This cohort study uses commercial claims data to estimate rates of cascade events and associated spending following screening breast magnetic resonance imaging (MRI) vs mammography among commercially insured women in the US.
Key Points
Question
What is the prevalence of cascades of medical services and new diagnoses following screening breast magnetic resonance imaging (MRI) vs mammography among commercially insured US women?
Findings
In this cohort study of 9208 women receiving MRI and 9208 propensity score–matched women receiving mammography, breast MRI recipients had higher rates of downstream mammary and extramammary visits, total and out-of-pocket spending, and for mammary findings specifically, higher rates of imaging tests, procedures, hospitalizations, and new diagnoses.
Meaning
Screening breast MRI recipients experienced more mammary and extramammary cascade events and spending relative to mammogram recipients, with potential for harms to these patients and the health system.
Abstract
Importance
Increasing use of screening breast magnetic resonance imaging (MRI), including among women at low or average risk of breast cancer, raises concerns about resulting mammary and extramammary cascades (downstream services and new diagnoses) of uncertain value.
Objective
To estimate rates of cascade events (ie, laboratory tests, imaging tests, procedures, visits, hospitalizations, and new diagnoses) and associated spending following screening breast MRI vs mammography among commercially insured US women.
Design, Setting, and Participants
This cohort study used 2016 to 2018 data from the MarketScan research database (IBM Corporation), which includes claims and administrative data from large US employers and commercial payers. Participants included commercially insured women aged 40 to 64 years without prior breast cancer who received an index bilateral screening breast MRI or mammogram between January 1, 2017, and June 30, 2018. We used propensity scores based on sociodemographic, clinical, and utilization variables to match MRI recipients to mammogram recipients in each month of index service use. Data were analyzed from October 8, 2020, to October 28, 2021.
Exposures
Breast MRI vs mammography.
Main Outcomes and Measures
Mammary and extramammary cascade event rates and associated total and patient out-of-pocket spending in the 6 months following the index test.
Results
In this study, 9208 women receiving breast MRI were matched with 9208 women receiving mammography (mean [SD] age, 51.4 [6.7] years). Compared with mammogram recipients, breast MRI recipients had 39.0 additional mammary cascade events per 100 women (95% CI, 33.7-44.2), including 5.0 additional imaging tests (95% CI, 3.8-6.2), 17.3 additional procedures (95% CI, 15.5-19.0), 13.0 additional visits (95% CI, 9.4-17.2), 0.34 additional hospitalizations (95% CI, 0.18-0.50), and 3.0 additional new diagnoses (95% CI, 2.5-3.6). For extramammary cascades, breast MRI recipients had 19.6 additional events per 100 women (95% CI, 8.6-30.7) including 15.8 additional visits (95% CI, 10.2-21.4) and no statistically significant differences in other events. Breast MRI recipients had higher total spending for mammary events ($564 more per woman; 95% CI, $532-$596), extramammary events ($42 more per woman; 95% CI, $16-$69), and overall ($1404 more per woman; 95% CI, $1172-$1636). They also had higher overall out-of-pocket spending ($31 more per woman; 95% CI, $6-$55).
Conclusions and Relevance
In this cohort study of commercially insured women, breast MRI was associated with more mammary and extramammary cascade events and spending relative to mammography. These findings can inform cost-benefit assessments and coverage policies to ensure breast MRI is reserved for patients for whom benefits outweigh harms.
Introduction
Breast magnetic resonance imaging (MRI) is increasingly performed for breast cancer screening, typically in addition to mammographic screening.1,2,3 Breast MRI is more sensitive than mammography at identifying cancers,4 and although studies have not yet demonstrated that breast MRI screening reduces breast cancer mortality, in 2007, the American Cancer Society recommended screening MRI for all women with a lifetime risk of breast cancer exceeding 20%.5 Additionally, widespread breast density legislation in the US requires that patients be notified about their mammographic breast density and the potential role of supplemental imaging, such as MRI, despite uncertainty about whether supplemental MRI improves long-term outcomes for women with dense breasts. Such legislation, along with mandated insurance coverage for this supplemental imaging in some states,6,7 has further driven use of screening breast MRI.8,9 While the Society of Surgical Oncology, the American College of Physicians, and other groups recommend against breast MRI screening in women with low to average risk for breast cancer (eAppendix in the Supplement),10 such use may be common. A 2017 registry-based study in community settings found that 83% of screening breast MRI test were discordant with these guidelines.11
The increasing use of screening breast MRI, especially among women with low or average-risk for breast cancer, raises concerns about the extent to which these studies trigger downstream cascades of care that may have limited value and entail potential harm for patients.12,13,14,15,16,17 Breast MRI is less specific than mammography for breast pathology,4,18 resulting in more false-positive results and unnecessary biopsies.19,20,21 In addition, unlike mammography, breast MRI can reveal extramammary findings that may similarly prompt follow-up imaging and other services of unclear benefit. Single-site studies estimate that 10% to 34% of breast MRI tests show extramammary incidental findings.17,22,23,24 However, to our knowledge, no larger studies have examined the prevalence of or spending on downstream services that may follow. Further, extramammary cascades are rarely considered in breast MRI trials, cost-effectiveness analyses, guidelines, or shared decision-making discussions about breast cancer screening.25,26
Given growing use of screening breast MRI and the potential impact on patients and the health care system, it is important to characterize the national scope and associated spending on cascades following both mammary and extramammary findings. We used commercial claims data from across the US to compare women receiving breast MRI vs mammography screening on rates of downstream laboratory tests, imaging tests, procedures, visits, hospitalizations, and new diagnoses that could potentially follow mammary and extramammary findings on breast MRI, building on methods developed in prior work.12,13,27 We also compared total and patient out-of-pocket spending on cascade services and overall.
