Abstract
The Crown-shaped, severe acute respiratory syndrome-Coronavirus-2 (SARS-CoV-2) triggered the globally fatal illness of Coronavirus disease-2019 (COVID-19). This infection is known to be initially reported in bats and has been causing major respiratory challenges. The primary symptoms of COVID-19 include fever, fatigue and dry cough. As progressed the complications may lead to acute respiratory distress syndrome (ADRS), arrhythmia and shock. This review illustrates the neurological and neuropsychiatric impairments due to COVID-19 infection. The SARS-CoV-2 virus enters via the hematogenous or neural route, spreads to the Central Nervous System (CNS), causing a blood-brain barrier (BBB) dysfunction. Recent scientific articles have reported that SARS-CoV-2 causes several neurological issues such as encephalitis, seizures, acute stroke, delirium, meningoencephalitis and Guillain-Barré Syndrome (GBS). As a long-term effect of this disease certain neuropsychiatric conditions are witnessed such as depression and anxiety. Invasion into followed by degeneration takes place causing an uncontrolled immune response. Transcription factors like NF-κB (nuclear factor kappa light chain enhancer of activated B cells), which modulate genes responsible for inflammatory response gets over expressed. Nrf2 (nuclear factor erythroid 2- related factor 2) counterpoises the inflammation by antioxidant response towards COVID-19 infection. Like every other infection, the severity of this infection leads to deterioration of major organ systems and even leads to death. By the columns of this review, we elaborate on the neurological aspects of this life-threatening infection.
Keywords: COVID-19, SARS-CoV-2, Central nervous system, Neurological disorders, Neuropsychiatric disorders, ACE2, TMPRESS2
Graphical Abstract
1. Introduction
World Health Organisation initially reported several cases of viral pneumonia which was later identified as COVID-19. SARS-CoV-2 was reported as the causative virus behind the current worldwide pandemic. This highly infectious disease has caused uncountable casualties across the globe. Coronavirus is a Latin word that means a crown. The club-like spikes that are projected on the outermost surface help the virus to adhere on to the host, thus giving it a Crown-shaped structure. Based on its Crown-shaped structure the name Coronavirus was suggested (Velavan and Meyer, 2020). The Coronaviruses are the part Nidovirales order (Pal et al., 2020). Coronaviruses are members of the Coronaviridae family, which has two significant subfamilies, Coronavirinae and Torovirinae (Fehr and Perlman, 2015). These are further divided into four genres, Alphacoronavirus, Betacoronavirus, Gammacoronavirus and Deltacoronavirus (Pal et al., 2020). The Betacoronavirus can be divided into five sub-genre Nobecovirus, Hibecovirus, Embecovirus, Sarbecovirus and Merbecovirus (Cui et al., 2019, Dong et al., 2020, Wong et al., 2019). Many variants of SARS-CoV-2 have been reported such as B.1.617.1, B.1.617.3 and B.1.616 (WHO, 2021). The first defined coronavirus was the Avian Infectious Bronchitis virus which was isolated in 1937 (El-Sayed et al., 2021). The severe acute respiratory syndrome first emerged in China in 2002 (Zhu et al., 2020). The second emergence of this virus was in the form of Middle Eastern Respiratory Syndrome (MERS) that caused small outbreaks in 2012 initially in Saudi Arabia (Ramadan and Shaib, 2019). The third outbreak of SARS-CoV-2 appeared in Wuhan China in 2019 (Zhu et al., 2020). WHO, together with national authorities, institutions and researchers, frequently examines if variants of SARS-CoV-2 develop changes in transmissibility, clinical presentation and severity. The execution of public health and social measures (PHSM) by national health authorities is also followed up for changes in the variants. Systems are established to detect signals of potential Variants of Concern (VOCs) or Variants of Interest (VOIs). The amount of threat these variants pose is determined by evaluating the signals. WHO in collaboration with GISAID, a global collective of scientists monitors relative variant genome frequency per region at an exponential rate by sequencing the genome of different variants (WHO, 2021).
SARS-CoV-2, betacoronavirus has a single-stranded positive-sense RNA and has a diameter between 80 and 220 nm. The viral envelope is made up of a lipid bilayer in which tiny envelopes (E) glycoprotein, spike (S) glycoprotein, nucleocapsid (N) protein and membrane (M) glycoprotein are the major structural proteins. These major structural proteins help the virus as an anchor to the host (Bhandari et al., 2021). The viral pathogen spreads though respiration, sneezing or by close contact with the infected person (Ge et al., 2020). The droplets from an infected person tend to adhere to a surface which may cause transmission as the touch can carry the virus to mouth or nose. The incubation period for this infection varies from 2 to 14 days. The primary symptoms of COVID-19 include headache, fever, fatigue and dry cough. As the severity of the infection progress, it may lead to acute respiratory distress syndrome (ADRS), arrhythmia and shock. A lesser percentage shows signs of muscle ache, confusion, sore throat, headache, diarrhoea, chest pain, nausea and vomiting (Bhandari et al., 2021). Diminishing responsiveness, anosmia, hyposmia, hypogeusia and dysgeusia are the early manifestations of COVID-19 infection. Complete or incomplete anosmia and ageusia are the basic fringes in the sensory system appearances. Diffuse corticospinal tract signs with enhanced tendon reflexes, ankle clonus and bilateral extensor plantar reflexes were also accounted for in some patients. RNA of SARS-CoV-2 is detected in the cerebrospinal fluid (CSF) specimen (Z. Zhou et al., 2020). The most common and possible route to enter CNS is via the olfactory route (Ellul et al., 2020). The olfactory receptor neurons (ORNs) are the sole known neurons within the body that are directly in touch with the external medium, and that they show a high employee turnover and directly project within the CNS to the olfactory glomeruli of the olfactory bulb. On this basis, the likelihood that human coronavirus might invade the neural structure, and, in turn, the CNS began to be investigated (Oliviero et al., 2021). SARS-CoV-2 has also succeeded to invade the nervous system via the respiratory route. Precursor astrocyte and Oligodendrocyte cells have a significant number of receptors in major cells of the cerebral artery such as Angiotensin-converting enzyme 2 (ACE2) and Transmembrane Protease Serine 2 (TMPRESS2). This brain vascular network can help prevent SARS-CoV-2 from entering CNS (Garg, 2020). But, the presence of SARS-CoV-2 in CNS have been supported by a large number or research.
2. Background
The SARS-CoV-2 virus has a spike protein that mediates its entry into the host cells. The S gene of SARS-CoV-2 is highly variable from SARS-CoV, the nucleotide identity shared is less than 75% (Harrison et al., 2020). The receptor-binding domain present in the spike protein mediates its direct contact with a cellular receptors ACE2 and an S1 / S2 polybasic cleavage site that is proteolytically cleaved by cellular cathepsin L and TMPRSS2 (Hoffmann et al., 2020; F. Wu et al., 2020; P. Zhou et al., 2020). ACE2 receptor has proven to be the reason by which SARS-CoV-2 enters the host cell (Yan et al., 2020, Yang et al., 2020). The lung's epithelial cells (alveolar) and the small intestine (enterocytes) have ACE2 expressed in abundant. Viruses in oral, nasal and nasopharynx mucosa are also known to be present. Endothelium and smooth muscle of vascular cells can have the expression of the ACE2 receptor in the brain (Hamming et al., 2004). TMPRSS2 facilitates entry of the virus through the plasma membrane surface where the cathepsin L activates the spike protein of SARS-CoV-2 in the endosomes. Now, this can counterbalance the entry of the virus into the cells that do not have TMPRSS2 (Hoffmann et al., 2020). The genome is now released into the host cytosol, open reading frame, ORF1a and ORF1b are translated into viral replication protein and cleaved into individual non-structural proteins leading to the formation of RNA dependent RNA polymerase (Perlman and Netland, 2009). The endoplasmic reticulum is rearranged into double-membrane vesicles (DMVs), to facilitate viral replication of genomic and sub-genomic RNA. These DMVs are translated later into accessory and viral structural proteins that lead to virus particle formation (Snijder et al., 2006, Wu and Brian, 2010).
