Abstract
Background:
Identifying neural predictors of worsening subthreshold hypomania severity can help identify risk of progression to BD . While diffusion Magnetic Resonance Imaging (dMRI) studies reported white matter microstructural abnormalities in tracts supporting emotional regulation in individuals with BD, it remains unknown whether similar patterns of white matter microstructure predict worsening of subthreshold hypomania severity in non-BD individuals.
Methods:
dMRI data were collected in: 81 non-BD individuals recruited across a range of subthreshold depression and hypomania, and followed for six months; and independent samples of 75 BD and 58 healthy individuals. All individuals were assessed using standardized diagnostic assessments, mood and anxiety symptom rating scales. Global probabilistic tractography and a tract-profile approach examined fractional anisotropy(FA), a measure of fiber collinearity, in tracts supporting emotional regulation shown to have abnormalities in BD: forceps minor (FMIN), anterior thalamic radiation (ATR), cingulum bundle (CB), and uncinate fasciculus (UF).
Results:
Lower FA in left CB (middle,β=−0.22,P=0.022; posterior,β=−0.32,P<0.001), right CB (anterior,β=−0.30,P=0.003; posterior,β=−0.27,P=0.005), and right UF (frontal,β=−0.29,P=0.002; temporal,β=−0.40,P<0.001) predicted worsening of subthreshold hypomania severity in non-BD individuals. BD versus healthy individuals showed lower FA in several of these segments: middle left CB (F=8.7,P=0.004), anterior right CB (F=9.8,P=0.002), and frontal right UF (F=7.0,P=0.009). Non-BD individuals with worsening 6-month hypomania had lower FA in these three segments versus HC and non-BD individuals without worsening hypomania, but similar FA to BD individuals.
Limitations:
Relatively short follow-up.
Conclusions:
White matter predictors of worsening subthreshold hypomania in non-BD individuals parallel abnormalities in BD individuals, and can guide early risk identification and interventions.
Keywords: Bipolar Disorder, hypomania, risk, neural markers, white matter, dMRI
INTRODUCTION
Bipolar disorder (BD) is a lifelong condition and a major public health problem, marked by high levels of morbidity and mortality (Grande et al., 2016; Organization, 2011). Previous studies have reported that early adulthood (18-25 years), family history of mania/hypomania, and persistent subthreshold manic, depressive, and/or anxiety symptoms are important risk factors for the development of BD (Axelson et al., 2015; Birmaher et al., 2018; Craddock and Jones, 1999; Grande et al., 2016; Kerner, 2014). These subthreshold symptoms, in particular, have been associated with severe psychological distress and may persist for years prior reaching duration and severity criteria for BD in the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) (Faedda et al., 2015; Regier et al., 2013; SAMHSA, 2007; Skjelstad et al., 2010; Ward et al., 2016). Among these symptoms, hypomania, even when subthreshold, has been particularly associated with subsequent progression to BD (Axelson et al., 2015; Fiedorowicz et al., 2011; Hafeman et al., 2016). Identifying markers of these subthreshold hypomania severity can thus facilitate earlier detection of risk for BD, and aid the development of new interventions to delay, or even prevent, worsening of symptoms, and, potentially, BD. Neuroimaging techniques provide an unique opportunity to identify objective, neural markers reflecting the neurobiological processes underlying worsening of subthreshold symptoms and whether they are generalizable or particular for hypomanic, depression, or anxiety symptoms.
One neuroimaging technique, diffusion Magnetic Resonance Imaging (dMRI), has reliably identified white matter abnormalities in BD (Barnea-Goraly et al., 2009; Benedetti et al., 2011; Caseras et al., 2015; Emsell et al., 2013; Foley et al., 2018; Ha et al., 2011; Lin et al., 2011; Linke et al., 2013; McIntosh et al., 2008; Rozovsky et al., 2021; Santos et al., 2021; Sarrazin et al., 2014; Versace et al., 2014). By measuring the diffusivity properties of water in the brain, dMRI assesses the white matter microstructure that supports complex neurobiological processes, and can thereby provide insights into neural mechanisms associated with BD (Barnea-Goraly et al., 2009; Benedetti et al., 2011; Caseras et al., 2015; Emsell et al., 2013; Foley et al., 2018; Ha et al., 2011; Jones and Leemans, 2011; Lin et al., 2011; Linke et al., 2013; McIntosh et al., 2008; Rozovsky et al., 2021; Santos et al., 2021; Sarrazin et al., 2014; Versace et al., 2014). Most dMRI studies employed Fractional anisotropy (FA), an index of water directionality reflecting the integrity and/or collinearity of the fibers in white matter tracts (Jones and Leemans, 2011). Existing findings indicate lower FA relative to healthy individuals in white matter tracts connecting prefrontal cortical and other cortical regions important for emotional regulation (Barnea-Goraly et al., 2009; Benedetti et al., 2011; Caseras et al., 2015; Emsell et al., 2013; Foley et al., 2018; Ha et al., 2011; Lin et al., 2011; Linke et al., 2013; McIntosh et al., 2008; Santos et al., 2021; Sarrazin et al., 2014; Versace et al., 2014), including the forceps minor (FMIN) (Barnea-Goraly et al., 2009; Versace et al., 2014), anterior thalamic radiation (ATR) (Lin et al., 2011; Rozovsky et al., 2021), cingulum bundle (CB) (Benedetti et al., 2011; Emsell et al., 2013; Santos et al., 2021; Sarrazin et al., 2014; Versace et al., 2014), and uncinate fasciculus (UF) (Benedetti et al., 2011; Caseras et al., 2015; Foley et al., 2018; Ha et al., 2011; Lin et al., 2011; Linke et al., 2013; McIntosh et al., 2008; Versace et al., 2014). A study published by the ENIGMA network used Tract-Based Spatial Statistics (TBSS) to compare BD and healthy participants in over 40 regions of interest using FA. This study showed that BD participants had lower FA in 29 regions relative to healthy controls, with the CB and FMIN being among the regions with the largest effect sizes (Favre et al., 2019). Other studies have also shown that FA abnormalities are present in relatives of those with BD, which further highlights FA as a reasonable measure to study BD (Frazier et al., 2007; Ganzola et al., 2018; Versace et al., 2010). Diffusion properties are not uniform along tracts, however. In contrast to TBSS, tract-profile approaches (Wasserthal et al., 2018a; Wasserthal et al., 2018b; Yeatman et al., 2012) can identify which tract segments are most associated with outcomes of interest, to inform understanding of the specific gray matter regions whose connections run through these white matter segments, and, in turn, provide more focal neural targets for novel neuromodulation interventions (e.g., Theta Burst Stimulation, TBS, or transcranial Direct Current Stimulation, tDCS) (Dondé et al., 2017; Ferrarelli and Phillips, 2021; Safadi et al., 2018). Using tract-profile approaches, we showed that individuals with BD had lower FA in the anterior segment of right CB relative to healthy individuals (Rozovsky et al., 2021). It remains unknown, however, whether these neural abnormalities predict worsening of subthreshold hypomania. While there are some reports of functional neural predictors of worsening of subthreshold hypomania in individuals (Eckstrand et al., 2021), no study to our knowledge has determined the extent to which focal or widespread abnormalities in white matter tracts supporting emotional regulation predict worsening of these symptoms. Thus, knowledge of the white matter predictors of worsening of subthreshold hypomania remains limited.
