Abstract
Helicobacter pylori infection in humans is associated with chronic type B gastritis, peptic ulcer disease, and gastric carcinoma. A high intake of carotenoids and vitamin C has been proposed to prevent development of gastric malignancies. The aim of this study was to explore if the microalga Haematococcus pluvialis rich in the carotenoid astaxanthin and vitamin C can inhibit experimental H. pylori infection in a BALB/cA mouse model. Six-week-old BALB/cA mice were infected with the mouse-passaged H. pylori strain 119/95. At 2 weeks postinoculation mice were treated orally once daily for 10 days (i) with different doses of algal meal rich in astaxanthin (0.4, 2, and 4 g/kg of body weight, with the astaxanthin content at 10, 50, and 100 mg/kg, respectively), (ii) with a control meal (algal meal without astaxanthin, 4 g/kg), or (iii) with vitamin C (400 mg/kg). Five mice from each group were sacrificed 1 day after the cessation of treatment, and the other five animals were sacrificed 10 days after the cessation of treatment. Culture of H. pylori and determination of the inflammation score of the gastric mucosae were used to determine the outcome of the treatment. Mice treated with astaxanthin-rich algal meal or vitamin C showed significantly lower colonization levels and lower inflammation scores than those of untreated or control-meal-treated animals at 1 day and 10 days after the cessation of treatment. Lipid peroxidation was significantly decreased in mice treated with the astaxanthin-rich algal meal and vitamin C compared with that of animals not treated or treated with the control meal. Both astaxanthin-rich algal meal and vitamin C showed an inhibitory effect on H. pylori growth in vitro. In conclusion, antioxidants may be a new strategy for treating H. pylori infection in humans.
The human gastric pathogen Helicobacter pylori causes type B chronic gastritis and is associated with peptic ulcer disease as well as gastric cancer (7, 9, 38). Some epidemiological studies have shown the difference between the prevalence of H. pylori-associated diseases and intake of certain vitamins and antioxidants in various populations (10, 28, 33) and that a high intake of vitamin C, α-tocopherol, or β-carotene in food reduces the risk of gastric carcinoma (2, 30). H. pylori infection in humans is characterized by a marked infiltration of neutrophilic leukocytes of the gastric mucosa, and the generation of reactive oxygen metabolites (ROMs) may play a part in the development of severe chronic type B gastritis (15, 26).
Astaxanthin is a carotenoid found in many different organisms in nature, but the main dietary sources for humans are found in crustaceans and other seafood, especially salmon. Astaxanthin is a powerful lipid-soluble antioxidant in vitro (8, 16, 19) and was shown to be most effective in stimulating immune defenses when different carotenoids were compared (12, 13, 23). Vitamin C is a water-soluble antioxidant required for many biological functions such as the normal synthesis of hormones, neurotransmitters, collagen, and carnitine and the absorption of iron and other substances (17, 27). Vitamin C is a dietary antioxidant which, at a normal physiologic concentration, scavenges ROMs to provide protection against oxidative DNA damage (6, 21). The aim of this study was to explore how the antioxidant astaxanthin from the alga Haematococcus pluvialis and vitamin C affect H. pylori infection in a BALB/cA mouse model.
MATERIALS AND METHODS
Chemicals.
Homogenized and dried cells of the unicellular green alga Haematococcus pluvialis rich in astaxanthin (2 to 3% [wt/wt]) and algal meal without astaxanthin as the control meal were obtained from AstaCarotene AB (Gustavsberg, Sweden). Vitamin C (l-ascorbic acid) was purchased from ICN Biomedicals Inc. (Lund, Sweden). Algal meal and control meal were suspended in distilled water and vitamin C was dissolved in distilled water just before use.
Bacterial strains.
The H. pylori mouse-passaged strain 119/95 was grown on GAB-Camp agar (Gc Agar Base; Becton Dickinson, Lund, Sweden) supplemented with 10% horse serum and incubated for 48 h at 37°C under microaerophilic conditions (35). The cells were harvested in phosphate-buffered saline (PBS), centrifuged at 2,800 × g for 10 min, and resuspended in PBS to a final concentration of 109 CFU/ml.
Animals.
Six- to eight-week-old conventional BALB/cA mice were used in this study (B&K Universal Company, Stockholm, Sweden). Mice were housed on a 12-h light–12-h dark schedule and fed with rat and mouse standard diet no. 2 (B&K Universal) (34) and water ad libitum.