Methods
This cohort study was considered exempt from review by the Harvard Medical School and Mass General Brigham institutional review boards. Informed consent was waived because patient data were deidentified. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline. We used enrollment, outpatient, and inpatient data from January 1, 2016, to December 31, 2018, from the MarketScan research database (IBM Corporation), which includes medical claims from large employers and commercial payers in the US.
Study Cohorts
Breast MRI and Mammography Cohorts
We identified all female members who had a bilateral breast MRI claim between January 1, 2017, and June 30, 2018; analysis was limited to the first MRI received during the study period. We further selected women who were aged 40 to 64 years on the date of the MRI, were continuously enrolled in their plan from 12 months before the MRI through 6 months after the MRI, and did not have another breast MRI claim or diagnostic mammography claim in the preceding 12 months. We further excluded women with any claim including a breast cancer diagnosis code in the preceding 12 months of the index MRI or on the date of the index MRI (eTable 1 in the Supplement), and women with any claim including a diagnostic code for managing breast implants, any cancer surveillance, or diagnostic imaging on the date of the index MRI. We took these steps based on prior literature to optimize the likelihood of identifying a screening MRI since bilateral breast MRI procedure codes do not specify screening vs diagnostic indication.28,29 Among women who did not have a breast MRI, we identified those who had a bilateral screening mammogram claim between January 1, 2017, and June 30, 2018; were aged 40 to 64 years on the date of the mammogram; were continuously enrolled from 12 months before the mammogram through 6 months after the mammogram; did not have a breast MRI claim in the preceding 12 months; and did not have a claim with a breast cancer diagnosis code in the preceding 12 months or on the date of the index mammogram.
Propensity Score Matching
We used propensity scores to match women who received breast MRI with similar women who received mammography based on variables observable in the data that might influence the decision to obtain MRI, informed by published literature, clinical guidelines, and experience.11,19,28,30 Specifically, we used 1:1 greedy matching without replacement to match 1 mammogram recipient to each MRI recipient with the same month of index service use based on the propensity score for that month using models that included: (1) age (continuous); (2) US census division (out of 9 total)31; (3) combined comorbidity score in the year before the index test32; (4) prior utilization (total outpatient health care spending in the prior 12 months); (5) health plan type (Table 1), a binary indicator for whether the index test occurred on or after breast density legislation was enacted in the member’s state of residence33; and (6) any diagnosis or procedure code in the prior 12 months suggesting higher than average risk of breast cancer or of breast MRI receipt (ie, family history of breast cancer, BRCA1 or BRCA2 mutation, dense breasts, history of chest irradiation, high-risk nonmalignant breast lesion, benign breast disease, or breast biopsy) (eTable 2 in the Supplement). We used standardized differences to assess the balance between the MRI and mammography cohorts before and after propensity matching.34
Table 1. Characteristics of the Study Population Before and After Propensity Matching.
| Characteristic | Before matching | After matching | ||||
|---|---|---|---|---|---|---|
| Patients, No. (%) | Standardized differencea | Patients, No. (%) | Standardized differencea | |||
| Breast MRI | Mammography | Breast MRI | Mammography | |||
| No. | 9351 | 1 841 424 | 9208 | 9208 | ||
| Age, mean (SD) | 51.5 (6.6) | 52.7 (6.8) | −0.190 | 51.5 (6.6) | 51.3 (6.9) | 0.020 |
| Census division | ||||||
| New England | 583 (6.2) | 72 889 (4.0) | 0.103 | 575 (6.2) | 587 (6.4) | −0.005 |
| Middle Atlantic | 1529 (16.4) | 208 252 (11.3) | 0.146 | 1487 (16.2) | 1557 (16.9) | −0.020 |
| East North Central | 1266 (13.5) | 269 560 (14.6) | −0.032 | 1238 (13.4) | 1231 (13.4) | 0.002 |
| West North Central | 378 (4.0) | 89 830 (4.9) | −0.041 | 373 (4.1) | 383 (4.2) | −0.006 |
| South Atlantic | 1786 (19.1) | 399 932 (21.7) | −0.065 | 1766 (19.2) | 1690 (18.4) | 0.021 |
| East South Central | 378 (4.0) | 116 691 (6.3) | −0.104 | 374 (4.1) | 392 (4.3) | −0.010 |
| West South Central | 478 (5.1) | 160 245 (8.7) | −0.142 | 474 (5.2) | 470 (5.1) | 0.002 |
| Mountain | 426 (4.6) | 70 284 (3.8) | 0.037 | 423 (4.6) | 443 (4.8) | −0.010 |
| Pacific | 1164 (12.5) | 165 723 (9.0) | 0.112 | 1149 (12.5) | 1178 (12.8) | −0.009 |
| Unknown census division | 1363 (14.6) | 288 018 (15.6) | −0.030 | 1349 (14.7) | 1277 (13.9) | 0.022 |
| Breast density law present in state | 7633 (81.6) | 1 448 138 (78.6) | 0.075 | 7514 (81.6) | 7546 (82.0) | −0.009 |
| Breast diagnoses | ||||||
| Benign breast disease | 3070 (32.8) | 64 523 (3.5) | 0.823 | 2945 (32.0) | 3042 (33.0) | −0.023 |
| Histologic high-risk benign breast lesion | 695 (7.4) | 3773 (0.20) | 0.384 | 632 (6.9) | 514 (5.6) | 0.053 |
| History of chest irradiation | 49 (0.52) | 1697 (0.09) | 0.078 | 48 (0.52) | 68 (0.74) | −0.028 |
