Abstract
Common chronic diseases represent the greatest driver of rising healthcare costs, as well as declining function, independence, and quality of life. Geroscience‐guided approaches seek to delay the onset and progression of multiple chronic conditions by targeting fundamental biological pathways of aging. This approach is more likely to improve overall health and function in old age than treating individual diseases, by addressing aging the largest and mostly ignored risk factor for the leading causes of morbidity in older adults. Nevertheless, challenges in repurposing existing and moving newly discovered interventions from the bench to clinical care have impeded the progress of this potentially transformational paradigm shift. In this article, we propose the creation of a standardized process for evaluating FDA‐approved medications for their geroscience potential. Criteria for systematically evaluating the existing literature that spans from animal models to human studies will permit the prioritization of efforts and financial investments for translating geroscience and allow immediate progress on the design of the next Targeting Aging with MEtformin (TAME)‐like study involving such candidate gerotherapeutics.
Keywords: aging, clinical trials, drug repurposing, geroscience, preclinical studies
Geroscience‐guided approaches seek to delay the onset and progression of chronic conditions by targeting the pathways of aging. This approach is likely to improve overall health in old age by addressing aging—the largest risk factor for the leading causes of morbidity. Here, we propose the creation of a standardized process for evaluating FDA‐approved drugs for their geroscience potential, permitting the prioritization of efforts and investments for translating geroscience discoveries.
Abbreviations
- ACEi
angiotensin‐converting enzyme inhibitor
- ACS
Acute Coronary Syndrome
- AD
Alzheimer’s disease
- ADAS‐cog
Alzheimer's Disease Assessment Scale–Cognitive Subscale
- ARB
angiotensin receptor blocker
- BCC
basal cell carcinoma
- BEZ235
dactolisib
- BMD
bone mineral density
- BP
blood pressure
- CAD
Coronary Artery Disease
- cIMT
coronary (artery) intimal media thickness
- CKD
chronic kidney disease
- CRC
colorectal cancer
- CV
cardiovascular
- CVA
cerebrovascular accident
- DM
diabetes mellitus
- ESRD
end‐stage renal disease
- GFR
glomerular filtration rate
- HF
heart failure
- HR
hazard ratio
- HRCT
high‐resolution chest CT
- HTN
hypertension
- IFN
interferon
- ILD
interstitial lung disease
- IPF
idiopathic pulmonary fibrosis
- IRR
incidence rate ratio
- MACE
major adverse cardiovascular event
- MCI
mild cognitive impairment
- MI
myocardial infarction
- MMSE
mini mental status evaluation
- OR
odds ratio
- RAD001
everolimus
- RCT
randomized controlled trial
- RR
relative risk
- SASP
senescence‐associated secretory phenotype
- SRR
standardized rate ratio
- SU
sulfonylurea
- T2D
diabetes mellitus, type 2
1. INTRODUCTION
Geroscience represents a novel paradigm whereby biological aging is recognized as the major modifiable driver of age‐related diseases and other late‐life conditions (Burch et al., 2014; Kennedy et al., 2014; López‐Otín et al., 2013). Widespread clinical use of geroscience‐guided interventions could transform the public health landscape because the ability to target biological aging as a risk factor could simultaneously delay the onset and progression of multiple conditions, thereby enhancing health, function, and independence in late‐life. A corollary is that targeting this biology will affect human healthspan (the portion of lifespan free of major disease and disability) most profoundly, and with a better prognosis than the current model of addressing one disease at a time. Geroscientists agree on several criteria defining distinct yet interrelated molecular and cellular hallmarks of aging that include (1) the measurable systemic or tissue‐specific biological process should be altered during aging; (2) its disruption should have negative consequences in both lifespan and healthspan; and (3) when positively modulated, it should extend both lifespan and healthspan in preclinical models (Huffman et al., 2016; Kritchevsky & Justice, 2020). Currently approved and accepted tools to target aging in humans are restricted to diet and exercise, which seem to influence multiple hallmarks of aging. On the contrary, advances in geroscience are spurring the development of gerotherapeutics (pharmacological interventions that promote health by directly or indirectly targeting the aging hallmarks, in addition to the disease itself) which show efficacy toward multiple age‐related conditions. Interventions that influence these hallmarks at the cellular level toward more youthful function appear to lead to similarly favorable functional changes at the organ and systemic level.
While aging is unequivocally the major risk factor for age‐related diseases, regulatory bodies around the world, such as the FDA or EMA, do not yet recognize geroscience‐guided clinical outcomes as a path to regulatory approval. This is in part because the processes for validating specific compounds or combinations of compounds for their ability to delay the onset and progression of multiple chronic diseases have not yet been delineated in humans. Without regulatory approval, insurers will not pay for such treatments, which disincentivizes pharmaceutical companies from developing geroscience‐guided approaches, simply because there is no path for them to develop a viable business plan. Therefore, there is an urgent need to demonstrate, in a well‐designed clinical trial, that a cluster of age‐related diseases can be significantly delayed by repurposing existing or developing novel gerotherapeutics.
Targeting Aging with MEtformin (TAME) is such a study that has been under development for the last few years, and whose basic principles have been developed in consultation with the FDA (Barzilai et al., 2016). Metformin was initially used in the 1950s to prevent influenza and malaria, but it was also noted to lower glucose levels in people with diabetes without triggering hypoglycemia, leading to it becoming the treatment of choice for type 2 diabetes (T2D). In addition to its well‐documented benefits on diabetes, clinical and observational studies have linked metformin to beneficial effects on multiple age‐related diseases, including cardiovascular disease (CVD), cancer, Alzheimer's disease, and mild cognitive impairment, as well as reduced mortality (Barzilai et al., 2016; Campbell et al., 2018; Novelle et al., 2016). TAME was designed by a group of geroscientists as a robust proof‐of‐principle study, whereby the primary outcome of the placebo‐controlled, double‐blinded trial will be the “time to event” of a cluster of age‐related diseases consisting of cardiovascular events, cancer, cognitive decline, dementia, and death. In this manner, TAME is planned to provide a template for the design of future studies evaluating other FDA‐approved drugs that are repurposed as gerotherapeutics (Espeland et al., 2017; Justice et al., 2018).
We believe that efforts to test and repurpose existing, safe gerotherapeutics should be extended beyond TAME, not only to increase the number of drugs potentially available to target aging in humans but also to mitigate the risks to the field, should any such trials fail to reach their desired outcome. The purpose of this paper is to gather available evidence in the literature that supports and ranks potential gerotherapeutics and to help clinical investigators, geroscientists, not‐for‐profit foundations, and governments to accelerate research and clinical trials to test their efficacy and safety. Importantly, our mission is for such drug candidates to become clinically available relatively soon, and thus, we limit our analyses only to potential gerotherapeutics that are already FDA‐approved for other clinical indications.
We sought to identify such FDA‐approved drugs or classes of drugs that had at least one publication showing extension of lifespan in rodents and data in humans suggesting the highest chance of success if tested in a well‐controlled TAME‐like clinical trial. We developed a 12‐point prioritization scale that assigns equal points for the preclinical and clinical evidence for each of these candidates. Points on the preclinical side were assigned for effects on the hallmarks of aging, improvement in healthspan and extension of lifespan in rodents as part of the NIA’s Interventions Testing Program (ITP), a well‐characterized, multicentered study to evaluate gerotherapeutics, as well as non‐ITP rodent lifespan studies (Harrison et al., 2014; Nadon et al., 2017). On the clinical side, points were assigned for observed healthspan outcomes extending beyond the diseases targeted by the drug, and mortality from any cause or off‐target diseases, with a scale where interventional studies weigh more than observational studies. Here, we outline the nature of the process and priority scoring designed to assess the likelihood that a specific gerotherapeutic may be successful in a clinical study.
2. METHODS
2.1. Identification of potential gerotherapeutics among FDA‐approved drugs
We conducted mining of the DrugAge (Build 3) database for drugs that extend lifespan (Barardo et al., 2017; detailed in Figure 1). Results were then filtered for rodents (mice and rats) in studies reporting statistically significant increased median and/or maximum lifespan as the inclusion criteria (n = 41 drugs or supplements). This approach meant that drugs that have shown some clinical benefits but have not shown extension of lifespan in preclinical rodent models were excluded from further analysis. These findings were then updated with drugs tested in the ITP since 2019 (DrugAge Build 3 was released in July 2019). Nutraceutical/supplemental products were excluded, and only FDA‐approved drugs were included (n = 9 drugs). Search was then split into preclinical (more detail on lifespan, measures of healthspan and hallmarks of aging) and clinical (healthspan and mortality) using the search terms and datasets as described in Figure 1. The search terms for clinical outcomes were based on the non‐communicable diseases among the 10 leading causes of death in persons age 65 and older in the US (CDC, 2017).
FIGURE 1.
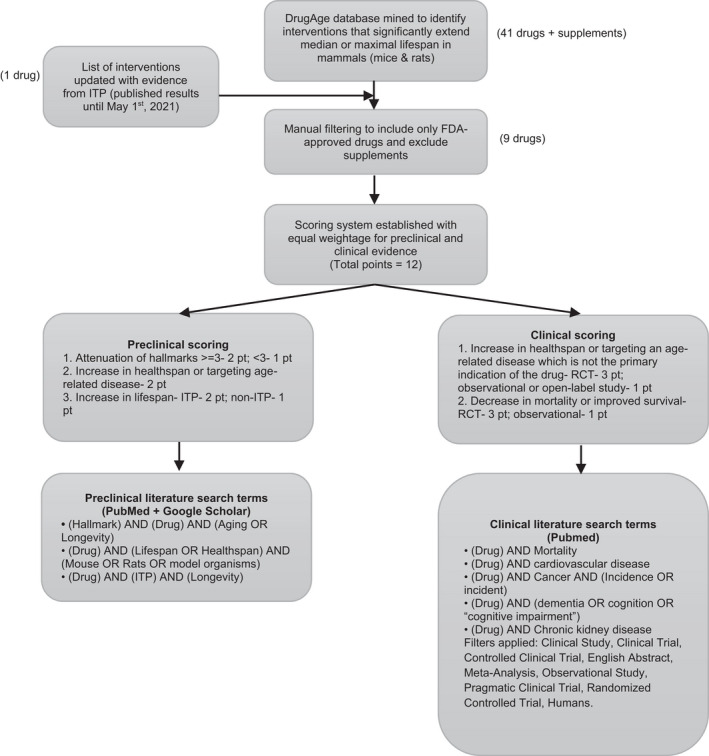
Workflow to select candidate gerotherapeutics and evaluate preclinical and clinical evidence for future geroscience‐guided clinical trials. Number of interventions after each step of selection are indicated in parentheses
2.2. Ranking gerotherapeutics
An ordinal 12‐point scale was equally divided between basic and clinical studies (6 points each). Thereby, promising gerotherapeutics that have significant basic geroscience rationale, yet currently lack supportive data from human subject studies were not penalized.
2.3. Preclinical scoring
Of the 6 points evaluating basic or preclinical factors, up to 2 points were assigned each for targeting hallmarks of aging, improving preclinical healthspan and preclinical lifespan each, with the breakdown as follows: (i) 1 point assigned for less than 3 hallmarks, 2 points assigned for 3 or more hallmarks; (ii) 2 points assigned for effect on healthspan parameters; (iii) 1 point assigned for lifespan tested outside ITP, 2 points assigned for a significant increase in lifespan within ITP (López‐Otín et al., 2013).
2.4. Clinical scoring
Out of the 6 points assessing clinical considerations, up to 3 points were assigned to healthspan and 3 points for mortality data, as follows: (i) Healthspan: drug needed to demonstrate that it targeted at least one age‐related disease/pathologic process which it was not intended to treat, with 1 point assigned for observational studies and 3 points for interventional, randomized controlled trials (RCTs); (ii) mortality: drug needed to demonstrate that it reduced all‐cause mortality or death from a disease which it was not intended to treat, with 1 point assigned for observational studies and 3 points assigned for RCTs.
