Abstract
Objectives
Our aim in this study was to compare rates of anthropometric, blood pressure (BP) and glycated hemoglobin (A1C) measurements and laboratory screening for hypothyroidism, nephropathy and dyslipidemia in children and youth with type 1 diabetes (T1D), 1 year before and after the onset of COVID-19.
Methods
Clinical data were analyzed from a voluntary registry of children and youth with T1D followed at the BC Children’s Hospital between March 2019 and 2021. Logistic and Poisson mixed-effect models were used.
Results
Four hundred forty patients with a median (interquartile range) age and time since diagnosis of 12.7 (9.5 to 15.4) and 4.7 (2.6 to 7.9) years, respectively, were included. Clinic visits were all in-person before March 2020, and 99% via telemedicine afterward. The median number of visits per patient was 2 (interquartile range, 2 to 3), with a 6% increase during the pandemic (relative risk [RR], 1.06; 95% confidence interval [CI], 1.01 to 1.10). There was a substantial decrease in height, weight and BP measurements (RR, 0.32; 95% CI, 0.28 to 0.36; RR, 0.34, 95% CI, 0.31 to 0.38; RR, 0.005, 95% CI, 0.002 to 0.014, respectively); only 49% of patients had anthropometric data and 1% had BP data during the pandemic year, compared with >97% before the pandemic. A1C measurements dropped from 3 (interquartile range, 2 to 4) to 1 (interquartile range, 1 to 2) per patient per year (RR, 0.53; 95% CI, 0.48 to 0.57). Rates of screening investigations were suboptimal before the pandemic, and these rates continued to decline.
Conclusions
Shifting to telemedicine allowed ongoing care during the pandemic, but the frequency of anthropometric, BP and A1C measurements decreased dramatically. A combined telemedicine/in-person model may be needed to ensure adequate care for this population.
Keywords: children, COVID-19, telemedicine, type 1 diabetes
Résumé
Objectifs
L’objectif de notre étude était de comparer le nombre de mesures anthropométriques, de la pression artérielle (PA) et de l’hémoglobine glyquée (A1c) au taux de dépistage en laboratoire de l’hypothyroïdisme, de la néphropathie et de la dyslipidémie chez les enfants et les jeunes atteints du diabète de type 1 (DT1) 1 an avant et après l’apparition de la COVID-19.
Méthodes
Nous avons analysé les données cliniques d’un registre volontaire d’enfants et jeunes atteints du DT1 suivis au BC Children’s Hospital entre mars 2019 et 2021. Nous avons utilisé des modèles mixtes de régression logistique et de régression de Poisson.
Résultats
Nous avons sélectionné 440 patients, dont l’âge médian (écart interquartile) et la durée depuis le diagnostic étaient respectivement de 12,7 (de 9,5 à 15,4) et de 4,7 (de 2,6 à 7,9) ans. Avant mars 2020, les consultations cliniques avaient lieu en personne. Par la suite, 99 % des consultations se sont déroulées en télémédecine. Le nombre de consultations par patient était de 2 (de 2 à 3), avec une augmentation de 6 % durant la pandémie (risque relatif [RR], 1,06; intervalle de confiance [IC] à 95 %, de 1,01 à 1,10). Il y a eu une diminution substantielle des mesures de la taille, du poids et de la pression artérielle (RR, 0,32; IC à 95 %, de 0,28 à 0,36; RR, 0,34, IC à 95 %, de 0,31 à 0,38; RR, 0,005, IC à 95 %, de 0,002 à 0,014, respectivement); seuls 49 % des patients ont eu des données anthropométriques, et seul 1 % ont eu des données sur la pression artérielle, durant l’année de la pandémie, contre > 97 % avant la pandémie. Les mesures de l’A1c sont passées de 3 (de 2 à 4) à 1 (1 à 2) par patient par année (RR, 0,53; IC à 95 %, de 0,48 à 0,57). Les taux d’examens de dépistage qui étaient sous-optimaux avant la pandémie n’ont pas cessé de diminuer.
Conclusions
La transition vers la télémédecine a permis de poursuivre les soins durant la pandémie, mais la fréquence des mesures anthropométriques, de la pression artérielle et de l’A1c a considérablement diminué. Un modèle combiné de consultations en personne et en télémédecine peut s’avérer nécessaire pour que cette population reçoive des soins adéquats.
Mots clés: enfants, COVID-19, télémédecine, diabète de type 1
Key Messages.
-
•
An unprecedented shift to telemedicine allowed continued provision of care to children with type 1 diabetes during the COVID-19 pandemic.
-
•
This study shows a dramatic drop in growth, blood pressure and glycated hemoglobin measurements 1 year into the pandemic.
-
•
There are still many challenges with telemedicine for pediatric diabetes care. Hybrid care models and innovative solutions are needed.
Introduction
Delivery of care for patients with different chronic conditions has undergone a dramatic change because of the COVID-19 pandemic (1,2). Particularly during the early stages of this pandemic, there has been a substantial decrease in the use of in-person outpatient health-care services (3,4). In this setting, telemedicine, defined as the use of electronic information and communication technologies to provide health care when distance separates the participants (5), has been an incredibly valuable tool (6,7).
During this challenging time, the feasibility and effectiveness of telemedicine have been documented in the management of hospitalized patients with type 1 diabetes (T1D) and the provision of diabetes education (8,9). Furthermore, there is evidence supporting the efficacy of telemedicine interventions for outpatient diabetes care (10, 11, 12). There have been discrepant findings regarding glycated hemoglobin (A1C) trends in children and youth with T1D during the lockdown due to the pandemic, with some studies showing increased levels and others reporting no significant changes (13, 14, 15, 16). However, it has been proposed that telemedicine and diabetes technology have been key to maintaining or even improving glycemic management (16). This model is now standard practice in many centres worldwide, and it seems likely that it will become a permanent component of diabetes care (17,18).