Experimental design.
Sixty mice were inoculated orally through a feeding tube three times at 2-day intervals with 0.1 ml of an H. pylori suspension containing 109 CFU/ml, and 10 mice were inoculated with PBS as a negative control group. The H. pylori-inoculated mice were divided into six groups. Five groups were orally treated with three doses of algal meal rich in astaxanthin (0.4, 2, and 4 g/kg of body weight, in which the astaxanthin content was 10, 50, and 100 mg/kg, respectively), a control algal meal (4 g/kg), or vitamin C (400 mg/kg) once daily for 10 days at 2 weeks postinoculation (p.i.). The infected and normal uninfected control mice were given distilled water through a feeding tube. Half of the animals from each group were sacrificed 1 day after the cessation of treatment, and the other half were sacrificed 10 days after the cessation of treatment.
Mice were killed with carbon dioxide, and their stomachs were collected. The stomachs were opened through the longer curvature using sterile surgical instruments. One-third of the stomachs and duodena, covering all subtypes of mucosa, were used for histopathology. One-third of the stomachs were used for culturing H. pylori. The remaining part of the gastric tissue was used for determination of the astaxanthin concentration.
Culture.
One-third of stomach biopsies were placed in 0.5 ml of PBS in a 1.5-ml Eppendorf tube and homogenized with a Pellet Pestle Mixer from KEBO Laboratories (Lund, Sweden). The homogenized sample was serially diluted 10-fold. Each 0.1 ml of homogenate was plated on GAB-Camp agar and incubated at 37°C for 5 to 10 days under microaerophilic conditions (36). The H. pylori colonies were counted and calculated as log10 CFU per milliliter of homogenate. The presence of H. pylori on the culture plates was confirmed by urease, catalase, and oxidase testing, Gram staining, and PCR analysis with H. pylori urease primers (20).
Carotenoid and astaxanthin analysis.
Stomach samples were homogenized and extracted in acetone (25). The acetone extracts were pooled and mixed vigorously with cyclohexane (1:1) and approximately 200 to 400 μl of distilled water to obtain phase separation. The samples were then centrifuged, and the concentrations of carotenoids recovered in the hexane phase were determined by measuring the absorbency at 474 nm with a Spectronic 601 spectrophotometer (Milton Roy Co.). An extinction coefficient measured in micrograms per kilogram was used for calculations.
The carotenoid composition was determined by high-pressure liquid chromatography after evaporation of the cyclohexane extract to dryness with nitrogen and dissolving of the carotenoids in chloroform-methanol (2:1). The high-pressure liquid chromatography system (Merck-Hitachi) consisted of a model L6200A Intelligent pump, a model D-2000 injector, and a model L4200 visible light-UV detector set at a wavelength of 474 nm and with 0.1 absorbency unit at full scale. External and internal carotenoid standards (astaxanthin and canthaxanthin, 99% pure; Hoffman-La Roche Ltd., Hvidovre, Denmark) were employed to check the recovery of carotenoid during extraction and the reproducibility of the results of the analytical methods applied. All the solvents and chemicals used were of analytical grade and purchased from Merck, Darmstadt, Germany.
Histopathology.
Murine stomach tissues were fixed in 10% buffered formalin and embedded in paraffin, and 4-μm-thick sections were prepared and stained with hematoxylin and eosin by standard procedures. The degree of inflammation was scored in a blind manner on a scale of 0 to 3 for body, antrum, and duodenum (36).
Lipid peroxidation assay.
Mice stomach tissues were homogenized in 20 mM Tris-HCl, pH 7.4, to a concentration of 10% (wt/vol). Homogenate supernatants (200 μl) were tested for malondialdehyde (MDA) and 4-hydroxy-2(E)-nonenal with a lipid peroxidation assay kit from Calbiochem (Lund, Sweden). The colorimeters were measured at an absorbency at 586 nm, and tissue lipid peroxidation was calculated as micromoles per gram of tissue.
In vitro inhibition test.