3. RESULTS
Using the process detailed in Figure 1, we were able to prioritize 9 drug classes (Table 1). SGLT2 inhibitors (SGLT2i), a relatively new drug class, was the only one to receive the maximum score, owing to not only its robust effects on improving rodent healthspan and lifespan (including ITP) but also strong evidence for the extension of healthspan and reduction of mortality in humans. Metformin was next on the list, and it received a submaximal score, due to negative findings for rodent lifespan extension in ITP. Acarbose, rapamycin/rapalogs, and methylene blue (MB) all had strong preclinical data and promising findings for human healthspan (the latter being the most robust for acarbose), but sparse clinical data for human mortality. Angiotensin‐converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARBs) were found to extend preclinical healthspan and lifespan (outside of ITP) and had robust effects on extending human healthspan, but the studies on human mortality, while abundant, were predominantly negative. The last three drugs on our list, senolytics‐ Dasatinib + Quercetin (D + Q), aspirin, and N‐acetyl cysteine (NAC), all had strong preclinical data, but their effects on human healthspan and mortality have not yet been assessed in clinical studies or appropriate doses/populations. Obviously, future studies may change the priority order for drugs that did not receive points due to the paucity of clinical data.
TABLE 1.
Ranking of FDA‐approved drugs as potential gerotherapeutics based on scoring (out of 12) for preclinical and clinical evidence
| Gerotherapeutics | Hallmarks of aging | Preclinical healthspan | Preclinical lifespan | Human healthspan | Human mortality | Score (out of 12) |
|---|---|---|---|---|---|---|
| SGLT‐2 inhibitors | 2 | 2 | 2 | 3 | 3 | 12 |
| Metformin | 2 | 2 | 1 | 3 | 3 | 11 |
| Acarbose | 2 | 2 | 2 | 3 | 0 (Not assessed) | 9 |
| Rapamycin/rapalogs | 2 | 2 | 2 | 3* | 0 (Not assessed) | 9 |
| Methylene blue | 2 | 2 | 2 | 3* | 0 (Not assessed) | 9 |
| ACEi/ARB | 2 | 2 | 1 | 3 | 0 | 8 |
| Dasatinib + (quercetin) | 2 | 2 | 1 | 1 | 0 (Not assessed) | 6 |
| Aspirin | 2 | 2 | 2 | 0 (Not assessed) | 0 (Not assessed) | 6 |
| N‐acetyl cysteine | 1 | 2 | 2 | 0 (Not assessed) | 0 (Not assessed) | 5 |
3.1. Preclinical results
3.1.1. Rodent lifespan
Gerotherapeutics in preclinical models were primarily evaluated for their ability to extend rodent lifespan (Table 2). Robust evidence from ITP suggests that SGLT2i (canagliflozin), acarbose, rapamycin, MB, and aspirin extend either median and maximum lifespan or median lifespan alone in genetically heterogeneous UM‐HET3 mice, generally in a sexually dimorphic manner (Harrison et al., 2014; Nadon et al., 2017). SGLT2i canagliflozin extended median survival by 14% and the age for 90th percentile survival by 9% in male mice (Miller et al., 2020). Acarbose extended median lifespan by 22% in males and only 5% in females, while rapamycin exhibits a dose‐dependent response by increasing median lifespan by 16%–26% in females and 13%–23% in males (Harrison et al., 2014, 2019; Miller et al., 2011, 2014; Strong et al., 2016, 2020). Although less rigorous than ITP, individual non‐ITP studies have also identified metformin, ACEi/ARB, senolytics D + Q to have a lifespan‐extending effect in mice (Martin‐Montalvo et al., 2013; Santos et al., 2009; Xu et al., 2018).
TABLE 2.
Preclinical evidence for candidate gerotherapeutics in improving lifespan and healthspan and attenuating hallmarks of aging
| Gerotherapeutics | Effects on model organism lifespan | Effects on healthspan and age‐related diseases in preclinical models | Hallmarks of Aging | |||
|---|---|---|---|---|---|---|
| Macromolecular Damage / Adaptation to Stress | Epigenetic effects / Stem Cell renewal and regeneration | Proteostasis / Inflammation / Senescence | Metabolism | |||
| SGLT2 inhibitors | ITP: Canagliflozin ↑ median lifespan by 14% in males (Miller et al., 2020) | Dapagliflozin ↓ atherosclerosis with macrophage infiltration in diabetic ApoE −/− mice (Leng et al., 2016) | Dapagliflozin restores Calcium uptake and prevents age‐associated Calcium build up in the mitochondria of cardiomyocytes (Olgar et al., 2020) | No applicable studies | Empagliflozin reactivates glomerular autophagy in db/db mice (Korbut et al., 2020) |
|
| Metformin (pre‐2020 studies summarized previously (Kulkarni et al., 2020)) | 5.83% ↑ in mean lifespan of 84‐weeks‐old males (Martin‐Montalvo et al., 2013) |
|
|
Regulation of UPR via AMPK/ERK1/2 pathway to attenuate age‐related hearing loss, cell apoptosis and neurodegeneration in old rats (Cai et al., 2020) | ||
| Acarbose | ITP: 22% ↑ in median lifespan (males) and 5% ↑ in (females) (Harrison et al., 2014, 2019; Strong et al., 2016) |
|
No applicable studies | ↓ PDX−1 methylation in beta‐cells reverting T2DM in db/db mice (D. Zhou et al., 2021) | ||
| Rapamycin and Rapalogs | ITP: Median lifespan‐ 13–23% ↑ (dose‐dependent) in male mice; 16–26% ↑ (dose‐dependent) in female mice; Max lifespan‐ 8% ↑ in male mice; 5–11% ↑ in female mice (Miller et al., 2014) |
|
|
|||
| Methylene Blue | ITP: Median lifespan‐ 0.7% ↑ and max lifespan‐ 6% ↑ in females (Harrison et al., 2014) | Improved mitochondrial respiration and ↓ reactive oxygen species and oxidative stress in diabetic rat hearts (Duicu et al., 2017; Xiong et al., 2016) | No applicable studies | Delays cellular senescence (Atamna et al., 2008; Xiong et al., 2017) | No applicable studies | |
| ACEi and ARBs | ↑ rat lifespan with long‐term enalapril treatment (Santos et al., 2009) | No applicable studies | No applicable studies | No applicable studies | ||
| Dasatinib + Quercetin (Senolytics) | Biweekly treatment in 24‐month‐old mice ↑ median lifespan by 36% (Xu et al., 2018) |
|
No applicable studies | ↑ insulin sensitivity and glucose tolerance in obese mice (Sierra‐Ramirez et al., 2020) | ||
| Aspirin (dose‐specific effect) | ITP: (21 mg/kg)‐ Median lifespan 8% ↑ in males (Strong et al., 2008) | Suppression of age‐related and CC‐hypermethylation in colon (Noreen et al., 2020) |
|
↑ AMPK, ↓ mTOR signaling and recapitulating metabolic effects of caloric restriction (Pietrocola et al., 2018) | ||
| N‐acetyl Cysteine | ITP effect only in UM mice(could be due to inadvertent dietary restriction) (Flurkey et al., 2010) | ↓ age‐related hearing loss, memory decline, spatial memory deficits and oocyte aging in mice (Costa et al., 2016; Liu et al., 2012; Marie et al., 2018; More et al., 2018) (Marie et al., 2018; Costa et al., 2016; Lamming et al., 2012; More et al., 2018) | ↓ oxidative stress and neurodegeneration in rat brain (Garg et al., 2018) | No applicable studies | No applicable studies | No applicable studies |
3.1.2. Rodent healthspan
In addition to lifespan effects, we evaluated the evidence of potential gerotherapeutics on improving healthspan in preclinical models of aging and age‐related diseases, where the action of the drug was attributed to their non‐primary target or mechanism. Metformin displayed strong evidence in targeting conditions that were beyond its antihyperglycemic function (Barzilai et al., 2016; Kulkarni et al., 2018, 2020). In osteoarthritic mice, it moderated cartilage degeneration and chondrocyte aging (Feng et al., 2020). Similarly, SGLT2i and acarbose demonstrated effects beyond their glucose‐lowering potential by lowering the incidence of atherosclerosis in diabetic ApoE −/− mice with the former, and that of lung tumors, liver degeneration, and glomerulosclerosis with the latter (Han et al., 2017; Harrison et al., 2019; Liu et al., 2021). In mouse models of neurodegeneration, metformin, acarbose, rapamycin, MB, aspirin, and NAC improved cognitive and motor functions and attenuated age‐related memory impairments and behavioral changes (Cao et al., 2012; Chandra et al., 2018; Hosokawa et al., 2012; Kaeberlein & Galvan, 2019; Kodali et al., 2021; Lamming et al., 2012; Martínez et al., 2000; Ryu et al., 2020; Spilman et al., 2010; Tong et al., 2015; Yan et al., 2015; Zakaria et al., 2016).
Complementary to its effects on lifespan, rapamycin improved mouse healthspan by reducing the incidence of retinopathy, myocardial alterations, liver degeneration, and endometrial hyperplasia while also scavenging reactive oxygen species (ROS) in corneal endothelial cells (Lamming et al., 2012; Shin et al., 2011).
Rapamycin, metformin, and ACEi enalapril are also shown to impact indicators of frailty, including motor and physical performance, grip strength, stride length, resistance to muscle fatigue as well as decreased incidence of kyphosis index and cataracts in middle‐aged and older mice (Correia‐Melo et al., 2019; Keller et al., 2019; Palliyaguru et al., 2019).
The effects of senolytics D + Q are primarily attributed to lowering the burden of senescent cells or downregulating inflammation in older mice and thereby preventing aging‐related cognitive, mobility declines, frailty, vasomotor, uterine dysfunction, or fibrosis (Cavalcante et al., 2020; Farr et al., 2017; Roos et al., 2016; Schafer et al., 2017; P. Zhang et al., 2019).
3.1.3. Attenuation of hallmarks of aging
To assess the molecular utility of gerotherapeutics in targeting fundamental mechanisms of aging, we subsequently evaluated their actions on hallmarks of aging. By grouping similar pathways together, the seven pillars of aging were classified into four (1) Macromolecular damage and adaptation to stress; (2) epigenetic effects, stem cell renewal and regeneration; (3) proteostasis, inflammation (and senescence); and (4) metabolism (Kennedy et al., 2014; López‐Otín et al., 2013). Importantly, drugs well‐studied from the aging perspective such as metformin, rapamycin, and aspirin have substantial evidence of targeting hallmarks of aging, as compared to relatively less studied or newly approved drugs including SGLT2i, senolytics, and NAC.
We have previously elaborated on metformin's impact on attenuating hallmarks of aging (Kulkarni et al., 2020). Further investigation on other gerotherapeutics indicates that they target common mechanisms within each hallmark of aging, which includes scavenging of ROS and thereby oxidative stress by metformin in human immune cells, rapamycin in erythrocytes, MB in rat hearts, aspirin in endothelial cells, and NAC in the brain (Duicu et al., 2017; Garg et al., 2018; Hartwig et al., 2021; Jorda et al., 2020; Singh et al., 2016). Moreover, metformin, rapamycin, and aspirin further protect against DNA damage either via lowering DNA‐damage accumulation (rapamycin and aspirin) or upregulating DNA‐damage response (metformin) (Chen et al., 2011; Kulkarni et al., 2020; Rahman et al., 2021; Saha et al., 2014). SGLT2i improves mitochondrial function by preventing calcium buildup, while metformin and MB regulate the electron transport chain (Chen et al., 2021; Duicu et al., 2017; Olgar et al., 2020; Xiong et al., 2017).
Many gerotherapeutics exhibit anti‐inflammatory, senolytic/senomorphic, and proteostasis‐regulating properties. Metformin, rapamycin/rapalogs, ARBs, and aspirin increased autophagy in a tissue‐specific manner, SGLT2i empagliflozin reactivates glomerular autophagy in db/db mice (Bharath et al., 2020; Castoldi et al., 2020; Kodali et al., 2021; Korbut et al., 2020; Lin et al., 2018; Pietrocola et al., 2018; Schinaman et al., 2019; Woo & Jung, 2017; Zhao et al., 2020). Metformin, rapamycin, senolytics, and aspirin are seen to lower peripheral and tissue‐specific pro‐inflammatory cytokines (Jorda et al., 2020; Kodali et al., 2021; Kulkarni et al., 2020; Laberge et al., 2015; Paschoal et al., 2017; Saccon et al., 2021; Zhang et al., 2019). Recent evidence also suggests the role of metformin and acarbose in modulating the gut microbiome, thereby suppressing inflammation (Ahmadi et al., 2020; Smith et al., 2019). In addition to senolytics, metformin, rapamycin, MB, and aspirin have also been shown to lower senescence‐associated secretory phenotype (SASP) levels (Atamna et al., 2008; Feng et al., 2019; Moiseeva et al., 2013; Wang et al., 2017; Xiong et al., 2017).
