Abstract
The hemolytic antimicrobial peptide dermaseptin S4 was recently shown to exert antimalarial activity. In this study, we attempted to understand the underlying mechanism(s) and identify derivatives with improved antimalarial activity. A number of dermaseptin S4 derivatives inhibited parasite growth with a 50% inhibitory concentration (IC50) in the micromolar range. Among these, the substituted S4 analog K4K20-S4 was the most potent (IC50 = 0.2 μM), while its shorter version, K4-S4(1–13)a, retained a considerable potency (IC50 = 6 μM). Both K4K20-S4 and K4-S4(1–13)a inhibited growth of the parasites more at the trophozoite stage than at the ring stage. Significant growth inhibition was observed after as little as 1 min of exposure to peptides and proceeded with nearly linear kinetics. The peptides selectively lysed infected red blood cells (RBC) while having a weaker effect on noninfected RBC. Thus, K4K20-S4 lysed trophozoites at concentrations similar to those that inhibited their proliferation, but trophozoites were >30-fold more susceptible than normal RBC to the lytic effect of K4K20-S4, the most hemolytic dermaseptin. The same trend was observed with K4-S4(1–13)a. The d isomers of K4K20-S4 or K4-S4(1–13)a were as active as the l counterparts, indicating that antimalarial activity of these peptides, like their membrane-lytic activity, is not mediated by specific interactions with a chiral center. Moreover, dissipation of transmembrane potential experiments with infected cells indicated that the peptides induce damage in the parasite's plasma membrane. Fluorescence confocal microscopy analysis of treated infected cells also indicated that the peptide is able to find its way through the complex series of membranes and interact directly with the intracellular parasite. Overall, the data showed that dermaseptins exert antimalarial activity by lysis of infected cells. Dermaseptin derivatives are also able to disrupt the parasite plasma membrane without harming that of the host RBC.
Antimicrobial peptides are an essential defense component of both invertebrates and vertebrates (5, 18, 39). They display a large amount of heterogeneity in primary and secondary structures but share common features that seem to underlie their cytotoxic function, such as amphipathy and net positive charge. Although the precise mechanism of their action is not fully understood, antimicrobial peptides are believed to kill target cells by disrupting the membrane structure. Antimicrobial peptides are potentially active against a large spectrum of microorganisms, yet they are generally nontoxic to normal mammalian cells. The molecular basis for this selectivity is also ill defined, but it is believed to result from differences in the membrane fluidity and negative charge density in target microbial cells versus non-target cells, which result from differences in the lipid composition (3, 4, 11, 12, 14, 16, 31, 42, 46, 53). Isomers composed of all d-amino acids display a potency identical to that of all the l counterparts, which implies that the mechanism of their antimicrobial activity is not mediated by interaction with specific receptors.
The dermaseptins are a large family of antimicrobial peptides of between 28 and 34 amino acids, produced by the skin of tree frogs belonging to the genus Phyllomedusidae (32, 34, 36). The dermaseptins are linear polycationic peptides, with an amphipathic α-helical structure in apolar solvents (33). They have a cytolytic activity in vitro against a broad spectrum of pathogenic microorganisms (bacteria, protozoa, and filamentous fungi) and spoilage yeast (7, 8, 16, 19, 32–36). Dermaseptins are also potent killers of nongrowing or slow-growing bacteria (25), suggesting a potential use in the eradication of bacteria placed in a dormant state and/or subject to low oxygen tension. In contrast, most conventional bactericidal or bacteriostatic antibiotics are not effective against dormant bacteria or against those under conditions of low oxygen.
The dermaseptins are known to bind avidly to the membranes of model liposomes and cells. For instance, the partition coefficient (Kp) of some dermaseptins in the presence of phospholipids was calculated to be in the range of 106 M−1 (53). Binding of this class of peptides is believed to represent an early step in a series of events that ultimately leads to a polymerization of the membrane-bound peptide that destabilizes the microbial membrane structure, resulting in cell permeabilization and death. The selective antimicrobial action of these peptides was demonstrated to be mediated by selective interaction of the amphipathic α-helix moiety with the plasma membrane phospholipids (8, 16, 19, 35, 42, 53). Thus, at antimicrobial concentrations, the dermaseptins are essentially nontoxic to erythrocytes, with the exception of dermaseptin S4, which is particularly toxic to erythrocytes (16). To identify peptides derived from dermaseptin S4 that have a reduced hemolytic activity, a library of substitution and deletion peptides derived from dermaseptin S4 was recently investigated (12). Several dermaseptin S4 derivatives were identified as peptides that display both enhanced antibacterial potency and reduced hemolytic activity and hence possess a potential interest as antimalarial products.
Malaria constitutes a major human health problem in most of the tropical areas of the world. Hundreds of millions of people, mostly in Third World countries, are affected by the disease, and 1.5 million to 2 million children die every year in Africa alone. The total population at risk is estimated at 2.2 billion. The efficacy of classical antimalarial drugs and insecticides is declining with increasing resistance of parasites and their vectors, respectively. Since the hopes for an antimalarial vaccine have not been realized as yet, it seems that control of this fatal (for children and nonimmune adults) and debilitating (for semi-immune adults in areas of endemicity) disease will have to rely on chemotherapy in the foreseeable future.
The maturation of the intraerythrocytic malaria parasite results in a series of extensive and dramatic changes in the functional and structural properties of the infected red blood cell (RBC) (reviewed in references 6, 16, 40, 45, 48, and 50). These changes include the loss of the normal discoid shape of the host cell and the appearance of hundreds of electron-dense knobs that mediate the adherence of infected cells to the walls of blood microvessels (20). Appearance of caveola-vesicle complexes on the surface of an infected erythrocyte characterizes the membrane, whereas membranous clefts, vesicles, and aggregates of membrane proteins appear in the cytoplasm of the host cell (1). Studies on the host cell membrane, involving spin-labeled analogs, fluorescent lipids, immunological techniques, and a variety of biochemical techniques, all indicate major disturbances in membrane structure and function (17). These are expressed in alterations of lipid (22) and protein (2, 24, 27) composition, enhanced membrane fluidity (49, 55), increased surface charge (21), reduced deformability (37, 38, 41), enhanced transbilayer mobility of phospholipids (51, 56), and increased levels of heme and lipid peroxides (52). These membrane alterations would predispose the host cell membrane to differential interactions with lytic compounds, such as the dermaseptins. Indeed, an antimalarial effect of dermaseptin S4 was previously observed (16). However, due to the peptide's high hemolytic activity, no conclusions could be drawn as to the mechanism of the antimalarial effect.
In this study, we investigated the antimalarial activities of various dermaseptin S4 derivatives, since their interaction with the altered membranes of the infected cells and their supposed ability to penetrate these cells could constitute specific means for affecting the intraerythrocytic parasite. This research's objective is thus twofold: (i) to assess the effect of dermaseptin S4 and its analogs on the malaria parasite Plasmodium falciparum grown in culture, in order to find the most active antimalarial peptide, and (ii) to investigate the mechanism of antimalarial action.
MATERIALS AND METHODS
Synthesis of dermaseptin S4 derivatives.