Despite the benefits of telemedicine, some aspects of in-person visits cannot be replaced easily. As per current standards of care, children and youth living with T1D should have regular physical exams, with height, weight and blood pressure (BP) measurements, and laboratory investigations including A1C, albumin-to-creatinine ratio (ACR), thyrotropin (TSH) and lipid profile (19, 20, 21, 22). Adhering to these recommendations may be challenging with telemedicine, especially in the current context of the pandemic (23). A large national laboratory in the United States reported a marked decline in A1C test volumes during March and April 2020 (24). Also, a recent study in Latin America showed that children with T1D had fewer A1C measurements in 2020 compared with 2019 and 2018 (25). To our knowledge, there are no similar studies among children and youth living with diabetes in North America.
Early in the pandemic, in accordance with provincial recommendations, the diabetes clinic at the BC Children’s Hospital (BCCH) underwent a rapid shift for all routine visits from in person to telemedicine. In this context, we set out to evaluate the comprehensiveness of care of children and youth with T1D by comparing the rates of measurement of height, weight, BP, A1C and laboratory screening for hypothyroidism, dyslipidemia and nephropathy, 1 year before and after the onset of the COVID-19 pandemic.
Methods
Setting
The BCCH is the only pediatric tertiary care hospital in British Columbia (BC), Canada. The diabetes clinic at the BCCH serves approximately 700 (of the >2,000) children and youth living with diabetes from across the province (26). Before the COVID-19 pandemic, most patients were seen, on average, 2 times per year, in person, by a multidisciplinary diabetes team (i.e. doctor, nurse, dietitian and social worker). During this time, only a small fraction of patients living in rural/remote areas or far away from BCCH had telemedicine visits, in which both local care providers and the BCCH diabetes clinic team participated. In-person visits were scheduled for approximately 90 minutes and included a physical exam, anthropometric and BP measurements and a point-of-care A1C measurement. Families were advised to have A1C measurements in between clinic visits if the visits were >3 months apart. They also received requisitions for laboratory screening investigations: TSH levels yearly, lipid profile (total cholesterol, low-density lipoprotein, high-density lipoprotein and triglycerides) at age 10 and 18 years or before transition to adult care, and ACR yearly, starting 5 years after the initial diagnosis in youth ≥12 years of age. These indications for screening were agreed upon by a multidisciplinary team, based on international clinical practice guidelines (19, 20, 21, 22, 23). Investigations could be done at the BCCH laboratory immediately after a clinic visit or later at a community-based laboratory.
With the onset of the COVID-19 pandemic, all routine diabetes clinic visits were shifted to telemedicine. Visits were conducted using video-conferencing platforms (Skype for Business or Zoom) or, alternatively, by phone. Patients and caregivers received instructions to perform height and weight measurements at home, and BP measurements were not requested. Orders for laboratory investigations (A1C and other screening tests, when indicated) were sent via postal mail, and families were responsible for booking outpatient appointments at their closest laboratory. To ensure all patients could be seen, telemedicine visits were scheduled for 30 minutes and only with a physician. A nurse educator, social worker and/or dietitian would join these visits upon request. In many cases, patients themselves did not participate in the visits, but rather only their parents or caregivers (27). In-person visits were arranged on a case-by-case basis, when a physical exam was considered urgent or in-person education was required.
Study design
This was a retrospective review of data from the BC Pediatric Diabetes Registry (BC-PDR). The BC-PDR is a voluntary registry, which collects de-identified clinical data of patients with diabetes <19 years of age, to support quality improvement research. By January 2021, 590 of 682 potential participants at the BCCH Diabetes Clinic (86.5%) had provided consent to be part of the BC-PDR (28).
The BC-PDR includes patient-level and visit-level information. Most data are recorded on a paper medical chart by the physician during clinic visits and transcribed into the electronic database by a research assistant. In addition, results from laboratory tests are extracted from the electronic health records and manually entered into the database. Comprehensive training of research assistants, standardization of data collection, data validation and automatic data checks in the database have been implemented to optimize data quality (28).
Children and youth with T1D followed at the BCCH, aged 0 to 19 years, whose families consented to be part of the BC-PDR, and who had at least 1 clinic visit between March 19, 2019, and March 18, 2021, were included in this study. The following were considered exclusion criteria: i) having had a telemedicine visit before March 18, 2020; ii) having been diagnosed with T1D during the study period; and iii) being ≥18 years old by March 19, 2020, with no clinic visits after this date, indicating transition to adult care before the onset of COVID-19.
Exposure
We defined 2 study periods: study period 1, or “pre-COVID pandemic,” from March 19, 2019 until March 18, 2020; and study period 2, or “during the COVID pandemic,” from March 19, 2020, to March 18, 2021.
Outcomes
The following outcomes were considered: height, weight and BP measurements; A1C; TSH; ACR; and lipid profile (total cholesterol, high-density lipoprotein and triglycerides) testing. These were analyzed by patient and by visit, as count (number of measurements/tests) or as binary variables (performed: yes/no), for each study period.
Indications for screening investigations were defined according to our clinic’s usual procedures, considering the patient’s age at the beginning of each study period ±6 months. It is important to note that heights and weights during the pandemic were predominantly measured at home by a caregiver and self-reported. Because of studies suggesting that A1C levels could be estimated from mean glucose levels using continuous glucose monitoring (29), sensor use at each clinic visit (yes/no) was assessed and was included for adjustment in our models.
Data analysis
Descriptive statistics are presented as frequency and percentage for nominal variables, and median and interquartile range (IQR) for continuous variables. Regarding outcomes analyzed per patient, logistic and Poisson mixed-effect models were used to evaluate the adjusted associations between study periods and binary and count outcomes, respectively. Results are expressed as odds ratio (OR) and rate ratio (RR) with 95% confidence interval (CI) and as adjusted figures (conditional rates/possibilities of outcomes at most common covariate values). Patients’ characteristics (age, sex, time since diagnosis of T1D, last laboratory-measured A1C value before COVID-19 onset, pump use and sensor use) were included in the models for adjustment. Age, time since diagnosis and last A1C value before COVID-19 onset were centred to the means. Patients were categorized as pump and/or sensor users, for each study period, if they reported using these technologies at ≥50% of their visits.