The aims of this longitudinal study were twofold. First, we aimed to identify white matter predictors of worsening subthreshold hypomania severity in non-BD individuals. To this end, we used global probabilistic tractography and a tract-profile approach to examine FA in white matter tracts supporting emotional regulation previously shown to be abnormal in BD, the FMIN, ATR, CB and UF. Second, we evaluated the extent to which these white matter markers differentiated non-BD, BD and healthy individuals, to draw parallels between white matter predictors of worsening of subthreshold hypomania and abnormalities characterizing BD. This approach allowed us to identify specific neural markers of BD risk that were also associated with BD, and thus likely reflected neural marker predictors of future progression to BD. We hypothesized that: 1. lower fiber collinearity in frontal segments of white matter tracts supporting emotional regulation would predict worsening of subthreshold hypomania severity six months later in non-BD individuals; and 2. individuals with BD, and non-BD individuals showing worsening hypomania severity six months later, would show lower fiber collinearity in these segments relative to a separate group of healthy individuals, while BD and these non-BD individuals would show no such differences.
METHODS AND MATERIALS
Participants.
Eighty-one non-BD individuals (ages=18-30, mean[SD]=21.9[2.7] years) were recruited through the Dimensions of Affect, Mood, and Neural Activity Associated with Distress (DIAMOND) study (R37MH100041, PI: Phillips), across a range of subthreshold hypomanic (and depressive) symptoms. All individuals were right-handed. Bipolar spectrum disorders were excluded using the Structured Clinical Interview for DSM-5 (SCID) (First, 2014; First et al., 2015).
Seventy-five individuals with BD (ages=8-30, mean[SD]=21.5[5.3] years) were recruited through the Course and Outcome of Bipolar Youth (COBY, R01MH059929; PIs: Birmaher, Phillips, Versace), Bipolar Offspring Study (BIOS; R01MH060952; PIs: Birmaher and Phillips) studies and DIAMOND. COBY and BIOS are both longitudinal studies, with COBY examining neural predictors of different clinical courses of BD from childhood to adulthood, while BIOS assessed neural predictors of BD or psychopathology in general in offspring of BD and non-BD parents. Fifty-eight age and sex-matched healthy individuals (HC; ages=10-35, mean[SD]=21.6[6.2] years) with no Axis-I diagnoses were also recruited across these studies. BD and HC individuals were right-handed. Demographic and clinical characteristics are in Table 1 (Supplementary information for exclusion criteria).
Table 1.
Demographic and clinical characteristics
| Demographic and clinical information for Non-BD individuals (N=81) | |||
|---|---|---|---|
| Variable | Baseline | 6-month | Baseline vs 6- month testing |
| Sexa | |||
| Female, No. (%) | 59 (72.8%) | - | - |
| Male, No. (%) | 22 (27.1%) | - | - |
| Age (years) - mean (SD)b | 21.9 (2.7) | - | - |
| YMRS - mean score (SD)c | 1.44 (1.6) | 1.94 (2.2) | χ2=4.9 (P=0.026) |
| HAM-D - mean score (SD)c | 8.27 (8.6) | 8.21 (6.1) | χ2=2.0 (P=0.157) |
| HAM-A - mean score (SD)c | 6.89 (7.5) | 6.19 (6.2) | χ2=0.5 (P=0.473) |
| Taking medications | |||
| Antidepressants, No. (%) | 1 (1.2%) | 10 (12.3%) | - |
| Antipsychotics, No. (%) | 0 (0.0%) | 0 (0.0%) | - |
| Mood stabilizers, No. (%) | 0 (0.0%) | 1 (1.2%) | - |
| Demographic and clinical information for BD and healthy individuals | |||
| Variable | BD (N=75) | HC (N=58) | BD vs HC testing |
| Sexa | |||
| Female, No. (%) | 33 (44.0%) | 25 (43.1%) | χ2<0.1 (P=0.999) |
| Male, No. (%) | 42 (56.0%) | 33 (56.9%) | |
| Age (years) - mean (SD)b | 21.5 (5.3) | 21.6 (6.3) | t=0.1(P=0.913) |
| Age of BD onset – mean (SD) | 10.4 (4.7) | - | - |
| BD illness duration – mean (SD) | 11.2 (7.0) | - | - |
| Lifetime history of non-BD psychiatric disorders | |||
| Depression, No. (%) | 14 (18.7%) | 0 (0.0%) | - |
| Anxiety, No. (%) | 51 (68.0%) | 0 (0.0%) | - |
| ADHD, No. (%) | 29 (38.7%) | 0 (0.0%) | - |
| Taking medications at scan | |||
| Antidepressants, No. (%) | 19 (25.3%) | 0 (0%) | - |
| Antipsychotics, No. (%) | 21 (28.0%%) | 0 (0%) | - |
| Mood stabilizers, No. (%) | 27 (36.0%) | 0 (0%) | - |
Abbreviations: BD, bipolar disorder; HC, healthy control; YMRS, Young Mania Rating Scale; HAM-D, Hamilton Depression Rating Scale; HAM-A, Hamilton Anxiety Rating Scale.
There was a significant sex difference between non-BD and HC (χ2=11.2, P<0.001) and non-BD and BD (χ2=12.2, P<0.001).
There was no age difference between non-BD and BD (t=0.6, P=0.534) or non-BD and HC (t=0.4, P=0.721).
Friedman’s test was used for these analyses.
Data in subsamples of DIAMOND, COBY and BIOS were previously published in separate studies focusing on different questions (Acuff et al., 2019; Greenberg et al., 2021; Rozovsky et al., 2021; Santos et al., 2021), but no study combined data from these studies under the same tractography approach to determine whether white matter predictors of worsening of subthreshold hypomania severity in individuals parallel white matter abnormalities in individuals with established BD.
All studies obtained approval from the Office of Research Protections at the University of Pittsburgh.
Clinical Assessments.
Non-BD individuals were assessed at the time of the scan (baseline) and 6-month follow-up to assess clinically-relevant changes in mood symptoms (Excellence, 2009). At both time points, the SCID-5 was used to determine diagnostic status, and the Young Mania Rating Scale (YMRS) (Young et al., 1978) and the Hamilton Depression Rating Scale (HAM-D) (HAMILTON, 1960), to assess hypomanic and depressive symptom severity. The Hamilton Anxiety Rating Scale (HAM-A) (Hamilton, 1959) was used to evaluate anxiety severity, as anxiety also precedes BD (Duffy et al., 2010). Medication details were noted at scan and 6-month follow-up.
In BD and HC individuals, information (yes/no) on antidepressant, antipsychotic, and mood stabilizer usage was collected at scan. Age of BD onset and lifetime history of non-BD psychiatric disorders (Depression, Anxiety, and Attention Deficit Hyperactivity Disorder - ADHD) was also evaluated using the SCID for DSM-4 (Supplementary information for other clinical assessments in BD and HC individuals) (First and Gibbon, 2004).
