Abstract
Pathogen virulence factors and inflammation are responsible for tissue injury associated with respiratory failure in bacterial pneumonia, as seen in the bovine lung infected with Pasteurella haemolytica. Tilmicosin is a macrolide antibiotic used for the treatment of bovine bacterial pneumonia. Recent evidence suggests that tilmicosin-induced neutrophil apoptosis may have anti-inflammatory effects. Using bovine leukocytes, we sought to define whether live P. haemolytica affected tilmicosin-induced neutrophil apoptosis, assessed the proapoptotic effects of tilmicosin in comparison with other drugs, and characterized its impact on phagocytic uptake of neutrophils by macrophages. Induction of apoptosis in the presence or absence of P. haemolytica was assessed by using an enzyme-linked immunosorbent assay for apoptotic nucleosomes. In addition, fluorescent annexin-V staining identified externalized phosphatidylserine in neutrophils treated with tilmicosin, penicillin, ceftiofur, oxytetracycline, or dexamethasone. Neutrophil membrane integrity was assessed by using propidium iodide and trypan blue exclusion. As phagocytic clearance of apoptotic neutrophils by macrophages contributes to the resolution of inflammation, phagocytosis of tilmicosin-treated neutrophils by esterase-positive cultured bovine macrophages was assessed with light microscopy and transmission electron microscopy. Unlike bovine neutrophils treated with penicillin, ceftiofur, oxytetracycline, or dexamethasone, neutrophils exposed to tilmicosin became apoptotic, regardless of the presence or absence of P. haemolytica. Tilmicosin-treated apoptotic neutrophils were phagocytosed at a significantly greater rate by bovine macrophages than were control neutrophils. In conclusion, tilmicosin-induced neutrophil apoptosis occurs regardless of the presence or absence of live P. haemolytica, exhibits at least some degree of drug specificity, and promotes phagocytic clearance of the dying inflammatory cells.
Pneumonic pasteurellosis is a pulmonary disease of domestic animals and is particularly prominent in calves placed in feedlots (4, 11, 40). Pasteurella haemolytica A1 is the most common etiologic agent of this acute fibrinous pneumonia (15, 27). The pathogenesis of pneumonic pasteurellosis involves both host inflammation and bacterial virulence factors which, together, lead to respiratory failure and death. Bacterial factors include endotoxins (44) and leukotoxins (34). P. haemolytica leukotoxins are known to lyse macrophages and polymorphonuclear neutrophils (PMNs), which in turn impairs the host's antibacterial defense and promotes further infiltration of PMNs (11, 34, 36). Local accumulation of PMNs and leukotriene B4 at the site of inflammation plays a central role in the pathogenesis of bovine pneumonic pasteurellosis (6, 11, 17, 24, 42). PMNs release large amounts of reactive oxygen species and proteolytic enzymes that target the invading bacteria but concurrently damage the bronchial epithelium. These host products, compounded with the effects of leukotoxins, contribute to delayed elimination of P. haemolytica and subsequent uncontrolled self-perpetuating inflammation.
Eukaryotic cells, such as PMNs, may die via two distinct processes: apoptosis or necrosis (7, 12, 18, 35, 43). When PMNs die via necrosis, the cells swell and burst, spilling proteolytic compounds into surrounding tissues and amplifying local inflammatory injury. Throughout the apoptotic process, the cytoplasmic organelles remain intact, and nuclear chromatin condenses and is cleaved into mono- and oligonucleosomes (43). The detection of these fragments is a reliable marker of apoptosis. Preservation of membrane integrity in apoptotic PMNs helps minimize self-perpetuation of inflammation and subsequent tissue injury (35, 43). Moreover, apoptotic cells are quickly phagocytosed by neighboring cells such as macrophages (29, 43), a phenomenon known to be mediated by phosphatidylserine which is translocated onto the outer cell membrane leaflet during apoptosis. Indeed, apoptotic cells lose normal membrane asymmetry, leading to the externalization of phosphatidylserine (10, 21, 30, 31). One mechanism by which macrophages recognize apoptotic PMNs, prior to phagocytosis, is through the phosphatidylserine receptor (15, 44). This process ensures that the contents of these cells and organelles are not released into the extracellular space (16, 43). Therefore, the phagocytosis of apoptotic PMNs by macrophages plays a key role in the resolution of inflammation in a number of systems, including the respiratory tract (8, 16, 29, 31, 32).
The clinical efficacy of tilmicosin in the treatment of pneumonic pasteurellosis has been attributed to its pharmacodynamics in appropriate tissues (13, 23, 24, 33) and low inhibitory concentrations (15). A recent study suggested that tilmicosin may reduce pulmonary inflammation in calves with pneumonia (24). Previous studies have indicated that some macrolides may have anti-inflammatory properties by reducing accumulation of inflammatory cells such as PMNs, mononuclear leukocytes, and lymphocytes; decreasing the secretory functions of airway secretory cells; increasing epithelial airway ciliary motility; and decreasing epithelial synthesis of proinflammatory cytokines such as interleukin-6 (14, 25, 28, 37–39, 41). Recent evidence suggests that erythromycin and other macrolides may induce PMN apoptosis in vitro (1), but the physiological significance of this observation needs to be further assessed. Recently, using calves infected with live P. haemolytica, we demonstrated that bronchoalveolar PMNs recovered from tilmicosin-treated animals exhibit elevated levels of apoptosis and that the synthesis of proinflammatory leukotriene B4 in the infected lungs of these animals is reduced (5). As P. haemolytica alone has been shown to induce PMN apoptosis (36), additional experiments are warranted to clearly distinguish the apparent anti-inflammatory benefits of tilmicosin from its antibacterial properties. Further, this model represents a useful system in which to investigate whether induction of PMN apoptosis confers anti-inflammatory benefits to an antibiotic. In an attempt to improve our understanding of the physiological significance of tilmicosin-induced PMN apoptosis, the aims of the present study were as follows: (i) to determine the effects of tilmicosin on PMN apoptosis in the presence or in the absence of live P. haemolytica, (ii) to compare the proapoptotic effects of tilmicosin in PMNs with those of other drugs, and (iii) to assess the effects of tilmicosin-induced apoptosis on phosphatidylserine translocation and subsequent macrophage phagocytosis of PMNs.
