Abstract
Background:
Platelet-rich plasma (PRP) exerts its effect through the release of growth factors and cytokines from the platelet concentrate. Certain medications may affect platelet count or function, resulting in decreased efficacy of PRP injections.
Purpose:
To systematically review the literature regarding common medications and their effects on platelets to establish guidelines for which medications should be stopped before obtaining a PRP injection.
Study Design:
Systematic review; Level of evidence, 2.
Methods:
This review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. A search for studies assessing the effect of common medications on platelet count or platelet function was performed of the PubMed, Cochrane Library, Web of Science, and OpenGrey databases. Inclusion criteria were as follows: drug studied was aspirin, acetaminophen, a nonsteroidal anti-inflammatory drug (NSAID), a statin, or gabapentin; human participants; and article in the English language. Risk of bias was assessed using the Cochrane Risk of Bias tool and the Risk of Bias in Non-randomised Studies—of Interventions tool.
Results:
A total of 1711 studies were identified through the initial search, with 20 studies meeting all inclusion criteria. No studies involving gabapentin met all inclusion criteria. Patients treated with aspirin (268 patients) or acetaminophen (13 patients) showed a significant decrease in platelet aggregation. Statin therapy (73 patients) did not result in a significant decrease in platelet aggregation. Patients who took NSAIDs (172 patients) demonstrated significantly decreased platelet aggregation only when treated with nonselective formulations. Those treated with cyclooxygenase (COX)-2–selective NSAIDs showed no significant difference in platelet aggregation. Treatment with aspirin, acetaminophen, statins, or NSAIDs did not lead to a significant decrease in platelet count.
Conclusion:
Aspirin, acetaminophen, and nonselective NSAIDs should be considered for suspension before a PRP injection because of their potential to diminish the effects of the injection. COX-2–selective NSAIDs and statins do not need to be withheld before a PRP injection.
Keywords: platelet-rich plasma, aspirin, acetaminophen, statin, NSAID, platelet count, platelet function
Platelet-rich plasma (PRP) is an autologous blood product containing a patient’s own concentrated platelets in a small volume of plasma that is used for the treatment of a variety of tendinous and ligamentous injuries as well as augmentation of healing after surgical procedures. 4,7 –9 PRP exerts its effect on the surrounding tissues through the release of an assortment of growth factors and cytokines from the platelet concentrate. 1
Platelet count and platelet aggregation are 2 factors that have been shown to affect PRP efficacy. No consensus exists for a standardized concentration of platelets in PRP, but studies have shown that too low or too high of a platelet count can inhibit PRP efficacy. 42 Importantly, Taniguchi et al 38 found that platelet count positively correlated with growth factor concentration, showing that platelet count may play an important role in determining the ability of PRP to exert its downstream effects. Along the same lines, platelet aggregation is necessary for platelets to release their growth factors through a process of intracellular protein phosphorylation. 1,45
While there has been an abundance of research on the different preparations and methods of activation of PRP on its efficacy, 5,24 there is a relative dearth of literature on how patient-related variables can affect the efficacy of PRP injections. One author identified this gap in the literature and sought to identify patient-specific variables that could influence the effectiveness of PRP. 18 Specifically, Kuffler 18 identified medications, blood pressure, mental and physical stress levels, smoking status, and alcohol consumption as patient-specific variables that must be considered because of their capacity to influence the analgesic efficacy of PRP. Nonetheless, there are currently few studies on patient medications and their effects on PRP. In addition, specific guidelines do not exist regarding which medications patients should stop before getting a PRP injection.
The purpose of this study was to evaluate some of the most commonly prescribed medications in the United States and their effects on platelets so that guidelines could be established for which medications should be stopped before obtaining a PRP injection. The specific medications included in this review were selected because of their prevalent use in the United States based on pharmaceutical literature as well as the authors’ own experiences with patients and medication use at their institution. 12
Methods
This systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. 27 A search for studies assessing the effect of common medications on platelet count or platelet function was performed of the PubMed, Cochrane Library, Web of Science, and OpenGrey databases. Searches were performed using the following terms: (“Nonsteroidal Anti-inflammatory Drugs” OR aspirin OR acetaminophen OR gabapentin OR statin) drug effect AND (“Platelet Function” OR “platelet quality” OR “platelet count”) AND (“platelet aggregation” OR “platelet activation”). Searches were performed in April 2020.
Inclusion criteria for the review were as follows: drug studied was aspirin, acetaminophen, a nonsteroidal anti-inflammatory drug (NSAID; both nonselective and cyclooxygenase [COX] 2–selective), a statin, or gabapentin; human participants; and article in the English language. Studies were excluded if there was not a placebo or “no treatment” comparator. Studies that did not report main outcomes as listed above were also excluded.
