This randomized clinical trial evaluates the effect of ixekizumab on self-reported and objective measures of case severity, itch, adverse events, and other outcomes in children with plaque psoriasis.
Key Points
Question
What is the long-term (up to 108 weeks) efficacy and safety of ixekizumab for pediatric patients with moderate to severe psoriasis?
Findings
In this randomized clinical trial of 139 pediatric patients with plaque psoriasis, those who completed treatment with ixekizumab through week 108 achieved improvement in the Psoriasis Area and Severity Index as well as static Physician’s Global Assessment scores, and these results were sustained through week 108. There were no new safety findings, including no new cases of inflammatory bowel disease or candidal infection.
Meaning
Findings of this trial show that the safety of ixekizumab in this pediatric population was consistent with previously reported data and the known safety profile of this treatment.
Abstract
Importance
About 1% of children and adolescents worldwide are affected by plaque psoriasis.
Objective
To evaluate the long-term efficacy and safety of ixekizumab for pediatric patients with moderate to severe psoriasis.
Design, Setting, and Participants
This multicenter randomized clinical trial (IXORA-PEDS) evaluated pediatric patients with plaque psoriasis. Participants were aged 6 years to younger than 18 years; had moderate to severe psoriasis, which was defined as Psoriasis Area and Severity Index (PASI) of 12 or higher, static Physician’s Global Assessment (sPGA) score of 3 or higher, and psoriasis-affected body surface area of 10% or greater at screening and baseline; were candidates for phototherapy or systemic therapy; or had psoriasis that was not adequately controlled by topical therapies. Data analysis, which followed the intention-to-treat principle, was conducted from May to October 2021.
Interventions
Pediatric patients were randomized 2:1 to receive either a weight-based dose of ixekizumab every 4 weeks or placebo. After a 12-week placebo-controlled period, patients entered a 48-week, open-label ixekizumab maintenance period (weeks 12-60), followed by an extension period that lasted through 108 weeks. A substudy evaluated the randomized withdrawal of ixekizumab after week 60.
Main Outcomes and Measures
Efficacy outcomes at week 108 included the percentage of patients achieving 75% (PASI 75), 90% (PASI 90), or 100% (PASI 100) improvement from baseline; an sPGA score of 0 or 1 or score of 0; and improvement of 4 points or higher from baseline in the Itch Numeric Rating Scale. Safety outcomes included assessments of adverse events (AEs), including treatment-emergent AEs, serious AEs, and AEs of special interest, as well as improvement from baseline in a range of challenging body areas. Missing data for categorical outcomes were imputed using modified nonresponder imputation.
Results
A total of 171 patients (mean [SD] age, 13.5 [3.04] years; 99 female children [57.9%]) were randomized to either ixekizumab (n = 115) or placebo (n = 56). Of 166 patients who entered the maintenance period, 139 (83.7%) completed week 108 of the trial. Primary and gated secondary end points were sustained through week 108, with patients achieving PASI 75 (91.7% [n = 86]), PASI 90 (79.0% [n = 74]), PASI 100 (55.1% [n = 52]), sPGA 0 or 1 (78.3% [n = 74]), and sPGA 0 (52.4% [n = 49]). Fifty-five patients (78.5%) reported an Itch Numeric Rating Scale improvement of 4 points or higher. In patients who received ixekizumab, at week 108, clearance of nail psoriasis was reported in 68.1% (n = 28), clearance of palmoplantar psoriasis was reported in 90.0% (n = 10), clearance of scalp psoriasis was reported in 76.2% (n = 83), and clearance of genital psoriasis was reported in 87.5% (n = 24). There were no new safety findings during weeks 48 to 108 of the trial, including no new cases of inflammatory bowel disease or candida infection.
Conclusions and Relevance
Results of this study showed improvements across patient-reported outcomes and objective measures of complete skin clearance of psoriasis among pediatric patients who received ixekizumab, and these response rates were sustained through week 108 of the trial. Safety of ixekizumab was consistent with previously reported findings in this population and the known safety profile of this treatment.
Trial Registration
ClinicalTrials.gov Identifier: NCT03073200
Introduction
Pediatric plaque psoriasis affects approximately 1% of children and adolescents,1,2 with a median age at onset of 7 to 10 years.3 This condition impairs the quality of life4 of pediatric patients4 and their parents,5 interfering with self-esteem, family and social relationships, school, and work-life balance.6,7 Psoriasis in certain locations, such as the face, scalp, palms and soles, nails, and genital region, has a disproportionately greater effect on a patient’s quality of life because of its high visibility or challenge in treating given the involvement of more sensitive areas that may be more recalcitrant to topical agents.8 Similar to adults, pediatric patients with psoriasis can have comorbidities, including psoriatic arthritis, obesity, Crohn disease, hypertension, diabetes, and psychiatric disorders.9
Phototherapy, methotrexate, and potent topical therapies have long been the mainstays of treatment for moderate to severe psoriasis and have been prescribed off-label for pediatric plaque psoriasis. However, 5 biologics (etanercept, adalimumab, ustekinumab, secukinumab, and ixekizumab) for pediatric psoriasis have recently been approved by the European Medicines Agency, 4 of which (etanercept, ustekinumab, secukinumab, and ixekizumab) have also been approved by the US Food and Drug Administration.10,11 These biologics are highly efficacious with excellent tolerance and safety profiles.4
Ixekizumab, which is approved as a first-line option for treatment of moderate to severe psoriasis in children aged 6 years to younger than 18 years,1,4 was found to be superior to placebo after 12 weeks of treatment, and these responses were sustained through week 48 in the IXORA-PEDS (Multicenter, Double-Blind, Randomized, Placebo-Controlled Study to Evaluate Safety, Tolerability, and Efficacy of Ixekizumab in Patients From 6 to Less Than 18 Years of Age With Moderate-to-Severe Plaque Psoriasis) trial, a phase 3 randomized clinical trial.1 In this trial, we evaluated the long-term efficacy and safety of ixekizumab for pediatric patients with moderate to severe psoriasis up to 108 weeks.