Additionally, gerotherapeutics are shown to regulate metabolism by targeting evolutionarily conserved nutrient‐sensing pathways across multiple species, primarily AMPK signaling by metformin, mTOR signaling by rapamycin and rapalogs, and insulin‐IGF1 signaling by acarbose (Barzilai et al., 2012; Tong et al., 2015). Glucose tolerance was improved with SGLT2i dapagliflozin while senolytics D + Q, acarbose reduced postprandial glucose levels and improved insulin sensitivity (Durak et al., 2018; Harrison et al., 2019; Sierra‐Ramirez et al., 2020). SGLT2i empagliflozin increased the AMP/ATP ratio in a cardiac injury model, induced renoprotection in diabetic mouse kidneys via inhibition of mTORC1 hyperactivation, while aspirin recapitulated metabolic effects of caloric restriction (Pietrocola et al., 2018; Tomita et al., 2020; H. Zhou et al., 2018).
3.2. Clinical results
3.2.1. Mortality
To assess the effects of gerotherapeutics on mortality, we focused on all‐cause mortality and deaths from off‐target diseases, that is, those that were not the primary target for the drug (Table 3). SGLT2i demonstrated the most robust clinical evidence for reduction of death from any cause and CVD, in patients with or without T2D, heart failure, and chronic kidney disease (CKD) (Cavender et al., 2018; Heerspink et al., 2020; Neal et al., 2017; Packer et al., 2020; Perkovic et al., 2019). Clinical mortality data for metformin are less abundant, but UK Prospective Diabetes Study (UKPDS), an RCT that followed people with T2D for >10 years, found that metformin reduced all‐cause mortality compared to diet and other glucose‐lowering drugs (“Effect of Intensive Blood‐Glucose Control with Metformin on Complications in Overweight Patients with Type 2 Diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group,” 1998). Ample observational evidence supports survival benefits in people with diabetes treated with metformin, including when compared to people without diabetes (Bannister et al., 2014; Campbell et al., 2017; Jong et al., 2019; Ritsinger et al., 2020).
TABLE 3.
Clinical evidence for candidate gerotherapeutics in targeting human healthspan and mortality in observational and interventional studies
| Gerotherapeutics | Observational Healthspan | Interventional Healthspan | Observational Mortality | Interventional Mortality |
|---|---|---|---|---|
| SGLT−2 inhibitors |
|
↓all‐cause mortality (HR = 0.49 [0.41–0.57]) (Kosiborod et al., 2017) | ||
| Metformin |
|
|
|
|
| Acarbose |
|
|
||
| Rapamycin/Rapalogs |
|
|
No applicable studies. | No applicable studies. |
| Methylene Blue |
|
|
No applicable studies | No applicable studies |
| ACEi/ARB |
|
Meta‐analysis: No Δ in CRC mortality (Dai et al., 2015) |
|
|
| Dasatinib + (quercetin) | No applicable studies |
|
No applicable studies | No applicable studies |
| Aspirin | No applicable studies. | No applicable studies. | No applicable studies | No applicable studies. |
| N‐Acetyl Cysteine | No applicable studies. | No applicable studies. | No applicable studies. | No applicable studies. |
Except for ACEi/ARB, mortality data in appropriate dose/populations of the other evaluated drugs remain limited. One RCT of acarbose vs. placebo predefined the all‐cause and cardiovascular mortality as secondary outcomes and found no difference (Holman et al., 2017). Most studies analyzing mortality (and healthspan) with rapamycin/rapalogs focused on organ transplant recipients, a context that does not permit an assessment of gerotherapeutic benefits for several reasons: (1) the doses used were high and targeted to achieve immunosuppression, (2) these high‐risk patients are not representative of the general aging population, and (3) rapamycin/rapalogs were compared to other, more toxic immunosuppressants. Rapalogs are also used to coat intravascular stents, but systemic doses delivered by this route are merely a fraction of a single daily dose used to achieve immunomodulation (Wiemer et al., 2008). In small clinical trials, MB improved survival post cardiac surgery, owing to its vasoconstrictive effect, but it was not studied outside of the critical care setting (Levin et al., 2004). Numerous studies have investigated the role of aspirin in human lifespan and healthspan but, unlike animal studies, the administered doses were in the range at which aspirin displays anti‐platelet, not anti‐inflammatory activity. The use of anti‐inflammatory doses in humans would likely be prohibited by the risk of bleeding (ASCEND Study Collaborative Group, 2018; Hansson et al., 1998; McNeil et al., 2018; Ridker et al., 2005).
While abundant, clinical mortality data for ACEi/ARB in non‐high‐risk hypertensive populations (i.e., without heart failure, acute coronary syndromes, and organ transplants) are conflicted, at least in part due to marked variability in study populations, agents, and dosages used, comparators (placebo or active comparators), open‐label addition of other agents and achieved blood pressure goals (Hansson et al., 1999; Lithell et al., 2003, 2004; Oparil et al., 2013). Overall, the findings were predominantly negative, as illustrated in a meta‐analysis of 20 RCTs which found a borderline reduction in all‐cause mortality, driven by ACEi, while individual examination of the included studies shows that only 3 out of 20 showed mortality benefits, all with perindopril (van Vark et al., 2012).
3.2.2. Human healthspan
In examining human healthspan, we focused on the ability of gerotherapeutics to prevent age‐related disease or reduce the progression of off‐target age‐related pathologies. SGLT2i, originally intended for glucose‐lowering, in RCTs demonstrated particularly strong and consistent cardioprotective and renoprotective effects, while metformin had beneficial effects across the widest range of healthspan outcomes (Heerspink et al., 2020; Neal et al., 2017; Perkovic et al., 2019; Wiviott et al., 2019). In RCTs, metformin prevented T2D and cardiovascular events (“Effect of Intensive Blood‐Glucose Control with Metformin on Complications in Overweight Patients with Type 2 Diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group,” 1998; Hong et al., 2013; Knowler et al., 2002). Pilot trials suggest that metformin may improve memory and executive function, while observational studies and meta‐analyses reported up to 81% reduction in incident dementia (Campbell et al., 2018; Cheng et al., 2014; Koenig et al., 2017; Luchsinger et al., 2017; Samaras et al., 2020; Zhou et al., 2020). By contrast, one small observational study reported impaired cognitive performance with metformin, but it did not adjust for glycemic control, duration of diabetes, or concomitant glucose‐lowering therapy (Moore et al., 2013). Another case–control study reported that metformin use was associated with increased odds for prevalent Alzheimer's disease but without consistent dose–response relationship or replication of findings with metformin monotherapy (Imfeld et al., 2012). Observational evidence further suggests that metformin may reduce overall cancer incidence by 31%, including colon, liver, pancreas, lung, and ovarian cancer (Gandini et al., 2014; Lee et al., 2011; Shi et al., 2019; Tseng, 2012; Xiao et al., 2020; Zhou et al., 2016).
Other drugs have been less studied in appropriate doses/populations, except for ACEi/ARB. In RCTs, acarbose prevented T2D in individuals with glucose intolerance (Chiasson et al., 2002; Holman et al., 2017). The STOP‐NIDDM RCT found a 49% reduction in major cardiovascular events with acarbose, while two RCTs in higher‐risk populations reported conflicted findings (Chiasson et al., 2003; Holman et al., 2017; Yun et al., 2016). Rapalogs have shown promise for boosting the immune response to vaccination and reducing respiratory infections in older individuals (Mannick et al., 2014, 2018). Recent phase 2b and phase 3 studies of RTB101 (also named BEZ235, dactolisib) reported a reduction in laboratory‐confirmed respiratory infections and upregulation of IFN‐induced antiviral genes in older participants. However, since RTB101 is not an FDA‐approved agent, we excluded these studies from grading (Mannick et al., 2021). MB and its derivatives show promise in reducing cognitive decline in mild and moderate Alzheimer's disease, while D+Q have shown beneficial effects in small open‐label pilot clinical trials in idiopathic pulmonary fibrosis and CKD (Hickson et al., 2019; Justice et al., 2019; Wilcock et al., 2018; Wischik et al., 2015).
ACEi/ARB prevented diabetes in RCTs (Bangalore et al., 2016; NAVIGATOR Study Group, 2010; Niklason et al., 2004; Os et al., 2008; Savarese et al., 2013; Song et al., 2012; Tocci et al., 2011). Most RCTs analyzing incident cardiovascular events in hypertensive populations were negative, but meta‐analyses reported cardioprotective effects (Akioyamen et al., 2016; Asselbergs et al., 2004; Brugts et al., 2015; DREAM Trial Investigators et al., 2008; NAVIGATOR Study Group, 2010; Haller et al., 2011; Lithell et al., 2003; Nakao et al., 2012; Thomopoulos et al., 2015). ACEi/ARB are also renoprotective, which is explained by the known beneficial effects of the renin–angiotensin–aldosterone system blockade on blood pressure and renal hemodynamics, rather than gerotherapeutic properties. Intriguingly, observational data suggest that centrally active ACEi may reduce cognitive decline, while non‐centrally active ACEi might increase the risk for dementia (Lithell et al., 2004; Sink et al., 2009). ACEi/ARB might also reduce the risk of pneumonia and pneumonia‐related death, reduce bone loss and hip fracture, and improve physical function (Caldeira et al., 2012; Onder et al., 2002; Rianon et al., 2017; Shea & Witham, 2020; Sumukadas et al., 2007).
4. DISCUSSION
The primary objective of the nascent field of geroscience is to translate the discoveries from basic research on the biology of aging to human studies and ultimately clinical care (Sierra et al., 2021). Current approaches to achieving this goal range from continuing robust research in preclinical models to testing candidate gerotherapeutics in clinical trials against individual age‐related diseases. Vigorous efforts into the development of possible phase‐III trials are needed and repurposing already FDA‐approved drugs represents a promising avenue offering accelerated evidence for geroscience at only a fraction of the cost and a faster rate of return than validating new compounds. A critical issue, therefore, is the identification of the best possible candidates for this purpose. Here, we identify several drugs that, like metformin, are relatively low‐cost molecular entities with a well‐established safety profile, representing possible candidates for inclusion in a geroscience‐guided clinical trial modeled after TAME, and aimed at probing whether a predefined cluster of age‐related diseases or well‐characterized loss of function can be significantly delayed using an approach based on targeting aging biology.
We provide a rigorous assessment of the literature concerning both preclinical and clinical status for several FDA‐approved potential gerotherapeutics. We developed a scoring scale that equally values (6 points each) the preclinical and clinical evidence supporting the claims for each of the nine potential gerotherapeutics selected. Surprisingly, with a score of 12/12, the best evidence was found for a relatively new class of drugs, the SGLT2i, which showed better promise than other known potential gerotherapeutics such as metformin, rapamycin, or acarbose.
The profound effects of SGLT2i on healthspan and lifespan in both animal models and humans may result from the pleiotropic effects beyond renal glucose and sodium handling, including improved mitochondrial function, restoration of autophagy, and promotion of ketogenesis‐dependent dampening of mTORC1 hyperactivation (Durak et al., 2018; Korbut et al., 2020; Tomita et al., 2020). We also identify several drugs that, like metformin, are relatively low‐cost molecular entities with a well‐established safety profile, representing possible candidates for inclusion in a geroscience‐guided clinical trial modeled after TAME, and aimed at probing whether a predefined cluster of age‐related diseases or well‐characterized loss of function can be significantly delayed using an approach based on targeting aging biology. Moreover, our geroscience‐guided literature‐driven approach is concordant with previously proposed data‐driven drug repurposing approaches using evidence from gene expression or model organism studies, with the overlap of rapamycin and senolytics identified as top candidate drugs across these (Dönertaş et al., 2018; Ziehm et al., 2017).
For our analysis, we decided to assign an equal number of points to knowledge derived from geroscience (i.e., preclinical) and to findings from humans (i.e., clinical—both observational and interventional). Although for translational purposes, knowledge from human studies is likely to have more weight than studies in rodents, we gave both equal values for several reasons: (1) The literature on potential gerotherapeutics in rodents is quite extensive and, while many of these studies are too recent to have been considered for clinical trials yet, their potential should not be dismissed based solely on their “novelty”; (2) some mouse studies, like the ITP, are quite robust, thus enhancing their predictive value as potential gerotherapeutics; (3) because of their highly controlled nature, studies in mice often have fewer caveats than similar studies in humans. Thus, while it could be argued that preclinical geroscience knowledge becomes less relevant if a drug has solid data on decreasing all‐cause mortality in humans, we decided it was not appropriate at this time to penalize potential drugs that have strong preclinical evidence.