| Dense breasts | 1796 (19.2) | 25 436 (1.4) | 0.614 | 1699 (18.5) | 1514 (16.4) | 0.053 |
| Family history of breast cancer or genetic susceptibility | 4747 (50.8) | 26 414 (1.4) | 1.358 | 4611 (50.1) | 4661 (50.6) | −0.011 |
| History of breast biopsy | 527 (5.6) | 7045 (0.38) | 0.312 | 473 (5.1) | 443 (4.8) | 0.015 |
| Prior outpatient spending, mean (SD) | 6808.57 (12 626.30) | 4559.35 (12 298.96) | 0.180 | 6742.28 (12 639.67) | 7776.66 (18 710.75) | −0.060 |
| Plan type | ||||||
| Consumer-driven health plan | 984 (10.5) | 259 621 (14.1) | −0.109 | 974 (10.6) | 971 (10.6) | 0.001 |
| Comprehensive plan | 274 (2.9) | 59 071 (3.2) | −0.016 | 269 (2.9) | 291 (3.2) | −0.014 |
| Exclusive provider organization plan | 71 (0.76) | 15 023 (0.82) | −0.007 | 68 (0.74) | 89 (0.97) | −0.025 |
| High-deductible health plan | 787 (8.4) | 163 477 (8.9) | −0.016 | 781 (8.5) | 759 (8.2) | 0.009 |
| Health maintenance organization plan | 1022 (10.9) | 213 729 (11.6) | −0.022 | 1010 (11.0) | 1064 (11.6) | −0.019 |
| Point-of-Service Plan | 606 (6.5) | 115 378 (6.3) | 0.009 | 597 (6.5) | 614 (6.7) | −0.008 |
| Capitated point-of-service plan | 170 (1.8) | 23 188 (1.3) | 0.45 | 168 (1.8) | 161 (1.8) | 0.005 |
| Preferred provider organization plan | 5239 (56.0) | 957 983 (52.0) | 0.081 | 5153 (56.0) | 5044 (54.8) | 0.024 |
| Unknown | 198 (2.1) | 33 954 (1.8) | 0.020 | 188 (2.0) | 215 (2.3) | −0.020 |
| Combined comorbidity score, mean (SD) | 0.41 (1.1) | 0.26 (1.00) | 0.150 | 0.41 (1.05) | 0.45 (1.2) | −0.030 |
Standardized differences measure the balance in baseline covariates between 2 groups. Values less than 0.10 demonstrate that the groups are well-balanced.
Index and Cascade Events
We defined the index event as the index breast MRI (for the breast MRI cohort) or the index mammogram (for the mammography cohort). We defined the cascade period as the 6 months starting the day after the index event. We used literature15,16,17,23 and clinical expert review (S.R., I.G., N.K., L.P.) to identify potential cascade events: all laboratory tests, imaging tests, procedures, visits, hospitalizations, and new diagnoses that may plausibly follow from a mammary or extramammary finding on breast MRI (eTables 3-7 in the Supplement). We categorized laboratory tests, imaging tests, procedures, visits, and diagnoses as related to mammary or nonmammary findings; since billing codes for the laboratory tests likely to follow mammary findings (ie, pathology and cytology) do not have sufficient specificity to the breast, we grouped these events as a separate category (pathology and cytology). For laboratory tests (including pathology and cytology), imaging tests, and procedures, we counted only 1 event within a given subcategory on a given day of service (eg, if there were 2 billing codes for breast ultrasonography on the same day, we counted 1 ultrasonography test, but if there were billing codes for breast ultrasonography and breast MRI on the same day, we counted 2 unique events). Hospitalizations were identified based on having a cascade-relevant principal diagnosis (based on new diagnoses described above). The authors grouped diagnosis codes into subcategories (eg, renal cyst), and each given subcategory could only be counted once. A diagnosis was defined as new if it was included on any inpatient claim during hospitalization or on 2 outpatient visit claims during the cascade period but not during the 12 months before the index event.
Spending
Using inpatient and outpatient files, we measured total spending (ie, all allowable payments) and out-of-pocket spending (ie, the sum of copayments, coinsurance, and deductibles) for mammary and extramammary cascade-attributable services during the 6-month cascade period. We then assessed total spending and out-of-pocket spending for all services received during the cascade period. We estimated mean total and out-of-pocket spending on the index breast MRI and index mammograms among the respective recipients. To account for the non-independence of index and cascade service out-of-pocket spending (eg, index event out-of-pocket spending could affect cascade out-of-pocket spending by contributing to deductible limits), we also estimated out-of-pocket spending for mammary cascades, extramammary cascades, and all services while including the cost of the index MRI or mammogram.
Statistical Analysis
We estimated cascade event rates and spending for the breast MRI and mammography groups, Winsorizing estimates at 1%. We then created Poisson regression models to estimate cascade event rates and log-linear regression models to estimate spending during the cascade period for each cohort. The Poisson models were adjusted for potential overdispersion by scaling according to the Pearson residuals. We then estimated cascade event rate and spending differences between the MRI and mammography groups, using t values to determine CIs. Since mammogram findings are limited to the breast, extramammary events following index mammograms were captured to represent baseline service rates and permit quantification of extramammary cascades in the MRI group. In our primary analysis, we did not count mammograms that occurred during the cascade period as mammary cascade events since they may instead represent standalone subsequent screening events; we performed a sensitivity analysis in which we repeated our estimates of mammary cascades including mammograms as cascade events.
