Abstract
Different ofloxacin-loaded unilamellar vesicles were prepared by the extrusion technique, and their antimicrobial activities were determined in comparison to those of the free drug by means of MIC determinations with both American Type Culture Collection standards and wild-type bacterial strains (six strains of Enterococcus faecalis, seven strains of Escherichia coli, six strains of Staphylococcus aureus, and six strains of Pseudomonas aeruginosa). The accumulation of ofloxacin and liposome-ofloxacin was measured by determining the amount of the drug inside the bacteria as a function of time. Encapsulated fluoroquinolone yielded MICs which were at least twofold lower than those obtained with the free drug. In particular, liposomes made up of dimyristoylphosphatidylcholine-cholesterol-dipalmitoylphosphatidylserine and dimyristoylphosphatidylcholine-cholesterol-dihexadecylphosphate (4:3:4 molar ratio) provided the best improvement in antimicrobial activity against the various bacterial strains investigated. The liposome formulation produced higher intracellular fluoroquinolone concentrations than those achieved simultaneously with the free drug in both E. coli and P. aeruginosa.
Since the discovery of the original DNA gyrase inhibitor nalidixic acid, numerous structural modifications have been carried out to the quinolone nucleus to increase antimicrobial activity and improve pharmacokinetic performance (12, 22, 29, 46).
The efficacy of fluoroquinolone antibiotics has led to their proposed use for the treatment and prophylaxis of different bacterial diseases: therapy for the respiratory tract, skin structure, and bone and gastrointestinal infections, as well as urinary tract infections (20, 21, 23, 36, 41). However, many studies were developed to improve the potency and spectrum, to achieve sustained blood levels, and to reduce as much as possible drug interactions with various metabolic pathways and physiological processes. Particularly, the use of antibiotic “carrier/delivery systems” would result in enhanced concentrations of the antimicrobial agent at the site of infection. In fact, delivery systems can contribute to (i) targeting of the drug to the infected tissues, (ii) increasing intracellular antibiotic concentrations, and (iii) reducing toxicity of potentially toxic drugs resulting from the targeting to the infectious organisms.
Liposomes are possible carriers for controlled drug delivery and targeting by the intravenous route. As with most drug carriers, liposomes have been extensively used in an attempt to improve the selective delivery and the therapeutic index of antimicrobial agents (3, 47). Liposomes, artificial phospholipid membranes, are usually produced from naturally occurring, biodegradable, and nontoxic lipids, such as lecithin, cholesterol, and phosphatidylserine.
The aim of the study described here was to investigate the antimicrobial activity against and accumulation in bacteria of ofloxacin-loaded liposomes (of different lipid compositions) in comparison to those of free drugs. Our preliminary experiments demonstrated that ofloxacin-loaded liposomes showed an antibacterial activity that was the same as or better than that of the free drug (45). Moreover, the liposome formulation was able to deliver ofloxacin into McCoy cells in a greater amount (2.6-fold) than the free drug, improving antibiotic accumulation (16).
MATERIALS AND METHODS
Chemicals.
Dipalmitoyl-dl-α-phosphatidyl-l-serine (PS), cholesterol (Chol), lipopolysaccharide (LPS) obtained by trichloroacetic acid extraction, and l-α-phosphatidyl-dl-glycerol (PG) were obtained from Sigma Chemical Co. (St. Louis, Mo.). Dihexadecyl hydrogen phosphate (DP), 1,2-dimyristoyl-sn-glycero-phosphocholine monohydrate (MC), 1,2-dipalmitoyl-sn-glycero-phosphoethanolamine (PE), 1,2-dipalmitoyl-sn-glycero-phosphatidic acid sodium salt (PA), dipicolinic acid (DPA; pyridine-2,6-dicarboxylic acid), and terbium(III) chloride were obtained from Fluka Chemical Co. (Buchs, Switzerland). Before use, the lipid purity (greater than 99%) was assayed by two-dimensional thin-layer chromatography on silica gel plates (E. Merck, Darmstadt, Germany) (14). Ofloxacin was a gift from Sigma-Tau S.p.A. (Pomezia, Italy). The purity of the drug was greater than 99.5%, as assayed by high-performance liquid chromatography (HPLC) analysis. Double-distilled water was used throughout. All other materials and solvents (Carlo Erba, Milano, Italy) were of analytical grade.
Strains.
Escherichia coli ATCC 25922, E. coli ATCC 35218, Enterococcus faecalis ATCC 29212, Staphylococcus aureus ATCC 29213, Pseudomonas aeruginosa ATCC 27853, and 20 recent clinical isolates (5 strains of E. faecalis, 5 strains of E. coli, 5 strains of S. aureus, and 5 strains of P. aeruginosa resistant to quinolones) were investigated for this study.
Liposome preparation.
The desired amount (50 mg) of lipid components of the liposome matrix were solubilized in chloroform (5 ml) in a round-bottom flask in the presence of 10 g of glass beads (mean diameter, 2 to 3 mm) (Carlo Erba). The organic solvent was evaporated off at 30°C on a rotating evaporator under a nitrogen stream and stored overnight under high vacuum to form a thin lipid film to be deposited on the inner wall of glass vials and on the glass bead surface. Liposomes were prepared by dispersing the lipid component films of the various liposomal formulations in an isotonic phosphate buffer solution (1 ml) containing ofloxacin (20 mg). This procedure was carried out at 55°C under mechanical stirring. The liposome suspension was submitted to 10 cycles of freezing (liquid nitrogen) and thawing (40°C in a water bath) and was then extruded through two (stacked) polycarbonate membranes (diameter, 25 mm; Nucleopore Corp., Pleasanton, Calif.) at 50°C at a pressure of 4,100 kPa. For the extrusion, a stainless steel extrusion device (Lipex Biomembranes, Vancouver, British Columbia, Canada) was used (13). To obtain small unilamellar vesicles, the extrusion procedure used consisted of 10 passages through 400-nm-pore-size filters, followed by another cycle of 10 passages through 200-nm-pore-size membrane filters. Different unilamellar liposome formulations were prepared. Liposomes were made up of MC, Chol, and a charged phospholipid (PS, DP, PE, or PA) at a molar ratio of 4:3:4, respectively.