MICs were determined as described elsewhere (22) such that each preculture containing 103 cells was plated onto GAB-Camp agar with or without various concentrations of algal meal (0.3125 to 20 mg/ml), with algal meal without astaxanthin (5 mg/ml), or with vitamin C (0.5 to 4 mg/ml). The surviving cells were counted on the agar as colonies, and the MIC was defined as the concentration leaving no survivors after 5 to 10 days of incubation under microaerophilic conditions. Ten strains of H. pylori were tested, and MICs were shown as a range.
Statistical analysis.
The Mann-Whitney U test was used for analysis of colonization and inflammation distribution. The level of significance was chosen to be a P of <0.05.
RESULTS
Total carotenoid and astaxanthin analysis.
The mouse stomachs showed correspondingly high total carotenoid and astaxanthin contents when they were treated with various concentrations of astaxanthin (Table 1). Significant differences were noted between the treated and untreated group, especially for animals just posttreatment. Mice treated with the highest dose of astaxanthin demonstrated a higher astaxanthin content in their stomachs than those of the animals treated with lower doses.
TABLE 1.
Concentrations of total carotenoids and astaxanthin in mouse stomachs
| Treatment group (concn of astaxanthin [mg/kg]) | Mean concn ± SD (μg/kg)
|
|||
|---|---|---|---|---|
| 1 day after cessation of treatment
|
10 days after cessation of treatment
|
|||
| Total carotenoid | Astaxanthin | Total carotenoid | Astaxanthin | |
| Normal | 58 ± 8 | 0 | 51 ± 3 | 0 |
| H. pylori | 98 ± 28 | 0 | 58 ± 5 | 0 |
| H. pylori + algal meal (100) | 5,806 ± 1,162 | 516 ± 192 | 164 ± 34 | 49 ± 10a |
| H. pylori + algal meal (50) | 5,225 ± 1,344 | 344 ± 101 | 107 ± 13 | 26 ± 3 |
| H. pylori + algal meal (10) | 2,926 ± 668 | 120 ± 29b | 116 ± 34 | 34 ± 15 |
P < 0.05 versus results from the group treated with 50 mg of astaxanthin per kg.
P < 0.05 versus results from the groups treated with 100 and 50 mg of astaxanthin per kg.
Culture.
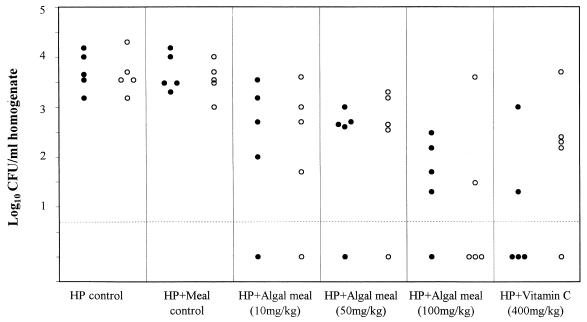
All noninoculated mice were H. pylori negative in culture. Both astaxanthin-rich algal meal (dose from 10 to 100 mg/kg) and vitamin C significantly reduced the number of H. pylori organisms in gastric tissue 1 day after the cessation of treatment (3.5 weeks p.i.) compared with the numbers recoverable from the untreated mice and the control mice treated with meal lacking astaxanthin (P < 0.05) (Fig. 1). At 10 days after the cessation of treatment (5 weeks p.i.), the numbers of H. pylori organisms in the groups treated with astaxanthin-rich algal meal and vitamin C were again significantly lower than the numbers in the groups not treated or treated with the control meal (P < 0.05) (Fig. 1). However, the astaxanthin-rich algal meal (100 mg/kg) and vitamin C (400 mg/kg) treatment groups had more numbers of H. pylori-negative animals (40%) than the astaxanthin-rich algal meal (10 and 50 mg/kg) treatment groups (20%). There were no significant differences among the groups treated with three doses of astaxanthin-rich algal meal and vitamin C.
FIG. 1.
Effects of different doses of astaxanthin-rich algal meal or vitamin C on the recovery of H. pylori from BALB/cA mice. Treatment was started 2 weeks p.i., and samples were taken 3.5 (●) and 5 (○) weeks p.i. Each dot or circle represents the bacterial count from one animal, and the dashed line indicates the limit of detection. HP, H. pylori.
Histopathology.