To test the assumption that additional cascade events in the breast MRI group are plausibly causally linked to the index events rather than due to selection effects (eg, if women who receive breast MRI also use more health care overall), we performed a falsification test in which we repeated the cascade analyses with a service, knee x-ray, that would not plausibly follow from a breast MRI. Finally, we estimated the proportion of breast MRI recipients who had any potential mammary cascade event, any potential extramammary cascade event, both, or neither. We described these as potential cascade events because they included some commonly performed medical services, and we could not confirm in this observational, claims-based analysis whether the event followed causally from the index screening test.
We performed analyses using SAS software, version 9.4 (SAS Institute, Inc). Reported P values were 2 sided and P < .05 represented statistical significance. Data were analyzed from October 8, 2020, to October 28, 2021.
Results
Before matching, women receiving breast MRI were more likely to be younger, to have prior breast-related diagnoses (such as benign breast disease) and prior breast biopsy, and to reside in New England or Middle Atlantic regions than women receiving mammography (Table 1). After propensity score matching, there were 18 416 women in our cohort (mean [SD] age, 51.4 [6.7] years). Characteristics of the breast MRI (n = 9208) and mammography (n = 9208) groups were well-balanced, with standardized differences for all covariates less than 0.1.
Cascade Event Rates
When examining mammary cascade events, breast MRI recipients had 39.0 additional cascade events per 100 women (95% CI, 33.7 to 44.2) (Table 2). These included additional imaging tests (5.0 per 100 women; 95% CI, 3.8 to 6.2), procedures (17.3; 95% CI, 15.5 to 19.0), specialist visits (13.0; 95% CI, 9.4 to 17.2), hospitalizations (0.34; 95% CI, 0.18 to 0.50), and new diagnoses (3.0; 95% CI, 2.5 to 3.6) compared with mammogram recipients (Table 2). Breast MRI recipients also had higher rates of pathology and cytology tests (14.1; 95% CI, 12.3 to 15.9). In the sensitivity analysis including mammograms as cascade events, the rate of additional imaging tests for MRI recipients was higher (eTable 8 in the Supplement). When examining extramammary cascade events, breast MRI recipients had 19.6 additional services per 100 women (95% CI, 8.6 to 30.7), including 15.8 additional visits (95% CI, 10.2 to 21.4). There were no statistically significant differences between the breast MRI and mammography groups in rates of laboratory tests, imaging tests, procedures, hospitalizations, and new diagnoses. The falsification test showed that there were 4.2 knee x-rays per 100 in the MRI cohort vs 4.3 knee x-rays per 100 in the mammogram cohort, with a nonsignificant difference of −0.13; (95% CI, −0.81 to 0.55).
Table 2. Cascade Event Rates Among Screening Breast MRI vs Mammography Recipients in the 6 Months Following the Screening Test.
| Characteristics | Event rate per 100 members | Breast MRI cascade-attributable event rate per 100 members (95% CI) | |
|---|---|---|---|
| Breast MRI (n = 9208) | Mammography (n = 9208) | ||
| Mammary | |||
| All mammary cascade events | 134.1 | 95.1 | 39.0 (33.7 to 44.2) |
| Imaging tests | 18.1 | 13.1 | 5.0 (3.8 to 6.2) |
| Procedures | 22.4 | 5.1 | 17.3 (15.5 to 19.0) |
| Visits | 89.3 | 76.0 | 13.3 (9.4 to 17.2) |
| Hospitalizations | 0.38 | 0.04 | 0.34 (0.18 to 0.50) |
| New diagnoses | 3.9 | 0.9 | 3.0 (2.5 to 3.6) |
| Extramammary | |||
| All extramammary cascade events | 304.5 | 284.8 | 19.6 (8.6 to 30.7) |
| Laboratory tests | 114.9 | 111.8 | 3.2 (−2.2 to 8.5) |
| Imaging tests | 33.3 | 33.7 | −0.45 (−2.7 to 1.8) |
| Procedures | 10.3 | 9.4 | 0.97 (−0.08 to 2.0) |
| Visits | 141.0 | 125.2 | 15.8 (10.2 to 21.4) |
| Hospitalizations | 0.27 | 0.38 | −0.11 (−0.28 to 0.06) |
| New diagnoses | 4.7 | 4.4 | 0.29 (−0.53 to 1.11) |
| Othera | |||
| Pathology/cytology | 35.8 | 21.7 | 14.1 (12.3 to 15.9) |
Abbreviation: MRI, magnetic resonance imaging.
Since billing codes for the laboratory tests likely to follow mammary findings (ie, pathology and cytology) do not have sufficient specificity to identify breast vs other tissue sites for biopsy or cytology samples, we grouped these events as a separate category and did not include them in counts of aggregate cascade event or spending estimates.