Peptides were synthesized by the solid phase method, applying Fmoc (9-fluorenylmethoxycarbonyl) active ester chemistry as described previously (12). After removal of the Fmoc from the N-terminal amino acid, the peptide was cleaved from the resin with an 85:5:5:5 mixture of trifluoroacetic acid, para-cresol, H2O, and thioanisole (10 mg of resin-bound peptide in 1 ml of mixture). The trifluoroacetic acid was then evaporated, and the peptide was precipitated with ether and then washed six times with ether. The crude peptides were extracted from the resin with 30% acetonitrile in water and purified to chromatographic homogeneity in the range of 98 to >99% by reverse-phase high-pressure liquid chromatography (Alliance-Waters) with an automatic injector, photodiode array UV detector, and Millennium integration software. High-pressure liquid chromatography runs were performed on a semipreparative C4 column using a linear gradient of acetonitrile in water (1%/min), both solvents containing 0.1% trifluoroacetic acid. The purified peptides were subjected to amino acid analysis and electrospray mass spectrometry in order to confirm their composition. Peptides were stored as a lyophilized powder at −20°C. Prior to experimentation, fresh solutions were prepared in water, vortexed, sonicated, and centrifuged, and the supernatant was diluted in the appropriate medium.
Peptide labeling.
Peptide labeling with rhodamine at the N-terminal amino acid was performed by treating 10 mg of resin-bound peptide with 0.8 ml of dimethylformamide (DMF) containing 20% piperidine in an Eppendorf test tube in order to remove the Fmoc protecting group of the N-terminal amino acid of the linked peptide. The mixture was agitated for 10 min and then centrifuged, and the supernatant was discarded. The resin-bound peptide was rinsed 3 times in DMF before addition of 0.3 ml of a solution of Lissamine rhodamine chloride (10 mg/ml) in dry DMF containing 7% (vol/vol) diisopropylethylamine. After 24 h of incubation (stirred in the dark at room temperature [RT]), the resin-bound peptide was washed thoroughly three times with DMF and diethyl ether/dichloromethane (1:1) and dried at 40°C (4 h). The peptide was then cleaved from the resin, precipitated with ether, extracted, and purified as described above.
Confocal microscopy.
Confocal microscope images of samples of nonfixed cells treated with rhodaminated dermaseptins were taken using an MRC 1024 confocal imaging system (Bio-Rad, United Kingdom). The microscope (Axiovert 135M; Zeiss, Oberkochen, Germany) is equipped with a 63× objective (Apoplan; numerical aperture, 1,4). For rhodamine excitation, an Argon ion laser adjusted at 514 nm (Em, 580 ± 16 nm) was used.
Parasite cultivation and drug sensitivity testing.
The FCR3 strain was used throughout this investigation. Other strains (W2 and HB3) were used for some critical experiments in order to detect possible strain-specific effects of dermaseptins. Parasites were cultivated by the Jensen and Trager technique as modified in our laboratory (47). Cultures were synchronized by the sorbitol method (29), and infected cells were enriched from culture by gelatin flotation (23) or by Percoll-alanine gradient centrifugation.
Antimalarial assay.
Synchronized cultures at the ring stage were cultured at 1% hematocrit and 2% parasitemia in the presence of increasing concentrations of dermaseptins. After 18 h of incubation, [3H]hypoxanthine was added (final concentration, 2 μCi/ml), and cells were harvested 24 h later. The cell-associated radioactivity was determined and inhibition of growth was calculated by comparison with controls (without peptide). The 50% inhibitory concentration (IC50) was determined by nonlinear regression fitting of the data using the Sigmaplot computer program.
To compare hemolytic and growth inhibition activities, infected cells at the young trophozoite stage were separated from uninfected RBC using the Percoll-alanine gradient and incubated for a 2-h recovery period in culture medium at 37°C. To assess parasite proliferation, infected cells (about 90% parasitemia, determined on Giemsa-stained thin blood smears) were suspended at 0.2% hematocrit in culture medium containing increasing amounts of dermaseptins. After 2 h of incubation, [3H]hypoxanthine was added, and cells were harvested 4 h later. To assess the peptides' hemolytic activity, two additional sets of cultures of infected (95% parasitemia) and normal RBC were pelleted after 2 and 6 h of exposure to peptides, and the absorbance of hemoglobin in the supernatant was determined by absorption spectroscopy at 405 nm. Full lysis was obtained by lysing the same number of cells in an equal volume of distilled water.
For the time-dependence study, parasites at the ring stage (100% parasitemia) or trophozoite stage (100% parasitemia) were exposed at 2% hematocrit to K4K20-S4 or K4-S4(1–13)a at their respective IC50 for 1, 5, 20, 60, 120, 180, and 1,440 min. At the end of the incubation, the peptide was washed off, and parasites returned to culture conditions with [3H]hypoxanthine; parasite-associated radioactivity was measured after 6 h and compared to the control (28).
Measuring the dissipation of parasite membrane potential by the peptides.
Whole culture at the trophozoite stage in modified growth medium (10 mM bicarbonate and 7% plasma) at 0.5% hematocrit was incubated in the presence of 1 μM rhodamine 123 (R123) for 30 min at 37°C. R123 accumulates inside cells in proportion to the membrane potential (Δϕ) and has been shown to respond to the dissipation of Δϕ in P. falciparum (54). Aliquots of this culture were then exposed to different peptides and to a mixture of nigericin (K+:H+ exchanger) and monensin (Na+:H+ exchanger) to dissipate the ion gradients across membranes (positive control), and samples were taken at different time intervals. Cells were pelleted by centrifugation, washed in phosphate-buffered saline (PBS), and resuspended in PBS (original volume of the sample). Aliquots of 150 μl were placed in a 96-well plate, and the fluorescence was read in a Bio-Tek FL600 microplate fluorescence reader (excitation wavelength [λex] = 530 nm; emission wavelength [λem] = 585 nm). Relative fluorescence (as a percentage of that of the untreated control at the same time) was plotted against the time of incubation.
RESULTS
To identify peptides with an improved antimalarial activity, substitution, deletion, and combined substitution and deletion peptides derived from dermaseptin S4 were investigated (Table 1). These included a set of substitution derivatives in which Asp (D) replaced Met (M) in position 4 and replaced Asn (N) in position 20 or both positions and where the same positions were substituted with Lys (K), i.e., six substitution derivatives in total. A second set of deletion derivatives of dermaseptin S4 was prepared, wherein the primary structure of dermaseptin S4 was sequentially shortened from the N and/or C termini. A third set of substitution and deletion dermaseptin S4 derivatives, composed of substituted shortened versions of dermaseptin S4, was also prepared.
TABLE 1.
Sequences of dermaseptin S4 and derivatives
| Peptidea | Amino acid sequenceb |
|---|---|
| S4 | ALWMTLLKKVLKAAAKAALNAVLVGANA |
| Substitution derivatives | |
| D20–S4 | –––––––––––––––––––D–––––––– |
| D4–S4 | –––D–––––––––––––––––––––––– |
| D4D20–S4 | –––D–––––––––––––––D–––––––– |
| K20–S4 | –––––––––––––––––––K–––––––– |
| K4–S4 | –––K–––––––––––––––––––––––– |
| K4K20–S4 | –––K–––––––––––––––K–––––––– |
| Deletion derivatives | |
| S4(1–20) | –––––––––––––––––––– |
| S4(1–16) | –––––––––––––––– |
| S4(1–12) | –––––––––––– |
| S4(5–16) | –––––––––––– |
| S4(5–28) | –––––––––––––––––––––––– |
| S4(9–28) | –––––––––––––––––––– |
| S4(13–28) | –––––––––––––––– |
| Substitution and deletion derivatives | |
| K4–S4(1–16) | –––K–––––––––––– |
| K4–S4(1–16)a | –––K––––––––––––NH2 |
| K4–S4(1–15)a | –––K–––––––––––NH2 |
| K4–S4(1–13)a | –––K–––––––––NH2 |
| K4–S4(1–10)a | –––K––––––––NH2 |
a, amide.
