Abstract
Magnetic resonance imaging is widely used for different diagnostic examinations involving autistic patients. The noisy, narrow, isolating magnetic resonance imaging environment and long scan times may not be suitable for autistic individuals, given their communication challenges, sensory sensitivities and often heightened anxiety. This systematic review aims to reveal any reasonable and feasible radiography-based adjustments to facilitate magnetic resonance imaging scanning without the use of sedation or general anaesthesia. Nine electronic databases were systematically searched. Out of 4442 articles screened, 53 were deemed directly relevant; when assessed against eligibility criteria, only 21 were finally included in this systematic review. Customising communication was found to be a key adjustment, as well as scan-based optimisation and environmental adaptations. The importance of distraction techniques and use of technology for familiarisation with the processes was also highlighted. The results of this study can inform recommendations to improve magnetic resonance imaging practice and patient experience, without the use of sedation or anaesthesia, where feasible. They can also inform the basis of dedicated training for magnetic resonance imaging radiographers.
Lay abstract
Autistic patients often undergo magnetic resonance imaging examinations. Within this environment, it is usual to feel anxious and overwhelmed by noises, lights or other people. The narrow scanners, the loud noises and the long examination time can easily cause panic attacks. This review aims to identify any adaptations for autistic individuals to have a magnetic resonance imaging scan without sedation or anaesthesia. Out of 4442 articles screened, 53 more relevant were evaluated and 21 were finally included in this study. Customising communication, different techniques to improve the environment, using technology for familiarisation and distraction have been used in previous studies. The results of this study can be used to make suggestions on how to improve magnetic resonance imaging practice and the autistic patient experience. They can also be used to create training for the healthcare professionals using the magnetic resonance imaging scanners.
Keywords: adjustment, autism, MRI, person-centred, systematic review
Introduction
Around 1%–2% of the general population is autistic, with a twofold male predominance observed (Park et al., 2016).
There is considerable heterogeneity in the severity and onset of autism spectrum disorder (ASD) manifestations across the lifespan (Martinez-Pedraza & Carter, 2009). Delayed non-verbal interactions, often challenging peer relationships or different ways of expressing emotions, different range of communicative gestures, delayed or repetitive language and lack of active visual exploration have all been previously described (Bryson et al., 2004; Martinez-Pedraza & Carter, 2009; Park et al., 2016). The communication challenges can range from very mild to severe (Llaneza et al., 2010). Autistic children are the need for non-verbal or minimally verbal in 25%–30% of the cases (Brignell et al., 2018).
Autism is often associated with increased anxiety levels (Vasa & Mazurek, 2015). Recent research shows altered sensory processing in autism, including hyper and hypo-sensitivities to different external sensory stimuli. Also, specific sensory stimuli may cause distress, aggressive or self-injurious behaviour and withdrawal in many autistic individuals (Krakowski & Ickowicz, 2018; Marco et al., 2011). Altered sensory responses were recently added as a Diagnostic and Statistical Manual of Mental Disorders (5th ed.; DSM-V) recognised parameter (Green et al., 2016; Weiland et al., 2020). Sensory over-responsivity is increased compared to neurotypical individuals, while sensory issues are often correlated with the severity of autism in children (Kern et al., 2007; Tavassoli et al., 2014). Recent research suggests the need for sensory integration, but stronger evidence is needed (Siemann et al., 2020).
The role of magnetic resonance imaging in ASD
It is estimated that the general population requires at least one medical imaging examination in their lifetime. An annual increase in medical imaging examination requests, including magnetic resonance imaging (MRI), is underpinned by the need to establish diagnoses, suggest optimal treatment pathways and monitor efficacy of treatments (Smith-Bindman et al., 2019).
Autistic individuals may need to undergo an MRI examination for common clinical concerns, such as recurring low back pain, persistent headaches, injury or trauma. In addition, some medical conditions may be more common in autistic adults, including immune conditions, gastrointestinal and sleep disorders, epilepsy, obesity, dyslipidemia, hypertension and diabetes. Similarly, many autistic individuals will present with an increased incidence of psychological and mental health comorbidities (Shaltout et al., 2020).
Furthermore, autistic individuals are more likely to be scanned with MRI for research studies to facilitate understanding of the pathophysiological correlates of autism. Structural MRI can help in the detection and follow-up of brain structural changes (Chen et al., 2011). Functional MRI is widely used in the radiological investigation of epilepsy in autistic individuals, where it may demonstrate altered brain connectivity (Buckley & Holmes, 2016).
MRI environment implications for autistic patients
The MRI environment may be challenging for neurotypical and autistic patients alike, since it involves relatively long scan times (20–60 min), depending on clinical condition and imaging protocol. The MRI scanner often has a narrow bore, which can exacerbate claustrophobia and panic attacks (Iwan et al., 2021). Indeed, 10% of scanned neurotypical patients may experience claustrophobia (Napp et al., 2017), which could impact patient experience, increase MRI scan repeats and cancellations.
Noise from the scanner can often reach 100 db, depending on the examination, often has no pattern and can change without warning. While patients are offered ear protection, sound-effects are not fully mitigated as the headphones have to be pneumatic as MR-compatible. There are also many other background noises, including the pumping sound sustaining the cryomagnet.
Sudden examination table movements and vibrations, the need for patient immobilisation, proximity of equipment such as imaging cameras and a relatively colder environment, to prevent over-heating, might also be challenging for some autistic individuals.
In addition to these MRI-related characteristics, sensitivity to sensory stimuli, altered communication and increased anxiety compared to neurotypical patients (Smith et al., 2019) can make MRI a daunting experience for autistic individuals.
While there are currently many studies using MRI to further our understanding about autism, there is a paucity of studies to assess how autistic individuals experience MRI; often reasonable adjustments are based on generalised assumptions about autistic experience and behaviour. Furthermore, many adjustments are targeted to make the autistic person ‘MR-compatible’ and not making the MRI environment truly ‘autism/patient friendly’ (Nicolaidis et al., 2015; Nordahl et al., 2016).
Often sedation or general anaesthesia may be used to facilitate MRI in autistic patients (Ahmed et al., 2014; Kamat et al., 2018), while around 23% of the outpatient MRI procedures in children use anaesthesia (Xu et al., 2020). Anaesthetics and sedatives carry a risk of neurotoxicity and may impact neurodevelopmental outcome in young children (Tith et al., 2012; Walkden et al., 2019). Also, some parents of autistic children are hesitant to allow anaesthesia (Nordahl et al., 2016). However, their side effects in adults have not been fully explored. Hence, sedation or general anaesthesia, should only be reserved for those patients who cannot co-operate and the benefits of sedation/anaesthesia outweigh the risks.
Rationale for the study
This is the first study to summarise the range of reasonable radiographic adjustments offered for autistic individuals undergoing MRI scanning, whether for adults or children.
The aim of our study is to understand current radiographic practice in MRI for autistic people excluding sedation/anaesthesia. This can help inform future best practice recommendations for autism-friendly MRI scans with improved patient experience.
The research question, therefore, is: ‘What are the reasonable and feasible adjustments to facilitate person-centred MRI scanning for autistic patients?’
Based on the PICO framework, the population (P) of this systematic review is ‘autistic patients’, including both adults and children. The intervention (I) is ‘reasonable and feasible adjustments’. The outcome (O) is ‘to facilitate person-centred MRI scanning’. No comparisons were made.
Methods
Protocol
This systematic review was conducted in alignment with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Moher et al., 2009).
The research protocol was submitted to PROSPERO at: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020196864 (Stogiannos et al., 2020).
The search for this review was performed between July 2020 and October 2020 by two experienced researchers. An updated search was conducted shortly before submission of this review in July 2021.
Sources of information
The following databases were searched: PubMed, Google Scholar, Wiley Online Library, ProQuest, Embase, CINAHL, Web of Science, Joanna Briggs Institute EBP Database, The Cochrane Library.
Eligibility criteria
Explicit eligibility criteria were applied to ensure the inclusion of all relevant studies (Table 1). Non-relevant studies were excluded after thorough screening of titles and abstracts by two independent reviewers. There were no restrictions regarding the design of the selected studies. To maintain methodological rigour, studies were excluded if there was heterogeneity in the sample size, for example, studies including autistic patients and patients with other neurodevelopmental disorders.
Table 1.
Inclusion criteria applied to this search.
| Inclusion criteria |
| Studies published from 1 January 2010 to present |
| Studies written in English language |
| Full-text studies |
| Only peer-reviewed studies |
| Studies with any research design |
| Exclusion criteria |
| Sedation or anaesthesia used during MRI scans |
MRI: magnetic resonance imaging.
Search strategy
Specific keywords were used (Table 2), combined with Boolean operators ‘AND’ and ‘OR’ to generate more precise results. Truncations were also applied to obtain results from synonyms and variant spellings. When searching in PubMed, the relevant Medical Subject Heading (MeSH) terms were used in conjunction with free-text keywords.