In evaluating the preclinical data for specific drugs, we only considered rodent models. This approach excludes a sizable amount of literature on lifespan extension in lower organisms such as yeast, nematodes, and flies, but since the interventions have not yet been tested in mammals, approval for a trial in humans would likely take decades. Furthermore, in evaluating data on mouse longevity (a surrogate for all‐cause mortality) we gave more weight to studies that have been validated by the ITP, a well‐characterized, multicentered study that is widely accepted in the field to determine the translatability of gerotherapeutics, based on their rigor and their use of a genetically heterogeneous model that attempts to approximate the diversity in human populations. From the clinical side, we considered drugs that affect either all‐cause mortality (the most relevant parameter) or have a positive effect on unrelated diseases not intended to be the primary target of the drug. Thus, we were interested, for example, in a diabetes drug known to affect molecular pathways involved in aging, and that has been shown to also have a positive effect on incident off‐target age‐related diseases, such as cancer, dementia, or CVD.
Our approach restricted potential gerotherapeutics that affect hallmarks of aging without known effects on healthspan, as well as those that have not been tested for lifespan in animals. Two potential gerotherapeutics that failed in this test are colchicine (an anti‐inflammatory agent) and bisphosphonates (used in the treatment of osteoporosis). Interestingly, several studies show that bisphosphonates can decrease mortality even within critical care settings, where their action is clearly not related to their usual therapeutic use in osteoporosis. So, while these and possibly other FDA‐approved drugs might indeed show great potential as gerotherapeutics, they have not met our inclusion criteria, and their analysis is beyond the scope of this paper. The available data suggests that both colchicine and bisphosphonates should be rapidly tested in the ITP or in other centers, to confirm improved healthspan and lifespan in mammals.
In addition to the classes of drugs discussed above, another type of molecular entity that was not considered in our analysis deserves special mention; we did not consider nutraceuticals, several of which have indeed been shown to positively affect both healthspan and lifespan (e.g., NAD + replenishment). They not only lack robust pharmacological and clinical data, but also fail to serve our main purpose of identifying FDA‐approved drugs with the goal of repurposing them as gerotherapeutics that can reduce the rate of deterioration accompanying aging.
One important point that was also not considered in our analysis involves potential sexually dimorphic effects of gerotherapeutics. This is an important issue as we try to move geroscience into the clinical realm since many of the positive results published by the ITP are sex‐specific, increasing longevity either solely, or at least preferentially in one sex. For example, the ITP has clearly shown that acarbose has convincingly male‐specific effects on longevity (Nadon et al., 2017). Much more work is required in this domain, but we did not consider this variable because, in most human studies, data are adjusted for sex, so that potential differences are obscured, and in many cases, the size of the studies precludes the ability to perform a separate bona fide sex‐specific analysis. Another issue that is relevant for translating findings from geroscience‐guided animal studies into humans is the question of dosing. For instance, the doses of rapamycin or rapalogs used both in mice (ITP studies) and human (Mannick et al, 2014; Mannick et al, 2018; Mannick et al, 2021) studies are several times lower than doses commonly used in clinical practice to induce immunotolerance in transplant patients, or as an adjuvant to therapy in stage IV cancer. Furthermore, in human studies aimed at geroprotection (Mannick et al, 2014; Mannick et al, 2018; Mannick et al, 2021), these drugs have been used intermittently and/or for short periods, unlike in clinical practice where they are used chronically. As a result, especially in human studies, different dosage regimens result in different outcomes, that is, stimulation of the immune response in gerotherapeutic studies with intermittent exposure to low doses vs. immunosuppression in clinical settings with chronic administration of high doses. Investigating these dose‐specific differences will allow for minimizing side effects in gerotherapy clinical trials.
Recent advances in geroscience and aging biology research show that the hallmarks and pillars of aging described almost a decade ago interact strongly with each other, often in an additive or even a synergistic manner (Kennedy et al., 2014; Kulkarni et al., 2020; López‐Otín et al., 2013). It is therefore possible to imagine that future gerotherapies will encompass combinations of various gerotherapeutics (perhaps in lower doses and thus with better safety profile) or, in cases where a patient is already afflicted by a disease, a combination of a gerotherapeutic along with a disease‐specific intervention. For example, elimination of senescent cells in mice can improve the response to chemotherapy, both by further reducing tumor number and size, but also by decreasing side effects such as fatigue. Further research will be needed; however, since while some combinations (e.g., rapamycin +metformin) might result in further benefits than each drug alone, other combinations could result in either blunting of established geroprotective strategies (e.g., metformin or rapamycin with exercise) or worsening risk of toxicity often seen with polypharmacy in older adults (Strong et al., 2016; Walton et al., 2019).
Delaying aging to prevent multiple age‐related diseases is an obviously more exciting and promising approach than attacking one disease at a time, where because of comorbidities, the outcome is a no‐gain exchange of one disease for another. The benefits for health and fulfillment are obvious. However, there are also powerful political, economic, and societal gains to be attained, as framed in The Longevity Dividend (Olshansky, 2016). The economic value that can be derived from applying geroscience principles to healthcare has been recently reassessed showing that a slowdown in aging that increases life expectancy by just one year is worth $38 trillion a year, due to complementary gains in health and longevity (Scott et al., 2021). This is a very conservative estimate since the available data suggest that, for example, metformin has the potential to increase life span by ~3 years (Barzilai et al., 2016; Scott et al., 2021). Thus, targeting aging with gerotherapeutics offers potentially larger economic gains than eradicating individual diseases, and for both quality of life and economic reasons, we cannot afford to forfeit this opportunity for a truly transformational approach to healthcare.
CONFLICT OF INTEREST
ASK is an employee of AbbVie, but his recent employment did not influence the work reviewed or presented in this manuscript. SA, DB, FS, GAK, and NB declare no competing interests.
AUTHOR CONTRIBUTIONS
ASK, SA, and NB conceived the idea for the manuscript. All authors (ASK, SA, DB, FS, GAK, and NB) contributed to the literature search, writing, and editing of the manuscript.
ACKNOWLEDGMENT
This work was supported by The Nathan Shock Center of Excellence for the basic Biology of Aging (P30AG038072) (NB) and the Einstein‐Paul Glenn Foundation for Medical Research Center for the Biology of Human Aging (NB), and NIH grants P30AG067988 and R33AG061456 (GAK).
Kulkarni, A. S. , Aleksic, S. , Berger, D. M. , Sierra, F. , Kuchel, G. A. , & Barzilai, N. (2022). Geroscience‐guided repurposing of FDA‐approved drugs to target aging: A proposed process and prioritization. Aging Cell, 21, e13596. 10.1111/acel.13596
Ameya S. Kulkarni and Sandra Aleksic contributed equally to this work.
Contributor Information
Ameya S. Kulkarni, Email: ameyak225@gmail.com.
Nir Barzilai, Email: nir.barzilai@einsteinmed.org.
REFERENCES
- Ahmadi, S. , Razazan, A. , Nagpal, R. , Jain, S. , Wang, B. O. , Mishra, S. P. , Wang, S. , Justice, J. , Ding, J. , McClain, D. A. , Kritchevsky, S. B. , Kitzman, D. , & Yadav, H. (2020). Metformin reduces aging‐related leaky gut and improves cognitive function by beneficially modulating gut microbiome/goblet cell/mucin axis. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 75(7), e9–e21. 10.1093/gerona/glaa056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akioyamen, L. , Levine, M. , Sherifali, D. , O'Reilly, D. , Frankfurter, C. , Pullenayegum, E. , Goeree, R. , & Tsoi, B. (2016). Cardiovascular and cerebrovascular outcomes of long‐term angiotensin receptor blockade: Meta‐analyses of trials in essential hypertension. Journal of the American Society of Hypertension: JASH, 10(1), 55–69.e1. 10.1016/j.jash.2015.11.005 [DOI] [PubMed] [Google Scholar]
- ASCEND Study Collaborative Group (2018). Effects of aspirin for primary prevention in persons with diabetes mellitus. New England Journal of Medicine, 379(16), 1529–1539. 10.1056/NEJMoa1804988 [DOI] [PubMed] [Google Scholar]
- Asselbergs, F. W. , Diercks, G. F. H. , Hillege, H. L. , van Boven, A. J. , Janssen, W. M. T. , Voors, A. A. , de Zeeuw, D. , de Jong, P. E. , van Veldhuisen, D. J. , & van Gilst, W. H. (2004). Effects of fosinopril and pravastatin on cardiovascular events in subjects with microalbuminuria. Circulation, 110(18), 2809–2816. 10.1161/01.CIR.0000146378.65439.7A [DOI] [PubMed] [Google Scholar]
- Atamna, H. , Nguyen, A. , Schultz, C. , Boyle, K. , Newberry, J. , Kato, H. , & Ames, B. N. (2008). Methylene blue delays cellular senescence and enhances key mitochondrial biochemical pathways. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 22(3), 703–712. 10.1096/fj.07-9610com [DOI] [PubMed] [Google Scholar]
- Bangalore, S. , Fakheri, R. , Toklu, B. , Ogedegbe, G. , Weintraub, H. , & Messerli, F. H. (2016). Angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers in patients without heart failure? Insights from 254,301 patients from randomized trials. Mayo Clinic Proceedings, 91(1), 51–60. 10.1016/j.mayocp.2015.10.019 [DOI] [PubMed] [Google Scholar]
- Bannister, C. A. , Holden, S. E. , Jenkins‐Jones, S. , Morgan, C. L. , Halcox, J. P. , Schernthaner, G. , Mukherjee, J. , & Currie, C. J. (2014). Can people with type 2 diabetes live longer than those without? A comparison of mortality in people initiated with metformin or sulphonylurea monotherapy and matched, non‐diabetic controls. Diabetes, Obesity & Metabolism, 16(11), 1165–1173. 10.1111/dom.12354 [DOI] [PubMed] [Google Scholar]