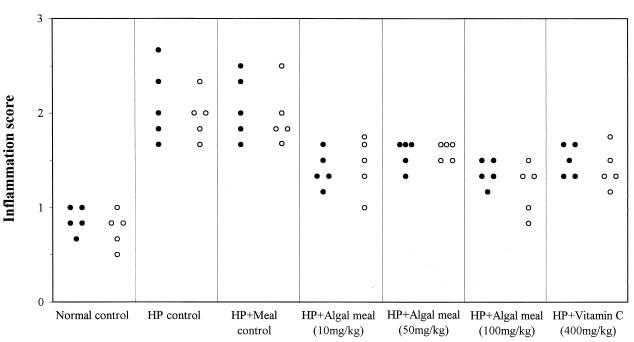
Mice treated with astaxanthin-rich algal meal or vitamin C showed significantly lower inflammation scores than control mice infected with H. pylori or treated with meal lacking astaxanthin 1 day and 10 days after the cessation of treatment (P < 0.05) (Fig. 2). The control-meal-treated mice developed gastritis as severe as that of the untreated control animals, and their inflammation scores were significantly higher than those of the non-H. pylori-inoculated mice (P < 0.01). The mice treated with the highest dose of astaxanthin (100 mg/kg) in algal meal showed significantly lower inflammation scores than the mice treated with 50 mg of astaxanthin per kg (P < 0.05).
FIG. 2.
Inflammation scores for gastritis in BALB/cA mice treated with astaxanthin-rich algal meal and vitamin C at 3.5 (●) and 5 (○) weeks p.i. Each dot or circle represents the inflammation score from one animal. HP, H. pylori.
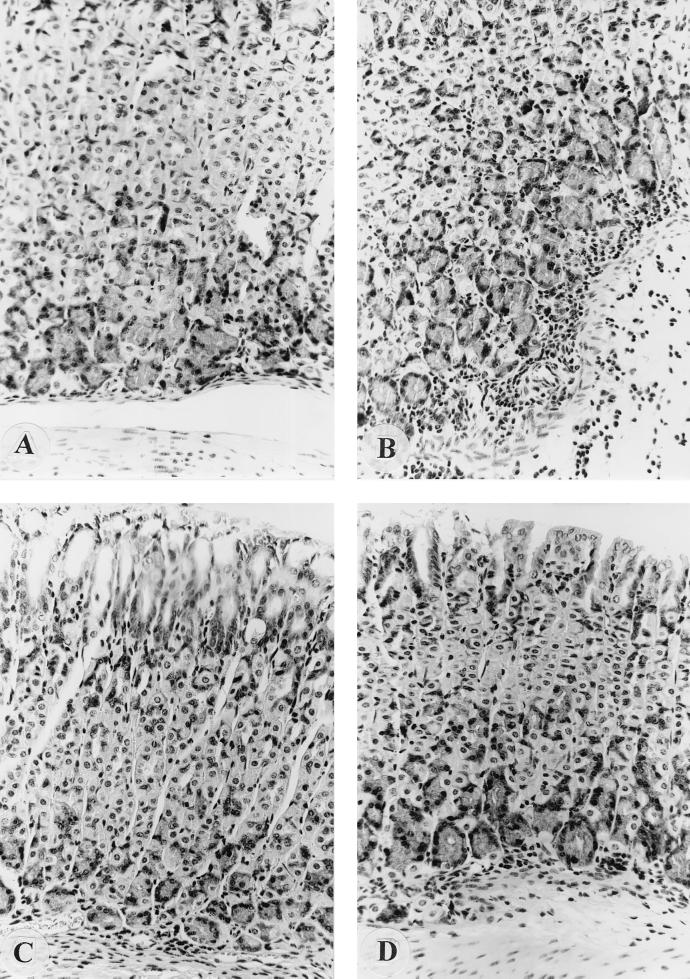
Normal noninfected control mice showed normal fundic mucosae (Fig. 3A). Mice treated with astaxanthin-rich algal meal (100 mg/kg) or vitamin C (400 mg/kg) showed fewer inflammatory cells in their mucosae than infected control mice (Fig. 3B to D).
FIG. 3.
(A) Normal fundic mucosa from an uninfected control mouse; (B) tissue from an H. pylori-infected mouse with a large amount of acute inflammatory cell infiltration within the mucosa and along the lamina muscular mucosa; (C and D) less inflammation (small amount of inflammatory cell infiltration) in mice treated with astaxanthin-rich algal meal and vitamin C, respectively.