The following main outcomes were assessed between studies: platelet count; platelet aggregation as measured by maximal aggregation percentage or rate of aggregation (slope) using light transmission aggregometry (LTA); platelet adhesion as measured by closure time using platelet function assay (PFA-100); and thromboxane metabolites thromboxane B2 (TxB2) and 11-dehydro-TxB2 as measured by enzyme immunoassay. These outcome measures were selected because they represent varied and validated methods of assessing platelet function for the clotting cascade, with LTA serving as the gold standard. 31 Platelet aggregation, or the ability of platelets to clump, serves as a measure of platelet function, as is determined by measuring the change in optical density in a sample of PRP before and after activation. 45 Similarly, closure time represents platelet function and measures the time necessary for platelets to form a clot over a small aperture in a cartridge where primary hemostasis is simulated in vitro. 31 Thromboxane metabolites are major products of platelet metabolism and serve as a surrogate for platelet function as well. 31
Risk of bias for randomized controlled trials (RCTs) was assessed using the Cochrane Risk of Bias tool, and risk of bias for nonrandomized studies was assessed using the Risk of Bias in Non-randomised Studies—of Interventions tool. 35,36
Two authors (D.S.K. and S.W.Z.) independently performed the initial title and abstract screening, full-text screening, data extraction, and risk-of-bias assessments. Disagreements were resolved through discussion.
Results
Study Selection
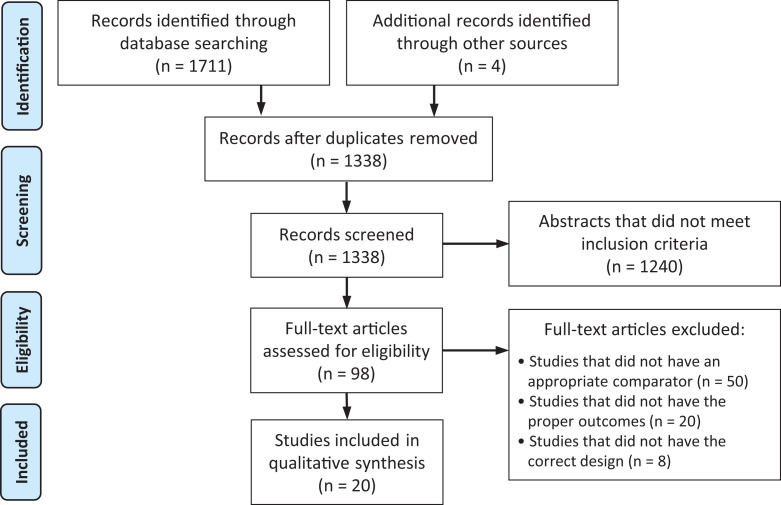
A total of 1711 studies were identified through the initial search, with 20 studies meeting all inclusion criteria (Figure 1). No studies involving gabapentin met all inclusion criteria. Eleven studies involving aspirin were identified. ‡ One study involved acetaminophen. 29 Eight studies involved NSAIDs. 14,15,17,20,26,30,32,33 Two studies involved statins. 2,34 Of the 20 studies that met all inclusion criteria, 15 were RCTs and 5 were nonrandomized cohort studies. Seven of the 15 randomized studies had a “low” risk of bias, while 8 studies had “some concern” for risk of bias (Figure 2). Of the 5 nonrandomized studies, all 5 were found to have a “moderate” risk of bias (Figure 3). 25
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram detailing the search and selection process for studies included in the systematic review.
Figure 2.
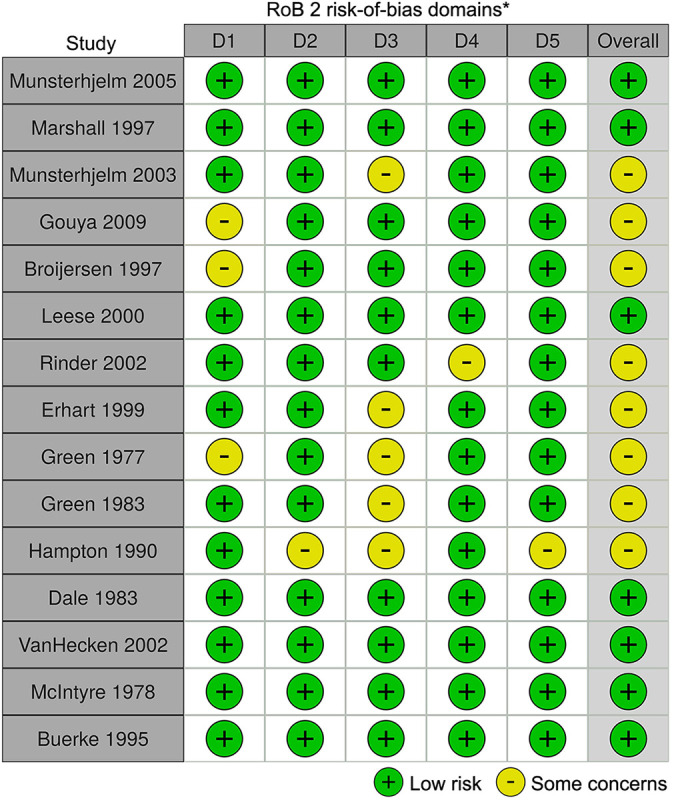
Risk of bias for randomized controlled trials using the Cochrane Risk of Bias tool (RoB 2). *Domains: D1 = bias arising from the randomization process; D2 = bias due to deviations from intended intervention; D3 = bias due to missing outcome data; D4 = bias in measurement of the outcome; D5 = bias in selection of the reported result.
Figure 3.