2.1. Pathogenesis associated with neurological complications
The transcription factor family of NF-κB is a pleiotropic regulator involved in many cell signalling pathways. Its stimuli cause inflammation and influence the development of axons and dendrites in their initiating state (Gutierrez and Davies, 2011). COVID-19 infection causes NF-κB activation, leading to stroke or brain thrombosis neuropathy. The cytokine (IL-6, IL-10, IFM-μ and TNF-α) storm is typical of the increased reaction in the colony of granulocytes. The genetic induction of adaptive immunogenic cells appears to have an impact on NF-κB. The unregulated activation results in an autoimmune T-cell response coupled with inflammasome being released (Guisado-Vasco et al., 2020). When parthenolide, an inhibitor of NF-κB was administered there was a reduction in the COVID-19 infection. NF-κB was reported as a transcript of IκB degradation, a complex kinase enzyme phosphorylated by a protein kinase, induced by mitogens (DeDiego et al., 2014). Excess cytokines and species of reactive oxygen cause brain injury along with dysregulation of the neurotransmitter due to high pro-inflammatory genes (Welcome and Mastorakis, 2021).
Nrf2 is a part of a leucine transcription factor that expresses antioxidant genes under the influence of oxidative stress (Cecchini and Cecchini, 2020). It can be useful for COVID-19 since it works against similar illnesses (Bhandari et al., 2021). In order to alleviate reactive stresses, Nrf2 is released, stabilised and translocated. Nrf2 generates an internal antioxidant alleviation during the process when the oxidative stress is switched on/off (Bhandari et al., 2021, Singh and Devasahayam, 2020).
If a virus starts replicating within the cell, inflammatory mediators are triggered to combat inflammatory cytokines and the species of reactive oxygen. Particularly the levels of macrophages and dendritic cells gets elevated. Respiratory failure creates radicals of superoxide and hydrogen peroxides that cause oxidative stress. The cytokine storm causes serious tissue damage upon activation of NF-κB (Cecchini and Cecchini, 2020). This over-articulation was observed to be agitated in Nrf2 knockout astrocytes (Pan et al., 2012). NF-κB monitors Nrf2 mediated response element expression, the antioxidant response element (ARE). NF-κB basic component, p65, obstructs the antioxidant response element (ARE) gene expression (Bhandari et al., 2021).
2.2. SARS-CoV-2 encounter with the central nervous system
The structural proteins of SARS-CoV-2 plays an important role in determining the approach, multiplication and inclusion of the virions into a host body (Satarker and Nampoothiri, 2020a). It has also been reported that during the second week of COVID-19 infection and acute brainstem dysfunction is observed (Wong et al., 2020). Olfactory sustentacular epithelial cells are important for smell and olfactory neuronal metabolism. Also, there is a significant amount of ACE2 and TMPRSS2. The SARS-CoV-2 virus, therefore, penetrates CNS and creates olfactory system abnormalities (Bilinska et al., 2020). The olfactory receptor neurons (ORNs) are the main neurons in the body that are immediately in touch with the outside medium. They show a high turnover rate and straightforwardly project inside the CNS to the olfactory glomeruli of the olfactory bulb (Oliviero et al., 2021). A study reported that human pluripotent stem cells obtain mixed neurones which in turn suggested that ACE2 is expressed in huge amounts in the neuronal cell bodies but comparatively less in the axons and dendrites (Xu and Lazartigues, 2020). Another study to second the previous results suggested that SARS-CoV-2 tends to infect the neural progenitor cells (B. Z. Zhang et al., 2020). The S protein of SARS-CoV-2 possesses an ACE2 binding protein of 10–20 times the binding capacity of SARS-CoV. A transformation takes place in the S1 subunit of the receptor-binding domain to enhance ACE2 receptor binding as well as viral binding (Wrapp et al., 2020). Now, the S2 subunit is transformed into a post-fusion mode (Satarker and Nampoothiri, 2020b). To help in membrane fusion the activation and priming of the spike (S) protein is important, for which the ACE2 is expressed alongside TMPRESS2 (Glowacka et al., 2011).
Facilitated viral replication in the host body produces a high amount of N protein in the initial stage of infection (Surjit and Lal, 2008). SARS-CoV RNA has N protein bound to it, which bundles them into a ribonucleoprotein complex (Huang et al., 2004). The N-terminal domain helps in RNA binding, the serine-rich linker region promotes phosphorylation and the C-terminal domain facilitates oligomerization (Kang et al., 2020). The small E proteins play a crucial role in viral duplication, deform minor hydrophobic viroporins that help in the removal and assemble the viral particles. They even facilitate cytotoxicity and pathogenic pathways (Ye and Hogue, 2007). Neuronal virulence and degradation are some of the major functions of E proteins (Stodola et al., 2018). It is even known to enhance the inflammation-mediated effect by increasing inflammatory responses in the host (Wang and Liu, 2016).
Another structural protein, the most abundantly present in coronavirus is the M protein (Alsaadi and Jones, 2019). This protein when associated with protein E, facilitates the adherence of spike protein over the exterior of M protein. The elongated structure of M protein is bent to produce an enveloped layer around the ribonucleoprotein (Neuman et al., 2011). Hence, we can conclude that such functions of structural proteins present in the SARS-CoV-2 play an important role in infecting the host cells (Satarker and Nampoothiri, 2020b).
3. Neurological and neuropsychiatric complications
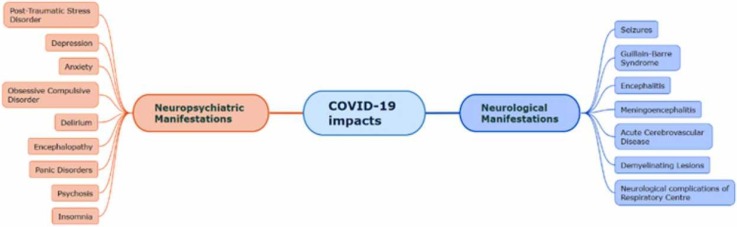
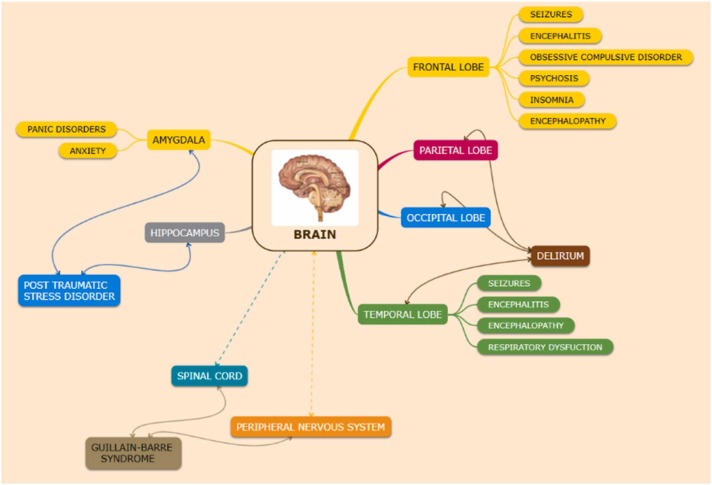
COVID-19 adversities along with the respiratory dysfunction extend to the central as well as the peripheral nervous system, causing neurological and neuropsychiatric complications. The infection affects different regions of the nervous system (as shown in Fig. 1).
Fig. 1.
Neurological and neuropsychiatric manifestations due to COVID-19 affecting different regions of the brain. The frontal lobe is affected by many neurological abnormalities such as seizures, encephalitis, obsessive-compulsive disorder, psychosis, insomnia, encephalopathy. Disorders like delirium affect the parietal, occipital as well as the temporal lobe. The temporal lobe is also affected by seizures, encephalitis, encephalopathy and respiratory dysfunction. Neuropsychiatric disorders such as pain disorders and anxiety affect the amygdala. Post-traumatic stress disorder affects the hippocampus as well as the amygdala. Guillain-Barré syndrome affects the peripheral nervous system and the spinal cord region.