The untrapped drug was separated from the liposome colloidal suspension by gel permeation chromatography, as previously described (16). Liposome drug loading was evaluated by HPLC analysis (32), after destruction of the vesicular carrier with a mixture of methanol-acetonitrile (8:2 [vol/vol]). Results are expressed as drug content and encapsulation capacity (5). The encapsulation parameter, which is defined as the encapsulation capacity (milliliters/millimoles), is a function of the liposome size and the number of lamellae per vesicle. The encapsulation capacity is calculated as follows: encapsulation capacity = Cf/(C0 × C1), where C0 is the initial drug concentration, Cf is the final drug concentration, and C1 is the lipid concentration in the liposome suspension. The various concentrations are expressed as millimoles/milliliter. The drug content depends on the encapsulation capacity and on the lipid concentration in the vesicles. This parameter is calculated as follows: drug content = [D1/(D1 × L1)] × 100, where D1 is the amount of drug found in the liposomes and L1 is the amount of liposome lipids.
Morphological characterization of liposomes.
Photon correlation spectroscopy (PCS) (Zetamaster; Malvern Instruments Ltd., Sparing Lane South, Worcestershire, England) was used to determine the vesicle size. The experiments were carried out using a solid-state laser as a light source. The laser was a nominal 4.5-mW laser diode with a maximum output of 5 mW at 670 nm. The PCS measurements were carried out at a scattering angle of 90°. The correlation functions were performed by a Malvern PCS submicron particle analyzer and a third-order cumulant fitting (6, 11) with a dilation of 1.20 to obtain the mean diameter and polydispersity. In particular, the polydispersity index gives information on the size distribution of the colloidal sample; values lower than 0.3 are attributed to a narrow size distribution, and the lower the value the more homogeneous the colloidal population. The real and imaginary refractive indexes were set at 1.59 and 0.0, respectively. The following parameters were used for experiments: medium refractive index, 1.330; medium viscosity, 1.0, and dielectric constant, 79. The samples were suitably diluted with filtered water (Sartorius membrane filters, 0.22-μm pore size) to avoid multiscattering phenomena and were placed in a quartz cuvette. Thirty measurements were obtained for each sample.
The morphological characterization was carried out by freeze-fracture electron microscopy using the propane-jet technique (35). The samples were fractured at −165°C, and platinum-carbon replicas were observed in a Philips EM 301 electron microscope at 100 kV.
The liposome lamellarity was evaluated by 31P-nuclear magnetic resonance (31P-NMR) spectroscopy (49). Mn2+ was added to the vesicle colloidal suspension (2 ml; 50 μmol of phospholipid per ml in an NMR tube) at levels high enough (5 mM) to eliminate the 31P-NMR signal arising from those phospholipids facing the external medium. Proton-decoupled 31P-NMR spectra were obtained with a GN500 MHz spectrometer operating at 202.45 MHz. Trimethyl phosphate (10% in D2O) was used as a reference and set at 0.0 ppm. The average number of bilayers (N) was calculated from the following expression: N = 100/(2 × RLOS), where RLOS is the percentage of the relative loss of signal found before and after Mn2+ addition (18).
Vesicle fusion experiments.
The fusogenic properties of the various liposome formulations were investigated by means of assays for mixing aqueous vesicle contents by using MC-PG-LPS (5:3:2 molar ratio) vesicles as a model system of bacterial outer membranes (53). This kind of study is based on the interaction between Tb(III) and DPA forming the fluorescent Tb(DPA)33− complex (51). The fluorescence intensity of Tb(III) in aqueous solution on its own was very low (4, 51), whereas it became higher, up to 104-fold, following the interaction with DPA (55). In order to evaluate the fusogenic properties, Tb(III) was encapsulated in MC-PG-LPS vesicles, and DPA in liposomes was made up of various phospholipid mixtures. The complex Tb(DPA)33− was excited at 276 nm, and the emission fluorescence was measured at 492 and 554 nm. Tb(III)-loaded vesicles were prepared in the presence of 2.5 mM TbCl3 and 50 mM sodium citrate, following the freeze-and-thaw procedure, and then extruded through 1.2-μm-pore-size membranes, as described above. Liposomes (mean diameter, 200 nm) made up of various phospholipid mixtures were prepared in the presence of 50 mM DPA. The free probe molecules were removed by gel-permeation chromatography. The fluorescence intensity of the various vesicles with entrapped DPA or Tb(III) was measured after making a 1:5 dilution with isotonic phosphate buffer to avoid fluorescence quenching (λex, 276 nm). The fluorescence data represent the measured fluorescence intensity divided by the fluorescence intensity obtained after complete release of the vesicle-entrapped components following cholate addition (to yield a 3% [wt/vol] concentration). Experiments were carried out at 37 ± 0.05°C (Haake F3-R).
Susceptibility test procedure.
The antimicrobial activity of ofloxacin-loaded liposomes was determined in comparison with that of the free drug, with MICs determined by using the standard broth microdilution assay (37). The liposome suspension containing ofloxacin was added in order to obtain an equivalent drug concentration with respect to that of the free drug solution, thus making the direct comparison of the results possible to evaluate the effectiveness of the liposome carrier. Mueller-Hinton broth was replaced by Iso-Sensitest broth (Oxoid, Basingstoke, United Kingdom) as previously described (45). Stock solutions of liposomo-ofloxacin (2,560 μg/ml in Iso-Senstitest broth) and ofloxacin (512 μg/ml in Iso-Sensitest from an original stock in water at 5,120 μg/ml) were prepared and diluted as proposed by National Committee for Clinical Laboratory Standards (37). A total of 11 concentrations of each sample were prepared. A suspension of organisms (1 μl of a suspension containing 107 CFU/ml) was added to each well. A positive control (growth) consisting of organisms in broth, a negative control (sterility) consisting of uninoculated broth, drug control consisting of broth containing the highest concentrations of drug, and drug-free liposomes (concentrations 1, 10, and 100 times higher than those used throughout the experiments) were included for each bacterial strain tested. Plates were sealed with transparent acetate and incubated at 37°C under atmospheric conditions for up to 18 h. Each assay was repeated six times with each antimicrobial agent formulation and six additional times on a different day with all formulations to ensure reproducibility of results.
Drug uptake.