Table 2.
Keywords and search strings for this study.
| 1. Autis*, ASD, autistic disorder, spectrum disorder |
| 2. Magnetic Resonance Imag*, MRI, scan* |
| 3. Adjust*, interven*, distract*, adapt*, prepar* |
| 4. Patient-centred, patient car*, patient experience, patient engag*, person-centred |
| 5. Image quality |
| 6. Anxiety, claustrophobi*, motion, movement, distress, sens*, fear |
| 7. Sedation, anaesthesia, anesthesia, hypnosis, relax*, calm* |
ASD: autism spectrum disorder; MRI: magnetic resonance imaging.
An example of the full search strategy for one database is described in supplementary material (Supplemental Appendix 1).
Study selection and synthesis
Two independent researchers performed a thorough screening of the titles and abstracts of each study. The selected studies had to include different types of adjustments performed before, during or after the MRI examinations, as per eligibility criteria. Papers where solely sedation or anaesthesia was employed were excluded from this systematic review.
The final selection of studies was based primarily on consensus between the researchers and input from everyone on the research team. Finally, a third researcher performed a percentage search (10%) to ensure agreement of identified studies was high.
Any identified practice adjustments were classified accordingly, and thematic analysis was used to synthesise the results (Braun & Clarke, 2006).
Risk of bias assessment
This review used the Critical Appraisal Skills Programme (CASP, 2018 a–e) checklists for risk of bias assessment. Each checklist consists of a set of questions and the possible answers can be ‘yes’, ‘no’ or ‘can’t tell’. A classification system depending on the total number of ‘yes’ and ‘no’ answers for each article, similar to Skelton et al. (2020) was established. The papers were then categorised as ‘low’, ‘moderate’ or ‘high’ risk of bias, where a higher number of ‘yes’, similar number of ‘yes’ and ‘no’ and lower number of ‘yes’ was identified, respectively (Table 3).
Table 3.
Summary of the included studies and their main characteristics.
| Title | Author(s) | Year | Country | Objectives | Research design | Participants and imaging protocol | Outcomes | Risk of bias |
|---|---|---|---|---|---|---|---|---|
| A neuroimaging preparation protocol tailored for autism | Tziraki M et al. | 2021 | UK | To prepare an imaging preparation protocol with high transferability to the whole autism spectrum | Cross-sectional | 31 children with neurofibromatosis 1 and autism (aged 4–10 years), without severe learning disability. Imaging protocol: A T1-weighted MPRAGE sequence, a T2-weighted SE sequence, DTI, Arterial spin labelling, MRS using a MEGA-PRESS sequence and an rs-fMRI sequence |
Ability to obtain high-quality multi-parametric MRI data from the vast majority of participants | Low |
| The feasibility of magnetic resonance imaging in a non-selective comprehensive clinical trial in pediatric autism spectrum disorder | DeMayo MM et al. | 2021 | Australia | To include MRI in unsedated autistic children, without the use of adaptive functioning or IQ score restrictions | Clinical Trial | 71 autistic children (aged 3–12 years). No IQ-related or functioning restrictions. Imaging protocol: A T1-weighted sequence, DWI and MR Spectroscopy using PRESS and MEGA-PRESS sequences |
Only one-third of the participants completed the baseline MRI session. Children with higher IQ scores had increased rates of success | Moderate |
| Low-motion fMRI data can be obtained in pediatric participants undergoing a 60 minute scan protocol | Horien C et al. | 2020 | USA | To achieve low-motion, high-quality fMRI data in paediatric participants undergoing a 60-min scan session, using a mock scan protocol and other in-scanner techniques | Cross-sectional | 35 children (aged 7–17 years), 5 of them being autistic. All of them with IQ scores > 70. Imaging protocol: A T1-weighted MPRAGE sequence, a T1-weighted FLASH sequence and T2-weighted SPACE, followed by fMRI using a multi-band EPI sequence (60 min) |
It is possible to obtain low-motion fMRI data after long scan protocols in these populations | Low |
| Individualised MRI training for paediatric neuroimaging: A child-focused approach | Pua EPK et al. | 2020 | Australia | To provide an individualised MRI training procedure to help autistic children tolerate MRI | Cross-sectional | 12 monozygotic twins (aged 5–18 years), autistic (high-functioning) or not. Imaging protocol: A T1-weighted MEMPRAGE sequence and fMRI using two multi-band EPI sequences |
All the participants completed the procedure. These strategies could improve image quality |
Low |
| Participant-driven simulation protocol with a mock scanner for pediatric magnetic resonance neuroimaging preparation without sedation | Yamada K et al. | 2020 | Japan | Use of a simulation protocol with a mock scanner for paediatric MRI neuroimaging studies | Cross-sectional | 241 children (139 with neurodevelopmental disorders) aged 4–17 years. Imaging protocol: T2-weighted sequences, DTI, fMRI and MRS (30–60 min) |
Acceptable quality for children with neurodevelopmental disorders | Low |
| A protocol for sedation free MRI and PET imaging in adults with autism spectrum disorder |
Smith CJ et al. | 2019 | USA | To share a training protocol for low-functioning autistic adults |
Review | 19 autistic adults (mean age 24.2 ± 5.2), both low- and high-functioning. Imaging protocol: A high-resolution GRASS sequence and fMRI using EPI sequences |
Facilitate the inclusion of autistic individuals in neuroimaging studies |
Moderate |
| Autism spectrum disorder: patient care strategies for medical imaging | Hayes, LM | 2018 | USA | To identify evidence-based support strategies for autistic patients going to medical imaging | Review | Autistic patients undergoing medical imaging. Imaging protocol: Not specified |
Increase quality of care. Decrease anxiety | Moderate |
| Functional MRI connectivity of children with autism and low verbal and cognitive performance | Gabrielsen TP et al. | 2018 | USA | To complete non-sedated structural and functional MRI scans using behavioural and anxiety reduction techniques, questionnaires, video modelling, active noise-cancelling headphones and the Inscapes movie paradigm | Cross-sectional | 62 children and adolescents (aged 7–17 years), both with low and high verbal and cognitive performance. Of them, 42 were autistic. Imaging protocol: A 3D T1-weighted MPRAGE sequence and fMRI using multi-band EPI sequences with high temporal resolution |
Many autistic children with low verbal and cognitive performance can successfully complete functional MRI scans with moderate support | Low |
| Robust motion correction strategy for structural MRI in unsedated children demonstrated with three-dimensional radial MPnRAGE. | Kecskemeti S et al. | 2018 | USA | To develop and evaluate a retrospective method to minimise motion artefacts in structural MRI | Cross-sectional | 44 children (32 autistic and 12 controls) aged 5–17.8 years. Imaging protocol: Isotropic, whole-brain MPnRAGE sequence |
Improved image quality of T1-W images in unsedated children | Low |
| Using a motion-tracking device to facilitate motion control in children with ASD for neuroimaging | Sandbank M, Cascio C | 2018 | USA | To examine whether a motion-tracking system could facilitate motion | Qualitative | Two autistic children (aged 6 and 8 years). ADOS scores of 13 and 18. Imaging protocol: DTI scan |
Behavioural shaping techniques combined with real-time visual feedback can help autistic children increase motion control | Low |
| Autism and research using magnetic resonance imaging | Johnson N et al. | 2017 | USA | To explore the experiences of participating in a research study using MRI. To assess the feasibility of an iPad application |
Cross-sectional | Five autistic children (aged 14.8 ± 1.2) and five typically developing children (aged 14.2 ± 3.2), each of them with a parent. Verbal IQ scores were > 70. Imaging protocol: Not specified |
Children had fewer challenging behaviours. Most of them completed the actual MRI examination | Low |
| Establishing motion control in children with autism and intellectual disability: Applications for anatomical and functional MRI | Cox A et al. | 2017 | New Zealand | To establish tolerance to the MRI environment and a level of motion control | Cross-sectional | Seven autistic children (aged 6–13 years), five of them with intellectual disability. Imaging protocol: An fMRI sequence (5 min) |
Autistic children could learn to tolerate the MRI scanner and suppress body motion | Moderate |