3.1. Neuronal damages in respiratory centre
Pulmonary malfunction is caused by aberrant muscular breathing, including chronic obstructive pulmonary disease (COPD) leading to a malfunctioning of the lungs. Pneumonia with ground glass opacities in the central and inferior lobes of the lungs is a characteristic indication of this infection. This along with acute respiratory distress syndrome and septic shock leads to deterioration in respiration (Wu and McGoogan, 2020). This condition of lung damage arises in ARDS patients (Scala and Heunks, 2018). In studies conducted on animals, it was observed that SARS-CoV-2 particles enter the brain via the olfactory nerves and circulate into the brainstem as well as in the thalamus. The virus replicated to a high number in the lungs of Tg mice. An extensive virus replication was also detected in the brain. The virus was not detected to a significant extent in CNS at day 2, but by day 4, a large fraction of cells, predominantly neurons expressed viral antigen. This extensive brain infection was postulated to be a critical condition about aspirational pneumonia. SARS-CoV-infected K18-hACE2 Tg mice were studied to further address the aspects of SARS-CoV infection of the brain, including sites of viral entry into the CNS and factors responsible for a lethal outcome. (Netland et al., 2008). In light of a recent study conducted by Zazhytska et al. the SARS-CoV-2 infection instigates extensive olfactory receptor (OR) and signalling component downregulation. This non-cell autonomous action is accompanied by a significant remodelling of neuronal nuclear architecture, resulted in the disintegration of genomic compartments housing OR genes. SARS-CoV-2 induced anosmia has a molecular explanation where the virus may change the integrity of cells without the passageway receptors. Because the virus only infects a limited percentage of cells, its capacity to modify the OSN transcriptome is constrained. The non-cell-autonomous, broad, and persistent downregulation of OR and OR signalling genes is thus the most plausible explanation for COVID-19 patients' olfactory abnormalities. The fact that UV-neutralized serum from infected hamsters causes considerable and swift alterations in OSN nuclear architecture imply that SARS CoV-2 infection influences the anatomy and function of cells that the virus cannot infect (Zazhytska et al., 2022). ACE2 receptors have been depicted in sections of the brainstem, in the paraventricular nucleus (PVN), also in the nucleus of the tractus solitarius (NTS) and even in the rostral ventrolateral medulla (Calcagno et al., 2020). The nucleus of the solitary tract in association with the nucleus ambiguus commands the respiration process of the lungs (Zoccal et al., 2014). The receptors in the respiratory tract and lung regions transmit sensory data into the solitary tract nucleus and nucleus ambiguus. The intrusions of efferent fibres emerge out of all the two nuclei leading to the respiratory system (Li et al., 2020). SARS-CoV-2 advances in the direction of the neurons of the solitary tract nucleus in the medulla oblongata and cause damage to the rhythmic centre of respiration. It also affects the action potentials in the neuron which regulates the mechanism of respiration (inspiration and expiration) (Tassorelli et al., 2020). A primary oscillator named pre-Bötzinger complex (PBC) present in the brain stem is vital for the process of respiration. As the secondary oscillator, it contains the retrotrapezoid nucleus or the parafacial respiratory group (RTN/pFRG). SARS-CoV-2 could penetrate the CNS and infect PBC (Gandhi et al., 2020). This could lead to cardiorespiratory damages responsible for causing ARDS COVID-19 infected patients with respiratory failure (Acharya et al., 2020).
3.2. Acute cerebrovascular disease (ACVD)
Acute cerebrovascular disease is caused by various complications in the cerebral blood vessels. The flow of cerebral blood and the supply of oxygen to the cerebral region is compromised in ACVD. Ischemic stroke, due to blockage of cerebral blood flow causing reduced oxygen supply leads to ACVD (Satarker and Nampoothiri, 2020b). A case study was presented with the symptoms of COVID-19 along with acute onset aphasia and weakness in the right side of the brain. A proximal middle cerebral artery thrombus and territorial infarct with local mass effect was confirmed by a computerized tomography (CT) Scan of the brain. The CT angiogram displayed a saddle pulmonary embolism, which is most likely to be managed with split-dose low molecular weight heparin. The activation of the coagulation system leads to the circulation of a peptide degradation product in the bloodstream, known as the D-dimer. D-dimer in access can be reduced by anticoagulation (Paterson et al., 2020) i.e., higher levels of D dimer in the systemic circulation system could suggest higher chances of thrombosis which may lead to stroke (Zhang et al., 2018). COVID patients with severe infection having an acute cerebrovascular disease were suspected with a higher level of the D-dimer (Mao et al., 2020). Markers like hypersensitive C reactive protein (hsCRP), procalcitonin (PCT), erythrocytes sedimentation rate (ESR) and D dimer level are activated along with the coagulation system in COVID-19 patients (Yu et al., 2020).
3.3. Seizures
A seizure is a rapid, occasional, abrupt, uncontrolled electrical disturbance in the nerve cell activity due to high discharge from neurones in the brain. It causes modifications in behaviour, movements and level of consciousness. They are characterized by loss of attention, impaired or loss of consciousness, skeletal muscle contraction as partial and generalized seizures (Stafstrom CE, 2015). It has been observed that seizures occur in the earlier stage of infection, and the ones with hypoxia have a high risk to manifest seizures (Lu et al., 2020). Frontal sharp waves in an EEG (Electroencephalography) test run in a COVID-19 patient suggested sporadic epileptic abnormality, a frontal epileptogenic dysfunction concluding SARS-CoV-2 invasion into the brain through the olfactory route (Galanopoulou et al., 2020).
3.4. Guillain- Barré syndrome
Guillain-Barré Syndrome (GBS) is an acute generalized polyradiculoneuropathy autoimmune neurologic disease of the peripheral nervous system. GBS is mainly caused by an immune response towards the antigen that causes the demyelination and injury to the axons (Satarker and Nampoothiri, 2020b). The nerves affecting muscle movement leading to Areflexia in the limbs, bilateral weakness in the facial muscles, bladder or bowel dysfunction, acute inflammatory demyelinating polyneuropathy, acute motor axonal neuropathy are some characteristics of GBS (Willison et al., 2016). Zika virus, Middle East Respiratory Syndrome (MERS-CoV) severe acute respiratory syndrome (SARS-CoV) have been known to trigger GBS (Cao-Lormeau et al., 2016, Kim et al., 2017, Ng Kee Kwong et al., 2020). GBS can be diagnosed under clinical, electrophysiology or nerve conduction studies. In some studies, a real time reverse transcription–polymerase chain reaction (RT-PCR) assay of the CSF was used to confirm the presence of SARS-CoV-2. The test turned out to be negative and, in some studies, antibodies against ganglioside were observed to be absent. Shreds of evidence conclude that exposure to SARS-CoV-2 in the nervous system can result in the part of the peripheral nervous system being attacked by the immune system (Catanzaro et al., 2020). This abnormal activation of immune response might provoke GBS by occupying glycoconjugate, particularly the gangliosides are the neural target (Cutillo et al., 2020). Sialic acid is used as an attachment factor for cell entry, they are linked to gangliosides. The S protein containing two components the S1 and S2 subunits mediates the association and access of viral particles in the host cells(Alejandra Tortorici et al., 2019; Matrosovich, Herrler G, 2015). The communication between axons and glia and the receptor signal transduction is assisted by several gangliosides (Yamashita et al., 2005). Gangliosides are susceptible to an antibody-mediated attack due to their presence on the plasma membrane of the cells (Cutillo et al., 2020). COVID-19 triggers a cytokine storm by removing cytokines directly or encouraging a transition to a more anti-inflammatory cytokine profile (Caress et al., 2020).
3.5. Encephalitis
Encephalitis mediated by a viral (even bacterial) infection causes inflammation in the brain leading to neurological dysfunction (Willison et al., 2016). Patients with disturbed consciousness, seizures, fever, arthralgia symptoms and even respiratory malfunctions could be manifested with viral encephalitis (Costa, da, Sato, 2020). The presence of inflammatory lesions in the brain parenchyma is a characteristic clinical feature of COVID-19 existing side-by-side with meningitis (Dogan et al., 2020, Ye et al., 2020). The intrusion of the virus into the CSF is a supportive hypothesis proved by a COVID-19 patient manifested with encephalitis (Huang et al., 2020). Some authors suspect that encephalitis might be the result of anti-NMDA (N-methyl D-aspartate) receptor antibodies inducing functional destruction of glutamatergic signalling in the CNS (Panariello et al., 2020). Neuroinflammation leading to encephalitis is a consequence of the glial system being influenced by the immunological response of the brain. (Asadi-Pooya and Simani, 2020, Mudgal et al., 2019, Natoli et al., 2020). However, the association of clotting and infarction with the presence of ACE2 receptor in the vascular endothelium imply the possibility of another mechanism. (Satarker and Nampoothiri, 2020b).
3.6. Meningoencephalitis
Meningoencephalitis occurs when there is an inflammation in the meninges (three layers of membranes known to protect the brain and spinal cord) and the brain tissue. This leads to the symptoms like neck muscle stiffness, fever and headache. Meningoencephalitis may even involve inflammation in the brain parenchyma causing cortical dysfunction and aphasia coupled with hemiparesis (Sapra and Singhal, 2019). As the traces of SARS-CoV-2 were detected in the CSF of a COVID 19 patient, this suggest the association of COVID-19 with meningoencephalitis. (Moriguchi et al., 2020). The absence of a strong immune system leads the pathogen to multiply rapidly (Satarker and Nampoothiri, 2020b). In many prognoses white matter hyperintensities, anomalous occurrence in the medial temporal lobe and sacculus haemorrhage were observed. Increased level of proinflammatory cytokines IL-6, ferritin levels in CSF without pleocytosis is evident enough to elucidate the meningoencephalitis manifestation in COVID-19 patients (Dogan et al., 2020). Numerous neurological manifestations of meningoencephalitis are the result of an immune-mediated reaction to the aggressive virus (Satarker and Nampoothiri, 2020b).