MATERIALS AND METHODS
Bacteria.
P. haemolytica A serotype 1 (strain B122) isolated from a steer that died from pneumonic pasteurellosis was grown overnight on Columbia blood agar (Difco Laboratories, Detroit, Mich.) containing 5% sterile defibrinated sheep blood at 35°C in a microaerophilic environment (5% CO2). Cells were harvested and suspended in pyrogen-free phosphate-buffered saline (PBS) (pH 7.2, 0.15 M NaCl). A bacterial concentration of 3 × 108 cells per ml was prepared for use in the experimental protocol. The bacterial concentrations were estimated by using McFarland nephelometry and were confirmed by CFU enumeration on Columbia blood agar.
PMN purification.
Peripheral blood was drawn into ACD vacutainers (ACD solution A; Becton Dickinson Vacutainer Systems, Franklin Lakes, N.J.) from the jugular veins of healthy Holstein calves. The blood was pooled in two 50-ml polypropylene centrifuge tubes and spun at 1,171 × g in an IEC Centra-7R swinging bucket centrifuge (IEC 210 rotor; International Equipment Company, Needham Heights, Mass.) for 20 min at room temperature without braking. The plasma, buffy coat, and the top one-half of the erythrocyte pack were removed down to the 10-ml level. Then 20 ml of cold hypotonic lysis solution (10.6 mM Na2HPO4, 2.7 mM NaH2PO4) was added to the cell pack and gently mixed for 1 min. Isotonicity was restored through the addition of 10 ml of 3× hypertonic solution (10.6 mM Na2HPO4, 2.7 mM NaH2PO4, 462 mM NaCl). The mixture was centrifuged at 650 × g for 10 min, and the supernatant was discarded. The lysing procedure was repeated once again. After the final lysing step, the leukocyte cell pellet was resuspended in 10 ml of PBS (8.1 mM Na2HPO4, 1.47 mM KH2PO4, 2.68 mM KCl, 136.9 mM NaCl). The cell solution was poured into a 15-ml centrifuge tube and then centrifuged at 650 × g for 10 min. The supernatant was discarded, and the pellet was resuspended in 10 ml of 25 mM HEPES-buffered RPMI 1640 cell culture medium (Sigma Chemical Co., St. Louis, Mo.) supplemented with 10 mM l-glutamine. A cell concentration of 106 cells/ml in 45 ml was obtained through the use of a hemacytometer. The hemacytometer was also used to assess the percentage of cells that excluded trypan blue (Flow Laboratories Inc., McLean, Va.) to assess viability. Differential cell counts were performed on cytospin preparations stained with Diff Quik (Baxter Healthcare Corp., Miami, Fla.) to assess PMN purity. PMN populations were >95% pure.
Nucleosome quantification.
For the analysis of apoptosis in cell populations, the purified PMNs (106 cells/ml) were incubated with 5 or 0.5 μg of tilmicosin (Micotil; Provel, Guelph, Ontario, Canada) per ml at 37°C and 5% CO2 in the presence or absence of 107 P. haemolytica (B122)/ml for 2 h. These concentrations of tilmicosin are consistent with serum pharmacological levels measured in vivo (24). PMN apoptosis was measured by using a cell death detection enzyme-linked immunosorbent assay (ELISA) kit (Roche Molecular Biochemicals, Laval, Quebec, Canada) according to the manufacturer's instructions. This quantitative sandwich enzyme immunoassay specifically measures the histone region (H1, H2A, H2B, H3, and H4) of mono- and oligonucleosomes which are released during apoptosis. Photometric development was monitored kinetically by reading the plate (THERMOmax microplate reader; Molecular Devices Corp., Menlo Park, Calif.) (405 nm) at various times: 1, 3, 6, 9, 12, 15, 38, and 53 min. Apoptosis was measured in triplicate from 105 PMNs of each group and expressed as the absorbance ratios of the experimental cell lysates versus absorbances from unmanipulated controls arbitrarily set at 1.0 as done previously (5). The detection limit for this ELISA is 102 apoptotic cells. Cells incubated with 1× PBS were used for comparisons of PMNs incubated with tilmicosin only. Cells incubated with P. haemolytica were compared to PMNs incubated with both tilmicosin and bacteria.
Translocation of phosphatidylserine.