| Management of children with autism spectrum disorder in the anesthesia and radiographic context | Berglund IG et al. | 2017 | Sweden | To develop guidelines for autistic children going to radiologic procedures | Cross-sectional | 21 experts (aged 38–67 years) working with autistic children in anaesthesia and radiology departments in Sweden. Imaging protocol: Not specified |
Guidelines should be locally applied for autistic children during radiology procedures | Low |
| Peri-radiographic guidelines for children with autism spectrum disorder: a nationwide survey in Sweden | Bjorkman B et al. | 2017 | Sweden | To investigate the guidelines and routines used when autistic children are examined in a radiology department |
Cross-sectional | 86 radiology departments in Sweden. Imaging protocol: Not specified |
Recommendations for the development of guidelines to increase support and decrease anxiety | Low |
| Real-time motion analytics during brain MRI improve data quality and reduce costs | Dosenbach N et al. | 2017 | USA | To develop a software for real-time head motion analytics | Cross-sectional | 1134 children (aged 7.2–17.8 years). Of them, 84 were autistic. Imaging protocol: T1-weighted MPRAGE sequence and T2*-weighted EPI sequences for rs-fMRI |
Cost and time reduction by 50% | Low |
| Methods for acquiring MRI data in children with autism spectrum disorder and intellectual impairment without the use of sedation | Nordahl CW et al. | 2016 | USA | To develop a protocol for acquiring MRI scans in autistic children with intellectual impairment, using ABA | Cohort study | 17 autistic children (aged 9–13 years) with MSEL overall development quotient score < 70. Imaging protocol: A 3D T1-weighted MPRAGE sequence and DWI |
High-quality scans were acquired from all the participants | Moderate |
| Effect of a social script iPad application for children with autism going to imaging | Johnson NL et al. | 2014 | USA | To test the effectiveness of an iPad® application for autistic children going to imaging | Randomised controlled trial | 32 autistic children/parent dyads (children aged 3–18 years). Imaging protocol: Not specified |
Lower levels of anxiety for patients and parents. Fewer challenging behaviours |
Low |
| Social script iPad application versus usual care before undergoing medical imaging: two case studies of children with autism | Johnson NL, Bree OA | 2014 | USA | To describe the process of the social script intervention | Case study | Two low-functioning autistic children (aged 8 and 16 years), each one with a parent. Imaging protocol: Not specified |
Less challenging behaviours. Decreased scan length and parent anxiety |
Moderate |
| Using picture schedules in medical settings for patients with an autism spectrum disorder | Chebuhar A et al. | 2013 | USA | To examine the use of picture schedules in medical settings | Cross-sectional | Six nurses, one child-life specialist, nine parents, one medical assistant. Imaging protocol: Not specified |
Decreased anxiety, improved communication | Low |
| Brief report: approaches to P-MRS in awake, non-sedated children with and without autism spectrum disorder. | Erickson LC et al. | 2012 | USA | To facilitate successful 31P-MRS scans in unsedated autistic patients | Case study | Six autistic children (aged 6–18 years) and six age-matched controls. Imaging protocol: Brain and muscle 31P-MRS |
All subjects successfully completed at least one scan without the need for sedation or mock scanning | Low |
| Distraction strategies used in obtaining an MRI in pediatrics: a review of the evidence | Netzke-Doyle V | 2010 | USA | To review distraction strategies for brain MRI without sedation | Systematic review | Children between 5 and 7 years old. Imaging protocol: Not specified |
Parent/patient satisfaction, elimination of the risks of sedation and decreased costs | Low |
MPRAGE: magnetisation prepared rapid acquisition gradient echo; DTI: diffusion tensor imaging; MRS: magnetic resonance spectroscopy; MRI: magnetic resonance imaging; IQ: intelligent quotient; DWI: diffusion-weighted imaging; EPI: echo planar imaging; MEMPRAGE: multi-echo MPRAGE; PET: positron emission tomography; ASD: autism spectrum disorder; ADOS: Autism Diagnostic Observation Schedule; rs-fMRI: resting-state functional MRI; MSEL: Mullen Scales of Early Learning.
Community involvement
This systematic review is part of a larger project, led by City, University of London, to address inequalities for medical imaging services provision for autistic service users. The autistic community has been consulted at all cases in the conceptualisation, design, implementation and write-up of the work involved in this wider project. This included autistic consultants from the National Autistic Society (NAS), individual service users from the Twitter autistic community and personal communications with autistic individuals. Drafts of the specific systematic review were read and approved by autistic individuals.
Results
Study selection
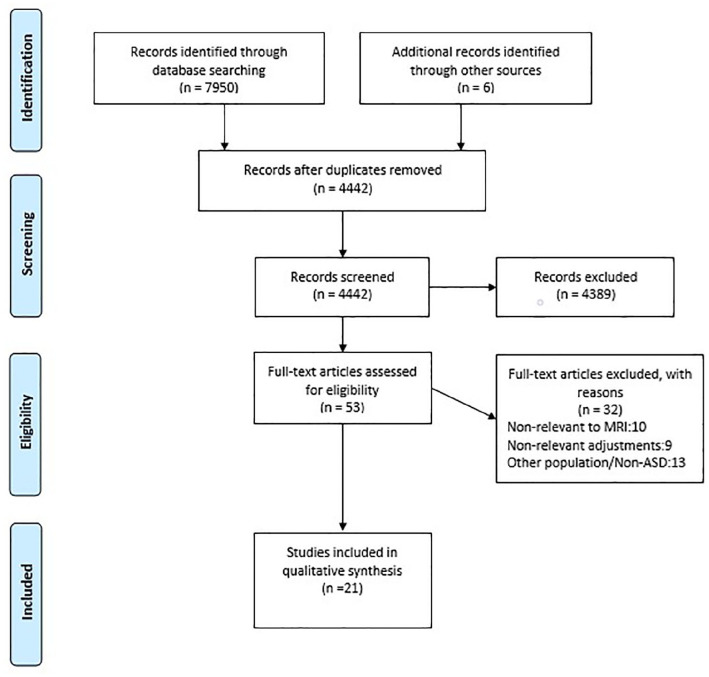
Of the initial 4442 identified studies, only 53 studies were deemed eligible. Of those, 32 studies were further excluded for reasons outlined in the PRISMA diagram (Figure 1). No studies were excluded based on risk of bias assessment. The extracted categories and data of the final 21 studies are summarised in Table 3.
Figure 1.
The PRISMA flow diagram of the study.
The following PRISMA flow diagram (Figure 1) depicts the total number of included and excluded studies, as well as the main reasons for exclusion.
Study characteristics
Of the 21 studies included in this review, 2 were randomised controlled trials, 1 was a systematic review, 2 were narrative reviews, 13 were cross-sectional studies, 2 were case studies and 1 was a cohort study. The publication years range from 2010 to 2021. The included studies were from USA (n = 14), Australia (n = 2), Sweden (n = 2), Japan (n = 1), UK (n = 1) and New Zealand (n = 1). In total, 1812 individuals were included in these studies, 94.4% (n = 1711) were children and 5.6% (n = 101) were adults. Two studies investigated autistic adults (Hayes, 2018; Smith et al., 2019). Thirteen of the included studies recruited only autistic children (Cox et al., 2017; DeMayo et al., 2021; Dosenbach et al., 2017; Erickson et al., 2012; Gabrielsen et al., 2018; Horien et al., 2020; Kecskemeti et al., 2018; Netzke-Doyle, 2010; Nordahl et al., 2016; Pua et al., 2020; Sandbank & Cascio, 2019; Tziraki et al., 2021; Yamada et al., 2020). In addition to children, parents were also recruited in three of the included studies (Johnson & Bree, 2014; Johnson et al., 2014, 2017).
Reasonable adjustments
There were six types of reasonable adjustments in MRI for autistic patients. The identified themes are as follows: (a) communication, (b) psychology-based interventions, (c) sensory-easing adjustments, (d) simulation and familiarisation (including use of technology), (e) distraction (including use of technology) and (f) scan-based optimisation. These themes are described below and also depicted in Table 4 (Supplemental Appendix 2).
Communication
Optimisation of communication was found to be vital for an autism-friendly MRI service. Specifically, contacting autistic individuals and their parents/carers prior to the examination to provide customised patient care before the MRI scan was widely seen as useful practice (Berglund et al., 2017; DeMayo et al., 2021; Erickson et al., 2012; Nordahl et al., 2016; Björkman et al., 2017; Pua et al., 2020; Tziraki et al., 2021; Yamada et al., 2020), as was encouraging radiographers to wear staff ID cards to help patients with face–name associations (Pua et al., 2020). Also, pre-scan interviews with autistic children and their parents play an important role in pre-MRI communication (Gabrielsen et al., 2018). Sending information letters or flyers prior to the scan also allowed patients to familiarise with the examination and its requirements and encouraged them to ask questions (Erickson et al., 2012). The implementation of a flowchart with visual cues to guide the patient through the procedure has also been recommended (DeMayo et al., 2021; Pua et al., 2020). In addition, extra time should be allocated to communicate with autistic patients, to explain the procedure in detail and answer any queries (Berglund et al., 2017; Horien et al., 2020; Nordahl et al., 2016; Björkman et al., 2017; Tziraki et al., 2021). Prior notification to radiographers of any examinations scheduled for autistic patients was vital (Berglund et al., 2017), as was the need to equip radiographers with the specific knowledge and skills to support and manage autistic patients (Björkman et al., 2017).