A dash indicates that at the specified position, the peptide contains the amino acid identical to that of dermaseptin S4, listed above.
Antimalarial activity of the dermaseptin S4 derivatives against the FCR3 strain.
Antimalarial activity was assessed by measuring the incorporation of [3H]hypoxanthine into the parasite's nucleic acids of P. falciparum-infected human RBC. Synchronized cultures at the ring stage were cultured in the presence of peptide and [3H]hypoxanthine. Then the cell-associated radioactivity was determined and inhibition of growth was calculated from controls (without peptide).
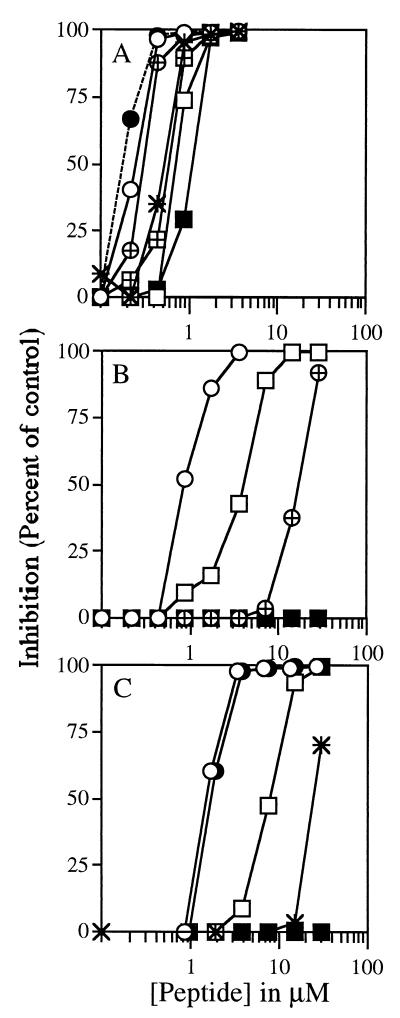
As shown in Fig. 1, various dermaseptin S4 derivatives inhibited parasite growth with an IC50 in the micromolar range. Dermaseptin S4 and its substituted analogs were among the most active. Inhibition of growth was found to depend on the nature of the peptide, in that the highly charged species were the most active. Thus, analogs whose positive charge was increased were more potent (K4K20-S4 was the most potent), while analogs whose positive charge was decreased were less potent.
FIG. 1.
Antimalarial activity of dermaseptin S4 and of various derivatives. Synchronized cultures at the ring stage were cultured at 1% hematocrit and 2% parasitemia in the presence of increasing concentrations of dermaseptins. After 18 h of incubation, [3H]hypoxanthine was added, and cells were harvested 24 h later. The cell-associated radioactivity was determined and inhibition of growth was calculated by comparison with controls (without peptide). (A) Star, S4; empty circle, K4-S4; crossed circle, K20-S4; filled circle, K4K20-S4; empty rectangle, D4-S4; crossed rectangle, D20-S4; filled rectangle, D4D20-S4. (B) Star, S4(5–16); empty circle, S4(1–20); crossed circle, S4(1–16); filled circle, S4(1–12); empty rectangle, S4(5–28); crossed rectangle, S4(9–28); filled rectangle, S4(13–28). (C) Star, K4-S4(1–16); empty circle, K4-S4(1–16)a; filled circle, K4-S4(1–15)a; empty rectangle, K4-S4(1–13)a; filled rectangle, K4-S4(1–10)a.
Similarly, among the active derivatives from the set of C-terminal deletions, S4(1–20) and S4(1–16) were respectively 2- and 20-fold less active than dermaseptin S4, while S4(5–28) was 10-fold less active. All other deletions resulted in inactivity up to the highest concentration assayed (30 μM).
Among the last set of derivatives (set of substitution and deletion) K4-S4(1–16) was 50-fold less active than dermaseptin S4. Interestingly, however, amidation of the C-terminal carboxyl of K4-S4(1–16) resulted in an increase in potency of more than 10-fold. Further truncation of the C-terminal residue while preserving the C-terminal amide did not affect potency, since K4-S4(1–15)a was as active as K4-S4(1–16)a. Finally, K4-S4(1–13)a was two- to threefold more active than K4-S4(1–16), but K4-S4(1–10)a displayed no activity up to a 30 μM concentration.
Antimalarial activities against other malarial strains.
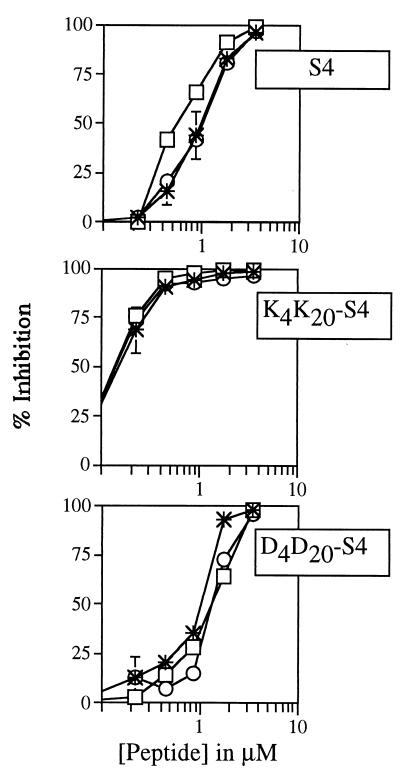
In order to detect possible strain-specific effects, the potencies of three dermaseptin peptides (S4, K4K20-S4 and D4D20-S4) were assessed simultaneously against three different parasite strains: the chloroquine-sensitive HB3, the partially resistant FCR3, and the chloroquine-resistant W2. No major strain specificity could be observed. The dose-response profiles obtained (depicted in Fig. 2) were practically identical.
FIG. 2.
Antimalarial activity of three dermaseptins on three different malarial strains. The experimental procedure was as described for Fig. 1. Star, FCR3 strain; circle, HB3 strain; rectangle, W2 strain.
Kinetic studies.
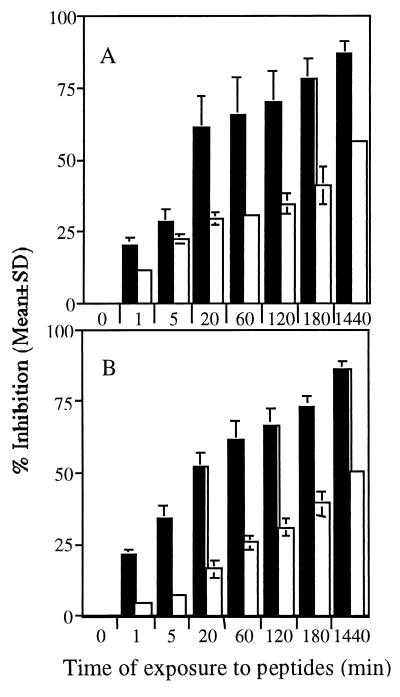
The time dependence of the antimalarial effect of two dermaseptin S4 derivatives was investigated using synchronized cultures that were essentially at the ring stage. Parasites were exposed either to K4K20-S4 or to K4-S4(1–13)a—at their respective IC50s—for various incubation periods. In addition, to verify a possible stage-dependent susceptibility to the peptides, the experiment was repeated using synchronized cultures at the trophozoite stage.
