Abstract
Hemocompatibility of cardiovascular implants represents a major clinical challenge and, to date, optimal antithrombotic properties are lacking. Next-generation tissue-engineered heart valves (TEHVs) made from human-cell-derived tissue-engineered extracellular matrices (hTEMs) demonstrated their recellularization capacity in vivo and may represent promising candidates to avoid antithrombotic therapy. To further enhance their hemocompatibility, we tested hTEMs pre-endothelialization potential using human-blood-derived endothelial-colony-forming cells (ECFCs) and umbilical vein cells (control), cultured under static and dynamic orbital conditions, with either FBS or hPL. ECFCs performance was assessed via scratch assay, thereby recapitulating the surface damages occurring in transcatheter valves during crimping procedures. Our study demonstrated: feasibility to form a confluent and functional endothelium on hTEMs with expression of endothelium-specific markers; ECFCs migration and confluency restoration after crimping tests; hPL-induced formation of neo-microvessel-like structures; feasibility to pre-endothelialize hTEMs-based TEHVs and ECFCs retention on their surface after crimping. Our findings may stimulate new avenues towards next-generation pre-endothelialized implants with enhanced hemocompatibility, being beneficial for selected high-risk patients.
Keywords: human cell-derived tissue-engineered matrices, endothelial colony forming cell, anti-coagulation, hemocomaptibility, scratch assay, HPL, transcatheter tissue-engineered valve, endothelial progenitor cell
Introduction
Thromboembolic events associated with currently available cardiovascular implants still remain a major problem in daily clinical routine. Thus, long-term anticoagulation or anti-platelet therapy is often required, which however permanently increases the risk for bleeding and decreases the overall patient’s quality of life. Therefore, a good hemocompatibility profile represents a key requirement in the development of novel cardiovascular implants (van Oeveren, 2013; Carnicelli et al., 2016).
Next-generation tissue-engineered (TE) replacements with regenerative capacities (Dahl et al., 2011; Weber et al., 2013; Driessen-Mol et al., 2014; Syedain et al., 2014; Syedain et al., 2015; Reimer et al., 2017; Syedain et al., 2017; Emmert et al., 2018; Lintas et al., 2018; Motta et al., 2018; Biermann et al., 2019; Boethig et al., 2019; Kirkton et al., 2019; Motta et al., 2019; Gerdisch et al., 2020; Fioretta et al., 2021; Syedain et al., 2021) have demonstrated their strong potential in numerous preclinical studies and first clinical pilot trials (Fioretta et al., 2021), and may therefore represent an ideal candidate to overcome the limitations of current prostheses. As one promising TE approach, we have recently introduced a biomimetic acellular tissue-engineered matrix (TEM), that is, manufactured from a polymer composite and an in vitro grown extracellular matrix (ECM), which can be engineered from different (human) cell sources (Emmert et al., 2018; Fioretta et al., 2021).
Importantly, prior to implantation, the TEM is decellularized leaving a cell-free construct which is thereby conceptually applicable to every patient (Dijkman et al., 2012). Preclinical evaluations of such decellularized TEMs in the context of heart valves and blood vessels have demonstrated their strong remodeling, and recellularization capacity, including continuous endothelialization over time (Weber et al., 2013; Driessen-Mol et al., 2014; Emmert et al., 2018; Lintas et al., 2018; Motta et al., 2018; Motta et al., 2019; Motta et al., 2020; Fioretta et al., 2021). As part of the remodeling process, the blood coagulation cascade is initiated immediately upon implantation of the TEM (Lynn et al., 2004; van Loon et al., 2013), where host cells are able to rapidly infiltrate to finally allow for the gradual formation of a functional endothelium, thereby ensuring a sufficient and safe performance of the TEM. However, the exact mechanisms of the endothelialization process (e.g., blood-borne and/or migration from the adjacent tissue) in such TEMs remain to be elucidated, especially as their endothelialization capacity may also be influenced by the patient’s individual regenerative potential. This aspect may be particularly relevant when using TEMs in the high-pressure circulation of the left heart (e.g., transcatheter aortic valve implantation, TAVI) which is known to carry a high risk for thromboembolic events.
Hence, from a translational perspective, the concept of TEM in vitro pre-endothelialization prior to implantation may be warranted for certain indications or specific patient populations with a high risk for thromboembolic events and may represent a good strategy to further enhance their overall hemocompatibility. Therefore, we here tested the hypothesis whether in vitro pre-endothelialization of human cell-based TEMs (hTEMs) using an endothelial progenitor cell (EPC)-based approach is feasible. We particularly focused on hTEM in vitro pre-endothelialization in the setting of transcatheter heart valves as these implants are prone to microstructural damage due to the required crimping maneuvers during delivery (Kiefer et al., 2011; Amahzoune et al., 2013; Alavi et al., 2014). Our group has previously developed several (h)TEM-based transcatheter valves (Weber et al., 2011; Weber et al., 2013; Driessen-Mol et al., 2014; Emmert et al., 2018; Lintas et al., 2018; Motta et al., 2018; Motta et al., 2019; Fioretta et al., 2021), and therefore we investigated if the integrity of an in vitro pre-endothelialized hTEM can also be preserved after such crimping procedures.
To test these hypotheses, we first evaluated the general in vitro pre-endothelialization capacity of EPC-seeded hTEMs in a high-throughput patch culture experiment. In a next step, we assessed the regenerative capacity of human blood-derived EPCs or human-derived umbilical vein endothelial cells (HUVECs) seeded hTEMs in a scratch assay experiment (Liang et al., 2007) under static and dynamic orbital conditions, which allowed us to mimic potential endothelial damages that may occur due to crimping of transcatheter valves. Thereafter, we generated hTEM-based transcatheter tissue engineered sinus valves (TESVs) which were then in vitro pre-endothelialized before they underwent crimping procedures to assess whether the endothelium remains stable. Finally, to enhance their translational relevance, the hTEM pre-endothelialization capacity was also assessed under xenogeneic-free culture conditions (i.e., supplemented with human platelet lysate (hPL)).
Results
Macroscopic Evaluation of Human Cell-Derived Tissue-Engineered Extracellular Matrices
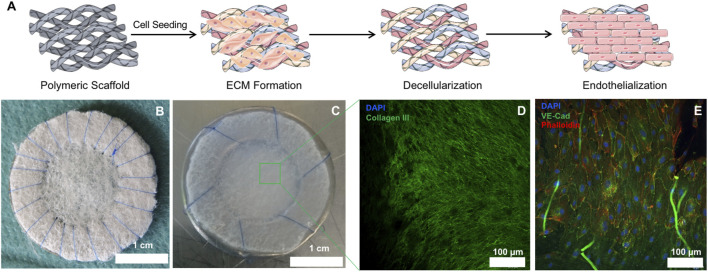
Upon in vitro culture and decellularization of PGA/P4HB patches (Figures 1A,B), the resulting hTEMs displayed shiny and homogeneous ECM formation (Figure 1C). Newly formed tissue was evident in all hTEMs and confirmed by the expression of abundant Collagen III (Figure 1D). Successful decellularization procedure was confirmed by the absence of cell nuclei indicating a cell-free matrix (Figure 1D).
FIGURE 1.
Macroscopic and microscopic appearance of hTEMs before and after in vitro culture. (A): Schematic representation of the hTEM production procedure (Images adapted from Servier Medical Art under a creative common attribution 3.0 unported license). Briefly, PGA/P4HB scaffolds were sutured on stainless-steel metal ring and seeded with hDFs. After 4 weeks culture, patches were decellularized and finally endothelialized in vitro. (B): Macroscopic image of the PGA/P4HB scaffold sutured onto a stainless-steel metal ring before cell seeding. (C): Macroscopic appearance of the hTEM resulting from 4 weeks of tissue culture and subsequent decellularization. The hTEM is characterized by a shiny and smooth surface, indicating the presence of ECM. (D): Confocal microscope image of the hTEM stained for collagen III (green) and DAPI (blue) reveals the presence of a dense and homogeneous collagenous matrix with complete absence of cell nuclei. (E): Confocal microscope image showing homogeneous endothelialization on the hTEM surface.