To compare the induction of PMN apoptosis by various drugs and to assess the effects of these drugs on phosphatidylserine translocation, purified PMNs (106 cells/ml) were coincubated with similar concentrations of tilmicosin (0.5 μg of Micotil per ml) (Provel), penicillin G (Sigma), ceftiofur sodium (Excenel; The Upjohn Company, Animal Health Division, Orangeville, Ontario, Canada), or oxytetracycline (Sigma) for 2 h at 37°C and 5% CO2. Penicillin, ceftiofur, and oxytetracycline are commonly used to treat pneumonic pasteurellosis. Concentrations of penicillin, ceftiofur, and oxytetracycline were selected to match the concentration of tilmicosin. Controls were set up using 1× PBS instead of the antibiotics. An additional control was exposed to dexamethasone (10−8 M Azium; Schering-Plough Animal Health, Pointe-Claire, Quebec, Canada). The concentration of dexamethasone was selected based on previous observations which showed that 10−8 M dexamethasone may inhibit apoptosis in human PMNs (20). PMN apoptosis in individual cells was determined by using an Annexin-V-FLUOS staining kit (Roche) according to the manufacturer's instructions. This staining kit specifically measures phosphatidylserine which is translocated from the inner side of the plasma membrane to the external surface of the cell during apoptosis and necrosis. Apoptotic cells can be differentiated from necrotic cells by using annexin-V fluorescein, which has high affinity for phosphatidylserine, and propidium iodide, which binds DNA. Briefly, the incubated cells were pelleted at 200 × g for 5 min, were washed with 1 ml of pyrogen-free 1× PBS, were warmed at room temperature, and were centrifuged again at 200 × g for 5 min (Baxter Canlab Biofuge A; Heraeus Sepatech GmbH, Hanau, Germany). The supernatant was discarded, and the pellet was resuspended with 0.1 ml of a staining solution containing 20 μl of annexin-V fluorescein, 20 μl of propidium iodide, and 1 ml of HEPES buffer. The cells were incubated in the dark for 15 min at room temperature. After incubation, the cells were washed with 0.1 ml of HEPES buffer warmed to room temperature and were centrifuged at 200 × g for 5 min. A wet mount of 15 μl of the stained cells was prepared and was visualized under fluorescent microscopy. The cells were visualized by fluorescence microscopy by using a Zeiss Axiovert 25 CFL inverted microscope where apoptotic cells appeared solid green (fluorescein isothiocyanate filter, excitation = 450 nm, emission = 490 nm) and necrotic cells appeared green (fluorescein isothiocyanate filter) with red nuclei (Cy-3 filter, excitation = 535 nm, emission = 550 nm). The percentages of apoptotic and necrotic cells in each sample were obtained from 10 randomly selected fields under 400× magnification.
Membrane integrity.
The effects of the various drugs on PMN membrane integrity were further assessed in the presence or absence of P. haemolytica. The same experimental design as above was used, and additional groups were prepared where the PMNs were coincubated with the drugs and with 108 P. haemolytica cells/ml. After incubation, PMNs were exposed to 0.1% trypan blue to assess membrane leakage.
Macrophages.
Bovine blood was obtained and pooled as described previously. The pooled blood was centrifuged at 1,200 × g in a Beckman J-6B centrifuge (Beckman Instruments, Palo Alto, Calif.) for 20 min at room temperature without braking. The mononuclear layer and some of the adjacent erythrocyte layer were collected and diluted 1:1 with sterile saline. The blood-saline mixture was layered onto a polysucrose and sodium diatrizoate gradient (Histopaque 1077; Sigma) and centrifuged at 1,500 × g for 40 min. The cell suspension was collected, was washed in 1× Hank's balanced salt solution (HBSS) (Gibco BRL, Life Technologies Inc., Grand Island, N.Y.), and was centrifuged at 250 × g for 10 min. Contaminating erythrocytes were eliminated by resuspending the pellet in pyrogen-free sterile water three times for 1 min each suspension (MTC Pharmaceuticals, Cambridge, Ontario, Canada) and restoring osmolarity by adding 2× HBSS. Following an additional wash in 1× HBSS, purified cells were resuspended in Iscove's modified Dulbecco's medium (Gibco) to a final concentration of either 6 × 106 cells/ml for phagocytosis assays and morphological cell differentiation counts or 2 × 106 cells/ml for nonspecific esterase staining. Cells were counted in a hemacytometer, and viability was assessed by 0.1% trypan blue exclusion. Differential cell counts were performed on cytospin preparations stained with Diff Quik (Baxter) to assess monocyte purity. Cells were incubated (37°C, 5% CO2) for 60 min in either well plates (Costar, Cambridge, Mass.) for phagocytosis assays or Labtek chamber slides (Nalge Nunc International, Naperville, Ill.) for nonspecific esterase staining. Nonadherent cells were removed by washing three times with 37°C 1× HBSS. Monocytes were incubated (37°C, 5% CO2) in Iscove's modified Dulbecco's medium containing 10% fetal bovine serum (Sigma), 0.9% penicillin-streptomycin (Sigma), and 0.9% tylosin (Sigma) for 7 days and were allowed to differentiate into macrophages. The medium was replaced every 2 days.
Macrophage differentiation.
Cells were stained in their wells with Diff Quik (Baxter) on days 2, 5, and 7. The percentage of monocytes that had morphologically differentiated into macrophages was determined by light microscopy. Cells with large and vacuolated cytoplasm were counted as macrophages while small cells with undifferentiated cytoplasm were counted as undifferentiated monocytes. Nonspecific esterase staining was performed as previously described by Partridge and Dransfield to confirm macrophage differentiation (26). Briefly, fixed cells were incubated for 45 min at 37°C in a buffered esterase substrate solution containing hexazoatized paraosalanine (Sigma) and α-naphthyl acetate (Sigma). Cells were counterstained with 1% methyl green acetate (Sigma) for 2 min. The percentage of esterase-positive cells was determined with light microscopy.
PMN phagocytosis by macrophages.
PMNs (2.5 × 107 cells/ml) were incubated (37°C, 5% CO2) for 2 h in either PBS (pH 7.1, control) or 0.5 μg of tilmicosin (Micotil; Provel) per ml and then centrifuged at minimum speed for 5 min (Hermle Z180M; National Labnet Co., Woodbridge, N.J.). The pellet was washed twice in 1× PBS and was resuspended to a volume of 300 μl. PMNs were added to wells containing mature macrophages from the same steer at an approximately 50:1 PMN-macrophage ratio in keeping with the high PMN-macrophage ratio found in the bronchoalveolar space of the inflamed lung (5, 42). Cells were coincubated (37°C, 5% CO2) for either 0.5, 1, or 2 h. The wells were washed three times with 37°C 1× PBS, were fixed, and were stained with Diff Quik (Baxter). The percentage of macrophages that had completely engulfed at least one PMN was determined under light microscopy from 300 macrophages in each preparation.