Lipid peroxidation.
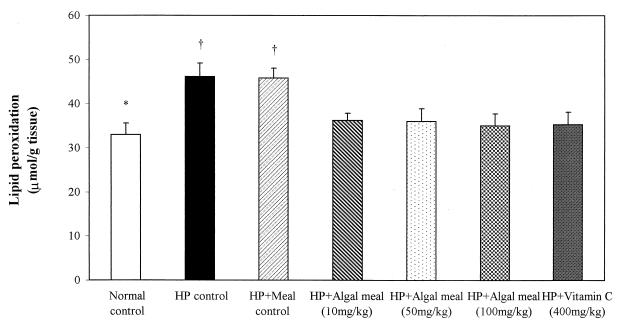
The concentrations of MDA and 4-hydroxyalkenals in murine stomachs (in micromoles per gram of tissue) were significantly increased in H. pylori-infected untreated and control-meal (algal meal without astaxanthin)-treated mice compared with concentrations in normal control animals (P < 0.01). All astaxanthin-rich algal meal- or vitamin C-treated mice showed significant decreases in lipid peroxidation compared with levels in the untreated and control-meal-treated animals (P < 0.05) (Fig. 4).
FIG. 4.
The concentrations of MDA and 4-hydroxyalkenals in murine stomachs were significantly increased in H. pylori-infected untreated and control-meal-treated mice compared to those in normal control animals (∗, P < 0.01). All antioxidant-treated mice showed significant decreases in lipid peroxidation compared to the levels in untreated and control-meal-treated mice († P < 0.05).
In vitro inhibition.
Astaxanthin-rich algal meal inhibited H. pylori growth at 0.3125 to 2.5 mg/ml (astaxanthin content, 6.25 to 50 μg/ml, pH 7.2), while algal meal without astaxanthin did not show this effect at 5 mg/ml. Vitamin C inhibited the growth of H. pylori at concentrations of 0.5 to 2 mg/ml (pH 7.2).
DISCUSSION
We have shown that antioxidants such as algal meal rich in astaxanthin as well as vitamin C inhibit H. pylori infection in BALB/cA mice. Among the three doses of astaxanthin tested, the highest dose (100 mg/kg) showed the best effect in reducing bacterial load and gastric inflammation. This finding is to our knowledge the first demonstration of an antimicrobial activity of astaxanthin-rich algal meal against H. pylori and associated gastric inflammation.
H. pylori infection has been associated with a decreased level of vitamin C and of major antioxidants (e.g., β-carotene) in human gastric tissue (5, 26). We found that vitamin C reduced bacterial colonization in the murine stomach and decreased the inflammation score. Interestingly, Jarosz et al. (11) reported that a high daily dose of vitamin C for 4 weeks (5 g per day) given to H. pylori-infected patients with chronic gastritis resulted in apparent H. pylori eradication in 30% of treated patients. In those patients the highly significant rise in total vitamin C concentration in the gastric juice persisted for at least 4 weeks posttreatment. Vitamin C not only seems to be an antioxidant and a free radical scavenger (17, 21, 26) but also shows antimicrobial activity against H. pylori both in vitro and in a Mongolian gerbil infection model (39).
Epidemiological evidence and clinical experiments suggest that vitamin C may exert a protective effect against the development of H. pylori-associated gastric carcinoma (4, 6, 37), but the mechanisms involved are not so clear.
The carotenoid astaxanthin has been established to be a powerful antioxidant in vitro (15, 24) and was previously shown to be able to prevent oral carcinogenesis in an experimental rat model (32). However, this carotenoid has not previously been shown to have an antimicrobial activity. We found that algal meal rich in astaxanthin has an inhibitory effect on H. pylori growth in vitro and also colonization in mouse stomach. BALB/cA mice treated with astaxanthin-rich algal meal showed decreased lipid peroxidation and granulocyte infiltration in their gastric mucosae.
H. pylori-infected individuals show high oxidative stress and high levels of ROMs in their gastric mucosae and an increased gastric antioxidative capacity after the eradication of H. pylori (14). A recent study of the formation of pro- and antioxidants to H. pylori infection in a Mongolian gerbil model showed an increase in the level of lipid peroxidation and activated glutathione turnover (31).