Human Cell-Derived Tissue-Engineered Extracellular Matrix Pre-endothelialization Potential
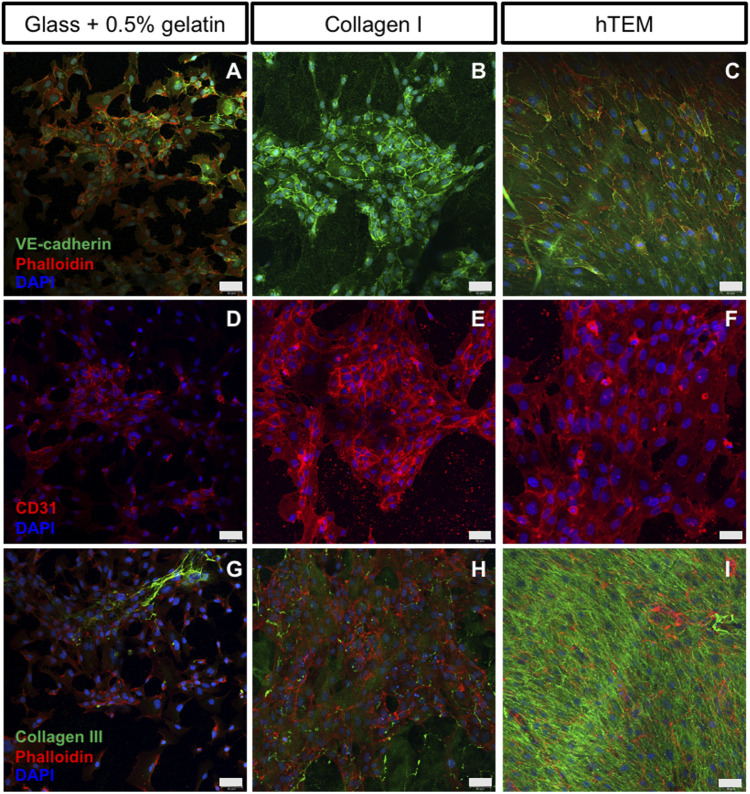
hTEM pre-endothelialization potential of ECFCs and HUVECs (Figure 1E) was compared to two control substrates (0.5% gelatin-coated glass, and Collagen I gel) and assessed by immunofluorescence (Figure 2). ECFCs were characterized by flow cytometry (Supplementary Figure S1). After 5 days of ECFC culture, gelatin-coated glass (Figures 2A,D,G) and Collagen I substrates (Figures 2B,E,H) showed incomplete and inhomogeneous (spot-dependent) endothelialization of the surfaces, with poor ECFC confluency, limited cell-cell contacts, and therefore limited expression of CD31 and VE-cad. To the contrary, a confluent endothelium was observed on the hTEMs (Figures 2C,F,I), with ECFCs displaying the typical cobblestone morphology and expressing the characteristic endothelial cell-cell contact proteins VE-Cad (Figure 2C) and CD31 (Figure 2F). Similar results were observed for hTEMs seeded with control HUVECs (Supplementary Figure S2A).
FIGURE 2.
Confocal images of ECFCs cultured on gelatin-coated glass, collagen I gels, and hTEMs at 5 days. (A–C): ECFCs stained for VE-cad (green), phalloidin (red), and DAPI (blue). (D–F): ECFCs stained for CD31 (red) and DAPI (blue). (G–I): Staining for Collagen III (green), phalloidin (red), and DAPI (blue). (A,D,G): glass covered with 0.5% gelatin show limited pre-endothelialization potential with poor ECFCs confluency after 5 days of culture, limited cell-cell contact, and low expression of CD31 and VE-cad. (B,E,H): Collagen I gel show spot-dependent pre-endothelialization. ECFCs are organized in colonies with presence of cell-cell contacts and expression of CD31 and VE-cad. (C,F,I): ECFCs seeded onto hTEM substrates demonstrate good pre-endothelialization potential, by achieving complete confluency after 5 days of culture (50 µm scale bars).
Characterization of Endothelial-Colony-Forming Cells Markers Expressed on Human Cell-Derived Tissue-Engineered Extracellular Matrices
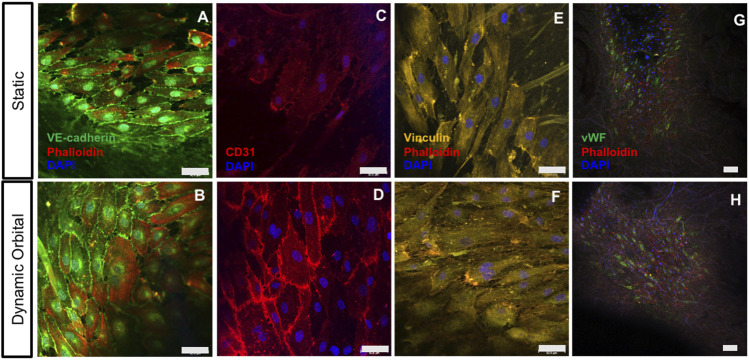
After 14 days of static or dynamic orbital in vitro culture, ECFCs (n = 2 donors) seeded on the hTEMs were tested for specific Endothelial cells (ECs) marker expression. ECFCs formed a stable and confluent endothelium retaining the expression of characteristic ECs markers such as VE-cad (Figures 3A,B), CD31 (Figures 3C,D), and vWF (Figures 3G,H). Cell adhesion to the substrate was confirmed by the abundant presence of focal adhesion points as stained by Vinculin (Figures 3E,F) in both static and dynamic culture conditions.
FIGURE 3.
ECFCs cultured on hTEMs express characteristic EC markers after 14 days. (A–B): ECFCs stained for VE-CAD (green), Phalloidin (red), and DAPI (blue) show confluent endothelialization and expression of cell-cell surface markers under both static and dynamic orbital conditions. (C–D): ECFCs stained for CD31 (red) and DAPI (blue) show the expression of ECFCs intercellular junctions under static and dynamic flow conditions. (E–I): Staining for Vinculin (yellow), Phalloidin (red) and DAPI (blue) demonstrating the expression of focal adhesions by ECFCs. (G–H): Staining for vWF (green), Phalloidin (red), and DAPI (blue) demonstrate endothelium functionality (50 µm scale bars).
Scratch Assay and Experimental Crimping Tests
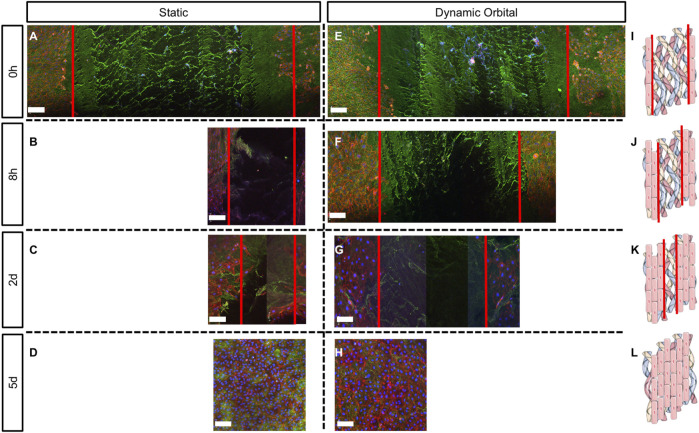
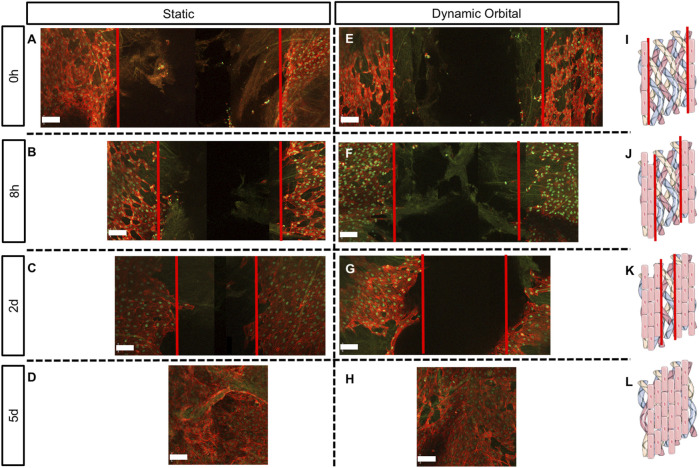
Scratch assay was used to assess the ability of ECFCs to migrate and restore a confluent endothelium (Figures 4, 5) once seeded onto hTEMs using HUVECs as a control (Supplementary Figure S2B). After performing a damage of about 2 mm in width in the middle of the patches (Figures 4A,E, 5A,E, and Supplementary Figure S2B, red lines), results showed that ECFCs, as well as HUVECs, were able to regenerate the endothelium by restoring its integrity within 5 days of culture with FBS under both static and dynamic orbital conditions (Figure 4). By using hPL as medium supplement, comparable results were observed, demonstrating good migration potential within 5 days after scratch for both static and dynamic orbital conditions (Figure 5). Additionally, ECFCs cultured on hTEMs with hPL as medium supplement were able to spontaneously form microvessel-like structures (Supplementary Figure S3, dotted circles). Importantly, and independently from the culture conditions used, ECFCs were able to restore a homogeneous collagenous matrix composed of Collagen III (Figures 4D–H, 5D–H) and Collagen IV (Supplementary Figure S4).
FIGURE 4.
Scratch assay performed on hTEMs seeded with ECFCs and cultured with FBS as medium supplement. Scratch was performed in the middle of the hTEM (red lines). ECFCs were cultured for up to 5 days under static (A–D) or dynamic orbital conditions (E–H), and finally stained for Collagen III (green), phalloidin (red) and DAPI (blue) at four different time points (0 h, 8 h, 2 days, and 5 days). (A,E): Control scratch performed at time 0, under static and dynamic orbital conditions, respectively. (B,F): ECFCs migration at 8 h timepoint under static and dynamic orbital conditions, respectively. (C,G): ECFCs migration at 2 days timepoint under static and dynamic orbital conditions, respectively. (D,H): ECFCs reached confluency after 5 days from scratch. (I–L): Schematic representation of the scratch assay test on hTEMs (Images adapted from Servier Medical Art under a creative common attribution 3.0 unported license) (500 µm scale bars).
FIGURE 5.