Methods
Study Design
The IXORA-PEDS trial was conducted from March 28, 2017, to March 23, 2021, in accordance with the ethical principles of the Declaration of Helsinki.12 Local investigators or appropriate representatives at each of the 68 participating sites provided documentation of its ethical review board approval of the protocol, and the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use assent form was provided to Eli Lilly before the study began at the site. Parents or legal guardians provided written informed consent, and patients provided written assent before the study assessments, examinations, and/or procedures. We followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
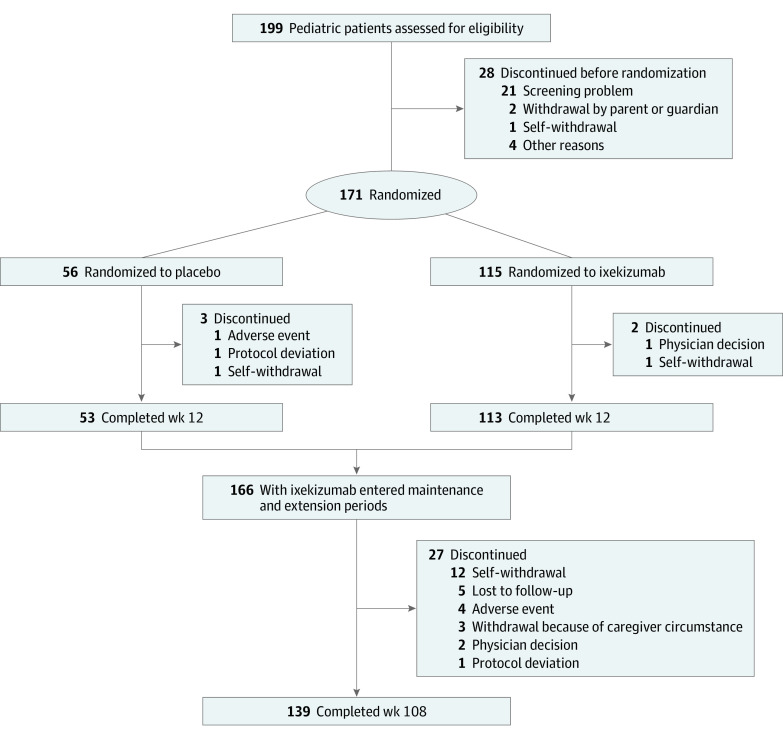
The full study methods of the IXORA-PEDS trial have been published.1 During a 12-week, double-blind treatment period, patients were randomized 2:1 to receive either a subcutaneous, weight-based dose of ixekizumab every 4 weeks or placebo (Figure 1). After this 12-week period, all patients entered a 48-week maintenance period in which they received open-label ixekizumab every 4 weeks (weeks 12-60). This period was followed by an extension period that lasted through week 108 (see the trial protocol in Supplement 1 and the primary study design in eAppendixes 1 and 2 and eFigure 1 in Supplement 2). Patients who changed weight category during the trial remained in their original categorization for the initial 12 weeks and then changed categories according to their updated weight.
Figure 1. CONSORT Diagram Through Week 108 of the IXORA-PEDS Trial.
As part of a European Union protocol addendum, an etanercept reference group was included in the IXORA-PEDS trial to demonstrate the efficacy of ixekizumab in comparison to an approved therapy for pediatric psoriasis.1 In addition, participants with severe psoriasis (defined as Psoriasis Area and Severity Index [PASI] of ≥20 or static Physician’s Global Assessment [sPGA] score of ≥4) from European Union countries who met the response criterion (defined as sPGA score of 0 or 1) at week 60 were rerandomized 1:1 to either ixekizumab or placebo from weeks 60 to 108 during the double-blind, randomized withdrawal period (eAppendix 1 and eFigure 2 in Supplement 2). During this withdrawal period, the time to first clinical relapse was calculated as follows: (date of first sPGA score ≥2 during the 48-week double-blind, randomized withdrawal period) – (date of week 60 rerandomization +1). If a patient had not experienced relapse by completion or early discontinuation of the withdrawal period, the patient was censored at the date of their last visit during the withdrawal period.
Patients
Study participants were aged 6 years to younger than 18 years and had moderate to severe plaque psoriasis, which was defined as PASI of 12 or higher, sPGA score of 3 or higher, and psoriasis-affected body surface area of 10% or greater at screening and baseline. These patients were also candidates for phototherapy or systemic therapy, or they had psoriasis that was determined by a trial investigator (including A.S.P., M.M.B.S., G.A.M., A.P., J.C.C., and K.A.P.) to be not adequately controlled by topical therapies.
Race data were self-reported by participants to the study investigators at each site. The categories represented were American Indian or Alaska Native, Asian, Black or African American, White, and multiple; 4 patients did not report the data.
Efficacy and Safety Parameters
The objective of the present trial was to evaluate the efficacy and safety of treatment throughout a long-term extension period. The efficacy end points at week 108 included percentage of patients who achieved at least 75% (PASI 75), 90% (PASI 90), or 100% (PASI 100) improvement from baseline in PASI; an sPGA score of 0 or 1 (sPGA 0 or 1, with 0 indicating clear or no signs of psoriasis [postinflammatory hyperpigmentation may be present], and 1 indicating almost clear or intermediate between mild and clear signs of psoriasis) or score of 0; and improvement of 4 points or higher from baseline in the Itch Numeric Rating Scale (NRS; with a score of 0 indicating no itch) in patients with a baseline score of 4 points or higher. For patients who had baseline scalp, nail, or palmoplantar involvement, efficacy was evaluated by the percentage of patients who achieved complete resolution based on the Psoriasis Scalp Severity Index (PSSI; score range: 0-72, with 0 indicating no psoriasis and higher scores indicating more severe disease13), Nail Psoriasis Severity Index (NAPSI; score range for each nail: 0-8; NAPSI score range: 0-8014), and the Palmoplantar Psoriasis Area and Severity Index (PPASI; score range, 0-72, with 0 indicating no psoriasis and higher scores indicating more severe disease13), respectively. The 11-point Itch NRS (score range, 0-10, with 0 indicating no itch and 10 indicating worst itch imaginable) evaluated quality of life in patients with baseline Itch NRS score higher than 0. Clearance of genital psoriasis (in patients with baseline genital psoriasis) was also evaluated.
