Abstract
Dendrimers are large highly branched macromolecules synthesized from a polyfunctional core. They have shown a variety of biological properties, including, in some instances, antiviral activity. In this study, five dendrimers were evaluated for in vitro activity against herpes simplex virus (HSV) types 1 and 2 by cytopathic effect (CPE) inhibition and plaque reduction (PR) assay in human foreskin fibroblast cells. All of the compounds were active against both virus types in the CPE inhibition assay, in which drug was added to the cells prior to the addition of virus. Antiviral activity was reduced or lost in the PR assays, in which the cells were incubated with the virus before the drug was added. The prophylactic efficacy suggested that the dendrimers might have potential as topical microbicides, products intended to be applied to the vaginal or rectal mucosa to protect against sexually transmitted infections. Three dendrimers were evaluated for this application against genital HSV infection in mice. Two of the compounds, BRI-2999 and BRI-6741, significantly reduced infection rates when 15 μl of a 100-mg/ml solution was administered immediately prior to intravaginal challenge, and the most effective compound, BRI-2999, provided significant protection even when applied 30 min before challenge. This is the first report of microbicidal activity by dendrimers in vivo.
Dendrimers are large highly branched macromolecules that are synthesized from a polyfunctional core (e.g., ammonia or ethylenediamine) which dictates the three-dimensional shape of the molecule. To this are added repeat units (e.g., polyamidoamine [PAMAM], polyamino acids, polyphenyls, polyporphyrins, and polyethers) that completely react with the functional groups of the core, in turn leaving terminal functional groups that can react again. As each layer of repeat units is added to the molecule, it reacts with the functional groups of the previous layer, producing branching and an increasing number of surface functional units. In the final step, the dendrimer is capped with a layer that provides the desired surface chemical properties of the molecule. Thus, by altering the nature of the core and repeating units, the number of layers, and the composition of the surface layer, it is possible to synthesize a single polymeric molecule of defined three-dimensional structure and size that possesses predictable chemical properties.
Dendrimers have found a number of biological applications—chiefly as complexing and carrier molecules, e.g., for radiolabelling antibodies (13), delivery of boron 10 in neutron capture therapy of tumors (2), and as DNA transfection delivery systems (3). However, recently it has been shown that they can also have biological activity in their own right. Dendrimer molecules have been synthesized that contain functional groups in the surface layer that can form complexes with cell or viral receptors, disrupting normal virus-cell interactions, including the initial binding of virus to the cell. Some of these molecules have shown activity in vitro against a variety of viruses, including influenza virus, respiratory syncytial virus (RSV), measles virus, and human immunodeficiency virus (HIV) (1, 17; D. L. Barnard, J. E. Matheson, A. Morrison, R. W. Sidwell, J. H. Huffman, B. Matthews, and G. Holan, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. H-76, 1998). The ability of dendrimers to disrupt virus-cell binding suggested to us that they might be used as topical microbicides, compounds applied to the vaginal or rectal mucosa to reduce the spread of sexually transmitted infections.
In the studies presented here, we examined the activity of five dendrimers in vitro against herpes simplex virus type 1 (HSV-1) and HSV-2. Subsequently three of the compounds were further evaluated in vivo to examine their protective efficacy when used as topical microbicides in a mouse model of HSV-2-induced genital herpes infection.
MATERIALS AND METHODS
Viruses and cells.
HSV-1 strain E-3777 and HSV-2 strain MS, used in in vitro assays, and HSV-2 strain 186, used in in vivo studies, were prepared by culture in low-passage primary rabbit kidney (RK) cells. Virus stocks were maintained frozen (−80°C). RK cells were prepared as previously described (15) and were maintained in minimal essential medium (MEM; Mediatech, Inc., Herndon, Va.) supplemented with 10% (vol/vol) fetal bovine serum (FBS; Hyclone, Inc., Logan, Utah). Primary human foreskin fibroblast (HFF) cells were prepared from newborn human foreskins obtained from the University of Alabama—Birmingham or Brookwood Hospital (Birmingham, Ala.) as previously described (9) and maintained in MEM with 10% FBS, vancomycin, and amphotericin B (Fungizone).
Dendrimers.