Transmission electron microscopy.
Phagocytosis of PMNs by macrophages was confirmed with transmission electron microscopy. Purified PMNs (1.46 × 107 cells/ml) were incubated (37°C, 5% CO2) for 2 h with 0.5 μg of tilmicosin per ml in PBS. After incubation, PMNs were centrifuged at 1,500 × g for 10 min. The pellet was washed once with PBS and then resuspended to a final concentration of 1.17 × 107 cells/ml in PBS. PMNs (4.0 ml) were added to a flask of 7-day-old macrophages and were incubated (37°C, 5% CO2) for 1 h. The medium was aspirated to remove nonadherent cells, and attached macrophages were washed three times with PBS prewarmed to 37°C. Cells were fixed in 5% glutaraldehyde (Electron Microscopy Sciences, Fort Washington, Pa.) in the flasks, were scraped into 15-ml centrifuge tubes, were transferred into BEEM capsules (JBS Supplies, JB EM Services Inc., Dorval, Quebec, Canada), were postfixed in 1% osmium tetroxide (JBS Supplies), were dehydrated in acetone, and were embedded in Spurr low-viscosity medium (Electron Microscopy Sciences). Thin sections (90 nm) were stained with saturated uranyl acetate in 50% aqueous ethanol and 0.04% (wt/vol) lead citrate. Photomicrographs were obtained with a Hitachi H-7000 transmission electron microscope at an acceleration voltage of 75 kV. Macrophages with phagocytosed PMNs and PMNs with signs of apoptosis (i.e., intact blebbed membranes, condensed nuclear chromatin, nuclear delamination, condensed perinuclear cytoplasm, and intact organelles) were identified.
Statistical analysis.
Results were expressed as means ± standard errors of the means and compared by one-way analysis of variance, followed by Tukey's test for multiple-comparison analysis where applicable. When results were expressed as percentages, all values underwent an arcsine transformation prior to statistical comparison and were transformed back to percentages for the expression of the means ± standard errors of the means. For the comparison of two lines, linear regression and one-way analysis of variance were performed, followed by Tukey's test for pairwise multiple-comparison analysis. P values of less than 0.05 were considered significant.
RESULTS
PMN apoptosis and phosphatidylserine translocation.
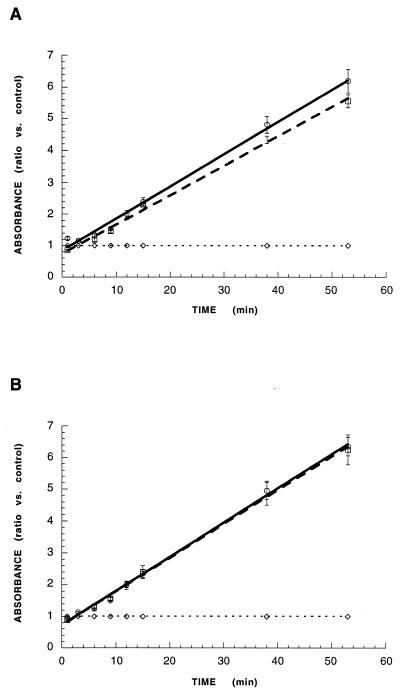
The first experiments assessed the effects of live P. haemolytica on tilmicosin-induced PMN apoptosis. The production of apoptotic nucleosomes by peripheral PMNs incubated with 5 or 0.5 μg of tilmicosin per ml, concentrations which are consistent with the pharmacokinetic profile of this compound (24), was measured in the presence or absence of P. haemolytica using quantitative ELISA. After 2 h of incubation, tilmicosin at either concentration, in the presence or the absence of P. haemolytica, significantly increased the induction of PMN apoptosis compared to controls (Fig. 1). There was no significant difference between the degree of induction of PMN apoptosis by tilmicosin alone and that of tilmicosin plus P. haemolytica at either drug concentration.
FIG. 1.
Apoptosis in peripheral bovine PMNs incubated with 5 μg (A) or 0.5 μg (B) of tilmicosin in the presence (□) or the absence (○) of P. haemolytica. In both A and B, values from 53 min of ELISA development were calculated as absorbance ratios versus values measured in PMNs from controls incubated with 1× PBS (--◊--) arbitrarily set at 1.0. Values are means ± standard errors of the means; n = 4 per experimental group. Induction of PMN apoptosis by tilmicosin is not different in the presence or absence of P. haemolytica, at either concentration of the drug.
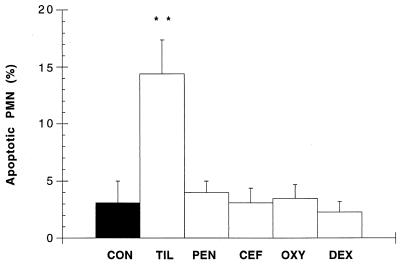
Another series of experiments determined whether tilmicosin-induced PMN apoptosis was associated with phosphatidylserine translocation and whether this effect was drug specific. Tilmicosin (17.7 ± 3.4), but not penicillin (7.6 ± 1.3), ceftiofur (6.8 ± 1.6), oxytetracycline (6.1 ± 1.3), or dexamethasone (4.8 ± 0.6), significantly increased phosphatidylserine translocation in PMNs when compared to controls (7.1 ± 1.5). The deduction of necrotic cells from these numbers confirmed that tilmicosin-induced phosphatidylserine translocation reflected a significantly (P < 0.01) increased incidence of apoptotic PMNs (Fig. 2). Neither penicillin, ceftiofur, oxytetracycline, nor dexamethasone affected apoptosis in PMNs (Fig. 2).
FIG. 2.
