Abstract
We describe the in vitro antiproliferative effects of the new triazole derivative UR-9825 against the protozoan parasite Trypanosoma (Schizotrypanum) cruzi, the causative agent of Chagas' disease in Latin America. The compound was found to be extremely active against the cultured (epimastigote) form of the parasite, equivalent to that present in the reduviid vector, with a MIC of 30 nM, a concentration 33-fold lower than that required with the reference compound ketoconazole. At that MIC, growth arrest coincided with depletion of the parasite's 4,14-desmethyl endogenous sterols (ergosterol, 24-ethylcholesta-5,7,22-trien-3b-ol, and precursors) and their replacement by methylated sterols (lanosterol, 24-methylenedihydrolanosterol, and obtusifoliol), as revealed by high-resolution gas chromatography coupled with mass spectrometry. This indicated that the primary mechanism of action of UR-9825 was inhibition of the parasite's sterol C14α demethylase, as seen with other azole derivatives. The phospholipid composition of growth-arrested epimastigotes was also altered, when compared to controls, with a significant increase in the content of phosphatidylethanolamine and phosphatidylserine and a concomitant reduction of the content of phosphatidylcholine. The clinically relevant intracellular amastigote form, grown in cultured Vero cells at 37°C, was even more sensitive to UR-9825, with a MIC of 10 nM, comparable to that for ketoconazole. The results showed that UR-9825 is among the most potent azole derivatives tested against this parasite and support in vivo studies with this compound.
The largest parasitic disease burden on the American continent is Chagas' disease, caused by the kinetoplastid protozoon Trypanosoma (Schizotrypanum) cruzi, with 16 to 18 million people infected and an estimated annual loss of 2.7 disability-adjusted years (41). The parasite develops intracellularly in its mammalian hosts, leading to severe tissue damage, particularly in the heart and gastrointestinal tract, which, coupled with intense inflammatory reactions, underlies the pathogenesis of the disease (1, 5). Although >90% of those infected survive the initial acute phase, 30 to 40% of individuals with chronic T. cruzi infection will develop, over years or decades, irreversible heart and gastrointestinal lesions which are frequently lethal (1, 6, 23, 25). Current chemotherapeutic approaches for the treatment of this condition, based on nitrofurans and nitroimidazoles, are unsatisfactory due to limited efficacy, particularly for the prevalent chronic form of the disease, and frequent toxic side effects (8, 10, 23, 25). Recently, great advances have been made in the control of both vectorial and transfusional transmission of the disease, particularly through the Southern Cone and Andean Initiatives (41). However, transmission continues in other parts of the continent and the problem of those already infected, most of them in the undetermined chronic phase, remains a challenge (8, 28, 29).
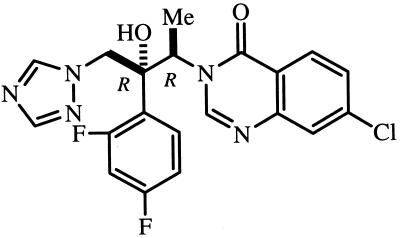
Sterol biosynthesis inhibitors (SBI) are currently the most advanced and widely used drugs in the treatment of fungal diseases (26, 38, 39). Although T. cruzi requires specific endogenous sterols for cell viability and proliferation and thus is extremely sensitive to SBI in vitro (reviewed in references 27, 28, and 30), currently available SBI are not powerful enough to eradicate T. cruzi from experimentally infected animals or human patients (4, 12, 20, 22). However, we have recently shown that new triazole derivatives such as D08070 (Zeneca Pharmaceuticals) and SCH 56592 (Schering-Plough), both selective inhibitors of the parasite's sterol C14α demethylase with high intrinsic anti-T. cruzi activity and special pharmacokinetic properties (long terminal half-lives and large volumes of distribution), are capable of inducing radical parasitological cure of both acute and chronic experimental Chagas' disease (21, 28, 30, 34, 35). UR-9825 [(1R,2R)-7-chloro-3-[2-(2,4-difluoropenyl) - 2 - hydroxyl - 1 - methyl - 3 - (1H - 1,2,4 - triazol - 1 - yl)propyl]quinazolin-4(3H)-one] (Uriach & Cia) (Fig. 1) is a new triazole derivative with potent and broad-spectrum antifungal activity and a long terminal life in dogs and cynomolgous monkeys (3, 24; J. Bartolí, E. Turmo, M. Algueró, E. Boncompte, M. L. Vericat, L. Conte, J. Ramis, J. García-Rafanell, and J. Forn, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. E-67, 1997). In this article we describe the results of a study on the in vitro anti-T. cruzi activity of UR-9825, which suggest that it is a good candidate for in vivo chemotherapeutic studies in appropriate animal models of Chagas' disease.