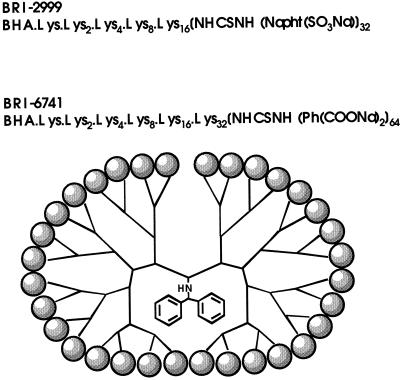
All of the dendrimer molecules used in these studies were synthesized at the Biomolecular Research Institute (Clayton, Victoria, Australia). BRI-6039, BRI-2784, and BRI-2923 are PAMAM dendrimers. BRI-2999 and BRI-6741 are polylysine dendrimers. Detailed structures of the two polylysine dendrimer molecules are shown in Fig. 1.
FIG. 1.
Molecular structure of the polylysine dendrimers BRI-2999 and BRI-6741. The molecules are synthesized by the addition of polylysine repeat units to a central benhydrylamine core group. The capping layer is indicated as  . For BRI-2999, the capping layer is naphthyl disodium disulfonate (n = 32); for BRI-6741, the capping layer is phenyl disodium dicarbonxylate (n = 64).
. For BRI-2999, the capping layer is naphthyl disodium disulfonate (n = 32); for BRI-6741, the capping layer is phenyl disodium dicarbonxylate (n = 64).
In vitro antiviral activity assays.
In vitro antiviral activity was evaluated by cytopathic effect (CPE) inhibition and plaque reduction (PR) assays by previously described methods (12). Briefly, these methods were performed as follows.
(i) CPE inhibition.
Low-passage HFF cells were trypsinized, counted, and seeded into 96-well plates at a concentration of 2.5 × 104 cells/0.1 ml of MEM supplemented with 10% FBS. The cells were incubated for 24 h at 37°C in 5% CO2–95% air in a 90% humidified atmosphere. The medium was removed, and 100 μl of MEM containing 2% FBS was added to all but the first row. In the first row, 125 μl of medium containing the experimental drug was added in triplicate wells. Medium alone was added to both cell and virus control wells. The drug in the first row of wells was then diluted serially 1:5 throughout the remaining wells. The plates were incubated for 1 h, and 100 μl containing 1,000 PFU of HSV-1 or HSV-2 was added to each well, excluding cell control wells, which received 100 μl of MEM. The plates were incubated at 37°C in a CO2 incubator for 3 days. After the incubation period, the medium was aspirated and the cells were stained with a 0.1% crystal violet solution for 4 h, washed to remove excess stain, and allowed to dry for 24 h. The amount of CPE in each row was determined with a BioTek Multiplate Autoreader set at 620 nm. The 50% effective concentration (EC50) and 50% cytotoxic concentration (CC50) were determined by comparing drug-treated and untreated cells by using a MacSynergy II computer software program.
(ii) PR assay.
Two days prior to use, HFF cells were trypsinized, counted, and plated into six-well plates and incubated at 37°C with 5% CO2 and 90% humidity. On the day of assay, the drug was prepared in MEM with 2% FBS and serially diluted 1:5 in MEM to give six concentrations ranging from 100 to 0.03 μg/ml. Pooled human globulin (Baxter Health Care Corporation, Deerfield, Ill.) was diluted 1:500 and added to the medium that the drug was diluted in to limit the extracellular spread of virus through the medium. The virus was diluted in MEM containing 10% FBS to a concentration which gave 20 to 30 PFU/well. The medium was aspirated from the wells, and 0.2 ml of virus was added to each well in triplicate, with 0.2 ml of medium being added to drug toxicity wells. The plates were incubated for 1 h with shaking every 15 min. The medium containing drug was added to the appropriate wells either 1 h before (PR-Pre) or 1 h after (PR-Post) the addition of the virus, and the cultures were incubated for 72 h. The overlay medium was removed, 1 ml of crystal violet stain (1% crystal violet, 20% methanol) was added to each well, and the wells were incubated for 10 min. The stain was aspirated, the wells were washed with saline, and viral plaques were enumerated with a stereomicroscope. The EC50s were calculated by comparing drug-treated and untreated wells by using the computer software program indicated above.
Cytotoxicity assays.