- Barardo, D. , Thornton, D. , Thoppil, H. , Walsh, M. , Sharifi, S. , Ferreira, S. , Anžič, A. , Fernandes, M. , Monteiro, P. , Grum, T. , Cordeiro, R. , De‐Souza, E. A. , Budovsky, A. , Araujo, N. , Gruber, J. , Petrascheck, M. , Fraifeld, V. E. , Zhavoronkov, A. , Moskalev, A. , & de Magalhães, J. P. (2017). The DrugAge database of aging‐related drugs. Aging Cell, 16(3), 594–597. 10.1111/acel.12585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai, N. , Crandall, J. P. , Kritchevsky, S. B. , & Espeland, M. A. (2016). Metformin as a tool to target aging. Cell Metabolism, 23(6), 1060–1065. 10.1016/j.cmet.2016.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai, N. , Huffman, D. M. , Muzumdar, R. H. , & Bartke, A. (2012). The critical role of metabolic pathways in aging. Diabetes, 61(6), 1315–1322. 10.2337/db11-1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharath, L. P. , Agrawal, M. , McCambridge, G. , Nicholas, D. A. , Hasturk, H. , Liu, J. , Jiang, K. , Liu, R. , Guo, Z. , Deeney, J. , Apovian, C. M. , Snyder‐Cappione, J. , Hawk, G. S. , Fleeman, R. M. , Pihl, R. M. F. , Thompson, K. , Belkina, A. C. , Cui, L. , Proctor, E. A. , … Nikolajczyk, B. S. (2020). Metformin enhances autophagy and normalizes mitochondrial function to alleviate aging‐associated inflammation. Cell Metabolism, 32(1), 44–55.e6. 10.1016/j.cmet.2020.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny, M. V. (2019). Rapamycin for longevity: Opinion article. Aging, 11(19), 8048–8067. 10.18632/aging.102355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode‐Böger, S. M. , Martens‐Lobenhoffer, J. , Täger, M. , Schröder, H. , & Scalera, F. (2005). Aspirin reduces endothelial cell senescence. Biochemical and Biophysical Research Communications, 334(4), 1226–1232. 10.1016/j.bbrc.2005.07.014 [DOI] [PubMed] [Google Scholar]
- Brugts, J. J. , van Vark, L. , Akkerhuis, M. , Bertrand, M. , Fox, K. , Mourad, J.‐J. , & Boersma, E. (2015). Impact of renin‐angiotensin system inhibitors on mortality and major cardiovascular endpoints in hypertension: A number‐needed‐to‐treat analysis. International Journal of Cardiology, 181, 425–429. 10.1016/j.ijcard.2014.11.179 [DOI] [PubMed] [Google Scholar]
- Bulckaen, H. , Prévost, G. , Boulanger, E. , Robitaille, G. , Roquet, V. , Gaxatte, C. , Garçon, G. , Corman, B. , Gosset, P. , Shirali, P. , Creusy, C. , & Puisieux, F. (2008). Low‐dose aspirin prevents age‐related endothelial dysfunction in a mouse model of physiological aging. American Journal of Physiology. Heart and Circulatory Physiology, 294(4), H1562–1570. 10.1152/ajpheart.00241.2007 [DOI] [PubMed] [Google Scholar]
- Burch, J. B. , Augustine, A. D. , Frieden, L. A. , Hadley, E. , Howcroft, T. K. , Johnson, R. , Khalsa, P. S. , Kohanski, R. A. , Li, X. L. , Macchiarini, F. , Niederehe, G. , Oh, Y. S. , Pawlyk, A. C. , Rodriguez, H. , Rowland, J. H. , Shen, G. L. , Sierra, F. , & Wise, B. C. (2014). Advances in geroscience: Impact on healthspan and chronic disease. The Journals of Gerontology: Series A, 69(Suppl_1), S1–S3. 10.1093/gerona/glu041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai, H. , Han, B. , Hu, Y. , Zhao, X. , He, Z. , Chen, X. , Sun, H. , Yuan, J. , Li, Y. , Yang, X. , Kong, W. , & Kong, W. J. (2020). Metformin attenuates the D‐galactose‐induced aging process via the UPR through the AMPK/ERK1/2 signaling pathways. International Journal of Molecular Medicine, 45(3), 715–730. 10.3892/ijmm.2020.4453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldeira, D. , Alarcão, J. , Vaz‐Carneiro, A. , & Costa, J. (2012). Risk of pneumonia associated with use of angiotensin converting enzyme inhibitors and angiotensin receptor blockers: Systematic review and meta‐analysis. BMJ (Clinical Research Ed.), 345, e4260. 10.1136/bmj.e4260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, J. M. , Bellman, S. M. , Stephenson, M. D. , & Lisy, K. (2017). Metformin reduces all‐cause mortality and diseases of ageing independent of its effect on diabetes control: A systematic review and meta‐analysis. Ageing Research Reviews, 40, 31–44. 10.1016/j.arr.2017.08.003 [DOI] [PubMed] [Google Scholar]
- Campbell, J. M. , Stephenson, M. D. , de Courten, B. , Chapman, I. , Bellman, S. M. , & Aromataris, E. (2018). Metformin use associated with reduced risk of dementia in patients with diabetes: A systematic review and meta‐analysis. Journal of Alzheimer’s Disease: JAD, 65(4), 1225–1236. 10.3233/JAD-180263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, L. , Li, L. , & Zuo, Z. (2012). N‐acetylcysteine reverses existing cognitive impairment and increased oxidative stress in glutamate transporter type 3 deficient mice. Neuroscience, 220, 85–89. 10.1016/j.neuroscience.2012.06.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castoldi, F. , Humeau, J. , Martins, I. , Lachkar, S. , Loew, D. , Dingli, F. , Durand, S. , Enot, D. , Bossut, Noëlie , Chery, A. , Aprahamian, F. , Demont, Y. , Opolon, P. , Signolle, N. , Sauvat, A. , Semeraro, M. , Bezu, L. , Baracco, E. E. , Vacchelli, E. , … Pietrocola, F. (2020). Autophagy‐mediated metabolic effects of aspirin. Cell Death Discovery, 6(1), 1–17. 10.1038/s41420-020-00365-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalcante, M. B. , Saccon, T. D. , Nunes, A. D. C. , Kirkland, J. L. , Tchkonia, T. , Schneider, A. , & Masternak, M. M. (2020). Dasatinib plus quercetin prevents uterine age‐related dysfunction and fibrosis in mice. Aging (Albany NY), 12(3), 2711–2722. 10.18632/aging.102772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavender, M. A. , Norhammar, A. , Birkeland, K. I. , Jørgensen, M. E. , Wilding, J. P. , Khunti, K. , Fu, A. Z. , Bodegård, J. , Blak, B. T. , Wittbrodt, E. , Thuresson, M. , Fenici, P. , Hammar, N. , Kosiborod, M. , Kosiborod, M. , Cavender, M. A. , Fu, A. Z. , Wilding, J. P. , Khunti, K. , … Murphy, B. (2018). SGLT‐2 inhibitors and cardiovascular risk. Journal of the American College of Cardiology, 71(22), 2497–2506. 10.1016/j.jacc.2018.01.085 [DOI] [PubMed] [Google Scholar]
- CDC (2017). Deaths, percent of total deaths, and death rates for the 15 leading causes of death in selected age groups, by race and sex: United States, 2015 (No. LCWK3_2015). Atlanta, GA: Centers for Disease Control and Prevention. https://www.cdc.gov/nchs/data/dvs/LCWK3_2015.pdf [Google Scholar]
- Chandra, S. , Jana, M. , & Pahan, K. (2018). Aspirin induces lysosomal biogenesis and attenuates amyloid plaque pathology in a mouse model of Alzheimer’s disease via PPARα. Journal of Neuroscience, 38(30), 6682–6699. 10.1523/JNEUROSCI.0054-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C. , Liu, Y. , Liu, Y. , & Zheng, P. (2010). Mammalian target of rapamycin activation underlies HSC defects in autoimmune disease and inflammation in mice. The Journal of Clinical Investigation, 120(11), 4091–4101. 10.1172/JCI43873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, H. , Ma, Z. , Vanderwaal, R. P. , Feng, Z. , Gonzalez‐Suarez, I. , Wang, S. , Zhang, J. , Roti Roti, J. L. , Gonzalo, S. , & Zhang, J. (2011). The mTOR inhibitor rapamycin suppresses DNA double‐strand break repair. Radiation Research, 175(2), 214–224. 10.1667/rr2323.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Q. , Thompson, J. , Hu, Y. , & Lesnefsky, E. J. (2021). Chronic metformin treatment decreases cardiac injury during ischemia‐reperfusion by attenuating endoplasmic reticulum stress with improved mitochondrial function. Aging, 13(6), 7828–7845. 10.18632/aging.202858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. , Li, H. , Ye, Z. , Găman, M.‐A. , Tan, S. C. , & Zhu, F. (2020). The effect of metformin on carotid intima‐media thickness (CIMT): A systematic review and meta‐analysis of randomized clinical trials. European Journal of Pharmacology, 886, 173458. 10.1016/j.ejphar.2020.173458 [DOI] [PubMed] [Google Scholar]
- Cheng, C. , Lin, C.‐H. , Tsai, Y.‐W. , Tsai, C.‐J. , Chou, P.‐H. , & Lan, T.‐H. (2014). Type 2 diabetes and antidiabetic medications in relation to dementia diagnosis. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 69(10), 1299–1305. 10.1093/gerona/glu073 [DOI] [PubMed] [Google Scholar]
- Chiasson, J.‐L. , Josse, R. G. , Gomis, R. , Hanefeld, M. , Karasik, A. , Laakso, M. , & STOP‐NIDDM Trail Research Group (2002). Acarbose for prevention of type 2 diabetes mellitus: The STOP‐NIDDM randomised trial. Lancet (London, England), 359(9323), 2072–2077. 10.1016/S0140-6736(02)08905-5 [DOI] [PubMed] [Google Scholar]
- Chiasson, J.‐L. , Josse, R. G. , Gomis, R. , Hanefeld, M. , Karasik, A. , Laakso, M. , & STOP‐NIDDM Trial Research Group (2003). Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: The STOP‐NIDDM trial. JAMA, 290(4), 486–494. 10.1001/jama.290.4.486 [DOI] [PubMed] [Google Scholar]
- Correia‐Melo, C. , Birch, J. , Fielder, E. , Rahmatika, D. , Taylor, J. , Chapman, J. , & Passos, J. F. (2019). Rapamycin improves healthspan but not inflammaging in nfκb1‐/‐ mice. Aging Cell, 18(1), e12882. 10.1111/acel.12882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa, M. , Bernardi, J. , Fiuza, T. , Costa, L. , Brandão, R. , & Pereira, M. E. (2016). N‐acetylcysteine protects memory decline induced by streptozotocin in mice. Chemico‐Biological Interactions, 253, 10–17. 10.1016/j.cbi.2016.04.026 [DOI] [PubMed] [Google Scholar]
- Cryer, D. R. , Nicholas, S. P. , Henry, D. H. , Mills, D. J. , & Stadel, B. V. (2005). Comparative outcomes study of metformin intervention versus conventional approach the COSMIC Approach Study. Diabetes Care, 28(3), 539–543. 10.2337/diacare.28.3.539 [DOI] [PubMed] [Google Scholar]
- da Cunha, V. , Tham, D. M. , Martin‐McNulty, B. , Deng, G. , Ho, J. J. , Wilson, D. W. , Rutledge, J. C. , Vergona, R. , Sullivan, M. E. , & Wang, Y.‐X. (2005). Enalapril attenuates angiotensin II‐induced atherosclerosis and vascular inflammation. Atherosclerosis, 178(1), 9–17. 10.1016/j.atherosclerosis.2004.08.023 [DOI] [PubMed] [Google Scholar]
- Dai, Y.‐N. , Wang, J.‐H. , Zhu, J.‐Z. , Lin, J.‐Q. , Yu, C.‐H. , & Li, Y.‐M. (2015). Angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers therapy and colorectal cancer: A systematic review and meta‐analysis. Cancer Causes & Control: CCC, 26(9), 1245–1255. 10.1007/s10552-015-0617-1 [DOI] [PubMed] [Google Scholar]
- de Cavanagh, E. M. , Fraga, C. G. , Ferder, L. , & Inserra, F. (1997). Enalapril and captopril enhance antioxidant defenses in mouse tissues. American Journal of Physiology‐Regulatory, Integrative and Comparative Physiology, 272(2), R514–R518. 10.1152/ajpregu.1997.272.2.R514 [DOI] [PubMed] [Google Scholar]
- Debarba, L. K. , Mulka, A. , Lima, J. , Didyuk, O. , Fakhoury, P. , Koshko, L. , Awada, A. A. , Zhang, K. , Klueh, U. , & Sadagurski, M. (2020). Acarbose protects from central and peripheral metabolic imbalance induced by benzene exposure. Brain, Behavior, and Immunity, 89, 87–99. 10.1016/j.bbi.2020.05.073 [DOI] [PubMed] [Google Scholar]