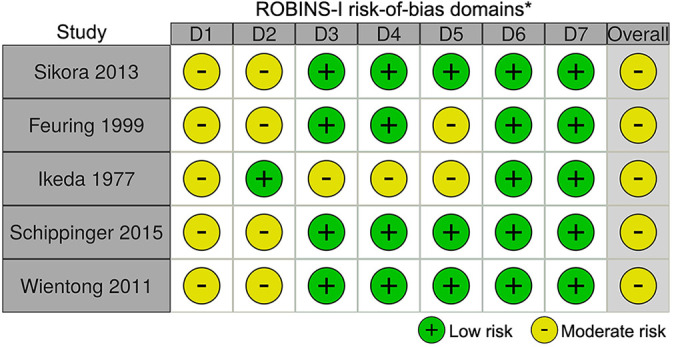
Risk of bias for nonrandomized studies using the Risk of Bias in Non-randomised Studies—of Interventions (ROBINS-I) tool. *Domains: D1 = bias due to confounding; D2 = bias due to selection of participants; D3 = bias in classification of interventions; D4 = bias due to deviations from intended interventions; D5 = bias due to missing data; D6 = bias in measurement of outcomes; D7 = bias in selection of the reported result.
Patient Characteristics
This review included a total of 792 patients (387 male, 194 female, 211 not specified) with ages ranging from 18 to 88 years (Appendix Table A1). Most patients included in the study were healthy (449/792). One hundred twenty-nine patients had type 2 diabetes mellitus, 50 patients had type 2 hyperlipidemia, 15 had isolated hypercholesterolemia, 8 had combined hyperlipoproteinemia, 66 had coronary artery disease, 49 had a history of thromboembolic transient ischemic attack, 15 had osteoarthritis, and 11 had a history of orthopaedic surgery.
Assessment of Outcomes
Appendix Table A1 provides a summary of the studies included in this review.
Aspirin
Patients who were randomized to differing doses of aspirin were consistently shown to exhibit decreased platelet aggregation when compared with patients randomized to placebo. 3,6,13 –15,22,32 A cohort study by Wientong et al 43 similarly found that aspirin therapy led to a decrease in platelet aggregation compared with control patients. This study showed that patients taking 60-mg/d aspirin had a mean platelet aggregation of 18% compared with 79% in control patients. On the other hand, Feuring et al 11 found that daily treatment with 100-mg/d aspirin failed to significantly decrease platelet aggregation. Using PFA-100, they showed a mean closure time of 129 seconds in the treatment group compared with 92 seconds in healthy control patients. Aspirin administration reliably lowered serum TxB2 levels. 40,43 Three studies found that aspirin treatment did not lead to a significant change in platelet count. 6,15,16 Interestingly, Erhart et al 10 found that healthy patients treated with 250-mg/d aspirin for 7 days displayed an increased platelet count.
Acetaminophen
Munsterhjelm et al 29 found that acetaminophen inhibited platelet aggregation in a dose-dependent manner when compared with placebo. Patients treated with 0, 15, 22.5, and 30 mg/kg of acetaminophen showed increasingly diminished platelet aggregation as measured by area under the curve, with values decreasing from 25.7 × 103 to 22.8 × 103 to 4.1 × 103 to 3.6 × 103 units of area, respectively.
Nonselective NSAIDs
Patients treated with ibuprofen or indomethacin exhibited decreased platelet aggregation but no change in platelet count. 17,26,32 Munsterhjelm et al 30 found that a single intravenous infusion of diclofenac led to decreased platelet aggregation when compared with placebo, with TxB2 levels dropping to 1.6% of baseline 5 minutes after infusion with diclofenac versus 97.6% with placebo. Likewise, Schippinger et al 33 found that PRP samples from patients who took dexibuprofen or diclofenac after recent orthopaedic surgery demonstrated substantial inhibition of platelet aggregation. Using LTA, they found that platelets from patients who took NSAIDs only showed 2.2% aggregation as opposed to 80% in the control group. 33 Healthy men and women treated with naproxen of 500 mg twice a day for 10 days similarly showed a decrease in both platelet aggregation and serum TxB2 concentration. 20 Patients treated with a single dose of sulindac showed almost complete inhibition of platelet aggregation, which resolved by 24 hours. With continued daily administration for 8 days, aggregation remained inhibited. 15 Administration of diflunisal resulted in a dose-dependent inhibition of platelet aggregation. 14
COX-2–Selective NSAIDs
Interestingly, Rinder et al 32 found that administration of meloxicam, a COX-2–selective NSAID, did not lead to decreased platelet aggregation, while treatment with indomethacin, a nonselective NSAID, did lead to decreased platelet aggregation, as has previously been shown. Platelet aggregation after daily administration of 15-mg meloxicam for 8 days was 77%, compared with 71.7% with placebo. Treatment with an 8-day course of 75-mg indomethacin led to a decrease in platelet aggregation to 2.9% compared with placebo. 32 Treatment with meloxicam did not cause a significant change in platelet count. 32 Along the same lines, Leese et al 20 showed that administration of 600-mg celecoxib for 10 days resulted in neither a significant decrease in platelet aggregation nor a decrease in serum TxB2 concentration.