3.7. Demyelinating lesions
Autopsy reports have confirmed the presence of the demyelination and SARS-CoV viral particles along with genomics sequence in the brain (Gu et al., 2005, Zanin et al., 2020). Via the trans-lamina cribrosa, the neurotropic virus may reach the brain through the olfactory tract (Baig et al., 2020). The virus can infiltrate into the neuronal cells due to the intercommunication between the spike protein S1 and the ACE2 receptor of the host cell. (Wrapp et al., 2020). The delayed damage in the CNS appears to be controlled by the immune system (Klein et al., 2017). The development of virus-induced systemic inflammatory response syndrome (SIRS) also known as SIRS-like immune disorder is intricately linked to severe viral infection (Zanin et al., 2020). The proinflammatory state induced by the cytokine storm mainly prolonged by IL-1, IL-6 and TNF α, could be responsible for the activation of glial cells with successive demyelination. In other words the brain and spine Magnetic resonance imaging (MRI) of the patient exhibited a new onset of multiple, non-enhancing demyelinating lesions. It was assumed that following SARS-CoV-2 infection, the pro-inflammatory environment induced by the cytokine storm might be responsible for the activation of glial cells with subsequent demyelination as reported by (Mehta et al., 2020, Shabani, 2021). As a para-infective or post-infective phenomenon, the virus could trigger the activation of antibodies against the glial cells (Zanin et al., 2020). SARS-CoV-2 might act as an operative infective trigger like the one of Epstein-Barr virus in multiple sclerosis. Severe pneumonia leading to hypoxia in the CNS causing an increase in anaerobic metabolism is required to trigger neurological damage. SARS-CoV-2 in the CSF sample are not a genuine confirmation as the neurological damage could be persistent due to an overdue immune response that occurs following the viremia. CSF clearance of virus is a low sensibility method to reduce lesions (Helms et al., 2020, Panciani et al., 2020, Ye et al., 2020).
3.8. Neuropsychiatric consequence
The neuropsychiatric sequelae are an emergent concern about the neuropsychiatric burden suffered by severely infected patients. Trauma-related symptoms such as depression, anxiety have been post-COVID 19 observations. But, the cause being viral infections or the host immune response is uncertain (Troyer et al., 2020). COVID infection associated with a history of mood disorder or with suicidal tendencies is another apprehension (Okusaga et al., 2011). Many COVID survivors were diagnosed with post-traumatic stress disorder, depression, pain disorder, panic disorder and obsessive-compulsive disorder (Lam et al., 2017). Another set of neuropsychiatric manifestations includes encephalopathy, delirium, mild cognitive impairment, mood swings, insomnia, suicidal tendencies and psychosis (Alkeridy et al., 2020, Duong et al., 2020, Helms et al., 2020, Huarcaya-Victoria et al., 2020, Moriguchi et al., 2020, Poyiadji et al., 2020, Troyer et al., 2020, Valdés-Florido et al., 2020, Ye et al., 2020, Yin et al., 2020).
The underlying biological basis of anosmia and the potential link between COVID-19 and Parkinson's disease remains obscure. SARS-CoV-2 infection has been predicted as a possible risk factor for developing Parkinsonism-related symptoms during a good portion of patients and survivors. Parkinson's disease with olfactory dysfunctions are characterized by impaired neurogenesis in olfactory bulb and olfactory epithilium (Rethinavel et al., 2021).
COVID-19 patients with prior mental illness are recommended to be treated with psychotropic drugs simultaneously with the usual treatment for the infection. Based on the tolerability and minimal drug interactions the ones that are considered safe are Benzodiazepines (oxazepam and lorazepam), antidepressants (citalopram and escitalopram), antipsychotics (olanzapine), and mood stabilizers (valproate) (K. Zhang et al., 2020). Delirium is another one of the uncharacteristic presentations of COVID-19 infection (Alkeridy et al., 2020). Many of the atypical antipsychotics are forbidden in this treatment as they may cause metabolic syndrome and worsen pre-existing/disease-related hyperglycaemia. It is recommended to steer clear of Benzodiazepines. For the elderly among antipsychotics, quetiapine is preferred or as alternative oral haloperidol (0.5–1 mg) (di Giacomo et al., 2020; National Institute for Health and Care Excellence (NICE) in collaboration with NHS England and NHS Improvement., 2020). Recent Findings suggest that over 30% of patients hospitalized with COVID-19 may exhibit cognitive impairment, depression, and anxiety that persist for months after discharge from the hospital. These symptoms are even more common in patients who required intensive care for the severe effects of the virual infection. In addition to the pandemic-related psychological stress, multiple biological mechanisms are proposed to know the neuropsychiatric symptoms observed with COVID-19. Given the limited information about the virus, no concrete proof can support such findings (Nakamura et al., 2021).
4. Mechanism hypothesis
There could be two major routes for coronavirus to enter the CNS: hematogenous or neural retrograde dissemination. The spread of COVID-19 infection in CNS could happen through the olfactory pathway and continuing towards the neuronal territory (Y. Wu et al., 2020). A direct invasion by the SARS-CoV2 virus might be possible through the activation of the ACE2 receptor, expressed in both capillary and neural endothelium (Hamming et al., 2004; Y. Wu et al., 2020). To trigger chronic neuropsychiatry sequelae, secondary immune alterations are hypothesized (Chuan et al., 2017, Needham et al., 2020, Severance et al., 2011). Excessive inflammation is a crucial manifestation in severity of the infection that is caused due to dysregulated immune response (Chuan et al., 2017, Toljan, 2020). Patients may also exhibit increased prothrombin time and coagulopathy that may contribute to thrombosis or haemorrhage (Wang et al., 2020). Stress during the virus outbreaks is consequential to activating the hypothalamic-pituitary-adrenal axis, releasing increased levels of steroids. These steroids once released impair immune system functioning. This event also precipitates the infection or worsens the severity, leading to neurasthenia and chronic fatigue. Psychotic symptoms could also manifest as a secondary side effect of drugs such as oseltamivir, corticosteroids and interferons which are used to treat COVID-19 infection, (Dinakaran et al., 2020, Russell et al., 2020, Ueda et al., 2015).
The hypothesis that COVID-19 virus follows hematogenous route explains another plausible path. The virus can infect endothelial cells of the BBB to gain access or infect leukocytes to disseminate into the CNS (Desforges et al., 2019, Desforges et al., 2014, Swanson and McGavern, 2015). It has been noticed that SARS-CoV infect endothelial cells of the BBB following viremia, giving a direct passage across the BBB into the CNS (Guo et al., 2008). The ACE2 expressed in the capillary endothelium of the BBB has SARS-CoV-2 bound to gain access into the CNS (Baig et al., 2020). To pass through the blood-brain barrier SARS-CoV can infect monocytes and macrophages (Gu et al., 2005). The other proposed major route for entry into the CNS is by infecting the neurons in the peripheral and using the axonal transportation machinery to enter inside the CNS (Desforges et al., 2019, Desforges et al., 2014, Swanson and McGavern, 2015). For SARS-CoV-2 another potential route of entry into the CNS is via the cranial nerve, ACE2 receptor is broadly expressed on the epithelial cell of the oral mucosa (Xu et al., 2020). The olfactory nerve serves as the shortest route for several viruses to enter the CNS. The olfactory receptor neurones protrude into the nasal cavity and stretch the axons to the cerebriform plate into the olfactory bulb of the brain (Riel et al., 2015). Hyposmia, the olfactory neurological manifestation was reported in patients who tested positive in the nasopharyngeal swab test (Z. Zhou et al., 2020). SARS-CoV-2 patients manifest cytokine storm, elevated D-dimer levels and thrombocytopenia suggesting neurological infringement (Mehta et al., 2020, Zhang et al., 2020).
5. Peripheral nervous system
Under a case study, COVID-19 patients with GBS as well as the onset of neurological symptoms, developed a brachial plexopathy after COVID-19 symptoms. GBS patients were treated with IVIG, with brachial plexopathy they received corticosteroid and gained partial recovery at the time of writing (Paterson et al., 2020).
