group.
P < 0.05, any treatment versus control.
P < 0.05, FLC versus AmB.
P < 0.05, sequential therapy versus FLC.
P < 0.05, sequential therapy versus AmB.
P < 0.05, any treatment versus control.
P < 0.05, AmB at 1.5 mg/kg/day versus AmB at 0.5 mg/kg/day.
P < 0.05, sequential therapy versus AmB.
DISCUSSION
To date, with the exception of AmB and flucytosine used in combination for the treatment of several systemic mycoses, few data are available on the interaction between antifungal compounds. Use of a combination of a polyene and an azole has always been questioned because of the potential for antagonism. However, recent experimental data have demonstrated that the effects of an azole antifungal agent on the efficacy of AmB are either drug or fungus specific (11, 16–21, 23–26). Little is known about the interaction between azoles and AmB against C. neoformans (1, 11, 16, 17).
Although standard therapy for cryptococcosis remains AmB with or without flucytosine, FLC is sometimes used in combination with AmB, mainly for seriously ill patients. The effects of this combination therapy were first investigated in vitro against a large number of clinical isolates of C. neoformans. The procedure used in the present study is a checkerboard titration broth microdilution-based method that adheres to the recommendations of NCCLS (13). We found that the combination resulted in a synergistic interaction against only one isolate (7%), while FLC combined with AmB yielded additive (67%) or indifferent (26%) interactions against the majority of the isolates. Although synergy against our series of isolates was a rare event, the geometric mean MICs of both drugs dropped dramatically when they were used in combination: the geometric mean MIC of AmB dropped from 0.73 to 0.07 μg/ml, and the geometric mean MIC of FLC dropped from 4.1 to 1.8 μg/ml. The effects of this combination therapy in vitro were extended by including two other triazoles: ITC and the new investigational triazole SCH. We found that the combination of ITC and AmB resulted in a synergistic interaction against one isolate (7%). On the other hand, SCH combined with the polyene yielded a synergistic interaction against five isolates (33%). It must be noted, however, that the definition of synergy is dependent upon the methodology used and to some degree is arbitrary. Although in recent reports (15) the synergy between antibacterial or antifungal drugs has been defined as an FIC index of <1.0, in this study we selected a more stringent criterion for the definition of synergy. The findings that antagonism was not observed are encouraging. One of the main reasons for the use of combination antifungal therapy is that nontoxic amounts of two antifungal agents can be used when toxic doses of a single drug would be required. Our in vitro data suggest that these combination therapies would allow the use of lower doses of AmB without the loss of a clinical response.
The in vitro results were confirmed by studying the effects of concomitant FLC-AmB therapy in a model of systemic murine cryptococcosis. Survival studies showed that combination therapy was more effective than FLC alone and was at least as effective as AmB given as a single drug. Tissue burden results mirrored the results of the survival study. In addition, they showed that the degree of beneficial effects depends on the organ considered. Actually, we found an additive effect against the fungal burdens in the brain, lung, and kidney, while the combination yielded a synergistic effect against the fungal burdens in the liver and spleen. Our data agree with those previously reported by Perfect and Durak (16). Those investigators used a rabbit model of experimental cryptococcal meningitis and found that AmB and ketoconazole had an additive effect. Similarly, Albert et al. (1) showed a lack of antagonism between the triazole SCH 39304 and AmB in a model of murine cryptococcal meningitis. Thus far, FLC-AmB combination therapy has mainly been investigated with experimental models of murine candidiasis. Sugar and colleagues (23, 25) showed that this combination was at least as effective as AmB alone in both immunocompetent and immunosuppressed mice. Similarly, Sanati et al. (19) showed that AmB monotherapy or combination therapy significantly decreased the fungal densities in a rabbit model of endocarditis due to Candida albicans.