Statins
Patients treated with simvastatin 20 mg/d for 10 to 12 weeks exhibited urinary 11-dehydro-TxB2 levels and platelet counts comparable to those of participants treated with placebo. 2 Day and night values of 11-dehydro-TxB2 in patients treated with placebo versus patients treated with simvastatin were 218 and 177 ng/mmol versus 216 and 163 ng/mmol, respectively, while platelet counts in patients treated with placebo and those treated with simvastatin were 216 × 109/L versus 226 × 109/L. 2 Similarly, Sikora et al 34 found that there was no significant difference in maximal platelet aggregation in patients treated with an 8-week course of 10-mg/d atorvastatin, 20-mg/d simvastatin, or 20-mg/d pravastatin when compared with control patients. Interestingly, they did find a significant decrease in aggregation rate in patients treated with pravastatin, with a drop from 37.2% T/min to 24.9% T/min, but not with atorvastatin or simvastatin. 34
Discussion
Most patients treated with differing doses of aspirin (268 patients) showed a significant decrease in platelet aggregation. Surprisingly, there was a cohort of patients treated with aspirin (48 patients) that failed to exhibit decreased platelet aggregation. 11 This unexpected finding might be explained by these patients’ coronary artery disease, which may have confounded the results. In addition, this cohort was significantly older than those in other studies. A previous study by Vaturi et al 41 showed that age was independently correlated with decreased aspirin responsiveness in patients with coronary artery disease and that patients aged 75 years or older exhibited higher rates of aspirin resistance. The smaller dosage of aspirin (100 mg/d) used compared with some other studies was likely not the cause of the surprising results, as other studies using even smaller doses of aspirin showed that treatment resulted in decreased platelet aggregation. 3,40,43
A single study involving acetaminophen (13 patients) showed that the drug inhibited platelet inhibition in a dose-dependent manner, presumably as a result of its ability to weakly inhibit COX-1. 29 Few other studies exist that describe the effects of acetaminophen on platelets, and the ones that do show conflicting findings. 19,39
Statin therapy (73 patients) did not result in a significant decrease in maximal platelet aggregation. Although there was not a significant difference in platelet aggregation with statin treatment when compared with control patients, treatment with statins did trend toward a significant decrease in aggregation. Pravastatin in particular trended most strongly toward having a significant effect on maximal aggregation, and actually did have a significant effect on rate of platelet aggregation. 34 Further studies into the effect of statins, especially pravastatin, should be done to elucidate their effects on platelet aggregation.
Patients who took NSAIDs (172 patients) demonstrated significantly decreased platelet aggregation only when treated with nonselective formulations. Interestingly, those treated with COX-2–selective NSAIDs showed no significant difference in platelet aggregation, showing that COX selectivity plays a crucial role in determining whether NSAID use causes platelet dysfunction. This finding coincides with the knowledge that COX-1 plays a significant role in thrombosis, whereas COX-2 plays a more significant role in the inflammation cascade. 28 Treatment with aspirin, acetaminophen, statins, or NSAIDs did not lead to a significant decrease in platelet count.
To our knowledge, this is the first review of its kind that looks at medication effects on platelets and the role they play in PRP therapy. To date, there have been few studies that have evaluated patient factors and their role in the efficacy of PRP injections. One study discussed the possible role of blood pressure, diet, and physical and mental stress on the efficacy of PRP in providing chronic pain relief. 18 Research shows that the initiation of the platelet aggregation cascade is crucial to the release of platelet growth factors such as platelet-derived growth factor and vascular endothelial growth factor. 21,22 Consequently, PRP obtained from patients taking platelet-inhibiting medications may not be able to provide the same burst in growth factors that allow PRP to exert its effects. This review helped to identify common medications that inhibit the platelet aggregation cascade. Future research should help to qualify and quantify the guidelines set forth in this study.
To date, there is no evidence that stopping the medications studied in this review before a PRP injection would affect the efficacy of the injection. RCTs and other high-level studies are needed to see whether the concerns proposed in this review are borne out in the data. Another factor that needs to be considered is the potential risk related to medication suspension. As an example, a large Swedish study showed that discontinuation of low-dose aspirin in the absence of major surgery or bleeding led to an increased risk of cardiovascular events. 37 The increase in risk of cardiovascular events, however small, may outweigh any potential benefit in PRP efficacy.
Limitations
The limitations of our review include the high heterogeneity of the studies regarding medication dosages, formulations, agonist used for platelet aggregation, outcome measurements, and limited number of patients studied for some medications. The heterogeneity within the studies precluded any meta-analysis of the data. Furthermore, only certain medications were studied in this review. Other commonly prescribed medications can certainly affect platelet and PRP function, and this study should serve as a stepping-stone to further research that provides more comprehensive guidelines. This study also fails to provide data regarding the length of time each medication needs to be discontinued before a PRP injection and when the medication can be resumed. Individual studies included in this review also used varying agonists for platelet aggregation, including arachidonic acid, epinephrine, adenosine diphosphate, and collagen. Different agonists produced distinct changes in the platelet aggregation response to the drugs studied. It is beyond the scope of this study to tease out the differences in each agonist and how it affects the platelet response, but it should be noted that the specific agonist used is an important variable in platelet function testing and could affect the results of the current study. 45
It should also be noted that dosages of medications studied in the trials might not accurately correlate with clinically appropriate doses. For example, Green et al 14 randomized patients to a maximum dose of 1300-mg aspirin twice a day. This dose is significantly higher than what is generally prescribed. Another important factor to consider was that most of the patients studied within this review were male. Amounts of growth factors within PRP have been shown to be different based on patient sex. 44 This could potentially limit the applicability of the study’s results to both sexes. Lastly, there is a risk that relevant articles were missed during the database search and therefore not included in the review. Specifically, only 20 studies met all inclusion criteria, with no studies involving gabapentin meeting all criteria. Ideally, more studies involving each medication would have been included in the review.