- Dodds, S. G. , Parihar, M. , Javors, M. , Nie, J. , Musi, N. , Dave Sharp, Z. , & Hasty, P. (2020). Acarbose improved survival for Apc+/Min mice. Aging Cell, 19(2), e13088. 10.1111/acel.13088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dönertaş, H. M. , Valenzuela, M. F. , Partridge, L. , & Thornton, J. M. (2018). Gene expression‐based drug repurposing to target aging. Aging Cell, 17(5), e12819. 10.1111/acel.12819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DREAM Trial Investigators , Dagenais, G. R. , Gerstein, H. C. , Holman, R. , Budaj, A. , Escalante, A. , & Yusuf, S. (2008). Effects of ramipril and rosiglitazone on cardiovascular and renal outcomes in people with impaired glucose tolerance or impaired fasting glucose: Results of the Diabetes REduction Assessment with ramipril and rosiglitazone Medication (DREAM) trial. Diabetes Care, 31(5), 1007–1014. 10.2337/dc07-1868 [DOI] [PubMed] [Google Scholar]
- Duicu, O. M. , Privistirescu, A. , Wolf, A. , Petruş, A. , Dănilă, M. D. , Raţiu, C. D. , Muntean, D. M. , & Sturza, A. (2017). Methylene blue improves mitochondrial respiration and decreases oxidative stress in a substrate‐dependent manner in diabetic rat hearts. Canadian Journal of Physiology and Pharmacology, 95(11), 1376–1382. 10.1139/cjpp-2017-0074 [DOI] [PubMed] [Google Scholar]
- Durak, A. , Olgar, Y. , Degirmenci, S. , Akkus, E. , Tuncay, E. , & Turan, B. (2018). A SGLT2 inhibitor dapagliflozin suppresses prolonged ventricular‐repolarization through augmentation of mitochondrial function in insulin‐resistant metabolic syndrome rats. Cardiovascular Diabetology, 17(1), 144. 10.1186/s12933-018-0790-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekström, N. , Svensson, A.‐M. , Miftaraj, M. , Franzén, S. , Zethelius, B. , Eliasson, B. , & Gudbjörnsdottir, S. (2016). Cardiovascular safety of glucose‐lowering agents as add‐on medication to metformin treatment in type 2 diabetes: Report from the Swedish National Diabetes Register. Diabetes, Obesity & Metabolism, 18(10), 990–998. 10.1111/dom.12704 [DOI] [PubMed] [Google Scholar]
- Espeland, M. A. , Crimmins, E. M. , Grossardt, B. R. , Crandall, J. P. , Gelfond, J. A. L. , Harris, T. B. , Kritchevsky, S. B. , Manson, J. A. E. , Robinson, J. G. , Rocca, W. A. , Temprosa, M. , Thomas, F. , Wallace, R. , & Barzilai, N. (2016). Clinical trials targeting aging and age‐related multimorbidity. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 72(3), 355–361. 10.1093/gerona/glw220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr, J. N. , Xu, M. , Weivoda, M. M. , Monroe, D. G. , Fraser, D. G. , Onken, J. L. , Negley, B. A. , Sfeir, J. G. , Ogrodnik, M. B. , Hachfeld, C. M. , LeBrasseur, N. K. , Drake, M. T. , Pignolo, R. J. , Pirtskhalava, T. , Tchkonia, T. , Oursler, M. J. , Kirkland, J. L. , & Khosla, S. (2017). Targeting cellular senescence prevents age‐related bone loss in mice. Nature Medicine, 23(9), 1072–1079. 10.1038/nm.4385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, M. , Kim, J. , Field, K. , Reid, C. , Chatzistamou, I. , & Shim, M. (2019). Aspirin ameliorates the long‐term adverse effects of doxorubicin through suppression of cellular senescence. FASEB BioAdvances, 1(9), 579–590. 10.1096/fba.2019-00041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, X. , Pan, J. , Li, J. , Zeng, C. , Qi, W. , Shao, Y. , Liu, X. , Liu, L. , Xiao, G. , Zhang, H. , Bai, X. , & Cai, D. (2020). Metformin attenuates cartilage degeneration in an experimental osteoarthritis model by regulating AMPK/mTOR. Aging, 12(2), 1087–1103. 10.18632/aging.102635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flurkey, K. , Astle, C. M. , & Harrison, D. E. (2010). Life extension by diet restriction and N‐acetyl‐L‐cysteine in genetically heterogeneous mice. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 65(12), 1275–1284. 10.1093/gerona/glq155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandini, S. , Puntoni, M. , Heckman‐Stoddard, B. M. , Dunn, B. K. , Ford, L. , DeCensi, A. , & Szabo, E. (2014). Metformin and cancer risk and mortality: A systematic review and meta‐analysis taking into account biases and confounders. Cancer Prevention Research (Philadelphia, Pa.), 7(9), 867–885. 10.1158/1940-6207.CAPR-13-0424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg, G. , Singh, S. , Singh, A. K. , & Rizvi, S. I. (2018). N‐acetyl‐l‐cysteine attenuates oxidative damage and neurodegeneration in rat brain during aging. Canadian Journal of Physiology and Pharmacology, 96(12), 1189–1196. 10.1139/cjpp-2018-0209 [DOI] [PubMed] [Google Scholar]
- Haller, H. , Ito, S. , Izzo, J. L. , Januszewicz, A. , Katayama, S. , Menne, J. , Mimran, A. , Rabelink, T. J. , Ritz, E. , Ruilope, L. M. , Rump, L. C. , & Viberti, G. (2011). Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. New England Journal of Medicine, 364(10), 907–917. 10.1056/NEJMoa1007994 [DOI] [PubMed] [Google Scholar]
- Han, J. H. , Oh, T. J. , Lee, G. , Maeng, H. J. , Lee, D. H. , Kim, K. M. , Choi, S. H. , Jang, H. C. , Lee, H. S. , Park, K. S. , Kim, Y.‐B. , & Lim, S. (2017). The beneficial effects of empagliflozin, an SGLT2 inhibitor, on atherosclerosis in ApoE ‐/‐ mice fed a western diet. Diabetologia, 60(2), 364–376. 10.1007/s00125-016-4158-2 [DOI] [PubMed] [Google Scholar]
- Hansson, L. , Lindholm, L. H. , Niskanen, L. , Lanke, J. , Hedner, T. , Niklason, A. , Luomanmäki, K. , Dahlöf, B. , de Faire, U. , Mörlin, C. , Karlberg, B. E. , Wester, P. O. , & Björck, J.‐E. (1999). Effect of angiotensin‐converting‐enzyme inhibition compared with conventional therapy on cardiovascular morbidity and mortality in hypertension: The Captopril Prevention Project (CAPPP) randomised trial. Lancet (London, England), 353(9153), 611–616. 10.1016/s0140-6736(98)05012-0 [DOI] [PubMed] [Google Scholar]
- Hansson, L. , Zanchetti, A. , Carruthers, S. G. , Dahlöf, B. , Elmfeldt, D. , Julius, S. , Ménard, J. , Rahn, K. H. , Wedel, H. , & Westerling, S. (1998). Effects of intensive blood‐pressure lowering and low‐dose aspirin in patients with hypertension: Principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet (London, England), 351(9118), 1755–1762. 10.1016/s0140-6736(98)04311-6 [DOI] [PubMed] [Google Scholar]
- Harrison, D. E. , Strong, R. , Alavez, S. , Astle, C. M. , DiGiovanni, J. , Fernandez, E. , Flurkey, K. , Garratt, M. , Gelfond, J. A. L. , Javors, M. A. , Levi, M. , Lithgow, G. J. , Macchiarini, F. , Nelson, J. F. , Sukoff Rizzo, S. J. , Slaga, T. J. , Stearns, T. , Wilkinson, J. E. , & Miller, R. A. (2019). Acarbose improves health and lifespan in aging HET3 mice. Aging Cell, 18(2), e12898. 10.1111/acel.12898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison, D. E. , Strong, R. , Allison, D. B. , Ames, B. N. , Astle, C. M. , Atamna, H. , Fernandez, E. , Flurkey, K. , Javors, M. A. , Nadon, N. L. , Nelson, J. F. , Pletcher, S. , Simpkins, J. W. , Smith, D. , Wilkinson, J. E. , & Miller, R. A. (2014). Acarbose, 17‐α‐estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell, 13(2), 273–282. 10.1111/acel.12170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig, J. , Loebel, M. , Steiner, S. , Bauer, S. , Karadeniz, Z. , Roeger, C. , Skurk, C. , Scheibenbogen, C. , & Sotzny, F. (2021). Metformin attenuates ROS via FOXO3 activation in immune cells. Frontiers in Immunology, 12, 581799. 10.3389/fimmu.2021.581799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerspink, H. J. L. , Stefánsson, B. V. , Correa‐Rotter, R. , Chertow, G. M. , Greene, T. , Hou, F.‐F. , Mann, J. F. E. , McMurray, J. J. V. , Lindberg, M. , Rossing, P. , Sjöström, C. D. , Toto, R. D. , Langkilde, A.‐M. , & Wheeler, D. C. (2020). Dapagliflozin in patients with chronic kidney disease. New England Journal of Medicine, 383(15), 1436–1446. 10.1056/NEJMoa2024816 [DOI] [PubMed] [Google Scholar]
- Herrera, J. J. , Louzon, S. , Pifer, K. , Leander, D. , Merrihew, G. E. , Park, J. H. , Szczesniak, K. , Whitson, J. , Wilkinson, J. E. , Fiehn, O. , MacCoss, M. J. , Day, S. M. , Miller, R. A. , & Garratt, M. (2020). Acarbose has sex‐dependent and ‐independent effects on age‐related physical function, cardiac health, and lipid biology. JCI Insight, 5(21), 137474. 10.1172/jci.insight.137474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickson, L. T. J. , Langhi Prata, L. G. P. , Bobart, S. A. , Evans, T. K. , Giorgadze, N. , Hashmi, S. K. , Herrmann, S. M. , Jensen, M. D. , Jia, Q. , Jordan, K. L. , Kellogg, T. A. , Khosla, S. , Koerber, D. M. , Lagnado, A. B. , Lawson, D. K. , LeBrasseur, N. K. , Lerman, L. O. , McDonald, K. M. , McKenzie, T. J. , … Kirkland, J. L. (2019). Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine, 47, 446–456. 10.1016/j.ebiom.2019.08.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman, R. R. , Coleman, R. L. , Chan, J. C. N. , Chiasson, J.‐L. , Feng, H. , Ge, J. , Gerstein, H. C. , Gray, R. , Huo, Y. , Lang, Z. , McMurray, J. J. , Rydén, L. , Schröder, S. , Sun, Y. , Theodorakis, M. J. , Tendera, M. , Tucker, L. , Tuomilehto, J. , Wei, Y. , … Chen, J. (2017). Effects of acarbose on cardiovascular and diabetes outcomes in patients with coronary heart disease and impaired glucose tolerance (ACE): A randomised, double‐blind, placebo‐controlled trial. The Lancet Diabetes & Endocrinology, 5(11), 877–886. 10.1016/S2213-8587(17)30309-1 [DOI] [PubMed] [Google Scholar]
- Hong, J. , Zhang, Y. , Lai, S. , Lv, A. , Su, Q. , Dong, Y. , Zhou, Z. , Tang, W. , Zhao, J. , Cui, L. , Zou, D. , Wang, D. , Li, H. , Liu, C. , Wu, G. , Shen, J. , Zhu, D. , Wang, W. , Shen, W. , & Ning, G. (2013). Effects of metformin versus glipizide on cardiovascular outcomes in patients with type 2 diabetes and coronary artery disease. Diabetes Care, 36(5), 1304–1311. 10.2337/dc12-0719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa, M. , Arai, T. , Masuda‐Suzukake, M. , Nonaka, T. , Yamashita, M. , Akiyama, H. , & Hasegawa, M. (2012). Methylene blue reduced abnormal tau accumulation in P301L tau transgenic mice. PLoS One, 7(12), e52389. 10.1371/journal.pone.0052389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman, D. M. , Justice, J. N. , Stout, M. B. , Kirkland, J. L. , Barzilai, N. , & Austad, S. N. (2016). Evaluating health span in preclinical models of aging and disease: Guidelines, challenges, and opportunities for geroscience. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 71(11), 1395–1406. 10.1093/gerona/glw106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imfeld, P. , Bodmer, M. , Jick, S. S. , & Meier, C. R. (2012). Metformin, other antidiabetic drugs, and risk of Alzheimer’s disease: A population‐based case‐control study. Journal of the American Geriatrics Society, 60(5), 916–921. 10.1111/j.1532-5415.2012.03916.x [DOI] [PubMed] [Google Scholar]
- Iske, J. , Seyda, M. , Heinbokel, T. , Maenosono, R. , Minami, K. , Nian, Y. , Quante, M. , Falk, C. S. , Azuma, H. , Martin, F. , Passos, J. F. , Niemann, C. U. , Tchkonia, T. , Kirkland, J. L. , Elkhal, A. , & Tullius, S. G. (2020). Senolytics prevent mt‐DNA‐induced inflammation and promote the survival of aged organs following transplantation. Nature Communications, 11(1), 4289. 10.1038/s41467-020-18039-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong, C.‐B. , Chen, K.‐Y. , Hsieh, M.‐Y. , Su, F.‐Y. , Wu, C.‐C. , Voon, W.‐C. , Hsieh, I.‐C. , Shyu, K.‐G. , Chong, J.‐T. , Lin, W.‐S. , Hsu, C.‐N. , Ueng, K.‐C. , & Lai, C.‐L. (2019). Metformin was associated with lower all‐cause mortality in type 2 diabetes with acute coronary syndrome: A Nationwide registry with propensity score‐matched analysis. International Journal of Cardiology, 291, 152–157. 10.1016/j.ijcard.2019.03.021 [DOI] [PubMed] [Google Scholar]