Scratch assay performed on hTEMs seeded with ECFCs and cultured with hPL as medium supplement. Scratch was performed in the middle of the hTEM (red lines). ECFCs were cultured for up to 5 days under static (A–D) or dynamic orbital conditions (E–H) and finally stained for Collagen III (green), phalloidin (red) and DAPI (blue). (A,E): Control scratch performed at time 0, under static and dynamic orbital conditions, respectively. (B,F): ECFCs migration at 8 h timepoint under static and dynamic orbital conditions, respectively. (C,G): ECFCs migration at 2 days timepoint under static and dynamic orbital conditions, respectively. (D,H): ECFCs reached confluency after 5 days from scratch. (I–L): Schematic representation of the scratch assay test on hTEMs (Images adapted from Servier Medical Art under a creative common attribution 3.0 unported license) (500 µm scale bars).
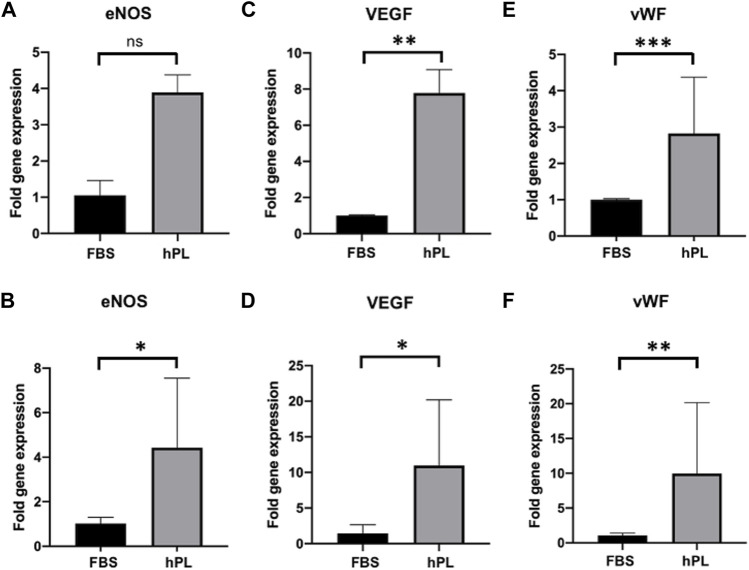
Gene Expression Profile of Endothelial-Colony-Forming Cells on Human Cell-Derived Tissue-Engineered Extracellular Matrices
The gene expression profile of hTEMs seeded with ECFCs and HUVECs under static and dynamic orbital conditions was evaluated via rtPCR after 5 days of culture (Figure 6 and Supplementary Figure S5). The reported graphs show differences between the FBS and hPL cultured hTEMs, with a significantly higher expression of ECFCs marker genes such as nitric oxide synthase (eNOS, Figures 6A,B), vascular endothelial growth factor (VEGF, Figures 6C,D) and von Willebrand Factor (vWF, Figures 6E,F) in the hPL cultured group compared to the FBS, under both static and dynamic orbital conditions. For HUVECs gene expression profile more variability was observed depending on the culture conditions used (Supplementary Figure S5). In fact, if cultured under static conditions HUVECs showed comparable expression of eNOS and vWF when supplemented with either FBS or hPL. Remarkably and similarly to ECFCs, expression profiles of the genes eNOS, VEGF, and vWF were upregulated when HUVECs were supplemented with hPL under dynamic orbital conditions (Supplementary Figures S5B,D,F).
FIGURE 6.
Gene expression profile of hTEMs seeded with ECFCs under static and dynamic orbital conditions using FBS or hPL as medium supplement after 5 days of culture. The gene expression is displayed in dCt and normalized on the average expression level of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). (A,B): eNOS expression under static (A) and dynamic orbital (B) conditions. (C,D): VEGF expression under static (C) and dynamic orbital (D) conditions. (E,F): vWF expression under static (E) and dynamic orbital (F) conditions. The cutoff for statistical significance was considered to be p < 0.05 (*p < 0.05; **p < 0.01; ***p < 0.001). ns = non-significant.
Pre-Endothelialization and Crimping of an Human Cell-Derived Tissue-Engineered Extracellular Matrix-Based Tissue Engineered Sinus Valve
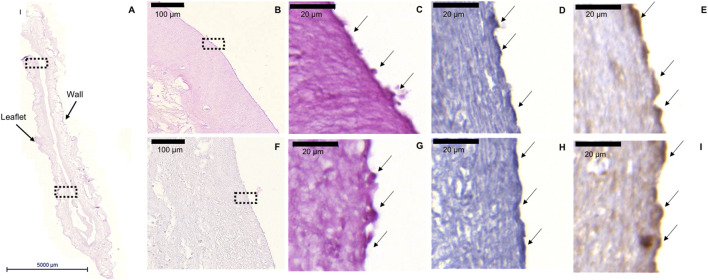
In vitro pre-endothelialization of hTEM-based TESVs (n = 4) was confirmed via histological analysis (Figures 7A–I). After a crimping procedure of 20 min (Supplementary Figures S6A–F), TESVs morphology was preserved, pre-seeded ECFCs were detected at the valve surface and their presence (H&E and CD31) as well as functionality (vWF) was confirmed by (immuno) histological stainings of the upper (Figures 7B–E) and lower (Figures 7F–I) TESVs leaflet. Control TESVs seeded with HUVECs also showed the presence of ECs (Supplementary Figure S2C), whereas non-seeded TESV displayed an acellular leaflet surface (Supplementary Figures S8A–E).
FIGURE 7.
ECFCs retention after TESV crimping procedure. Representative images of the H&E, CD31, and vWF stainings performed on the cross section of the valve. (A): H&E staining shows general morphology of TESV (5000 µm scale bar). (B,F): ×20 magnification pictures of the H&E staining of the upper (B) and lower (F) leaflet of the valve showing the presence of ECFCs on the surface (100 µm scale bars). (C,G): ×100 magnification pictures of H&E staining of the upper (C) and lower (G) leaflet marked by the dotted rectangles in panels (B,F) respectively (20 µm scale bars). (D,H): ×100 magnification pictures of CD31 staining shows ECFCs presence in the upper (D) and lower (H) leaflet (20 µm scale bars). (E,I): ×100 magnification pictures of vWF staining expressed by ECFCs in the upper (E) and lower (I) leaflet (20 µm scale bars).
Discussion
The hemocompatibility profile of current cardiovascular implants remains a major clinical challenge. To this end, ongoing research studies (e.g., GALILEO and ATLANTIS trials) are evaluating the impact of thrombosis on valve hemodynamics, valve durability, and its progression (Chakravarty et al., 2020). To prevent thromboembolic events related to the implantation of such substitutes, the routine use of anticoagulation or anti-platelet therapies is still required in many patients, while on the other hand such therapies come at a price of a permanently increased risk of bleeding complications (Leiria et al., 2011). In addition, especially in newborns and children, the use of such therapies is limited due to the risk of severe adverse events (e.g., heparin-induced thrombocytopenia) (Dabbous et al., 2014; Newall et al., 2018). Hence, when considering the significant patient population born with congenital cardiovascular malformations requiring treatment with currently-available cardiovascular implants (DiBardino and Jacobs, 2014; Jacobs et al., 2014), this still represents a significant and unresolved clinical problem around the globe. In addition, due to the embryonic toxicity of anticoagulation therapy (e.g., warfarin), also young pregnant women are not eligible for such therapies; and thus cannot be treated with a mechanical heart valve prosthesis (Alshawabkeh et al., 2016; Ayad et al., 2016).
hTEMs may represent a promising option to overcome the limitations of current cardiovascular prostheses. In fact, in numerous animal studies we have shown that (h)TEMs are mechanically mature enough to be safely applied in the cardiovascular system but, on the other hand, are still premature enough to allow for significant recellularization allowing the host cells to invade into their specific niche thereby continuously remodeling the hTEM into native-like tissue. However, despite their strong in vivo remodeling potential, the hemocompatibility profile of hTEMs could be further enhanced by using autologous EPC-based in vitro pre-endothelialization approaches, which may be particularly beneficial in vulnerable patient cohorts (e.g., patients with diabetes, children, athletes, pregnant women Weber et al., 2012).
A particular focus in our study was to test the feasibility of pre-endothelialize acellular hTEMs in the context of transcatheter heart valve applications as these are particularly exposed to surface damages due to the required crimping maneuver during valve delivery. Our study demonstrates that hTEMs may provide an ideal microstructural basis for the effective and stable in vitro formation, retention, and potential repair (in case of damage) of a confluent and functional human EPC-derived endothelium under both, static, and dynamic orbital conditions (e.g., pulmonary arterial shear forces). In our dedicated in vitro system, the application of shear forces did not have a negative impact on the protein expression in EPCs (i.e., cell-cell contact proteins, secreted factors, and matrix components). These findings suggest that hTEMs are able to sustain a functional endothelium under dynamic conditions. As suggested by previous studies (Dardik et al., 2005), laminar shear stress is believed to have an atheroprotective effect on ECs, whereas orbital (oscillatory) shear stress often correlates with areas of atherosclerosis. In vitro models of “activated” ECs are usually replicated by means of low magnitude oscillatory shear stress platforms, such as an orbital shaker, as the shear stress is not homogeneously applied throughout the culture well. We demonstrated that the hTEM functionally sustains the growth of EPCs when exposed to low shear magnitudes in an orbital shaker. Additionally, recent computational modeling analyses performed on TEMs-based TEHVs demonstrated that increasing pressure and shear stress conditions (i.e., aortic shear forces) corresponded to an improved preservation of valve functionality (Loerakker et al., 2016; Emmert et al., 2018). We could therefore hypothesize, that at higher magnitudes EPCs should positively respond and do not show major signs of activation. Our data further highlight the migration properties of EPCs on hTEMs in an in vitro simulated crimping procedure (i.e., scratch assay). In fact, we hypothesize that the particular composition of our hTEM may promote the adhesion properties of EPCs by allowing the expression of cell-matrix contact proteins (e.g., Vinculin), which in turn may enhance cell retention in situ. Moreover, vWF is a well-known mediator for inflammation, which in case of pre-endothelialized hTEMs, may be helpful to further stimulate native host cell recruitment in situ.