Conclusion
Aspirin, acetaminophen, and nonselective NSAIDs should be considered for suspension before a PRP injection because of their potential to diminish the effects of the injection. COX-2–selective NSAIDs and statins do not need to be withheld before a PRP injection.
APPENDIX
Table A1.
Summary of Studies Included in the Review a
| Lead Author (Year) | Drug(s) Studied | Treatment Duration | Study Design | Treatment Breakdown | Patient Characteristics | Outcome Measured | Summary of Findings |
|---|---|---|---|---|---|---|---|
| Broijersen (1997) 2 | Simvastatin 20 mg/d | 10-12 wk | RCT crossover | 23 Simvastatin, 23 placebo | 23 M, ages not given, 15 with isolated hypercholesterolemia, 8 with familial combined hyperlipidemia | Platelet count, urinary 11-dehydro-TxB2 | No change in platelet count, no difference in urinary 11-dehydro-TxB2 excretion |
| Buerke (1995) 3 | Aspirin 40, 100 mg/d | 14 d | RCT | 56 Aspirin (differing doses), 8 placebo | 64 M, ages 22-34 y (mean, 28 y), healthy | Concentration for 50% aggregation | Significant decrease with aspirin |
| Dale (1983) 6 | Aspirin 250, 1000 mg | Single dose | RCT | 12 Aspirin, 6 placebo | 18 M, 21-65 y (mean not given), “most with coronary artery disease” | Maximal aggregation, platelet count | No difference in platelet count; significant decrease in aggregation |
| Erhart (1999) 10 | Aspirin 250 mg/d | 7 d | RCT | 10 Aspirin, 10 placebo | 20 M, ages 29-47 y (mean not given), healthy | Platelet count | Significant increase in platelet count with aspirin treatment |
| Feuring (1999) 11 | Aspirin 100 mg/d | ≥7 d | Prosp cohort | 48 Aspirin, 10 control | 40 M/18 F, ages 44-88 y (mean, 67 y), all with coronary artery disease, healthy volunteers ages 26-88 y (mean, 64 y) | Closure time | No change in CT with aspirin treatment |
| Gouya (2009) 13 | Aspirin 160 mg/d | 7-10 d | RCT | 17 Aspirin, 20 placebo | 37 M, ages 23-42 y (median, 28 y), healthy | Closure time | Significant increase in CT with aspirin vs placebo |
| Green (1977) 15 | Aspirin 600 mg, sulindac 200 mg | 8 d | RCT | 6 Aspirin, 6 sulindac, 5 placebo | 17 Patients (age/sex not listed), healthy | Platelet count; platelet aggregation | No change in platelet count; both aspirin and sulindac resulted in decreased platelet aggregation |
| Green (1981) 14 | Diflunisal 250, 500, 1000 mg BID; aspirin 650, 1300 BID | 8 d | RCT | 15 Placebo, 13 aspirin, 26 diflunisal | 28 M/28 F, ages 21-40 y (mean not given), healthy | Aggregation index | Decreased aggregation with aspirin; decreased aggregation with diflunisal at high doses |
| Hampton (1990) 16 | Aspirin 300 mg/d, 600 mg BID | 9 mo–4 y | RCT | 33 Aspirin, 16 placebo | 35 M/14 F, ages 45-80 y (median, 59 y), with history of transient ischemic attack | Platelet count, aggregation | No change in platelet count with aspirin treatment; decreased aggregation with aspirin treatment |
| Ikeda (1977) 17 | Ibuprofen 300/900 mg/d, 300 mg BID or TID | Single dose | Prosp cohort | 25 Ibuprofen, 16 control | 41 Patients; 26 healthy, 15 with arthritis | Aggregation | Decrease in maximal aggregation with ibuprofen treatment |
| Leese (2000) 20 | Celecoxib 600 mg BID, naproxen 500 mg BID | 10 d | RCT | 8 Celecoxib, 8 naproxen, 8 placebo | 12 M/12 F, ages 18-55 y (mean, 30 y), healthy | Aggregation and serum TxB2 | Significant decrease in aggregation with naproxen but not celecoxib; significant decrease in serum TxB2 with naproxen but not celecoxib |
| Marshall (1997) 23 | Aspirin 750 mg TID | 5 d | RCT crossover | 12 Aspirin, 12 placebo | 12 M, ages 27-50 y (mean not given), healthy | Closure time and concentration for 50% aggregation | Increase in CT with aspirin, decrease in aggregation |
| McIntyre (1978) 26 | Ibuprofen 600 mg/d | Single dose | RCT | 12 Ibuprofen, 12 placebo | 24 Healthy men and women | Platelet count | No change in platelet count with ibuprofen treatment |
| Munsterhjelm (2003) 30 | IV diclofenac (1.1 mg/kg) | Single dose | RCT crossover | 10 Diclofenac, 10 placebo | 10 M, ages 21-30 y (mean not given), healthy | Closure time and AUC | Significant decrease in aggregation with diclofenac vs placebo; significant increase in CT with diclofenac |
| Munsterhjelm (2005) 29 | IV acetaminophen (15, 22.5, 30 mg/kg) | Single dose | RCT crossover | 13 Acetaminophen, 13 placebo | 13 M, ages 19-26 y (mean not given), healthy nonsmokers | AUC | Significant decrease in aggregation with acetaminophen treatment vs placebo |