FIG. 1.
Chemical structure of UR-9825.
MATERIALS AND METHODS
Parasite.
The EP (11) and Y stocks of T. cruzi were used in this study with indistinguishable results. Handling of live T. cruzi was done according to established guidelines (13).
In vitro studies.
The epimastigote form of the parasite was cultivated in liver infusion tryptose (LIT; see reference 11) medium, supplemented with 10% newborn calf serum (Gibco) at 28°C with strong agitation (120 rpm). The cultures were initiated with a cell density of 2 × 106 epimastigotes per ml, and the drugs were added at a cell density of 0.5 × 107 to 1 × 107 epimastigotes per ml. Cell densities were measured with an electronic particle counter (model ZBI; Coulter Electronics Inc., Hialeah, Fla.) and by direct counting with a hemocytometer. Cell viability was followed by trypan blue exclusion using light microscopy. Amastigotes were cultured in Vero cells maintained in minimal essential medium supplemented with 1% fetal calf serum in a humidified 95% air–5% CO2 atmosphere at 37°C as previously described (18, 31–33, 36). Briefly, the cells were infected with 10 tissue culture-derived trypomastigotes per cell for 2 h and then washed three times with phosphate-buffered saline to remove nonadherent parasites; fresh medium with and without drugs was added and the cells were incubated for 96 h with a medium change at 48 h. Quantification of the number of infected cells and of the number of parasites per cell by use of light microscopy and statistical analysis of the results were carried out as described previously (18, 31–34, 36).
Studies of lipid composition.
For the analysis of the effects of drugs on the lipid composition of the epimastigotes, total lipids from control and drug-treated cells were extracted and fractionated into neutral and polar lipid fractions by silicic acid column chromatography and gas-liquid chromatography (18, 34–37). The neutral lipid fractions were first analyzed by thin-layer chromatography (on Merck 5721 silica gel plates with heptane–isopropyl ether–glacial acetic acid [60:40:4] as the developing solvent) and conventional gas-liquid chromatography (isothermic separation in a 4-m glass column packed with 3% OV-1 on Chromosorb 100/200 mesh, with nitrogen as the carrier gas at 24 ml/min and flame ionization detection in a Varian 3700 gas chromatograph). For quantitative analysis and structural assignments the neutral lipids were separated in a capillary high resolution column (25 m by 0.20 mm [inside diameter] Ultra-2 column; 5% phenyl-methyl-siloxane; 0.33-μm film thickness) in a Hewlett-Packard 5890 series II gas chromatograph equipped with an HP5971A mass sensitive detector. The lipids were injected in ethyl acetate; the column was kept at 50°C for 1 min and then the temperature was increased to 270°C at a rate of 25°C · min−1 and finally to 300°C at a rate of 1°C · min−1. The carrier gas (He) flow was kept constant at 1.0 ml · min−1. The injector temperature was 250°C, and the detector was kept at 280°C. The polar lipid fraction (containing mostly phospholipids) was analyzed as described before (7); briefly, the lipid fractions eluted from the silicic acid column with chloroform-methanol at 1:1 (vol/vol) were pooled and further fractionated by thin-layer chromatography on Merck 5721 silica gel plates using chloroform–methanol–32.5% ammonia (wt/vol) (17:7:1 by volume) as the developing solvent (9). The phospholipid spots were visualized using iodine and scraped, and the total organic phosphorous was measured using the method of Ames and Dubin (2).