Percentage of apoptotic peripheral bovine PMNs incubated for 2 h with either 1× PBS (CON), 0.5 μg of tilmicosin per ml (TIL), 0.5 μg of penicillin per ml (PEN), 0.5 μg of ceftiofur per ml (CEF), 0.5 μg of oxytetracycline per ml (OXY), or 10−8 M dexamethasone (DEX). Values are means ± standard errors of the means; n = 5 per experimental group. ∗∗, P < 0.01 versus control.
The percentages of necrotic cells positive for both phosphatidylserine and propidium iodide were not significantly different between any groups (control, 4.0 ± 1.0; tilmicosin, 3.4 ± 0.7; penicillin, 3.6 ± 0.6; ceftiofur, 3.7 ± 0.5; oxytetracycline, 2.7 ± 0.7; dexamethasone, 2.6 ± 0.7). Similarly, trypan blue exclusion was not significantly different between any groups (data not shown).
Macrophage differentiation.
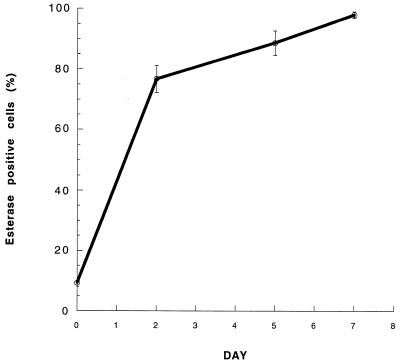
Phagocytic clearance of proinflammatory products in the bronchoalveolar spaces of the lung is carried out by fully differentiated alveolar macrophages. Therefore, circulating bovine monocytes used in this study had to be allowed to mature in vitro prior to use in the phagocytic assays. Based on morphological appearance (overall cell size, cytoplasm-to-nucleus volume ratio, presence of differentiated cytoplasmic vacuoles), the percentage of monocytes that had differentiated into mature macrophages significantly increased over the course of 7 days of incubation and reached 77.5% ± 3.9 on day 2, 89.7% ± 2.4 on day 5, and 96.5% ± 0.9 on day 7. Nonspecific esterase staining confirmed this increase in macrophage maturity over the 7-day period (76.6% ± 4.5 on day 2, 88.5% ± 4.1 on day 5, and 97.7% ± 1.1 on day 7) (Fig. 3).
FIG. 3.
Differentiation of bovine monocytes into macrophages, as determined by nonspecific esterase staining. Values are means ± standard errors of the means; n = 8 (days 0, 2, and 5) or 7 (day 7) per experimental group.
Macrophage phagocytosis assay.
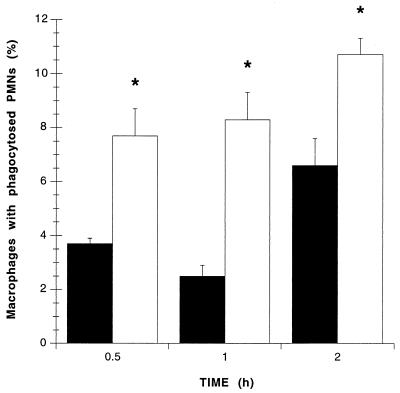
As phagocytosis of apoptotic PMNs by macrophages is an important contributor to the resolution of inflammation, additional experiments assessed the effects of tilmicosin-induced apoptosis on PMN phagocytosis by macrophages. Rates of macrophage phagocytosis were significantly enhanced if PMNs had been exposed to tilmicosin for 2 h when compared to controls (Fig. 4). Macrophage phagocytic indices for PMNs were increased over threefold after 1 h of coincubation, and this increase was detectable after 0.5, 1, or 2 h of coincubation (Fig. 4).
FIG. 4.
Percentages of macrophages that phagocytosed at least one apoptotic PMN after 0.5, 1, and 2 h of coincubation. PMNs were pretreated for 2 h with tilmicosin (□) or PBS (control; ■). Values are means ± standard errors of the means; n = 6 (0.5 and 2 h) and 5 (1 h) per experimental group. ∗, P < 0.01 versus respective control.
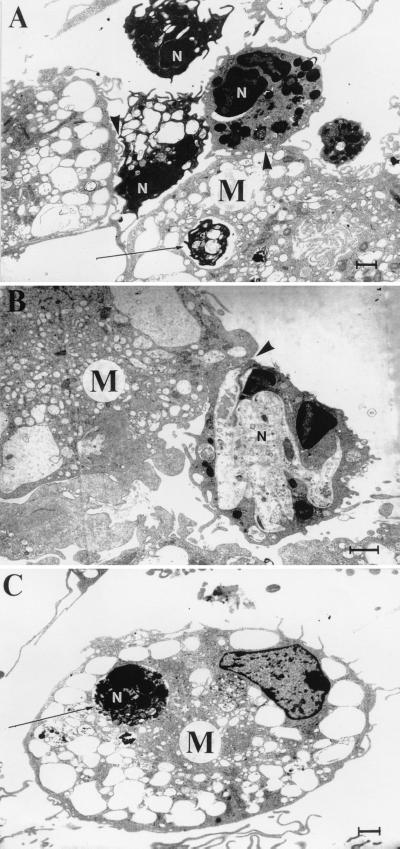
Coincubated cells were prepared for transmission electron microscopy to verify that our observations truly reflected complete phagocytic ingestion of apoptotic PMNs. Tilmicosin-treated PMNs exhibited ultrastructural evidence of apoptosis at various stages of cell death (Fig. 5). Apoptotic cells were characterized by the presence of a blebbed but intact plasma membrane, nuclear membrane delamination, chromatin condensation, cytoplasmic vacuolation, and maintenance of organelle integrity. Apoptotic bodies were also observed. A large number of apoptotic PMNs were either found within phagocytic vacuoles of macrophages (Fig. 5A and C) or had their plasma membranes in close apposition to that of an adjacent macrophage (Fig. 5A and B).
FIG. 5.