The accumulation of ofloxacin and ofloxacin-loaded liposomes was measured as previously described by us (15), by a method derived from those described by Chapman and Georgopapadakou (10), Mortimer and Piddock (34), and Asuquo and Piddock (2). In the same way as for the susceptibility test, the amount of ofloxacin added to the bacterial cell suspensions as free drug or entrapped within liposomes was equal. E. coli was grown in Iso-Sensitest broth with shaking at 37°C up to an optical density of 0.8 at 660 nm, while P. aeruginosa was grown in the same conditions, but at 35°C and up to an optical density of 0.65 at 470 nm (2, 34). Bacteria were harvested by centrifugation at 4,470 × g for 10 min at 20°C. For E. coli, the pellet was resuspended in 50 mM cold sodium phosphate buffer (pH 7.0), washed, and concentrated 20-fold in the same buffer. For P. aeruginosa, the pellet was resuspended in 30 mM cold sodium phosphate buffer (pH 7.0) containing 5% glucose, washed, and concentrated 20-fold in the same buffer (2, 15, 34). Aliquots of 15 ml were poured into a sterile bottle containing a stir bar and allowed to equilibrate at 37°C in a water bath on a magnetic stirrer for 10 min. A zero-time sample for each strain was removed, and the two formulations of ofloxacin were added to yield a final concentration of 10 μg/ml. At predetermined intervals, 0.5 ml of the solution was withdrawn and transferred to microcap centrifuge tubes containing 1 ml of chilled buffer; this mixture was immediately centrifuged at 12,000 × g for 5 min at 4°C. The pellet was washed once in 1 ml of buffer at 4°C and resuspended in 1 ml of 0.1 M glycine hydrochloride (pH 3.0) and incubated at 20°C overnight to lyse the bacterial cells. The suspension was then centrifuged twice at 20°C for 5 min to remove any cell debris. The concentration of the antibiotic was estimated by HPLC analysis. A Hewlett-Packard model 1050 system (Milan, Italy) equipped with a Hewlett-Packard 1046A fluorescence detector was used for liquid chromatography. A Rheodyne model 7125 loading injection valve (Cotati, Calif.) with a 100-μl loop was also used. The chromatographic apparatus was connected to a Hewlett-Packard model 3395 reporting integrator. The excitation and emission wavelengths were 286 and 452 nm, respectively. Chromatographic separation was carried out at room temperature with a Hypersil C18 cartridge column (5-μm-particle-size column, 125 by 4.6 mm [inner diameter]; Alltech, Milan, Italy), equipped with a direct-connect guard column. The eluent mixture consisted of acetonitrile and pH 4.5 buffer mixture (40:60 [vol/vol]). The mobile phase flow rate was 1 ml/min with a mean pressure of 85 atm. The eluent was filtered through a 0.2-μm-pore-size Teflon membrane (Spartan-3; Schleicher & Schuell, Keene, N.H.) and deaerated by ultrasonication prior to use, and Enoxacin was used as internal standard. The HPLC assay reproducibility was evaluated by repetitive analysis of bacterial cell suspensions spiked with a known amount of ofloxacin. The within-day reproducibility was 99.1% ± 1.7% (assay accuracy ± relative standard deviation; n = 9). The day-to-day reproducibility was 98.3% ± 2.5%. The lower detection limit of ofloxacin in biological samples was 3 ng/ml with a signal/noise ratio of 4:1. In the chromatographic analysis, no interference was observed from the other components present in the various samples. The results are expressed as nanograms of drug per milligram of protein (2, 15, 34).
RESULTS AND DISCUSSION
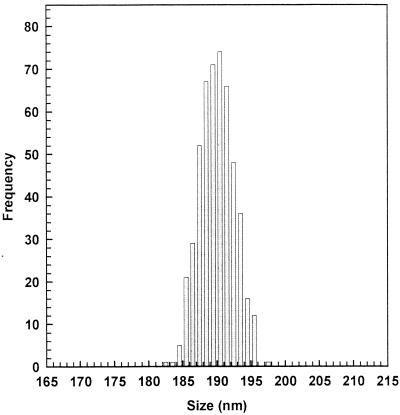
The mean size of a colloidal drug carrier is an important requisite that can influence the delivery device's biological effectiveness (26, 42). In order to obtain liposome colloidal suspensions with a reduced mean size, the extrusion through polycarbonate filters was carried out. Before extrusion, the various liposome suspensions were characterized by liposomes with a mean diameter greater than 1.5 μm and a polydispersity index value of ∼0.8, showing the presence of large vesicles with a very large size distribution. The extrusion procedure provided liposome suspensions with vesicles with a mean diameter of ∼190 nm, as evidenced by light-scattering experiments (Fig. 1). This procedure also allowed the formation of liposome suspensions with a very narrow size distribution having a polydispersity index of 0.01. The extrusion process also determined a significative reduction of the number of lamellae per vesicle, as shown by 31P-NMR spectroscopy (data not reported). In fact, the hydration of the lipid films, followed by freezing and thawing, led to the formation of multilamellar systems with a mean of 11 lamellae per vesicle; after the extrusion, the number of lamellae per vesicle was reduced to 2. Thus, a conversion from multilamellar to oligolamellar systems was achieved.
FIG. 1.
Size distribution of oligolamellar liposome suspension (MC-Chol-DP, 4:3:4 molar ratio) obtained by extrusion through polycarbonate filters (200-nm pore size). The sample was diluted to achieve the most suitable optical density for light-scattering analysis. The distribution function was determined by the Laplace inversion transform. Similar results were obtained with other liposome colloidal suspensions.
Before extrusion, large multilamellar vesicles were submitted to a freeze-and-thaw process to achieve a high drug-entrapping efficiency within liposomes (33). As reported in Table 1, the freeze-and-thaw procedure elicited an increase of ofloxacin loading capacity within various formulations of about three times compared to that of simple large multilamellar vesicles. It is noteworthy that the extrusion determined no variation of drug content and encapsulation capacity values of frozen and thawed liposomes. Enhanced colloidal properties and high drug carrier capacity represent two important parameters for an effective drug delivery system to be proposed in antibacterial chemotherapy.
TABLE 1.
Encapsulation parameters of the various liposome formulations as a function of the preparation procedure and lipid matrix compositiona
| Liposomesb | MLVc
|
FATMLVd
|
LUVETe
|
|||
|---|---|---|---|---|---|---|
| DCf | ECg | DC | EC | DC | EC | |
| MC-Chol-DP | 8.1 ± 2.4 | 5.8 ± 1.6 | 22.7 ± 3.8 | 16.3 ± 2.7 | 21.8 ± 2.6 | 15.7 ± 1.9 |
| MC-Chol-PS | 9.2 ± 2.7 | 6.5 ± 2.1 | 28.9 ± 4.3 | 20.8 ± 3.1 | 29.2 ± 5.2 | 21.0 ± 3.7 |
| MC-Chol-PE | 5.9 ± 1.8 | 4.3 ± 1.1 | 12.8 ± 3.5 | 9.2 ± 2.5 | 12.4 ± 3.1 | 8.9 ± 2.2 |
| MC-Chol-PA | 5.1 ± 2.9 | 3.6 ± 2.2 | 10.1 ± 2.9 | 7.1 ± 1.8 | 10.2 ± 3.3 | 7.3 ± 2.4 |
Each value is the average of five different experiments ± standard deviation.
MC, 1,2-dimyristoyl-sn-glycero-phosphocholine monohydrate; Chol, cholesterol; DP, dihexadecyl hydrogen phosphate; PS, dipalmitoyl-dl-α-phosphatidyl-l-serine; PE, 1,2-dipalmitoyl-sn-glycero-phosphoethanolamine; PA, 1,2-dipalmitoyl-sn-glycero-phosphatidic acid sodium salt.