Spending
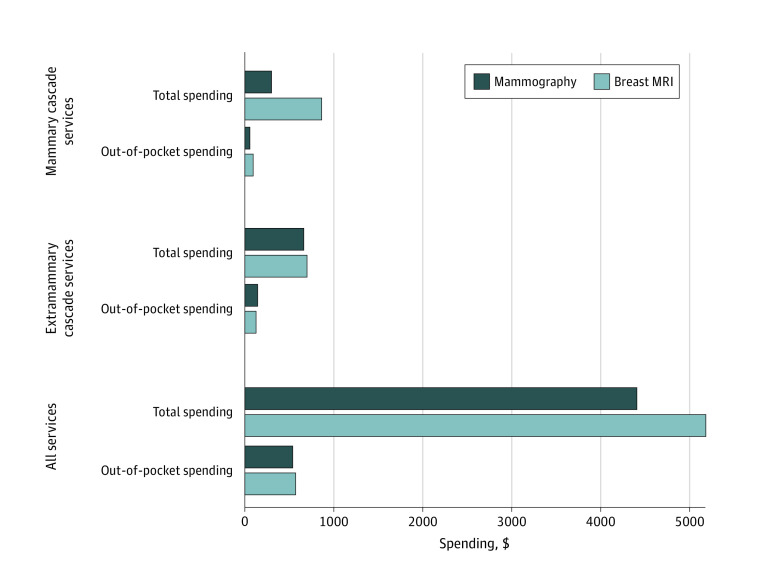
Breast MRI recipients had higher total spending on mammary cascades ($564; 95% CI, $532-$596) and on extramammary cascades ($42; 95% CI, $16-$69) (Figure). Breast MRI recipients had higher out-of-pocket spending on mammary cascades and slightly lower out-of-pocket spending on extramammary cascades. However, when we included the cost of the index event in the cascade estimates (because index event out-of-pocket spending for MRI and mammography could affect cascade out-of-pocket spending by contributing to deductible limits), we found that breast MRI recipients had higher out-of-pocket spending for both mammary services ($305; 95% CI, $297-$313) and extramammary services ($254; 95% CI, $244-$263) (eTable 9 in the Supplement). When considering spending on all services received in the cascade period, breast MRI recipients had higher mean total overall spending ($1404 more per woman; 95% CI, $1172-$1636) and higher mean total out-of-pocket spending ($31 more per woman; 95% CI, $6-$55) (Figure).
Figure. Total and Out-of-Pocket Spending on Mammary Cascade Services, Extramammary Cascade Services, and All Services Among Screening Breast MRI vs Mammography Recipients in the 6 Months Following the Screening Test.
Among the 7015 (76.2%) breast MRI recipients with any potential cascade event (which included services that are commonly performed for a variety of indications), 338 (3.7%) had a potential mammary cascade event, 1949 (21.2%) had a potential extramammary cascade event, and 4728 (51.4%) had both.
Discussion
In this national study of commercially insured women aged 40 to 64 years, we found that relative to women undergoing breast cancer screening with mammography only, women undergoing screening breast MRI experienced additional cascade services related to both mammary and extramammary findings. Breast MRI recipients had additional mammary imaging tests, procedures, visits, hospitalizations, and new diagnoses, additional extramammary visits, and additional pathology and cytology tests, with relatively modest associated additional overall and out-of-pocket spending.
We found that breast MRI was associated with substantially higher rates of cascades from mammary findings than mammograms, which would be expected given MRI’s higher sensitivity and lower specificity for breast cancer detection relative to mammography and well-documented higher rates of false-positive results and biopsies, especially among women without known breast cancer.19,21 Our results further demonstrate higher rates of office visits and hospitalizations for mammary diagnoses following MRI, which to our knowledge have not been previously shown.
Our estimates of nonmammary cascades following breast MRI in a large, population of women who were commercially insured build on single institution studies on this topic.17,22,23,24 European studies have documented incidental findings in 10% to 34% of breast MRI tests.23,24 In a US study of 327 breast cancer patients, Padia et al17 found that 11% experienced 1 or more incidental extramammary findings, with many of those women receiving additional imaging (51%), laboratory testing (9%), physician referrals (6%), and biopsies (6%). None of those incidental findings turned out to be malignancies. Niell et al22 noted extramammary findings in 17% of 2324 examined breast MR images, of which 4% were recommended for follow up imaging—most often for hepatic lesions—and only 0.4% led to a clinically important finding. Our study showed that women receiving MRI had higher rates of total extramammary events and specialist visits than women receiving mammograms, while small differences in other extramammary events did not reach statistical significance.
We also found that MRI receipt was associated with more cascade-attributable and overall total and out-of-pocket spending than mammography receipt. When considering extramammary cascade spending (for which mammogram recipients represented baseline spending rates on these services), compared with mammogram recipients, MRI recipients had $42 additional total spending and $9 less out-of-pocket spending.22 This negative result is likely explained by MRI recipients being more likely to reach their deductible because of the MRI itself. In support of this possibility, when we included index event cost in the comparison of extramammary cascade spending, MRI recipients spent an additional $254 out of pocket. These results use actual payer reimbursements and build on prior single-site studies that estimated spending based on standardized Medicare reimbursement rates. Niell et al22 estimated a mean of $16 in spending across all MRI recipients for within-site follow-up imaging tests alone for extramammary findings. Padia et al17 estimated a mean of $328 spent on downstream extramammary laboratory tests, imaging, and biopsies across all women who had incidental findings.