Neuroimaging data.
dMRI acquisition protocols for each study and preprocessing steps are described in the Supplementary information. In brief, diffusion-weighted images were corrected for eddy current, subject motion, and echo planar imaging distortion using topup and eddy (Andersson et al., 2003; Smith et al., 2004). After preprocessing, dMRI bundle-specific tractography for seven white matter tracts in emotion regulation circuitries (FMIN and left and right ATR, CB, and UF) was performed using TractSeg (Wasserthal et al., 2018a; Wasserthal et al., 2018b), a convolutional neural network (CNN) approach that segments white matter tracts using fiber orientation distribution function (fODF) peaks, derived using Mrtrix-3 (Tournier et al., 2019). CNN is a state-of-art deep learning model used for the identification and classification of complex patterns in images (Wasserthal et al., 2018a; Wasserthal et al., 2019; Wasserthal et al., 2018b). Overall mean FA and nodal values were extracted for each tract as measures of the collinearity of the fibers across (mean) and along (tract-profile) the tract, using 10 consecutive nodes, each node covering 10% of the entire tract. To explore the contribution of other white matter tracts in predicting hypomania severity at 6 months, 39 additional, non a priori tracts were also reconstructed (Supplementary information for additional details). The effect of movements (translation and rotation) on mean FA for all reconstructed tracts was examined using Multivariate analysis of covariance (MANCOVA) and, in case of a significant effect, average translation and/or rotation were included as covariates in the statistical analyses. In addition, to help interpret FA findings, Axial Diffusivity (AD) and Radial Diffusivity (RD) maps were also calculated, as they reflect the displacement of water molecules along (AD) or perpendicular (RD) to the principal diffusion direction (Jones and Leemans, 2011), with higher RD in particular thought to reflect greater dispersion of fibers and/or myelin damage (Aung et al., 2013). The effects of different scanner protocols in FA, AD, and RD metrics were harmonized using the ComBat toolbox, as previously done (Fortin et al., 2017; Jalbrzikowski et al., 2021; Versace et al., 2021). ComBat is batch-effect correction tool that minimize the variance introduced by different MRI protocols (Core Team, 2017; Fortin et al., 2017). After harmonization, the effect of scanner protocol was negligible (P>0.050) in the distressed non-BD sample (F=0.6, P=0.778) and BD/HC sample (F=0.6, P=0.689).
Statistical analyses.
We used R to perform a two-level analytic approach, as described below (Core Team, 2017). Evaluation of clinical scores (YMRS, HAM-D, and HAM-A) revealed a non-normal distribution (Shapiro-Wilk test, P<0.05) in the non-BD sample. Consequently, to test our main hypothesis, we performed Friedman’s tests instead of standard ANOVA and negative binomial regression instead of linear regression (Core Team, 2017). Given the known effects of age (Kochunov et al., 2012; Lebel et al., 2012; Westlye et al., 2010) and sex (Simmonds et al., 2014) on white matter, these variables were included as covariates. In addition, mean translation was included as a covariate as it showed a main effect on mean FA of reconstructed tracts (Supplementary information) (Yendiki et al., 2014). False Discovery Rate (FDR P < 0.05) (Benjamini and Hochberg, 1995) accounted for multiple testing.
Level 1 analysis.
To identify the neural predictors of worsening subthreshold hypomania severity in non-BD individuals, we first assessed whether there was a significant change in YMRS scores over time (baseline to 6 months later). Then, after accounting for age, sex, mean translation, and severity of subthreshold hypomania severity at baseline (independent variables), seven independent negative binomial regression models were used to assess the main effect of mean FA on hypomania severity 6 months later (dependent variable), with one model for each tract of interest (FMIN and left and right ATR, CB, UF).
Tract-profile analyses.
For those tracts in which, after FDR correction, there was a significant relationship between mean FA (across the entire tract) and hypomania severity 6 months later, nodal FA values (tract-profile; 10 consecutive nodes) were used in negative binomial regression models to determine if the above findings were focal or widespread. If two or more consecutive nodes survived FDR correction (across 10 nodes) in each tract, mean FA across these nodes was used to identify node clusters for use in analyses in Level 2. Mean AD and RD were also extracted from these identified node clusters to determine whether AD and RD in these node clusters predicted hypomania severity 6 months later, using models as above.
Controlling for other symptoms at baseline.
To evaluate the effects of baseline depressive and anxiety symptoms in the hypomania prediction models performed above, depressive (HAM-D) and anxiety (HAM-A) symptom severity at baseline were included as covariates in additional regression models.
Level 2 analysis.
Analysis of covariance (ANCOVA) compared FA between BD and HC individuals in node clusters in tracts identified in Level 1 analysis. Covariates were: age, sex, and mean translation.
To determine the extent to which non-BD individuals with increasing versus those with non-increasing hypomania severity differed from either HC or BD individuals in FA in node clusters showing significant differences in FA between these two groups, non-BD individuals were first divided into two groups: 1) those who showed an increase in hypomania severity over 6 months, and 2) those who showed no change or decreasing hypomania severity over 6 months. A four (group) x one (FA in node cluster) ANCOVA then compared FA between groups in each node cluster showing differences between BD and HC individuals. Covariates were: age, sex, and mean translation. FDR accounted for multiple testing.
Sensitivity analyses
To assess whether FA in node clusters predicted other symptoms, depressive (HAM-D) or anxiety (HAM-A) symptom severity 6 months later were dependent variables in additional regression models described in Level 1 analysis.
Additional analyses also evaluated the effect of starting new medications between visits on hypomania, depression, and anxiety severity 6 months later. Baseline symptom severity was a covariate. These analyses were performed excluding non-BD individuals who started medications between visits.
Comparison of FA between BD and HC (and among BD, HC and non-BD) groups in node clusters showing differences in FA between BD and HC (and among BD, HC and non-BD) groups in the main analyses above was also performed including only individuals older than 18 years.
Exploratory analyses
In non-BD individuals, additional analyses explored the effects of age and sex on hypomania severity at 6 months.
In individuals with BD, analyses evaluated relationships among FA in node clusters showing abnormal FA relative to HC individuals (as well as with FA in node clusters showing relationships with future hypomania or other symptom severity) and medication classes (yes/no), lifetime history of non-BD diagnoses (depression, anxiety, and ADHD), hypo/manic, depressive and anxiety symptoms, age of onset, and BD illness duration.
In all three main groups, analyses explored the effects of age and sex on FA in the above node clusters.
RESULTS
Level 1 analysis.
Friedman’s test revealed a significant increase in YMRS scores over time (Table 1). Regression models revealed that lower mean FA of three white matter tracts were associated with worsening subthreshold hypomania 6 months later: left CB, right CB, and right UF (Table 2).
Table 2.
White matter predictors of worsening of subthreshold hypomania severity at 6-month follow-up.
| Level 1 analysis - Tracts a | ||||||
|---|---|---|---|---|---|---|
| White matter tract | β | SE | IRR | 95% CI | Pb | FDR Pb,c |
| FMIN | −0.19 | 0.10 | 0.83 | 0.67 - 1.01 | 0.062 | 0.072 |
| Left ATR | −0.21 | 0.10 | 0.81 | 0.66 - 0.99 | 0.044 | 0.063 |
| Right ATR | −0.13 | 0.11 | 0.88 | 0.71 - 1.07 | 0.199 | 0.199 |
| Left CB | −0.22 | 0.10 | 0.80 | 0.67 - 0.96 | 0.017 | 0.047 |
| Right CB | −0.25 | 0.10 | 0.78 | 0.64 - 0.92 | 0.004 | 0.028 |
| Left UF | −0.20 | 0.10 | 0.82 | 0.67 - 0.99 | 0.045 | 0.063 |
| Right UF | −0.24 | 0.10 | 0.79 | 0.64 - 0.96 | 0.020 | 0.047 |
| Level 1 analysis - Node clusters a | ||||||
| Cluster | β | SE | IRR | 95% CI | Pb | FDR Pb,c |
| Left CB - Middle Cluster (size=30%) | −0.22 | 0.09 | 0.80 | 0.67 - 0.97 | 0.022 | 0.022 |
| Left CB - Posterior cluster (size=20%) | −0.32 | 0.09 | 0.73 | 0.61 - 0.86 | <0.001 | 0.001 |
| Right CB - Anterior Cluster (size=30%) | −0.30 | 0.10 | 0.74 | 0.61 - 0.91 | 0.003 | 0.004 |
| Right CB - Posterior cluster (size=20%) | −0.27 | 0.10 | 0.76 | 0.62 - 0.93 | 0.005 | 0.007 |
| Right UF - Frontal cluster (size=10%) | −0.29 | 0.10 | 0.75 | 0.56 - 0.90 | 0.002 | 0.004 |
| Right UF - Temporal cluster (size=30%) | −0.40 | 0.10 | 0.67 | 0.55 - 0.81 | <0.001 | <0.001 |
Abbreviations: SE, Standard error; IRR, Incidence-rate ratios; CI, Confidence interval; FDR, False discovery rate; ATR, anterior thalamic radiation; FMIN, forceps minor; CB, cingulum bundle; UF, uncinate fasciculus.