The principal feasibility to pre-endothelialize matrices such as polymers and xenogeneic or allogenic substitutes has been successfully demonstrated in several preclinical and clinical studies (Zilla et al., 1994; Shinoka et al., 1995; Lehner et al., 1997; Cebotari et al., 2006; Hibino et al., 2010; Roh et al., 2010; Quint et al., 2011; Tudorache et al., 2013; Meier et al., 2014; Melchiorri et al., 2015; Theodoridis et al., 2015; Ma et al., 2017; Drews et al., 2020; Schwarz et al., 2021). In comparison to non-endothelialized counterparts, they showed less neointima and thrombus formation. However, on the other hand, human ECs are also known to have limited capabilities to grow and expand across anastomotic regions (Berger et al., 1972; Zilla et al., 2007), thereby limiting the overall in vivo endothelialization capacity of tissue-engineered constructs once implanted.
hTEMs and other, conceptually comparable acellular technologies have demonstrated strong over time endothelialization following implantation in recent preclinical studies (Syedain et al., 2017; Biermann et al., 2019),. However, despite this potential, evidence in the human setting remains preliminary (Shirzad et al., 2017) and a systematical investigation in larger clinical studies are needed. Therefore, to date, precautionary anticoagulation or anti-platelet therapy would be still advisable (Lawson et al., 2016; Tudorache et al., 2016; Bobylev et al., 2019; Boethig et al., 2019; Kirkton et al., 2019).
Recently, hPL has been validated and suggested as a clinically relevant alternative to the FBS used for standard cell culture procedures, especially in the setting of wound healing (Bieback, 2013; Martínez et al., 2015; Shirzad et al., 2017). In fact, recent studies reported reduced inflammation, improved bone regeneration, and improved hard and soft tissue wound healing when hPL was added as medium supplement (Chiara Barsotti et al., 2013; Ramos-Torrecillas et al., 2014), further highlighting the potential of hPL for clinical application.
In our study, EPCs pre-endothelialization of hTEMs cultured with hPL demonstrated the capacity to form microvessel-like structures, suggesting the ability of EPCs to rearrange and reorganize to induce angiogenesis (Fortunato et al., 2016). This finding might be attributable to specific factors that are present in hPL such as vascular endothelial growth factor (VEGF, a sub-family of platelet-derived growth factors), which is able to induce the formation of blood vessels (Shanskii et al., 2013; Ramos-Torrecillas et al., 2014). Interestingly, the results presented in this study suggest an anti-thrombotic effect of EPC seeded hTEMs. Here, ECFCs gene expression showed an upregulation of the genes involved in vascular tone control (eNOS), in angiogenesis (VEGF), and hemostasis and EPCs activation (vWF).
Finally, we were able to demonstrate that the concept of EPCs pre-endothelialized hTEMs is also feasible in the setting of a transcatheter heart valve geometry, and in particular, that such pre-endothelialized hTEM-based transcatheter valves can safely undergo crimping maneuvers which are mandatory for transcatheter valve delivery.
This is an important finding, as to date, only surgical tissue-engineered heart valves have been investigated as possible starter matrices for pre-endothelialization studies (Zilla et al., 2008; Stassen et al., 2017). Moreover, due to the increasing clinical relevance of transcatheter valve replacements, their forthcoming suitability for low-risk and young patients (Arora et al., 2017; Kenny and Hijazi, 2017; Fioretta et al., 2018), and our recent advances in the development of transcatheter tissue-engineered heart valves, our results are of high translational relevance, as it is of key importance to understand whether such prostheses could cope with in vitro pre-endothelialization procedures.
Crimping maneuvers are indeed very frequent “folding techniques” in order to enable transcatheter delivery of cardiovascular prostheses such as stented heart valves, vascular conduits or occluders (Jung and Choi, 2018; Mack et al., 2019). Crimping of current cardiovascular substitutes down to diameters of 14–18 Fr has been demonstrated to cause microstructural damages which could potentially result in an accelerated failure of the prosthesis (Kiefer et al., 2011; Amahzoune et al., 2013; Alavi et al., 2014). Notably, the non-regenerative nature of the fixed xenogeneic bioprostheses may not enable the complete recovery of the initial surface structure, thereby favoring platelets adhesion and long-term calcifications. In our study, we have successfully demonstrated that 1) pre-endothelialized hTEMs can be adjusted to a TESV geometry; and 2) TESVs sustained human EPCs adhesion following the crimping maneuver. Importantly, due to the strong regenerative potential of EPCs together with their migration capacity once seeded onto our hTEMs, we may hypothesize that potential damages that may have occurred during crimping may be repaired in vivo following implantation.
Limitations
This study focused on the in vitro evaluation of EPC behavior when seeded onto hTEMs, by means of simplified in vitro techniques (e.g., scratch assay). In vivo model system showing the direct functionality of pre-endothelialized hTEMs once in contact with animal/human blood was not assessed and was beyond the scope of the study. Future studies should investigate how hTEMs pre-endothelialized with EPCs will impact on the mechanical and functional cardiovascular environment, also taking into consideration blood flow hemodynamics and immune cell response. In this context, in-depth understanding of EPCs anti-thrombotic molecular pathways over time could be analyzed to better predict EPCs behavior in vivo. In addition, taking into account the impact of the cell source (i.e., allogenic or autologous) and the existing inter-patient variability (e.g., age, health status, environmental factors) to better understand the dynamic of EPCs behavior in vivo and improve their translation to clinical application, an increased patient number could be implemented, also including EPCs isolated from diseased patients.
Conclusion
This study demonstrates the feasibility to develop an EPCs pre-endothelialized hTEM-based construct as starting matrix for cardiovascular applications. EPCs were successfully retained on hTEMs and showed over time migration properties as well as expression of typical endothelial cells markers such as VE-Cad, CD31, and vWF, under both static and dynamic orbital conditions. The translational potential of such matrices was further proven by the implementation of xenogeneic-free culture protocols and the application of physiological pulmonary shear forces with the characterization of a mature endothelium. Additionally, proof-of-concept pre-endothelialization of clinically-relevant TESVs was successfully demonstrated and the retention of EPCs endured also after crimping procedure, which opens up new avenues towards next-generation pre-endothelialized TESVs, that may provide an enhanced hemocompatibility profile, which may therefore be particularly beneficial in certain indications and for selected patients at high risk for thromboembolic events. Together, our findings provide further important insight into the potential of hTEMs as a next-generation technology for the development of cardiovascular implants.
Materials and Methods
Preparation and Culture of Human Cell-Derived Tissue Engineered Matrices
Human cell-based TEMs (hTEMs, n = 160) were fabricated using 6 cm2 patches of non-woven polyglycolic-acid meshes (PGA; thickness 1.0 mm; specific gravity 70 mg/cm3; Cellon, Bereldange) coated with 1% poly-4-hydroxybutyrate (MW 1 × 106: P4HB; TEPHA Inc.) in liquid tetrahydrofuran (Fluka Sigma-Aldrich) (Figure 1A). After overnight drying, the patches were sutured onto stainless steel rings (28 mm in diameter, RVS Paleis) by using continuous sutures (Yavo, PVDF, non-resorbable, 6/0 USP) (Figure 1B). The scaffolds were finally sterilized using a 70% EtOH solution for 30 min and then rinsed twice with phosphate buffer saline (PBS, Sigma-Aldrich) supplemented with 2% amphotericin (Gibco, Invitrogen AG) for 30 min. Finally, scaffolds were incubated overnight at 37°C with advanced Dulbecco’s Modified Eagle Medium (DMEM, Gibco, Invitrogen AG), supplemented with 10% fetal bovine serum (FBS, Gibco, Invitrogen AG), 1% GlutaMax (Gibco, Invitrogen AG), 1% penicillin-streptomycin (Lonza) and L-ascorbic acid 2-phosphate (0.25 mg/ml; Sigma-Aldrich). Human dermal fibroblasts (hDFs) were purchased by CellSystems Biotechnology (Vertrieb GmbH) and expanded in advanced DMEM medium, supplemented with 10% FBS, 1% GlutaMax, and 1% penicillin-streptomycin, and cultured in a cell incubator containing 5% CO2 at 37°C. Afterwards, hDFs (1.0 × 106 cells/cm2) were seeded onto the scaffolds using fibrin as cell carrier (Mol et al., 2005a) (Figure 1A). Medium was replaced every 2–3 days. After 4 weeks of culturing, patches were decellularized as previously described (Dijkman et al., 2012) (Figures 1A–D). Shortly, patches were washed in PBS and incubated over-night with a detergent solution (PBS supplemented with 0.25% Triton-X-100 (Merck), 0.25% sodium-deoxycholate (Sigma-Aldrich) and 0.02% ethylenediaminetetraacetic acid (Sigma-Aldrich) at 37°C, followed by incubation for 5–8 h with a Benzonase Nuclease (Novagen, Merk) treatment solution (TRIS-HCl buffer, pH = 8) at decreasing concentrations (first treatment: 100 U/ml; second treatment: 80 U/ml; third treatment: 20 U/ml) at 37°C. After washing in PBS, patches were stored at 4°C in DMEM medium.