Multilamellar vesicles (MLV) prepared by lipid film dispersion in isotonic phosphate buffer.
Freeze-and-thaw MLV (FATMLV).
Large unilamellar vesicles (LUVET) obtained from FATMLV by an extrusion technique through polycarbonate membranes.
Drug content (DC) as percentage of drug entrapped within liposomes.
Encapsulation capacity (EC) as microliters of aqueous solution entrapped per micromole of lipids.
Liposome formulations which are able to deliver their content directly into the cell cytoplasm by fusion with the plasma membrane could have a special advantage in terms of intracellular transport and hence antibacterial effectiveness. For this reason, the fusogenic properties of the various liposome formulations with respect to a bacterial outer membrane model were investigated by evaluating the increase of the fluorescence intensity at 492 and 554 nm due to the Tb(DPA)33− complex, which was formed following the fusion between Tb(III)-loaded MC-PG-LPS (5:3:2 molar ratio) membranes and DPA-loaded phospholipid mixture membranes.
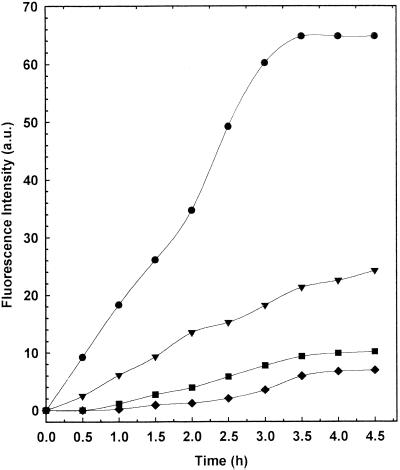
Membrane fusion leads to the formation of the Tb(DPA)33− complex, resulting in an increase in the fluorescence intensity of both bands as a function of the incubation time. As shown in Fig. 2, a noticeable increase in the fluorescence emission intensity at 492 and 554 nm was observed for liposomes presenting PS in their lipid composition. The other liposome formulations showed a lower fluorescence intensity increase (Fig. 2).
FIG. 2.
Fluorescence intensity profiles at 492 nm of the various liposome formulations. Symbols: ●, MC-Chol-PS (4:3:4 molar ratio); ▾, MC-Chol-DP (4:3:4 molar ratio); ■, MC-Chol-PA (4:3:4 molar ratio); ⧫, MC-Chol-PE (4:3:4 molar ratio). The experiments were carried out at 37 ± 0.05°C (mean ± standard deviation).
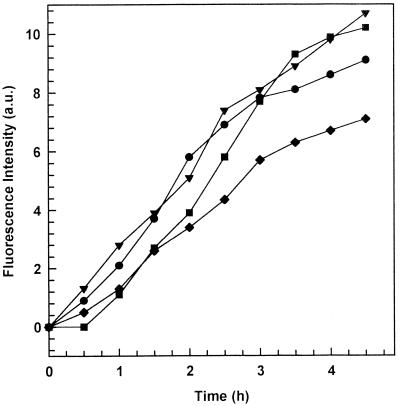
The formation of the highly fluorescent Tb(DPA)33− complex can be triggered not only by vesicle fusion but also by molecular transfer through the bulk aqueous phase or upon contact release. This possibility was evaluated by physically separating the MC-PG-LPS (5:3:2 molar ratio) bacterial outer membrane model from the various liposome formulations with a dialysis membrane. This experiment showed that the molecular transfer phenomenon also took place to a certain extent for all liposome formulations (Fig. 3). Only 13% of the fluorescence intensity increase can be attributed to a molecular transfer rather than to a fusion process for liposomes containing PS. For other formulations, taking into account data reported in Fig. 2 and 3, the molecular transfer phenomenon seemed to be the main process determining the fluorescence increase.
FIG. 3.
Fluorescence intensity profiles at 492 nm of the various liposome formulations as a function of time. The liposome formulations were separated from the bacterial outer membrane model made up of MC-PG-LPS (5:3:2 molar ratio) by means of a dialysis bag (molecular weight cutoff, 25,000). Symbols: ●, MC-Chol-PS (4:3:4 molar ratio); ▾, MC-Chol-DP (4:3:4 molar ratio); ■, MC-Chol-PA (4:3:4 molar ratio); ⧫, MC-Chol-PE (4:3:4 molar ratio). The experiments were carried out at 37 ± 0.05°C (mean ± standard deviation).
The in vitro susceptibilities to free drug and ofloxacin-loaded liposomes are shown for comparison. Ofloxacin-loaded liposomes yielded MICs that were different from those obtained with the free drug (Table 2). Differences were observed between the four liposome formulations. Unilamellar liposomes made up of MC-Chol-PS and MC-Chol-DP yielded MICs lower than those obtained with the free drug. In particular, a twofold MIC decrease was obtained with respect to that for P. aeruginosa, while the highest MIC decrease was achieved in the case of E. faecalis (16-fold decrease). MICs obtained with MC-Chol-PE and MC-Chol-PA matched those obtained with the free drug or were 1 dilution higher or lower. Free liposomes showed no activity against all the bacteria tested up to a concentration 100 times higher than that used throughout our experiments.
TABLE 2.
In vitro activity of different liposome formulations containing ofloxacin against reference strains
| Bacterial strains | MIC (μg/ml)
|
||||
|---|---|---|---|---|---|
| Free drug | MC-Chol-DP | MC-Chol-PS | MC-Chol-PE | MC-Chol-PA | |
| E. coli ATCC 25992 | 0.12 | 0.03 | 0.03 | 0.06 | 0.25 |
| E. coli ATCC 35218 | 0.12 | 0.03 | 0.03 | 0.12 | 0.25 |
| E. faecalis ATCC 29212 | 2 | 0.12 | 0.25 | 2 | 2 |
| S. aureus ATCC 29213 | 1 | 0.25 | 0.12 | 0.5 | 1 |
| P. aeruginosa ATCC 27853 | 1 | 0.5 | 0.5 | 2 | 1 |
Our findings show that the liposome composition is an important parameter that is able to influence the antibacterial effectiveness of the drug-loaded delivery devices. The presence of a negatively charged component able to interact with bacterial cell surfaces, i.e., PS and DP, in the constitution of the liposome matrix seems to be a fundamental requisite to ensure a certain improvement of the antibacterial effectiveness (PE component is positively charged). In fact, negatively charged phospholipids, such as PS and DP, can form hydrogen bonds and/or ionic interactions with various components which constitute the outer bacterial membranes, i.e., saccharide moieties of various natures, phospholipids (phosphatidylglycerol and diphytanoyl-phosphatidylcholine), glycosphingolipids, lipopolysaccharides, and peptidoglycan (30, 31).