The cytotoxicity produced by dendrimers in stationary HFF cell cultures was determined by microscopic inspection of cells not exposed to the virus in PR assays. In addition, cell proliferation and neutral red uptake assays were performed as previously described (12). Briefly, these assays were performed as follows.
(i) Cell proliferation.
One day prior to assay, HFF cells were seeded in six-well plates at 2.5 × 104 cells/well in MEM containing 10% FBS. On the day of assay, dendrimers were diluted serially in the same medium at increments of 1:5 covering a range from 100 to 0.03 mg/ml. The medium was aspirated from the wells, and 2 ml of each dendrimer concentration was added to each well. Plates were incubated in a CO2 incubator at 37°C for 72 h. The medium was aspirated from each well, and the cells were washed. Trypsin-EDTA (0.25%, 1 ml) was added to each well, and the plate was incubated until the cell monolayer detached. The cell-medium mixture was pipetted vigorously to break up the cell suspension, and 0.2 ml of the mixture was added to 9.8 ml of Isoton III (Coulter Corp., Hialeah, Fla.) and counted with a Coulter counter. Each sample was counted three times with two replicate wells per sample. The 50% inhibitory concentrations (IC50s) were calculated by a computer program.
(ii) Neutral red uptake.
One day prior to assay, HFF cells were plated into 96-well plates at a concentration of 2.5 × 104 cells/well. On the day of assay, the medium was aspirated, and medium containing the dendrimer was added to the first row of wells and serially diluted as described above for the CPE assay. The plates were incubated in a CO2 incubator at 37°C for 7 days. The medium or drug was removed, the cells were washed, and 0.2 ml of 0.01% neutral red in phosphate-buffered saline (PBS) was added per well for 1 h. The dye was aspirated, and the cells were washed with a Nunc plate washer. After the wash was removed, 0.2 ml of 50% ethanol–1% aqueous acetic acid was added per well. The plates were placed on a rotating shaker for 15 min, and optical densities at 540 nm were read to determine CC50s.
Mouse model of genital HSV-2 infection.
In vivo activity was determined by using a mouse model of genital HSV-2 infection (4–6). Briefly, female Swiss Webster mice (body weight, 18 to 21 g) (Harlan, Indianapolis, Ind.), progesterone pretreated to increase susceptibility to vaginal HSV infection (11), were administered 15 μl of a 100-mg/ml dendrimer solution in sterile PBS intravaginally 20 s to 30 min before instillation of 15 μl of a suspension containing 104 PFU of HSV-2 strain 186. Vaginal swab samples were collected from all animals on day 2 postinoculation (p.i.) and stored frozen (−80°C) until assayed for the presence of virus by culture on susceptible RK cell monolayers. Mice were evaluated to day 21 p.i. for symptomatic infection (including hair loss and erythema around the perineum, chronic urinary incontinence, hind-limb paralysis, and mortality). Animals that did not develop symptoms were defined as infected if virus was isolated from vaginal swab samples collected on day 2 p.i.
Statistics.
Incidence data were compared by Fisher's exact test. All comparisons were two tailed.
RESULTS
In vitro studies.
Three PAMAM and two polylysine dendrimers were evaluated in vitro for antiviral activity against HSV-1 and HSV-2 in CPE inhibition assays. All of the compounds showed activity against both viruses when applied to target cells prior to the addition of virus (Table 1). However, in PR assays in which the dendrimers were not added until the virus had been incubated with target cells for 1 h (PR-Post), only BRI-2999 (against HSV-1 and HSV-2) and BRI-6741 (against HSV-2) displayed antiviral activity, and only in the case of BRI-2999 against HSV-2 was the EC50 comparable to that seen in CPE inhibition assays (Table 1). When the PR assay was modified so that the drug was present before the virus was added to the cells (PR-Pre), antiviral activity was restored, suggesting that the compounds act at an early stage of the virus infection process (Table 1). None of the compounds were cytotoxic at the concentrations used in in vitro studies, as determined by microscopic inspection and neutral red uptake assay. BRI-2999 did display some toxicity in the cell proliferation assay (Table 1), but only at concentrations that were considerably higher than those required for antiviral activity.
TABLE 1.