There are clinical circumstances, such as the worsening of a cryptococcal infection occurring during FLC suppression therapy, in which therapy is switched from an azole to AmB. Therefore, we performed additional experiments to see whether the anticryptococcal activity of the polyene maintains its initial efficacy after FLC exposure. Similar to the findings of Vazquez et al. (27, 28) for several species of Candida, we showed that the anticryptococcal activity of AmB was clearly reduced in vitro after the cells were exposed to FLC at 50 μg/ml. Since the in vitro model or models that most closely mimic clinical infections are not known, we performed killing experiments by using both replicating and nonreplicating cells. Although both systems confirmed that AmB had reduced anticryptococcal activity against cells preexposed to the triazole, this phenomenon was particularly evident for nonreplicating cells. It seems likely that a process of adaptation to FLC may occur and that this process can protect the cells during exposure to AmB. Although the mechanism by which preexposure to FLC reduces the activity of AmB was not investigated, one can speculate that the membrane ergosterol is replaced by a methylated sterol derivative that does not interact with AmB (6, 10). However, results of our in vivo studies of sequential therapy did not correlate with data from in vitro studies. AmB given after FLC therapy (or FLC prophylaxis) was shown to be at least as effective as AmB alone. In addition, these studies showed that a synergistic interaction upon sequential therapy was not a rare event. In particular, in study 3 sequential therapy was superior to both monotherapies in reducing the fungal burdens in the lung, brain, and spleen, while in study 4 AmB administered after prophylaxis with FLC was shown to be more effective than AmB alone in reducing the fungal burden in the brain. In the latter study we found serum FLC levels of 33.3 and 1.1 μg/ml 2 and 24 h after administration of the last dose of FLC, respectively. It has been reported that 70 to 80% of the FLC in serum penetrates the cerebrospinal fluid (5). It seems likely that a high, even transient concentration of FLC in cerebral tissue produced a large decrease in the fungal burden and that the remaining fungi were more easily eliminated by the high AmB dose used in this experiment. The reason why a similar effect was not observed for the remaining four organs is difficult to explain. It can be hypothesized that the low protein concentration in the cerebrospinal fluid makes the bioavailabilities and, consequently, the effectiveness of both drugs greater than those in other body compartments. Differences in experimental approaches to sequential therapy can account for the contradictory data observed between the in vitro and in vivo results. While in vitro the cells were exposed to high dose of FLC (50 μg/ml), the same concentration was not tested in vivo.
It must be noted that our in vivo experiments were performed with one clinical isolate of C. neoformans. Due to the considerable degree of variation among isolates of C. neoformans with respect to genetic background, the effects of such combination therapies in animal models should be further explored with multiple strains. In addition, the potential effect of the immune system on the interaction of AmB and FLC (or other triazoles) must be considered. AmB is an important immunomodulator that may mediate some of the immune system effects by increasing macrophage function (22). Whether the effects of AmB and FLC combined differ depending on the status of the host immune system merits further investigation.
In conclusion, the results of the present study demonstrated that the combination of triazoles and AmB is significantly more active than either drug alone against C. neoformans in vitro. Our in vivo data confirmed that combination therapy with FLC and AmB is not antagonistic and is at least additive. This finding suggests that this combination therapy should be widely explored in clinical studies. Although under our in vitro experimental conditions cells preexposed to FLC were found to be less susceptible to AmB, the same phenomenon was not observed in vivo. Rather, when the two drugs were used sequentially for the treatment of murine systemic cryptococcosis, a reciprocal potentiation was often observed.
ACKNOWLEDGMENTS
This work was supported in part by grants from Istituto Superiore di Sanità, Rome (II AIDS project, no. 50B.36) and from MURST, Rome Italy.