Safety outcomes included assessments of adverse events (AEs), including treatment-emergent AEs (TEAEs), serious AEs (SAEs), and AEs of special interest. Data on suspected inflammatory bowel disease (IBD) were adjudicated by an external clinical events committee (eAppendix 4 in Supplement 2). A list of all end points is provided in eAppendix 3 in Supplement 2.
Statistical Analysis
Analyses were provided for the week 108 final database lock. Efficacy for the combined treatment periods was summarized using descriptive statistics for all patients randomized to ixekizumab at week 0 who received ixekizumab throughout their trial participation through week 108. Efficacy was also analyzed according to baseline weight category (<25 kg, ≥25 kg to ≤50 kg, and >50 kg). In addition, efficacy was summarized using descriptive statistics for the randomized withdrawal period.
Missing data in categorical outcomes for the combined treatment periods were imputed using modified nonresponder imputation unless otherwise specified. Specifically, only patients who discontinued treatment because of AEs or lack of efficacy were imputed as a nonresponder; otherwise, patients were imputed using multiple imputation. Missing data in categorical outcomes for the withdrawal period were imputed using nonresponder imputation. Continuous outcomes were analyzed using a mixed model for repeated measures analysis, including treatment, affected region, baseline sPGA score, baseline weight category, baseline value, visit, and treatment-by-visit and baseline-by-visit interactions as fixed factors. A 2-tailed t was used to test if least-squares mean (LSM) change from baseline value was significantly different than 0.
Safety was summarized using descriptive statistics up to the week 108 final database lock for the all-ixekizumab safety population. This population comprised all patients who received at least 1 dose of ixekizumab, including patients who were initially randomized to receive placebo or etanercept.
Statistical analyses were performed in SAS, version 9.4 (SAS Institute). A 2-sided P < .05 was considered statistically significant. Data analysis, which followed the intention-to-treat principle, was conducted from May to October 2021.
Results
A total of 171 patients were randomized to either ixekizumab every 4 weeks (n = 115) or placebo (n = 56). These patients had a mean (SD) age of 13.5 (3.04) years and consisted of 99 female (57.9%) and 72 male (42.1%) children; most patients (83.8% [n = 140 of 167]) were of White race; 4 patients did not report their race and thus this calculation was based on 167 patients. Of the 166 patients who entered the maintenance and extension periods, 139 (83.7%) completed week 108 and 27 (16.3%) discontinued their participation (Figure 1). Baseline demographics and disease characteristics were similar between treatment groups (Table 1).1 For results imputed by modified nonresponders imputation, response rates were obtained through the average response rate of imputation data and are presented as percentages with the number of patients in the analysis.
Table 1. Baseline Demographics and Disease Characteristics.
| Characteristic | Patients, No. (%) | |
|---|---|---|
| Placebo group (n = 56) | Ixekizumab group (n = 115) | |
| Age, mean (SD), y | 13.1 (2.8) | 13.7 (3.1) |
| Sex | ||
| Female | 36 (64.3) | 63 (54.8) |
| Male | 20 (35.7) | 52 (45.2) |
| Racea | ||
| American Indian or Alaska Native | 0 | 2 (1.8) |
| Asian | 2 (3.8) | 4 (3.5) |
| Black or African American | 3 (5.7) | 3 (2.6) |
| White race | 45 (84.9) | 95 (83.3) |
| Multiple | 3 (5.7) | 10 (8.8) |
| Weight, mean (SD), kg | 60.3 (20.3) | 63.9 (24.9) |
| <25 | 1 (2.0) | 2 (2.0) |
| ≥25 to ≤50 | 14 (25.0) | 29 (25.0) |
| >50 | 41 (73.0) | 84 (73.0) |
| BMI, mean (SD) | 23.5 (5.6) | 24.1 (6.8) |
| Duration of psoriasis since diagnosis, mean (SD), y | 4.7 (3.0) | 4.7 (3.3) |
| Previous psoriasis treatment | ||
| Nonbiologic systemic | 15 (27.0) | 39 (34.0) |
| Biologic | 2 (4.0) | 5 (4.0) |
| Phototherapy | 13 (23.0) | 25 (22.0) |
| BSA, mean (SD), % | 27.1 (17.3) | 27.1 (18.6) |
| sPGA score, mean (SD) | 3.5 (0.6) | 3.6 (0.6) |
| 3 | 31 (55.0) | 57 (50.0) |
| 4 | 21 (38.0) | 51 (44.0) |
| 5 | 4 (7.0) | 7 (6.0) |
| PASI, mean (SD) | 19.7 (8.0) | 19.8 (7.5) |
| NAPSI, mean (SD)b | 24.5 (20.9) | 33.9 (29.5) |
| >0 | 12.0 (21.0) | 34.0 (30.0) |
| PSSI, mean (SD)c | 29.7 (17.2) | 27.3 (17.0) |
| >0 | 50.0 (89.0) | 102.0 (89.0) |
| PPASI, mean (SD)d | 15.4 (21.1) | 8.2 (8.7) |
| >0 | 9.0 (16.0) | 17.0 (15.0) |
| Itch NRS score, mean (SD) | 5.0 (2.5) | 5.4 (2.8) |
| ≥4 | 40.0 (71.0) | 83.0 (72.0) |
| CDLQI, mean (SD)e | 7.4 (4.8) | 8.5 (5.5) |
| DLQI, mean (SD)f | 10.2 (5.42) | 9.3 (4.9) |
| PatGA score, mean (SD) | 3.5 (0.9) | 3.6 (1.1) |
| >0 | 56 (100) | 115 (100) |
| Presence of genital psoriasis | 14 (25.0) | 41 (36.0) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BSA, body surface area; CDLQI, Children’s Dermatology Life Quality Index; DLQI, Dermatology Life Quality Index; NAPSI, Nail Psoriasis Severity Index; NRS, Numeric Rating Scale; PASI, Psoriasis Area and Severity Index; PatGA, Patient’s Global Assessment of Disease Severity; PPASI, Palmoplantar Psoriasis Area and Severity Index; PSSI, Psoriasis Scalp Severity Index; sPGA, static Physician’s Global Assessment.