Activity of dendrimers against HSV-1 and HSV-2 in vitro
| Drug name | Assay type | EC50 (μg/ml) | CC50 (μg/ml) | Safety index (CC50/EC50) | IC50 (μg/ml) |
|---|---|---|---|---|---|
| BRI-2999 | HSV-1 CPE | 0.44 | >100 | >227 | |
| HSV-1 PR-Post | 63.4 | >100 | >1.6 | ||
| HSV-1 PR-Pre | 1.9 | >100 | >52.6 | ||
| HSV-2 CPE | 1.5 | >100 | >66.7 | ||
| HSV-2 PR-Post | 3.9 | >100 | >25.6 | ||
| HSV-2 PR-Pre | 2.4 | >100 | >41.6 | ||
| Neutral red | >100 | ||||
| Cell proliferation | 46.5 | ||||
| BRI-6039 | HSV-1 CPE | 0.6 | >100 | >167 | |
| HSV-1 PR-Post | >100 | >100 | 0 | ||
| HSV-1 PR-Pre | 1.9 | >100 | >52.6 | ||
| HSV-2 CPE | 0.39 | >100 | >256 | ||
| HSV-2 PR-Post | >100 | >100 | 0 | ||
| HSV-2 PR-Pre | 2.0 | >100 | >50 | ||
| Neutral red | >100 | ||||
| Cell proliferation | >100 | ||||
| BRI-6741 | HSV-1 CPE | 2.2 | >100 | >45.4 | |
| HSV-1 PR-Post | >100 | >100 | 0 | ||
| HSV-2 CPE | 1.0 | >100 | >100 | ||
| HSV-2 PR-Post | 89.0 | >100 | >1.1 | ||
| Neutral red | >100 | ||||
| Cell proliferation | >100 | ||||
| BRI-2784 | HSV-1 CPE | 11.3 | >100 | >8.8 | |
| HSV-1 PR-Post | >100 | >100 | 0 | ||
| HSV-2 CPE | 3.3 | >100 | >30.3 | ||
| HSV-2 PR-Post | >100 | >100 | 0 | ||
| Neutral red | >100 | ||||
| Cell proliferation | >100 | ||||
| BRI-2923 | HSV-1 CPE | 1.4 | >100 | >71.4 | |
| HSV-1 PR-Post | >100 | >100 | 0 | ||
| HSV-1 PR-Pre | 1.1 | >100 | >87.7 | ||
| HSV-2 CPE | 1.1 | >100 | >87.7 | ||
| HSV-2 PR-Post | >100 | >100 | 0 | ||
| HSV-2 PR-Pre | 2.0 | >100 | >50 | ||
| Neutral red | >100 | ||||
| Cell proliferation | >100 |
In vivo studies.
The PAMAM dendrimer BRI-6039 and the two polylysine dendrimers BRI-2999 and BRI-6741 were evaluated in vivo for activity as topical microbicides in a mouse model of genital HSV-2 infection. In initial studies, all three unformulated dendrimers significantly reduced the incidence of disease compared to that in PBS-treated controls when applied intravaginally 20 s prior to virus challenge, but only two of the compounds, BRI-2999 and BRI-6741, also provided significant protection against infection (Table 2, study 1; P < 0.001 each). In repeat studies with BRI-2999 and BRI-6741, both compounds again significantly reduced the incidence of disease and infection (Table 2, studies 2 and 3). However, BRI-6741 proved less effective than in the initial study, with 50% of the animals becoming infected and developing disease. Because BRI-2999 had proven the most effective of the dendrimers in preliminary studies, it was selected for use in subsequent evaluations.
TABLE 2.
Effect of dendrimers on genital HSV-2 in mice
| Groupa | n | No. (%) of mice protected against:
|
|
|---|---|---|---|
| Disease | Infectionb | ||
| Study 1 | |||
| PBS | 15 | 0 (0) | 0 (0) |
| BRI-2999 | 16 | 15 (94)c | 15 (94)c |
| BRI-6039 | 16 | 7 (44)d | 3 (19) |
| BRI-6741 | 16 | 14 (88)c | 13 (81)c |
| Study 2 | |||
| PBS | 16 | 0 (0) | 0 (0) |
| BRI-2999 | 16 | 16 (100)c | 16 (100)c |
| Study 3 | |||
| PBS | 16 | 0 (0) | 0 (0) |
| BRI-6741 | 16 | 8 (50)d | 8 (50)d |
BRI-2999, BRI-6039, and BRI-6741 were each given at 100 mg/ml 20 s before virus inoculation.