| Rinder (2002) 32 | Meloxicam 7.5, 15, 30 mg/d; indomethacin 75 mg/d | 8 d | RCT | 17 Indomethacin, 16 placebo, 49 meloxicam | 25 M/57 F, ages 18-55 y (mean, 36 y), healthy | Aggregation | Significant decrease in aggregation with indomethacin but not meloxicam; no differences in platelet count |
| Schippinger (2015) 33 | Diclofenac 75 mg BID, dexibuprofen 400 mg BID | 2-5 d | Retrosp cohort | 11 NSAID, 10 control | 10 M/11 F, ages 24-65 y (mean, 41 y), 10 healthy, 11 with orthopaedic surgeries | Platelet aggregation, AUC | Significant decrease in aggregation with NSAID |
| Sikora (2013) 34 | Statins (atorvastatin 10 mg/d, simvastatin 20 mg/d, pravastatin 20 mg/d) | 8 wk | Prosp cohort | 50 Total: 20 atorvastatin, 18 simvastatin, 12 pravastatin | 32 M/38 F; mean age, 58 y; 50 with type 2 hyperlipidemia, 20 healthy | Maximal aggregation, aggregation velocity | Trend toward decreased aggregation with statin, but none statistically significant vs control (pravastatin significant for decrease in velocity) |
| Van Hecken (2002) 40 | Aspirin 81 mg/d | 7 d | RCT | 12 Aspirin, 12 placebo | 8 M/16 F, ages 18-55 y (mean, 24.6 y), healthy | Serum TxB2 and platelet aggregation | Significant decrease in aggregation with aspirin; significant inhibition of serum TxB2 with aspirin |
| Wientong (2011) 43 | Aspirin 60-325 mg/d | >14 d | Prosp cohort | 97 Aspirin, 32 control | 129 men/women with type 2 diabetes | Mean aggregation and serum TxB2 | Decrease in aggregation with aspirin, decrease in TxB2 with aspirin |
a AUC, area under the curve; BID, twice per day; CT; closure time; F, female; IV, intravenous; M, male; NSAID, nonsteroidal anti-inflammatory drug; Prosp, prospective; RCT, randomized controlled trial; Retrosp, retrospective; TID, 3 times per day; TxB2, thromboxane B2.
Footnotes
Final revision submitted January 3, 2022; accepted January 21, 2022.
One or more of the authors has declared the following potential conflict of interest or source of funding: A.R.V. has received grant support from DJO, education payments from Arthrex and Smith & Nephew, and hospitality payments from DePuy and RTI Surgical. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
References
- 1. Alsousou J, Thompson M, Hulley P, Noble A, Willett K. The biology of platelet-rich plasma and its application in trauma and orthopaedic surgery. J Bone Joint Surg Br. 2009;91(8):987–996. doi:10.1302/0301-620X.91B8.22546 [DOI] [PubMed] [Google Scholar]
- 2. Broijersen A, Eriksson M, Leijd B, Angelin B, Hjemdahl P. No influence of simvastatin treatment on platelet function in vivo in patients with hypercholesterolemia. Arterioscler Thromb Vasc Biol. 1997;17(2):273–278. doi:10.1161/01.atv.17.2.273 [DOI] [PubMed] [Google Scholar]
- 3. Buerke M, Pittroff W, Meyer J, Darius H. Aspirin therapy: optimized platelet inhibition with different loading and maintenance doses. Am Heart J. 1995;130(3 Pt 1):465–472. doi:10.1016/0002-8703(95)90353-4 [DOI] [PubMed] [Google Scholar]
- 4. Carr AJ, Murphy R, Dakin SG, et al. Platelet-rich plasma injection with arthroscopic acromioplasty for chronic rotator cuff tendinopathy: a randomized controlled trial. Am J Sports Med. 2015;43(12):2891–2897. [DOI] [PubMed] [Google Scholar]
- 5. Cavallo C, Roffi A, Grigolo B, et al. Platelet-rich plasma: the choice of activation method affects the release of bioactive molecules. Biomed Res Int. 2016;2016:6591717. doi:10.1155/2016/6591717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dale J, Thaulow E, Myhre E, Parry J. The effect of a thromboxane synthetase inhibitor, dazoxiben, and acetylsalicylic acid on platelet function and prostaglandin metabolism. Thromb Haemost. 1983;50(3):703–706. [PubMed] [Google Scholar]
- 7. de Almeida AM, Demange MK, Sobrado MF, Rodrigues MB, Pedrinelli A, Hernandez AJ. Patellar tendon healing with platelet-rich plasma: a prospective randomized controlled trial. Am J Sports Med. 2012;40(6):1282–1288. doi:10.1177/0363546512441344 [DOI] [PubMed] [Google Scholar]
- 8. de Jonge S, de Vos RJ, Weir A, et al. One-year follow-up of platelet-rich plasma treatment in chronic Achilles tendinopathy: a double-blind randomized placebo-controlled trial. Am J Sports Med. 2011;39(8):1623–1629. doi:10.1177/0363546511404877 [DOI] [PubMed] [Google Scholar]