When interacting with autistic patients, it was found that radiographers adapting the communication style to the patient’s needs achieved a truly individualised approach (Berglund et al., 2017; Erickson et al., 2012; Hayes, 2018; Pua et al., 2020; Yamada et al., 2020); also, giving clear instructions in a calming and reassuring tone better supported their needs (Hayes, 2018). Finally, but very importantly, it was vital to allow parents/carers to be involved in the procedure, as they helped alleviate patient’s anxiety. Either inside the MRI room or the control room, they were able to better communicate with their children and facilitate a successful examination (DeMayo et al., 2021; Gabrielsen et al., 2018; Nordahl et al., 2016), always following MRI safety checks, as required.
Psychology-based interventions
Regarding psychology-based interventions, applied behavioural analysis (ABA) for autistic children, conducted by certified behaviour analysts was found to be beneficial (Nordahl et al., 2016; Smith et al., 2019). In addition, positive reinforcement learning strategies have also been widely used to facilitate MRI procedures with autistic patients (Cox et al., 2017; Gabrielsen et al., 2018; Hayes, 2018; Smith et al., 2019; Tziraki et al., 2021). A customised approach for autistic children using stimulus fading sequences in conjunction with progressive reinforcement helped them familiarise with the procedure in a mock scanner before the actual MRI examination (Cox et al., 2017; DeMayo et al., 2021; Horien et al., 2020). Individualised anxiety reduction plans should be employed when scanning autistic children (Gabrielsen et al., 2018).
Sensory-easing adjustments
These not only refer to the MRI environment modifications but also to patient comfort inside the scanner. An optimised schedule was found to be extremely important for reducing waiting times, as autistic patients usually face many challenges during waiting periods (Berglund et al., 2017; Hayes, 2018). In addition, it was beneficial if MRI departments reduced the number of staff involved to a minimum, where feasible, to minimise noise and distractions that may unnecessarily accentuate anxiety (Berglund et al., 2017). Moreover, it was useful to provide a dedicated quiet area within the facility, ideally equipped with adjustable lights (Berglund et al., 2017; Hayes, 2018), to serve as a waiting room or relaxation room for these patients in case they became overwhelmed. For autistic children, using a visit map to serve as a visual aid (Pua et al., 2020) was helpful for orientation during their visit to the MRI facility, while a visual timer placed within the MRI room has also been recommended to highlight the remaining examination time and time per scan as well (Nordahl et al., 2016; Yamada et al., 2020).
This study identified some important strategies to increase physical comfort of autistic patients while in the MRI scanner and hence minimise any motion-induced artefacts. The use of specifically designed weighted blankets has been a well-established technique to calm autistic children inside the MRI scanner (DeMayo et al., 2021; Erickson et al., 2012; Horien et al., 2020; Nordahl et al., 2016). Except for blankets, some dedicated gentle immobilisation aids, such as pads, cushions, foam positioners and sandbags have also been widely used to increase comfort and reduce bulk patient motion (Erickson et al., 2012; Gabrielsen et al., 2018; Yamada et al., 2020). Moreover, the provision of headphones (also active, noise-cancelling) or earplugs to reduce the acoustic noise proved effective in the included studies (DeMayo et al., 2021; Erickson et al., 2012; Gabrielsen et al., 2018; Horien et al., 2020; Nordahl et al., 2016; Tziraki et al., 2021; Yamada et al., 2020). Another suggested physical comfort and patient safety facilitating strategy was to encourage autistic patients to wear MR-safe cotton clothes without pockets when coming to the MRI department (Gabrielsen et al., 2018; Nordahl et al., 2016).
Simulation for familiarisation
Simulated, or mock, MRI scanning sessions were widely used for familiarisation with the MRI environment (Cox et al., 2017; DeMayo et al., 2021; Horien et al., 2020; Johnson et al., 2017; Nordahl et al., 2016; Pua et al., 2020; Sandbank & Cascio, 2019; Smith et al., 2019; Yamada et al., 2020). This was in addition or instead of a pre-visit to the MRI department (Berglund et al., 2017; Björkman et al., 2017; Yamada et al., 2020), where an orientation session was added to explain the day’s schedule and the whole procedure (Pua et al., 2020). Live modelling, or peer-modelling, was also a useful strategy to explain the procedure to autistic children (DeMayo et al., 2021; Gabrielsen et al., 2018; Hayes, 2018; Nordahl et al., 2016). Other familiarisation techniques for autistic children undergoing MRI scans included (a) using an illustrated storybook, in the form of a social story (DeMayo et al., 2021; Netzke-Doyle, 2010; Nordahl et al., 2016; Pua et al., 2020; Tziraki et al., 2021), (b) picture schedules to understand the different elements of MRI scanning and reduce their anxiety (Berglund et al., 2017; Chebuhar et al., 2013; Hayes, 2018), (c) some technology-enabled mobile applications (Pua et al., 2020), (d) alongside some iPad applications (Johnson & Bree, 2014; Johnson et al., 2014, 2017), (e) while distraction with virtual reality tools has been previously proposed with some success (Netzke-Doyle, 2010). Audiovisual material sent for use at home has been broadly recommended (DeMayo et al., 2021; Erickson et al., 2012; Gabrielsen et al., 2018; Nordahl et al., 2016; Pua et al., 2020; Tziraki et al., 2021). In addition, a home-based MRI simulation tool has been trialled, in conjunction with pre-recorded MR noises to simulate the procedure (Erickson et al., 2012). Finally, photographs of the healthcare workers involved in the care of autistic individuals have been distributed to them before the examination, allowing them to gain familiarity with the person doing their scan prior to the actual examination (Berglund et al., 2017; Tziraki et al., 2021).
Distraction techniques
Regarding distraction techniques, the most widely used approach was the provision of audiovisual material (music, videos or movies) for use during the scan provided by the department (DeMayo et al., 2021; Erickson et al., 2012; Gabrielsen et al., 2018; Horien et al., 2020; Netzke-Doyle, 2010; Nordahl et al., 2016; Pua et al., 2020; Sandbank & Cascio, 2019; Smith et al., 2019; Tziraki et al., 2021; Yamada et al., 2020) or by the patients themselves (Berglund et al., 2017; Erickson et al., 2012; Hayes, 2018; Kecskemeti et al., 2018; Yamada et al., 2020). Before the examination, autistic patients were provided with coping kits, which proved effective for use during waiting times. These could be video games, stress balls, music devices or sound-producing toys (Hayes, 2018), while MR-safe stuffed animals have been also used for in-scanner help (Gabrielsen et al., 2018). When scanning children and depending on their age, coordinating the scan with their regular sleeping and/or feeding routines was beneficial to keep them calm during the examination (Netzke-Doyle, 2010).
Scan-based optimisation
The implementation of fast acquisition protocols, in conjunction with ‘soft tone’ sequences to reduce acoustic noise, were identified in the literature (Tziraki et al., 2021). High temporal resolution scan protocols, using multiband echoplanar imaging (EPI) has been proved to be beneficial during fMRI acquisitions (Gabrielsen et al., 2018). A magnetisation-prepared rapid acquisition gradient echo (MPRAGE) sequence with motion correction to obtain T1-weighted images in unsedated children has been also used (Kecskemeti et al., 2018), as inherently motion resistant. In addition, various prospective motion correction approaches have been introduced (Pua et al., 2020). These included the use of motion-tracking devices with real-time feedback (Horien et al., 2020; Sandbank & Cascio, 2019; Smith et al., 2019). The Framewise Integrated Real-time MRI Monitoring (FIRMM) software as real-time quality control display (Dosenbach et al., 2017) was used, giving the operators the ability to have real-time access to motion analytics and a continuous update in the slice positioning and field of view for motion correction (Pua et al., 2020). Furthermore, the use of a video inside the MRI room which blacked out, or froze when the patient moved, offered real-time feedback and helped patients keep still for longer duration (DeMayo et al., 2021; Horien et al., 2020; Nordahl et al., 2016). Finally, the Automatic Removal Of Motion Artifacts (AROMA) method has been used to remove motion-related components from fMRI data (Tziraki et al., 2021).
Discussion
The main themes emerging from this systematic review will be discussed below in relation to current practice and related evidence.
Communication
Only when effective communication takes place, can radiographers and other healthcare providers identify individual patient needs and preferences, and support them and their carers during what might be a challenging but necessary medical imaging examination. This is true for all patients; however, autistic patients may face specific challenges with communication, and have increased anxiety and sensitivity to certain sensory stimuli (Marco et al., 2011; Nimmo-Smith et al., 2020). Communication with autistic individuals is vital at any healthcare setting, as previous studies have shown (Calleja et al., 2020; Simpson, 2020).
A key step in this process is communication between the referring consultant or family doctor and the medical imaging team. This is fundamental for arranging reasonable adjustments before the patient arrives in the MRI department, allowing time for preparation and planning. Radiology departments can choose to engage experienced radiographers or special educational needs (SEN) and play specialists, to facilitate a successful MRI examination offering an optimal patient experience. In patients with more complex communication challenges as assessed by specialists, speech-generating devices, picture books, sign language, graphic symbols or graphic gestures could all be used to achieve a better outcome (Brignell et al., 2018). Being friendly and compassionate towards an autistic individual might simply not be enough; radiographers must be both technically skilled and aware of the patient’s preferences to provide optimal care (Björkman et al., 2017).