For outcomes analyzed by visit, interrupted time series (ITS) logistic mixed-effect models were further applied to examine the adjusted associations between study periods and the presence of screening investigations over time. ITS is a quasi-experimental design that quantifies the effect of an exposure or policy change implemented at a specific time point (30,31). We fit a segmented regression model including time (months since the beginning of the study) and interaction between time and period (before/during pandemic) to assess the possible immediate and lagged effects of the pandemic. Models were further adjusted for patients’ characteristics. Results were summarized as marginal predicted effects and displayed graphically (i.e. adjusted figures demonstrating conditional possibilities of outcomes over time at most common covariate values). ORs for the level change and during pandemic trend were also estimated.
In addition, a subgroup analysis with a logistic mixed-effect model was conducted only among data from period 2 to determine whether any patients’ characteristics at the start of this period were associated with per-visit outcomes during the pandemic. For the latter analysis, lipid profile was not included, as patients would be expected to have this laboratory investigation done only once in the whole study period.
Statistical significance was set at p<0.05. Because of the potential for type I error due to multiple comparisons, findings should be interpreted as exploratory. Data were analyzed using SAS version 9.4 (SAS Institute, Cary, North Carolina, United States).
Ethics approval for the BC-PDR was obtained from the Children & Women’s Research Ethics Board at the University of British Columbia.
Results
A total of 440 patients were included in our investigation. Patients’ characteristics are summarized in Table 1 . Over the 2 study periods, 1,791 clinic visits were analyzed: all visits in period 1 were in person (n=910), whereas 874 of 881 (99%) in period 2 were telemedicine visits.
Table 1.
Baseline characteristics of patients (N=440) ∗
| Characteristics | Value |
|---|---|
| Gender male, n (%) | 236 (54) |
| Age, median (IQR) | 12.7 (9.47–15.42) |
| Time since diagnosis, median (IQR) | 4.7 (2.55–7.88) |
| A1C, median of patient medians † (IQR) | 7.9 (7.2–8.7) |
| Pump use †, n (%) | 200 (46) |
| Sensor use †, n (%) | 196 (45) |
A1C, glycated hemoglobin; IQR, interquartile range.
Total=393 patients with visits in both study periods, with 41 and 6 patients seen only in period 1 and period 2, respectively.
During study period 1.
Per-patient analysis
The median number of clinic visits per patient was 2 (IQR, 2 to 3) in both periods. After adjustment for patients’ characteristics, there was a 6% increase in the number of visits per patient in period 2, compared with period 1 (RR, 1.06; 95% CI, 1.01 to 1.10; p=0.01).
During period 1, 100% of children and youth included in this study had their height and weight assessed, and 97% had their BP measured at least once. In period 2, the percentage of patients with height and weight data decreased to 53% and 49%, respectively, and only 1% had BP measurements. The median number of measurements per patient in both periods and the corresponding RRs are presented in Table 2 .
Table 2.
Anthropometric, BP and A1C measurements per patient
| Number of measurements, median (IQR) |
||||
|---|---|---|---|---|
| Pre-COVID onset | During pandemic | Unadjusted RR (95% CI), p value | Adjusted RR (95% CI), p value ∗ | |
| Height | 2 (2–3) | 1 (0–1) | 0.32 (0.29–0.36), <0.0001 | 0.32 (0.28–0.36), <0.0001 |
| Weight | 2 (2–3) | 1 (0–1) | 0.35 (0.32–0.39), <0.0001 | 0.34 (0.31–0.38), <0.0001 |
| BP | 2 (2–2) | 0 (0) | 0.005 (0.002–0.013), <0.0001 | 0.005 (0.002–0.014), <0.0001 |
| A1C | 3 (2–4) | 1 (1–2) | 0.52 (0.48–0.57), <0.0001 | 0.52 (0.48–0.57), <0.0001 |
A1C, glycated hemoglobin; BP, blood pressure; CI, confidence interval; IQR, interquartile range; RR, relative risk.
Estimated RR from Poisson model, after adjustment for age, sex, time since diagnosis, A1C before COVID onset, pump use and continuous glucose monitor use.
There was also a substantial decrease in the number of A1C measurements, from a median of 3 yearly per patient (IQR, 2 to 4) in period 1, to 1 yearly A1C (IQR, 1 to 2) in period 2. Before March 2020, 26% of patients had quarterly A1C measurements, in line with clinical practice recommendations (19, 20, 21, 22), and this proportion fell to 5% during the pandemic. Also, 22% of patients had no A1C measurements documented in period 2.
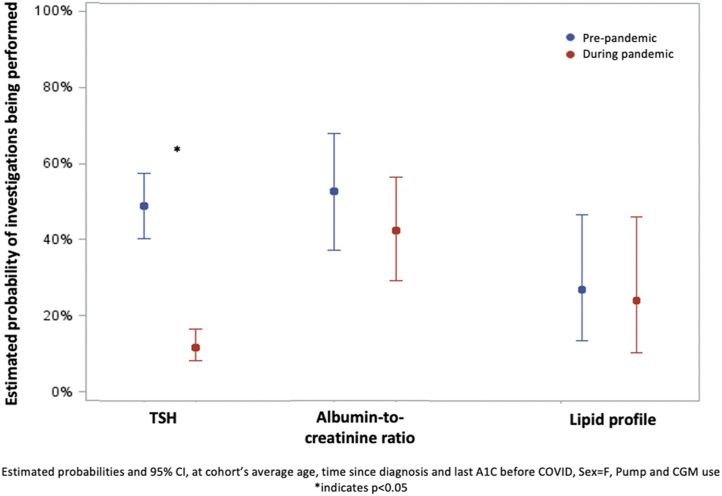
Figure 1 represents the probability of patients having laboratory screening investigations in both study periods. Screening rates for hypothyroidism dropped from 52% in period 1 to 13% in period 2 (OR, 0.14; 95% CI, 0.10 to 0.20; p<0.001). Among patients with indication (19, 20, 21, 22), 53% had screening for nephropathy in period 1, compared with 43% in period 2 (OR, 0.66; 95% CI, 0.40 to 1.01; p=0.10). For dyslipidemia, screening rates declined slightly from 33% to 29% of eligible patients over the 2 periods (OR, 0.87; 95% CI, 0.40 to 1.91; p=0.72).