Race was self-reported by participants to the trial investigators at each site; 4 patients did not report the data. Data were calculated with 53 patients in the placebo group and 114 patients in the ixekizumab group.
Assessed for patients with nail psoriasis at baseline (as reported by the investigator). No. of patients with nonmissing values: n = 13, placebo group; n = 34, ixekizumab group.
Assessed for patients with scalp psoriasis at baseline (as reported by the investigator). No. of patients with nonmissing values: n = 50, placebo group; n = 103, ixekizumab group.
Assessed for patients with palmoplantar psoriasis at baseline (as reported by the investigator). No. of patients with nonmissing values: n = 9, placebo group; n = 18, ixekizumab group.
Assessed in patients aged 6 to 16 years. No. of patients with nonmissing values: n = 48, placebo group; n = 86, ixekizumab group.
Assessed in patients 17 years of age or older. No. of patients with nonmissing values: n = 6, placebo group; n = 26, ixekizumab group.
Efficacy
Primary and gated secondary end points that were achieved by week 12 were sustained through week 108, with patients achieving or maintaining PASI 75 (91.7% [n = 86]), PASI 90 (79.0% [n = 74]), PASI 100 (55.1% [n = 52]), sPGA 0 or 1 (78.3% [n = 74]), and sPGA 0 (52.4% [n = 49]) (Figure 2; Table 2). At week 60, 90.0% of patients (n = 94) attained PASI 75 and 80.3% of patients (n = 94) attained PASI 90 (Table 2). In addition, 78.5% of patients (n = 55) with a baseline Itch NRS score of 4 or higher reported an Itch NRS improvement of 4 points or higher at week 108. Children’s Dermatology Life Quality Index or Dermatology Life Quality Index (score range: 0-30, with higher scores indicating more impact on quality of life15) of 0 or 1 was achieved by 60.6% of patients (n = 57), and Patient’s Global Assessment of Disease Severity16 score of 0 or 1 was achieved by 83.5% of patients (n = 79), and these results were sustained through week 108 (Table 2).
Figure 2. Proportion of Patients Achieving End Points and Observed and Imputed Response Rates.
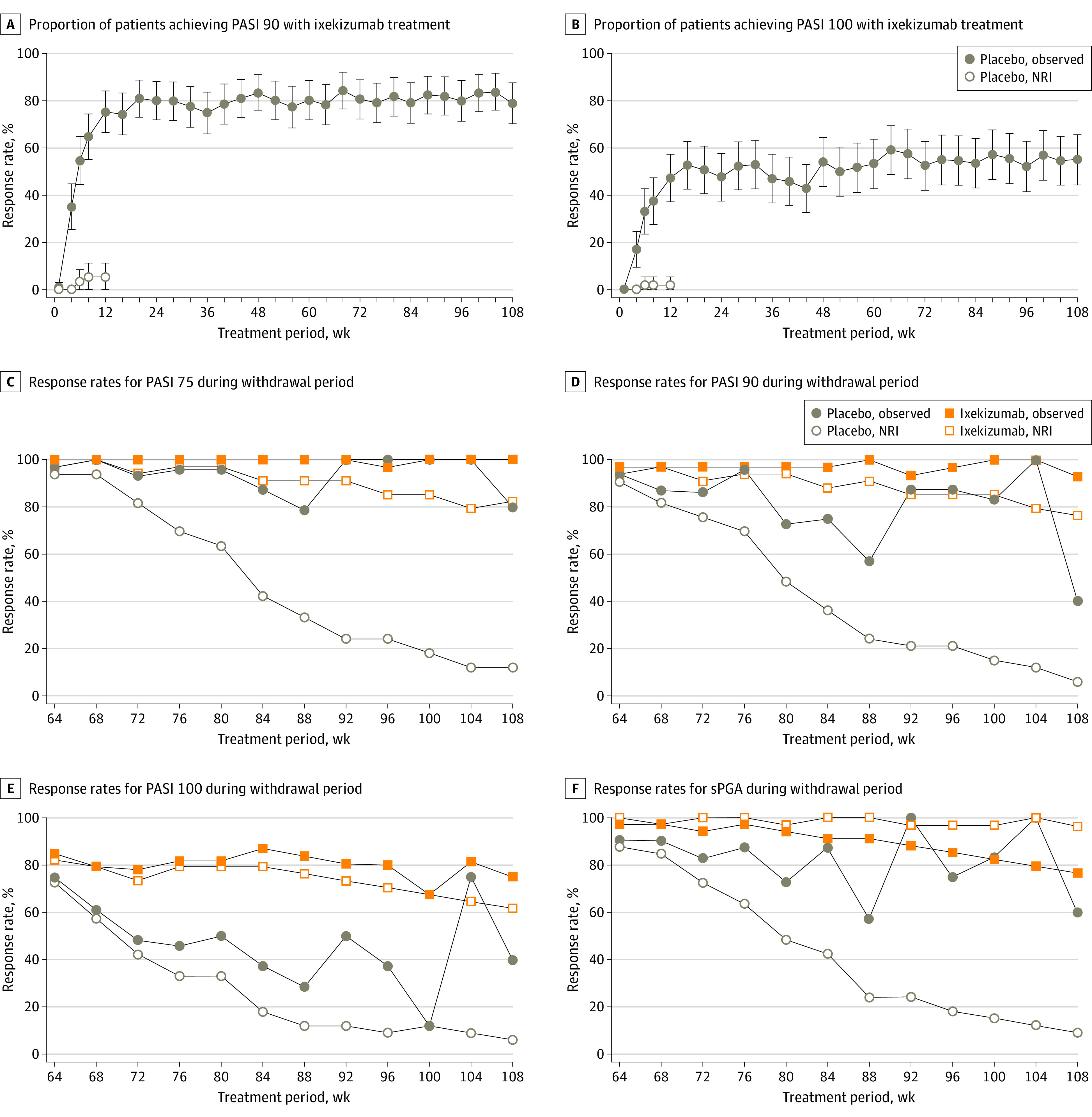
The numbers of responders at milestone intervals were as follows: Psoriasis Area and Severity Index (PASI) 90: 71 at week 12, 79 at week 48, 76 at week 60, and 74 at week 108; PASI 100: 44 at week 12, 51 at week 48, 50 at week 60, and 52 at week 108. NRI indicates nonresponder imputation; sPGA, static Physician’s Global Assessment.