6. Long term consequences
The long-term neurological consequences are because of various neurological manifestations such as encephalopathy, encephalitis, stroke, acute peripheral nerve disease, demyelination, axonal GBS, Miller Fisher syndrome, Kawasaki like multisystem inflammatory syndromes were primarily reported. Magnitudes of brain damage were caused either by a direct viral infection or by intravascular coagulation. The endothelial dysfunction or cerebral ischemia or severe immunological response and cytokines storm leading to autoimmune events were also some manifestations. Lewy bodies accumulated and localised initially in the olfactory pathway prior to extending themselves to other parts of the brain. Such disorders can even occur due to prolonged stressful situations and may induce posttraumatic stress disorder and other neuropsychiatric syndromes (El-Sayed et al., 2021).
7. Conclusion
COVID-19 infection caused by SARS-CoV-2 has been known to engender severe respiratory complications. The SARS-CoV-2 virus has been biased towards the inferior respiratory tract leading to the most common manifestations such as fever and dry cough. In mild conditions, headache, altered smell and taste, cough, asthenia and myalgia are commonly noted in COVID-19 patients. There have been reports of encephalopathy, encephalitis, meningitis, stroke, seizures, neuromuscular disorders, GBS and other neuropathies. Symptoms involving the CNS include dizziness, headache, altered and sensorium cerebrovascular events. The peripheral nervous system manifestations include diminished sensation towards taste and smell. The ACE2 receptor, which binds to the spike protein of Coronavirus along with TMPRESS2 are known to facilitate the entry of SARS-CoV-2 into the host. By infecting the endothelial cells via the BBB SARS-CoV-2 enables its entry into the CNS. The Nrf2 mediated antioxidant response element expression is regulated by NF-κB. The involvement of NF-κB and Nrf2 in cytokine storm and oxidative stress are the characteristic of COVID-19. For the infection to disseminate into the neurological territory immunological detriment is crucial. This neuro-invasion can cause complications such as GBS, SIRS-like immune disorders, demyelinating lesions and more. To weigh these complications as significant as any, is a step to doctor the infection.
Conflict of interest
None.
Acknowledgements
Funding: This work is not supported by any funding agency.
References
- Acharya A., Kevadiya B.D., Gendelman H.E., Byrareddy S.N. SARS-CoV-2 infection leads to neurological dysfunction. J. Neuroimmune Pharmacol. 2020;15:167–173. doi: 10.1007/s11481-020-09924-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alejandra Tortorici M., Walls A.C., Lang Y., Wang C., Li Z., Koerhuis D., Boons G.J., Bosch B.J., Rey F.A., de Groot R.J., Veesler D. Structural basis for human coronavirus attachment to sialic acid receptors. Nat. Struct. Mol. Biol. 2019;26:481–489. doi: 10.1038/s41594-019-0233-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkeridy W.A., Almaghlouth I., Alrashed R., Alayed K., Binkhamis K., Alsharidi A., Liu-Ambrose T. A unique presentation of delirium in a patient with otherwise asymptomatic COVID-19. J. Am. Geriatr. Soc. 2020;68:1382–1384. doi: 10.1111/jgs.16536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsaadi E.A.J., Jones I.M. Membrane binding proteins of coronaviruses. Future Virol. 2019;14:275–286. doi: 10.2217/fvl-2018-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asadi-Pooya A.A., Simani L. Central nervous system manifestations of COVID-19: a systematic review. J. Neurol. Sci. 2020;413 doi: 10.1016/j.jns.2020.116832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig A.M., Khaleeq A., Ali U., Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem. Neurosci. 2020;11:995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- Bhandari R., Khanna G., Kaushik D., Kuhad A. Divulging the intricacies of crosstalk between NF-Kb and Nrf2-Keap1 pathway in neurological complications of COVID-19. Mol. Neurobiol. 2021 doi: 10.1007/s12035-021-02344-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilinska K., Jakubowska P., Von Bartheld C.S., Butowt R. Expression of the SARS-CoV-2 entry proteins, ACE2 and TMPRSS2, in cells of the olfactory epithelium: identification of cell types and trends with age. ACS Chem. Neurosci. 2020;11:1555–1562. doi: 10.1021/acschemneuro.0c00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcagno N., Colombo E., Maranzano A., Pasquini J., Keller Sarmiento I.J., Trogu F., Silani V. Rising evidence for neurological involvement in COVID-19 pandemic. Neurol. Sci. 2020;41:1339–1341. doi: 10.1007/s10072-020-04447-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao-Lormeau V.M., Blake A., Mons S., Lastère S., Roche C., Vanhomwegen J., Dub T., Baudouin L., Teissier A., Larre P., Vial A.L., Decam C., Choumet V., Halstead S.K., Willison H.J., Musset L., Manuguerra J.C., Despres P., Fournier E., Mallet H.P., Musso D., Fontanet A., Neil J., Ghawché F. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French polynesia: a case-control study. Lancet. 2016;387:1531–1539. doi: 10.1016/S0140-6736(16)00562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caress J.B., Castoro R.J., Simmons Z., Scelsa S.N., Lewis R.A., Ahlawat A., Narayanaswami P. COVID-19–associated Guillain-Barré syndrome: the early pandemic experience. Muscle and Nerve. 2020;62:485–491. doi: 10.1002/mus.27024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catanzaro M., Fagiani F., Racchi M., Corsini E., Govoni S., Lanni C. Immune response in COVID-19: addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Signal Transduct. Target. Ther. 2020:5. doi: 10.1038/s41392-020-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchini R., Cecchini A.L. SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression. Med. Hypotheses. 2020;143 doi: 10.1016/j.mehy.2020.110102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuan Qin, Zhou L., Hu Z., Zhang S. Dysregulation of immune response in patients with COVID-19 in Wuhan, China Chuan. J. Chem. Inf. Model. 2017;53:1689–1699. [Google Scholar]
- Costa B.K., da, Sato D.K. Viral encephalitis: a practical review on diagnostic approach and treatment. J. Pediatr. (Rio. J) 2020;96:12–19. doi: 10.1016/j.jped.2019.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J., Li F., Shi Z.L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutillo G., Saariaho A.H., Meri S. Physiology of gangliosides and the role of antiganglioside antibodies in human diseases. Cell. Mol. Immunol. 2020;17:313–322. doi: 10.1038/s41423-020-0388-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeDiego M.L., Nieto-Torres J.L., Regla-Nava J.A., Jimenez-Guardeno J.M., Fernandez-Delgado R., Fett C., Castano-Rodriguez C., Perlman S., Enjuanes L. Inhibition of NF- B-mediated inflammation in severe acute respiratory syndrome coronavirus-infected mice increases survival. J. Virol. 2014;88:913–924. doi: 10.1128/jvi.02576-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desforges M., Le Coupanec A., Dubeau P., Bourgouin A., Lajoie L., Dubé M., Talbot P.J. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2019;12:1–28. doi: 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desforges M., Le Coupanec A., Stodola J.K., Meessen-Pinard M., Talbot P.J. Human coronaviruses: viral and cellular factors involved in neuroinvasiveness and neuropathogenesis. Virus Res. 2014;194:145–158. doi: 10.1016/j.virusres.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Giacomo E., Bellelli G., Peschi G., Scarpetta S., Colmegna F., de Girolamo G., Clerici M. Management of older people during the COVID-19 outbreak: Recommendations from an Italian experience. Int. J. Geriatr. Psychiatry. 2020;35:803–805. doi: 10.1002/gps.5318. [DOI] [PubMed] [Google Scholar]
- Dinakaran D., Manjunatha N., Naveen Kumar C., Suresh B.M. Neuropsychiatric aspects of COVID-19 pandemic: a selective review. Asian J. Psychiatr. 2020;53 doi: 10.1016/j.ajp.2020.102188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogan L., Kaya D., Sarikaya T., Zengin R., Dincer A., Akinci I.O., Afsar N. Plasmapheresis treatment in COVID-19–related autoimmune meningoencephalitis: Case series. Brain. Behav. Immun. 2020;87:155–158. doi: 10.1016/j.bbi.2020.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong N., Yang X., Ye L., Chen K., Chan E.W.-C., Chen S. Genomic and protein structure modelling analysis depicts the origin and pathogenicity of 2019-nCoV, a new coronavirus which caused a pneumonia outbreak in Wuhan, China. F1000Research. 2020;9:121. doi: 10.12688/f1000research.22357.2. [DOI] [Google Scholar]