Figure 1.
Laboratory screening for diabetes-related complications. Estimated rates and 95% confidence intervals (CIs), adjusted for age, sex, time since diagnosis, glycated hemoglobin before COVID onset, and pump and sensor use are shown. Albumin-to-creatinine ratio and lipid profile measurements were only considered among patients with indication for screening. ∗ p<0.05. A1C, glycated hemoglobin; TSH, thyrotropin.
Per-visit analysis
When outcomes were analyzed per visit, there was a similar drop in anthropometric, BP and A1C measurements. The proportion of clinic visits with height and weight data fell from 99% in study period 1 to 31% and 33%, respectively, in period 2. A BP measurement was documented in 93% of clinic visits in period 1 and in <1% (4 of 881) in period 2. An A1C result was recorded in 99% of visits in period 1, compared with 30% in period 2. Across all visits in period 2, 34% did not have a laboratory-measured A1C or documented use of a continuous glucose monitor.
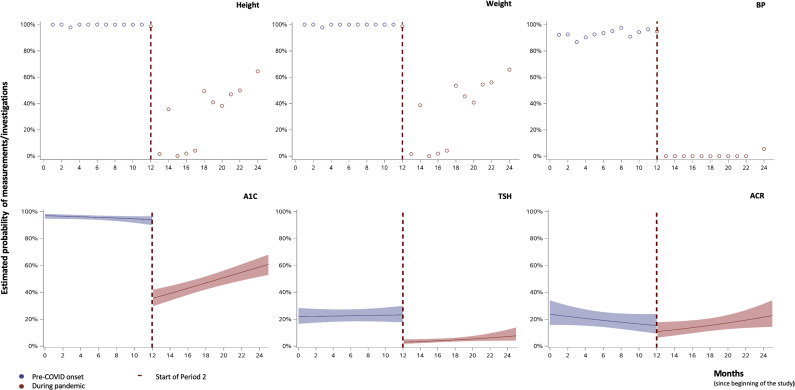
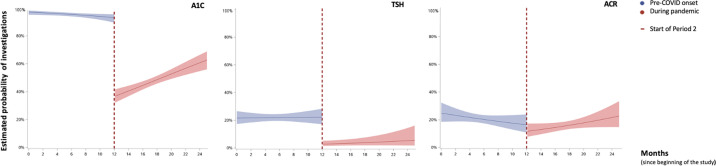
Figure 2 illustrates the change in the probability of having anthropometric measurements, A1C and screening investigations over time during the 2 study periods. The ITS models for weight, height and BP measurements were unable to be fit due to the complete or quasi-complete separation issue. However, as shown in the unadjusted figures, there was a drastic drop in measurements in period 2. After fitting the ITS logistic mixed-effect model, we observed an immediate level drop in period 2 for both A1C (OR, 0.04; 95% CI, 0.02 to 0.06; p<0.0001) and TSH (OR, 0.10; 95% CI, 0.05 to 0.18; p<0.0001) measurements. Supplementary Figure 1 represents the unadjusted models for laboratory investigations.
Figure 2.
Anthropometric, blood pressure (BP) and glycated hemoglobin (A1C) measurements and screening investigations over time. Estimated rates and 95% confidence intervals, adjusted for age, sex, time since diagnosis, A1C before COVID onset, pump and sensor use (for A1C, thyrotropin [TSH] and albumin-to-creatinine ratio [ACR] measurements). For weight, height and BP measurements, unadjusted data are plotted due to complete or quasi-complete separation between the 2 periods.
Period 2 subgroup analysis
When assessing the data during period 2 only, when other variables were held constant, later visit dates during the pandemic were associated with a higher odds of having self-reported growth measurements (height: OR, 1.28; 95% CI, 1.22 to 1.33; p<0.0001; weight: OR, 1.27; 95% CI, 1.20 to 1.34; p<0.0001; per 1-month increase in visit date). Likewise, later visits during the pandemic were associated with a higher odds of having an A1C measurement (OR, 1.08; 95% CI, 1.05 to 1.12; p<0.0001). There was a similar trend over time for hypothyroidism and nephropathy screening, although estimates had increased uncertainty (TSH: OR, 1.08; 95% CI, 1.00 to 1.17; p=0.06; ACR: OR, 0.67; 95% CI, 0.32 to 1.39; p=0.28).
Finally, in terms of the relationship between patients’ characteristics and outcomes during the pandemic, the likelihood of having height measurements was higher for boys (OR, 1.64; 95% CI, 1.12 to 2.39; p=0.01) and for younger children (OR, 0.93; 95% CI, 0.88 to 0.98; p=0.01, per 1-year increase in age). The proportion of patients using continuous glucose monitoring increased to 57% during this period (from 46% pre-pandemic), however, there was no association between the use of continuous glucose monitoring and the likelihood of having laboratory A1C measurements. None of the patients’ characteristics included in our models were associated with the likelihood of having weight, A1C, TSH or ACR measurements (Supplementary Table 1). BP measurements were not included in the analysis because of the small number available for period 2.