Furthermore, the liposome bilayer fluidity and fusogenic properties seem to be other important factors in determining biological effectiveness. Fusogenic properties can cause the liposome carrier to fuse with the bacterial outer membrane, thus delivering its content into bacterial cells. Bilayer fluidity can be particularly important in the case of gram-positive bacteria, which have the peptidoglycan barrier hampering the direct contact with liposomes. Liposomes having a bilayer membrane with a certain fluidity can release their content after the interaction with the external peptidoglycan barrier, whereas liposomes which have a rigid bilayer structure release their content very slowly, thus being less effective against bacterial cells (25, 52). In particular, the PA component presents a polar head with a negative charge as well, but does not confer either a certain fluidity as DP does or fusogenic properties to the bilayer structure as PS does (13, 17, 28).
Liposome formulations made up of MC-Chol-PE and MC-Chol-PA were not investigated against fresh isolates because of poor improvement of antibacterial activity shown in the case of the reference strains assayed. The in vitro susceptibilities of 20 fresh isolates to both MC-Chol-DP and MC-Chol-PS liposome formulations are shown in Table 3. Similarly to previous results obtained with standard strains, MICs were lower than those obtained with the free drug. Four- and eightfold improvements were gained with E. coli and S. aureus, respectively, and up to 32-fold improvements were gained with E. faecalis. Differences were observed between the two formulations, namely that MC-Chol-DP was more active than MC-Chol-PS. In the case of P. aeruginosa, a lower MIC improvement was obtained. In fact, the best MIC improvement against P. aeruginosa was a fourfold decrease for the MC-Chol-DP liposome mixture.
TABLE 3.
In vitro activity of different liposome formulations containing ofloxacin against freshly isolated bacteria
| Bacteria | MIC (μg/ml)
|
||
|---|---|---|---|
| Free drug | MC-Chol-DP | MC-Chol-PS | |
| E. coli | 0.12 | 0.03 | 0.03 |
| E. coli | 0.12 | 0.03 | 0.03 |
| E. coli | 0.12 | 0.03 | 0.03 |
| E. coli | 0.12 | 0.03 | 0.03 |
| E. coli | 0.06 | 0.015 | 0.03 |
| E. faecalis | 4 | 0.25 | 0.5 |
| E. faecalis | 8 | 0.25 | 1 |
| E. faecalis | 4 | 0.12 | 0.5 |
| E. faecalis | 1 | 0.06 | 0.12 |
| E. faecalis | 1 | 0.06 | 0.12 |
| S. aureus | 0.12 | 0.03 | 0.03 |
| S. aureus | 0.5 | 0.12 | 0.06 |
| S. aureus | 0.5 | 0.06 | 0.06 |
| S. aureus | 0.5 | 0.12 | 0.06 |
| S. aureus | 1 | 0.25 | 0.12 |
| P. aeruginosa | 32 | 8 | 32 |
| P. aeruginosa | 32 | 8 | 32 |
| P. aeruginosa | 8 | 4 | 8 |
| P. aeruginosa | 16 | 8 | 8 |
| P. aeruginosa | 8 | 4 | 8 |
Three different entrance routes have been proposed for fluoroquinolones to penetrate cell envelopes of gram-negative bacteria: (i) the hydrophilic pathway through porin channels (40), (ii) the hydrophobic pathway through the membrane bilayer matrix (27), and (iii) the self-promoted uptake pathway (10). The first two entrance pathways seem to be influenced by some drug properties, such as hydrophobicity and the size and structure of fluoroquinolones (J. Pace, A. Bertasso, and N. H. Georgopadakou, Abstr. 91st Annu. Meet. Am. Soc. Microbiol. 1991, p. 16, 1991). Meanwhile, the self-promoted uptake route is based on the displacement of divalent cations (mainly Mg2+ and Ca2+) from the outer membrane lipopolysaccharides. How a vesicular carrier (unilamellar liposomes) can influence the penetration of a fluoroquinolone agent (ofloxacin) has been investigated.
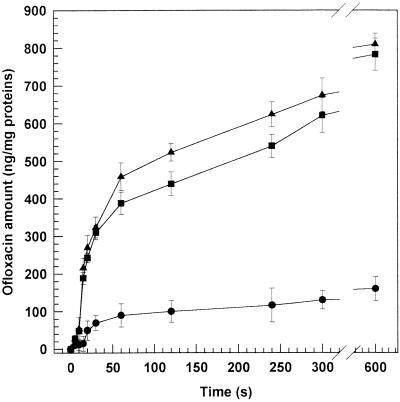
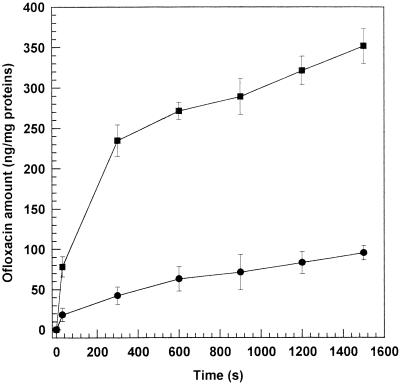
Accumulation of ofloxacin at a fixed concentration, both free and liposome entrapped, by a strain of E. coli (ATCC 25922) and a strain of P. aeruginosa (ATCC 27853) was examined. Owing to the lower MICs, only MC-Chol-DP liposomes were investigated. The results of the accumulation are shown in Fig. 4 and 5. In E. coli strains, a gradual drug entry followed the rapid phase of accumulation. Similar profiles were observed for P. aeruginosa. In this case, the accumulation time course was extended up to 25 min due to the slower and lower ofloxacin accumulation into P. aeruginosa (2). The liposome formulations reached significantly (P < 0.001) higher intracellular accumulations than the free drug with the two strains tested.
FIG. 4.
Intrabacterial accumulation of ofloxacin-loaded MC-Chol-DP (4:3:4 molar ratio) unilamellar liposomes within E. coli ATCC 25922 (■) and E. coli ATCC 35218 (▴) versus the free drug (E. coli ATCC 35218 accumulation) (●) as a function of time. Free drug accumulation within both E. coli strains was very similar (data not reported). The experiments were carried out at 37°C. Each point represents the mean value of five different experiments ± standard deviation.
FIG. 5.
Intrabacterial accumulation of ofloxacin-loaded MC-Chol-DP (4:3:4 molar ratio) unilamellar liposomes within P. aeruginosa ATCC 27853 (■) versus the free drug (●) as a function of time. The experiments were carried out at 37°C. Each point represents the mean value of five different experiments ± standard deviation.