While our estimates of total additional spending are modest, they add up across the population. A claims-based study28 showed about 0.18% of all US women aged 40 to 64 received screening MRI in 2016 (0.19% among women aged 40-49; 0.17% among women aged 50-64), with current rates likely to be higher. Extrapolating to 52.6 million women aged 40 to 64 based on 2020 US census data, this would entail about $53.4 million in additional total spending on mammary cascades and $4.0 million on extramammary cascades nationwide. Cost-effectiveness analyses of breast MRI and mammography to date have not included the downstream costs of MRI extramammary findings in their assessments and are overall less comprehensive in their examination of events.25,35,36,37 Differences in out-of-pocket spending from cascades are critical to share with women when making decisions about breast cancer screening approaches, especially given growing enrollment in high deductible health plans.29,38
Our findings about additional mammary and extramammary cascade events associated with MRI contribute to understanding the potential benefits and harms of these studies. MRI screening has been associated with earlier-stage breast cancer diagnoses and may have value in women at elevated breast cancer risk. However, MRI also prompts overdiagnosis and overtreatment of breast cancer.39 Repeated exposure to gadolinium contrast is another concern that is not well understood.40 Our findings regarding downstream cascades and spending associated with MRI further underscore the importance of identifying patients who are most likely to derive benefit from these studies.
Limitations
This study has limitations inherent to claims-based analyses. Although use of administrative data allowed us to examine a large population across the US, these data lacked clinical detail to confirm intentions behind billed services or to determine if downstream events followed causally from the index event. Specifically, bilateral breast MRI codes do not distinguish between screening and diagnostic indications, although we took several analytic steps to exclude diagnostic MRI consistent with prior literature.28,29 In addition, potential cascade events necessarily include services commonly performed for various indications, so while we report these rates, our main conclusions focus on additional event rates in the breast MRI group compared with the mammography group. The falsification test offers further reassurance that the additional cascade events reported may plausibly be associated with the index event. We also could not account for all potential confounders associated with breast MRI receipt, such as detailed risk scores, race, facility or clinician characteristics, or socioeconomic status.41 For example, clinicians may be more likely to use billing codes for breast cancer risk factors when ordering MRI to facilitate insurance coverage, and thus these risk factors may be less often identified for the mammography cohort. We expect such differences would bias our results to the null.
Additionally, we did not observe the clinical impact of the cascade events and do not place judgments on the value of individual cascade events. While prior work suggests that some of these downstream services had minimal benefit for patients,17,22 some incidental extramammary findings from breast MRI—such as a newly diagnosed lung cancer—could have improved health. Regarding mammary services following breast MRI, although some women will benefit from earlier detection of breast cancer, the proportion experiencing low-value mammary services from false-positive results is likely to be high in the substantial number of women receiving MRI who do not have elevated breast cancer risk.11 Finally, we studied women aged 40 to 64 years with commercial insurance and further study is needed to determine whether our results are generalizable to older women or women with other types of health insurance.
Conclusions
Our study provides a detailed national picture of downstream services and new diagnoses following breast MRI relative to mammography, including extramammary events and spending that have not been well-characterized previously. The additional testing and related insurer and out-of-pocket spending has important implications for understanding the benefits and harms of MRI screening at the population level and for assisting women in shared decision making about MRI screening. Our findings underscore the importance of avoiding screening breast MRI in low or average-risk women for whom potential harms from screening outweigh potential benefits.
eAppendix. Relevant Choosing Wisely Recommendation
eTable 1. Diagnosis and Procedure Codes Used in MRI and Mammography Cohort Selection
eTable 2. Diagnosis and Procedure Codes for Propensity to Receive Breast MRI
eTable 3. Procedure Codes for Cascade Laboratory Tests
eTable 4. Procedure Codes for Cascade Imaging Tests
eTable 5. Procedure Codes for Cascade Procedures
eTable 6. Procedure Codes for Cascade Visits and Specialty Codes for Cascade Specialty Visits
eTable 7. Diagnosis Codes for Cascade New Diagnoses
eTable 8. Cascade Event Rates and Total and Out-of-Pocket Spending on Mammary Cascade Services Among Screening Breast MRI vs Mammography Recipients in the 6 Months Following the Screening Test, Sensitivity Analysis Including Mammograms as Cascade Events
eTable 9. Total and Out-of-Pocket Spending on Mammary Cascade Services, Extra-Mammary Cascade Services, and All Services Among Screening Breast MRI vs Mammography Recipients in the 6 Months Following the Screening Test, Including Spending on the Index Event
References