- Duong L., Xu P., Liu A. Meningoencephalitis without respiratory failure in a young female patient with COVID-19 infection in Downtown Los Angeles, early April 2020. Brain. Behav. Immun. 2020;87:33. doi: 10.1016/j.bbi.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sayed A., Aleya L., Kamel M. COVID-19: a new emerging respiratory disease from the neurological perspective. Environ. Sci. Pollut. Res. 2021 doi: 10.1007/s11356-021-12969-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellul M.A., Benjamin L., Singh B., Lant S., Michael B.D., Easton A., Kneen R., Defres S., Sejvar J., Solomon T. Neurological associations of COVID-19. Lancet Neurol. 2020;19:767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Coronaviruses Methods Protoc. 2015:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanopoulou A.S., Ferastraoaru V., Correa D.J., Cherian K., Duberstein S., Gursky J., Hanumanthu R., Hung C., Molinero I., Khodakivska O., Legatt A.D., Patel P., Rosengard J., Rubens E., Sugrue W., Yozawitz E., Mehler M.F., Ballaban-Gil K., Haut S.R., Moshé S.L., Boro A. EEG findings in acutely ill patients investigated for SARS-CoV-2/COVID-19: a small case series preliminary report. Epilepsia Open. 2020;5:314–324. doi: 10.1002/epi4.12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi S., Srivastava A.K., Ray U., Tripathi P.P. Is the collapse of the respiratory center in the brain responsible for respiratory breakdown in COVID-19 patients? ACS Chem. Neurosci. 2020;11:1379–1381. doi: 10.1021/acschemneuro.0c00217. [DOI] [PubMed] [Google Scholar]
- Garg R. Spectrum of neurological manifestations in Covid-19: a review. Neurol. India. 2020;68:560–572. doi: 10.4103/0028-3886.289000. [DOI] [PubMed] [Google Scholar]
- Ge H., Wang X., Yuan X., Xiao G., Wang C., Deng T., Yuan Q., Xiao X. The epidemiology and clinical information about COVID-19. Eur. J. Clin. Microbiol. Infect. Dis. 2020;39:1011–1019. doi: 10.1007/s10096-020-03874-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowacka I., Bertram S., Muller M.A., Allen P., Soilleux E., Pfefferle S., Steffen I., Tsegaye T.S., He Y., Gnirss K., Niemeyer D., Schneider H., Drosten C., Pohlmann S. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J. Virol. 2011;85:4122–4134. doi: 10.1128/jvi.02232-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J., Gong E., Zhang B., Zheng J., Gao Z., Zhong Y., Zou W., Zhan J., Wang S., Xie Z., Zhuang H., Wu B., Zhong H., Shao H., Fang W., Gao D., Pei F., Li X., He Z., Xu D., Shi X., Anderson V.M., Leong A.S.Y. Multiple organ infection and the pathogenesis of SARS. J. Exp. Med. 2005;202:415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guisado-Vasco P., Cano-Megías M., Rodríguez-López M., de-Luna-Boquera I.M., Carnevali-Ruiz D. COVID-19 and metabolic syndrome: NF-κB activation. crossroads. Trends Endocrinol. Metab. 2020;31:802–803. doi: 10.1016/j.tem.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y., Korteweg C., McNutt M.A., Gu J. Pathogenetic mechanisms of severe acute respiratory syndrome. Virus Res. 2008;133:4–12. doi: 10.1016/j.virusres.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez H., Davies A.M. Regulation of neural process growth, elaboration and structural plasticity by NF-κB. Trends Neurosci. 2011;34:316–325. doi: 10.1016/j.tins.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming I., Timens W., Bulthuis M.L.C., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison A.G., Lin T., Wang P. Mechanisms of SARS-CoV-2 Transmission and Pathogenesis. Trends Immunol. 2020;41:1100–1115. doi: 10.1016/j.it.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C., Collange O., Boulay C., Fafi-Kremer S., Ohana M., Anheim M., Meziani F. Neurologic features in severe SARS-CoV-2 infection. N. Engl. J. Med. 2020;382:2268–2270. doi: 10.1056/nejmc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(271–280) doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q., Yu L., Petros A.M., Gunasekera A., Liu Z., Xu N., Hajduk P., Mack J., Fesik S.W., Olejniczak E.T. Structure of the N-terminal RNA-binding domain of the SARS CoV nucleocapsid protein. Biochemistry. 2004;43:6059–6063. doi: 10.1021/bi036155b. [DOI] [PubMed] [Google Scholar]
- Huang Y.H., Jiang D., H.J SARS-CoV-2 detected in cerebrospinal fluid by PCR in a case of COVID-19 encephalitis. Brain Behav. Immun. 2020 doi: 10.1016/j.bbi.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huarcaya-Victoria J., Herrera D., Castillo C. Psychosis in a patient with anxiety related to COVID-19: a case report. Psychiatry Res. 2020;289 doi: 10.1016/j.psychres.2020.113052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S., Yang M., Hong Z., Zhang L., Huang Z., Chen X., He S., Zhou Ziliang, Zhou Zhechong, Chen Q., Yan Y., Zhang C., Shan H., Chen S. Crystal structure of SARS-CoV-2 nucleocapsid protein RNA binding domain reveals potential unique drug targeting sites. Acta Pharm. Sin. B. 2020;10:1228–1238. doi: 10.1016/j.apsb.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.E., Heo J.H., Kim H.O., Song S.H., Park S.S., Park T.H., Ahn J.Y., Kim M.K., Choi J.P. Neurological complications during treatment of middle east respiratory syndrome. J. Clin. Neurol. 2017;13:227–233. doi: 10.3988/jcn.2017.13.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R.S., Garber C., Howard N. Infectious immunity in the central nervous system and brain function. Nat. Immunol. 2017;18:132–141. doi: 10.1038/ni.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam M.H., Wing Y.K., Yu M.W., Leung C.M., Ma R.C., Kong A.P., So W.Y., Fong S.Y., L.S. Mental Morbidities and Chronic Fatigue in Severe Acute Respiratory Syndrome Survivors. 2017;169:2142–2147. doi: 10.1001/archinternmed.2009.384. [DOI] [PubMed] [Google Scholar]
- Li Y.C., Bai W.Z., Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J. Med. Virol. 2020;92:552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Xiong W., Liu D., Liu J., Yang D., Li N., Mu J., Guo J., Li W., Wang G., Gao H., Zhang Y., Lin M., Chen L., Shen S., Zhang H., Sander J.W., Luo J., Chen S., Zhou D. New onset acute symptomatic seizure and risk factors in coronavirus disease 2019: a retrospective multicenter study. Epilepsia. 2020;61:e49–e53. doi: 10.1111/epi.16524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D., Miao X., Li Y., Hu B. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrosovich M., Herrler G K.H. Sialic acid receptors of viruses. Top Curr. Chem. 2015 doi: 10.1007/128_2013_466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi T., Harii N., Goto J., Harada D., Sugawara H., Takamino J., Ueno M., Sakata H., Kondo K., Myose N., Nakao A., Takeda M., Haro H., Inoue O., Suzuki-Inoue K., Kubokawa K., Ogihara S., Sasaki T., Kinouchi H., Kojin H., Ito M., Onishi H., Shimizu T., Sasaki Y., Enomoto N., Ishihara H., Furuya S., Yamamoto T., Shimada S. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudgal J., Nampoothiri M., Basu Mallik S., Kinra M., Hall S., Grant G., Anoopkumar-Dukie S., Rao C.M., Arora D. Possible involvement of metformin in downregulation of neuroinflammation and associated behavioural changes in mice. Inflammopharmacology. 2019;27:941–948. doi: 10.1007/s10787-019-00638-w. [DOI] [PubMed] [Google Scholar]
- Nakamura Z.M., Nash R.P., Laughon S.L., Rosenstein D.L. Neuropsychiatric complications of COVID-19. Curr. Psychiatry Rep. 2021:23. doi: 10.1007/s11920-021-01237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence (NICE) in collaboration with NHS England and NHS Improvement Managing COVID-19 symptoms (including at the end of life) in the community: summary of NICE guidelines. BMJ. 2020:369. doi: 10.1136/bmj.m1461. [DOI] [PubMed] [Google Scholar]