Transmission electron micrographs illustrating apoptotic tilmicosin-treated PMNs (N) in close apposition to (arrowheads) or within (arrows) phagocytic vacuoles of bovine macrophages (M). PMNs exhibit ultrastructural features characteristic of apoptosis, including nuclear membrane delamination, chromatin condensation, intact cytoplasmic granules, and vacuolation of the cytoplasm. Bar = 1 μm.
DISCUSSION
Results from this study confirm our previous observation that tilmicosin induces apoptosis in bovine PMNs. The results also indicate that tilmicosin-induced PMN apoptosis is independent of the presence or absence of live P. haemolytica. Furthermore, this effect appears to be antibiotic specific, at least to some degree, as neither penicillin, ceftiofur, nor oxytetracycline increased PMN apoptosis in sham conditions. Finally, tilmicosin-induced apoptosis in bovine PMNs is associated with increased translocation of phosphatidylserine and enhanced phagocytic uptake of these cells by macrophages.
Recruitment of PMNs serves as a protective mechanism against P. haemolytica (42). However, pathological accumulation of PMNs associated with high levels of local leukotriene B4 has been implicated in the propagation of tissue injury during pneumonic pasteurellosis (6, 17, 24, 42). During a P. haemolytica infection, a soluble heat-labile leukotoxin released by the bacteria destroys PMNs, promoting the release of proinflammatory products in surrounding tissues and further amplifying inflammation (2, 3, 34). We recently demonstrated that tilmicosin treatment of calves infected with live P. haemolytica significantly increases apoptosis in bronchoalveolar PMNs and reduces the synthesis of proinflammatory leukotriene B4 in the lung (5). We speculated that induction of PMN apoptosis by tilmicosin may confer anti-inflammatory properties on this macrolide and hence contribute to its clinical efficacy. Consistent with this observation, pulmonary inflammation is significantly reduced in tilmicosin-treated calves experimentally infected with P. haemolytica when compared with sham-treated animals (24). Experiments described in the present study further assessed the proapoptotic effects of tilmicosin on bovine peripheral PMNs. The findings indicate that tilmicosin equally induces apoptosis in bovine PMNs in the presence or absence of live P. haemolytica. This observation suggests that tilmicosin may exert its proapoptotic property regardless of the variable bacterial loads of an infected lung.
In addition, experiments measuring the externalization of phosphatidylserine on the cell membrane of bovine PMNs were used to further characterize tilmicosin-induced PMN apoptosis. Translocation of phosphatidylserine occurs early in apoptotic cells (21) and signals macrophages for phagocytic elimination of these cells via ligand binding to the phosphatidylserine receptor (10, 32). This in turn prevents the release of proinflammatory contents in situ by the dying cells and hence significantly contributes to the resolution of inflammation (10, 29, 32). PMN apoptosis and exteriorization of phosphatidylserine were examined in bovine PMNs and compared with the effects of exposure to an anti-inflammatory corticosteroid (dexamethasone) or other antibiotics (penicillin, ceftiofur, oxytetracycline) commonly used for the treatment of bovine pneumonic pasteurellosis. Results show that tilmicosin, but not the other drugs, induces PMN apoptosis. As opposed to findings from other studies on human PMNs (9, 20, 22), dexamethasone did not inhibit spontaneous apoptosis in bovine PMNs. Apart from species differences, a possible explanation for this observation is that inhibition of apoptosis by dexamethasone was observed after 24 h in previous experiments (9, 20, 22), whereas the 2-h incubation used in this study may not have been sufficient to significantly inhibit apoptosis. Indeed, recent findings have shown similar results with human PMNs exposed to another macrolide azalide, azithromycin (19). Tilmicosin-induced phosphatidylserine externalization was associated with a significant enhancement of PMN phagocytosis by macrophages. With transmission electron microscopy, further observation of tilmicosin-treated PMNs cocultured with macrophages clearly revealed PMNs at various stages of phagocytosis, from tight apposition on the surface of a neighboring macrophage to full enclosure within phagocytic vacuoles. In the light of the role played by phagocytic elimination of apoptotic PMNs by macrophages in the resolution of local inflammation, the findings reported here are consistent with our hypothesis that induction of PMN apoptosis may confer anti-inflammatory properties to tilmicosin.
Additional experiments using propidium iodide or trypan blue exclusion assays confirmed that tilmicosin-induced PMN apoptosis did not compromise membrane integrity with this experimental design. Treatment of bovine PMNs with the other drugs included in this study did not affect cell membrane integrity either.
In summary, the findings from this study show that, unlike penicillin, ceftiofur, oxytetracycline, or dexamethasone, tilmicosin induces PMN apoptosis. Induction of PMN apoptosis by tilmicosin occurs independently of the presence or absence of live P. haemolytica, increases phosphatidylserine translocation, and promotes the phagocytic ingestion of PMNs by macrophages. We postulate that, in addition to its antibacterial properties, induction of PMN apoptosis by an antibiotic such as tilmicosin may provide significant clinical benefits in the treatment of bacterial infections associated with severe inflammation.
ACKNOWLEDGMENTS
This work was supported by grants from the Natural Sciences and Engineering Research Council of Canada, the Alberta Agricultural Research Institute, and Provel Division Eli Lilly Canada Inc. A. Buret was funded by the Margaret Gunn Endowment for Animal Health Research.
We thank Herb Loeman, Crystal Koch, David Esteban, and Liz Middlemiss for technical assistance.