Discussion
As a result of the COVID-19 pandemic, routine diabetes care has been transformed to be predominantly delivered via telemedicine (32, 33, 34). In March 2020, the diabetes clinic at the BCCH underwent an unprecedented shift to telemedicine (27), as has been the case in many centres worldwide. In our study, we evaluated the comprehensiveness of care provided to children and youth with T1D in the only tertiary care centre in the Canadian province of BC, 1 year before and after the onset of the pandemic. We found a dramatic decline in the frequency of anthropometric, BP and A1C measurements as well as laboratory screening tests for diabetes-related comorbidities and complications during the pandemic.
Having continued to provide ambulatory care during the pandemic by telemedicine is undoubtedly positive. A recent study in children with T1D from 4 Latin American countries (N=227) showed that those with access to telemedicine had more medical visits in 2020 compared to those without access (25). We showed a slight increase in the number of clinic visits per patient during the pandemic, compared with the pre-pandemic year. This may be related to the advantages of telemedicine eliminating barriers to accessing care, such as cost, time required for travel, time off work or need for childcare (35,36). However, there was a proportion of patients in our study who did not have visits during the pandemic, and it is possible that some of them were lost to follow-up due to difficulties accessing or engaging with telemedicine. Unfortunately, our registry does not include data on cancelled or missed appointments, so the frequency of these events during the 2 study periods could not be compared. Also, there is no information on relocation or transfer to other health-care teams, which could explain this apparent loss to follow-up.
During the early stages of the pandemic, certain medical exams and interventions were safely deferred based on individual patient characteristics and risks. However, prolonged postponement of necessary evaluations could carry long-term detrimental implications for patients’ health and well-being, as shown in other populations (37). Thus, as the pandemic continues, the definition and frequency of “essential” medical exams will need further consideration.
Evaluating the height and weight of children and youth with T1D is key to providing optimal care (19, 20, 21, 22, 23). Inadequate growth can indicate suboptimal glycemic management (38,39) or comorbid conditions, such as hypothyroidism or celiac disease. Obesity and overweight are highly prevalent among children and youth with T1D (40), and these conditions are associated with increased risk of complications (41). Our study has shown that there was not enough information to assess the growth trajectory or body mass index of most patients during the pandemic, and a substantial proportion did not have any anthropometric data during the whole year. Also, even when home-measured heights and weights were performed, they could be significantly less accurate than those measured by a health-care provider in a clinic visit (42). A lack of growth data may have led to missed opportunities to identify patients with poor glycemic management, diagnose comorbid conditions or counsel on healthy lifestyle.
Similarly, hypertension is more prevalent in children and youth with T1D than in the general population (43). Early identification and appropriate treatment are critical to prevent the development of micro- and macrovascular complications (44,45). Nevertheless, families in our diabetes clinic were not routinely advised to have BP measurements in preparation for their telemedicine visits. Not surprisingly, only a handful of patients had documented BP levels during the pandemic year.
Laboratory investigations are another crucial component of diabetes care (19, 20, 21, 22, 23). A1C levels are a strong predictor of the risk of complications, and could serve as a tool to optimize management (46). Studies have shown beneficial effects of rapidly available A1C results (obtained by point-of-care testing) on glycemic management and patient satisfaction (46,47). Also, infrequent A1C monitoring is associated with worse glycemic outcomes (48,49). Moreover, whereas A1C could be estimated based on continuous glucose monitoring data (29), there may be significant discordance in a real-world setting, reinforcing the value of laboratory-measured A1C (50). At our clinic, we noted a dramatic decline in the frequency of A1C testing during the pandemic, as reported in other studies (24,25). Only a small fraction of patients had recent A1C results to review at their telemedicine visits, and a substantial proportion were not wearing a continuous glucose monitor. Furthermore, the likelihood of having an A1C measurement at a clinic visit was not affected by the use of a sensor. This would certainly make it challenging to assess long-term glycemic trends and provide appropriate treatment.
Clinical practice guidelines provide evidence-based recommendations to guide appropriate timing and testing for diabetes-related complications and comorbidities, aiming to standardize and improve delivery of care (51). Among adults with diabetes, adherence to these screening recommendations is associated with lower morbidity and mortality rates (52). In keeping with previous findings (53), the frequency of laboratory screening in our study population was already suboptimal pre-pandemic, when visits were in person. With the onset of the COVID-19 pandemic, laboratory testing was deferred and rates of screening continued to decline.
Our study supports consideration of a hybrid model of in-person and telemedicine visits to ensure appropriate anthropometric, BP and A1C measurements are completed. Early in the pandemic, families followed in our diabetes clinic had expressed their desire to continue having telemedicine visits in the future (27). Still, based on our results, it appears that the current model of almost all visits being done by telemedicine may not be appropriate, as standards of care have not been met (19, 20, 21, 22). Redesigning our model of pediatric diabetes care will require attention to raising awareness among patients, families and clinicians of the importance of a proportion of clinic visits being in person so that evidence-based, high-quality care can be achieved. Although we did not find specific patient characteristics that would predict the likelihood of having measurements and investigations during the pandemic, future research could help identify characteristics of families who would benefit most from continuing telemedicine exclusively vs those who will need an element of in-person care when this becomes possible.
Interestingly, children and youth with T1D in our study were more likely to have growth and A1C measurements available at telemedicine clinic visits that occurred later during the pandemic, which is not in concordance with the provincial trend in COVID-19 cases and restrictions (54). The reasons for this may include evolving views on the risk associated with attending health care, as well as changes in the perceived need for these evaluations by families and their providers, as time went by since the last in-person visit. Further studies assessing families’ perspectives on barriers to completing measurements and laboratory testing will be essential to create innovative, patient-centred solutions.