- Natoli S., Oliveira V., Calabresi P., Maia L.F., Pisani A. Does SARS-Cov-2 invade the brain? Translational lessons from animal models. Eur. J. Neurol. 2020;27:1764–1773. doi: 10.1111/ene.14277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham E.J., Chou S.H.Y., Coles A.J., Menon D.K. Neurological implications of COVID-19 infections. Neurocrit. Care. 2020;32:667–671. doi: 10.1007/s12028-020-00978-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netland J., Meyerholz D.K., Moore S., Cassell M., Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J. Virol. 2008;82:7264–7275. doi: 10.1128/jvi.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman B.W., Kiss G., Kunding A.H., Bhella D., Baksh M.F., Connelly S., Droese B., Klaus J.P., Makino S., Sawicki S.G., Siddell S.G., Stamou D.G., Wilson I.A., Kuhn P., Buchmeier M.J. A structural analysis of M protein in coronavirus assembly and morphology. J. Struct. Biol. 2011;174:11–22. doi: 10.1016/j.jsb.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng Kee Kwong K.C., Mehta P.R., Shukla G., Mehta A.R. COVID-19, SARS and MERS: a neurological perspective. J. Clin. Neurosci. 2020;77:13–16. doi: 10.1016/j.jocn.2020.04.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okusaga O., Yolken R.H., Langenberg P., Lapidus M., Arling T.A., Dickerson F.B., Scrandis D.A., Severance E., Cabassa J.A., Balis T., Postolache T.T. Association of seropositivity for influenza and coronaviruses with history of mood disorders and suicide attempts. J. Affect. Disord. 2011;130:220–225. doi: 10.1016/j.jad.2010.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliviero A., de Castro F., Coperchini F., Chiovato L., Rotondi M. COVID-19 pulmonary and olfactory dysfunctions: is the chemokine CXCL10 the common denominator? Neuroscientist. 2021;27:214–221. doi: 10.1177/1073858420939033. [DOI] [PubMed] [Google Scholar]
- Pal M., Berhanu G., Desalegn C., Kandi V. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): an update. Cureus. 2020 doi: 10.7759/cureus.7423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H., Wang H., Wang X., Zhu L., Mao L. The absence of Nrf2 enhances NF-κB-dependent inflammation following scratch injury in mouse primary cultured astrocytes. Mediators Inflamm. 2012:2012. doi: 10.1155/2012/217580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panariello A., Bassetti R., Radice A., Rossotti R., Puoti M., Corradin M., Moreno M., Percudani M. Anti-NMDA receptor encephalitis in a psychiatric Covid-19 patient: a case report. Brain. Behav. Immun. 2020;87:179–181. doi: 10.1016/j.bbi.2020.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panciani P.P., Saraceno G., Zanin L., Renisi G., Signorini L., Battaglia L., Fontanella M.M. SARS-CoV-2: “Three-steps” infection model and CSF diagnostic implication. Brain. Behav. Immun. 2020;87:128–129. doi: 10.1016/j.bbi.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson R.W., Brown R.L., Benjamin L., Nortley R., Wiethoff S., Bharucha T., Jayaseelan D.L., Kumar G., Raftopoulos R.E., Zambreanu L., Vivekanandam V., Khoo A., Geraldes R., Chinthapalli K., Boyd E., Tuzlali H., Price G., Christofi G., Morrow J., McNamara P., McLoughlin B., Lim S.T., Mehta P.R., Levee V., Keddie S., Yong W., Trip S.A., Foulkes A.J.M., Hotton G., Miller T.D., Everitt A.D., Carswell C., Davies N.W.S., Yoong M., Attwell D., Sreedharan J., Silber E., Schott J.M., Chandratheva A., Perry R.J., Simister R., Checkley A., Longley N., Farmer S.F., Carletti F., Houlihan C., Thom M., Lunn M.P., Spillane J., Howard R., Vincent A., Werring D.J., Hoskote C., Jäger H.R., Manji H., Zandi M.S. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143:3104–3120. doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman S., Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat. Rev. Microbiol. 2009;7:439–450. doi: 10.1038/nrmicro2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyiadji N., Shahin G., Noujaim D., Stone M., Patel S., Griffith B. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: imaging features. Radiology. 2020;296:E119–E120. doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadan N., Shaib H. Middle east respiratory syndrome coronavirus (MERS-COV): a review. Germs. 2019;9:35–42. doi: 10.18683/germs.2019.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rethinavel H.S., Ravichandran S., Radhakrishnan R.K., Kandasamy M. COVID-19 and Parkinson’s disease: Defects in neurogenesis as the potential cause of olfactory system impairments and anosmia. J. Chem. Neuroanat. 2021:115. doi: 10.1016/j.jchemneu.2021.101965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riel D.Van, Verdijk R., Kuiken T. The olfactory nerve: a shortcut for influenza and other viral diseases into the central nervous system. J. Pathol. 2015;235:277–287. doi: 10.1002/path.4461. [DOI] [PubMed] [Google Scholar]
- Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapra H., Singhal V. Managing meningoencephalitis in indian icu. Indian J. Crit. Care Med. 2019;23:S124–S128. doi: 10.5005/jp-journals-10071-23189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satarker S., Nampoothiri M. Structural proteins in severe acute respiratory syndrome coronavirus-2. Arch. Med. Res. 2020;51:482–491. doi: 10.1016/j.arcmed.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satarker S., Nampoothiri M. Involvement of the nervous system in COVID-19: The bell should toll in the brain. Life Sci. 2020;262 doi: 10.1016/j.lfs.2020.118568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scala R., Heunks L. Highlights in acute respiratory failure. Eur. Respir. Rev. 2018;27:8–11. doi: 10.1183/16000617.0008-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance E.G., Dickerson F.B., Viscidi R.P., Bossis I., Stallings C.R., Origoni A.E., Sullens A., Yolken R.H. Coronavirus immunoreactivity in individuals with a recent onset of psychotic symptoms. Schizophr. Bull. 2011;37:101–107. doi: 10.1093/schbul/sbp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabani Z. Demyelination as a result of an immune response in patients with COVID-19. Acta Neurol. Belg. 2021;121:859–866. doi: 10.1007/s13760-021-01691-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh E., Devasahayam G. Neurodegeneration by oxidative stress: a review on prospective use of small molecules for neuroprotection. Mol. Biol. Rep. 2020;47:3133–3140. doi: 10.1007/s11033-020-05354-1. [DOI] [PubMed] [Google Scholar]
- Snijder E.J., van der Meer Y., Zevenhoven-Dobbe J., Onderwater J.J.M., van der Meulen J., Koerten H.K., Mommaas A.M. Ultrastructure and origin of membrane vesicles associated with the severe acute respiratory syndrome coronavirus replication complex. J. Virol. 2006;80:5927–5940. doi: 10.1128/jvi.02501-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafstrom CE C.L. Seizures and epilepsy: an overview for neuroscientists. Cold Spring Harb Perspect Med. 2015 doi: 10.1101/cshperspect.a022426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stodola J.K., Dubois G., Le Coupanec A., Desforges M., Talbot P.J. The OC43 human coronavirus envelope protein is critical for infectious virus production and propagation in neuronal cells and is a determinant of neurovirulence and CNS pathology. Virology. 2018;515:134–149. doi: 10.1016/j.virol.2017.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surjit M., Lal S.K. The SARS-CoV nucleocapsid protein: a protein with multifarious activities. Infect. Genet. Evol. 2008;8:397–405. doi: 10.1016/j.meegid.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson P.A., McGavern D.B. Viral diseases of the central nervous system. Curr. Opin. Virol. 2015;11:44–54. doi: 10.1016/j.coviro.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassorelli C., Mojoli F., Baldanti F., Bruno R., Benazzo M. COVID-19: what if the brain had a role in causing the deaths? Eur. J. Neurol. 2020;27:e41–e42. doi: 10.1111/ene.14275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toljan K. Vol. 11. 2020. Letter to the Editor Regarding the Viewpoint “evidence of the COVID-19 Virus Targeting the CNS: Tissue Distribution, Host-Virus Interaction, and Proposed Neurotropic Mechanism.”; pp. 1192–1194. (ACS Chem. Neurosci). [DOI] [PubMed] [Google Scholar]
- Troyer E.A., Kohn J.N., Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain. Behav. Immun. 2020;87:34–39. doi: 10.1016/j.bbi.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda N., Umetsu R., Abe J., Kato Y., Nakayama Y., Kato Z., Kinosada Y., Nakamur M. Analysis of neuropsychiatric adverse events in patients treated with oseltamivir in spontaneous adverse event reports. Biol. Pharm. Bull. 2015;38:1638–1644. doi: 10.1248/bpb.b15-00253. [DOI] [PubMed] [Google Scholar]