Drugs.
UR-9825 was provided by the Research Center, Uriach & Cia, Barcelona, Spain, while ketoconazole was provided by Janssen Pharmaceutica, Caracas, Venezuela. The drugs were added as dimethyl sulfoxide solutions; the final dimethyl sulfoxide concentration in the culture media never exceeded 1% (vol/vol) and had no effect by itself on the proliferation of the parasites or Vero cells.
RESULTS AND DISCUSSION
Effects on epimastigotes.
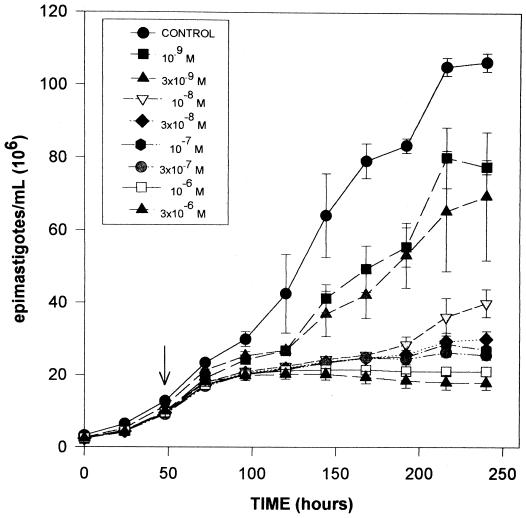
The data presented in Fig. 2 show the effects of UR-9825 on the proliferation of the epimastigote form of T. cruzi, equivalent to that present in its reduviid vectors, grown in LIT medium at 28°C (11). The response observed is characteristic of all SBI tested against this organism, with no significant effects on the growth rate for at least one generation after the drug addition, followed, at effective doses, by complete growth arrest and cell lysis after three to four generations (verified by light microscopy and trypan blue exclusion). Just before lysis, epimastigotes became rounded and large cell aggregates were observed, as seen before with other SBI (16, 17, 40). The minimal concentration of UR-9825 required to induce this “delayed lytic effect” after 144 h (MIC) was 30 nM, which is 33 times lower than that of ketoconazole (7, 16, 31, 33), D0870 (18, 35), or itraconazole (results not shown) and only comparable to that of SCH 56592, the most potent SBI known against this parasite (34).
FIG. 2.
Effects of UR-9825 on the proliferation of T. cruzi epimastigotes. Epimastigotes were cultured in modified LIT medium at 28°C as described in Materials and Methods. The arrow indicates the time of addition of the drug, at the indicated concentrations. Each experimental point corresponds to the mean of three independent cultures; each full bar represents two standard deviations.
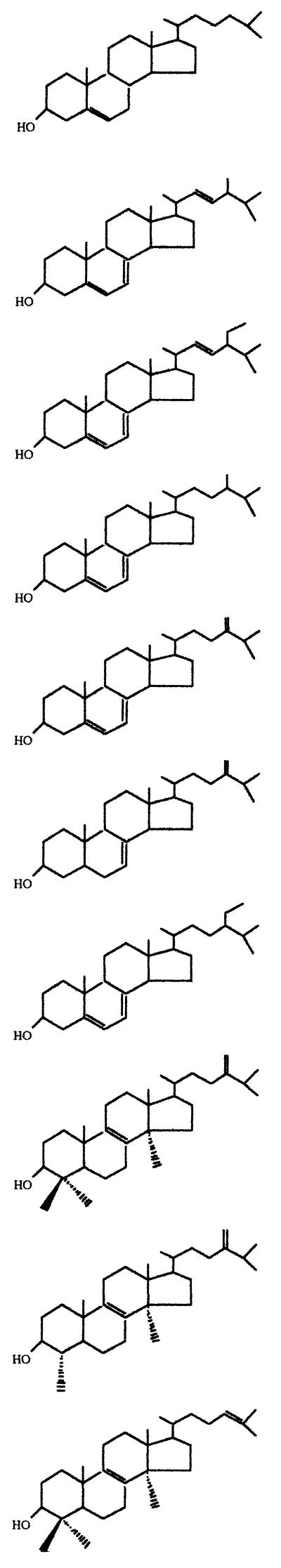
The onset of growth arrest and subsequent cell lysis induced by SBI in T. cruzi have been associated with the complete depletion of the parasite's endogenous sterols (18, 32–37). This was confirmed in epimastigotes treated with UR-9825 by analysis of the neutral lipid fraction using high-resolution gas-liquid chromatography coupled with mass spectrometry. We present in Table 1 the free sterol composition of epimastigotes incubated for 120 h with concentrations of UR-9825 or ketoconazole equal to or above the MIC (30 nM and 1 μM, respectively), together with that of control (untreated) organisms. It can be seen that under those conditions essentially all the endogenous 4,14-desmethyl sterols (ergosterol, 24-ethyl-cholesta-5,7,22-trien-3β-ol, and precursors) were replaced by 4,14-dimethyl and trimethyl sterols such as lanosterol, 24-methylene-dihydrolanosterol, and 4,14-dimethylergosta-8,24(241)-dien-3β-ol (obtusifoliol). These results strongly suggest that the primary target of UR-9825 in T. cruzi is the cytochrome P-450-dependent sterol C14α demethylase, as found for other azoles (18, 32–37).