Future quality improvement initiatives should aim to address gaps in care found in this study. Seeking support from local primary care providers to perform in-person measurements and exams could be an effective solution. Continuous glucose monitoring systems may also be an option to improve the availability of information on glycemic trends during clinic visits, but this may not completely replace the need for laboratory-measured A1C (29,52). Furthermore, current pediatric diabetes guidelines are based on the best available evidence before the onset of the pandemic (19, 20, 21, 22, 23). Updated guidelines could consider including recommendations on the need for physical exams and investigations for children and youth with T1D in this rapidly evolving context.
Strengths of this study include the large number of patients and clinic visits, which represent the vast majority of children and youth followed in the only pediatric tertiary care centre in the province of BC. There are several limitations. First, despite the high proportion of patients enrolled in the BC-PDR, it is still possible that the use of data from a voluntary registry could introduce selection bias. Also, the relative impact of this unprecedented shift to telemedicine care cannot be differentiated from the effects of the pandemic. Thus, findings may be difficult to generalize and integrate into planning future care. Moreover, investigations for other diabetes-related comorbidities and complications (celiac disease, retinopathy and neuropathy) were not considered. This was due to variable recommendations in terms of frequency of screening, as well as the lack of good quality data for retinopathy and neuropathy screening in our registry (19, 20, 21, 22). Also, because of the low rates of laboratory testing during the pandemic, it was not possible to assess the change in A1C levels. Finally, we did not evaluate the change in endpoints such as rates of hospitalization, emergency department visits, severe hypoglycemia and diabetic ketoacidosis or incidence of diabetes-related complications. Nevertheless, we believe our work presents useful information by showing the decrease in recommended anthropometric measurements and laboratory investigations, which are intended to prevent such complications among patients with T1D. Longer term studies are needed to evaluate the impact of these changes on patient outcomes.
In conclusion, the rapid and unprecedented shift from in-person to telemedicine care allowed for ongoing delivery of care during the COVID pandemic. However, the care provided to children and youth in our diabetes clinic has not been in keeping with current recommendations regarding anthropometric measurements and laboratory investigations. The number of height, weight, BP and A1C measurements showed a dramatic decline during the pandemic year, and screening for important diabetes-related comorbidities and complications also showed a downward trend. This has likely led to missed opportunities for early intervention and may result in negative long-term health outcomes in this population. We noted a positive trend over time during the pandemic, with increased frequency of height, weight and A1C measurements at later visits during this period. Still, as it is, the current model of primarily telemedicine care does not appear to be suitable for most children and youth with T1D. Currently at more than 2 years into the global COVID-19 pandemic, it is essential that we engage in health system redesign that includes novel technology, leverages primary care and combines in-person and telemedicine modalities, so that children and youth living with T1D receive comprehensive, high-quality care.
Acknowledgments
This study was funded by a research grant from the British Columbia Children’s Hospital Research Institute.
Author Disclosures
Conflicts of interest: None.
Author Contributions
C.S. and S.A. contributed to the conception and study design, interpretation of data and analysis and writing the manuscript. Q.Z and J.N.B. contributed to the statistical analysis, interpretation of data and assisted with drafting the manuscript. All authors reviewed and revised the manuscript and provided approval of the final version submitted for publication.
Supplementary Material
To access the supplementary material accompanying this article, visit the online version of the Canadian Journal of Diabetes at www.canadianjournalofdiabetes.com.
Supplementary data
Supplementary Figure 1.
References
- 1.Rosenbaum L. The untold toll---the pandemic’s effects on patients without Covid-19. N Engl J Med. 2020;382:2368–2371. doi: 10.1056/NEJMms2009984. [DOI] [PubMed] [Google Scholar]
- 2.Danhieux K., Buffel V., Pairon A., et al. The impact of COVID-19 on chronic care according to providers: A qualitative study among primary care practices in Belgium. BMC Fam Pract. 2020;21:255. doi: 10.1186/s12875-020-01326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whaley C.M., Pera M.F., Cantor J., et al. Changes in health services use among commercially insured US populations during the COVID-19 pandemic. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.24984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glazier R.H., Green M.E., Wu F.C., Frymire E., Kopp A., Kiran T. Shifts in office and virtual primary care during the early COVID-19 pandemic in Ontario, Canada. CMAJ. 2021;193:E200–E210. doi: 10.1503/cmaj.202303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.US Institute of Medicine . In: Telemedicine: A Guide to Assessing Telecommunications in Health Care. Field M.J., editor. National Academies Press; Washington, DC: 1996. Committee on Evaluating Clinical Applications of Telemedicine.https://www.ncbi.nlm.nih.gov/books/nbk45440 [PubMed] [Google Scholar]