Isolation of Endothelial-Colony-Forming Cells
Human peripheral blood mononuclear cells were isolated from 60 ml of blood of two healthy volunteers through gradient centrifugation using Histopaque (Sigma-Aldrich) as separation medium (Fioretta et al., 2012). The cells were isolated under Ethical agreement number 2016-00208 and approved by the cantonal authorities. To obtain ECFCs, peripheral blood cells were seeded onto 0.5% gelatin-coated culture plates and cultured with EGM-2 medium. For the first week after isolation, medium was changed every day. From the second week on, medium was refreshed every 2 days. After 27 days of culture, the first ECFCs colonies were visible, harvested and expanded.
Fluorescence Activated Cell Sorting Characterization of Endothelial-Colony-Forming Cells
Aliquots of 1 million isolated ECFCs from both donors were characterized by fluorescence activated cell sorting (FACS), and HUVECs were used as control. Briefly, cells were resuspended in cold 1% bovine serum albumin (BSA; pH = 7.0, Axonlab, in solution with PBS) and centrifuged at 300 g for 5 min at 4°C. The supernatant was discarded, and the cells again resuspended into 1% BSA/PBS at 4°C. 1% formaldehyde (FormaFix 4%, EBIS) was then added at the cells and the mixture was incubated for 10 min at 4°C. Cells were subsequently washed with 1% BSA/PBS and finally centrifuged. After resuspension with 1% BSA/PBS, the primary antibody (Pacific blue anti-human CD31 antibody, BioLegend, 2.0 µg per 1 M cells) was added and let incubated at 4°C for 30 min. Finally, ECFCs were again washed, centrifuged, and resuspended with 1% BSA/PBS. FACS was performed using High-end BD SORP flow cytometer (Fortessa, BD Biosciences) and BD FACSDiva software (BD Biosciences). Results were elaborated and analyzed with FlowJo software (FlowJo, LLC).
Seeding of Endothelial-Colony-Forming Cells and Human-Derived Umbilical Vein Endothelial Cells on Human Cell-Derived Tissue-Engineered Extracellular Matrices
ECFCs (n = 2 donors) or HUVECs were expanded in EGM-2 medium supplemented with FBS or human platelet lysate (hPL) and cultured in a cell incubator containing 5% CO2 at 37°C. Afterwards, cells were seeded onto hTEMs (n = 160, 15′000 cells/cm2), glass covered with 0.5% gelatin (n = 12, 15′000 cells/cm2), and Collagen I gels (Symatese, concentration of 1.5% in PBS) (n = 12, 15′000 cells/cm2) using pooled HUVECs (Thermofisher) as control. After seeding, cells on hTEMs, glass, and collagen I gels were left for 5 days in the incubator to allow for cell adhesion and endothelial cells (ECs) markers expression.
Orbital Conditioning, Scratch Assay and Experimental Wound Creation
Seeding of ECFCs (n = 40) and HUVECs (n = 40) on hTEMs was performed as previously described. From the second day after seeding, hTEMs samples were subdivided into a static (n = 20) and a dynamic (orbital shaker, n = 20) group for both ECFCs and HUVECs. The orbital shaker was set onto 170 rpm to mimic a pulmonary shear force (19.4 dynes/cm2) (Dardik et al., 2005; Kim et al., 2015). These conditions were used to assess hTEMs pre-endothelialization feasibility as well as resistance to shear forces.
Scratch assay (n = 4) enabled the analysis of migration and remodeling properties of ECFCs once seeded onto hTEMs. Briefly, after reaching confluency (5 days), an artificial gap (scratch) was performed in the central part of the hTEMs. The scratch was created with a p200 pipet tip as previously described (Liang et al., 2007). ECFCs on hTEMs were cultured under static and dynamic orbital (170 rpm) conditions with EGM-2 medium supplemented with FBS (n = 20) or hPL (n = 20) at 37°C and finally fixed with 4% formalin after different time-points (0 h, 8 h, 2, 5, and 14 days, duplicates per timepoint). Pooled HUVECs were used as control under the same conditions. For a schematic overview of samples and experimental design see Supplementary Table S1.
Immunofluorescent Staining
At the terminal time point, cells cultured either on glass, Collagen I, or hTEMs were washed with PBS and fixed in 4% formalin for 15–20 min. Afterwards, cells were permeabilized 10 min with 0.1% Triton-X-100 in PBS, again washed in PBS, and blocked with a blocking buffer composed of 2% BSA in PBS for 1 h. Primary antibody for Vascular Endothelial Cadherin (Ve-Cad), Collagen III, Von Willebrand Factor (vWF), Collagen IV, Phalloidin, and Vinculin (Supplementary Table S2) were solubilized in a 1% BSA/PBS solution and incubated for 1 h at room temperature. After washing with 0.1% Tween-20 in PBS and PBS, samples were incubated with the corresponding secondary antibodies (Supplementary Table S2) for 1 h at room temperature. Finally, cells were again washed with 0.1% Tween-20/PBS and PBS and embedded with the Fluoroshield Mounting Medium with DAPI (Abcam). The stained cells were visualized using a Leica SP8 inverse confocal laser scanning microscope (DMI6000 AFC). Image processing was performed using the LAS AF Lite Microscope software (Leica, version 4.0.11706).
RNA Isolation and Semi-quantitative Real-Time PCR (qRT-PCR)
ECFCs and HUVECs gene expression cultured in FBS or HPL supplemented medium was analyzed using qRT-PCR. RNA was isolated from hTEMs cultured under static (n = 3) or dynamic orbital (n = 3) conditions using the RNeasy mini extraction kit (Qiagen). Isolation was done according to the manufacturer’s instructions. RNA concentration was measured on NanoDrop spectrophotometer and diluted with water to obtain the same concentration for all samples. Purified RNA was then transcribed to cDNA (iScript cDNA synthesis kit, BIO-RAD) by mixing it with iScript Reverse transcriptase (1 μl) and 5x iScript Reaction mix (4 μl). Synthesis of cDNA was performed in 20 μl reaction volume in PCR-thermocycler (Biometra, Germany). The reaction protocol consisted of priming for 5 min at 25°C, reverse transcription for 20 min at 46°C, and reverse transcription inactivation for 1 min at 95°C. The relative expression of the genes of interest was quantified by using sequence-specific forward and reverse primers (Supplementary Table S3). Custom-designed primers and probes were synthetized by Microsynth (Balgach, Switzerland) whereas commercially available sets were purchased from Eurofins Genomics (Supplementary Table S3). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as housekeeping gene. qRT-PCR was performed in 10 μl reaction volume using QuantStudio™ 7 Flex Real-Time PCR System (Applied Biosystems™). The cDNA was diluted 1:10 with DEPC-treated water. A master mix for every primer, composed of Fast SYBR Green (Applied Biosystems™), the diluted primers (0.2 μM) and DEPC-treated water was prepared and mixed in a 384-well plate with 2 μl of cDNA. Samples were tested in triplicate for each gene. The cycling parameters were as follows: initial denaturation at 95°C for 2 min, followed by 40 cycles of 95°C for 10 s, annealing at 60°C for 15 s. Denaturation was then achieved at 95°C for 15 s, extension and read fluorescence was done at 60°C for 1 min. The double ddCT method was used to analyze the data in Excel and GraphPad Prism (Version 8.0).
Pre-Endothelialization of Tissue-Engineered Sinus Valves
Preparation and Culture of Tissue-Engineered Sinus Valves
Trileaflet tissue-engineered sinus valves (TESVs, n = 4) were fabricated from non-woven PGA scaffold sewn onto radially self-expandable nitinol stents (CARAG AG), as described elsewhere (Motta et al., 2019). Thereafter, the PGA scaffold was coated with 1% P4HB in liquid tetrahydrofuran, dried overnight and sterilized in ethanol. Meanwhile, hDFs (CellSystems Biotechnology Vertrieb GmbH) were expanded in medium and used as cell source to produce ECM during 4 weeks of in vitro culture. 1.0 × 106 cells/cm2 were seeded onto the TESV scaffold using fibrin as cell carrier. After seeding the valves were cultured into a Diastolic Pulse Duplicator System, which recapitulates the physiological environment of pulmonary heart valves (Mol et al., 2005b). Finally, TESVs were decellularized, as previously described (Dijkman et al., 2012).
Seeding of Endothelial-Colony-Forming Cells and Human-Derived Umbilical Vein Endothelial Cells on Tissue-Engineered Sinus Valves
ECFCs and HUVECs were expanded in EGM-2 medium and cultured in a cell incubator containing 5% CO2 at 37°C. Afterwards, cells were seeded onto TESVs (15′000 cells/cm2). After seeding, cells on TESVs (n = 4) were left for 5 days in the incubator to allow for cell adhesion and growth.