TABLE 1.
Free sterols present in T. cruzi epimastigotes (EP stock) grown in the absence or presence of UR-9825 or ketoconazolea
Sterols were extracted from T. cruzi epimastigotes cultured in LIT medium in the absence or in the presence of the indicated concentrations of UR-9825 or ketoconazole for 120 h; they were separated from polar lipids by silicic acid column chromatography and were analyzed by quantitative capillary gas-liquid chromatography and mass spectrometry as described in Materials and Methods. Results are expressed as mass percent.
Epimastigotes treated with UR-9825 concentrations which induced complete growth arrest also displayed an altered phospholipid composition when compared with control (untreated) cells. It can be seen in Table 2 that these cells exhibited a large increase in the content of phosphatidylethanolamine (PE) and a concomitant decrease in the content of phosphatidylcholine (PC) (Table 2). This effect has also been observed before with several other SBI and has been attributed to a sharp reduction in the activity of membrane-bound PC-PE-N-methyl transferase, due to the altered sterol composition (7). Thus, UR-9825 seemed to induce all the physiological alterations characterized before in SBI-treated epimastigotes, but at lower concentrations than conventional azoles.
TABLE 2.
Phospholipid composition of T. cruzi epimastigotes (EP stock) grown in the absence or presence of UR-9825 or ketoconazole for 120 ha
| Phospholipid species | Control (no drug) | UR-9825
|
Ketoconazole (1 μM) | |
|---|---|---|---|---|
| 0.03 μM | 1 μM | |||
| Phosphatidylcholine | 15.2 ± 1.8 | 13.7 ± 1.1 | 13.1 ± 0.8 | 11.7 ± 1.7 |
| Phosphatidylethanolamine | 23.9 ± 2.5 | 31.0 ± 2.8b | 33.8 ± 2.8b | 34.6 ± 2.9b |
| Phosphatidylinositol | 13.4 ± 1.9 | 12.8 ± 2.5 | 17.2 ± 2.1 | 14.2 ± 2.8 |
| Phosphatidylserine | 31.4 ± 2.9 | 28.4 ± 2.1 | 26.8 ± 3.0 | 28.2 ± 2.8 |
| Sphingomyelin | 16.1 ± 1.2 | 14.1 ± 1.2 | 9.1 ± 1.5 | 11.3 ± 1.9 |
Epimastigotes were grown in LIT medium with and without the indicated drug concentrations for 120 h, and polar lipids were separated from the total lipid extract by silicic acid column chromatography and thin-layer chromatography as described in Materials and Methods. The different phospholipid species present were quantitated by measuring organic phosphorous. The results are expressed as mole percent and the means and standard errors are based on at least three independent experiments.