- 1.Beatty JD, Porter BA. Contrast-enhanced breast magnetic resonance imaging: the surgical perspective. Am J Surg. 2007;193(5):600-605. doi: 10.1016/j.amjsurg.2007.01.015 [DOI] [PubMed] [Google Scholar]
- 2.Gundry KR. The application of breast MRI in staging and screening for breast cancer. Oncology (Williston Park). 2005;19(2):159-169. [PubMed] [Google Scholar]
- 3.Stout NK, Nekhlyudov L, Li L, et al. Rapid increase in breast magnetic resonance imaging use: trends from 2000 to 2011. JAMA Intern Med. 2014;174(1):114-121. doi: 10.1001/jamainternmed.2013.11958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warner E, Messersmith H, Causer P, Eisen A, Shumak R, Plewes D. Systematic review: using magnetic resonance imaging to screen women at high risk for breast cancer. Ann Intern Med. 2008;148(9):671-679. doi: 10.7326/0003-4819-148-9-200805060-00007 [DOI] [PubMed] [Google Scholar]
- 5.Saslow D, Boetes C, Burke W, et al. ; American Cancer Society Breast Cancer Advisory Group . American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75-89. doi: 10.3322/canjclin.57.2.75 [DOI] [PubMed] [Google Scholar]
- 6.Saulsberry L, Pace LE, Keating NL. The impact of breast density notification laws on supplemental breast imaging and breast biopsy. J Gen Intern Med. 2019;34(8):1441-1451. doi: 10.1007/s11606-019-05026-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DenseBreast-Info.org . Accessed November 15, 2021. https://densebreast-info.org/
- 8.Bakker MF, de Lange SV, Pijnappel RM, et al. ; DENSE Trial Study Group . Supplemental MRI Screening for women with extremely dense breast tissue. N Engl J Med. 2019;381(22):2091-2102. doi: 10.1056/NEJMoa1903986 [DOI] [PubMed] [Google Scholar]
- 9.Comstock CE, Gatsonis C, Newstead GM, et al. Comparison of abbreviated breast MRI vs digital breast tomosynthesis for breast cancer detection among women with dense breasts undergoing screening. JAMA. 2020;323(8):746-756. doi: 10.1001/jama.2020.0572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breast MRI to screen average risk women. Choosing Wisely. Accessed June 3, 2021. https://www.choosingwisely.org/clinician-lists/sso-breast-mri-to-screen-average-risk-women/
- 11.Hill DA, Haas JS, Wellman R, et al. Utilization of breast cancer screening with magnetic resonance imaging in community practice. J Gen Intern Med. 2018;33(3):275-283. doi: 10.1007/s11606-017-4224-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ganguli I, Lupo C, Mainor AJ, et al. Prevalence and cost of care cascades after low-value preoperative electrocardiogram for cataract surgery in fee-for-service Medicare beneficiaries. JAMA Intern Med. 2019;179(9):1211-1219. doi: 10.1001/jamainternmed.2019.1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ganguli I, Lupo C, Mainor AJ, et al. Assessment of prevalence and cost of care cascades after routine testing during the Medicare annual wellness visit. JAMA Netw Open. 2020;3(12):e2029891. doi: 10.1001/jamanetworkopen.2020.29891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma X, Wang R, Long JB, et al. The cost implications of prostate cancer screening in the Medicare population. Cancer. 2014;120(1):96-102. doi: 10.1002/cncr.28373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayes LM, Frebault JS, Landercasper J, et al. Extramammary findings in diagnostic breast magnetic resonance imaging among patients with known breast cancer: incidence and cost analysis. Am J Surg. 2016;212(6):1194-1200. doi: 10.1016/j.amjsurg.2016.09.020 [DOI] [PubMed] [Google Scholar]
- 16.Rinaldi P, Costantini M, Belli P, et al. Extra-mammary findings in breast MRI. Eur Radiol. 2011;21(11):2268-2276. doi: 10.1007/s00330-011-2183-6 [DOI] [PubMed] [Google Scholar]
- 17.Padia SA, Freyvogel M, Dietz J, Valente S, O’Rourke C, Grobmyer SR. False-positive extra-mammary findings in Breast MRI: another cause for concern. Breast J. 2016;22(1):90-95. doi: 10.1111/tbj.12524 [DOI] [PubMed] [Google Scholar]
- 18.Peters NHGM, Borel Rinkes IHM, Zuithoff NPA, Mali WPTM, Moons KGM, Peeters PHM. Meta-analysis of MR imaging in the diagnosis of breast lesions. Radiology. 2008;246(1):116-124. doi: 10.1148/radiol.2461061298 [DOI] [PubMed] [Google Scholar]
- 19.Buist DSM, Abraham L, Lee CI, et al. ; Breast Cancer Surveillance Consortium . Breast biopsy intensity and findings following breast cancer screening in women with and without a personal history of breast cancer. JAMA Intern Med. 2018;178(4):458-468. doi: 10.1001/jamainternmed.2017.8549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saadatmand S, Geuzinge HA, Rutgers EJT, et al. ; FaMRIsc study group . MRI versus mammography for breast cancer screening in women with familial risk (FaMRIsc): a multicentre, randomised, controlled trial. Lancet Oncol. 2019;20(8):1136-1147. doi: 10.1016/S1470-2045(19)30275-X [DOI] [PubMed] [Google Scholar]
- 21.Kuhl CK, Keulers A, Strobel K, Schneider H, Gaisa N, Schrading S. Not all false positive diagnoses are equal: on the prognostic implications of false-positive diagnoses made in breast MRI versus in mammography /digital tomosynthesis screening. Breast Cancer Res. 2018;20(1):13. doi: 10.1186/s13058-018-0937-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niell BL, Bennett D, Sharma A, Gazelle GS. Extramammary findings on breast MR examinations: frequency, clinical relevance, and patient outcomes. Radiology. 2015;276(1):56-64. doi: 10.1148/radiol.14141539 [DOI] [PubMed] [Google Scholar]
- 23.Iodice D, Di Donato O, Liccardo I, et al. Prevalence of extramammary findings on breast MRI: a large retrospective single-centre study. Radiol Med. 2013;118(7):1109-1118. doi: 10.1007/s11547-013-0937-8 [DOI] [PubMed] [Google Scholar]