These results can be due to the capability of the liposome carrier to interact with the bacterial cell outer membranes, altering the permeation conditions. In fact, liposomes presenting fusogenic properties, such as MC-Chol-PS vesicles, may fuse with the membrane, enhancing drug entry through the hydrophobic and/or self-promoted pathway. In fact, liposome fusion or vesicle-bacterial outer membrane phospholipid exchange may elicit an increase of disorder and fluidity of biological membrane bilayers, leading to greater membrane permeability. No particular effect should be exerted by the liposome carrier at the level of the porin entrance pathway, if for no other reason than due to the fact that an alteration of the membrane phospholipid matrix may trigger conformational changes of porin proteins.
Although ofloxacin bacterial outer membrane penetration and accumulation should correlate to some extent with differences in susceptibility, the latter is correlated not only to drug entrance (improved uptake) but also to the affinity with the drug to its target. In fact, our results show that liposome formulations can contribute to overcome bacterial resistance phenomena due to drug entrance (impermeability) (9, 48) by ensuring higher intrabacterial ofloxacin levels, but cannot be useful to avoid and/or circumvent DNA gyrase resistance (1, 24).
According to the literature (8), a susceptibility-drug accumulation relationship has also been observed in the case of P. aeruginosa. Namely, P. aeruginosa presents a poor outer membrane permeability (2), probably due to the presence of water-filled channels in this bacteria, such as porin F, that are substantially smaller than those of other gram-negative bacteria (55). This fact determines a lower permeability of hydrophilic antibiotics through the bacterial outer membrane. This intrinsic resistance, previously attributed only to the low permeability, is now recognized from the results of the synergy between broadly specific drug efflux pumps and low outer membrane permeability (39, 43, 50). Thus, an increased outer membrane permeability due to the liposome formulation is likely to contribute to the improved susceptibility of these organisms, as has been demonstrated also for other colloidal drug carriers (15).
Therefore, any condition determining a concentration gradient towards bacterial cell outer membranes could improve the drug permeation. Liposomes, besides having a direct interaction with the bacterial cell outer membrane, can also ensure “contact” and/or “juxtaproximal” release (38), that is, a massive drug release close to the bacterial cell surface (both outer membrane and peptidoglycan), allowing the formation of a drug concentration gradient and hence a higher rate of entrance than that of the free drug. The latter aspect is particularly important in the case of gram-positive bacteria, which present a peptidoglycan barrier that can hamper a direct contact with the cytoplasmic membrane. In this case, liposomes may interact with peptidoglycan and release ofloxacin, thus ensuring drug entrance. This possibility seems to be the more probable mechanism, rather than the liposome-bacterial membrane fusion, to explain the ofloxacin-loaded liposome activity greater than that of the free drug with respect to gram-positive bacteria (Table 3).
The initial step in the accumulation of fluoroquinolone antimicrobial agents, i.e., binding to cell surface components (7), is reduced by lowered pH and, under some conditions, by divalent cations (10). Fluoroquinolones with net charges (H2Q+, Q−) are not soluble in membrane structures; thus, accumulation would depend solely on the partition of the neutral forms (19, 43). Since inner pH is stable, the penetrating activities of quinolones in this model become simply a function of the outer pH. The uncharged species HQ0 is the best candidate to mediate passive diffusion for acidic quinolones, but the zwitterionic species (HQ+−) is very likely to be implicated in transmembrane diffusion as well (44). Therefore, considering these aspects and the fact that fluoroquinolones cross the cytoplasmic membrane by simple diffusion, ofloxacin-liposome encapsulation can also have the advantage of ensuring higher rates of drug entrance into the periplasmic space in spite of adverse environmental conditions (pH, presence of divalent cations, and drug physicochemical characteristics) and/or forms of bacterial resistance due to reduced drug permeability.
In conclusion, liposome drug delivery systems with suitable lipid compositions can improve the antibacterial effectiveness of loaded fluoroquinolone drugs, due to (i) a greater drug penetration within bacterial cells and (ii) protection against unfavorable environmental conditions. Liposome colloidal suspensions could be a suitable tool to improve selective drug delivery. In particular, liposomes can be used for the treatment of infection involving the mononuclear phagocyte systems, which take up colloidal carriers after systemic administration. Other body sites can be reached by modulating liposome size, lipid composition, and surface characteristics.
REFERENCES
- 1.Aoyama H, Sato K, Fujii T, Fugimaki K K, Inoue M, Mitsuhashi S. Purification of Citrobacter freundii DNA gyrase and inhibition of quinolones. Antimicrob Agents Chemother. 1988;32:104–109. doi: 10.1128/aac.32.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asuquo A E, Piddock L J V. Accumulation and killing of fifteen quinolones for Escherichia coli, Staphylococcus aureus and Pseudomonas aeruginosa. J Antimicrob Chemother. 1993;31:865–880. doi: 10.1093/jac/31.6.865. [DOI] [PubMed] [Google Scholar]
- 3.Bakker-Woundenberg I A J M, Lokerse A F, ten Kate M T, Melissen P M B, van Vianen W, van Ettenn E W M. Liposomes as carriers of antimicrobial agents or immunomodulatory agents in the treatment of infections. Eur J Clin Microbiol Infect Dis. 1993;12:61–67. doi: 10.1007/BF02389881. [DOI] [PubMed] [Google Scholar]
- 4.Barela T D, Sherry A D. A simple, one-step fluorometric method for determination of nanomolar concentrations of terbium. Anal Biochem. 1976;71:351–357. doi: 10.1016/s0003-2697(76)80004-8. [DOI] [PubMed] [Google Scholar]