REFERENCES
- 1.Aoshiba K, Nagai A, Konno K. Erythromycin shortens neutrophil survival by accelerating apoptosis. Antimicrob Agents Chemother. 1995;39:872–877. doi: 10.1128/aac.39.4.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baluyut C S, Simonson R R, Bemrick W J, Maheswaran S K. Interaction of Pasteurella haemolytica with bovine neutrophils: identification and partial characterization of cytotoxin. Am J Vet Res. 1981;42:1920–1926. [PubMed] [Google Scholar]
- 3.Berggren K A, Baluyut C S, Simonson R R, Bemrick W J, Maheswaran S K. Cytotoxic effects of Pasteurella haemolytica on bovine neutrophils. Am J Vet Res. 1981;42:1383–1388. [PubMed] [Google Scholar]
- 4.Brogden K A, Ackermann M R, Debey B M. Pasteurella haemolytica lipopolysaccharide-associated protein induces pulmonary inflammation after bronchoscopic deposition in calves and sheep. Infect Immun. 1995;63:3595–3599. doi: 10.1128/iai.63.9.3595-3599.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chin A C, Morck D W, Merrill J K, Ceri H, Olson M E, Read R R, Dick P, Buret A G. Anti-inflammatory benefits of tilmicosin in calves with Pasteurella haemolytica-infected lungs. Am J Vet Res. 1998;59:765–771. [PubMed] [Google Scholar]
- 6.Clinkenbeard K D, Clarke C R, Hague C M, Clinkenbeard P, Srikumaran S, Morton R J. Pasteurella haemolytica leukotoxin-induced synthesis of eicosanoids by bovine neutrophils in vitro. J Leukoc Biol. 1994;56:644–649. doi: 10.1002/jlb.56.5.644. [DOI] [PubMed] [Google Scholar]
- 7.Cohen J J, Duke R C, Fadok V A, Sellins K S. Apoptosis and programmed cell death in immunity. Annu Rev Immunol. 1992;10:267–293. doi: 10.1146/annurev.iy.10.040192.001411. [DOI] [PubMed] [Google Scholar]
- 8.Cox G, Crossley J, Xing Z. Macrophage engulfment of apoptotic neutrophils contributes to the resolution of acute pulmonary inflammation in vivo. Am J Respir Cell Mol Biol. 1995;12:232–237. doi: 10.1165/ajrcmb.12.2.7865221. [DOI] [PubMed] [Google Scholar]
- 9.Cox G, Austen R C. Dexamethasone-induced suppression of apoptosis in human neutrophils requires continuous stimulation of new protein synthesis. J Leukoc Biol. 1997;61:224–230. doi: 10.1002/jlb.61.2.224. [DOI] [PubMed] [Google Scholar]
- 10.Fadok V, Savill J S, Haslett C, Bratton D L, Doherty D E, Campbell P A. Different populations of macrophages use either the vitronectin receptor or the phosphatidylserine receptor to recognise and remove apoptotic cells. J Immunol. 1992;149:4029–4035. [PubMed] [Google Scholar]
- 11.Frank G H. Pasteurellosis of cattle. In: Adlam C, Rutter J M, editors. Pasteurella and pasteurellosis. San Diego, Calif: Academic Press Inc.; 1989. pp. 179–222. [Google Scholar]
- 12.Golstein P, Ojcius D M, Young J D-E. Cell death mechanisms and the immune system. Immun Rev. 1991;121:29–65. doi: 10.1111/j.1600-065x.1991.tb00822.x. [DOI] [PubMed] [Google Scholar]
- 13.Gorham P E, Carroll L H, McAskill J W, Watkins L E, Ose E E, Tonkinson L V, Merrill J K. Tilmicosin as a single injection treatment for respiratory disease of feedlot cattle. Can Vet J. 1990;31:826–829. [PMC free article] [PubMed] [Google Scholar]
- 14.Goswami S K, Kivity S, Marom Z. Erythromycin inhibits respiratory glycoconjugate secretion from human airways in vitro. Am Rev Respir Dis. 1990;141:72–78. doi: 10.1164/ajrccm/141.1.72. [DOI] [PubMed] [Google Scholar]
- 15.Hartman E G, Geryl J. Comparison between the minimal inhibitory concentration of tilmicosin and oxytetracycline for bovine pneumonic Pasteurella haemolytica isolates. Vet Q. 1993;15:184. doi: 10.1080/01652176.1993.9694404. [DOI] [PubMed] [Google Scholar]
- 16.Haslett C. Granulocyte apoptosis and inflammatory disease. Br Med Bull. 1997;53:669–683. doi: 10.1093/oxfordjournals.bmb.a011638. [DOI] [PubMed] [Google Scholar]
- 17.Henricks P A J, Binkhorst G J, Drijver A A, Nijkamp F P. Pasteurella haemolytica leukotoxin enhances production of leukotriene B4 and 5-hydroxyeicosatetraenoic acid by bovine polymorphonuclear leukocytes. Infect Immun. 1992;60:3238–3243. doi: 10.1128/iai.60.8.3238-3243.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kerr J F R, Wyllie A H, Currie A R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koch C C, Esteban D J, Chin A C, Olson M E, Read R R, Ceri H, Morck D W, Buret A G. Apoptosis, oxidative metabolism and interleukin-8 production in human neutrophils exposed to azithromycin: effects of Streptococcus pneumoniae. J Antimicrob Chemother. 2000;46:19–26. doi: 10.1093/jac/46.1.19. [DOI] [PubMed] [Google Scholar]
- 20.Liles W C, Dale D C, Klebanoff S J. Glucocorticoids inhibit apoptosis of human neutrophils. Blood. 1995;86:3181–3188. [PubMed] [Google Scholar]
- 21.Martin S J, Reutelingsperger C P M, McGahon A J, Rader J A, van Schie R C A A, LaFace D M, Green D R. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of bcl-2 and abl. J Exp Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meagher L C, Cousin J M, Seckl J R, Haslett C. Opposing effects of glucocorticoids on the rate of apoptosis in neutrophils and eosinophilic granulocytes. J Immunol. 1996;156:4422–4428. [PubMed] [Google Scholar]
- 23.Morck D W, Merrill J K, Thorlakson B E, Olson M E, Tonkinson L V, Costerton J W. Prophylactic efficacy of tilmicosin for bovine respiratory tract disease. J Am Vet Med Assoc. 1993;202:273–277. [PubMed] [Google Scholar]
- 24.Morck D W, Merrill J K, Gard M S, Olson M E, Nation P N. Treatment of experimentally induced pneumonic pasteurellosis of young calves with tilmicosin. Can J Vet Res. 1997;61:187–192. [PMC free article] [PubMed] [Google Scholar]
- 25.Morikawa K, Oseko F, Morikawa S, Iwamoto K. Immunomodulatory effects of three macrolides, midecamycin acetate, josamycin, and clarithromycin, on human T-lymphocyte function in vitro. Antimicrob Agents Chemother. 1994;38:2643–2647. doi: 10.1128/aac.38.11.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Partridge, L. J., and I. Dransfield. Isolation and characterization of mononuclear phagocytes, p. 91–118. In G. Gallagher, R. C. Rees, and C. W. Reynolds (ed.), Tumor immunobiology: a practical approach. IRL Press, New York, N.Y.