Age, sex, mean translation, and severity of hypomania severity at baseline were used as covariates.
P values ≤ 0.05 are reported in bold characters.
FDR corrected P values.
Tract-profile analyses.
Six focal segments were identified in which lower FA was associated with worsening subthreshold hypomania 6 months later: left CB middle and posterior clusters, right CB anterior and posterior clusters, right UF frontal and temporal clusters (Table 2, Figure 1, Supplementary Figure 1). There was no association between AD and RD and subthreshold hypomania severity 6 months later (Supplementary Table 1).
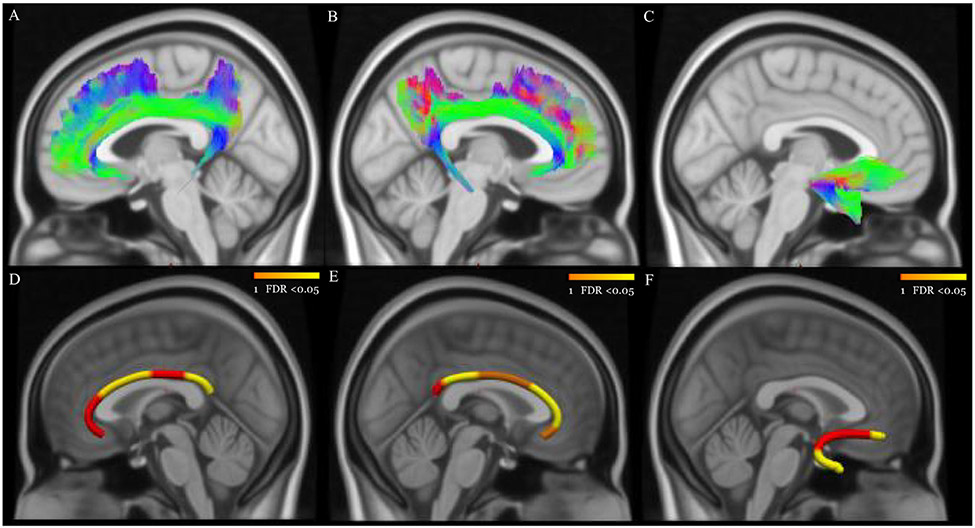
Figure 1. White matter predictors of worsening of subthreshold hypomania severity in non-BD individuals.
Panels A, B, and C show the reconstructed left CB, right CB, and right UF using TractSeg. Panels D, E, and F show node clusters (in yellow) in the left CB (middle, size=30% of the tract; posterior, size=20%), right CB (anterior, size=30% of the tract; posterior, size=20%), and right UF (frontal, size=10% of the tract; temporal, size=30%). The background is the standard MNI-152 1 mm brain. Red-Yellow color bar represents the range of p values after FDR correction.
Controlling for other symptoms at baseline.
After including baseline depression and anxiety severity as covariates, analyses revealed similar results, with all six node clusters being significantly associated with 6-month hypomania severity (Supplementary Table 2).
Level 2 analysis.
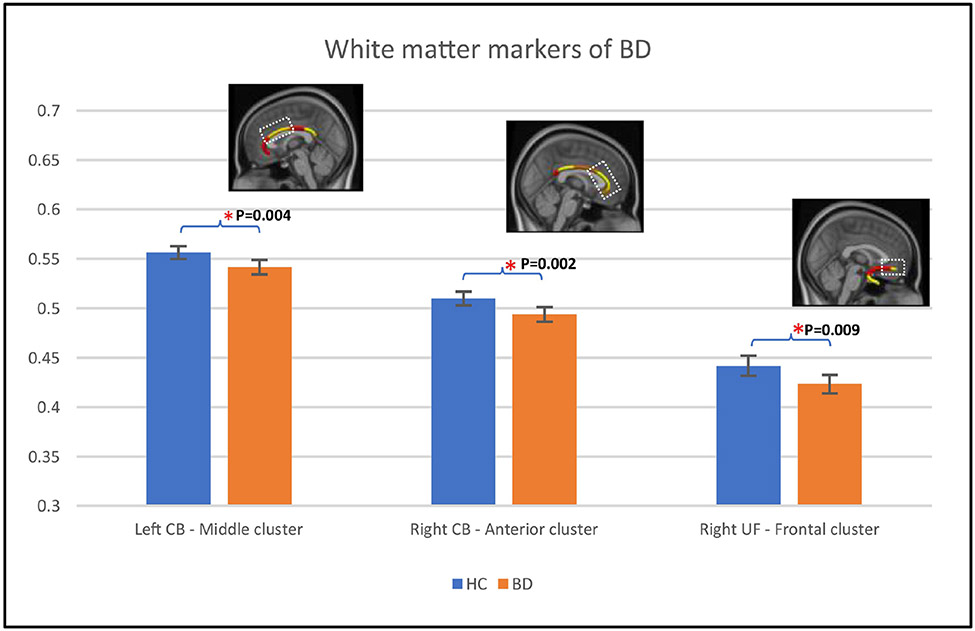
Relative to HC individuals, individuals with BD showed significantly lower FA in the left CB middle cluster, right CB anterior cluster, and right UF frontal cluster (Table 3A; Figure 2).
Table 3A-B.
Between group differences in FA (BD versus HC) using node clusters FA.
| Table 3A - BD x HC | |||
|---|---|---|---|
| Cluster | F | Pa | FDR Pa,b |
| Left CB - Middle Clusterc | 8.70 | 0.004 | 0.011 |
| Left CB - Posterior clusterc | 1.90 | 0.172 | 0.206 |
| Right CB - Anterior Clusterc | 9.80 | 0.002 | 0.011 |
| Right CB - Posterior clusterc | 4.60 | 0.035 | 0.052 |
| Right UF - Frontal clusterc | 7.00 | 0.009 | 0.018 |
| Right UF - Temporal clusterc | 0.40 | 0.513 | 0.513 |
| Table 3B - HC x Non-BD increasing hypomania severity x non-BD increasing hypomania severity x BD | |||
| Cluster | F | Pa | FDR Pa,b |
| Left CB - Middle Cluster | 3.00 | 0.034 | 0.037 |
| Right CB - Anterior Cluster | 2.90 | 0.037 | 0.037 |
| Right UF - Frontal cluster | 4.90 | 0.002 | 0.006 |
Abbreviations: BD, Bipolar Disorder; HC, healthy control; FDR, False discovery rate; CB, Cingulum bundle; UF, uncinate fasciculus.
P values ≤ 0.05 are reported in bold characters.
FDR corrected P values.
BD showed lower FA than HC individuals in this node cluster.
Figure 2. White matter differences between BD and HC individuals.
Figure 2 shows error-bar plots that depict the group difference upon FA in the 58 HC (blue color) and 75 BD individuals (orange color). Braces and asterisks show p-values that survived FDR correction. Panels show the node clusters. The background in the panels is the standard MNI-152 1 mm brain. Error bars show 95% confidence intervals. Abbreviations: CB, cingulum bundle; UF, uncinate fasciculus; HC, healthy control; BD, bipolar disorder; FA, Fractional Anisotropy; FDR, False Discovery Rate.