Animals were defined as infected if they developed symptoms or if virus was isolated from vaginal swabs collected on day 2 after inoculation.
P < 0.0001 versus PBS (Fisher's exact test).
P < 0.005 versus PBS (Fisher's exact test).
An effective microbicide will need not only to protect soon after application, but also to retain activity in the genital tract for a considerable period. Accordingly, we next examined the effect of time of application on efficacy. In Table 3, the results from study 1 show that when BRI-2999 was administered 5 min prior to virus challenge, the protection afforded was comparable to that seen in the previous studies at 20 s. Furthermore, when the interval between administration and challenge was extended to 30 min (Table 3, study 2), protection was reduced, but disease and infection rates remained significantly lower than in controls (P < 0.001 each). In this study, we also examined the effect of p.i. treatment showing that when BRI-2999 was administered 30 min after virus challenge, it failed to provide protection against either disease or infection.
TABLE 3.
Effect of time of application and dose of BRI-2999 on genital HSV-2 in mice
| Group (concn) | Time administereda | n | No. (%) of mice protected against:
|
|
|---|---|---|---|---|
| Disease | Infectionb | |||
| Study 1 | ||||
| PBS | −20 s | 16 | 0 (0) | 0 (0) |
| BRI-2999 (100 mg/ml) | −5 min | 16 | 16 (100)c | 16 (100)c |
| Study 2 | ||||
| PBS | −20 s | 16 | 0 (0) | 0 (0) |
| BRI-2999 (100 mg/ml) | −20 s | 10 | 10 (100)c | 10 (100)c |
| BRI-2999 (100 mg/ml) | −30 min | 15 | 11 (73)c | 11 (73)c |
| BRI-2999 (100 mg/ml) | +30 min | 15 | 1 (17) | 0 (0) |
| Study 3 | ||||
| PBS | −20 s | 16 | 0 (0) | 0 (0) |
| BRI-2999 (100 mg/ml) | −20 s | 16 | 16 (100)c | 16 (100)c |
| BRI-2999 (10 mg/ml) | −20 s | 16 | 14 (88)c | 14 (88)c |
Time relative to virus inoculation.
Animals were defined as infected if they developed symptoms or if virus was isolated from vaginal swabs collected on day 2 p.i.
P < 0.0001 versus PBS by Fisher's exact test.
Finally, we examined the protection afforded when the concentration of BRI-2999 used was reduced to 10 mg/ml and were able to show that even at the reduced concentration, there was significant protection against infection (Table 3, study 3).
DISCUSSION
In these studies, the five dendrimers tested all showed in vitro antiviral activity against both HSV-1 and HSV-2 in CPE reduction assays in which they were added to target cells prior to the addition of the virus. In contrast, with the exception of the activity of BRI-2999 against HSV-2, the compounds showed little activity when added to cells postexposure to the virus. Four of the compounds tested, BRI-2923, BRI-2784, BRI-2999, and BRI-6039, also have activity in CPE reduction assays against RSV (1), with BRI-2923 also being active in vitro against HIV both during virus adsorption and then during reverse transcription (17). These results suggest that the dendrimers' primary antiviral mechanism occurs early in the infection process, possibly blocking virus attachment to the cell or interfering with adsorption. However, certain compounds, such as BRI-2923 against HIV and BRI-2999 against HSV-2, also appear to have secondary mechanisms of action.
There are limited clinical applications for antimicrobial compounds that need to be present prior to or at the time of exposure to the pathogen. However, topical microbicides represent one such application. Topical microbicides are compounds that are applied directly to the genital tract or rectum prior to intercourse to protect against the acquisition of sexually transmitted infections. The lack of effective vaccines against sexually transmitted disease (STD) pathogens has stimulated interest in the development of topical microbicides as one means of curbing the epidemic of STDs (7, 8, 14, 16). The fact that microbicide use can be female initiated (if necessary, without partner consent) has added impetus to the search for safe and effective compounds, because it is recognized that females bear a disproportionate burden of STD infection and are frequently unable to negotiate condom use (7, 8, 14, 16).