- Valdés-Florido M.J., López-Díaz Á., Palermo-Zeballos F.J., Martínez-Molina I., Martín-Gil V.E., Crespo-Facorro B., Ruiz-Veguilla M. Reactive psychoses in the context of the COVID-19 pandemic: Clinical perspectives from a case series. Rev. Psiquiatr. Salud Ment. 2020;13:90–94. doi: 10.1016/j.rpsm.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velavan T.P., Meyer C.G. The COVID-19 epidemic. Trop. Med. Int. Heal. 2020;25:278–280. doi: 10.1111/tmi.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.Y., Li X.L., Yan Z.R., Sun X.P., Han J., Zhang B.W. Potential neurological symptoms of COVID-19. Ther. Adv. Neurol. Disord. 2020;13:1–2. doi: 10.1177/1756286420917830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Liu L. The membrane protein of severe acute respiratory syndrome coronavirus functions as a novel cytosolic pathogen-associated molecular pattern to promote beta interferon induction via a toll-like-receptor-related TRAF3-independent mechanism. MBio. 2016;7:1–14. doi: 10.1128/mBio.01872-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welcome M.O., Mastorakis N.E. Neuropathophysiology of coronavirus disease 2019: neuroinflammation and blood brain barrier disruption are critical pathophysiological processes that contribute to the clinical symptoms of SARS-CoV-2 infection. Inflammopharmacology. 2021:2. doi: 10.1007/s10787-021-00806-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . Vol. 43. 2021. COVID-19 Weekly Epidemiological; pp. 1–29. (World Heal. Organ). [Google Scholar]
- Willison H.J., Jacobs B.C., van Doorn P.A. Guillain-Barré syndrome. Lancet. 2016;388:717–727. doi: 10.1016/S0140-6736(16)00339-1. [DOI] [PubMed] [Google Scholar]
- Wong A.C.P., Li X., Lau S.K.P., Woo P.C.Y. Global epidemiology of bat coronaviruses. Viruses. 2019:11. doi: 10.3390/v11020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P.F., Craik S., Newman P., Makan A., Srinivasan K., Crawford E., Dev D., Moudgil H., Ahmad N. Lessons of the month 1: a case of rhombencephalitis as a rare complication of acute COVID-19 infection. Clin. Med. J. R. Coll. Physicians London. 2020;20:293–294. doi: 10.7861/clinmed.2020-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., Graham B.S., M.J Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020 doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G., Hu Y., Tao Z.W., Tian J.H., Pei Y.Y., Yuan M.L., Zhang Y.L., Dai F.H., Liu Y., Wang Q.M., Zheng J.J., Xu L., Holmes E.C., Zhang Y.Z. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, H.Y., Brian, D.A., 2010. Subgenomic messenger RNA amplification in coronaviruses. Proc. Natl. Acad. Sci. U. S. A. 107, 12257–12262. 10.1073/pnas.1000378107. [DOI] [PMC free article] [PubMed]
- Wu Y., Xu X., Chen Z., Duan J., Hashimoto K., Yang L., Liu C., Yang C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain. Behav. Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China. Jama. 2020;323:1239. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Xu H., Zhong L., Deng J., Peng J., Dan H., Zeng X., Li T., Chen Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020;12:1–5. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Lazartigues E. Expression of ACE2 in human neurons supports the neuro-invasive potential of COVID-19 virus. Cell. Mol. Neurobiol. 2020 doi: 10.1007/s10571-020-00915-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita, T., Wu, Y.P., Sandhoff, R., Werth, N., Mizukami, H., Ellis, J.M., Dupreell, J.L., Geyer, R., Sandhoff, K., Proia, R.L., 2005. Interruption of ganglioside synthesis produces central nervous system degeneration and altered axon-glial interactions. Proc. Natl. Acad. Sci. U. S. A. 102, 2725–2730. 10.1073/pnas.0407785102. [DOI] [PMC free article] [PubMed]
- Yan R., Yang Y., Zhang P., Zhong W., Wang Y., Wang Q., Xu Y., Li M., Li X., Zheng M., Chen L., Li H., Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., Graham B.S., McLellan J.S., Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q., Auerbach A., Brenk R., Schipani A., James D., Krasowski A., Gilbert I.H., Frearson J., Wyatt P.G., Shang J., Ye G., Shi K., Wan Y., Luo C., Aihara H., Geng Q., Auerbach A., Li F., Masaud Shah Bilal Ahmad, Sangdun Choi H.G.W., Capuzzi S.J., Muratov E.N., Tropsha A., Utomo R.Y., Ikawati M., Meiyanto E., Khaerunnisa S., Kurniawan H., Awaluddin R., Suhartati S., Global A., Global A., Global A., Global A. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;80(3):1–8. doi: 10.1126/science.abb2762. (-.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Yan, Xiao M.D.Meng, Zhang M.Sc. Shulan, Peng Xia M.D., Wei Cao M.D., Wei Jiang M.D., Huan Chen M.D., Xin Ding M.D., Hua Zhao M.D., Hongmin Zhang M.D., M.D C or r e sp ondence coagulopathy and antiphospholipid antibodies in patients with covid-19. Nejm. 2020;38:1–3. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Petitjean S.J.L., Koehler M., Zhang Q., Dumitru A.C., Chen W., Derclaye S., Vincent S.P., Soumillion P., Alsteens D. Molecular interaction and inhibition of SARS-CoV-2 binding to the ACE2 receptor. Nat. Commun. 2020:11. doi: 10.1038/s41467-020-18319-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M., Ren Y., Lv T. Encephalitis as a clinical manifestation of COVID-19. Brain. Behav. Immun. 2020;88:945–946. doi: 10.1016/j.bbi.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y., Hogue B.G. Role of the coronavirus e viroporin protein transmembrane domain in virus assembly. J. Virol. 2007;81:3597–3607. doi: 10.1128/jvi.01472-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin R., Feng W., Wang T., Chen G., Wu T., Chen D., Lv T., Xiang D. Concomitant neurological symptoms observed in a patient diagnosed with coronavirus disease 2019. J. Med. Virol. 2020;92:1782–1784. doi: 10.1002/jmv.25888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B., Li X., Chen J., Ouyang M., Zhang H., Zhao X., Tang L., Luo Q., Xu M., Yang L., Huang G., Liu X., Tang J. Evaluation of variation in D-dimer levels among COVID-19 and bacterial pneumonia: a retrospective analysis. J. Thromb. Thrombolysis. 2020;50:548–557. doi: 10.1007/s11239-020-02171-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanin L., Saraceno G., Panciani P.P., Renisi G., Signorini L., Migliorati K., Fontanella M.M. SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir. 2020;162:1491–1494. doi: 10.1007/s00701-020-04374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zazhytska M., Kodra A., Hoagland D.A., Frere J., Fullard J.F., Shayya H., McArthur N.G., Moeller R., Uhl S., Omer A.D., Gottesman M.E., Firestein S., Gong Q., Canoll P.D., Goldman J.E., Roussos P., tenOever B.R., Jonathan B.O., Lomvardas S. Non-cell-autonomous disruption of nuclear architecture as a potential cause of COVID-19-induced anosmia. Cell. 2022;185(1052–1064) doi: 10.1016/j.cell.2022.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B.Z., Chu H., Han S., Shuai H., Deng J., Hu Y. fan, Gong H. rui, Lee A.C.Y., Zou Z., Yau T., Wu W., Hung I.F.N., Chan J.F.W., Yuen K.Y., Huang J.D. Vol. 30. 2020. SARS-CoV-2 infects human neural progenitor cells and brain organoids; pp. 928–931. (Cell Res.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Song Y., Shan B., He M., Ren Q., Zeng Y., Liu Z., Liu H., Xu J. Elevated level of D-dimer increases the risk of stroke. Oncotarget. 2018;9:2208–2219. doi: 10.18632/oncotarget.23367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Zhou X., Liu H., Hashimoto K. Treatment concerns for psychiatric symptoms in patients with COVID-19 with or without psychiatric disorders. Br. J. Psychiatry. 2020;217:351. doi: 10.1192/bjp.2020.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X., Lou, Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R., Di, Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Kang H., Li S., Zhao X. Understanding the neurotropic characteristics of SARS-CoV-2: from neurological manifestations of COVID-19 to potential neurotropic mechanisms. J. Neurol. 2020;267:2179–2184. doi: 10.1007/s00415-020-09929-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z., Lian X., Su X., Wu W., Marraro G.A., Zeng Y. From SARS and MERS to COVID-19: a brief summary and comparison of severe acute respiratory infections caused by three highly pathogenic human coronaviruses. Respir. Res. 2020:21. doi: 10.1186/s12931-020-01479-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccal D.B., Furuya W.I., Bassi M., Colombari D.S.A., Colombari E. The nucleus of the solitary tract and the coordination of respiratory and sympathetic activities. Front. Physiol. 2014;5:1–12. doi: 10.3389/fphys.2014.00238. (JUN) [DOI] [PMC free article] [PubMed] [Google Scholar]