It must be noted that while other healthcare professions like nursing have published more in this area (Calleja et al., 2020; Nicolaidis et al., 2016; Simpson, 2020), evidence in medical imaging and radiography remains, sadly, still quite sparse and isolated. Validated toolkits for general use by primary care providers are already available and have shown improvements in patient–provider communication (Nicolaidis et al., 2016). However, further research is needed to develop dedicated, customised toolkits for managing autistic patients in Radiology departments.
Other adjustments related to communication trialled in different contexts, include implementation of augmentative and alternative communication (AAC) systems, exchange of useful information, ensuring that patients have a communication method with staff, increasing the communicative competence of staff and supporting carers in their roles (Hemsley & Balandin, 2014). These should be considered for medical imaging, where feasible.
Psychology-based interventions
Psychology-based interventions can facilitate a successful MRI examination, improve completion rates, minimise anxiety and reduce the use of sedation and/or anaesthesia. Although the added value of these interventions has been widely discussed and contemplated in the literature, it must be noted that few psychology-based interventions were identified through this systematic review. For instance, applied behaviour analysis (ABA) and reinforcement learning using certified behaviour analysts have been widely recommended (Weill et al., 2018). However, ABA is largely condemned by the autistic community as ‘cruel’ and ‘trying to bend the autistic person’ to fit neurotypical stereotypes, stealing away their unique autistic identity (Devita-Raeburn, 2016). There is lack of evidence this can work for MRI examinations; therefore, more research and further dialogue is needed with the autistic community that cautions against its use.
Sensory-easing interventions
Healthcare professionals should always consider the environment in which autistic patients are treated and managed, while the NHS has prioritised the development of accessible environments (Simpson, 2020). Therefore, MRI departments should opt to develop dedicated waiting rooms, with adjustable lighting and the capacity to reduce noise. Noise has been hailed as the single most important factor increasing patient anxiety in clinical environments (Muskat et al., 2015; Simpson, 2020). Visual, auditory, touch stimuli should be adjusted as much as possible. Asking the patient or their carer for their preferences and acting proportionately should be prioritised. In addition, reducing the number of staff involved, minimising waiting times for these patients and ensuring waiting rooms are more patient-centred, would have a great impact on reducing patient anxiety (Hlaing et al., 2015; Tugwell-Allsup & Pritchard, 2018).
Ensuring a patient’s physical comfort during the examination is vital for improving patient experience. This may reduce the likelihood of discomfort-related motion artefacts, which can impact the quality of imaging for diagnosis. Autistic children and adults avoid expressing their physical discomfort, which may lead to incorrect interpretation of pain from the caregivers (Allely, 2013). Also, some autistic individuals might have a higher threshold of pain and withstand an MRI examination without declaring it, so radiographers should always not only check with the patient and their carers, but also provisionally avoid imaging positions or use of imaging equipment, which might be uncomfortable for the patients. Reducing the acoustic noise during the MRI examination is also very important, as autistic patients may often exhibit hyperacusis (Riquelme et al., 2016). Hearing protection (headphones and earplugs) is mandatory for all patients undergoing MRI examinations. Silent scan technologies, various passive and active noise reduction techniques and optimal manipulation of scan parameters by the radiographer can result in quieter scans (Alibek et al., 2014; Baker, 2013). The encouragement of patients to wear cotton clothes without pockets was also identified as a good strategy to reduce the waiting times for pre-MRI safety screening and increase physical comfort. It will also remove the need to change from their own clothes to the hospital gown or scrubs, which can be challenging for them.
Simulation and familiarisation to prepare for scan (including use of technology)
Mock scanner sessions, a widely used technique before the actual examination takes place, offer access to MRI scanners typically built as exact copies of the real scanners, but without the magnetic field. The mock scanners used to simulate the examination can be also enriched by actual scan noises and a motion potentiometer to teach children how to remain still by receiving instant visual feedback (Nordahl et al., 2016). However, mock scanners are expensive and the alternative is offering a pre-visit to the MRI department for familiarising with the MRI environment (Berglund et al., 2017; Björkman et al., 2017; Yamada et al., 2020). This needs to be planned ahead at an optimal time for the patient, for example, quiet time at the end of the day.
Distraction (including use of technology)
The most widely used distraction strategy includes the provision of audiovisual material for use during the MRI examination; patients can listen to music or watch a video or movie when lying inside the scanner. Music-mediated healthcare delivery has been used in paediatrics, aiming to lower cortisol levels and relieve anxiety, during and after the healthcare intervention (Stegemann et al., 2019) and should also be considered for MRI scan preparation.
Image-based optimisation
There is a variety of motion-resistant and scan time–reducing MRI techniques, which are generally used in scans of patients likely to move (Malamateniou et al., 2013). Similarly, some prospective motion-correction techniques, as well as different motion-tracking devices could be integrated in the hardware and software of the scanner to serve as image optimisation techniques (Pua et al., 2020; Sandbank & Cascio, 2019; Smith et al., 2019).
Other important adaptations include image post-processing techniques, such as principal or independent component analysis, temporal censoring or regression of the global signal, to reduce motion-related artefacts (Bednarz & Kana, 2018), all of which can be recruited to ensure a quicker, more comfortable scan, often with minimal need for patient immobilisation.
Table 5 (Supplemental Appendix 3) includes additional adjustments identified from the literature from the originally 53 identified papers and classified under the emerging themes. Some of these additional adjustments are also referred to below where relevant, to enhance discussion. It must be noted that there are further adjustments, which have been drawn from the wider literature, however, these have not been tested in MRI of autistic individuals. Such interventions include the use of custom-milled head moulds (Lynch et al., 2021) or the provision of tactile feedback to alleviate head motion during fMRI studies (Krause et al., 2019), scanning of children during natural non-sedated sleep (Dean et al., 2014) or alternative headphone sets for PET-MRI studies (Tellmann et al., 2018).
Limitations
While systematic reviews represent an important summary of existing research evidence, they do have known limitations (Bartolucci & Hillegass, 2010) and there are different reasons where data or whole studies may be inadvertently missed (Higgins et al., 2021). Our study relied on two separate reviewers and followed all relevant guidelines, but it is possible that some studies were missed (Wang et al., 2020). The type of systematic review we conducted has a qualitative undertone in its approach, which relies on ‘reflexivity’ as an awareness of the researcher’s role in the practice of research, enabling the researcher to acknowledge the way in which they affect both the research process and outcomes (Haynes, 2012). This interpretative lens necessarily varies with the researcher’s educational background and work experience, and it is often guided by the aim of the research project. Our themes were aligned to the aim of the study conducted by a team of mainly imaging researchers and experts, seen through the lens of how to improve imaging practice further to allow for more accessible MRI scans for autistic service users. This type of ‘known bias’ that reflexivity allows and encourages is vital to meet the aim of the project.
The results of this systematic review cannot be generalised to the wider population of autistic individuals, given the differences in preferences and experiences they might have. Within the examined studies there is clearly complexity and heterogeneity of the spectrum of participants and techniques used. Personalised approaches are, therefore, vital; however, this study is offering a generalised framework of reasonable adjustments that have been used and worked for autistic populations of both adults and children. Therefore, the findings of this study can only serve as suggestions for MRI practitioners to improve and customise their practice according to their patients’ needs. In addition, some of the identified interventions can be applicable to a wider context, in patients with anxiety, who also find MRI scans very challenging.
Many of the studies cited here originate in North America, where recently there has been increased attention to accessibility for scanning and the consequences for imaging results and patient experience. We suggest the need for similar attention in imaging studies based in the United Kingdom and Europe, where autistic individuals are been increasingly scanned for either research studies or for common clinical concerns.
Despite these proposed adjustments, some autistic patients will inevitably require sedation/general anaesthesia to undergo MRI examinations, as this might be the safest and most effective way they can receive the required clinical care (Kamat et al., 2018; Seo et al., 2014).
Conclusion
Autistic individuals undergo MRI examinations as part of their diagnostic imaging pathway for autism or for common clinical concerns. However, MRI examinations can be impeded by suboptimal communication, poorly prepared clinical environments, lack of trained staff, lack of co-ordination of healthcare services, which in turn can make the whole experience challenging and often traumatic for autistic individuals and their carers. This systematic review provides an insight into the most widely used reasonable adjustments, which could facilitate MRI scanning of these patients, given the range of their sensory sensitivities and communication preferences and needs. These adjustments include efficient communication, simulation for familiarisation with the environment, distraction techniques, scan-based optimisation to name just a few. Radiographers need to be adequately trained to be able to care for these patients in MRI, offering a truly person-centred service. Further research is needed to develop dedicated toolkits for autistic individuals undergoing medical imaging examinations.