Both K4K20-S4 and K4-S4(1–13)a inhibited growth of the parasites more effectively at the trophozoite stage than at the ring stage, as shown in Fig. 3. Significant growth inhibition was observed after as little as 1 min of exposure to these peptides and proceeded with nearly linear kinetics, whereas the growth of ring stage parasites was inhibited by 50% only after overnight exposure, and the growth inhibition of trophozoites exceeded 50% after less than 20 min of exposure and reached >80% inhibition after overnight exposure. While the difference in inhibition rates may simply reflect the fact that trophozoites are more sensitive to the peptides' effect, the mutual progressive aspect of the inhibition may be the consequence of the gradual loss of the parasites' viability due to membrane permeabilization and the ensuing leakage of essential solutes. This may also be indicative of the peptides' ability to transfer from one cell to another and affect it, as previously observed (16).
FIG. 3.
Time and stage dependence of the antimalarial effect of K4K20-S4 (A) and K4-S4(1–13)a (B). Parasites at the ring (white columns) or trophozoite (black columns) stage were exposed to either K4K20-S4 or K4-S4(1–13)a at their respective IC50s for the indicated time periods. At the end of the incubation, the peptide was washed off, and parasites returned to culture conditions with [3H]hypoxanthine. Parasite-associated radioactivity was measured after 6 h and compared to the control.
Hemolytic activity versus antimalarial activity.
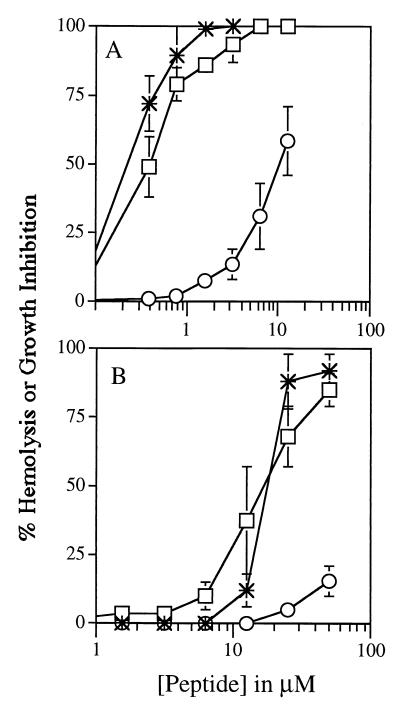
To probe the mechanism of antimalarial activity, K4K20-S4 and K4-S4(1–13)a were investigated simultaneously for their ability to induce hemolysis and to inhibit growth of infected cells. Infected cells at the young trophozoite stage were separated from normal RBC using a Percoll gradient. Infected (90% parasitemia) and noninfected cells were resuspended in culture medium containing increasing amounts of dermaseptins. After 2 h of incubation, [3H]hypoxanthine was added to one set of infected cultures, and cells were harvested 4 h later. Hemolysis was determined in two additional sets of cultured infected and normal RBC by measuring the specific absorptions of hemoglobin in the supernatants after 2 and 6 h of exposure to peptides.
As shown in Fig. 4, both K4K20-S4 (panels A) and K4-S4(1–13)a (panels B) selectively lysed infected RBC while having a weaker effect on noninfected RBC after 2 h of exposure. Exposure to peptides for 4 h yielded a slightly increased effect that paralleled the results for 2 h (not shown). Thus, trophozoites were >30-fold more susceptible than normal RBC to the lytic effect of K4K20-S4. The same trend was observed with K4-S4(1–13)a.
FIG. 4.
Hemolytic and growth inhibition activities of K4K20-S4 (A) and K4-S4(1–13)a (B). Infected cells at the trophozoite stage were isolated by Percoll gradient and resuspended in culture medium containing increasing amounts of dermaseptins for 2 h of incubation. To assess lytic activity, cells were pelleted, and the absorbance of hemoglobin in the supernatant was determined by absorption spectroscopy (405 nm). Full lysis was obtained by lysing the same number of cells in an equal volume of distilled water. To assess parasite growth, [3H]hypoxanthine was added after 2 h of exposure to peptides. Parasite-associated radioactivity was measured after 4 h of incubation. Stars, inhibition of trophozoites growth; rectangles, lysis of trophozoite-infected RBC; circles, lysis of normal RBC.
As is also shown in Fig. 4, an excellent correlation was found between the concentrations of K4K20-S4 that inhibit parasite proliferation and those that induce lysis of infected cells. Moreover, the data suggested that K4-S4(1–13)a is more selective than K4K20-S4, although both peptides fully inhibited parasite growth at concentrations at which no substantial hemolysis of uninfected cells could be observed.
d versus l isomers.
To verify the possibility that antimalarial activity may depend upon specific interactions of the dermaseptins with a chiral molecule (receptor, enzyme, etc.), the d-amino acid isomers of K4K20-S4 and K4-S4(1–13)a were assayed in parallel to their l-amino acid counterparts.
The d isomers of K4K20-S4 and K4-S4(1–13)a displayed a profile identical to that shown in Fig. 4 with respect to their ability to induce lysis of normal or infected cells or inhibition of parasite growth. This indicated that both activities are not mediated by specific peptide interaction with a chiral center.
Confocal microscopy.