Experimental Wound Creation by Crimping Procedure
TESVs were crimped for 20 min in a custom-made crimper device (CARAG AG) mimicking the total crimping time needed during a transcatheter valve implantation procedure (Kiefer et al., 2011; Amahzoune et al., 2013; Alavi et al., 2014). The test was performed in order to determine the damaging impact of such procedure on the endothelium.
Histology and Immunohistochemistry
TESVs were fixed in 4% formalin, embedded in paraffin and cut into 5 µm sections. To assess ECFCs and HUVECs presence and tissue composition, sections were stained for Hematoxylin-Eosin, CD31 (Biolegend), and vWF.
Statistical Analysis
Data in the text are represented as mean ± SD, unless stated otherwise. ECFCs (n = 3) and HUVECs (n = 3) gene expression were evaluated with non-parametric unpaired t-test. Prism software version 8 (GraphPad Software, Inc., San Diego, California) was used for the analyses.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the cantonal authorities of the Canton of Zurich under Ethical agreement number 2016-00208. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
SM and ME obtained the funding. SM designed the study concept. SM and PZ performed the experiments. SM analyzed the data. SM, PZ, EF, VL, CB, SH and ME drafted and/or revised the manuscript for critical content. SM wrote the manuscript. Administrative, technical, or supervisory tasks were handled by SM, SH and ME.
Funding
SM was partly supported by the University of Zurich Forschungskredit (FK-14-031), by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program, grant agreement no. 852814 (TAVI4Life), by the Swiss National Science Foundation, Spark grant no. CRSK-3_196717, and by the ETH Foundation under the umbrella of the Zurich Heart Project. PZ was supported by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program, grant agreement no. 852814 (TAVI4Life). EF was partly funded through the Swiss National Science Foundation (PZ00P3_180138). ME was supported by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program, grant agreement no. 852814 (TAVI4Life).
Conflict of Interest
SH is shareholder at Xeltis BV and LifeMatrix AG. ME is a shareholder at LifeMatrix AG.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2022.867877/full#supplementary-material
Abbreviations
BSA, bovine serum albumin; EC(s), endothelial cell(s); ECFC(s), endothelial colony forming cell(s); ECM, extracellular matrix; eNOS, nitric oxide synthase; EPC(s), endothelial progenitor cell(s); FBS, fetal bovine serum; hDF(s), human dermal fibroblast(s); hPL, Human Platelet Lysate; hTEM(s), human-derived tissue engineered matrix(-ces); HUVEC(s), human-derived umbilical vein cell(s); P4HB, poly-4-hydroxybutyrate; PBS, phosphate buffer saline; PGA, polyglycolic acid; TEHV(s), tissue engineered heart valve(-es); TEM(s), tissue engineered matrix(-ces); TESV(s), tissue engineered sinus valve(s); VE-Cad, vascular endothelial cadherin; vWF, von willebrand factor.
References
- Alavi S. H., Groves E. M., Kheradvar A. (2014). The Effects of Transcatheter Valve Crimping on Pericardial Leaflets. Ann. Thorac. Surg. 97, 1260–1266. 10.1016/j.athoracsur.2013.11.009 [DOI] [PubMed] [Google Scholar]
- Alshawabkeh L., Economy K. E., Valente A. M. (2016). Anticoagulation during Pregnancy. J. Am. Coll. Cardiol. 68, 1804–1813. 10.1016/j.jacc.2016.06.076 [DOI] [PubMed] [Google Scholar]
- Amahzoune B., Bruneval P., Allam B., Lafont A., Fabiani J.-N., Zegdi R. (2013). Traumatic Leaflet Injury during the Use of Percutaneous Valves: a Comparative Study of Balloon- and Self-Expandable Valved Stents. Eur. J. Cardio-Thoracic Surg. 43, 488–493. 10.1093/ejcts/ezs359 [DOI] [PubMed] [Google Scholar]
- Arora S., Misenheimer J. A., Ramaraj R. (2017). Transcatheter Aortic Valve Replacement: Comprehensive Review and Present Status. Tex. Hear. Inst. J. 44, 29–38. 10.14503/thij-16-5852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayad S. W., Hassanein M. M., Mohamed E. A., Gohar A. M. (2016). Maternal and Fetal Outcomes in Pregnant Women with a Prosthetic Mechanical Heart Valve. Clin. Med. Insights. Cardiol. 10, 11–17. 10.4137/CMC.S36740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger K., Sauvage L. R., Rao A. M., Wood S. J. (1972). Healing of Arterial Prostheses in Man. Ann. Surg. 175, 118–127. 10.1097/00000658-197201000-00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieback K. (2013). Platelet Lysate as Replacement for Fetal Bovine Serum in Mesenchymal Stromal Cell Cultures. Transfus. Med. Hemother 40, 326–335. 10.1159/000354061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biermann A. C., Marzi J., Brauchle E., Wichmann J. L., Arendt C. T., Puntmann V., et al. (2019). Improved Long-Term Durability of Allogeneic Heart Valves in the Orthotopic Sheep Model. Eur. J. Cardio-thoracic Surg. Off. J. Eur. Assoc. Cardio-thoracic Surg. 55, 484–493. 10.1093/ejcts/ezy292 [DOI] [PubMed] [Google Scholar]
- Bobylev D., Sarikouch S., Tudorache I., Cvitkovic T., Söylen B., Boethig D., et al. (2019). Double Semilunar Valve Replacement in Complex Congenital Heart Disease Using Decellularized Homografts. Interact. Cardiovasc. Thorac. Surg. 28, 151–157. 10.1093/icvts/ivy212 [DOI] [PubMed] [Google Scholar]
- Boethig D., Horke A., Hazekamp M., Meyns B., Rega F., Van Puyvelde J., et al. (2019). A European Study on Decellularized Homografts for Pulmonary Valve Replacement: Initial Results from the Prospective ESPOIR Trial and ESPOIR Registry Data†. Eur. J. Cardio-thoracic Surg. Off. J. Eur. Assoc. Cardio-thoracic Surg. 56, 503–509. 10.1093/ejcts/ezz054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicelli A. P., O'Gara P. T., Giugliano R. P. (2016). Anticoagulation after Heart Valve Replacement or Transcatheter Valve Implantation. Am. J. Cardiol. 118, 1419–1426. 10.1016/j.amjcard.2016.07.048 [DOI] [PubMed] [Google Scholar]
- Cebotari S., Lichtenberg A., Tudorache I., Hilfiker A., Mertsching H., Leyh R., et al. (2006). Clinical Application of Tissue Engineered Human Heart Valves Using Autologous Progenitor Cells. Circulation 114, I132–I137. 10.1161/CIRCULATIONAHA.105.001065 [DOI] [PubMed] [Google Scholar]
- Chakravarty T., Jilaihawi H., de la Rosa A., Makkar R. (2020). Anticoagulation Therapy after Transcatheter Aortic Valve Replacement. Curr. Cardiol. Rep. 22, 175. 10.1007/s11886-020-01425-8 [DOI] [PubMed] [Google Scholar]
- Chiara Barsotti M., Losi P., Briganti E., Sanguinetti E., Magera A., Al Kayal T., et al. (2013). Effect of Platelet Lysate on Human Cells Involved in Different Phases of Wound Healing. PLoS One 8, e84753. 10.1371/journal.pone.0084753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabbous M., Malaeb D., Sakr F. (2014). Anticoagulant Therapy in Pediatrics. J. Basic Clin. Pharma 5, 27–33. 10.4103/0976-0105.134947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl S. L., Kypson A. P., Lawson J. H., Blum J. L., Strader J. T., Li Y., et al. (2011). Readily Available Tissue-Engineered Vascular Grafts. Sci. Transl Med. 3, 68ra9. 10.1126/scitranslmed.3001426 [DOI] [PubMed] [Google Scholar]
- Dardik A., Chen L., Frattini J., Asada H., Aziz F., Kudo F. A., et al. (2005). Differential Effects of Orbital and Laminar Shear Stress on Endothelial Cells. J. Vasc. Surg. 41, 869–880. 10.1016/j.jvs.2005.01.020 [DOI] [PubMed] [Google Scholar]
- DiBardino D. J., Jacobs J. P. (2014). Current Readings: Long-Term Management of Patients Undergoing Successful Pediatric Cardiac Surgery. Semin. Thorac. Cardiovasc. Surg. 26, 132–144. 10.1053/j.semtcvs.2014.08.002 [DOI] [PubMed] [Google Scholar]
- Dijkman P. E., Driessen-Mol A., Frese L., Hoerstrup S. P., Baaijens F. P. T. (2012). Decellularized Homologous Tissue-Engineered Heart Valves as Off-The-Shelf Alternatives to Xeno- and Homografts. Biomaterials 33, 4545–4554. 10.1016/j.biomaterials.2012.03.015 [DOI] [PubMed] [Google Scholar]