Supplemental Material
Supplemental material, sj-docx-1-aut-10.1177_13623613211065542 for A systematic review of person-centred adjustments to facilitate magnetic resonance imaging for autistic patients without the use of sedation or anaesthesia by Nikolaos Stogiannos, Sarah Carlier, Jane M Harvey-Lloyd, Andrea Brammer, Barbara Nugent, Karen Cleaver, Jonathan P McNulty, Cláudia Sá dos Reis and Christina Malamateniou in Autism
Supplemental material, sj-docx-2-aut-10.1177_13623613211065542 for A systematic review of person-centred adjustments to facilitate magnetic resonance imaging for autistic patients without the use of sedation or anaesthesia by Nikolaos Stogiannos, Sarah Carlier, Jane M Harvey-Lloyd, Andrea Brammer, Barbara Nugent, Karen Cleaver, Jonathan P McNulty, Cláudia Sá dos Reis and Christina Malamateniou in Autism
Supplemental material, sj-docx-3-aut-10.1177_13623613211065542 for A systematic review of person-centred adjustments to facilitate magnetic resonance imaging for autistic patients without the use of sedation or anaesthesia by Nikolaos Stogiannos, Sarah Carlier, Jane M Harvey-Lloyd, Andrea Brammer, Barbara Nugent, Karen Cleaver, Jonathan P McNulty, Cláudia Sá dos Reis and Christina Malamateniou in Autism
Acknowledgments
We acknowledge Dr Ian Dale, Research lead of NAS, and Mr Stephen Humphreys, consultant from NAS for their input in the research design and Mr Keith Marais for reading and commenting on the manuscript’s final version. We would also like to thank Mrs Endang Scanlon, our dedicated librarian in the Department of Radiography, for providing essential help related to database searching and related guidance.
Footnotes
Authors’ note: This study uses identity-first language, as this is the preference of the autistic community.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Society and College of Radiographers CORIPS grant scheme (grant number SCoR 155-50011HY) and the City Radiography Research Fund (90020HY).
ORCID iDs: Nikolaos Stogiannos  https://orcid.org/0000-0003-1378-6631
https://orcid.org/0000-0003-1378-6631
Christina Malamateniou  https://orcid.org/0000-0002-2352-8575
https://orcid.org/0000-0002-2352-8575
Supplemental material: Supplemental material for this article is available online.
References
- Ahmed S. S., Unland T., Slaven J. E., Nitu M. E., Rigby M. R. (2014). Successful use of intravenous dexmedetomidine for magnetic resonance imaging sedation in autistic children. Southern Medical Journal, 107(9), 559–564. 10.14423/smj.0000000000000160 [DOI] [PubMed] [Google Scholar]
- Alibek S., Vogel M., Sun W., Winkler D., Baker C. A., Burke M., Gloger H. (2014). Acoustic noise reduction in MRI using Silent Scan: An initial experience. Diagnostic and Interventional Radiology, 20(4), 360–363. https://doi.org/10.5152%2Fdir.2014.13458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allely C. S. (2013). Pain sensitivity and observer perception of pain in individuals with autistic spectrum disorder. The Scientific World Journal, 13, Article 916178. 10.1155/2013/916178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M. A. (2013). Reduction of MRI acoustic noise achieved by manipulation of scan parameters-A study using veterinary MR sequences. Radiography, 19(1), 11–16. 10.1016/j.radi.2012.09.004 [DOI] [Google Scholar]
- Bartolucci A. A., Hillegass W. B. (2010). Overview, strengths, and limitations of systematic reviews and meta-analyses. In Chiappelli F., Brant X. M. C., Neagos N., Oluwadara O. O., Ramchandani M. H. (Eds.), Evidence-based practice: Toward optimizing clinical outcomes (pp. 17–33). Springer. [Google Scholar]
- Bednarz H. M., Kana R. K. (2018). Advances, challenges, and promises in pediatric neuroimaging of neurodevelopmental disorders. Neuroscience and Biobehavioral Reviews, 90, 50–69. 10.1016/j.neubiorev.2018.03.025 [DOI] [PubMed] [Google Scholar]
- Berglund I. G., Björkman B., Enskär K., Faresjö M., Huus K. (2017). Management of children with autism spectrum disorder in the anesthesia and radiographic context. Journal of Developmental and Behavioral Pediatrics, 38(3), 187–196. 10.1097/dbp.0000000000000432 [DOI] [PubMed] [Google Scholar]
- Björkman B., Berglund I. G., Enskär K., Faresjö M., Huus K. (2017). Peri-radiographic guidelines for children with autism spectrum disorder: A nationwide survey in Sweden. Child: Care, Health and Development, 43(1), 31–36. 10.1111/cch.12427 [DOI] [PubMed] [Google Scholar]
- Braun V., Clarke V. (2006). Using thematic analysis in psychology. Qualitative Research in Psychology, 3(2), 77–101. 10.1191/1478088706qp063oa [DOI] [Google Scholar]
- Brignell A., Chenausky K. V., Song H., Zhu J., Suo C., Morgan A. T. (2018). Communication interventions for autism spectrum disorder in minimally verbal children. The Cochrane Database of Systematic Reviews, 11(11), CD012324. 10.1002/14651858.cd012324.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryson S. E., Zwaigenbaum L., Roberts W. (2004). The early detection of autism in clinical practice. Paediatrics & Child Health, 9(4), 219–221. https://doi.org/10.1093%2Fpch%2F9.4.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley A. W., Holmes G. L. (2016). Epilepsy and autism. Cold Spring Harbor Perspectives in Medicine, 6(4), Article a022749. https://doi.org/10.1101%2Fcshperspect.a022749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calleja S., Islam F. M. A., Kingsley J., McDonald R. (2020). Healthcare access for autistic adults: A systematic review. Medicine, 99(29), Article E20899. https://doi.org/10.1097%2FMD.0000000000020899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chebuhar A., McCarthy A. M., Bosch J., Baker S. (2013). Using picture schedules in medical settings for patients with an autism spectrum disorder. Journal of Pediatric Nursing, 28(2), 125–134. 10.1016/j.pedn.2012.05.004 [DOI] [PubMed] [Google Scholar]
- Chen R., Jiao Y., Herskovits E. H. (2011). Structural MRI in autism spectrum disorder. Pediatric Research, 69, 63–68. https://doi.org/10.1203%2FPDR.0b013e318212c2b3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox A. D., Virues-Ortega J., Julio F., Martin T. L. (2017). Establishing motion control in children with autism and intellectual disability: Applications for anatomical and functional MRI. Journal of Applied Behavior Analysis, 50(1), 8–26. 10.1002/jaba.351 [DOI] [PubMed] [Google Scholar]
- Critical Appraisal Skills Programme. (2018. a). CASP Case Control Study Checklist. https://casp-uk.net/casp-tools-checklists/
- Critical Appraisal Skills Programme. (2018. b). CASP Cohort Study Checklist. https://casp-uk.net/casp-tools-checklists/
- Critical Appraisal Skills Programme. (2018. c). CASP Qualitative Checklist. https://casp-uk.net/casp-tools-checklists/
- Critical Appraisal Skills Programme. (2018. d). CASP Randomised Controlled Trial Checklist. https://casp-uk.net/casp-tools-checklists/
- Critical Appraisal Skills Programme. (2018. e). CASP Systematic Review Checklist. https://casp-uk.net/casp-tools-checklists/
- Dean D. C., Dirks H., O’Muircheartaigh J., Walker L., Jerskey B. A., Lehman K., Han M., Waskiewicz N., Deoni S. C. L. (2014). Pediatric neuroimaging using magnetic resonance imaging during non-sedated sleep. Pediatric Radiology, 44(1), 64–72. 10.1007/s00247-013-2752-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMayo M. M., Pokorski I., Song Y. J. C., Thapa R., Patel S., Ambarchi Z., Soligo D., Sadeli I., Thomas E. E., Hickie I. B., Guastella A. J. (2021). The feasibility of magnetic resonance imaging in a non-selective comprehensive clinical trial in pediatric autism spectrum disorder. Journal of Autism and Developmental Disorders. Advance online publication. 10.1007/s10803-021-05028-2 [DOI] [PubMed]
- Devita-Raeburn E. (2016, August 10). The controversy over autism’s more common therapy. https://www.spectrumnews.org/features/deep-dive/controversy-autisms-common-therapy/