Table 2. Efficacy Outcomes at Week 60 and Week 108.
| All ixekizumab populations at combined treatment perioda | ||
|---|---|---|
| Week 60 | Week 108 | |
| Response, No./total No. of responders (%) | ||
| PASI | ||
| 50 | 90/94 (95.7) | 89/94 (94.5) |
| 75 | 85/94 (90.0) | 86/94 (91.7) |
| 90 | 76/94 (80.3) | 74/94 (79.0) |
| 100 | 50/94 (53.2) | 52/94 (55.1) |
| sPGA score | ||
| 0 or 1 | 75/94 (80.0) | 74/94 (78.3) |
| 0 | 51/94 (54.2) | 49/94 (52.4) |
| Itch NRS score ≥4 | 58/70 (82.9) | 55/70 (78.5) |
| CDLQI or DLQI 0 or 1b | 63/80 (67.0) | 57/75 (60.6) |
| PatGA score 0 or 1 | 79/94 (83.9) | 79/94 (83.5) |
| NAPSI = 0 | 18/28 (65.3) | 19/28 (68.1) |
| PSSI = 0 | 61/83 (73.4) | 63/83 (76.2) |
| PPASI 100b | 9/11 (81.8) | 9/10 (90.0) |
| Clearance of genital psoriasisb | 24/27 (88.9) | 21/24 (87.5) |
| Response, LSM change from baseline (SE) [NX]c | ||
| PASI | −19.48 (0.4) [83] | −19.39 (0.4) [75]d |
| Itch NRS score | −3.46 (0.6) [83] | −3.21 (0.6) [75]d |
| NAPSI | −31.57 (2.3) [21] | −31.12 (2.2) [17]d |
| PSSI | −26.75 (1.4) [72] | −27.02 (1.3) [68]d |
| PPASI | −10.47 (0.1) [11] | −10.47 (0.1) [10]d |
Abbreviations: CDLQI, Children’s Dermatology Life Quality Index; DLQI, Dermatology Life Quality Index; LSM, Least Squares Mean; NAPSI, Nail Psoriasis Severity Index; NRS, Numeric Rating Scale; NX, No. of patients with data; PASI, Psoriasis Area and Severity Index; PatGA, Patient’s Global Assessment of Disease Severity; PPASI, Palmoplantar Psoriasis Area and Severity Index; PSSI, Psoriasis Scalp Severity Index; sPGA, static Physician’s Global Assessment.
All patients who were randomized to ixekizumab at week 0 (visit 2) and who received ixekizumab throughout their study participation. Missing data were imputed using modified nonresponder imputation, unless otherwise specified. For results imputed by modified nonresponders imputation, response rates were obtained through the average response rate of imputation data.
Observed data.
Continuous mixed model for repeated measures, a likelihood-based mixed-effects model, was used.
Significantly greater mean change from baseline at week 108 after ixekizumab treatment is denotated by P < .001.
At baseline, 26.9% of patients (n = 46) had NAPSI higher than 0, 88.9% of patients (n = 152) had PSSI higher than 0, 15.2% patients (n = 26) had PPASI higher than 0, and 32.2% patients (n = 55) had genital psoriasis. Clearance of nail psoriasis (NAPSI = 0) increased from 22.8% (n = 28) at week 12 to 68.1% (n = 28) at week 108. Similarly, clearance of palmoplantar psoriasis (PPASI 100) increased from 46.2% (n = 13) at week 12 to 90.0% (n = 10) at week 108, and clearance of scalp psoriasis (PSSI = 0) increased from 70.7% (n = 83) at week 12 to 76.2% (n = 83) at week 108 (Table 2; eFigure 3 in Supplement 2). Clearance of genital psoriasis was reported in 83.3% of patients (n = 25) who received ixekizumab at week 12 and increased slightly to 87.5% (n = 24) at week 108 (Table 2; eFigure 3 in Supplement 2).
Ixekizumab every 4 weeks resulted in a significantly greater LSM change from baseline at week 108 for Itch NRS score (LSM [SE], −3.21 [0.6]; P < .001), NAPSI (LSM [SE], −31.12 [2.2]; P < .001), PSSI (LSM [SE], −27.02 [1.3]; P < .001), and PPASI (LSM [SE], −10.47 [0.1]; P < .001) (Table 2).
In analyses by baseline weight, the overall responses were maintained through week 108 for PASI 75, PASI 90, PASI 100, sPGA 0 or 1, and sPGA 0 in the subgroups of patients in the weight categories of 25 kg or greater to 50 kg or less and greater than 50 kg. All ixekizumab responses were numerically similar between these 2 subgroups (eFigure 4 in Supplement 2). Comparison of responses in patients with baseline weight less than 25 kg was limited because of the small number of patients in this category (n = 1).