REFERENCES
- 1.Albert M M, Graybill J R, Rinaldi M G. Treatment of murine cryptococcal meningitis with an SCH 39304-amphotericin B combination. Antimicrob Agents Chemother. 1991;35:1721–1725. doi: 10.1128/aac.35.9.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barchiesi F, Arzeni D, Fothergill A W, Falconi Di Francesco L, Rinaldi M G, Scalise G. In vitro activities of the new antifungal triazole SCH 56592 against common and emerging yeast pathogens. Antimicrob Agents Chemother. 2000;44:226–229. doi: 10.1128/aac.44.1.226-229.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barchiesi F, Gallo D, Caselli F, Falconi Di Francesco L, Arzeni D, Giacometti A, Scalise G. In-vitro interactions of itraconazole with flucytosine against clinical isolates of Cryptococcus neoformans. J Antimicrob Chemother. 1999;44:65–70. doi: 10.1093/jac/44.1.65. [DOI] [PubMed] [Google Scholar]
- 4.Bennet J E, Dismukes W E, Duma R J, Medoff G, Sande M A, Gallis H, et al. A comparison of amphotericin B alone and combined with flucytosine in the treatment of criptococcal meningitis. N Engl J Med. 1979;301:126–131. doi: 10.1056/NEJM197907193010303. [DOI] [PubMed] [Google Scholar]
- 5.Como J A, Dismukes W E. Oral azole drugs as systemic antifungal therapy. N Engl J Med. 1994;330:263–272. doi: 10.1056/NEJM199401273300407. [DOI] [PubMed] [Google Scholar]
- 6.Currie B, Sanati H, Ibrahim A S, Edwards J E, Jr, Casedevall A, Ghannoum A. Sterol composition and susceptibilities to amphotericin B of environmental Cryptococcus neoformas isolates are changed by murine passage. Antimicrob Agents Chemother. 1995;39:1934–1937. doi: 10.1128/aac.39.9.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eliopoulos G M, Moellering R C. Antimicrobial combinations. In: Lorian V, editor. Antibiotics in laboratory medicine. 3rd ed. Baltimore, Md: The Williams & Wilkins Co.; 1991. pp. 432–492. [Google Scholar]
- 8.Galgiani J N, Lewis M L. In vitro studies of activities of the antifungal triazole SCH 56592 and itraconazole against Candida albicans, Cryptococcus neoformans, and other pathogenic yeasts. Antimicrob Agents Chemother. 1997;42:2467–2473. doi: 10.1128/aac.41.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.George D, Kordick D, Miniter P, Patterson T F, Andreole V T. Combination therapy in experimental invasive aspergillosis. J Infect Dis. 1993;168:692–698. doi: 10.1093/infdis/168.3.692. [DOI] [PubMed] [Google Scholar]
- 10.Ghannoum M A, Spelberg B J, Ibrahim A S, Ritchie J A, Currie B, Spitzer E D, Edwards J E, Jr, Casadevall A. Sterol composition of Cryptococcus neoformans in the presence and absence of fluconazole. Antimicrob Agents Chemother. 1994;38:2029–2033. doi: 10.1128/aac.38.9.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horne T J, Hollomon D, Loeffler R S T, Kelly S L. Cross-resistance to polyene and azole drugs in Cryptococcus neoformans. Antimicrob Agents Chemother. 1995;39:1526–1529. doi: 10.1128/aac.39.7.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchell T G, Perfect J R. Cryptococcosis in the era of AIDS—100 years after the discovery of Cryptococcus neoformans. Clin Microbiol Rev. 1995;8:515–548. doi: 10.1128/cmr.8.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Clinical Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 14.Ng T K, Chan R C, Adeyemy-Doro F A, Cheung S W, Cheng A F. Rapid high performance liquid chromatographic assay for antifungal agents in human sera. J Antimicrob Chemother. 1996;37(Suppl. 3):465–472. doi: 10.1093/jac/37.3.465. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen M H, Najvar L K, Yu Y C, Graybill J R. Combination therapy with fluconazole and flucytosine in murine model of cryptococcal meningitis. Antimicrob Agents Chemother. 1997;41:1120–1123. doi: 10.1128/aac.41.5.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perfect J R, Durak D T. Treatment of experimental cryptococcal meningitis with amphotericin B, 5-fluorocytosine and ketoconazole. J Infect Dis. 1982;146:429–435. doi: 10.1093/infdis/146.3.429. [DOI] [PubMed] [Google Scholar]