P < 0.05 versus controls.
Effects on amastigotes.
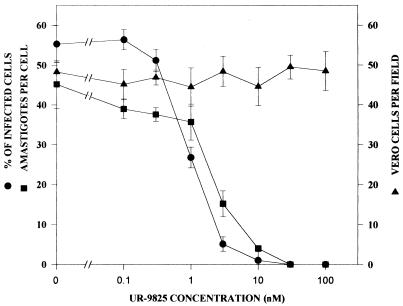
The clinically relevant intracellular amastigote forms, proliferating in cultured Vero cells at 37°C, were more susceptible to UR-9825 than epimastigotes: it can be seen in Fig. 3 that the MIC required to reduce infected cells by 99% and the IC50 (concentration of the drug required to reduce infected cells by 50%) were in this case 10 nM and 1 nM, respectively. The observation time was 96 h after infection, as at this time the intracellular development of the parasite is complete and nonproliferative trypomastigotes begin to be released by host cells. It can also be seen that no effects on the viability and proliferation of the host Vero cells were observed up to the highest concentration tested (100 nM), indicating a very specific antiparasitic activity. The MIC was essentially identical to that observed with ketoconazole (results not shown; see also references 31 and 33), D0870 (18, 35), or itraconazole (not shown), but higher than that previously reported for SCH 56592 (34).
FIG. 3.
Concentration dependence of the effects of UR9825 on the proliferation of T. cruzi amastigotes and Vero cells at 37°C. Shown are the percentage of infected cells (●), the number of amastigotes per cell (■), and the number of Vero cells per field (▴) after 96 h as a function of the drug concentration. Vero cells were infected with T. cruzi as described in Materials and Methods. Each full bar represents two standard deviations.
Conclusions.
The results presented above indicate that the in vitro activity of UR-9825 against T. cruzi is comparable to that of the most potent SBI tested against this organism, D0870 and SCH 56592, which have been shown to be able to induce radical parasitological cure in murine models of both acute and chronic Chagas' disease (34, 35). However, as discussed in detail elsewhere (18, 34, 35), effective in vivo activity of SBI against T. cruzi requires both high intrinsic (in vitro) antiparasitic activity and special pharmacokinetic properties, such as long terminal half-lives and large volumes of distribution. Thus, although the extremely short terminal half-life of UR-9825 in mice (3; Bartoli et al., 37th ICAAC) prevented us from testing this compound in our previously described murine models (19, 33–36), the long half-lives in dogs and cynomolgous monkeys (51 and 24 h, respectively) (Bartoli et al., 37th ICAAC) would suggest that this compound could have significant anti-T. cruzi activity in these animal models. Work is currently in progress to test this hypothesis in a dog model previously described by Lana et al. (14, 15).
ACKNOWLEDGMENTS
This work received financial support from the UNDP/World Bank/World Health Organization Programme for Research and Training in Tropical Diseases (grant 970297) and the Instituto Venezolano de Investigaciones Científicas.