- 27.Radostits O M, Blood D C, Gay C C. Veterinary medicine: a textbook of the diseases of cattle, sheep, pigs, goats, and horses. London, Great Britain: W. B. Saunders Co. Ltd.; 1994. p. 747. [Google Scholar]
- 28.Roche Y, Gougerot-Pocidalo M A, Fay M, Forest N, Pocidalo J J. Macrolides and immunity: effects of erythromycin and spiramycin on human mononuclear cell proliferation. J Antimicrob Chemother. 1986;17:195–203. doi: 10.1093/jac/17.2.195. [DOI] [PubMed] [Google Scholar]
- 29.Savill J S, Wyllie A H, Henson J E, Walport M J, Henson P M, Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Investig. 1989;83:865–875. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Savill J, Fadok V, Henson P, Haslett C. Phagocyte recognition of cells undergoing apoptosis. Immunol Today. 1993;14:131–136. doi: 10.1016/0167-5699(93)90215-7. [DOI] [PubMed] [Google Scholar]
- 31.Savill J. Apoptosis in resolution of inflammation. J Leukoc Biol. 1997;61:375–380. doi: 10.1002/jlb.61.4.375. [DOI] [PubMed] [Google Scholar]
- 32.Savill J. Recognition and phagocytosis of cells undergoing apoptosis. Br Med Bull. 1997;53:491–508. doi: 10.1093/oxfordjournals.bmb.a011626. [DOI] [PubMed] [Google Scholar]
- 33.Schumann F J, Janzen E D, McKinnon J J. Prophylactic tilmicosin medication of feedlot calves at arrival. Can Vet J. 1990;31:285–288. [PMC free article] [PubMed] [Google Scholar]
- 34.Shewen P E, Wilkie B N. Cytotoxin of Pasteurella haemolytica acting on bovine leukocytes. Infect Immun. 1982;35:91–94. doi: 10.1128/iai.35.1.91-94.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Squier M K T, Shenert A J, Cohen J J. Apoptosis in leukocytes. J Leukoc Biol. 1995;57:2–10. doi: 10.1002/jlb.57.1.2. [DOI] [PubMed] [Google Scholar]
- 36.Stevens P K, Czuprynski C J. Pasteurella haemolytica leukotoxin induces bovine leukocytes to undergo morphologic changes consistent with apoptosis in vitro. Infect Immun. 1996;64:2687–2694. doi: 10.1128/iai.64.7.2687-2694.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takeyama K, Tamaoki J, Chiyotani A, Tagaya E, Konno K. Effect of macrolide antibiotics on ciliary motility in rabbit airway epithelium in vitro. J Pharm Pharmacol. 1993;45:756–758. doi: 10.1111/j.2042-7158.1993.tb07104.x. [DOI] [PubMed] [Google Scholar]
- 38.Takizawa H, Desaki M, Ohtoshi T, Kikutani T, Okazaki H, Sato M, Akiyama N, Shoji S, Hiramatsu K, Ito K. Erythromycin suppresses interleukin 6 expression by human bronchial epithelial cells: a potential mechanism of its anti-inflammatory action. Biochem Biophys Res Commun. 1995;210:781–786. doi: 10.1006/bbrc.1995.1727. [DOI] [PubMed] [Google Scholar]
- 39.Tamaoki J, Noritaka S, Tagaya E, Konno K. Macrolide antibiotics protect against endotoxin-induced vascular leakage and neutrophil accumulation in rat trachea. Antimicrob Agents Chemother. 1994;38:1641–1643. doi: 10.1128/aac.38.7.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomson R G. A perspective on respiratory disease in feedlot cattle. Can Vet J. 1980;21:181–185. [PMC free article] [PubMed] [Google Scholar]
- 41.Umeki S. Anti-inflammatory action of erythromycin: its inhibitory effect on neutrophil NADPH oxidase activity. Chest. 1993;104:1191–1193. doi: 10.1378/chest.104.4.1191. [DOI] [PubMed] [Google Scholar]
- 42.Walker R D, Hopkins F M, Schultz T W, McCracken M D, Moore R N. Changes in leukocyte populations in pulmonary lavage fluids of calves after inhalation of Pasteurella haemolytica. Am J Vet Res. 1985;46:2429–2433. [PubMed] [Google Scholar]
- 43.Wyllie A H. Apoptosis: an overview. Br Med Bull. 1997;53:451–465. doi: 10.1093/oxfordjournals.bmb.a011623. [DOI] [PubMed] [Google Scholar]
- 44.Yoo H S, Maheswaran S K, Lin G, Townsend E L, Ames T R. Induction of inflammatory cytokines in bovine alveolar macrophages following stimulation with Pasteurella haemolytica lipopolysaccharide. Infect Immun. 1995;63:381–388. doi: 10.1128/iai.63.2.381-388.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]