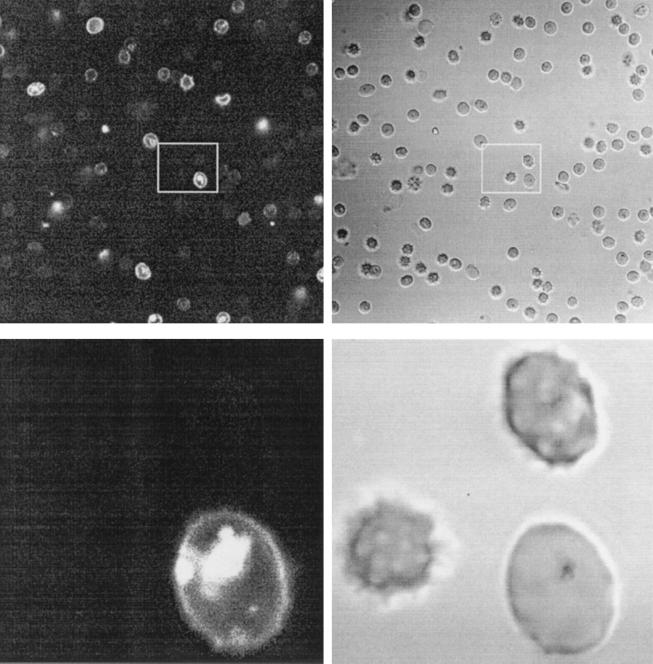
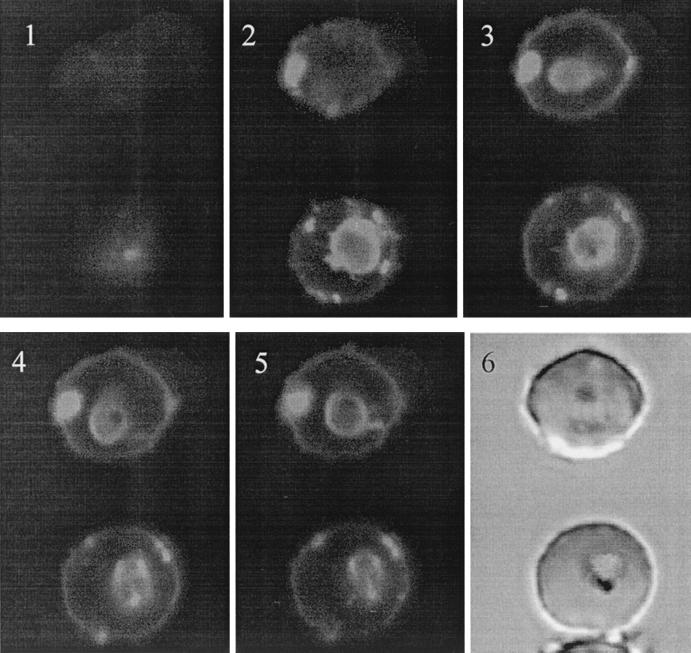
Towards the visualization of the peptides' interaction with the intracellular parasite, trophozoites were exposed to rhodaminated K4-S4(1–13)a and analyzed under a confocal fluorescence microscope. Figure 5 shows both low- and high-magnification images of a microscope field displaying a typical labeling pattern after a brief exposure to the peptide. A Z series of slices of two labeled infected cells are shown in Fig. 6. These pictures show that in infected cells, parasites are clearly labeled by the rhodaminated peptide. The peptide is not seen in the host cell cytosol, probably due to collisional quenching of the fluorescence by hemoglobin. Control cells analyzed under similar conditions after treatment with free lissamine rhodamine mixed with unlabeled peptide (1:1) did not label the cells (not shown). These results indicated that K4-S4(1–13)a is able to find its way through the complex series of host cell and parasite membranes and interact directly with the intracellular parasite. This also suggested that the peptide may affect the parasite's viability by disrupting its plasma membrane integrity, which is believed to be the general mechanism of antimicrobial action of such peptides.
FIG. 5.
Fluorescence microscopy of a treated malaria sample. Parasite cultures at the young trophozoite stage were exposed to rhodaminated K4-S4(1–13)a (1 μM; 1 to 2 min; RT), washed twice in culture medium, and observed unfixed under a microscope. Images were taken within 5 min. The upper left picture is an optical section (rhodamine filter) of a field of treated RBC. The upper right picture is the light transmission of the same field. Each white rectangle defines the zone enlarged, shown in the corresponding lower pictures.
FIG. 6.
Fluorescence confocal microscopy analysis of two labeled infected RBCs. Parasite cultures at the young trophozoite stage were exposed to rhodaminated K4-S4(1–13)a (1 μM; 1 to 2 min; RT), washed twice in culture medium, and observed unfixed under the microscope. Images were taken within 5 min. Images 1 to 5, Z series images (rhodamine filter) taken with 1-μm steps between each focal plane. Image 6, light transmission image.
Dissipation of the parasite plasma membrane potential.
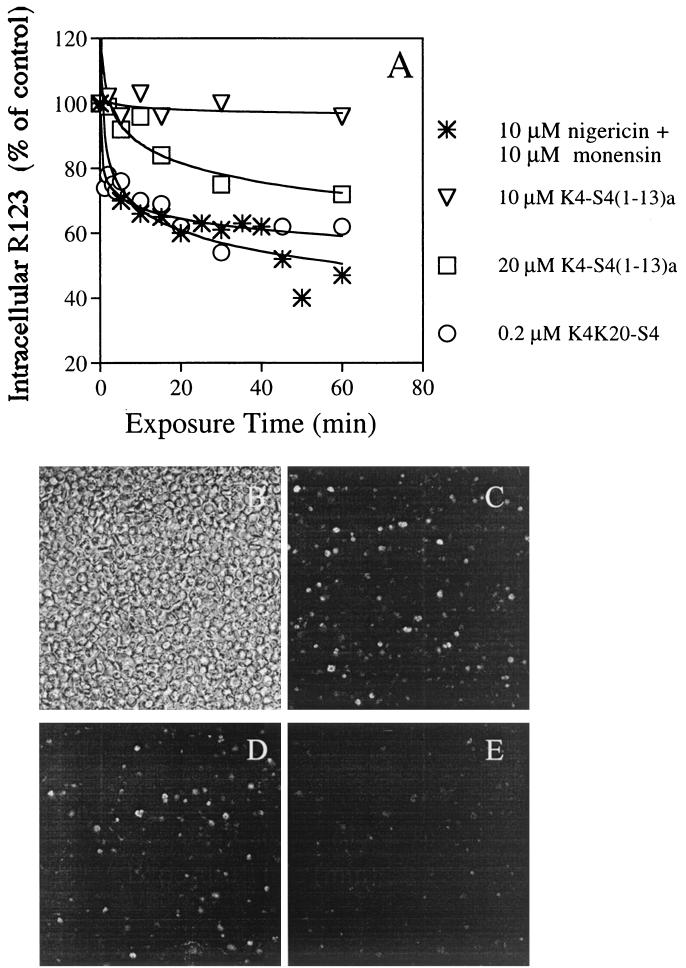
To assess whether the peptides can gain access to the parasite membrane, the dissipation of R123 fluorescence was monitored. It was first ascertained that fluorescence measurement does indeed dissipate Δϕ, as measured by reduced accumulation of the dye, using the ionophores nigericin and monensin (Fig. 7A). Then it was found that both K4-S4(1–13)a and K4K20-S4 depolarize the parasite membrane in a dose- and time-dependent manner. These results were obtained under conditions at which hemolysis that was measured in parallel was minimal (data not shown). These results clearly demonstrate that the peptides can cross the host cell membrane and interact with the parasite plasma membrane to permeabilize it.
FIG. 7.
Dissipation of the membrane potential resulting in leakage of R123 from infected cells, as analyzed using fluorescence spectroscopy (A) and confocal microscopy (B to E). (A) Trophozoites (0.5% hematocrit) that were preincubated with R123 were exposed to peptides or to a mixture of nigericin and monensin to dissipate the ion gradients across membranes. Samples—taken at the indicated time intervals—were washed and resuspended in PBS, and their fluorescence was read (λex = 530 nm; λem = 585 nm). Relative fluorescence (as a percentage of that of the untreated control at the same time) was plotted against the time of incubation. Panel B shows the light transmission image of a microscope field of the R123 treated infected cells. Panels C, D, and E show single optical sections (rhodamine filter) of the same field, 1 to 2 min after addition of 0, 10, and 20 μM K4-S4(1–13)a, respectively.
Observation of the treated cells under a confocal microscope confirmed the results obtained by fluorescence spectroscopy. As shown in Fig. 7B to E, addition of 10 to 20 μM K4-S4(1–13)a resulted in a progressive yet rapid reduction of the number of fluorescent cells.
DISCUSSION
The development of new antimalarial drugs is presently an urgent goal, since the available armamentarium of drugs is rapidly dwindling due to the evolution of drug resistance. In developing new drugs, one should aim at compounds that specifically and selectively affect the parasite while having minimal toxicity to the host. Peptide drugs are currently being actively developed and tested against various infectious diseases. There is no reason why antimalarial peptides could not be developed as well. In this respect, native dermaseptins may be regarded as lead compounds whose structure we attempted to modulate in order to optimize their action. Moreover, the fact that intravenous injection of high doses (up to 10 mg/kg of body weight) of the 13-residue dermaseptin, K4-S4(1–13), seems to be well tolerated by rats (unpublished results) suggests grounds for some optimism as to their potential toxicity in vivo.