REFERENCES
- 1.Acquatella H, Piras R. Chagas disease. Curr Opin Cardiol. 1993;8:463–472. [Google Scholar]
- 2.Ames B, Dubin D. The role of polyamines in the neutralization of deoxyribonucleic acid. J Biol Chem. 1960;235:769–775. [PubMed] [Google Scholar]
- 3.Bartrolí J, Turmo E, Algueró M, Boncompte E, Vericat M L, Conte L, Ramis J, Merlos M, García-Rafanell J, Forn J. New azole antifungals. 3. Synthesis and antifungal activity of 3-substituted-4(3H)-quinazolinones. J Med Chem. 1998;41:1869–1882. doi: 10.1021/jm9707277. [DOI] [PubMed] [Google Scholar]
- 4.Brener Z, Cançado J R, Galvão L M, da Luz Z M P, Filardi L d S, Pereira M E S, Santos L M T, Cançado C B. An experimental and clinical assay with ketoconazole in the treatment of Chagas disease. Mem Inst Oswaldo Cruz. 1993;88:149–153. doi: 10.1590/s0074-02761993000100023. [DOI] [PubMed] [Google Scholar]
- 5.Brener Z, Gazzinelli R T. Immunological control of Trypanosoma cruzi infection and pathogenesis of Chagas' disease. Int Arch Allergy Immunol. 1997;114:103–110. doi: 10.1159/000237653. [DOI] [PubMed] [Google Scholar]
- 6.Cançado J R. Terapêutica especifica. In: Pinto Dias J C, Coura J R, editors. Clínica e terapêutica da doença de Chagas. Rio de Janeiro, Brazil: Editora Fiocruz; 1997. pp. 323–351. [Google Scholar]
- 7.Contreras L M, Vivas J, Urbina J A. Altered lipid composition and enzyme activities of plasma membranes from Trypanosoma (Schizotrypanum) cruzi epimastigotes grown in the presence of sterol biosynthesis inhibitors. Biochem Pharmacol. 1997;53:697–704. doi: 10.1016/s0006-2952(96)00903-3. [DOI] [PubMed] [Google Scholar]
- 8.Croft S L, Urbina J A, Brun R. Chemotherapy of human leishmaniasis and trypanosomiasis. In: Hide G, Mottram J C, Coombs G H, Holmes P H, editors. Trypanomiasis and leishmaniasis. London, England: CAB International; 1997. pp. 245–257. [Google Scholar]
- 9.Cuzner M L, Davison A N. Quantitative thin layer chromatography of lipids. J Chromatogr. 1967;27:388–397. doi: 10.1016/s0021-9673(01)85895-7. [DOI] [PubMed] [Google Scholar]
- 10.de Castro S L. The challenge of Chagas disease chemotherapy: an update of drugs assayed against Trypanosoma cruzi. Acta Tropica. 1993;53:83–98. doi: 10.1016/0001-706x(93)90021-3. [DOI] [PubMed] [Google Scholar]
- 11.De Maio A, Urbina J A. Trypanosoma (Schizotrypanum) cruzi: terminal oxidases in two growth phases in vitro. Acta Cient Venez. 1984;35:136–141. [PubMed] [Google Scholar]
- 12.Fragata Filho A A, Boainain E, Mainfrino L B M. Itraconazole in treatment of chronic Chagas' disease. Mem Inst Oswaldo Cruz. 1993;88(Suppl. 1):243. [Google Scholar]
- 13.Hudson L, Grover F, Gutteridge W E, Klein R A, Peters W, Neal R A, Miles M A, Scott M T, Nourish R, Ager B P. Suggested guidelines for work with live Trypanosoma cruzi. Trans R Soc Trop Med Hyg. 1983;77:416–419. doi: 10.1016/0035-9203(83)90176-1. [DOI] [PubMed] [Google Scholar]
- 14.Lana M, Chiari E, Tafuri W L. Experimental Chagas' disease in dogs. Mem Inst Oswaldo Cruz. 1992;87:59–71. doi: 10.1590/s0074-02761992000100011. [DOI] [PubMed] [Google Scholar]
- 15.Lana M, Viera L M, Machado-Coelho G L L, Chiari E, Veloso V M, Tafuri W L. Humoral immune response in dogs experimentally infected with Trypanosoma cruzi. Mem Inst Oswaldo Cruz. 1991;86:471–476. doi: 10.1590/s0074-02761991000400019. [DOI] [PubMed] [Google Scholar]