- Drews J. D., Pepper V. K., Best C. A., Szafron J. M., Cheatham J. P., Yates A. R., et al. (2020). Spontaneous Reversal of Stenosis in Tissue-Engineered Vascular Grafts. Sci. Transl. Med. 12. 10.1126/scitranslmed.aax6919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen-Mol A., Emmert M. Y., Dijkman P. E., Frese L., Sanders B., Weber B., et al. (2014). Transcatheter Implantation of Homologous "Off-The-Shelf" Tissue-Engineered Heart Valves with Self-Repair Capacity. J. Am. Coll. Cardiol. 63, 1320–1329. 10.1016/j.jacc.2013.09.082 [DOI] [PubMed] [Google Scholar]
- Emmert M. Y., Schmitt B. A., Loerakker S., Sanders B., Spriestersbach H., Fioretta E. S., et al. (2018). Computational Modeling Guides Tissue-Engineered Heart Valve Design for Long-Term In Vivo Performance in a Translational Sheep Model. Sci. Transl. Med. 10. 10.1126/scitranslmed.aan4587 [DOI] [PubMed] [Google Scholar]
- Fioretta E. S., Fledderus J. O., Baaijens F. P., Bouten C. V. (2012). Influence of Substrate Stiffness on Circulating Progenitor Cell Fate. J. Biomech. 45, 736–744. 10.1016/j.jbiomech.2011.11.013 [DOI] [PubMed] [Google Scholar]
- Fioretta E. S., Dijkman P. E., Emmert M. Y., Hoerstrup S. P. (2018). The Future of Heart Valve Replacement: Recent Developments and Translational Challenges for Heart Valve Tissue Engineering J. Tissue Eng. Regen. Med. 12 (1), e323–e335. 10.1002/term.2326 [DOI] [PubMed] [Google Scholar]
- Fioretta E. S., Motta S. E., Lintas V., Loerakker S., Parker K. K., Baaijens F. P. T., et al. (2021). Next-generation Tissue-Engineered Heart Valves with Repair, Remodelling and Regeneration Capacity. Nat. Rev. Cardiol. 18, 92–116. 10.1038/s41569-020-0422-8 [DOI] [PubMed] [Google Scholar]
- Fortunato T. M., Beltrami C., Emanueli C., De Bank P. A., Pula G. (2016). Platelet Lysate Gel and Endothelial Progenitors Stimulate Microvascular Network Formation In Vitro: Tissue Engineering Implications. Sci. Rep. 6, 25326. 10.1038/srep25326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdisch M., Stelly T., Slaughter M., Rodriguez V. (2020). Early Results from the FDA Extracellular Matrix (ECM) Cylinder Valve Clinical Feasibility Trial. Struct. Heart 4, 102. 10.1080/24748706.2020.1717212 [DOI] [Google Scholar]
- Hibino N., McGillicuddy E., Matsumura G., Ichihara Y., Naito Y., Breuer C., et al. (2010). Late-term Results of Tissue-Engineered Vascular Grafts in Humans. J. Thorac. Cardiovasc. Surg. 139 (431–6), 431–432. 10.1016/j.jtcvs.2009.09.057 [DOI] [PubMed] [Google Scholar]
- Jacobs J. P., Mavroudis C., Quintessenza J. A., Chai P. J., Pasquali S. K., Hill K. D., et al. (2014). Reoperations for Pediatric and Congenital Heart Disease: an Analysis of the Society of Thoracic Surgeons (STS) Congenital Heart Surgery Database. Semin. Thorac. Cardiovasc. Surg. Pediatr. Card. Surg. Annu. 17, 2–8. 10.1053/j.pcsu.2014.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S. Y., Choi J. Y. (2018). Transcatheter Closure of Atrial Septal Defect: Principles and Available Devices. J. Thorac. Dis. 10, S2909–S2922. 10.21037/jtd.2018.02.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny D. P., Hijazi Z. M. (2017). Current Status and Future Potential of Transcatheter Interventions in Congenital Heart Disease. Circ. Res. 120, 1015–1026. 10.1161/circresaha.116.309185 [DOI] [PubMed] [Google Scholar]
- Kiefer P., Gruenwald F., Kempfert J., Aupperle H., Seeburger J., Mohr F. W., et al. (2011). Crimping May Affect the Durability of Transcatheter Valves: an Experimental Analysis. Ann. Thorac. Surg. 92, 155–160. 10.1016/j.athoracsur.2011.03.020 [DOI] [PubMed] [Google Scholar]
- Kim H., Yang K. H., Cho H., Gwak G., Park S. C., Kim J. I., et al. (2015). Different Effects of Orbital Shear Stress on Vascular Endothelial Cells: Comparison with the Results of In Vivo Study with Rats. Vsi 31, 33–40. 10.5758/vsi.2015.31.2.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkton R. D., Santiago-Maysonet M., Lawson J. H., Tente W. E., Dahl S. L. M., Niklason L. E., et al. (2019). Bioengineered Human Acellular Vessels Recellularize and Evolve into Living Blood Vessels after Human Implantation. Sci. Transl. Med. 11. 10.1126/scitranslmed.aau6934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson J. H., Glickman M. H., Ilzecki M., Jakimowicz T., Jaroszynski A., Peden E. K., et al. (2016). Bioengineered Human Acellular Vessels for Dialysis Access in Patients with End-Stage Renal Disease: Two Phase 2 Single-Arm Trials. The Lancet 387, 2026–2034. 10.1016/s0140-6736(16)00557-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner G., Fischlein T., Baretton G., Murphy J. G., Reichart B. (1997). Endothelialized Biological Heart Valve Prostheses in the Non-human Primate Model. Eur. J. Cardiothorac. Surg. 11, 498–504. 10.1016/s1010-7940(96)01096-2 [DOI] [PubMed] [Google Scholar]
- Leiria T. L. L., Lopes R. D., Williams J. B., Katz J. N., Kalil R. A. K., Alexander J. H. (2011). Antithrombotic Therapies in Patients with Prosthetic Heart Valves: Guidelines Translated for the Clinician. J. Thromb. Thrombolysis 31, 514–522. 10.1007/s11239-011-0574-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C.-C., Park A. Y., Guan J.-L. (2007). In Vitro scratch Assay: a Convenient and Inexpensive Method for Analysis of Cell Migration In Vitro . Nat. Protoc. 2, 329–333. 10.1038/nprot.2007.30 [DOI] [PubMed] [Google Scholar]
- Lintas V., Fioretta E. S., Motta S. E., Dijkman P. E., Pensalfini M., Mazza E., et al. (2018). Development of a Novel Human Cell-Derived Tissue-Engineered Heart Valve for Transcatheter Aortic Valve Replacement: an In Vitro and In Vivo Feasibility Study. J. Cardiovasc. Trans. Res. 11, 470–482. 10.1007/s12265-018-9821-1 [DOI] [PubMed] [Google Scholar]
- Loerakker S., Ristori T., Baaijens F. P. T. (2016). A Computational Analysis of Cell-Mediated Compaction and Collagen Remodeling in Tissue-Engineered Heart Valves. J. Mech. Behav. Biomed. Mater. 58, 173–187. 10.1016/j.jmbbm.2015.10.001 [DOI] [PubMed] [Google Scholar]
- Lynn A. K., Yannas I. V., Bonfield W. (2004). Antigenicity and Immunogenicity of Collagen. J. Biomed. Mater. Res. 71B, 343–354. 10.1002/jbm.b.30096 [DOI] [PubMed] [Google Scholar]
- Ma X., He Z., Li L., Liu G., Li Q., Yang D., et al. (2017). Development and In Vivo Validation of Tissue-Engineered, Small-Diameter Vascular Grafts from Decellularized Aortae of Fetal Pigs and Canine Vascular Endothelial Cells. J. Cardiothorac. Surg. 12, 101. 10.1186/s13019-017-0661-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack M. J., Leon M. B., Thourani V. H., Makkar R., Kodali S. K., Russo M., et al. (2019). Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 380, 1695–1705. 10.1056/nejmoa1814052 [DOI] [PubMed] [Google Scholar]
- Martínez C. E., Smith P. C., Palma Alvarado V. A. (2015). The Influence of Platelet-Derived Products on Angiogenesis and Tissue Repair: a Concise Update. Front. Physiol. 6, 290. 10.3389/fphys.2015.00290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier L. A., Syedain Z. H., Lahti M. T., Johnson S. S., Chen M. H., Hebbel R. P., et al. (2014). Blood Outgrowth Endothelial Cells Alter Remodeling of Completely Biological Engineered Grafts Implanted into the Sheep Femoral Artery. J. Cardiovasc. Trans. Res. 7, 242–249. 10.1007/s12265-013-9539-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchiorri A. J., Hibino N., Yi T., Lee Y. U., Sugiura T., Tara S., et al. (2015). Contrasting Biofunctionalization Strategies for the Enhanced Endothelialization of Biodegradable Vascular Grafts. Biomacromolecules 16, 437–446. 10.1021/bm501853s [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mol A., Driessen N. J. B., Rutten M. C. M., Hoerstrup S. P., Bouten C. V. C., Baaijens F. P. T. (2005). Tissue Engineering of Human Heart Valve Leaflets: a Novel Bioreactor for a Strain-Based Conditioning Approach. Ann. Biomed. Eng. 33, 1778–1788. 10.1007/s10439-005-8025-4 [DOI] [PubMed] [Google Scholar]