- Dosenbach N. U. F., Koller J. M., Earl E. A., Miranda-Dominguez O., Klein R. L., Van A. N., Snyder A. Z., Nagel B. J., Nigg J. T., Nguyen A. L., Wesevich V., Greene D. J., Fair D. A. (2017). Real-time motion analytics during brain MRI improve data quality and reduce costs. Neuroimage, 16, 180–193. 10.1016/j.neuroimage.2017.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson L. C., Scott-Van Zeeland A. A., Hamilton G., Lincoln A., Golomb B. A. (2012). Brief report: Approaches to 31P-MRS in awake, non-sedated children with and without autism spectrum disorder. Journal of Autism and Developmental Disorders, 42(6), 1120–1126. https://doi.org/10.1007%2Fs10803-011-1359-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielsen T. P., Anderson J. S., Stephenson K. G., Beck J., King J. B., Kellems R., Top D. N., Jr, Russell N. C. C., Anderberg E., Lundwall R. A., Hansen B., South M. (2018). Functional MRI connectivity of children with autism and low verbal and cognitive performance. Molecular Autism, 9, Article 67. 10.1186/s13229-018-0248-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D., Chandler S., Charman T., Simonoff E., Baird G. (2016). Brief report: DSM-5 sensory behaviours in children with and without an autism spectrum disorder. Journal of Autism and Developmental Disorders, 46(11), 3597–3606. 10.1007/s10803-016-2881-7 [DOI] [PubMed] [Google Scholar]
- Hayes A. L. (2018). Autism spectrum disorder: Patient care strategies for medical imaging. Radiologic Technology, 90(1), 31–47. [PubMed] [Google Scholar]
- Haynes K. (2012). Reflexivity in qualitative research. In Symon G., Cassell C. (Eds.), Qualitative organizational research: Core methods and current challenges (pp. 72–89). SAGE. [Google Scholar]
- Hemsley B., Balandin S. (2014). A metasynthesis of patient-provider communication in hospital for patients with severe communication disabilities: Informing new translational research. Augmentative and Alternative Communication, 30(4), 329–343. 10.3109/07434618.2014.955614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J. P. T., Thomas J., Chandler J., Cumpston M., Li T., Page M. J., Welch V. A. (2021). Cochrane handbook for systematic reviews of interventions version, 62. https://training.cochrane.org/handbook/current [Google Scholar]
- Hlaing S., Stutzman S. E., Supnet C., Olson D. M. (2015). Factors associated with increased anxiety in the MRI waiting room. Journal of Radiology Nursing, 34(3), 170–174. 10.1016/j.jradnu.2015.04.009 [DOI] [Google Scholar]
- Horien C., Fontenelle S., Joseph K., Powell N., Nutor C., Fortes D., Butler M., Powell K., Macris D., Lee K., Greene A. S., McPartland J. C., Volkmar F. R., Scheinost D., Chawarska K., Constable R. T. (2020). Low-motion fMRI data can be obtained in pediatric participants undergoing a 60-minute scan protocol. Scientific Reports, 10, Article 21855. 10.1038/s41598-020-78885-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwan E., Yang J., Enders J., Napp A. E., Rief M., Dewey M. (2021). Patient preferences for development in MRI scanner design: A survey of claustrophobic patients in a randomized study. European Radiology, 31(3), 1325–1335. 10.1007/s00330-020-07060-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson N. L., Bree O., Lalley E. E., Rettler K., Grande P., Gani M. O., Ahamed S. I. (2014). Effect of a social script iPad application for children with autism going to imaging. Journal of Pediatric Nursing, 29(6), 651–659. 10.1016/j.pedn.2014.04.007 [DOI] [PubMed] [Google Scholar]
- Johnson N. L., Bree O. A. (2014). Social script iPad application versus usual care before undergoing medical imaging: Two case studies of children with autism. Journal of Radiology Nursing, 33(3), 121–126. 10.1016/j.jradnu.2014.04.001 [DOI] [Google Scholar]
- Johnson N. L., Salowitz N., Van Abel M., Dolan B., Van Hecke A., Ahamed S. I. (2017). Autism and research using magnetic resonance imaging. Journal of Radiology Nursing, 36(4), 245–252. 10.1016/j.jradnu.2017.08.005 [DOI] [Google Scholar]
- Kamat P. P., Karaga M. K., Wisniewski B. L., McCracken C. E., Simon H. K., Sidhu R., Grunwell J. R. (2018). Outpatient procedural sedation of patients with autism spectrum disorders for magnetic resonance imaging of the brain using propofol. Journal of Child Neurology, 33(5), 313–319. 10.1177/0883073817753908 [DOI] [PubMed] [Google Scholar]
- Kecskemeti S., Samsonov A., Velikina J., Field A. S., Turski P., Rowley H., Lainhart J. E., Alexander A. L. (2018). Robust motion correction strategy for structural MRI in unsedated children demonstrated with three-dimensional radial MPnRAGE. Radiology, 289(2), 509–516. 10.1148/radiol.2018180180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern J. K., Trivedi M. H., Grannemann B. D., Garver C. R., Johnson D. G., Andrews A. A., Savla J. S., Mehta J. A., Schroeder J. L. (2007). Sensory correlations in autism. Autism, 11(2), 123–134. 10.1177/1362361307075702 [DOI] [PubMed] [Google Scholar]
- Krakowski A., Ickowicz A. (2018). Stimulant withdrawal in a child with autism spectrum disorder and ADHD: A case report. Journal of the Canadian Academy of Child and Adolescent Psychiatry, 27(2), 148–151. [PMC free article] [PubMed] [Google Scholar]
- Krause F., Benjamins C., Eck J., Luhrs M., van Hoof R., Goebel R. (2019). Active head motion reduction in magnetic resonance imaging using tactile feedback. Human Brain, Mapping, 40(14), 4026–4037. 10.1002/hbm.24683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llaneza D. C., DeLuke S. V., Batista M., Crawley J. N., Christodulu K. V., Frye C. A. (2010). Communication, interventions, and scientific advances in autism: A commentary. Physiology & Behavior, 100(3), 268–276. https://doi.org/10.1016%2Fj.physbeh.2010.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch C. J., Voss H. U., Silver B. M., Power J. D. (2021). On measuring head motion and effects of head molds during fMRI. NeuroImage, 225, Article 117494. 10.1016/j.neuroimage.2020.117494 [DOI] [PubMed] [Google Scholar]
- Malamateniou C., Malik S. J., Counsell S. J., Allsop J. M., McGuinness A. K., Hayat T., Broadhouse K., Nunes R. G., Ederies A. M., Hajnal J. V., Rutherford M. A. (2013). Motion-compensation techniques in neonatal and fetal MR imaging. American Journal of Neuroradiology, 34(6), 1124–1136. 10.3174/ajnr.a3128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco E. J., Hinkley L. B., Hill S. S., Nagarajan S. S. (2011). Sensory processing in autism: A review of neurophysiologic findings. Pediatric Research, 69(5 Pt. 2), 48–54. 10.1203/pdr.0b013e3182130c54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Pedraza F., Carter A. S. (2009). Autism spectrum disorders in young children. Child and Adolescent Psychiatric Clinics of North America, 18(3), 645–663. https://doi.org/10.1016%2Fj.chc.2009.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLOS Medicine, 6(7), Article e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muskat B., Burnham Riosa P., Nicholas D. B., Roberts W., Stoddart K. P., Zwaigenbaum L. (2015). Autism comes to the hospital: The experiences of patients with autism spectrum disorder, their parents and health-care providers at two Canadian paediatric hospitals. Autism, 19(4), 482–490. 10.1177/1362361314531341 [DOI] [PubMed] [Google Scholar]
- Napp A. E., Enders J., Roehle R., Diederichs G., Rief M., Zimmermann E., Martus P., Dewey M. (2017). Analysis and prediction of claustrophobia during MR imaging with the claustrophobia questionnaire: An observational prospective 18-month single-center study of 6500 patients. Radiology, 283(1), 148–157. 10.1148/radiol.2016160476 [DOI] [PubMed] [Google Scholar]
- Netzke-Doyle V. (2010). Distraction strategies used in obtaining an MRI in pediatrics: A review of the evidence. Journal of Radiology Nursing, 29(3), 87–90. 10.1016/j.jradnu.2009.12.005 [DOI] [Google Scholar]