- 24.Verslegers I, Van Goethem M, Hufkens G, Biltjes I, Parizel PM. Extramammary findings in T2-weighted MR breast images. Eur J Radiol. 2012;81(suppl 1):S181-S182. doi: 10.1016/S0720-048X(12)70074-8 [DOI] [PubMed] [Google Scholar]
- 25.Geuzinge HA, Obdeijn IM, Rutgers EJT, et al. ; Familial MRI Screening (FaMRIsc) Study group . Cost-effectiveness of breast cancer screening with magnetic resonance imaging for women at familial risk. JAMA Oncol. 2020;6(9):1381-1389. doi: 10.1001/jamaoncol.2020.2922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saadatmand S, Tilanus-Linthorst MMA, Rutgers EJT, et al. Cost-effectiveness of screening women with familial risk for breast cancer with magnetic resonance imaging. J Natl Cancer Inst. 2013;105(17):1314-1321. doi: 10.1093/jnci/djt203 [DOI] [PubMed] [Google Scholar]
- 27.Ganguli I, Cui J, Thakore N, et al. Downstream cascades of care following high-sensitivity troponin test implementation. J Am Coll Cardiol. 2021;77(25):3171-3179. doi: 10.1016/j.jacc.2021.04.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wernli KJ, Callaway KA, Henderson LM, et al. Trends in screening breast magnetic resonance imaging use among US women, 2006 to 2016. Cancer. 2020;126(24):5293-5302. doi: 10.1002/cncr.33140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan I-W, Oeffinger KC, Shih Y-CT. Cost-sharing and out-of-pocket cost for women who received MRI for breast cancer screening. J Natl Cancer Inst. 2022;114(2):254:262. doi: 10.1093/jnci/djab150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.American Cancer Society recommendations for the early detection of breast cancer. American Cancer Society. Published 2021. Accessed March 15, 2022. https://www.cancer.org/cancer/breast-cancer/screening-tests-and-early-detection/american-cancer-society-recommendations-for-the-early-detection-of-breast-cancer.html
- 31.Census regions and divisions of the United States. US Census Bureau. https://www2.census.gov/geo/pdfs/maps-data/maps/reference/us_regdiv.pdf
- 32.Sun JW, Rogers JR, Her Q, et al. Adaptation and validation of the combined comorbidity score for ICD-10-CM. Med Care. 2017;55(12):1046-1051. doi: 10.1097/MLR.0000000000000824 [DOI] [PubMed] [Google Scholar]
- 33.Durning M. Breast density notification laws by state: interactive map. Diagnostic Imaging. Published 2017. Accessed June 3, 2021. https://www.diagnosticimaging.com/view/breast-density-notification-laws-state-interactive-map
- 34.Huo J, Xu Y, Sheu T, Volk RJ, Shih YT. Complication rates and downstream medical costs associated with invasive diagnostic procedures for lung abnormalities in the community setting. JAMA Intern Med. 2019;179(3):324-332. doi: 10.1001/jamainternmed.2018.6277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phi XA, Greuter MJW, Obdeijn IM, et al. Should women with a BRCA1/2 mutation aged 60 and older be offered intensified breast cancer screening: a cost-effectiveness analysis. Breast. 2019;45:82-88. doi: 10.1016/j.breast.2019.03.004 [DOI] [PubMed] [Google Scholar]
- 36.Mango VL, Goel A, Mema E, Kwak E, Ha R. Breast MRI screening for average-risk women: a monte carlo simulation cost-benefit analysis. J Magn Reson Imaging. 2019;49(7):e216-e221. doi: 10.1002/jmri.26334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yeh JM, Lowry KP, Schechter CB, et al. Clinical benefits, harms, and cost-effectiveness of breast cancer screening for survivors of childhood cancer treated with chest radiation: a comparative modeling study. Ann Intern Med. 2020;173(5):331-341. doi: 10.7326/M19-3481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen RA, Martinez ME, Zammitti EP. Health insurance coverage: early release of estimates from the National Health Interview survey, January-March 2018. National Center for Health Statistics. Accessed March 10, 2022. https://www.cdc.gov/nchs/data/nhis/earlyrelease/insur202111.pdf
- 39.Evans A, Vinnicombe S. Overdiagnosis in breast imaging. Breast. 2017;31:270-273. doi: 10.1016/j.breast.2016.10.011 [DOI] [PubMed] [Google Scholar]
- 40.Drug Safety Podcast. FDA warns that gadolinium-based contrast agents (GBCAs) are retained in the body; requires new class warnings. US Food and Drug Administration. Accessed July 18, 2021. https://www.fda.gov/drugs/fda-drug-safety-podcasts/fda-drug-safety-podcast-fda-warns-gadolinium-based-contrast-agents-gbcas-are-retained-body-requires
- 41.Miles RC, Onega T, Lee CI. Addressing potential health disparities in the adoption of advanced breast imaging technologies. Acad Radiol. 2018;25(5):547-551. doi: 10.1016/j.acra.2017.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Relevant Choosing Wisely Recommendation
eTable 1. Diagnosis and Procedure Codes Used in MRI and Mammography Cohort Selection
eTable 2. Diagnosis and Procedure Codes for Propensity to Receive Breast MRI
eTable 3. Procedure Codes for Cascade Laboratory Tests
eTable 4. Procedure Codes for Cascade Imaging Tests
eTable 5. Procedure Codes for Cascade Procedures
eTable 6. Procedure Codes for Cascade Visits and Specialty Codes for Cascade Specialty Visits
eTable 7. Diagnosis Codes for Cascade New Diagnoses
eTable 8. Cascade Event Rates and Total and Out-of-Pocket Spending on Mammary Cascade Services Among Screening Breast MRI vs Mammography Recipients in the 6 Months Following the Screening Test, Sensitivity Analysis Including Mammograms as Cascade Events
eTable 9. Total and Out-of-Pocket Spending on Mammary Cascade Services, Extra-Mammary Cascade Services, and All Services Among Screening Breast MRI vs Mammography Recipients in the 6 Months Following the Screening Test, Including Spending on the Index Event