Native dermaseptin peptides were recently shown to exert antimalarial activity. In this study, we attempted to understand the mechanism(s) underlying this activity. Interestingly, export and targeting of parasite proteins is reminiscent in some functional details of that of gram-negative bacteria, with which some proteins are retained in the periplasmic space, some are inserted into the outer membrane, and others are exported outside the cell altogether (9). Bacteria are extremely sensitive to dermaseptins (46). While it is surmised that dermaseptins act by lysing the bacterial membrane(s), the first question that comes to mind is, could dermaseptin induce parasites' death by lysing their membranes?
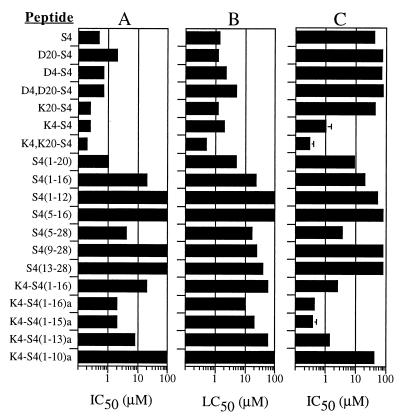
It appears from the reported results that antimalarial activity of dermaseptins obeys many of the rules governing their ability to disrupt biological membranes (these rules are discussed extensively in reference 12). This type of interaction is believed to be acutely influenced by the respective charges and amphipathies of the reactants. In fact, a comparison drawn between the peptides' ability to inhibit growth of malaria parasites and their ability to lyse RBC (Fig. 8A and B, respectively) demonstrates a remarkable parallelism in the way each modification affects both activities. Thus, S4 and all substitution derivatives are the most active, successive truncation of four residues from the C terminus leads to a gradual loss of activity, all N-terminal deletions result in inactivity except for S4(5–28), which retains partial activity, amidation of the C-terminal carboxyl increases potency of K4-S4(1–16), and successive C-terminal deletions of the substituted short derivatives gradually lead to inactivity.
FIG. 8.
Potency of dermaseptin S4 and various derivatives in inducing inhibition of growth of P. falciparum-infected RBC (A) (from this study) compared with their ability to induce hemolysis of normal RBC (assessed in PBS) and growth inhibition of Escherichia coli (B and C, respectively) (from reference 12). LC50 is the lowest peptide concentration that induced 50% lysis of erythrocytes.
Additional support of this view can be drawn from the results obtained with the kinetics experiments and the dissipation of the membrane potential experiments as well as from the fact that activity is rapid and is independent of a chiral center.
The parallelism in activity may furthermore be extended to the peptides' antibacterial activity (Fig. 8C), with the exception of five peptides (native S4, D4-S4, D20-S4, D4D20-S4, and K20-S4) that display high hemolytic and antimalarial activities but weak to no antibacterial activities. However, this may be due to the peptides' high aggregation state in solution, which was demonstrated to hamper their action against gram-negative bacteria. Indeed, peptides whose aggregation state was reduced (such as K4-S4, K4K20-S4, and all the substitution and deletion derivatives) recovered good to excellent antibacterial activity (12).
Thus, despite the fact that the parasite's membrane is well hidden within its host cell, we propose that the mode of antimalarial activity of these peptides might be based on selective membrane disruption. We speculate that due to differences in membrane composition, dermaseptins have a higher affinity to the membranes of infected RBC than to those of normal RBC (hence the higher hemolysis of infected cells) but have a still higher affinity to the parasite's membrane (hence the labeling of intracellular parasites in nonlysed infected RBC). Thus, when dermaseptin binds the membrane of a hosting RBC, the peptide somehow is able to transfer to the parasitic membrane in an affinity-driven manner and exerts its membrane-lytic activity upon the pathogen. How could such a transfer physically occur?
During their pathogenic stage, malaria parasites thrive and propagate inside the RBC of their host (15, 26). In correlation with parasite development, new permeability pathways (NPP) appear in the membrane of the host RBC. A wide variety of solutes, including sugars, polyols, amino acids, anions, cations, pyrimidines, and purines, as well as some peptides (44), penetrate rapidly into malaria-infected RBC through NPP which are induced by the parasite in the membrane of the host RBC. Since the evolution of the NPP is stage dependent, it could explain the greater sensitivity of the trophozoite stage compared to that of the ring stage. As the parasite is completely engulfed within a parasitophorous vacuole membrane (PVM), solutes which leave or enter the parasite must therefore traverse three membranes: that of the host RBC, the PVM, and the parasite membrane. An alternative (or parallel) pathway that has recently received attention and some (though controversial) experimental support consists of either a juxtaposition of the host RBC and the PVM or an extension of these two membranes in the form of a duct, which provides a “metabolic window” or direct access of the parasite to the extracellular milieu (30, 43). A tubovesicular membrane network extending from the PVM with Golgi-like properties has been described (10) and suggested as serving as an intermediary compartment for exported parasite proteins. It must, however, be stressed that the tubovesicular membrane-PVM system is absent from the ring stage, thus casting doubts about the functionality of this pathway in mediating the uptake of peptides.
From this description, it seems that dermaseptins could gain access to the parasite in malaria-infected cells. Experimental evidence for this was provided in the present study, using confocal microscopy analysis of labeled dermaseptins. The data showed that in infected cells, the labeled peptide reached and concentrated in internal compartments (parasite's membrane?). The resulting dissipation of potential is consistent with the view that direct peptide interaction damaged the parasite's membrane and hence parasite viability, as evidenced by the incorporation of hypoxanthine. Nevertheless, the differential distribution of rhodaminated peptides may also be due to an experimental artifact: the fluorescence may be collisionally quenched by hemoglobin, which is present only in the host cell compartment.
In conclusion, this study has identified potent antimalarial peptides of various compositions that may be useful in the design of new antimalarial drugs. In addition, this study provided experimental data—including direct and indirect evidence—that support the view that antimalarial activity of dermaseptin-based peptides proceeds via direct interaction between the peptide and the intracellular parasite. Future experiments will attempt to verify the hypothesis by exploring whether the driving force enabling the direct interaction is affinity based or not, namely by measuring the peptides binding to isolated parasites.
ACKNOWLEDGMENTS
This work was supported by the Israel Science Foundation, founded by the Academy of Sciences and Humanities—DOROT Science Fellowship Foundation.
The expert assistance of Naomi Melamed-Book and Josephina Silfen (Hebrew University of Jerusalem) in confocal microscopy and peptide synthesis, respectively, is gratefully acknowledged.