- Nicolaidis C., Raymaker D., McDonald K., Kapp S., Weiner M., Ashkenazy E., Gerrity M., Kripke C., Platt L., Baggs A. (2016). The development and evaluation of an online healthcare toolkit for autistic adults and their primary care providers. Journal of General Internal Medicine, 31(10), 1180–1189. 10.1007/s11606-016-3763-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaidis C., Raymaker D. M., Ashkenazy E., McDonald K. E., Dern S., Baggs A. E., Kapp S. K., Weiner M., Boisclair W. C. (2015). ‘Respect the way I need to communicate with you’: Healthcare experiences of adults on the autism spectrum. Autism, 19(7), 824–831. https://doi.org/10.1177%2F1362361315576221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmo-Smith V., Heuvelman H., Dalman C., Lundberg M., Idring S., Carpenter P., Magnusson C., Rai D. (2020). Anxiety disorders in adults with autism spectrum disorder: A population-based study. Journal of Autism and Developmental Disorders, 50(1), 308–318. 10.1007/s10803-019-04234-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordahl C. W., Mello M., Shen A. M., Shen M. D., Vismara L. A., Li D., Harrington K., Tanase C., Goodlin-Jones B., Rogers S., Abbeduto L., Amaral D. G. (2016). Methods for acquiring MRI data in children with autism spectrum disorder and intellectual impairment without the use of sedation. Journal of Neurodevelopmental Disorders, 8, Article 20. 10.1186/s11689-016-9154-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H. R., Lee J. M., Moon H. E., Lee D. S., Kim B. N., Kim J., Kim D. G., Paek S. H. (2016). A short review on the current understanding of autism spectrum disorders. Experimental Neurobiology, 25(1), 1–13. 10.5607/en.2016.25.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pua E. P. K., Barton S., Williams K., Craig J. M., Seal M. L. (2020). Individualised MRI training for paediatric neuroimaging: A child-focused approach. Developmental Cognitive Neuroscience, 41, Article 100750. 10.1016/j.dcn.2019.100750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riquelme I., Hatem S. M., Montoya P. (2016). Abnormal pressure pain, touch sensitivity, proprioception, and manual dexterity in children with autism spectrum disorders. Neural Plasticity, 2013, Article 1723401. 10.1155/2016/1723401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandbank M., Cascio C. (2019). Using a motion-tracking device to facilitate motion control in children with ASD for neuroimaging. Developmental Neurorehabilitation, 22(6), 365–375. 10.1080/17518423.2018.1502831 [DOI] [PubMed] [Google Scholar]
- Seo K. H., Jung H. S., Kang E. G., Kim C. J., Rhee H. Y., Jeon Y. S. (2014). Sedation using 5% lidocaine patches, midazolam and propofol in a combative, obese adolescent with severe autistic disorder undergoing brain magnetic resonance imaging: A case report. Korean Journal of Anesthesiology, 67(6), 421–424. 10.4097/kjae.2014.67.6.421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaltout E., Al-Dewik N., Samara M., Morsi H., Khattab A. (2020). Psychological comorbidities in autism spectrum disorder. Advances in Neurobiology, 24, 163–191. 10.1007/978-3-030-30402-7_6 [DOI] [PubMed] [Google Scholar]
- Siemann J. K., Veenstra-VanderWeele J., Wallace M. T. (2020). Approaches to understanding multisensory dysfunction in autism spectrum disorder. Autism Research, 13(9), 1430–1449. 10.1002/aur.2375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson S. (2020). Creating accessible healthcare environments for people with autism. Nursing Times, 116(1), 48–50. [Google Scholar]
- Skelton E., Drey N., Rutherford M., Ayers S., Malamateniou C. (2020). Electronic consenting for conducting research remotely: A review of current practice and key recommendations for using e-consenting. International Journal of Medical Informatics, 143, Article 104271. 10.1016/j.ijmedinf.2020.104271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. J., Bhanot A., Norman E., Mullett J. E., Bilbo S. D., McDougle C. J., Zurcher N. R., Hooker J. M. (2019). A protocol for sedation free MRI and PET imaging in adults with autism spectrum disorder. Journal of Autism and Developmental Disorders, 49(7), 3036–3044. 10.1007/s10803-019-04010-3 [DOI] [PubMed] [Google Scholar]
- Smith-Bindman R., Kwan M. L., Marlow E. C., Theis M. K., Bolch W., Cheng S. Y., Bowles E. J., Duncan J. R., Greenlee R. T., Kushi L. H., Pole J. D., Rahm A. K., Stout N. K., Weinmann S., Miglioretti D. L. (2019). Trends in use of medical imaging in US health care systems and in Ontario, Canada, 2000-2016. JAMA, 322(9), 843–856. 10.1001/jama.2019.11456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegemann T., Geretsegger M., Phan Quoc E., Riedl H., Smetana M. (2019). Music therapy and other music-based interventions in pediatric health care: An overview. Medicines, 6(1), Article 25. https://doi.org/10.3390%2Fmedicines6010025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stogiannos N., Carlier S., Harvey-Lloyd J., Brammer A., Cleaver K., McNulty J., Sa dos Reis C., Malamateniou C. (2020). A systematic review of patient-centred radiography practices to facilitate magnetic resonance imaging for patients with autism spectrum disorders (ASD) (PROSPERO CRD42020196864). https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020196864
- Tavassoli T., Miller L. J., Schoen S. A., Nielsen D. M., Baron-Cohen S. (2014). Sensory over-responsivity in adults with autism spectrum conditions. Autism, 18(4), 428–432. 10.1177/1362361313477246 [DOI] [PubMed] [Google Scholar]
- Tellmann L., Herzog H., Boers F., Lerche C., Shah N. J. (2018). Alternative headphones for patient noise protection and communication in PET-MR studies of the brain. EJNMMI Research, 8, Article 106. https://doi.org/10.1186%2Fs13550-018-0457-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tith S., Lalwani K., Fu R. (2012). Complications of three deep sedation methods for magnetic resonance imaging. Journal of Anaesthesiology Clinical Pharmacology, 28(2), 178–184. https://doi.org/10.4103%2F0970-9185.94837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugwell-Allsup J., Pritchard A. W. (2018). The experience of patients participating in a small randomised control trial that explored two different interventions to reduce anxiety prior to an MRI scan. Radiography, 24(2), 130–136. 10.1016/j.radi.2017.11.001 [DOI] [PubMed] [Google Scholar]
- Tziraki M., Garg S., Harrison E., Wright N. B., Hawkes R., Akhtar K., Green J., Stivaros S. (2021). A neuroimaging preparation protocol tailored for autism. Autism Research, 14(1), 65–74. 10.1002/aur.2427 [DOI] [PubMed] [Google Scholar]
- Vasa R. A., Mazurek M. O. (2015). An update on anxiety in youth with autism spectrum disorders. Current Opinion in Psychiatry, 28(2), 83–90. 10.1097/yco.0000000000000133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkden G. J., Pickering A. E., Gill H. (2019). Assessing long-term neurodevelopmental outcome following general anesthesia in early childhood: Challenges and opportunities. Anesthesia & Analgesia, 128(4), 681–694. https://doi.org/10.1213%2FANE.0000000000004052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Nayfeh T., Tetzlaff J., O’Blenis P., Murad M. H. (2020). Error rates of human reviewers during abstract screening in systematic reviews. PLOS ONE, 15(1), Article e0227742. https://doi.org/10.1371%2Fjournal.pone.0227742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland R. F., Polderman T., Hoekstra R. A., Smit D., Begeer S. (2020). The Dutch Sensory Perception Quotient-Short in adults with and without autism. Autism, 24(8), 2071–2080. https://doi.org/10.1177%2F1362361320942085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weill V., Zavodny S., Souders M. (2018). Autism spectrum disorder in primary care. The Nurse Practitioner, 43(2), 21–28. 10.1097/01.NPR.0000529670.62188.1a [DOI] [PubMed] [Google Scholar]
- Xu H. S., Cavaliere R. M., Min R. J. (2020). Transforming the imaging experience while decreasing sedation rates. Journal of the American College of Radiology, 17(1), 46–52. 10.1016/j.jacr.2019.08.005 [DOI] [PubMed] [Google Scholar]
- Yamada K., Suzuki Y., Ueki S., Itoh K., Watanabe M., Suzuki K., Igarashi H. (2020). Participant-driven simulation protocol with a mock scanner for pediatric magnetic resonance neuroimaging preparation without sedation. Clinical Simulation in Nursing, 47, 40–47. 10.1016/j.ecns.2020.07.002 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-aut-10.1177_13623613211065542 for A systematic review of person-centred adjustments to facilitate magnetic resonance imaging for autistic patients without the use of sedation or anaesthesia by Nikolaos Stogiannos, Sarah Carlier, Jane M Harvey-Lloyd, Andrea Brammer, Barbara Nugent, Karen Cleaver, Jonathan P McNulty, Cláudia Sá dos Reis and Christina Malamateniou in Autism
Supplemental material, sj-docx-2-aut-10.1177_13623613211065542 for A systematic review of person-centred adjustments to facilitate magnetic resonance imaging for autistic patients without the use of sedation or anaesthesia by Nikolaos Stogiannos, Sarah Carlier, Jane M Harvey-Lloyd, Andrea Brammer, Barbara Nugent, Karen Cleaver, Jonathan P McNulty, Cláudia Sá dos Reis and Christina Malamateniou in Autism
Supplemental material, sj-docx-3-aut-10.1177_13623613211065542 for A systematic review of person-centred adjustments to facilitate magnetic resonance imaging for autistic patients without the use of sedation or anaesthesia by Nikolaos Stogiannos, Sarah Carlier, Jane M Harvey-Lloyd, Andrea Brammer, Barbara Nugent, Karen Cleaver, Jonathan P McNulty, Cláudia Sá dos Reis and Christina Malamateniou in Autism