To determine whether dendrimers were effective when used as topical microbicides, we evaluated the ability of BRI-2999, BRI-6039, and BRI-6741 in vivo to prevent HSV-2 infection in a mouse model of genital herpes that we and others have used previously for this purpose (4–6, 18). BRI-6039 failed to provide protection, indicating as we have previously shown with other unformulated compounds that good in vitro activity is not always predictive of in vivo efficacy (4). The other two compounds both provided significant protection in initial studies, and the most effective compound, BRI-2999, was subsequently more extensively evaluated. It was found to have efficacy both when the time of application was extended to 30 min prior to virus challenge and at a reduced concentration. The latter observation is important because microbicides will be applied repeatedly, and so using the lowest effective concentration of the active ingredient will minimize the possibility of toxic effects to the host resulting from repeated use. In this regard, all of the dendrimers evaluated showed good in vitro safety profiles. Furthermore, there was no visible evidence of toxicity in mice after a single application of any of the compounds used. Studies to evaluate the effects of repeated applications on the vaginal mucosa and normal vaginal microflora are indicated.
Although BRI-2999 was active against HSV-2 when applied after the virus had been incubated with cells for 1 h in PR assays, it did not provide protection in vivo when administered post-virus challenge. While there are a number of possible reasons for this discrepancy, it suggests that in vivo protection is mainly a result of disruption of early virus-cell interactions such as attachment and that if dendrimers are to be used successfully as microbicides, they will indeed need to be applied before prophylactically.
A microbicide that could prevent genital herpes infection would be useful, particularly in HSV-discordant couples. However, for widespread use, a microbicide would need to be effective against a variety of STD pathogens, including HIV and Chlamydia trachomatis. There is already evidence that some dendrimers do have in vitro activity against HIV (17). Thus, further studies to determine whether dendrimers have activity against nonviral STD pathogens such as C. trachomatis appear warranted. It is also worth noting that the compounds evaluated here varied in the degree of protection afforded against disease and infection. Because dendrimer synthesis can be controlled, allowing accurate prediction of the size, three-dimensional shape, and surface properties of the molecule, the results of efficacy studies such as these may make it possible to determine structure-function relationships which will allow the synthesis of extended-spectrum compounds with increased efficacy against a variety of pathogens.
In summary, we have shown that dendrimers can have in vitro activity against HSV-1 and HSV-2. This activity generally required that the compound be present at or before the time that cells were exposed to virus. This suggested that the compounds might have utility as topical microbicides. This was evaluated in a mouse model of genital HSV-2 infection and represents the first demonstration that dendrimers are effective in an in vivo setting. The studies reported here were conducted with solutions of the dendrimers, and thus it is probable that with an appropriate formulation designed for vaginal delivery, efficacy could be improved. Further studies with these compounds appear warranted.
ACKNOWLEDGMENTS
We thank James Ireland, Frances Childs, and Emma Harden for excellent technical assistance and Toni Cunningham for manuscript preparation.