- 16.Lazardi K, Urbina J A, de Souza W. Ultrastructural alterations induced by two ergosterol biosynthesis inhibitors, ketoconazole and terbinafine, on epimastigotes and amastigotes of Trypanosoma (Schizotrypanum) cruzi. Antimicrob Agents Chemother. 1990;34:2097–2105. doi: 10.1128/aac.34.11.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lazardi K, Urbina J A, de Souza W. Ultrastructural alterations induced by ICI 195,739, a bis-triazole derivative with strong antiproliferative action against Trypanosoma (Schizotrypanum) cruzi. Antimicrob Agents Chemother. 1991;35:736–740. doi: 10.1128/aac.35.4.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liendo A, Lazardi K, Urbina J A. Antiproliferative effects and mechanism of action of D0870 and its S(−) enantiomer against Trypanosoma cruzi. J Antimicrob Chemother. 1998;41:197–205. doi: 10.1093/jac/41.2.197. [DOI] [PubMed] [Google Scholar]
- 19.Maldonado R A, Molina J, Payares G, Urbina J A. Experimental chemotherapy with combinations of ergosterol biosynthesis inhibitors in murine models of Chagas' disease. Antimicrob Agents Chemother. 1993;37:1353–1359. doi: 10.1128/aac.37.6.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCabe R E. Failure of ketoconazole to cure chronic murine Chagas disease. J Infect Dis. 1988;158:1408–1409. doi: 10.1093/infdis/158.6.1408. [DOI] [PubMed] [Google Scholar]
- 21.Molina J, Martins-Filho O, Brener Z, Romanha A J, Loebenberg D, Urbina J A. Activity of the triazole derivative SCH 56592 (posaconazole) against drug-resistant strains of the protozoan parasite Trypanosoma (Schizotrypanum) cruzi in immunocompetent and immunosuppressed murine hosts. Antimicrob Agents Chemother. 2000;44:150–155. doi: 10.1128/aac.44.1.150-155.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moreira A A B, DeSouza H B W T, Amato Neto V, Matsubara L, Pinto P L S, Tolezano J E, Nunes E V, Okumura M. Avaliacão da atividade terapêutica do itraconazol nas infecçoes crônicas, experimental e humana, pelo Trypanosoma cruzi. Rev Inst Med Trop Sao Paulo. 1992;34:177–180. [PubMed] [Google Scholar]
- 23.Quintas L E M, de Castro S L, Urbina J A, Borba-Santos J A, Pinto C N, Siqueira-Batista R, Miranda Filho N. Tratamento da doença de Chagas. In: Siqueira-Batista R, Corrêa A D, Higgins D W, editors. Molestia de Chagas. Rio de Janeiro, Brazil: Editora Cultura Medica; 1996. pp. 125–170. [Google Scholar]
- 24.Ramos G, Cuenca-Estrella M, Monzon A, Rodriguez-Tudela J L. In vitro comparative activity of UR-9825, itraconazole and fluconazole against clinical isolates of Candida spp. J Antimicrob Chemother. 1999;44:283–286. doi: 10.1093/jac/44.2.283. [DOI] [PubMed] [Google Scholar]
- 25.Rassi A, Luquetti A O. Therapy of Chagas disease. In: Wendel S, Brener Z, Camargo M E, Rassi A, editors. Chagas disease (American trypanosomiasis): its impact on transfusion and clinical medicine. São Paulo, Brazil: ISBT BRAZIL '92; 1992. pp. 237–247. [Google Scholar]
- 26.Ryder N S, Mieth H. Allylamine antifungal drugs. Curr Top Med Mycol. 1992;4:158–188. doi: 10.1007/978-1-4612-2762-5_6. [DOI] [PubMed] [Google Scholar]
- 27.Urbina J A. Lipid biosynthesis pathways as chemotherapeutic targets in kinetoplastid parasites. Parasitology. 1997;117:S91–S99. [PubMed] [Google Scholar]
- 28.Urbina J A. Chemotherapy of Chagas' disease: the how and the why. J Mol Med. 1999;77:332–338. doi: 10.1007/s001090050359. [DOI] [PubMed] [Google Scholar]
- 29.Urbina J A. Parasitological cure of Chagas disease: is it possible?, is it relevant? Mem Inst Oswaldo Cruz. 1999;94(Suppl. 1):349–355. doi: 10.1590/s0074-02761999000700068. [DOI] [PubMed] [Google Scholar]