- Mol A., van Lieshout M. I., Dam-de Veen C. G., Neuenschwander S., Hoerstrup S. P., Baaijens F. P. T., et al. (2005). Fibrin as a Cell Carrier in Cardiovascular Tissue Engineering Applications. Biomaterials 26, 3113–3121. 10.1016/j.biomaterials.2004.08.007 [DOI] [PubMed] [Google Scholar]
- Motta S. E., Fioretta E. S., Dijkman P. E., Lintas V., Behr L., Hoerstrup S. P., et al. (2018). Development of an Off-The-Shelf Tissue-Engineered Sinus Valve for Transcatheter Pulmonary Valve Replacement: a Proof-Of-Concept Study. J. Cardiovasc. Trans. Res. 11, 182–191. 10.1007/s12265-018-9800-6 [DOI] [PubMed] [Google Scholar]
- Motta S. E., Lintas V., Fioretta E. S., Dijkman P. E., Putti M., Caliskan E., et al. (2019). Human Cell-Derived Tissue-Engineered Heart Valve with Integrated Valsalva Sinuses: towards Native-like Transcatheter Pulmonary Valve Replacements. NPJ Regen. Med. 4, 14. 10.1038/s41536-019-0077-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta S. E., Fioretta E. S., Lintas V., Dijkman P. E., Hilbe M., Frese L., et al. (2020). Geometry Influences Inflammatory Host Cell Response and Remodeling in Tissue-Engineered Heart Valves In-Vivo Sci. Rep. 10, 19882. 10.1038/s41598-020-76322-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newall F., Branchford B., Male C. (2018). Anticoagulant Prophylaxis and Therapy in Children: Current Challenges and Emerging Issues. J. Thromb. Haemost. 16, 196–208. 10.1111/jth.13913 [DOI] [PubMed] [Google Scholar]
- Quint C., Kondo Y., Manson R. J., Lawson J. H., Dardik A., Niklason L. E. (2011). Decellularized Tissue-Engineered Blood Vessel as an Arterial Conduit. Proc. Natl. Acad. Sci. U.S.A. 108, 9214–9219. 10.1073/pnas.1019506108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Torrecillas J., De Luna-Bertos E., García-Martínez O., Ruiz C. (2014). Clinical Utility of Growth Factors and Platelet-Rich Plasma in Tissue Regeneration: a Review. Wounds 26, 207–213. [PubMed] [Google Scholar]
- Reimer J., Syedain Z., Haynie B., Lahti M., Berry J., Tranquillo R. (2017). Implantation of a Tissue-Engineered Tubular Heart Valve in Growing Lambs. Ann. Biomed. Eng. 45, 439–451. 10.1007/s10439-016-1605-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh J. D., Sawh-Martinez R., Brennan M. P., Jay S. M., Devine L., Rao D. A., et al. (2010). Tissue-engineered Vascular Grafts Transform into Mature Blood Vessels via an Inflammation-Mediated Process of Vascular Remodeling. Proc. Natl. Acad. Sci. U.S.A. 107, 4669–4674. 10.1073/pnas.0911465107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz E. L., Kelly J. M., Blum K. M., Hor K. N., Yates A. R., Zbinden J. C., et al. (2021). Hemodynamic Performance of Tissue-Engineered Vascular Grafts in Fontan Patients. NPJ Regen. Med. 6, 38. 10.1038/s41536-021-00148-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanskii Y. D., Sergeeva N. S., Sviridova I. K., Kirakozov M. S., Kirsanova V. A., Akhmedova S. A., et al. (2013). Human Platelet Lysate as a Promising Growth-Stimulating Additive for Culturing of Stem Cells and Other Cell Types. Bull. Exp. Biol. Med. 156, 146–151. 10.1007/s10517-013-2298-7 [DOI] [PubMed] [Google Scholar]
- Shinoka T., Breuer C. K., Tanel R. E., Zund G., Miura T., Ma P. X., et al. (1995). Tissue Engineering Heart Valves: Valve Leaflet Replacement Study in a Lamb Model. Ann. Thorac. Surg. 60, S513–S516. 10.1016/s0003-4975(21)01185-1 [DOI] [PubMed] [Google Scholar]
- Shirzad N., Bordbar S., Goodarzi A., Mohammad M., Khosravani P., Sayahpour F., et al. (2017). Umbilical Cord Blood Platelet Lysate as Serum Substitute in Expansion of Human Mesenchymal Stem Cells. Cell J 19, 403–414. 10.22074/cellj.2017.4886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stassen O. M. J. A., Muylaert D. E. P., Bouten C. V. C., Hjortnaes J. (2017). Current Challenges in Translating Tissue-Engineered Heart Valves. Curr. Treat. Options. Cardio Med. 19, 71. 10.1007/s11936-017-0566-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syedain Z. H., Graham M. L., Dunn T. B., O'Brien T., Johnson S. L., Schumacher R. J., et al. (2017). A Completely Biological "Off-The-Shelf" Arteriovenous Graft that Recellularizes in Baboons. Sci. Transl. Med. 9. 10.1126/scitranslmed.aan4209 [DOI] [PubMed] [Google Scholar]
- Syedain Z. H., Haynie B., Johnson S. L., Lahti M., Berry J., Carney J. P., et al. (2021). Pediatric Tri-tube Valved Conduits Made from Fibroblast-Produced Extracellular Matrix Evaluated over 52 Weeks in Growing Lambs. Sci. Transl. Med. 13. 10.1126/scitranslmed.abb7225 [DOI] [PubMed] [Google Scholar]
- Syedain Z. H., Meier L. A., Lahti M. T., Johnson S. L., Tranquillo R. T. (2014). Implantation of Completely Biological Engineered Grafts Following Decellularization into the Sheep Femoral Artery. Tissue Eng. A 20, 1726–1734. 10.1089/ten.tea.2013.0550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syedain Z., Reimer J., Schmidt J., Lahti M., Berry J., Bianco R., et al. (2015). 6-Month Aortic Valve Implantation of an Off-The-Shelf Tissue-Engineered Valve in Sheep. Biomaterials 73, 175–184. 10.1016/j.biomaterials.2015.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodoridis K., Tudorache I., Calistru A., Cebotari S., Meyer T., Sarikouch S., et al. (2015). Successful Matrix Guided Tissue Regeneration of Decellularized Pulmonary Heart Valve Allografts in Elderly Sheep. Biomaterials 52, 221–228. 10.1016/j.biomaterials.2015.02.023 [DOI] [PubMed] [Google Scholar]
- Tudorache I., Calistru A., Baraki H., Meyer T., Höffler K., Sarikouch S., et al. (2013). Orthotopic Replacement of Aortic Heart Valves with Tissue-Engineered Grafts. Tissue Eng. Part A 19, 1686–1694. 10.1089/ten.tea.2012.0074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudorache I., Horke A., Cebotari S., Sarikouch S., Boethig D., Breymann T., et al. (2016). Decellularized Aortic Homografts for Aortic Valve and Aorta Ascendens Replacement. Eur. J. Cardiothorac. Surg. 50, 89–97. 10.1093/ejcts/ezw013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loon S. L. M., M. Smits A. I. P., Driessen-Mol A., T. Baaijens F. P., C. Boute C. V. (2013). “The Immune Response in In Situ Tissue Engineering of Aortic Heart Valves,” in Calcific Aortic Valve Disease. Editor Aikawa E. (London: InTech; ). 10.5772/54354 [DOI] [Google Scholar]
- van Oeveren W. (2013). Obstacles in Haemocompatibility Testing. Scientifica (Cairo) 2013, 392584. 10.1155/2013/392584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber B., Dijkman P. E., Scherman J., Sanders B., Emmert M. Y., Grünenfelder J., et al. (2013). Off-the-shelf Human Decellularized Tissue-Engineered Heart Valves in a Non-human Primate Model. Biomaterials 34, 7269–7280. 10.1016/j.biomaterials.2013.04.059 [DOI] [PubMed] [Google Scholar]
- Weber B., Emmert M. Y., Hoerstrup S. P. (2012). Stem Cells for Heart Valve Regeneration Swiss Med. Wkly. 142, w13622. 10.4414/smw.2012.13622 [DOI] [PubMed] [Google Scholar]
- Weber B., Schoenauer R., Papadopulos F., Modregger P., Peter S., Stampanoni M., et al. (2011). Engineering of Living Autologous Human Umbilical Cord Cell-Based Septal Occluder Membranes Using Composite PGA-P4hb Matrices. Biomaterials 32, 9630–9641. 10.1016/j.biomaterials.2011.07.070 [DOI] [PubMed] [Google Scholar]
- Zilla P., Bezuidenhout D., Human P. (2007). Prosthetic Vascular Grafts: Wrong Models, Wrong Questions and No Healing. Biomaterials 28, 5009–5027. 10.1016/j.biomaterials.2007.07.017 [DOI] [PubMed] [Google Scholar]
- Zilla P., Brink J., Human P., Bezuidenhout D. (2008). Prosthetic Heart Valves: Catering for the Few. Biomaterials 29, 385–406. 10.1016/j.biomaterials.2007.09.033 [DOI] [PubMed] [Google Scholar]
- Zilla P., Deutsch M., Meinhart J., Puschmann R., Eberl T., Minar E., et al. (1994). Clinical In Vitro Endothelialization of Femoropopliteal Bypass Grafts: an Actuarial Follow-Up over Three Years. J. Vasc. Surg. 19, 540–548. 10.1016/s0741-5214(94)70083-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.