- Jorda, A. , Aldasoro, M. , Aldasoro, C. , Guerra‐Ojeda, S. , Iradi, A. , Vila, J. M. , Campos‐Campos, J. , & Valles, S. L. (2020). Action of low doses of Aspirin in Inflammation and Oxidative Stress induced by aβ1‐42 on Astrocytes in primary culture. International Journal of Medical Sciences, 17(6), 834–843. 10.7150/ijms.40959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice, J. N. , Nambiar, A. M. , Tchkonia, T. , LeBrasseur, N. K. , Pascual, R. , Hashmi, S. K. , Prata, L. , Masternak, M. M. , Kritchevsky, S. B. , Musi, N. , & Kirkland, J. L. (2019). Senolytics in idiopathic pulmonary fibrosis: Results from a first‐in‐human, open‐label, pilot study. EBioMedicine, 40, 554–563. 10.1016/j.ebiom.2018.12.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice, J. N. , Niedernhofer, L. , Robbins, P. D. , Aroda, V. R. , Espeland, M. A. , Kritchevsky, S. B. , Kuchel, G. A. , & Barzilai, N. (2018). Development of clinical trials to extend healthy lifespan. Cardiovascular Endocrinology & Metabolism, 7(4), 80–83. 10.1097/XCE.0000000000000159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein, M. , & Galvan, V. (2019). Rapamycin and Alzheimer’s disease: Time for a clinical trial? Science Translational Medicine, 11(476), eaar4289. 10.1126/scitranslmed.aar4289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller, K. , Kane, A. , Heinze‐Milne, S. , Grandy, S. A. , & Howlett, S. E. (2019). Chronic treatment with the ACE inhibitor enalapril attenuates the development of frailty and differentially modifies pro‐ and anti‐inflammatory cytokines in aging male and female C57BL/6 mice. The Journals of Gerontology: Series A, 74(8), 1149–1157. 10.1093/gerona/gly219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy, B. K. , Berger, S. L. , Brunet, A. , Campisi, J. , Cuervo, A. M. , Epel, E. S. , Franceschi, C. , Lithgow, G. J. , Morimoto, R. I. , Pessin, J. E. , Rando, T. A. , Richardson, A. , Schadt, E. E. , Wyss‐Coray, T. , & Sierra, F. (2014). Geroscience: Linking aging to chronic disease. Cell, 159(4), 709–713. 10.1016/j.cell.2014.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H. , Yu, M. R. , Lee, H. , Kwon, S. H. , Jeon, J. S. , Han, D. C. , & Noh, H. (2021). Metformin inhibits chronic kidney disease‐induced DNA damage and senescence of mesenchymal stem cells. Aging Cell, 20(2), e13317. 10.1111/acel.13317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowler, W. C. , Barrett‐Connor, E. , Fowler, S. E. , Hamman, R. F. , Lachin, J. M. , Walker, E. A. , Nathan, D. M. , & Diabetes Prevention Program Research Group (2002). Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. The New England Journal of Medicine, 346(6), 393–403. 10.1056/NEJMoa012512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodali, M. , Attaluri, S. , Madhu, L. N. , Shuai, B. , Upadhya, R. , Gonzalez, J. J. , Rao, X. , & Shetty, A. K. (2021). Metformin treatment in late middle age improves cognitive function with alleviation of microglial activation and enhancement of autophagy in the hippocampus. Aging Cell, 20(2), e13277. 10.1111/acel.13277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig, A. M. , Mechanic‐Hamilton, D. , Xie, S. X. , Combs, M. F. , Cappola, A. R. , Xie, L. , Detre, J. A. , Wolk, D. A. , & Arnold, S. E. (2017). Effects of the insulin sensitizer metformin in Alzheimer disease: Pilot data from a randomized placebo‐controlled crossover study. Alzheimer Disease and Associated Disorders, 31(2), 107–113. 10.1097/WAD.0000000000000202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korbut, A. I. , Taskaeva, I. S. , Bgatova, N. P. , Muraleva, N. A. , Orlov, N. B. , Dashkin, M. V. , Khotskina, A. S. , Zavyalov, E. L. , Konenkov, V. I. , Klein, T. , & Klimontov, V. V. (2020). SGLT2 inhibitor empagliflozin and DPP4 inhibitor linagliptin reactivate glomerular autophagy in db/db mice, a model of type 2 diabetes. International Journal of Molecular Sciences, 21(8), E2987. 10.3390/ijms21082987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosiborod, M. , Cavender, M. A. , Fu, A. Z. , Wilding, J. P. , Khunti, K. , Holl, R. W. , Norhammar, A. , Birkeland, K. I. , Jørgensen, M. E. , Thuresson, M. , Arya, N. , Bodegård, J. , Hammar, N. , Fenici, P. , & CVD‐REAL Investigators and Study Group (2017). Lower risk of heart failure and death in patients initiated on sodium‐glucose cotransporter‐2 inhibitors versus other glucose‐lowering drugs: The CVD‐REAL study (comparative effectiveness of cardiovascular outcomes in new users of sodium‐glucose cotransporter‐2 inhibitors). Circulation, 136(3), 249–259. 10.1161/CIRCULATIONAHA.117.029190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraig, E. , Linehan, L. A. , Liang, H. , Romo, T. Q. , Liu, Q. , Wu, Y. , Benavides, A. D. , Curiel, T. J. , Javors, M. A. , Musi, N. , Chiodo, L. , Koek, W. , Gelfond, J. A. L. , & Kellogg, D. L. (2018). A randomized control trial to establish the feasibility and safety of rapamycin treatment in an older human cohort: Immunological, physical performance, and cognitive effects. Experimental Gerontology, 105, 53–69. 10.1016/j.exger.2017.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritchevsky, S. B. , & Justice, J. N. (2020). Testing the geroscience hypothesis: Early days. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 75(1), 99–101. 10.1093/gerona/glz267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni, A. S. , Brutsaert, E. F. , Anghel, V. , Zhang, K. , Bloomgarden, N. , Pollak, M. , Mar, J. C. , Hawkins, M. , Crandall, J. P. , & Barzilai, N. (2018). Metformin regulates metabolic and nonmetabolic pathways in skeletal muscle and subcutaneous adipose tissues of older adults. Aging Cell, 17(2), e12723. 10.1111/acel.12723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni, A. S. , Gubbi, S. , & Barzilai, N. (2020). Benefits of metformin in attenuating the hallmarks of aging. Cell Metabolism, 32(1), 15–30. 10.1016/j.cmet.2020.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laberge, R.‐M. , Sun, Y. U. , Orjalo, A. V. , Patil, C. K. , Freund, A. , Zhou, L. , Curran, S. C. , Davalos, A. R. , Wilson‐Edell, K. A. , Liu, S. U. , Limbad, C. , Demaria, M. , Li, P. , Hubbard, G. B. , Ikeno, Y. , Javors, M. , Desprez, P.‐Y. , Benz, C. C. , Kapahi, P. , … Campisi, J. (2015). MTOR regulates the pro‐tumorigenic senescence‐associated secretory phenotype by promoting IL1A translation. Nature Cell Biology, 17(8), 1049–1061. 10.1038/ncb3195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamming, D. W. , Ye, L. , Katajisto, P. , Goncalves, M. D. , Saitoh, M. , Stevens, D. M. , Davis, J. G. , Salmon, A. B. , Richardson, A. , Ahima, R. S. , Guertin, D. A. , Sabatini, D. M. , & Baur, J. A. (2012). Rapamycin‐induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science (New York, N.Y.), 335(6076), 1638–1643. 10.1126/science.1215135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landman, G. W. D. , Kleefstra, N. , van Hateren, K. J. J. , Groenier, K. H. , Gans, R. O. B. , & Bilo, H. J. G. (2010). Metformin associated with lower cancer mortality in type 2 diabetes: ZODIAC‐16. Diabetes Care, 33(2), 322–326. 10.2337/dc09-1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, M.‐S. , Hsu, C.‐C. , Wahlqvist, M. L. , Tsai, H.‐N. , Chang, Y.‐H. , & Huang, Y.‐C. (2011). Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: A representative population prospective cohort study of 800,000 individuals. BMC Cancer, 11, 20. 10.1186/1471-2407-11-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng, W. , Ouyang, X. , Lei, X. , Wu, M. , Chen, L. , Wu, Q. , & Liang, Z. (2016). The SGLT‐2 inhibitor dapagliflozin has a therapeutic effect on atherosclerosis in diabetic ApoE‐/‐ mice. Mediators of Inflammation, 2016, 6305735. 10.1155/2016/6305735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leontieva, O. V. , Demidenko, Z. N. , & Blagosklonny, M. V. (2014). Rapamycin reverses insulin resistance (IR) in high‐glucose medium without causing IR in normoglycemic medium. Cell Death & Disease, 5, e1214. 10.1038/cddis.2014.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin, R. L. , Degrange, M. A. , Bruno, G. F. , Del Mazo, C. D. , Taborda, D. J. , Griotti, J. J. , & Boullon, F. J. (2004). Methylene blue reduces mortality and morbidity in vasoplegic patients after cardiac surgery. The Annals of Thoracic Surgery, 77(2), 496–499. 10.1016/S0003-4975(03)01510-8 [DOI] [PubMed] [Google Scholar]
- Lin, X. , Han, L. , Weng, J. , Wang, K. , & Chen, T. (2018). Rapamycin inhibits proliferation and induces autophagy in human neuroblastoma cells. Bioscience Reports, 38(6), BSR20181822. 10.1042/BSR20181822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lithell, H. , Hansson, L. , Skoog, I. , Elmfeldt, D. , Hofman, A. , Olofsson, B. , Trenkwalder, P. , & Zanchetti, A. , & SCOPE Study Group (2003). The Study on Cognition and Prognosis in the Elderly (SCOPE): Principal results of a randomized double‐blind intervention trial. Journal of Hypertension, 21(5), 875–886. 10.1097/00004872-200305000-00011 [DOI] [PubMed] [Google Scholar]
- Lithell, H. , Hansson, L. , Skoog, I. , Elmfeldt, D. , Hofman, A. , Olofsson, B. , Trenkwalder, P. , Zanchetti, A. , & SCOPE Study Group (2004). The Study on COgnition and Prognosis in the Elderly (SCOPE); outcomes in patients not receiving add‐on therapy after randomization. Journal of Hypertension, 22(8), 1605–1612. 10.1097/01.hjh.0000133730.47372.4c [DOI] [PubMed] [Google Scholar]
- Liu, J. , Liu, M. , Ye, X. , Liu, K. , Huang, J. , Wang, L. , Ji, G. , Liu, N. A. , Tang, X. , Baltz, J. M. , Keefe, D. L. , & Liu, L. (2012). Delay in oocyte aging in mice by the antioxidant N‐acetyl‐l‐cysteine (NAC). Human Reproduction (Oxford, England), 27(5), 1411–1420. 10.1093/humrep/des019 [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Xu, J. , Wu, M. , Xu, B. , & Kang, L. (2021). Empagliflozin protects against atherosclerosis progression by modulating lipid profiles and sympathetic activity. Lipids in Health and Disease, 20(1), 5. 10.1186/s12944-021-01430-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- López‐Otín, C. , Blasco, M. A. , Partridge, L. , Serrano, M. , & Kroemer, G. (2013). The hallmarks of aging. Cell, 153(6), 1194–1217. 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchsinger, J. A. , Ma, Y. , Christophi, C. A. , Florez, H. , Golden, S. H. , Hazuda, H. , Crandall, J. , Venditti, E. , Watson, K. , Jeffries, S. , Manly, J. J. , & Pi‐Sunyer, F. X. , & Diabetes Prevention Program Research Group (2017). Metformin, lifestyle intervention, and cognition in the diabetes prevention program outcomes study. Diabetes Care, 40(7), 958–965. 10.2337/dc16-2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchsinger, J. A. , Perez, T. , Chang, H. , Mehta, P. , Steffener, J. , Pradabhan, G. , Ichise, M. , Manly, J. , Devanand, D. P. , & Bagiella, E. (2016). Metformin in amnestic mild cognitive impairment: Results of a pilot randomized placebo controlled clinical trial. Journal of Alzheimer’s Disease: JAD, 51(2), 501–514. 10.3233/JAD-150493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannick, J. B. , Del Giudice, G. , Lattanzi, M. , Valiante, N. M. , Praestgaard, J. , Huang, B. , Lonetto, M. A. , Maecker, H. T. , Kovarik, J. , Carson, S. , Glass, D. J. , & Klickstein, L. B. (2014). mTOR inhibition improves immune function in the elderly. Science Translational Medicine, 6(268), 268ra179. 10.1126/scitranslmed.3009892 [DOI] [PubMed] [Google Scholar]