Double-blind Randomized Withdrawal Period
Time to Relapse
For those patients who were rerandomized to ixekizumab or placebo during the randomized withdrawal period, the median time to relapse in the placebo group was 149 (95% CI, 106-190) days. A total of 90.9% of patients (n = 30) who received placebo relapsed, compared with 17.6% of patients (n = 6) who were treated with ixekizumab (eFigure 5 in Supplement 2). Among patients who received ixekizumab, 100% achieved PASI 75, 92.9% achieved PASI 90, and 75% achieved PASI 100 at week 108, and 76.5% achieved an sPGA score of 0 or 1 (Figure 2C-F).
Safety
During the combined treatment period, in the all-ixekizumab safety population (n = 196) (defined as all patients who received at least 1 dose of ixekizumab, including patients initially randomized to placebo or etanercept), TEAEs were reported in 87.7% of patients (n = 172) who were treated with ixekizumab every 4 weeks. Of these patients, 41.3% (n = 81 of 196) experienced mild, 40.3% (n = 79) experienced moderate, and 6.1% (n = 12) experienced severe TEAEs (Table 3). Fifteen patients (7.7%) reported SAEs, including 2 with Crohn disease. In total, 5 patients (2.6%) discontinued study treatment because of an AE (including Crohn disease, astrocytoma, pityriasis rubra pilaris, and psoriasis) (Table 3). Treatment-emergent infections were reported in 74.0% of patients (n = 145), and no new cases of candidal infections were reported. A detailed list of infections is provided in eTable 1 in Supplement 2. No patient reported anaphylaxis, 10.2% (n = 20) reported allergic reactions or hypersensitivity, 1.5% (n = 3) reported cytopenia, 2.0% (n = 4) reported hepatic issues, 0.5% (n = 1) reported a malignant neoplasm, and 4.1% (n = 8) reported depression (Table 3). A breakdown of SAEs is included in eTable 2 in Supplement 2.
Table 3. Summary of Adverse Events From Ixekizumab Treatment at Week 108.
| All-ixekizumab safety populationa | ||
|---|---|---|
| Combined treatment periods, No. (%) | IR per 100 patient-years | |
| No. | 196 | NA |
| Total | NA | 342.81 |
| TEAEsb | 172 (87.7) | 50.2 |
| Mild | 81 (41.3) | 23.6 |
| Moderate | 79 (40.3) | 23.0 |
| Severe | 12 (6.1) | 3.5 |
| Discontinuation from AEs | 5 (2.6) | 1.5 |
| SAEs | 15 (7.7) | 4.4 |
| Death | 0 | 0 |
| AEs of special interest | ||
| Infections | 145 (74.0) | 42.3 |
| Candidal | 0 | 0 |
| Opportunistic | 2 (1.0) | 0.6 |
| Injection-site reactions | 40 (20.4) | 11.7 |
| Allergic reactions or hypersensitivity | 20 (10.2) | 5.8 |
| Potential anaphylaxis | 0 | 0 |
| Cytopeniac | 3 (1.5) | 0.9 |
| Hepaticd | 4 (2.0) | 1.2 |
| Malignant neoplasme | 1 (0.5) | 0.3 |
| Depression | 8 (4.1) | 2.3 |
| ILD | 0 | 0 |
| IBDf | 4 (2.0) | 1.2 |
Abbreviations: AE, adverse event; IBD, inflammatory bowel disease; ILD, interstitial lung disease; IR, incidence rate; NA, not applicable; SAE, serious AE; TEAE, treatment-emergent AE.
All patients who received at least 1 dose of ixekizumab, including patients who were initially randomized to receive placebo or etanercept. Patients with multiple occurrences of these categories were counted once for each category. Patients may be counted in more than 1 category.
TEAE was an event that first occurred or worsened in severity after baseline and on or before the date of the last visit within current ixekizumab treatment period. The association of the TEAE with study treatment was judged by the investigator. Patients with multiple occurrences of the same event were counted under the highest severity.
Included broad and narrow grades of neutropenia and leukopenia.
Included broad and narrow liver investigations; signs and symptoms; and hepatic failure, fibrosis and cirrhosis, and other liver damage–related conditions.
Malignant neoplasm in this patient was an astrocytoma.
IBD included 2 cases of Crohn disease.
Discussion
In pediatric patients who received ixekizumab, significant improvements in skin condition and itch were reported at week 121 and were sustained through week 108. Among patients who were treated with ixekizumab every 4 weeks, 91.7% achieved PASI 75, 79.0% achieved PASI 90, and 55.1% achieved PASI at week 108, a sustained response from week 12.1 The complete clearance rates (PASI 100) demonstrated in pediatric patients were somewhat higher than the rates observed in trials for adult patients with psoriasis after 5 years (46.3% in the UNCOVER-1 and UNCOVER-2 trials17 and 46.2% in the UNCOVER-3 trial18).
Although cross-trial comparison with other biologics approved for use in pediatric patients is not possible, ixekizumab demonstrated numerically greater long-term PASI 75 and PASI 90 responses at week 60 (90.0% and 80.3% of patients, respectively) and week 108 (91.7% and 79.0%, respectively) in the IXORA-PEDS trial, compared with etanercept (61% [PASI 75] and 30% [PASI 90] after 96 weeks),19 adalimumab (28%-47% [PASI 75] after 52 weeks),20 ustekinumab (88% [PASI 75] in patients aged 6-12 years and 71% [PASI 90] after 52 weeks),21 and secukinumab (80% [PASI 75] and 80% [PASI 90] after 52 weeks).22 To our knowledge, long-term PASI 100 responses have not been consistently reported. However, PASI 100 efficacy has been reported at 52 weeks for secukinumab, with 40.0% (low dose) and 46.5% (high dose) of patients achieving PASI 100 respectively.22 In this analysis, PASI 100 was achieved at week 108 by 55.1% of patients who received ixekizumab.