Astaxanthin acts as an antioxidant that protects against tissue damage induced by ROMs, and it may also inhibit infection through an altered immune response. As early as the 1930s it was discovered that β-carotene increases our natural resistance to bacterial and viral infections and it was proposed that vitamin A causes this effect (3). It is now well known that other carotenoids also improve the immune defense, and in comparative studies, astaxanthin was shown to be most effective (12, 13). Several studies have shown that strong T helper 1 (Th1) cellular immune responses contribute to Helicobacter-associated gastritis and that Th2 T lymphocytes producing interleukin 4 reduce the bacterial load of H. felis-infected mice (18, 29). We found recently that astaxanthin-rich algal meal induces a shift of the Th1-Th2 lymphocyte balance associated with an increased natural defense against H. pylori infection in this mouse model (1).
In conclusion, our results suggest that the use of antioxidants to combat H. pylori infection in humans is an attractive new treatment strategy. Further studies with different feeding formulas and delivery systems as well as prophylaxis studies of animal models and clinical studies are now in progress.
ACKNOWLEDGMENTS
This study was supported by grants from AstaCarotene AB in Gustavsberg, Sweden; the Swedish Medical Research Council (16X04723); the Swedish Forestry Agriculture Research Council (50.0497/98); the Kungliga fysiografiska sällskapet in Lund, Sweden; and the Medical Faculty, Lund University, Lund, Sweden.
REFERENCES
- 1.Bennedsen M, Wang X, Willén R, Wadström T, Andersen L P. Treatment of H. pylori infected mice with antioxidant astaxanthin reduces gastric inflammation, bacterial load and modulates cytokine release by splenocytes. Immunol Lett. 1999;70:185–189. doi: 10.1016/s0165-2478(99)00145-5. [DOI] [PubMed] [Google Scholar]
- 2.Buiatti E, Palli D, Decarli A, Amadori D, Avellini C, Bianchi S, Bonaguri C, Cipriani F, Cocco P, Giacosa A, et al. A case-control study of gastric cancer and diet in Italy. II. Association with nutrients. Int J Cancer. 1990;45:896–901. doi: 10.1002/ijc.2910450520. [DOI] [PubMed] [Google Scholar]
- 3.Chew B P. Role of carotenoids in the immune response. J Dairy Sci. 1993;76:2804–2811. doi: 10.3168/jds.S0022-0302(93)77619-5. [DOI] [PubMed] [Google Scholar]
- 4.Cohen M, Bhagavan H N. Ascorbic acid and gastrointestinal cancer. J Am Coll Nutr. 1995;14:565–578. doi: 10.1080/07315724.1995.10718545. [DOI] [PubMed] [Google Scholar]
- 5.Correa P, Malcom G, Schmidt B, Fontham E, Ruiz B, Bravo J C, Bravo L E, Zarama G, Realpe J L. Review article: antioxidant micronutrients and gastric cancer. Aliment Pharmacol Ther. 1998;12(Suppl. 1):73–82. doi: 10.1111/j.1365-2036.1998.00006.x. [DOI] [PubMed] [Google Scholar]
- 6.Drake I M, Davies M J, Mapstone N P, Dixon M F, Schorah C J, White K L, Chalmers D M, Axon A T. Ascorbic acid may protect against human gastric cancer by scavenging mucosal oxygen radicals. Carcinogenesis. 1996;17:559–562. doi: 10.1093/carcin/17.3.559. [DOI] [PubMed] [Google Scholar]
- 7.Ernst P. Review article: the role of inflammation in the pathogenesis of gastric cancer. Aliment Pharmacol Ther. 1999;13(Suppl. 1):13–18. doi: 10.1046/j.1365-2036.1999.00003.x. [DOI] [PubMed] [Google Scholar]
- 8.Fan L, Vonshak A, Zarka A, Boussiba S. Does astaxanthin protect Haematococcus against light damage? Z Naturforsch Sect C. 1998;53:93–100. doi: 10.1515/znc-1998-1-217. [DOI] [PubMed] [Google Scholar]