REFERENCES
- 1.Aikawa M. Variations in structure and function during the life cycle of malaria parasites. Bull W H O. 1977;55:139–150. [PMC free article] [PubMed] [Google Scholar]
- 2.Allred D, Gruenberg J, Sherman I W. Dynamic rearrangements of erythrocyte membrane internal architecture induced by infection with Plasmodium falciparum. J Cell Sci. 1986;81:1–16. doi: 10.1242/jcs.81.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Bessalle R, Gorea A, Shalit I, Metzger J W, Dass C, Desidero D M, Fridkin M. Structure-function study of amphiphilic peptides. J Med Chem. 1993;36:1203–1209. doi: 10.1021/jm00061a011. [DOI] [PubMed] [Google Scholar]
- 4.Blondelle S E, Houghten A H. Design of model peptides having potent antimicrobial activities. Biochemistry. 1992;31:12688–12694. doi: 10.1021/bi00165a020. [DOI] [PubMed] [Google Scholar]
- 5.Boman H G. Peptide antibiotics and their role in innate immunity. Annu Rev Immunol. 1995;13:61–92. doi: 10.1146/annurev.iy.13.040195.000425. [DOI] [PubMed] [Google Scholar]
- 6.Cabantchik Z. Properties of permeation pathways induced in the human red cell membrane by malaria parasites. Blood. 1989;74:1464–1471. [PubMed] [Google Scholar]
- 7.Coot P J, Holyoak C D, Bracey D, Ferdinando D P, Pearce J A. Inhibitory action of the amphibian skin peptide dermaseptin S3 on S. cerevisiae. Antimicrob Agents Chemother. 1998;42:2160–2170. doi: 10.1128/aac.42.9.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Lucca A J, Bland J M, Jacks T J, Grimm C, Walsh T J. Fungicidal and binding properties of the natural peptides cecropin B and dermaseptin S1. Med Mycol. 1998;36:291–298. [PubMed] [Google Scholar]
- 9.Duong F, Eichler J, Price A, Leonard M R, Wickner W. Biogenesis of the gram-negative bacterial envelope. Cell. 1997;91:567–573. doi: 10.1016/s0092-8674(00)80444-4. [DOI] [PubMed] [Google Scholar]
- 10.Elmendorf H G, Haldar K. Plasmodium falciparum exports the golgi marker sphingomyelin synthase into a tubovesicular network in the cytoplasm of mature erythrocytes. J Cell Biol. 1994;124:449–462. doi: 10.1083/jcb.124.4.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Epand R M, Shai Y, Segrest J P, Anantharamaiah G M. Mechanisms for the modulation of membrane bilayer properties by amphipathic helical peptides. Biopolymers. 1995;37:319–338. doi: 10.1002/bip.360370504. [DOI] [PubMed] [Google Scholar]
- 12.Feder R, Dagan A, Mor A. S.A.R. study of antimicrobial dermaseptin S4 showing the consequences of peptide oligomerization on selective cytotoxicity. J Biol Chem. 2000;275:4230–4238. doi: 10.1074/jbc.275.6.4230. [DOI] [PubMed] [Google Scholar]
- 13.Foley M, Tilley L. Home improvements: malaria and the red blood cell. Parasitol Today. 1995;11:436–439. doi: 10.1016/0169-4758(95)80032-8. [DOI] [PubMed] [Google Scholar]
- 14.Frohlich D R, Wells A W. Peptide amphipathy: a new strategy in design of potential insecticides. Int J Pept Protein Res. 1991;37:2–6. doi: 10.1111/j.1399-3011.1991.tb00725.x. [DOI] [PubMed] [Google Scholar]
- 15.Gero A M, Kirk K. Nutrient transport pathways in Plasmodium-infected erythrocytes: what and where are they? Parasitol Today. 1994;10:395–399. doi: 10.1016/0169-4758(94)90231-3. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh J K, Shaool D, Guillaud P, Ciceron L, Mazier D, Kustanovich I, Shay Y, Mor A. Selective cytotoxicity of dermaseptin S3 toward intraerythrocytic Plasmodium falciparium and the underlying molecular basis. J Biol Chem. 1997;267:6502–6509. doi: 10.1074/jbc.272.50.31609. [DOI] [PubMed] [Google Scholar]
- 17.Ginsburg H. Transport pathways in the malaria-infected erythrocyte—their characterization and their use as potential targets for chemotherapy. Biochem Pharmacol. 1994;48:1847–1856. doi: 10.1016/0006-2952(94)90582-7. [DOI] [PubMed] [Google Scholar]
- 18.Hancock R E W. Peptide antibiotics. Lancet. 1997;349:418–422. doi: 10.1016/S0140-6736(97)80051-7. [DOI] [PubMed] [Google Scholar]
- 19.Hernandez C, Mor A, Dagger F, Nicolas P, Hernandez A, Benedetti E L, Dunia I. Functional and structural damages in Leishmania mexicana exposed to the cationic peptide dermaseptin. Eur J Cell Biol. 1992;59:414–424. [PubMed] [Google Scholar]
- 20.Hommel M. Pathophysiology of clinical symptoms of malaria—role of cytokines, cytoadherence and premunition. Presse Med. 1996;25:70–76. [PubMed] [Google Scholar]
- 21.Howard R J. Alterations in the surface membrane of red blood cells during malaria. Immunol Rev. 1982;61:67–107. doi: 10.1111/j.1600-065x.1982.tb00374.x. [DOI] [PubMed] [Google Scholar]
- 22.Hsiao L L, Howard R J, Aikawa M, Taraschi T F. Modification of host cell membrane lipid composition by the intra-erythrocytic human malaria parasite Plasmodium falciparum. Biochem J. 1991;274:121–132. doi: 10.1042/bj2740121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensen J B. Concentration from continuous culture of erythrocytes infected with trophozoites and schizonts of Plasmodium falciparum. Am J Trop Med Hyg. 1978;27:1274–1276. doi: 10.4269/ajtmh.1978.27.1274. [DOI] [PubMed] [Google Scholar]
- 24.Johnson D, Gunther K, Ansorge I, Benting J, Kent A, Bannister L, Ridley R, Lingelbach K. Characterization of membrane proteins exported from Plasmodium falciparum into the host erythrocyte. Parasitology. 1994;109:1–9. doi: 10.1017/s0031182000077696. [DOI] [PubMed] [Google Scholar]
- 25.Jouenne T, Mor A, Bonato H, Junter G A. Antibacterial activity of synthetic dermaseptins against growing and non-growing E. coli cultures. J Antimicrob Chemother. 1998;42:87–90. doi: 10.1093/jac/42.1.87. [DOI] [PubMed] [Google Scholar]
- 26.Kirk K, Horner H A, Elford B C, Ellory J C, Newbold C I. Transport of diverse substrates into malaria-infected erythrocytes via a pathway showing functional characteristics of a chloride channel. J Biol Chem. 1994;269:3339–3347. [PubMed] [Google Scholar]
- 27.Knapp B, Hundt E, Lingelbach K R. Structure and possible function of Plasmodium falciparum proteins exported to the erythrocyte membrane. Parasitol Res. 1991;77:277–282. doi: 10.1007/BF00930901. [DOI] [PubMed] [Google Scholar]
- 28.Krugliak M, Ginsburg H. Studies on the antimalarial mode of action of quinoline-containing drugs—time-dependence and irreversibility of drug action, and interactions with compounds that alter the function of the parasite's food vacuole. Life Sci. 1991;49:1213–1219. doi: 10.1016/0024-3205(91)90133-v. [DOI] [PubMed] [Google Scholar]