Itch NRS improvement from baseline of 4 points or higher was reported in patients who were treated with ixekizumab every 4 weeks. Ixekizumab every 4 weeks provided meaningful improvements in itch for 78.5% of these patients at week 108. Patients who received ixekizumab every 4 weeks also reported significant improvements from baseline in challenging to treat body areas, including the scalp, nails, palms and soles, and genital region (Table 2; eFigure 3 in Supplement 2). For those patients with nail or palmoplantar involvement, extra time using the medication substantially increased the number of patients achieving clearance (NAPSI = 0 and PPASI 100), with numerically greater percentages observed at week 60 and week 108 (Table 2; eFigure 3 in Supplement 2). However, the small sample size for patients with palmoplantar involvement necessitated the use of observed data. This finding suggests that caution is needed when interpreting the PPASI 100 data. In addition, at week 108, responses with ixekizumab were generally consistent across weight subgroups, although analysis in patients with baseline weight less than 25 kg was limited by the small sample size (n = 1) (eFigure 4 in Supplement 2).
Details on TEAEs, SAEs, and AEs during the double-blind treatment period are reported in Paller et al.1 There were no new safety events during the period between week 48 and week 108. Consistent with previously reported data, this study found that infections and injection site reactions were the most frequently reported TEAEs. There were no new cases of IBD in the extension period, and no candidal infections were observed throughout the 108-week IXORA-PEDS trial. During the trial, most TEAEs were mild to moderate in severity, SAEs occurred in 7.7% of patients, and 2.6% of patients discontinued participation because of AEs. No deaths occurred. The safety profile of ixekizumab was similar in adult and pediatric patients, with the exception of IBD, which has a substantially higher background rate in patients with pediatric onset of psoriasis.3,23,24
Although this study showed that ixekizumab has a favorable efficacy and safety profile through 108 weeks, a number of open questions remain. Such questions include the effect of ixekizumab on additional patient-reported, health-related quality-of-life outcomes as well as the effectiveness and safety of ixekizumab for pediatric patients with psoriasis in the real world.
Limitations
This study has some limitations. First, the study lacked a comparator after week 12. Second, ixekizumab was administered on site, which may not be reflective of real-world administration for pediatric patients weighing more than 50 kg.
Conclusions
In pediatric patients with moderate to severe plaque psoriasis, ixekizumab provided rapid improvements across patient-reported outcomes and objective measures of complete skin clearance of psoriasis, including clearance of psoriasis, in challenging to treat body areas, and these results were sustained up to 108 weeks. Most patients achieved completely clear skin and nails. Safety findings were as previously observed in this population and consistent with the known safety profile of ixekizumab. No new cases of IBD were observed in the IXORA-PEDS trial, and there were no reported cases of candidal infection. Additional studies are warranted to address lingering questions, such as the effect of ixekizumab on additional patient-reported outcomes and the effectiveness and safety of ixekizumab for children with psoriasis in the real world.
Trial Protocol, Addendum, and Statistical Analysis Plan
eAppendix 1. Patients and Methods
eAppendix 2. Study Design, Patients, and Methods
eFigure 1. Primary Study Design: IXORA-PEDS [Aged 6 to <18 Years]
eFigure 2. EU Protocol Addendum: IXORA-PEDS [Aged 6 to <18 Years]
eAppendix 3. List of All Study Endpoints in IXORA-PEDS
eAppendix 4. Description of Efficacy Outcomes
eFigure 3. Challenging Body Areas: Proportion of Patients Achieving NAPSI = 0, PSSI = 0, PPASI 100 and no PsO on Genitalia at 108 Weeks
eFigure 4. PASI and sPGA Responses at Week 108 by Weight Category (mNRI)
eFigure 5. Time to Relapse to sPGA≥2
eTable 1. Infections
eTable 2. Serious Adverse Events
eReferences
Nonauthor Collaborators
Data Sharing Statement
References
- 1.Paller AS, Seyger MMB, Alejandro Magariños G, et al. ; IXORA-PEDS study group . Efficacy and safety of ixekizumab in a phase III, randomized, double-blind, placebo-controlled study in paediatric patients with moderate-to-severe plaque psoriasis (IXORA-PEDS). Br J Dermatol. 2020;183(2):231-241. doi: 10.1111/bjd.19147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Napolitano M, Megna M, Balato A, et al. Systemic treatment of pediatric psoriasis: a review. Dermatol Ther (Heidelb). 2016;6(2):125-142. doi: 10.1007/s13555-016-0117-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Augustin M, Glaeske G, Radtke MA, Christophers E, Reich K, Schäfer I. Epidemiology and comorbidity of psoriasis in children. Br J Dermatol. 2010;162(3):633-636. doi: 10.1111/j.1365-2133.2009.09593.x [DOI] [PubMed] [Google Scholar]
- 4.Libon F, Lebas E, De Schaetzen V, Sabatiello M, De Schepper S, Nikkels AF. Biologicals for moderate-to-severe plaque type psoriasis in pediatric patients. Expert Rev Clin Immunol. 2021;17(9):947-955. doi: 10.1080/1744666X.2021.1958675 [DOI] [PubMed] [Google Scholar]
- 5.Gånemo A, Wahlgren CF, Svensson Å. Quality of life and clinical features in Swedish children with psoriasis. Pediatr Dermatol. 2011;28(4):375-379. doi: 10.1111/j.1525-1470.2010.01292.x [DOI] [PubMed] [Google Scholar]