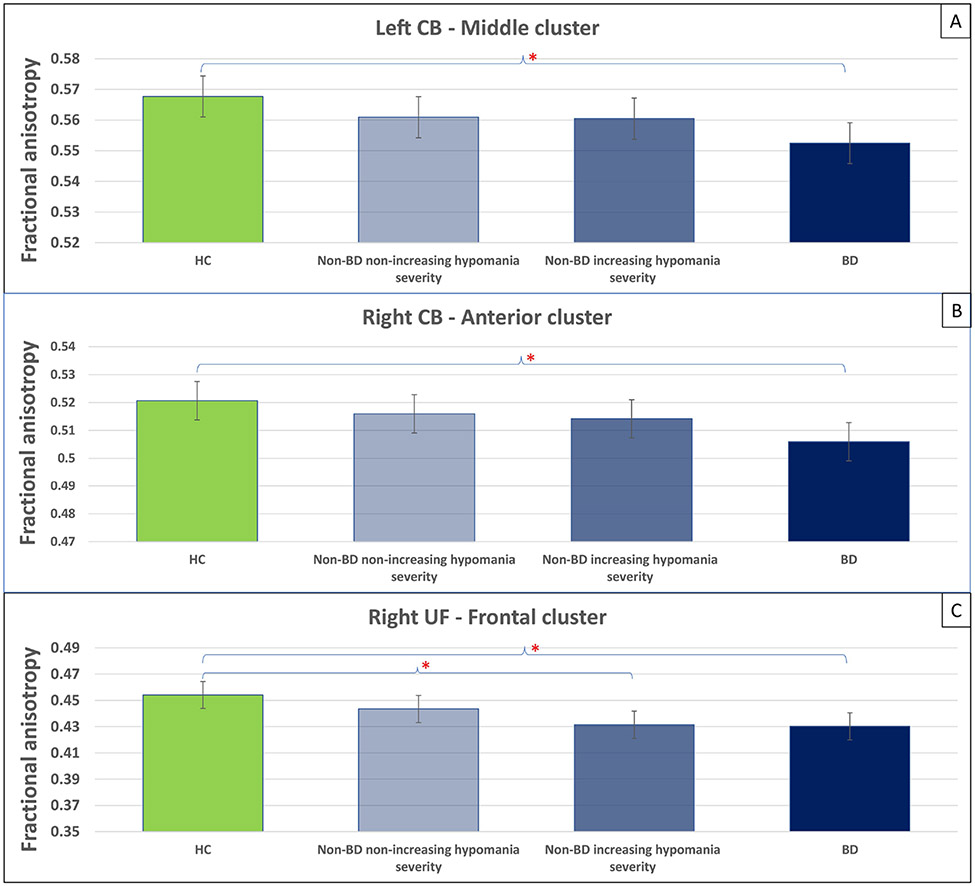
Thirty-four non-BD individuals showed an increase in hypomania severity over time, while 47 showed a decrease or no change. There was a significant main effect of group (non-BD non-increasing hypomania severity, non-BD increasing hypomania severity, BD, and HC) on FA in the 3 node clusters showing differences in FA between BD and HC individuals (Table 3B). Across these 3 node clusters, HC showed the highest FA, followed by non-BD with decreasing or no changes in hypomania severity, non-BD with increasing hypomania severity, and BD (Figure 3; Supplementary Table 3).
Figure 3. Between-group differences in FA of node clusters identified in Level 2 analysis.
Panels A-C depict the between-group differences in FA of node cluster identified in Level 2 analysis (Panels A, Left CB ; Panels B, Right CB; Panels C, Right UF) including all participants. Fifty-eight HC (green color), 47 non-BD with decreasing or no changes in hypomania severity (light blue color), 34 non-BD with increasing hypomania severity (blue color), and 75 BD (dark blue color) were included in the analyses. Asterisks show significant p-values for indicated between-group comparisons. Error bars show 95% confidence intervals. Abbreviations: FA, fractional anisotropy; CB, cingulum bundle; UF, uncinate fasciculus.
Sensitivity analyses
There was no difference in HAM-D and HAM-A scores between visits (Table 1). There was no association between FA in node clusters and either depressive or anxiety symptom severity 6 months later (Supplementary Table 4).
In non-BD individuals, those who started taking antidepressants between visits (N=9) showed higher hypomania severity 6 months later (mean[SD]=3.7[2.8]; P=0.009) than those who were not taking antidepressants (N=71, mean[SD]=1.7[2.1]). One participant reported taking antidepressants at both visits. There was no association between starting antidepressants and depressive (P=0.161) or anxiety (P=0.221) severity 6 months later. Sensitivity analyses excluding individuals who were taking antidepressants showed findings consistent with those reported in Level 1 analysis (Supplementary Table 5).
Sensitivity analyses including only BD, non-BD and HC individuals older than 18 years revealed a similar trend of reduced FA in BD (Supplementary Table 6 and 7).
Exploratory analyses
In non-BD individuals, younger age at scan was associated with worsening hypomania severity 6 months later (P=0.002). Sex showed no association with symptom severity 6 months later (Supplementary Table 8).
In individuals with BD, there was a significant effect of antidepressants on FA of three node clusters, indicating that BD individuals taking versus those not taking antidepressants had lower FA in these node clusters; however, these findings did not survive FDR correction (Supplementary Table 9). There was no effect of lifetime history of non-BD psychiatric disorders on FA in node clusters (Supplementary Table 10). There were trend negative correlations between hypo/mania severity, and BD illness duration, and FA in the right CB anterior cluster; and a positive correlation between depression severity and FA in the right UF frontal cluster, which did not survive FDR correction (Supplementary Tables 11 and 12). There were no relationships between age of onset (Supplementary Table 13) and FA in node clusters.
There was no effect of sex and age on FA of node clusters in all groups (Supplementary Table 14).
DISCUSSION
The current study employed global probabilistic tractography and a tract-profile approach to identify white matter predictors of worsening of subthreshold hypomania severity in non-BD individuals, and the extent to which these neural predictors paralleled abnormalities in an independent sample of individuals with BD. In support of our first hypothesis, lower collinearity of the fibers in the left/right CB and right UF was associated with worsening subthreshold hypomania severity six months later. These abnormalities were focal, including clusters in the middle and posterior left CB, anterior and posterior right CB, and frontal and temporal right UF. These relationships were specific to hypomania, and were not shown for changes in depression or anxiety. In support of our second hypothesis, individuals with BD showed lower FA in three of the six clusters (middle left CB, anterior right CB, and frontal right UF). Furthermore, the overall pattern of FA in these three clusters was for non-BD individuals with increasing hypomania severity at 6 months to have lower FA in these three clusters than HC individuals and non-BD individuals with decreasing/no change in hypomania severity at 6 months, demonstrating a parallel between white matter predictors of worsening subthreshold hypomania severity in non-BD individuals and white matter abnormalities associated with BD.