- 6.Bokolo A., Jr. Use of telemedicine and virtual care for remote treatment in response to COVID-19 pandemic. J Med Syst. 2020;44:132. doi: 10.1007/s10916-020-01596-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doraiswamy S., Abraham A., Mamtani R., Cheema S. Use of telehealth during the COVID-19 pandemic: Scoping review. J Med Internet Res. 2020;22 doi: 10.2196/24087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones M.S., Goley A.L., Alexander B.E., Keller S.B., Caldwell M.M., Buse J.B. Inpatient transition to virtual care during COVID-19 pandemic. Diabetes Technol Ther. 2020;22:444–448. doi: 10.1089/dia.2020.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garg S.K., Rodbard D., Hirsch I.B., Forlenza G.P. Managing new-onset type 1 diabetes during the COVID-19 pandemic: Challenges and opportunities. Diabetes Technol Ther. 2020;22:431–439. doi: 10.1089/dia.2020.0161. [DOI] [PubMed] [Google Scholar]
- 10.Pierce J.S., Gurnurkar S., Vyas N., Carakushansky M., Owens L., Patton S.R. Feasibility of implementing a pediatric diabetes clinic via telehealth. Diabetes Spectr. 2021;34:190–197. doi: 10.2337/ds20-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eberle C., Stichling S. Clinical improvements by telemedicine interventions managing type 1 and type 2 diabetes: Systematic meta-review. J Med Internet Res. 2021;23 doi: 10.2196/23244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Guzman K.R., Snoswell C.L., Taylor M.L., et al. A systematic review of pediatric telediabetes service models. Diabetes Technol Ther. 2020;22:623–628. doi: 10.1089/dia.2019.0489. [DOI] [PubMed] [Google Scholar]
- 13.Gayoso M., Lim W.Y., Mulekar M.S., Kaulfers A.D. Effect of Covid-19 quarantine on diabetes care in children. Clin Diabetes Endocrinol. 2021;7:9. doi: 10.1186/s40842-021-00122-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turan H., Güneş Kaya D., Tarçın G., Evliyaoğlu S.O. Effect of the COVID-19 quarantine on metabolic control in children and adolescents with type 1 diabetes. Endocrinol Diabetes Nutr (Engl Ed) 2021;8(69):201–208. doi: 10.1016/j.endinu.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nwosu B.U., Al-Halbouni L., Parajuli S., Jasmin G., Zitek-Morrison E., Barton B.A. COVID-19 pandemic and pediatric type 1 diabetes: No significant change in glycemic control during the pandemic lockdown of 2020. Front Endocrinol (Lausanne) 2021;12:703905. doi: 10.3389/fendo.2021.703905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Predieri B., Leo F., Candia F., et al. glycemic control improvement in italian children and adolescents with type 1 diabetes followed through telemedicine during lockdown due to the COVID-19 pandemic. Front Endocrinol (Lausanne) 2020;11:595735. doi: 10.3389/fendo.2020.595735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Regelmann M.O., Conroy R., Gourgari E., et al. Pediatric endocrinology in the time of COVID-19: Considerations for the rapid implementation of telemedicine and management of pediatric endocrine conditions. Horm Res Paediatr. 2020;93:343–350. doi: 10.1159/000513060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wittmeier K.D.M., Protudjer J.L.P., Wicklow B.A. Reflections on virtual care for chronic conditions during the COVID-19 pandemic. Can J Diabetes. 2021;45:1–2. doi: 10.1016/j.jcjd.2020.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wherrett D.K., Ho J., Huot C., Legault L., Nakhla M., Rosolowsky E. Diabetes Canada 2018 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada: Type 1 diabetes in children and adolescents. Can J Diabetes. 2018;42(Suppl):S234–S246. doi: 10.1016/j.jcjd.2017.10.036. [DOI] [PubMed] [Google Scholar]
- 20.American Diabetes Association Children and adolescents: Standards of medical care in diabetes---2021. Diabetes Care. 2021;44(Suppl):S180–S199. doi: 10.2337/dc21-S013. [DOI] [PubMed] [Google Scholar]
- 21.Mahmud F.H., Elbarbary N.S., Fröhlich-Reiterer E., et al. ISPAD Clinical Practice Consensus Guidelines 2018: Other complications and associated conditions in children and adolescents with type 1 diabetes. Pediatr Diabetes. 2018;19:275–276. doi: 10.1111/pedi.12740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donaghue K.C., Marcovecchio M.L., Wadwa R.P., et al. ISPAD Clinical Practice Consensus Guidelines 2018: Microvascular and macrovascular complications in children and adolescents. Pediatr Diabetes. 2018;19:262–274. doi: 10.1111/pedi.12742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.March C.A., Flint A., DeArment D., et al. Paediatric diabetes care during the COVID-19 pandemic: Lessons learned in scaling up telemedicine services. Endocrinol Diabetes Metab. 2020;21(4) doi: 10.1002/edm2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fragala M.S., Kaufman H.W., Meigs J.B., Niles J.K., McPhaul M.J. Consequences of the COVID-19 pandemic: Reduced hemoglobin A1c diabetes monitoring. Popul Health Manag. 2021;24:8–9. doi: 10.1089/pop.2020.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirschler V., Molinari C., Figueroa Sobrero A., et al. Influence of telemedicine on the number of visits and HbA1c determinations in Latin American children with type 1 diabetes. Diabetes Technol Ther. 2021;23:731–736. doi: 10.1089/dia.2021.0189. [DOI] [PubMed] [Google Scholar]
- 26.Fox D.A., Islam N., Sutherland J., Reimer K., Amed S. Type 1 diabetes incidence and prevalence trends in a cohort of Canadian children and youth. Pediatr Diabetes. 2018;19:501–505. doi: 10.1111/pedi.12566. [DOI] [PubMed] [Google Scholar]
- 27.Fung A., Irvine M., Ayub A., Ziabakhsh S., Amed S., Hursh B.E. Evaluation of telephone and virtual visits for routine pediatric diabetes care during the COVID-19 pandemic. J Clin Transl Endocrinol. 2020;22:100238. doi: 10.1016/j.jcte.2020.100238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ayub A., Ng C., Portales-Casamar E., Metzger D., Amed S. Towards building a provincial diabetes registry of children & youth living with diabetes in British Columbia, Canada. Can J Diabetes. 2022;46:346–352.e1. doi: 10.1016/j.jcjd.2021.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Bergenstal R.M., Beck R.W., Close K.L., et al. Glucose management indicator (GMI): A new term for estimating A1C from continuous glucose monitoring. Diabetes Care. 2018;41:2275–2280. doi: 10.2337/dc18-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kontopantelis E., Doran T., Springate D.A., Buchan I., Reeves D. Regression based quasi-experimental approach when randomisation is not an option: Interrupted time series analysis. BMJ. 2015;350:h2750. doi: 10.1136/bmj.h2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernal J.L., Cummins S., Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: A tutorial. Int J Epidemiol. 