- 6.Bronckers IM, Paller AS, van Geel MJ, van de Kerkhof PC, Seyger MM. Psoriasis in children and adolescents: diagnosis, management and comorbidities. Paediatr Drugs. 2015;17(5):373-384. doi: 10.1007/s40272-015-0137-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oostveen AM, de Jager ME, van de Kerkhof PC, Donders AR, de Jong EM, Seyger MM. The influence of treatments in daily clinical practice on the Children’s Dermatology Life Quality Index in juvenile psoriasis: a longitudinal study from the Child-CAPTURE patient registry. Br J Dermatol. 2012;167(1):145-149. doi: 10.1111/j.1365-2133.2012.10996.x [DOI] [PubMed] [Google Scholar]
- 8.Sarma N. Evidence and suggested therapeutic approach in psoriasis of difficult-to-treat areas: palmoplantar psoriasis, nail psoriasis, scalp psoriasis, and intertriginous psoriasis. Indian J Dermatol. 2017;62(2):113-122. doi: 10.4103/ijd.IJD_539_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahé E. Childhood psoriasis. Eur J Dermatol. 2016;26(6):537-548. doi: 10.1684/ejd.2016.2932 [DOI] [PubMed] [Google Scholar]
- 10.Nogueira M, Paller AS, Torres T. Targeted therapy for pediatric psoriasis. Paediatr Drugs. 2021;23(3):203-212. doi: 10.1007/s40272-021-00443-5 [DOI] [PubMed] [Google Scholar]
- 11.GlobeNewswire. Novartis Cosentyx receives FDA approval for treatment of children and adolescents with moderate to severe plaque psoriasis. June 1, 2021. Accessed October 6, 2021. https://www.globenewswire.com/news-release/2021/06/01/2239937/0/en/Novartis-Cosentyx-receives-FDA-approval-for-treatment-of-children-and-adolescents-with-moderate-to-severe-plaque-psoriasis.html
- 12.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 13.Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376(16):1551-1560. doi: 10.1056/NEJMoa1607017 [DOI] [PubMed] [Google Scholar]
- 14.Rich P, Scher RK. Nail Psoriasis Severity Index: a useful tool for evaluation of nail psoriasis. J Am Acad Dermatol. 2003;49(2):206-212. doi: 10.1067/S0190-9622(03)00910-1 [DOI] [PubMed] [Google Scholar]
- 15.van Geel MJ, Maatkamp M, Oostveen AM, et al. Comparison of the Dermatology Life Quality Index and the Children’s Dermatology Life Quality Index in assessment of quality of life in patients with psoriasis aged 16-17 years. Br J Dermatol. 2016;174(1):152-157. doi: 10.1111/bjd.14163 [DOI] [PubMed] [Google Scholar]
- 16.Perez-Chada LM, Salame NF, Ford AR, et al. Investigator and patient global assessment measures for psoriasis clinical trials: a Systematic Review on Measurement Properties from the International Dermatology Outcome Measures (IDEOM) initiative. Am J Clin Dermatol. 2020;21(3):323-338. doi: 10.1007/s40257-019-00496-w [DOI] [PubMed] [Google Scholar]
- 17.Leonardi C, Reich K, Foley P, et al. Efficacy and safety of ixekizumab through 5 years in moderate-to-severe psoriasis: long-term results from the UNCOVER-1 and UNCOVER-2 phase-3 randomized controlled trials. Dermatol Ther (Heidelb). 2020;10(3):431-447. doi: 10.1007/s13555-020-00367-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blauvelt A, Lebwohl MG, Mabuchi T, et al. Long-term efficacy and safety of ixekizumab: a 5-year analysis of the UNCOVER-3 randomized controlled trial. J Am Acad Dermatol. 2021;85(2):360-368. doi: 10.1016/j.jaad.2020.11.022 [DOI] [PubMed] [Google Scholar]
- 19.Paller AS, Siegfried EC, Eichenfield LF, et al. Long-term etanercept in pediatric patients with plaque psoriasis. J Am Acad Dermatol. 2010;63(5):762-768. doi: 10.1016/j.jaad.2010.04.004 [DOI] [PubMed] [Google Scholar]
- 20.Thaçi D, Papp K, Marcoux D, et al. Sustained long-term efficacy and safety of adalimumab in paediatric patients with severe chronic plaque psoriasis from a randomized, double-blind, phase III study. Br J Dermatol. 2019;181(6):1177-1189. doi: 10.1111/bjd.18029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Philipp S, Menter A, Nikkels AF, et al. Ustekinumab for the treatment of moderate-to-severe plaque psoriasis in paediatric patients (≥ 6 to < 12 years of age): efficacy, safety, pharmacokinetic and biomarker results from the open-label CADMUS Jr study. Br J Dermatol. 2020;183(4):664-672. doi: 10.1111/bjd.19018 [DOI] [PubMed] [Google Scholar]
- 22.Bodemer C, Kaszuba A, Kingo K, et al. Secukinumab demonstrates high efficacy and a favourable safety profile in paediatric patients with severe chronic plaque psoriasis: 52-week results from a phase 3 double-blind randomized, controlled trial. J Eur Acad Dermatol Venereol. 2021;35(4):938-947. doi: 10.1111/jdv.17002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390(10114):2769-2778. doi: 10.1016/S0140-6736(17)32448-0 [DOI] [PubMed] [Google Scholar]
- 24.Paller AS, Schenfeld J, Accortt NA, Kricorian G. A retrospective cohort study to evaluate the development of comorbidities, including psychiatric comorbidities, among a pediatric psoriasis population. Pediatr Dermatol. 2019;36(3):290-297. doi: 10.1111/pde.13772 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol, Addendum, and Statistical Analysis Plan
eAppendix 1. Patients and Methods
eAppendix 2. Study Design, Patients, and Methods
eFigure 1. Primary Study Design: IXORA-PEDS [Aged 6 to <18 Years]
eFigure 2. EU Protocol Addendum: IXORA-PEDS [Aged 6 to <18 Years]
eAppendix 3. List of All Study Endpoints in IXORA-PEDS
eAppendix 4. Description of Efficacy Outcomes
eFigure 3. Challenging Body Areas: Proportion of Patients Achieving NAPSI = 0, PSSI = 0, PPASI 100 and no PsO on Genitalia at 108 Weeks
eFigure 4. PASI and sPGA Responses at Week 108 by Weight Category (mNRI)
eFigure 5. Time to Relapse to sPGA≥2
eTable 1. Infections
eTable 2. Serious Adverse Events
eReferences
Nonauthor Collaborators
Data Sharing Statement