- 9.Goldstone A R, Quirke P, Dixon M F. Helicobacter pylori infection and gastric cancer. J Pathol. 1996;179:129–137. doi: 10.1002/(SICI)1096-9896(199606)179:2<129::AID-PATH504>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 10.Goodman K J, Correa P, Tengana A H J, DeLany J P, Collazos T. Nutritional factors and Helicobacter pylori infections in Colombian children. J Pediatr Gastroenterol. 1997;25:507–515. doi: 10.1097/00005176-199711000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Jarosz M, Dzieniszewski J, Dabrowska-Ufniarz E, Wartanowicz M, Ziemlanski S, Reed P I. Effects of high dose vitamin C treatment on Helicobacter pylori infection and total vitamin C concentration in gastric juice. Eur J Cancer Prev. 1998;7:449–454. doi: 10.1097/00008469-199812000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Jyonouchi H, Sun S, Mizokami M, Gross M D. Effects of various carotenoids on cloned, effector-stage T-helper cell activity. Nutr Cancer. 1996;26:313–324. doi: 10.1080/01635589609514487. [DOI] [PubMed] [Google Scholar]
- 13.Jyonouchi H, Zhang L, Gross M, Tomita Y. Immunomodulating actions of carotenoids: enhancement of in vivo and in vitro antibody production to T-dependent antigens. Nutr Cancer. 1994;21:47–58. doi: 10.1080/01635589409514303. [DOI] [PubMed] [Google Scholar]
- 14.Khaled M A, Sarker S A. Changes of oxidant and antioxidant status in humans due to H. pylori infection. Nutr Res. 1998;18:1463–1468. [Google Scholar]
- 15.Krinsky N I. Antioxidant function of carotenoids. Free Radic Biol Med. 1989;7:617–635. doi: 10.1016/0891-5849(89)90143-3. [DOI] [PubMed] [Google Scholar]
- 16.Kurashige M, Okimasu E, Inoue M, Utsumi K. Inhibition of oxidative injury of biological membranes by astaxanthin. Physiol Chem Physics Med NMR. 1990;22:27–38. [PubMed] [Google Scholar]
- 17.Levine M. New concepts in the biology and biochemistry of ascorbic acid. N Engl J Med. 1986;314:892–902. doi: 10.1056/NEJM198604033141407. [DOI] [PubMed] [Google Scholar]
- 18.Mohammadi M, Nedrud J, Redline R, Lycke N, Czinn S J. Murine CD4 T-cell response to Helicobacter infection: TH1 cells enhance gastritis and TH2 cells reduce bacterial load. Gastroenterology. 1997;113:1848–1857. doi: 10.1016/s0016-5085(97)70004-0. [DOI] [PubMed] [Google Scholar]
- 19.Morrow J D, Frei B, Longmire A W, Gaziano J M, Lynch S M, Shyr Y, Strauss W E, Oates J A, Roberts L J. Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. Smoking as a cause of oxidative damage. N Engl J Med. 1995;332:1198–1203. doi: 10.1056/NEJM199505043321804. [DOI] [PubMed] [Google Scholar]
- 20.Nilsson H O, Aleljung P, Nilsson I, Tyszkiewicz T, Wadström T. Immunomagnetic bead enrichment and PCR for detection of Helicobacter pylori in human stools. FEMS Immunol Med Microbiol. 1996;27:73–79. [Google Scholar]
- 21.Noroozi M, Angerson W J, Lean M E. Effects of flavonoids and vitamin C on oxidative DNA damage to human lymphocytes. Am J Clin Nutr. 1998;67:1210–1218. doi: 10.1093/ajcn/67.6.1210. [DOI] [PubMed] [Google Scholar]
- 22.Ohta R, Yamada N, Kaneko H, Ishikawa K, Fukuda H, Fujino T, Suzuki A. In vitro inhibition of the growth of Helicobacter pylori by oil-macerated garlic constituents. Antimicrob Agents Chemother. 1999;43:1811–1812. doi: 10.1128/aac.43.7.1811. . (Letter.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okai Y, Higashi-Okai K. Possible immunomodulating activities of carotenoids in in vitro cell culture experiments. Int J Immunopharmacol. 1996;18:753–758. doi: 10.1016/s0192-0561(97)85558-0. [DOI] [PubMed] [Google Scholar]
- 24.Palozza P, Krinsky N I. Astaxanthin and canthaxanthin are potent antioxidants in a membrane model. Arch Biochem Biophys. 1992;297:291–295. doi: 10.1016/0003-9861(92)90675-m. [DOI] [PubMed] [Google Scholar]