Other studies consistently reported lower FA in the CB and UF in BD relative to healthy individuals (Benedetti et al., 2011; Caseras et al., 2015; Lin et al., 2011; Versace et al., 2014; Von Der Heide et al., 2013). However, to the best of our knowledge, the present study is the first to show similarities between white matter predictors of worsening of subthreshold hypomania severity in non-BD individuals and white matter abnormalities in individuals with established BD. Furthermore, these predictive findings in non-BD individuals were associated only with worsening hypomania and not generalizable to changes in depressive and anxiety symptoms, suggesting that neural processes predisposing to worsening hypomania parallel those associated with BD. Our tract-profile findings further suggest that abnormalities in these tracts were focal rather than widespread. These segments include fibers connecting regions that have been associated with two main neural systems supporting emotional processing and regulation: ventral and dorsal systems (Heilbronner and Haber, 2014). While the dorsal system is largely important for the regulation of different emotional states and associated behaviors, the ventral system evaluates and evokes emotional responses in emotional contexts (Phillips et al., 2003a, b). These findings thus indicate that neural predictors of worsening of subthreshold hypomania in non-BD individuals dorsal (CG antero-middle clusters) and ventral systems (CG posterior clusters and UF clusters), likely reflecting dysregulated emotional responses and abnormal sensitivity to emotional contexts, respectively (Phillips et al., 2003a, b). Interestingly, only FA, and not AD or RD in these tract segments, predicted worsening of subthreshold hypomania, suggesting that neural processes predisposing to worsening hypomania involve lower fiber collinearity, and related connectivity, rather than greater fiber dispersion or myelin damage in these tract segments.
Only the antero-middle CB and frontal UF segments (Phillips et al., 2008) showed FA reductions in individuals with BD, paralleling our finding that white matter abnormalities in BD were restricted to the anterior segment of the right CB (Rozovsky et al., 2021). Non-BD and BD individuals showed no differences in FA in these segments. These tracts connect prefrontal cortical regions implicated in evaluation of emotional contexts (UF) and emotional regulation processes (Phillips et al., 2008). There were also non-significant trends for negative relationships between FA in the anterior right CB and hypo/mania severity (DIAMOND; BIOS) and illness duration (all studies) in BD individuals (Supplementary Tables 8 and 10). The absence of FA abnormalities in posterior CB and temporal UF in individuals with BD suggests that the predictive relationships among lower FA in these segments and worsening hypomania in non-BD individuals might reflect neurobiological processes related to emotional dysregulation in general rather than hypomania and BD specifically.
We used a novel combination of three study samples and data harmonization techniques, but there were limitations to the study. The design of this study aimed to examine the relationship between future subthreshold hypomania and white matter FA. However, our prediction model was not validated in an independent sample of non-BD participants. Future studies are needed to validate the prediction model identified in this study. Although non-BD individuals showed relatively low scores in the YMRS at baseline and six months, subthreshold symptoms, even when associated with low scores, have been identified as important predictors of BD onset (Fiedorowicz et al., 2011; Hafeman et al., 2017). In addition, this is an on-going longitudinal study and collection of additional timepoints is expected to capture larger changes in these symptoms. Thus, future studies with longer follow-ups are needed. Furthermore, while sensitivity analyses excluding individuals who were taking antidepressants showed findings consistent with our main findings, future longitudinal follow-up studies with larger numbers of individuals starting medications can evaluate the effects of such new medications on relationships among white matter markers and progression of symptoms While some BD participants and HC were younger than the non-BD individuals, similar patterns of abnormally reduced FA in BD participants were observed after excluding the younger BD and HC. Although BD and HC individuals were matched for sex, there was a higher proportion of females in non-BD individuals than in the other two groups. There was no effect of sex in predicting hypomania severity at 6 months, however. While younger age at scan predicted greater hypomania severity at 6 months, there was no significant effect of age on FA in white matter segment predictors of worsening of hypomania severity. Many individuals with BD were medicated. Antidepressants were associated with lower FA in some node clusters, indicating that such medication might have contributed to the pattern of lower FA in node clusters individuals with BD. It is, however, likely that individuals taking such medication had a more severe illness. Thus, the pattern of lower FA in these node clusters might reflect illness severity rather than antidepressant medication. Medication did not significantly contribute to the pattern of white matter abnormalities overall, however. Future studies with larger samples should evaluate the effect of family history of BD on relationships among these neural markers and progression of symptoms, as this was beyond the scope of the present study.
Our findings indicate that patterns of focal white matter abnormalities associated with BD predict worsening of subthreshold hypomania, an important predictor of risk for BD, in non-BD individuals, and thus might predispose to future BD. These neural predictors can provide targets to facilitate early BD diagnosis, monitor existing treatments, and aid the development of novel interventions, to help improve clinical and psychosocial outcomes in BD at-risk individuals.
Supplementary Material
HIGHLIGHTS.
Lower FA in CB and UF predicted worsening hypomania severity in non-BD individuals
Non-BD with worsening hypomania severity and BD showed lower FA than HC individuals
Non-BD with worsening hypomania severity showed similar FA patterns to BD individuals
ACKNOWLEDGMENTS
We acknowledge the participants for their contributions to this study.
FUNDING AND DISCLOSURE
This present study was supported by three studies funded by the National Institute of Mental Health: DIAMOND (R37MH100041, PI: Phillips), COBY (R01MH059929; PIs: Birmaher, Phillips, Versace) and BIOS (R01MH060952; PIs: Birmaher and Phillips). The funding agency was not involved in the design or conduct of the study, the collection, management, analysis, or interpretation of the data, or the preparation, review, or approval of the manuscript.
Dr. Birmaher declares royalties from book publications. Dr. Phillips declares a one-time honorarium from Sunovion in November 2016. Other authors declare no conflict of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Acuff HE, Versace A, Bertocci MA, Hanford LC, Ladouceur CD, Manelis A, Monk K, Bonar L, McCaffrey A, Goldstein BI, Goldstein TR, Sakolsky D, Axelsone D, Consortium L, Birmaher B, Phillips ML, 2019. White matter–emotion processing activity relationships in youth offspring of bipolar parents. Journal of affective disorders 243, 153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson JL, Skare S, Ashburner J, 2003. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage 20, 870–888. [DOI] [PubMed] [Google Scholar]