- 30.Urbina J A. Sterol biosynthesis inhibitors for Chagas disease. Curr Opin Anti-infect Inv Drugs. 2000;2:40–46. [Google Scholar]
- 31.Urbina J A, Lazardi K, Aguirre T, Piras M M, Piras R. Antiproliferative synergism of the allylamine SF-86327 and ketoconazole on epimastigotes and amastigotes of Trypanosoma (Schizotrypanum) cruzi. Antimicrob Agents Chemother. 1988;32:1237–1242. doi: 10.1128/aac.32.8.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Urbina J A, Lazardi K, Aguirre T, Piras M M, Piras R. Antiproliferative effects and mechanism of action of ICI 195,739, a novel bis-triazole derivative, on epimastigotes and amastigotes of Trypanosoma (Schizotrypanum) cruzi. Antimicrob Agents Chemother. 1991;35:730–735. doi: 10.1128/aac.35.4.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Urbina J A, Lazardi K, Marchan E, Visbal G, Aguirre T, Piras M M, Piras R, Maldonado R A, Payares G, DeSouza W. Mevinolin (lovastatin) potentiates the antiproliferative effects of ketoconazole and terbinafine against Trypanosoma (Schizotrypanum) cruzi: in vivo and in vitro studies. Antimicrob Agents Chemother. 1993;37:580–591. doi: 10.1128/aac.37.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urbina J A, Payares G, Contreras L M, Liendo A, Sanoja C, Molina J, Piras M M, Piras R, Perez N, Wincker P, Loebenberg D. Antiproliferative effects and mechanism of action of SCH 56592 against Trypanosoma (Schizotrypanum) cruzi: in vitro and in vivo studies. Antimicrob Agents Chemother. 1998;42:1771–1777. doi: 10.1128/aac.42.7.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Urbina J A, Payares G, Molina J, Sanoja C, Liendo A, Lazardi K, Piras M M, Piras R, Perez N, Wincker P, Ryley J F. Cure of short- and long-term experimental Chagas disease using D0870. Science. 1996;273:969–971. doi: 10.1126/science.273.5277.969. [DOI] [PubMed] [Google Scholar]
- 36.Urbina J A, Vivas J, Lazardi K, Molina J, Payares G, Piras M M, Piras R. Antiproliferative effects of Δ24(25) sterol methyl transferase inhibitors on Trypanosoma (Schizotrypanum) cruzi: in vitro and in vivo studies. Chemotherapy. 1996;42:294–307. doi: 10.1159/000239458. [DOI] [PubMed] [Google Scholar]
- 37.Urbina J A, Vivas J, Visbal G, Contreras L M. Modification of the sterol composition of Trypanosoma (Schizotrypanum) cruzi epimastigotes by Δ24(25)-sterol methyl transferase inhibitors and their combinations with ketoconazole. Mol Biochem Parasitol. 1995;73:199–210. doi: 10.1016/0166-6851(95)00117-j. [DOI] [PubMed] [Google Scholar]
- 38.Vanden Bossche H. Chemotherapy of human fungal infections. In: Lyr H, editor. Modern selective fungicides. Properties, applications, mechanism of action. Jena, Germany: Gustav Fisher Verlag; 1995. pp. 431–484. [Google Scholar]
- 39.Vanden Bossche H, Marichal P. Azole antifungals: mode of action. In: Yamaguchi H, Kobayashi G S, Takahashi H, editors. Recent progress in antifungal chemotherapy. New York, N.Y: Marcel Dekker; 1992. pp. 25–40. [Google Scholar]
- 40.Vivas J, Urbina J A, DeSouza W. Ultrastructural alterations in Trypanosoma (Schizotrypanum) cruzi induced by Δ24(25) sterol methyl transferase inhibitors and their combinations with ketoconazole. Intern J Antimicrob Agents. 1996;7:235–240. doi: 10.1016/s0924-8579(96)00325-1. [DOI] [PubMed] [Google Scholar]
- 41.World Bank. World development report 1993/1994: investing in health. Oxford, England: Oxford University Press; 1993. [Google Scholar]