- 9. Dragoo JL, Wasterlain AS, Braun HJ, Nead KT. Platelet-rich plasma as a treatment for patellar tendinopathy: a double-blind, randomized controlled trial. Am J Sports Med. 2014;42(3):610–618. doi:10.1177/0363546513518416 [DOI] [PubMed] [Google Scholar]
- 10. Erhart S, Beer JH, Reinhart WH. Influence of aspirin on platelet count and volume in humans. Acta Haematol. 1999;101(3):140–144. doi:10.1159/000040940 [DOI] [PubMed] [Google Scholar]
- 11. Feuring M, Haseroth K, Janson CP, Falkenstein E, Schmidt BM, Wehling M. Inhibition of platelet aggregation after intake of acetylsalicylic acid detected by a platelet function analyzer (PFA-100). Int J Clin Pharmacol Ther. 1999;37(12):584–588. [PubMed] [Google Scholar]
- 12. Fuentes AV, Pineda MD, Venkata KCN. Comprehension of top 200 prescribed drugs in the US as a resource for pharmacy teaching, training and practice. Pharm J Pharm Educ Pract. 2018;6(2). doi:10.3390/pharmacy6020043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gouya G, Jilma B, Niel M, Eichelberger B, Wolzt M, Panzer S. Cross validation of aspirin effect in healthy individuals by Impact-R and PFA-100: a double blind randomized placebo controlled trial. Platelets. 2009;20(3):171–176. doi:10.1080/09537100902745117 [DOI] [PubMed] [Google Scholar]
- 14. Green D, Davies RO, Holmes GI, et al. Effects of diflunisal on platelet function and fecal blood loss. Clin Pharmacol Ther. 1981;30(3):378–384. doi:10.1038/clpt.1981.176 [DOI] [PubMed] [Google Scholar]
- 15. Green D, Given KM, Ts’ao CH, Whippie JP, Rossi EC. The effect of a new non-steroidal anti-inflammatory agent, sulindac, on platelet function. Thromb Res. 1977;10(2):283–289. doi:10.1016/0049-3848(77)90009-3 [DOI] [PubMed] [Google Scholar]
- 16. Hampton KK, Cerletti C, Loizou LA, et al. Coagulation, fibrinolytic and platelet function in patients on long-term therapy with aspirin 300 mg or 1200 mg daily compared with placebo. Thromb Haemost. 1990;64(01):017–020. doi:10.1055/s-0038-1647146 [PubMed] [Google Scholar]
- 17. Ikeda Y. The effect of ibuprofen on platelet function in vivo. Keio J Med. 1977;26(4):213–222. doi:10.2302/kjm.26.213 [DOI] [PubMed] [Google Scholar]
- 18. Kuffler DP. Variables affecting the potential efficacy of PRP in providing chronic pain relief. J Pain Res. 2018;12:109–116. doi:10.2147/JPR.S190065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lages B, Weiss HJ. Inhibition of human platelet function in vitro and ex vivo by acetaminophen. Thromb Res. 1989;53(6):603–613. doi:10.1016/0049-3848(89)90150-3 [DOI] [PubMed] [Google Scholar]
- 20. Leese PT, Hubbard RC, Karim A, Isakson PC, Yu SS, Geis GS. Effects of celecoxib, a novel cyclooxygenase-2 inhibitor, on platelet function in healthy adults: a randomized, controlled trial. J Clin Pharmacol. 2000;40(2):124–132. doi:10.1177/00912700022008766 [DOI] [PubMed] [Google Scholar]
- 21. Linder BL, Chernoff A, Kaplan KL, Goodman DS. Release of platelet-derived growth factor from human platelets by arachidonic acid. Proc Natl Acad Sci U S A. 1979;76(8):4107–4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maloney JP, Silliman CC, Ambruso DR, Wang J, Tuder RM, Voelkel NF. In vitro release of vascular endothelial growth factor during platelet aggregation. Am J Physiol Heart Circ Physiol. 1998;275(3):H1054–H1061. doi:10.1152/ajpheart.1998.275.3.H1054 [DOI] [PubMed] [Google Scholar]
- 23. Marshall PW, Williams AJ, Dixon RM, et al. A comparison of the effects of aspirin on bleeding time measured using the Simplate™ method and closure time measured using the PFA-100™, in healthy volunteers. Br J Clin Pharmacol. 1997;44(2):151–155. doi:10.1046/j.1365-2125.1997.00639.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mazzocca AD, McCarthy MBR, Chowaniec DM, et al. Platelet-rich plasma differs according to preparation method and human variability. J Bone Joint Surg Am. 2012;94(4):308–316. doi:10.2106/JBJS.K.00430 [DOI] [PubMed] [Google Scholar]
- 25. McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2021;12(1):55–61. doi:10.1002/jrsm.1411 [DOI] [PubMed] [Google Scholar]
- 26. McIntyre BA, Philp RB, Inwood MJ. Effect of ibuprofen on platelet function in normal subjects and hemophiliac patients. Clin Pharmacol Ther. 1978;24(5):616–621. doi:10.1002/cpt1978245616 [DOI] [PubMed] [Google Scholar]