- Aung WY, Mar S, Benzinger TL, 2013. Diffusion tensor MRI as a biomarker in axonal and myelin damage. Imaging in medicine 5, 427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelson D, Goldstein B, Goldstein T, Monk K, Yu H, Hickey MB, Sakolsky D, Diler R, Hafeman D, Merranko J, Iyengar S, Brent D, Kupfer D, Birmaher B, 2015. Diagnostic precursors to bipolar disorder in offspring of parents with bipolar disorder: a longitudinal study. American Journal of Psychiatry 172, 638–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea-Goraly N, Chang KD, Karchemskiy A, Howe ME, Reiss AL, 2009. Limbic and corpus callosum aberrations in adolescents with bipolar disorder: a tract-based spatial statistics analysis. Biological psychiatry 66, 238–244. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Absinta M, Rocca MA, Radaelli D, Poletti S, Bernasconi A, Dallaspezia S, Pagani E, Falini A, Copetti M, Colombo C, Comi G, Smeraldi E, Filippi M, 2011. Tract-specific white matter structural disruption in patients with bipolar disorder. Bipolar disorders 13, 414–424. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal statistical society: series B (Methodological) 57, 289–300. [Google Scholar]
- Birmaher B, Merranko JA, Goldstein TR, Gill MK, Goldstein BI, Hower H, Yen S, Hafeman D, Strober M, Diler RS, Axelson D, Ryan ND, Keller MB, 2018. A risk calculator to predict the individual risk of conversion from subthreshold bipolar symptoms to bipolar disorder I or II in youth. Journal of the American Academy of Child & Adolescent Psychiatry 57, 755–763. e754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caseras X, Murphy K, Lawrence NS, Fuentes-Claramonte P, Watts J, Jones DK, Phillips ML, 2015. Emotion regulation deficits in euthymic bipolar I versus bipolar II disorder: a functional and diffusion-tensor imaging study. Bipolar disorders 17, 461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Core Team, R., 2017. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria: URL https://www.R-project.org/.[Google Scholar]. [Google Scholar]
- Craddock N, Jones I, 1999. Genetics of bipolar disorder. Journal of medical genetics 36, 585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dondé C, Amad A, Nieto I, Brunoni AR, Neufeld NH, Bellivier F, Poulet E, Geoffroy P-A, 2017. Transcranial direct-current stimulation (tDCS) for bipolar depression: A systematic review and metaanalysis. Progress in Neuro-Psychopharmacology and Biological Psychiatry 78, 123–131. [DOI] [PubMed] [Google Scholar]
- Duffy A, Alda M, Hajek T, Sherry SB, Grof P, 2010. Early stages in the development of bipolar disorder. Journal of affective disorders 121, 127–135. [DOI] [PubMed] [Google Scholar]
- Eckstrand KL, Forbes EE, Bertocci MA, Chase HW, Greenberg T, Lockovich J, Stiffler R, Aslam HA, Graur S, Bebko G, Phillips ML, 2021. Trauma Affects Prospective Relationships Between Reward-Related Ventral Striatal and Amygdala Activation and 1-Year Future Hypo/Mania Trajectories. Biological Psychiatry 89, 868–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsell L, Leemans A, Langan C, Van Hecke W, Barker GJ, McCarthy P, Jeurissen B, Sijbers J, Sunaert S, Cannon DM, McDonald C, 2013. Limbic and callosal white matter changes in euthymic bipolar I disorder: an advanced diffusion magnetic resonance imaging tractography study. Biological psychiatry 73, 194–201. [DOI] [PubMed] [Google Scholar]
- Excellence, N.I.f.C., 2009. Depression in adults: Recognition and management. Clinical guideline [CG90]. National Institute for Health and Clinical Excellence. Published October 28. [Google Scholar]
- Faedda GL, Marangoni C, Serra G, Salvatore P, Sani G, Vázquez GH, Tondo L, Girardi P, Baldessarini RJ, Koukopoulos A, 2015. Precursors of bipolar disorders: a systematic literature review of prospective studies. The Journal of clinical psychiatry 76, 0–0. [DOI] [PubMed] [Google Scholar]
- Favre P, Pauling M, Stout J, Hozer F, Sarrazin S, Abe C, Alda M, Alloza C, Alonso-Lana S, Andreassen OA, T. Baune B, Benedetti F, Busatto GF, Canales-Rodríguez EJ, Caseras X, Chaim-Avancini TM, Ching CRK, Dannlowski U, Deppe M, Eyler LT, Fatjo-Vilas M, Foley SF, Grotegerd D, Hajek T, Haukvik UK, Howells FM, Jahanshad N, Kugel H, Lagerberg TV, Lawrie SM, Linke JO, McIntosh A, Melloni EMT, Mitchell PB, Polosan M, Pomarol-Clotet E, Repple J, Roberts G, Roos A, Rosa PGP, Salvador R, Sarró S, Schofield PR, Serpa MH, Sim K, Stein DJ, Sussmann JE, Temmingh HS, Thompson PM, Verdolini N, Vieta E, Wessa M, Whalley HC, Zanetti MV, Leboyer M, Mangin J-F, Henry C, Duchesnay E, Houenou J, 2019. Widespread white matter microstructural abnormalities in bipolar disorder: evidence from mega-and meta-analyses across 3033 individuals. Neuropsychopharmacology 44, 2285–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrarelli F, Phillips ML, 2021. PFC neuromodulation with theta burst stimulation to impact behavior and neural network activity in schizophrenia and bipolar disorder. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedorowicz JG, Endicott J, Leon AC, Solomon DA, Keller MB, Coryell WH, 2011. Subthreshold hypomanic symptoms in progression from unipolar major depression to bipolar disorder. American Journal of Psychiatry 168, 40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, 2014. Structured clinical interview for the DSM (SCID). The encyclopedia of clinical psychology, 1–6. [Google Scholar]
- First MB, Gibbon M, 2004. The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) and the Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II). [Google Scholar]
- First MB, Williams JB, Karg RS, Spitzer RL, 2015. Structured clinical interview for DSM-5—Research version (SCID-5 for DSM-5, research version; SCID-5-RV). Arlington, VA: American Psychiatric Association, 1–94. [Google Scholar]
- Foley SF, Bracher-Smith M, Tansey KE, Harrison JR, Parker GD, Caseras X, 2018. Fractional anisotropy of the uncinate fasciculus and cingulum in bipolar disorder type I, type II, unaffected siblings and healthy controls. The British Journal of Psychiatry 213, 548–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin J-P, Parker D, Tunç B, Watanabe T, Elliott MA, Ruparel K, Roalf DR, Satterthwaite TD, Gur RC, Gur RE, Schultz RT, Verma R, Shinohara RT, 2017. Harmonization of multi-site diffusion tensor imaging data. Neuroimage 161, 149–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier JA, Breeze JL, Papadimitriou G, Kennedy DN, Hodge SM, Moore CM, Howard JD, Rohan MP, Caviness VS, Makris N, 2007. White matter abnormalities in children with and at risk for bipolar disorder. Bipolar disorders 9, 799–809. [DOI] [PubMed] [Google Scholar]
- Ganzola R, McIntosh AM, Nickson T, Sprooten E, Bastin ME, Giles S, Macdonald A, Sussmann J, Duchesne S, Whalley HC, 2018. Diffusion tensor imaging correlates of early markers of depression in youth at high-familial risk for bipolar disorder. Journal of Child Psychology and Psychiatry 59, 917–927. [DOI] [PubMed] [Google Scholar]
- Grande I, Berk M, Birmaher B, Vieta E, 2016. Bipolar disorder. The Lancet 387, 1561–1572. [DOI] [PubMed] [Google Scholar]
- Greenberg T, Bertocci MA, Versace A, Santos JPL, Chase HW, Siffler R, Aslam HA, Graur S, Bebko G, Lockovich JC, Phillips ML, 2021. Depression and anxiety mediate the relationship between frontotemporal white matter integrity and quality of life in distressed young adults. Journal of Psychiatric Research 132, 55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha TH, Her JY, Kim JH, Chang JS, Cho HS, Ha K, 2011. Similarities and differences of white matter connectivity and water diffusivity in bipolar I and II disorder. Neuroscience letters 505, 150–154. [DOI] [PubMed] [Google Scholar]
- Hafeman DM, Merranko J, Axelson D, Goldstein BI, Goldstein T, Monk K, Hickey MB, Sakolsky D, Diler R, Iyengar S, Brent D, Kupfer D, Birmaher B, 2016. Toward the definition of a bipolar prodrome: dimensional predictors of bipolar spectrum disorders in at-risk youths. American Journal of Psychiatry 173, 695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafeman DM, Merranko J, Goldstein TR, Axelson D, Goldstein BI, Monk K, Hickey MB, Sakolsky D, Diler R, Iyengar S, 2017. Assessment of a person-level risk calculator to predict new-onset bipolar spectrum disorder in youth at familial risk. JAMA psychiatry 74, 841–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M, 1959. The assessment of anxiety states by rating. British journal of medical psychology 32, 50–55. [DOI] [PubMed] [Google Scholar]