- 5.Benita S, Poly P A, Puisieux F, Delattre J. Radiopaque liposomes: effect of formulation conditions on encapsulation efficiency. J Pharm Sci. 1984;73:1751–1754. doi: 10.1002/jps.2600731223. [DOI] [PubMed] [Google Scholar]
- 6.Berne B, Pecora R. Dynamic light scattering. New York, N.Y: John Wiley and Sons; 1976. [Google Scholar]
- 7.Bryan L E, Bedard J. Impermeability to quinolones in Gram-positive and Gram-negative bacteria. Eur J Clin Microbiol Infect Dis. 1991;10:232–239. doi: 10.1007/BF01966995. [DOI] [PubMed] [Google Scholar]
- 8.Celesk R A, Robillard N J. Factors influencing the accumulation of ciprofloxacin in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1989;33:1921–1926. doi: 10.1128/aac.33.11.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chamberland S, Bayer A S, Scholiaardt T, Wong S A, Bryan L E. Characterization of mechanisms of quinolone resistance in Pseudomonas aeruginosa strains isolated in vitro and in vivo during experimental endocarditis. Antimicrob Agents Chemother. 1989;33:624–634. doi: 10.1128/aac.33.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chapman J S, Georgopapadakou N H. Routes of quinolone permeation in Escherichia coli. Antimicrob Agents Chemother. 1988;32:438–442. doi: 10.1128/aac.32.4.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chu B. Laser light scattering. New York, N.Y: Academic Press; 1974. [Google Scholar]
- 12.Ferrero L, Cameron B, Crouzet J. Analysis of gyrA and grlA mutations in stepwise-selected ciprofloxacin-resistant mutants of Staphylococcus aureus. Antimicrob Agents Chemother. 1995;39:1554–1558. doi: 10.1128/aac.39.7.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fresta M, Spadaro A, Cerniglia G, Ropero I M, Puglisi G, Furneri P M. Intracellular accumulation of ofloxacin-loaded liposomes in human synovial fibroblasts. Antimicrob Agents Chemother. 1995;39:1372–1375. doi: 10.1128/aac.39.6.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fresta M, Villari A, Puglisi G, Cavallaro G. 5-Fluorouracil various kinds of loaded liposomes: encapsulation efficiency, storage stability and fusogenic properties. Int J Pharm. 1993;99:146–157. [Google Scholar]
- 15.Fresta M, Wehrli E, Puglisi G. Enhanced therapeutic effect of cytidine-5I-diphosphate choline when associated with GM1 containing small liposomes as demonstrated in a rat ischemia model. Pharm Res. 1995;12:1769–1774. doi: 10.1023/a:1016234226404. [DOI] [PubMed] [Google Scholar]
- 16.Fresta M, Puglisi G, Giammona G, Cavallaro G, Micali N, Furneri P M. Pefloxacine mesilate- and ofloxacin-loaded polyethylcyanoacrylate nanoparticles: characterization of the colloidal drug carrier formulation. J Pharm Sci. 1995;84:895–902. doi: 10.1002/jps.2600840721. [DOI] [PubMed] [Google Scholar]
- 17.Fresta M, Furneri P M, Mezzasalma E, Nicolosi V M, Puglisi G. Correlation of trimethoprim and brodimoprim physicochemical and lipid membrane interaction properties with their accumulation in human neutrophils. Antimicrob Agents Chemother. 1996;40:2865–2873. doi: 10.1128/aac.40.12.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fresta M, Chillemi R, Spampinato S, Sciuto S, Puglisi G. Liposomal delivery of a 30-Mer antisense oligodeoxynucleotide to inhibit proopiomelanocortin expression. J Pharm Sci. 1999;87:616–625. doi: 10.1021/js9702978. [DOI] [PubMed] [Google Scholar]
- 19.Furet Y X, Deshusses J, Pechere J C. Transport of pefloxacin across the bacterial cytoplasmatic membrane in quinolone-susceptible Staphylococcus aureus. Antimicrob Agents Chemother. 1992;36:2506–2511. doi: 10.1128/aac.36.11.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gimeno C, Borja J, Navarro D, Valdes L, Garcia-Barbal J, Garcia-de-Lomas J. In vitro interaction between ofloxacin and cefotaxime against gram-positive and gram-negative bacteria involved in serious infections. Chemotherapy. 1998;44:94–98. doi: 10.1159/000007098. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein E J, Citron D M, Hudspeth M, Gerardo S H, Merriam C V. Trovafloxacin compared with levofloxacin, ofloxacin, ciprofloxacin, azithromycin and clarithromycin against unusual aerobic and anaerobic human and animal bite-wound pathogens. J Antimicrob Chemother. 1998;41:391–396. doi: 10.1093/jac/41.3.391. [DOI] [PubMed] [Google Scholar]
- 22.Gootz T D, Brighty K E. Fluoroquinolone antibacterials: SAR, mechanism of action, resistance, and clinical aspects. Med Res Rev. 1996;16:433–486. doi: 10.1002/(SICI)1098-1128(199609)16:5<433::AID-MED3>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 23.Guibert J, Kitzis M D, Acar J F. Antibacterial activity of ofloxacin in urine for 4 days after a single oral dose of 400 mg. Pathol Biol. 1998;46:656–660. [PubMed] [Google Scholar]
- 24.Hirai K, Suzue S, Irikura T, Iyobe S, Mitsuhashi S. Mutations producing resistance to norfloxacin in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1987;31:582–586. doi: 10.1128/aac.31.4.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ito Y, Cai H, Koizumi Y, Hori R, Terao M, Kimura T, Takagi S, Tomohiro M. Effects of lipid composition on the transcorneal penetration of liposomes containing a potential anti-cancer agent, in the rabbit. Biol Pharm Bull. 2000;23:327–333. doi: 10.1248/bpb.23.327. [DOI] [PubMed] [Google Scholar]
- 26.Karajgi J, Jain N K, Vyas S P. Passive vectoring of a colloidal carrier system for sodium stibogluconate: preparation, characterization and performance evaluation. J Drug Target. 1993;1:197–206. doi: 10.3109/10611869308996077. [DOI] [PubMed] [Google Scholar]
- 27.Kotera Y, Watanabe M, Yoshida S, Inoue M, Mitsuhashi S. Factors influencing the uptake of norfloxacin by Escherichia coli. J Antimicrob Chemother. 1991;27:733–739. doi: 10.1093/jac/27.6.733. [DOI] [PubMed] [Google Scholar]
- 28.La Rosa C, Grasso D, Fresta M, Ventura C, Puglisi G. Phospholipid vesicles as drug delivery system. Part II. A study on kinetic fusion between vesicles containing CDP-choline and dipalmitoylphosphatidylcholine vesicles. Thermochim Acta. 1992;198:181–190. [Google Scholar]
- 29.Lee S, Park T, Lee Y. Structure-activity relationship of fluoroquinolone in Escherichia coli. Arch Pharm Res. 1998;21:106–112. doi: 10.1007/BF02974013. [DOI] [PubMed] [Google Scholar]