- 27. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi:10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Morita I. Distinct functions of COX-1 and COX-2. Prostaglandins Other Lipid Mediat. 2002;68-69:165–175. doi:10.1016/S0090-6980(02)00029-1 [DOI] [PubMed] [Google Scholar]
- 29. Munsterhjelm E, Munsterhjelm NM, Niemi TT, Ylikorkala O, Neuvonen PJ, Rosenberg PH. Dose-dependent inhibition of platelet function by acetaminophen in healthy volunteers. Anesthesiology. 2005;103(4):712–717. doi:10.1097/00000542-200510000-00009 [DOI] [PubMed] [Google Scholar]
- 30. Munsterhjelm E, Niemi T, Syrjala M, Ylikorkala O, Rosenberg P. Propacetamol augments inhibition of platelet function by diclofenac in volunteers. Br J Anaesth. 2003;91(3):357–362. doi:10.1093/bja/aeg195 [DOI] [PubMed] [Google Scholar]
- 31. Paniccia R, Priora R, Alessandrello Liotta A, Abbate R. Platelet function tests: a comparative review. Vasc Health Risk Manag. 2015;11:133–148. doi:10.2147/VHRM.S44469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rinder HM, Tracey JB, Souhrada M, Wang C, Gagnier RP, Wood CC. Effects of meloxicam on platelet function in healthy adults: a randomized, double-blind, placebo-controlled trial. J Clin Pharmacol. 2002;42(8):881–886. doi:10.1177/009127002401102795 [DOI] [PubMed] [Google Scholar]
- 33. Schippinger G, Prüller F, Divjak M, et al. Autologous platelet-rich plasma preparations: influence of nonsteroidal anti-inflammatory drugs on platelet function. Orthop J Sports Med. 2015;3(6):2325967115588896. doi:10.1177/2325967115588896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sikora J, Kostka B, Marczyk I, Krajewska U, Chałubiński M, Broncel M. Effect of statins on platelet function in patients with hyperlipidemia. Arch Med Sci. 2013;4:622–628. doi:10.5114/aoms.2013.36905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi:10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi:10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 37. Sundström J, Hedberg J, Thuresson M, Aarskog P, Johannesen KM, Oldgren J. Low-dose aspirin discontinuation and risk of cardiovascular events: a Swedish nationwide, population-based cohort study. Circulation. 2017;136(13):1183–1192. doi:10.1161/CIRCULATIONAHA.117.028321 [DOI] [PubMed] [Google Scholar]
- 38. Taniguchi Y, Yoshioka T, Sugaya H, et al. Growth factor levels in leukocyte-poor platelet-rich plasma and correlations with donor age, gender, and platelets in the Japanese population. J Exp Orthop. 2019;6(1):4. doi:10.1186/s40634-019-0175-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Trettin A, Böhmer A, Suchy MT, et al. Effects of paracetamol on NOS, COX, and CYP activity and on oxidative stress in healthy male subjects, rat hepatocytes, and recombinant NOS. Oxid Med Cell Longev. 2014;2014:212576. doi:10.1155/2014/212576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Van Hecken A, Juliano ML, Depré M, et al. Effects of enteric-coated, low-dose aspirin on parameters of platelet function. Aliment Pharmacol Ther. 2002;16(9):1683–1688. doi:10.1046/j.1365-2036.2002.01332.x [DOI] [PubMed] [Google Scholar]
- 41. Vaturi M, Vaduganathan M, Bental T, Solodky A, Kornowski R, Lev EI. Relation of aspirin response to age in patients with stable coronary artery disease. Am J Cardiol. 2013;112(2):212–216. doi:10.1016/j.amjcard.2013.03.022 [DOI] [PubMed] [Google Scholar]
- 42. Weibrich G, Hansen T, Kleis W, Buch R, Hitzler WE. Effect of platelet concentration in platelet-rich plasma on peri-implant bone regeneration. Bone. 2004;34(4):665–671. doi:10.1016/j.bone.2003.12.010 [DOI] [PubMed] [Google Scholar]
- 43. Wientong P, Jongjarornprasert W, Panomvana D. Platelet aggregation and serum thromboxane B2 level after taking 60 mg/day of aspirin in type 2 diabetic Thai patients. Int J Pharm Pharm Sci. 2011;3(suppl 3):47–50. [Google Scholar]
- 44. Xiong G, Lingampalli N, Koltsov JCB, et al. Men and women differ in the biochemical composition of platelet-rich plasma. Am J Sports Med. 2018;46(2):409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhou L, Schmaier AH. Platelet aggregation testing in platelet-rich plasma: description of procedures with the aim to develop standards in the field. Am J Clin Pathol. 2005;123(2):172–183. doi:10.1309/Y9EC63RW3XG1V313 [DOI] [PubMed] [Google Scholar]