- HAMILTON M, 1960. A rating scale for depression. Journal of neurology, neurosurgery, and psychiatry 23, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronner SR, Haber SN, 2014. Frontal cortical and subcortical projections provide a basis for segmenting the cingulum bundle: implications for neuroimaging and psychiatric disorders. Journal of Neuroscience 34, 10041–10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalbrzikowski M, Hayes RA, Scully KE, Franzen PL, Hasler BP, Siegle GJ, Buysse DJ, Dahl RE, Forbes EE, Ladouceur CD, McMakin DL, Ryan ND, Silk JS, Goldstein TR, Soehner AM, 2021. Associations between brain structure and sleep patterns across adolescent development. Sleep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK, Leemans A, 2011. Diffusion tensor imaging, Magnetic resonance neuroimaging. Springer, pp. 127–144. [Google Scholar]
- Kerner B, 2014. Genetics of bipolar disorder. The application of clinical genetics 7, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Williamson DE, Lancaster J, Fox P, Cornell J, Blangero J, Glahn DC, 2012. Fractional anisotropy of water diffusion in cerebral white matter across the lifespan. Neurobiology of aging 33, 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C, 2012. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage 60, 340–352. [DOI] [PubMed] [Google Scholar]
- Lin F, Weng S, Xie B, Wu G, Lei H, 2011. Abnormal frontal cortex white matter connections in bipolar disorder: a DTI tractography study. Journal of affective disorders 131, 299–306. [DOI] [PubMed] [Google Scholar]
- Linke J, King AV, Poupon C, Hennerici MG, Gass A, Wessa M, 2013. Impaired anatomical connectivity and related executive functions: differentiating vulnerability and disease marker in bipolar disorder. Biological psychiatry 74, 908–916. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Maniega SM, Lymer GKS, McKirdy J, Hall J, Sussmann JE, Bastin ME, Clayden JD, Johnstone EC, Lawrie SM, 2008. White matter tractography in bipolar disorder and schizophrenia. Biological psychiatry 64, 1088–1092. [DOI] [PubMed] [Google Scholar]
- Organization, W.H., 2011. World report on disability 2011. [PubMed]
- Phillips ML, Drevets WC, Rauch SL, Lane R, 2003a. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biological psychiatry 54, 504–514. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R, 2003b. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biological psychiatry 54, 515–528. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC, 2008. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular psychiatry 13, 833–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regier DA, Kuhl EA, Kupfer DJ, 2013. The DSM-5: Classification and criteria changes. World psychiatry 12, 92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozovsky R, Versace A, Bonar LK, Bertocci M, Ladouceur CD, Fournier J, Monk K, Abdul-Waalee H, Bebko G, Hafeman D, Sakolsky D, Goldstein T, Birmaher B, Phillips ML, 2021. Differentiating white matter measures that protect against vs. predispose to bipolar disorder and other psychopathology in at-risk youth. Neuropsychopharmacology, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safadi Z, Grisot G, Jbabdi S, Behrens TE, Heilbronner SR, McLaughlin NC, Mandeville J, Versace A, Phillips ML, Lehman JF, Yendiki A, Haber SN, 2018. Functional segmentation of the anterior limb of the internal capsule: linking white matter abnormalities to specific connections. Journal of Neuroscience 38, 2106–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA, U., 2007. Results from the 2007 national survey on drug use and health: National findings. Rockville, MD: Office of Applied Studies, 1–290. [Google Scholar]
- Santos JPL, Brent D, Bertocci M, Mailliard S, Bebko G, Goldstein T, Kim T, Iyengar S, Hafeman D, Fenster-Ehrlich VC, Skeba A, Bonar L, Abdul-Waalee H, Gill M, Merranko J, Birmaher B, Phillips ML, Versace A, 2021. White matter correlates of suicidality in adults with bipolar disorder who have been prospectively characterized since childhood. Biological psychiatry: cognitive neuroscience and neuroimaging 6, 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarrazin S, Poupon C, Linke J, Wessa M, Phillips M, Delavest M, Versace A, Almeida J, Guevara P, Duclap D, Duchesnay E, Mangin J-F, Dudal KL, Daban C, Hamdani N, D'Albis M-A, Leboyer M, Houenou J, 2014. A multicenter tractography study of deep white matter tracts in bipolar I disorder: psychotic features and interhemispheric disconnectivity. JAMA psychiatry 71, 388–396. [DOI] [PubMed] [Google Scholar]
- Simmonds DJ, Hallquist MN, Asato M, Luna B, 2014. Developmental stages and sex differences of white matter and behavioral development through adolescence: a longitudinal diffusion tensor imaging (DTI) study. Neuroimage 92, 356–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skjelstad DV, Malt UF, Holte A, 2010. Symptoms and signs of the initial prodrome of bipolar disorder: a systematic review. Journal of affective disorders 126, 1–13. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, Stefano ND, Brady JM, Matthews PM, 2004. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23, S208–S219. [DOI] [PubMed] [Google Scholar]
- Tournier J-D, Smith R, Raffelt D, Tabbara R, Dhollander T, Pietsch M, Christiaens D, Jeurissen B, Yeh C-H, Connelly A, 2019. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. NeuroImage, 116137. [DOI] [PubMed] [Google Scholar]
- Versace A, Andreazza AC, Young L, Fournier JC, Almeida JR, Stiffler RS, Lockovich JC, Aslam HA, Pollock MH, Park H, Nimgaonkar VL, Kupfer DJ, Phillips ML, 2014. Elevated serum measures of lipid peroxidation and abnormal prefrontal white matter in euthymic bipolar adults: toward peripheral biomarkers of bipolar disorder. Molecular psychiatry 19, 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace A, Jones N, Joseph H, Lindstrom R, Wilson T, Santos JL, Gnagy E, Pelham W, Ladouceur C, Molina B, 2021. White matter abnormalities associated with ADHD outcomes in adulthood. Molecular Psychiatry, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace A, Ladouceur CD, Romero S, Birmaher B, Axelson DA, Kupfer DJ, Phillips ML, 2010. Altered development of white matter in youth at high familial risk for bipolar disorder: a diffusion tensor imaging study. Journal of the American Academy of Child & Adolescent Psychiatry 49, 1249–1259. e1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Der Heide RJ, Skipper LM, Klobusicky E, Olson IR, 2013. Dissecting the uncinate fasciculus: disorders, controversies and a hypothesis. Brain 136, 1692–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BW, Clarke TC, Nugent CN, Schiller JS, 2016. Early release of selected estimates based on data from the 2015 National Health Interview Survey. National Center for Health Statistics 46. [Google Scholar]
- Wasserthal J, Neher P, Maier-Hein KH, 2018a. Tractseg-fast and accurate white matter tract segmentation. NeuroImage 183, 239–253. [DOI] [PubMed] [Google Scholar]
- Wasserthal J, Neher PF, Hirjak D, Maier-Hein KH, 2019. Combined tract segmentation and orientation mapping for bundle-specific tractography. Medical image analysis 58, 101559. [DOI] [PubMed] [Google Scholar]
- Wasserthal J, Neher PF, Maier-Hein KH, 2018b. Tract orientation mapping for bundle-specific tractography, International Conference on Medical Image Computing and Computer-Assisted Intervention. Springer, pp. 36–44. [Google Scholar]
- Westlye LT, Walhovd KB, Dale AM, Bjornerud A, Due-Tonnessen P, Engvig A, Grydeland H, Tamnes CK, Ostby Y, Fjell AM, 2010. Life-span changes of the human brain white matter: diffusion tensor imaging (DTI) and volumetry. Cerebral cortex (New York, N.Y. : 1991) 20, 2055–2068. [DOI] [PubMed] [Google Scholar]
- Yeatman JD, Dougherty RF, Myall NJ, Wandell BA, Feldman HM, 2012. Tract profiles of white matter properties: automating fiber-tract quantification. PloS one 7, e49790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yendiki A, Koldewyn K, Kakunoori S, Kanwisher N, Fischl B, 2014. Spurious group differences due to head motion in a diffusion MRI study. Neuroimage 88, 79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA, 1978. A rating scale for mania: reliability, validity and sensitivity. The British Journal of Psychiatry 133, 429–435. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.