- 17.Polak A. Combination therapy of experimental candidiasis, cryptococcosis, aspergillosis and wangiellosis in mice. Chemotherapy (Basel) 1987;33:381–395. doi: 10.1159/000238524. [DOI] [PubMed] [Google Scholar]
- 18.Pore R S. Amphotericin B synergy testing by the FCST. Curr Microbiol. 1992;24:171–177. [Google Scholar]
- 19.Sanati H, Ramos C, Bayer A, Ghannoum M. Combination therapy with amphotericin B and fluconazole against invasive candidiasis in neutropenic-mouse and infective-endocarditis rabbit models. Antimicrob Agents Chemother. 1997;41:1345–1348. doi: 10.1128/aac.41.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schaffner A, Frick P G. The effect of ketoconazole on amphotericin B in a model of disseminated aspergillosis. J Infect Dis. 1985;151:902–909. doi: 10.1093/infdis/151.5.902. [DOI] [PubMed] [Google Scholar]
- 21.Scheven M, Schwegler F. Antagonistic interactions between azoles and amphotericin B with yeasts depend on azole lipophilia for special test conditions in vitro. Antimicrob Agents Chemother. 1995;38:371–373. doi: 10.1128/aac.39.8.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stevens D A. Combination immunotherapy and antifungal chemotherapy. Clin Infect Dis. 1998;26:1266–1269. doi: 10.1086/516362. [DOI] [PubMed] [Google Scholar]
- 23.Sugar A M. Interactions of amphotericin B and SCH 39304 in the treatment of experimental murine candidiasis: lack of antagonism of a polyene azole combination. Antimicrob Agents Chemother. 1991;35:1669–1671. doi: 10.1128/aac.35.8.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sugar A M. Use of amphotericin B with azole antifungal drugs: what are we doing? Antimicrob Agents Chemother. 1995;39:1907–1912. doi: 10.1128/aac.39.9.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sugar A M, Hitchcock C A, Troke P F, Picard M. Combination therapy of murine invasive candidiasis with fluconazole and anphotericin B. Antimicrob Agents Chemother. 1995;39:598–601. doi: 10.1128/AAC.39.3.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sugar A M, Liu X P. Interactions of itraconazole with amphotericin B in the treatment of murine invasive candidiasis. J Infect Dis. 1998;177:1660–1663. doi: 10.1086/515319. [DOI] [PubMed] [Google Scholar]
- 27.Vazquez J, Arganoza M, Vaishampayan J, Akins R. In vitro interaction between amphotericin B and azoles in Candida albicans. Antimicrob Agents Chemother. 1996;40:2511–2516. doi: 10.1128/aac.40.11.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vazquez J A, Arganoza M T, Boikov D B, Yoon S, Sobel J D, Akins R A. Stable phenotypic resistance of Candida species to amphotericin B conferred by preexposure to subinhibitory levels of azoles. J Clin Microbiol. 1998;36:2690–2694. doi: 10.1128/jcm.36.9.2690-2695.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wallace J E, Harris S C, Gallegos J, Foulds G, Chen T J, Rinaldi M G. Assay of fluconazole by high-performance liquid chromatography with a mixed-phase column. Antimicrob Agents Chemother. 1992;36:603–606. doi: 10.1128/aac.36.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zuger A, Louie E, Holzman R S, Simberkoff M S, Rahal J J. Cryptococcal disease in patients with the acquired immunodeficiency syndrome: diagnostic features and outcome of treatment. Ann Intern Med. 1986;104:234–240. doi: 10.7326/0003-4819-104-2-234. [DOI] [PubMed] [Google Scholar]