- 30.Lohner K, Prenner E J. Differential scanning calorimetry and X-ray diffraction studies of the specificity of the interaction of antimicrobial peptides with membrane-mimetic systems. Biochim Biophys Acta. 1999;1462:141–156. doi: 10.1016/s0005-2736(99)00204-7. [DOI] [PubMed] [Google Scholar]
- 31.Lohner K, Straudegger E, Prenner E J, Lewis R N, Kriechbaum M, Degovics G, McElhaney R N. Effect of staphylococcal δ-lysin on the thermotropic phase behavior and vesicle morphology of dimyristoylphosphatidylcholine lipid bilayer model membranes. Differential scanning calorimetric, 31P nuclear magnetic resonance and fourier transform infrared spectroscopic, and X-ray diffraction studies. Biochemistry. 1999;38:16514–16528. doi: 10.1021/bi9913101. [DOI] [PubMed] [Google Scholar]
- 32.Macek J, Ptacek P. Determination of ofloxacin in human plasma using high-performance liquid chromatography and fluorescence detection. J Chromatogr B. 1995;673:316–319. doi: 10.1016/0378-4347(95)00263-5. [DOI] [PubMed] [Google Scholar]
- 33.Mayer L D, Hope M J, Cullis P R, Janoff A S. Solute distribution and trapping efficiencies in freeze-thawed multilamellar vesicles. Biochim Biophys Acta. 1985;817:193–196. doi: 10.1016/0005-2736(85)90084-7. [DOI] [PubMed] [Google Scholar]
- 34.Mortimer P G S, Piddock L J V. A comparison of methods used for measuring the accumulation of quinolones by Enterobacteriaceae, Pseudomonas aeruginosa and Staphylococcus aureus. J Antimicrob Chemother. 1991;28:639–653. doi: 10.1093/jac/28.5.639. [DOI] [PubMed] [Google Scholar]
- 35.Müller M, Meister C, Moor H. Freezing in a propane jet and its application in freeze-fracturing. Mikroskopie. 1980;36:129–140. [PubMed] [Google Scholar]
- 36.Naber K G, Well M, Hollauer K, Kirchbauer D, Witte W. In vitro activity of enoxacin versus ciprofloxacin, lomefloxacin, ofloxacin, pefloxacin, and rufloxacin against uropathogens. Chemotherapy. 1998;44:77–84. doi: 10.1159/000007096. [DOI] [PubMed] [Google Scholar]
- 37.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically–5th ed. Approved standard M7-A5. Wayne, Pa: National Committee for Clinical Laboratory Standards; 2000. [Google Scholar]
- 38.New R R C, Black C D V, Parker R J, Puri A, Scherphof G L. Liposomes in biological systems. In: New R R C, editor. Liposomes, a practical approach. I.R.L. New York, N.Y: Press-Oxford University Press; 1990. pp. 221–252. [Google Scholar]
- 39.Nikaido H. Outer membrane barrier as mechanisms of antimicrobial resistance. Antimicrob Agents Chemother. 1989;33:1831–1836. doi: 10.1128/aac.33.11.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nikaido H, Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985;49:1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Onrust S V, Lamb H M, Balfour J A. Ofloxacin. A reappraisal of its use in the management of genitourinary tract infections. Drugs. 1998;56:895–928. doi: 10.2165/00003495-199856050-00015. [DOI] [PubMed] [Google Scholar]
- 42.Perez-Soler R, Khokhar A R. Lipophilic cisplatin analogues entrapped in liposomes: role of intraliposomal drug activation in biological activity. Cancer Res. 1992;52:6341–6347. [PubMed] [Google Scholar]
- 43.Piddock L J. Mechanisms of fluoroquinolone resistance: an update 1994–1998. Drugs. 1999;58:11–18. doi: 10.2165/00003495-199958002-00003. [DOI] [PubMed] [Google Scholar]
- 44.Piddock L J, Jin Y F, Ricci V, Asuquo A E. Quinolone accumulation by Pseudomonas aeruginosa, Staphylococcus and Escherichia coli. J Antimicrob Chemother. 1999;43:61–70. doi: 10.1093/jac/43.1.61. [DOI] [PubMed] [Google Scholar]
- 45.Puglisi G, Fresta M, Mazzone G, Furneri P M, Tempera G. Formulation parameters of fluoroquinolones-loaded liposomes and in vitro antimicrobial activity. Int J Pharm. 1995;118:65–76. [Google Scholar]
- 46.Ramon M S, Canton E, Peman J, Pastor A, Martinez J P. Mechanisms of action of quinolones against staphylococci and relationship with their in vitro bactericidal activity. Chemotherapy. 1999;45:175–182. doi: 10.1159/000007180. [DOI] [PubMed] [Google Scholar]
- 47.Richardson V J. Liposomes in antimicrobial chemotherapy. J Antimicrob Chemother. 1983;12:532–534. doi: 10.1093/jac/12.6.532. [DOI] [PubMed] [Google Scholar]
- 48.Robillard N J, Scarpa A L. Genetic and physiological characterization of ciprofloxacin resistance in Pseudomonas aeruginosa PAO. Antimicrob Agents Chemother. 1988;32:535–539. doi: 10.1128/aac.32.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwartz M A, McConnell H M. Surface areas of lipid membranes. Biochemistry. 1978;17:837–840. doi: 10.1021/bi00598a014. [DOI] [PubMed] [Google Scholar]
- 50.Skrikumar R, Kon T, Gotoh N, Poole K. Expression of Pseudomonas aeruginosa multidrug efflux pumps MexA-MexB-OprM and MexC-MexD-OprJ in a multidrug-sensitive Escherichia coli strain. Antimicrob Agents Chemother. 1998;42:65–71. doi: 10.1128/aac.42.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tas J, Westerneng G J. Fundamental aspects of the interaction of propidium diiodide with nuclei acids studied in a model system of polyacrylamide films. J Histochem Cytochem. 1981;29:929–936. doi: 10.1177/29.8.6168679. [DOI] [PubMed] [Google Scholar]
- 52.Thierry A R, Dritschilo A. Intracellular availability of unmodified, phosphorothioated and liposomally encapsulated oligodeoxynucleotides for antisense activity. Nucleic Acids Res. 1992;20:5691–5698. doi: 10.1093/nar/20.21.5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wiese A, Munstermann M, Gutsmann T, Lindner B, Kawahara K, Zahringer U, Seydel U. Molecular mechanisms of polymyxin B-membrane interactions: direct correlation between surface charge density and self-promoted transport. J Membr Biol. 1998;15:127–138. doi: 10.1007/s002329900350. [DOI] [PubMed] [Google Scholar]
- 54.Wilschut J, Duzgunes R F, Papahadjopoulos D. Studies on the mechanism of membrane fusion: kinetics of calcium ion induced fusion of phosphatidylserine vesicles followed by a new assay for mixing of aqueous vesicle contents. Biochemistry. 1980;19:6011–6021. doi: 10.1021/bi00567a011. [DOI] [PubMed] [Google Scholar]
- 55.Woodruff W A, Parr T R, Jr, Hancock R E W, Hanne L F, Nicas T I, Iglewski B H. Expression in Escherichia coli and function in Pseudomonas aeruginosa outer membrane porin protein F. J Bacteriol. 1986;167:473–479. doi: 10.1128/jb.167.2.473-479.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]