2017;46:348–355. doi: 10.1093/ije/dyw098. Erratum: Int J Epidemiol 2020;49:1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giani E., Dovc K., Dos Santos T.J., et al. Telemedicine and COVID-19 pandemic: The perfect storm to mark a change in diabetes care. Results from a world-wide cross-sectional web-based survey. Pediatr Diabetes. 2021;22:1115–1119. doi: 10.1111/pedi.13272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wake D.J., Gibb F.W., Kar P., et al. Endocrinology in the time of COVID-19: Remodelling diabetes services and emerging innovation. Eur J Endocrinol. 2020;183:67–77. doi: 10.1530/EJE-20-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee J.M., Carlson C., Albanese-O’Neill A., et al. Adoption of telemedicine for type 1 diabetes care during the COVID-19 pandemic. Diabetes Technol Ther. 2021;23:642–651. doi: 10.1089/dia.2021.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ladha S., Fox D., Bone J.N., Amed S. An analysis of self-reported barriers to type 1 diabetes care in a pediatric population in British Columbia, Canada. Can J Diabetes. 2021;45:383–389. doi: 10.1016/j.jcjd.2020.10.015. [DOI] [PubMed] [Google Scholar]
- 36.Iyengar V., Wolf A., Brown A., Close K. Challenges in diabetes care: Can digital health help address them? Clin Diabetes. 2016;34:133–141. doi: 10.2337/diaclin.34.3.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maringe C., Spicer J., Morris M., et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: A national, population-based, modelling study. Lancet Oncol. 2020;21:1023–1034. doi: 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonfig W., Kapellen T., Dost A., et al. Growth in children and adolescents with type 1 diabetes. J Pediatr. 2012;160:900–903.e2. doi: 10.1016/j.jpeds.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 39.Mitchell D.M. Growth in patients with type 1 diabetes. Curr Opin Endocrinol Diabetes Obes. 2017;24:67–72. doi: 10.1097/MED.0000000000000310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maffeis C., Birkebaek N.H., Konstantinova M., et al. Prevalence of underweight, overweight, and obesity in children and adolescents with type 1 diabetes: Data from the international SWEET registry. Pediatr Diabetes. 2018;19:1211–1220. doi: 10.1111/pedi.12730. [DOI] [PubMed] [Google Scholar]
- 41.Redondo M.J., Foster N.C., Libman I.M., et al. Prevalence of cardiovascular risk factors in youth with type 1 diabetes and elevated body mass index. Acta Diabetol. 2016;53:271–277. doi: 10.1007/s00592-015-0785-1. [DOI] [PubMed] [Google Scholar]
- 42.Esteban-Vasallo M.D., Galán I., Ortiz-Pinto M.A., et al. Accuracy of anthropometric measurements and weight status perceptions reported by parents of 4-year-old children. Public Health Nutr. 2020;23:589–598. doi: 10.1017/S1368980019003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dabelea D., Stafford J.M., Mayer-Davis E.J., et al. Association of type 1 diabetes vs type 2 diabetes diagnosed during childhood and adolescence with complications during teenage years and young adulthood. JAMA. 2017;317:825–835. doi: 10.1001/jama.2017.0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Orchard T.J., Forrest K.Y., Kuller L.H., Becker D.J. Pittsburgh Epidemiology of Diabetes Complications Study. Lipid and blood pressure treatment goals for type 1 diabetes: 10-year incidence data from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care. 2001;24:1053–1059. doi: 10.2337/diacare.24.6.1053. [DOI] [PubMed] [Google Scholar]
- 45.Downie M.L., Ulrich E.H., Noone D.G. An update on hypertension in children with type 1 diabetes. Can J Diabetes. 2018;42:199–204. doi: 10.1016/j.jcjd.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 46.Schnell O., Crocker J.B., Weng J. Impact of HbA1c testing at point of care on diabetes management. J Diabetes Sci Technol. 2017;11:611–617. doi: 10.1177/1932296816678263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petersen J.R., Finley J.B., Okorodudu A.O., Mohammad A.A., Grady J.J., Bajaj M. Effect of point-of-care on maintenance of glycemic control as measured by A1C. Diabetes Care. 2007;30:713–715. doi: 10.2337/dc06-1909. [DOI] [PubMed] [Google Scholar]
- 48.Driskell O.J., Holland D., Waldron J.L., et al. Reduced testing frequency for glycated hemoglobin, HbA1c, is associated with deteriorating diabetes control. Diabetes Care. 2014;37:2731–2737. doi: 10.2337/dc14-0297. [DOI] [PubMed] [Google Scholar]
- 49.Schwandt A., Best F., Biester T., et al. Both the frequency of HbA1c testing and the frequency of self-monitoring of blood glucose predict metabolic control: A multicentre analysis of 15 199 adult type 1 diabetes patients from Germany and Austria. Diabetes Metab Res Rev. 2017;33 doi: 10.1002/dmrr.2908. [DOI] [PubMed] [Google Scholar]
- 50.Perlman J.E., Gooley T.A., McNulty B., Meyers J., Hirsch I.B. HbA1c and glucose management indicator discordance: A real-world analysis. Diabetes Technol Ther. 2021;23:253–258. doi: 10.1089/dia.2020.0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McGee R.G., Cowell C.T., Arnolda G., et al. Assessing guideline adherence in the management of type 1 diabetes mellitus in Australian children: A population-based sample survey. BMJ Open Diabetes Res Care. 2020;8 doi: 10.1136/bmjdrc-2019-001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen Y., Sloan F.A., Yashkin A.P. Adherence to diabetes guidelines for screening, physical activity and medication and onset of complications and death. J Diabetes Complications. 2015;29:1228–1233. doi: 10.1016/j.jdiacomp.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Amed S., Nuernberger K., McCrea P., et al. Adherence to clinical practice guidelines in the management of children, youth, and young adults with type 1 diabetes---a prospective population cohort study. J Pediatr. 2013;163:543–548. doi: 10.1016/j.jpeds.2013.01.070. [DOI] [PubMed] [Google Scholar]
- 54.BC Centre for Disease Control . 2021. COVID-19: one year of the pandemic in BC.http://www.bccdc.ca/health-info-site/documents/covidbriefing_20210311.pdf [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.