- 29.Lambros C, Vanderberg J. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979;65:418–420. [PubMed] [Google Scholar]
- 30.Loyevsky M, Lytton S D, Mester B, Libman J, Shanzer A, Cabantchik Z I. The antimalarial action of desferal involves a direct access route to erythrocytic (Plasmodium falciparum) parasites. J Clin Investig. 1993;91:218–224. doi: 10.1172/JCI116174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsuzaki K, Sugishita K, Fujii N, Miyajima K. Molecular basis for membrane selectivity of an antimicrobial peptide, magainin 2. Biochemistry. 1995;34:3423–3429. doi: 10.1021/bi00010a034. [DOI] [PubMed] [Google Scholar]
- 32.Mor A, Nicolas P. Isolation and structure of novel vertebrate defensive peptides from frog skin. Eur J Biochem. 1994;219:145–154. doi: 10.1111/j.1432-1033.1994.tb19924.x. [DOI] [PubMed] [Google Scholar]
- 33.Mor A, Nicolas P. The NH2-terminal helical domain 1-18 of dermaseptin is responsible for antimicrobial activity. J Biol Chem. 1994;269:1934–1939. [PubMed] [Google Scholar]
- 34.Mor A, Amiche M, Nicolas P. Isolation, synthesis and activity of dermaseptin b, a novel vertebrate defensive peptide from frog skin: relationship with adenoregulin. Biochemistry. 1994;33:6642–6650. doi: 10.1021/bi00187a034. [DOI] [PubMed] [Google Scholar]
- 35.Mor A, Hani K, Nicolas P. The vertebrate peptide antibiotics dermaseptins have overlapping structural features but target specific microorganisms. J Biol Chem. 1994;269:31635–31641. [PubMed] [Google Scholar]
- 36.Mor A, Nguyen V H, Delfour A, Migliore S D, Nicolas P. Isolation, amino acid sequence and synthesis of dermaseptin, a novel antimicrobial peptide of amphibian skin. Biochemistry. 1991;3:8824–8830. doi: 10.1021/bi00100a014. [DOI] [PubMed] [Google Scholar]
- 37.Nash G, O'Brien E, Gordon-Smith E, Dormandy J. Abnormalities in the mechanical properties of red blood cells caused by Plasmodium falciparum. Blood. 1989;74:855–861. [PubMed] [Google Scholar]
- 38.Nash G B, Gratzer W B. Structural determinants of the rigidity of the red cell membrane. Biorheology. 1993;30:397–407. doi: 10.3233/bir-1993-305-611. [DOI] [PubMed] [Google Scholar]
- 39.Nicolas P, Mor A. Peptides as weapons against microorganisms in the chemical defense system of vertebrates. Annu Rev Microbiol. 1995;4:277–304. doi: 10.1146/annurev.mi.49.100195.001425. [DOI] [PubMed] [Google Scholar]
- 40.Pasloske B L, Howard R J. Malaria, the red cell, and the endothelium. Annu Rev Med. 1994;45:283–295. doi: 10.1146/annurev.med.45.1.283. [DOI] [PubMed] [Google Scholar]
- 41.Paulitschke M, Nash G B. Membrane rigidity of red blood cells parasitized by different strains of Plasmodium falciparum. J Lab Clin Med. 1993;122:581–589. [PubMed] [Google Scholar]
- 42.Pouny Y, Rapaport D, Mor A, Nicolas P, Shai Y. Interaction of antimicrobial dermaseptin and its fluorescently labeled analogues with phospholipid membranes. Biochemistry. 1992;31:12416–12423. doi: 10.1021/bi00164a017. [DOI] [PubMed] [Google Scholar]
- 43.Pouvelle B, Gormley J A, Taraschi T F. Characterization of trafficking pathways and membrane genesis in malaria-infected erythrocytes. Mol Biochem Parasitol. 1994;66:83–96. doi: 10.1016/0166-6851(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 44.Pouvelle B, Spiegel R, Hsiao L, Howard R J, Morris R L, Thomas A P, Taraschi T F. Direct access to serum macromolecules by intraerythrocytic malaria parasites. Nature. 1991;353:73–75. doi: 10.1038/353073a0. [DOI] [PubMed] [Google Scholar]
- 45.Roberts D J, Biggs B A, Brown G, Newbold C I. Protection, pathogenesis and phenotypic plasticity in Plasmodium falciparum malaria. Parasitol Today. 1993;9:281–286. doi: 10.1016/0169-4758(93)90121-u. [DOI] [PubMed] [Google Scholar]
- 46.Shai Y. Molecular recognition between membrane-spanning polypeptides. Trends Biochem Sci. 1995;20:460–464. doi: 10.1016/s0968-0004(00)89101-x. [DOI] [PubMed] [Google Scholar]
- 47.Shalmiev G, Ginsburg H. The susceptibility of the malarial parasite Plasmodium falciparum to quinoline-containing drugs is correlated to the lipid composition of the infected erythrocyte membranes. Biochem Pharmacol. 1993;46:365–374. doi: 10.1016/0006-2952(93)90511-t. [DOI] [PubMed] [Google Scholar]
- 48.Sherman I. Membrane structure and function of malaria parasites and the infected erythrocyte. Parasitology. 1985;91:606–645. doi: 10.1017/s0031182000062843. [DOI] [PubMed] [Google Scholar]
- 49.Sherman I W, Greenan J. Altered red cell membrane fluidity during schizogonic development of malarial parasites (Plasmodium falciparum and P. lophurae) Trans R Soc Trop Med Hyg. 1984;78:641–644. doi: 10.1016/0035-9203(84)90227-x. [DOI] [PubMed] [Google Scholar]
- 50.Sherman I W, Crandall I, Smith H. Membrane proteins involved in the adherence of Plasmodium falciparum-infected erythrocytes to the endothelium. Biol Cell. 1992;74:161–178. doi: 10.1016/0248-4900(92)90022-s. [DOI] [PubMed] [Google Scholar]
- 51.Simões A, Moll G, Slotboom A, Roelofsen B, Op den Kamp J. Selective internalization of choline-phospholipids in Plasmodium falciparum parasitized human erythrocytes. Biochim Biophys Acta. 1991;1063:45–50. doi: 10.1016/0005-2736(91)90351-8. [DOI] [PubMed] [Google Scholar]
- 52.Simões A P, Vandenberg J J, Roelofsen B, Opdenkamp J A. Lipid peroxidation in Plasmodium falciparum-parasitized human erythrocytes. Arch Biochem Biophys. 1992;298:651–657. doi: 10.1016/0003-9861(92)90462-6. [DOI] [PubMed] [Google Scholar]
- 53.Strahilevitz J, Mor A, Nicolas P, Shai Y. Spectrum of antimicrobial activity and assembly of dermaseptin-b and its precursor form in phospholipid membranes. Biochemistry. 1994;33:10951–10960. doi: 10.1021/bi00202a014. [DOI] [PubMed] [Google Scholar]
- 54.Tanabe K, Izumo A, Kato M, Miki A, Doi S. Stage-dependent inhibition of Plasmodium falciparum by potent Ca2+ and calmodulin modulators. J Protozool. 1989;36:139–143. doi: 10.1111/j.1550-7408.1989.tb01060.x. [DOI] [PubMed] [Google Scholar]
- 55.Taraschi T, Parashar A, Hooks M, Rubin H. Perturbation of red cell membrane structure during intracellular maturation of Plasmodium falciparum. Science. 1986;232:102–104. doi: 10.1126/science.3006251. [DOI] [PubMed] [Google Scholar]
- 56.Van der Schaft P H, Beaumelle B, Vial H, Roelofsen B, Op den Kamp J A F, Van Deenen L L M. Phospholipid organization in monkey erythrocytes upon Plasmodium knowlesi infection. Biochim Biophys Acta. 1987;901:1–14. doi: 10.1016/0005-2736(87)90250-1. [DOI] [PubMed] [Google Scholar]