- 25.Pettersson A, Lignell Å. Astaxanthin deficiency in eggs and fry of Baltic salmon (Salmo salar) with the M74 syndrome. Ambio. 1999;28:43–46. [Google Scholar]
- 26.Phull P S, Green C J, Jacyna M R. A radical view of the stomach: the role of oxygen-derived free radicals and antioxidants in gastroduodenal disease. Eur J Gastroenterol Hepatol. 1995;7:265–274. [PubMed] [Google Scholar]
- 27.Rebouche C J. Ascorbic acid and carnitine biosynthesis. Am J Clin Nutr. 1991;54(Suppl. 6):1147S–1152S. doi: 10.1093/ajcn/54.6.1147s. [DOI] [PubMed] [Google Scholar]
- 28.Shinchi K, Ishii H, Imanishi K, Kono S. Relationship of cigarette smoking, alcohol use and dietary habits with Helicobacter pylori infection in Japanese men. Scand J Gastroenterol. 1997;32:651–655. doi: 10.3109/00365529708996513. [DOI] [PubMed] [Google Scholar]
- 29.Sommer F, Faller G, Konturek P, Kirchner T, Hahn E G, Zeus J, Rollinghoff M, Lohoff M. Antrum- and corpus mucosa-infiltrating CD4+ lymphocytes in Helicobacter pylori gastritis display a Th1 phenotype. Infect Immun. 1998;66:5543–5546. doi: 10.1128/iai.66.11.5543-5546.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stahelin H B, Gey K F, Eichholzer M, Luden E. Beta-carotene and cancer prevention. Am J Clin Nutr. 1991;53:265S–269S. doi: 10.1093/ajcn/53.1.265S. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki H, Mori M, Seto K, Kai A, Kawaguchi C, Suzuki M, Suematsu M, Yoneta T, Miura S, Ishii H. Helicobacter pylori-associated gastric pro- and antioxidant formation in Mongolian gerbils. Free Radic Biol Med. 1999;26:679–684. doi: 10.1016/s0891-5849(98)00248-2. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka T, Makita H, Ohnishi M, Mori H, Satoh K, Hara A. Chemoprevention of rat oral carcinogenesis by naturally occurring xanthophylls, astaxanthin and canthaxanthin. Cancer Res. 1995;55:4059–4064. [PubMed] [Google Scholar]
- 33.Tsugane S, Tei Y, Takahashi T, Watanabe S, Sugano K. Salty food intake and risk of Helicobacter pylori infection. Jpn J Cancer Res. 1994;85:474–478. doi: 10.1111/j.1349-7006.1994.tb02382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang X, Sjunnesson H, Sturegård E, Wadström T, Willén R, Aleljung P. Dietary factors influence the recovery rates of Helicobacter pylori in a BALB/cA mouse model. Zentbl Bakteriol. 1998;288:195–205. doi: 10.1016/s0934-8840(98)80039-x. [DOI] [PubMed] [Google Scholar]
- 35.Wang X, Sturegård E, Rupar R, Nilsson H O, Aleljung P A, Carlen B, Willen R, Wadström T. Infection of BALB/cA mice by spiral and coccoid forms of Helicobacter pylori. J Med Microbiol. 1997;46:657–663. doi: 10.1099/00222615-46-8-657. [DOI] [PubMed] [Google Scholar]
- 36.Wang X, Willén R, Wadström T, Aleljung P. RAPD-PCR, histopathological and serological analysis of four mouse strains infected with multiple strains of Helicobacter pylori. Microb Ecol Health Dis. 1998;10:148–154. [Google Scholar]
- 37.Waring A J, Drake I M, Schorah C J, White K L, Lynch D A, Axon A T, Dixon M F. Ascorbic acid and total vitamin C concentrations in plasma, gastric juice, and gastrointestinal mucosa: effects of gastritis and oral supplementation. Gut. 1996;38:171–176. doi: 10.1136/gut.38.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Warren J R, Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;i:1273–1275. [PubMed] [Google Scholar]
- 39.Zhang H M, Wakisaka N, Maeda O, Yamamoto T. Vitamin C inhibits the growth of a bacterial risk factor for gastric carcinoma: Helicobacter pylori. Cancer. 1997;80:1897–1903. [PubMed] [Google Scholar]