These studies were supported by contracts N01-A1-85347 and N01-AI-65289 and grant PO1-AI-37940 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
REFERENCES
- 1.Barnard D L, Sidwell R W, Gage T L, Okleberry K M, Matthews B, Holan G. Anti-respiratory syncytial virus activity of dendrimer polyanions. Antivir Res. 1997;34:A88. [Google Scholar]
- 2.Barth R F, Soloway A H, Adams D M, Alam F. Delivery of Boron-10 for neutron capture therapy by means of monoclonal antibody-starburst dendrimer immunoconjugates. In: Allen B J, editor. Progress in neutron capture therapy for cancer. New York, N.Y: Plenum Press; 1992. pp. 265–268. [Google Scholar]
- 3.Bielinska A, Kukowska-Latallo J F, Johnson J, Tomalia D A, Baker J R., Jr Regulation of in vitro gene expression using antisense oligonucleotides or antisense expression plasmids transfected using starburst PAMAM dendrimers. Nucleic Acids Res. 1996;24:2176–2182. doi: 10.1093/nar/24.11.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourne K Z, Bourne N, Reising S F, Stanberry L R. Plant products as topical microbicide candidates: assessment of in vitro and in vivo activity against herpes simplex virus type 2. Antivir Res. 1999;42:219–226. doi: 10.1016/s0166-3542(99)00020-0. [DOI] [PubMed] [Google Scholar]
- 5.Bourne N, Bernstein D I, Ireland J, Sonderfan A J, Profy A T, Stanberry L R. The topical microbicide PRO 2000 protects against genital herpes infection in a mouse model. J Infect Dis. 1999;180:203–205. doi: 10.1086/314853. [DOI] [PubMed] [Google Scholar]
- 6.Bourne N, Ireland J, Stanberry L R, Bernstein D I. Effect of undecylenic acid as a topical microbicide against genital herpes infection in mice and guinea pigs. Antivir Res. 1999;40:139–144. doi: 10.1016/s0166-3542(98)00055-2. [DOI] [PubMed] [Google Scholar]
- 7.Elias C J, Coggins C. Female-controlled methods to prevent sexual transmission of HIV. AIDS. 1996;10(Suppl. 3):s43–s51. [PubMed] [Google Scholar]
- 8.Eng T R, Butler W T. The hidden epidemic: confronting sexually transmitted diseases. Washington, D.C.: National Academy Press; 1997. [PubMed] [Google Scholar]
- 9.Kern E R, Overall J C, Jr, Glasgow L A. Herpesvirus hominis infection in newborn mice. I. An experimental model and therapy with iododeoxyuridine. J Infect Dis. 1973;128:290–299. doi: 10.1093/infdis/128.3.290. [DOI] [PubMed] [Google Scholar]
- 10.Kukowska-Latallo J F, Bielinska A U, Johnson J, Spindler R, Tomalia D A, Baker J R., Jr Efficient transfer of genetic material into mammalian cells using starburst polyamidoamine dendrimers. Proc Natl Acad Sci USA. 1996;93:4897–4902. doi: 10.1073/pnas.93.10.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milligan G N, Bernstein D I. Interferon gamma (IFNγ) enhances resolution of herpes simplex virus type 2 (HSV-2) in the murine genital tract. Virology. 1997;229:259–268. doi: 10.1006/viro.1997.8441. [DOI] [PubMed] [Google Scholar]
- 12.Qiu Y-L, Ksebati M B, Ptak R G, Fan B Y, Breitenbach J M, Lin J-S, Cheng Y-C, Kern E R, Drach J C, Zemlicka J. (Z)- and (E)-2-(hydroxymethyl)cyclopropylidene)methyladenine and -guanine. New nucleoside analogues with a broad-spectrum antiviral activity. J Med Chem. 1998;41:10–23. doi: 10.1021/jm9705723. [DOI] [PubMed] [Google Scholar]
- 13.Roberts J C, Adams Y E, Tomalia D, Mercer-Smith J A, Lavallee D K. Using starburst dendrimers as linker molecules to radiolabel antibodies. Bioconjug Chem. 1990;1:305–308. doi: 10.1021/bc00005a001. [DOI] [PubMed] [Google Scholar]
- 14.Rosenthal S L, Cohen S S, Stanberry L R. Topical microbicides: current status and research considerations for adolescent girls. Sex Transm Dis. 1998;25:368–377. doi: 10.1097/00007435-199808000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Stanberry L R, Bernstein D I, Burke R L, Pachl C, Myers M G. Vaccination with recombinant herpes simplex virus glycoproteins: protection against initial and recurrent genital herpes. J Infect Dis. 1987;155:914–920. doi: 10.1093/infdis/155.5.914. [DOI] [PubMed] [Google Scholar]
- 16.Stein Z A. HIV prevention: the need for methods women can use. Am J Public Health. 1990;80:460–462. doi: 10.2105/ajph.80.4.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Witrouw M, Pannecouque C, Mathews B, Schols D, Andrei G, Snoeck R, Neyts J, Leyssen P, Desmyter J, Raff J, De Clercq E, Holan G. Dendrimers inhibit the replication of human immunodeficiency virus by a dual mechanism of action. Antivir Res. 1999;41:A25. [Google Scholar]
- 18.Zacharopoulos V R, Phillips D M. Vaginal formulations of carrageenan protect mice from herpes simplex virus infection. Clin Diagn Lab Immunol. 1997;4:465–468. doi: 10.1128/cdli.4.4.465-468.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]