- Mannick, J. B. , Morris, M. , Hockey, H.‐U. , Roma, G. , Beibel, M. , Kulmatycki, K. , Watkins, M. , Shavlakadze, T. , Zhou, W. , Quinn, D. , Glass, D. J. , & Klickstein, L. B. (2018). TORC1 inhibition enhances immune function and reduces infections in the elderly. Science Translational Medicine, 10(449), eaaq1564. 10.1126/scitranslmed.aaq1564 [DOI] [PubMed] [Google Scholar]
- Mannick, J. B. , Teo, G. , Bernardo, P. , Quinn, D. , Russell, K. , Klickstein, L. , Marshall, W. , & Shergill, S. (2021). Targeting the biology of ageing with mTOR inhibitors to improve immune function in older adults: Phase 2b and phase 3 randomised trials. The Lancet Healthy Longevity, 2(5), e250–e262. 10.1016/S2666-7568(21)00062-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie, A. , Meunier, J. , Brun, E. , Malmstrom, S. , Baudoux, V. , Flaszka, E. , Naert, G. , Roman, F. , Cosnier‐Pucheu, S. , & Gonzalez‐Gonzalez, S. (2018). N‐acetylcysteine treatment reduces age‐related hearing loss and memory impairment in the senescence‐accelerated prone 8 (SAMP8) mouse model. Aging and Disease, 9(4), 664–673. 10.14336/AD.2017.0930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez, M. , Hernández, A. I. , & Martínez, N. (2000). N‐Acetylcysteine delays age‐associated memory impairment in mice: Role in synaptic mitochondria. Brain Research, 855(1), 100–106. 10.1016/s0006-8993(99)02349-5 [DOI] [PubMed] [Google Scholar]
- Martin‐Montalvo, A. , Mercken, E. M. , Mitchell, S. J. , Palacios, H. H. , Mote, P. L. , Scheibye‐Knudsen, M. , Gomes, A. P. , Ward, T. M. , Minor, R. K. , Blouin, M.‐J. , Schwab, M. , Pollak, M. , Zhang, Y. , Yu, Y. , Becker, K. G. , Bohr, V. A. , Ingram, D. K. , Sinclair, D. A. , Wolf, N. S. , … de Cabo, R. (2013). Metformin improves healthspan and lifespan in mice. Nature Communications, 4(1), 2192. 10.1038/ncomms3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martyanov, V. , Kim, G.‐H. , Hayes, W. , Du, S. , Ganguly, B. J. , Sy, O. , Lee, S. K. , Bogatkevich, G. S. , Schieven, G. L. , Schiopu, E. , Marangoni, R. G. , Goldin, J. , Whitfield, M. L. , & Varga, J. (2017). Novel lung imaging biomarkers and skin gene expression subsetting in dasatinib treatment of systemic sclerosis‐associated interstitial lung disease. PLoS One, 12(11), e0187580. 10.1371/journal.pone.0187580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray, J. J. V. , Solomon, S. D. , Inzucchi, S. E. , Køber, L. , Kosiborod, M. N. , Martinez, F. A. , Ponikowski, P. , Sabatine, M. S. , Anand, I. S. , Bělohlávek, J. , Böhm, M. , Chiang, C.‐E. , Chopra, V. K. , de Boer, R. A. , Desai, A. S. , Diez, M. , Drozdz, J. , Dukát, A. , Ge, J. , … Langkilde, A.‐M. (2019). Dapagliflozin in patients with heart failure and reduced ejection fraction. New England Journal of Medicine, 381(21), 1995–2008. 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- McNeil, J. J. , Nelson, M. R. , Woods, R. L. , Lockery, J. E. , Wolfe, R. , Reid, C. M. , Kirpach, B. , Shah, R. C. , Ives, D. G. , Storey, E. , Ryan, J. , Tonkin, A. M. , Newman, A. B. , Williamson, J. D. , Margolis, K. L. , Ernst, M. E. , Abhayaratna, W. P. , Stocks, N. , Fitzgerald, S. M. , … Murray, A. M. (2018). Effect of aspirin on all‐cause mortality in the healthy elderly. New England Journal of Medicine, 379(16), 1519–1528. 10.1056/NEJMoa1803955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, R. A. , Harrison, D. E. , Allison, D. B. , Bogue, M. , Debarba, L. , Diaz, V. , Fernandez, E. , Galecki, A. , Garvey, W. T. , Jayarathne, H. , Kumar, N. , Javors, M. A. , Ladiges, W. C. , Macchiarini, F. , Nelson, J. , Reifsnyder, P. , Rosenthal, N. A. , Sadagurski, M. , Salmon, A. B. , … Strong, R. (2020). Canagliflozin extends life span in genetically heterogeneous male but not female mice. JCI Insight, 5(21), 140019. 10.1172/jci.insight.140019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, R. A. , Harrison, D. E. , Astle, C. M. , Baur, J. A. , Boyd, A. R. , de Cabo, R. , Fernandez, E. , Flurkey, K. , Javors, M. A. , Nelson, J. F. , Orihuela, C. J. , Pletcher, S. , Sharp, Z. D. , Sinclair, D. , Starnes, J. W. , Wilkinson, J. E. , Nadon, N. L. , & Strong, R. (2011). Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 66(2), 191–201. 10.1093/gerona/glq178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, R. A. , Harrison, D. E. , Astle, C. M. , Fernandez, E. , Flurkey, K. , Han, M. , Javors, M. A. , Li, X. , Nadon, N. L. , Nelson, J. F. , Pletcher, S. , Salmon, A. B. , Sharp, Z. D. , Van Roekel, S. , Winkleman, L. , & Strong, R. (2014). Rapamycin‐mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell, 13(3), 468–477. 10.1111/acel.12194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moiseeva, O. , Deschênes‐Simard, X. , St‐Germain, E. , Igelmann, S. , Huot, G. , Cadar, A. E. , & Ferbeyre, G. (2013). Metformin inhibits the senescence‐associated secretory phenotype by interfering with IKK/NF‐κB activation. Aging Cell, 12(3), 489–498. 10.1111/acel.12075 [DOI] [PubMed] [Google Scholar]
- Moore, E. M. , Mander, A. G. , Ames, D. , Kotowicz, M. A. , Carne, R. P. , Brodaty, H. , Woodward, M. , Boundy, K. , Ellis, K. A. , Bush, A. I. , Faux, N. G. , Martins, R. , Szoeke, C. , Rowe, C. , & Watters, D. A. (2013). Increased risk of cognitive impairment in patients with diabetes is associated with metformin. Diabetes Care, 36(10), 2981–2987. 10.2337/dc13-0229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- More, J. , Galusso, N. , Veloso, P. , Montecinos, L. , Finkelstein, J. P. , Sanchez, G. , Bull, R. , Valdés, J. L. , Hidalgo, C. , & Paula‐Lima, A. (2018). N‐acetylcysteine prevents the spatial memory deficits and the redox‐dependent RyR2 decrease displayed by an Alzheimer’s disease rat model. Frontiers in Aging Neuroscience, 10, 399. 10.3389/fnagi.2018.00399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadon, N. L. , Strong, R. , Miller, R. A. , & Harrison, D. E. (2017). NIA interventions testing program: Investigating putative aging intervention agents in a genetically heterogeneous mouse model. EBioMedicine, 21, 3–4. 10.1016/j.ebiom.2016.11.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao, Y. M. , Teramukai, S. , Tanaka, S. , Yasuno, S. , Fujimoto, A. , Kasahara, M. , Ueshima, K. , Nakao, K. , Hinotsu, S. , Nakao, K. , & Kawakami, K. (2012). Effects of renin‐angiotensin system blockades on cardiovascular outcomes in patients with diabetes mellitus: A systematic review and meta‐analysis. Diabetes Research and Clinical Practice, 96(1), 68–75. 10.1016/j.diabres.2011.11.025 [DOI] [PubMed] [Google Scholar]
- NAVIGATOR Study Group (2010). Effect of valsartan on the incidence of diabetes and cardiovascular events. New England Journal of Medicine, 362(16), 1477–1490. 10.1056/NEJMoa1001121 [DOI] [PubMed] [Google Scholar]
- Neal, B. , Perkovic, V. , Mahaffey, K. W. , de Zeeuw, D. , Fulcher, G. , Erondu, N. , Shaw, W. , Law, G. , Desai, M. , & Matthews, D. R. (2017). Canagliflozin and cardiovascular and renal events in type 2 diabetes. New England Journal of Medicine, 377(7), 644–657. 10.1056/NEJMoa1611925 [DOI] [PubMed] [Google Scholar]
- Niklason, A. , Hedner, T. , Niskanen, L. , & Lanke, J. , & Captopril Prevention Project Study Group (2004). Development of diabetes is retarded by ACE inhibition in hypertensive patients—A subanalysis of the Captopril Prevention Project (CAPPP). Journal of Hypertension, 22(3), 645–652. 10.1097/00004872-200403000-00029 [DOI] [PubMed] [Google Scholar]
- Noreen, F. , Chaber‐Ciopinska, A. , Regula, J. , Schär, P. , & Truninger, K. (2020). Longitudinal analysis of healthy colon establishes aspirin as a suppressor of cancer‐related epigenetic aging. Clinical Epigenetics, 12(1), 164. 10.1186/s13148-020-00956-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novelle, M. G. , Ali, A. , Diéguez, C. , Bernier, M. , & de Cabo, R. (2016). Metformin: A hopeful promise in aging research. Cold Spring Harbor Perspectives in Medicine, 6(3), a025932. 10.1101/cshperspect.a025932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olgar, Y. , Tuncay, E. , Degirmenci, S. , Billur, D. , Dhingra, R. , Kirshenbaum, L. , & Turan, B. (2020). Ageing‐associated increase in SGLT2 disrupts mitochondrial/sarcoplasmic reticulum Ca2+ homeostasis and promotes cardiac dysfunction. Journal of Cellular and Molecular Medicine, 24(15), 8567–8578. 10.1111/jcmm.15483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olshansky, S. J. (2016). Articulating the case for the longevity dividend. Cold Spring Harbor Perspectives in Medicine, 6(2), a025940. 10.1101/cshperspect.a025940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onder, G. , Penninx, B. W. J. H. , Balkrishnan, R. , Fried, L. P. , Chaves, P. H. M. , Williamson, J. , Carter, C. , Bari, M. D. , Guralnik, J. M. , & Pahor, M. (2002). Relation between use of angiotensin‐converting enzyme inhibitors and muscle strength and physical function in older women: An observational study. Lancet (London, England), 359(9310), 926–930. 10.1016/s0140-6736(02)08024-8 [DOI] [PubMed] [Google Scholar]
- Oparil, S. , Davis, B. R. , Cushman, W. C. , Ford, C. E. , Furberg, C. D. , Habib, G. B. , Haywood, L. J. , Margolis, K. , Probstfield, J. L. , Whelton, P. K. , & Wright, J. T. , & ALLHAT Collaborative Research Group (2013). Mortality and morbidity during and after antihypertensive and lipid‐lowering treatment to prevent heart attack trial: Results by sex. Hypertension (Dallas, Tex.: 1979), 61(5), 977–986. 10.1161/HYPERTENSIONAHA.111.00213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Os, I. , Franco, V. , Kjeldsen, S. E. , Manhem, K. , Devereux, R. B. , Gerdts, E. , Hille, D. A. , Lyle, P. A. , Okin, P. M. , Dahlöf, B. , & Oparil, S. (2008). Effects of losartan in women with hypertension and left ventricular hypertrophy: Results from the Losartan Intervention for Endpoint Reduction in Hypertension Study. Hypertension (Dallas, Tex.: 1979), 51(4), 1103–1108. 10.1161/HYPERTENSIONAHA.107.105296 [DOI] [PubMed] [Google Scholar]
- Ou, H.‐T. , Chang, K.‐C. , Li, C.‐Y. , & Wu, J.‐S. (2016). Risks of cardiovascular diseases associated with dipeptidyl peptidase‐4 inhibitors and other antidiabetic drugs in patients with type 2 diabetes: A nation‐wide longitudinal study. Cardiovascular Diabetology, 15, 41. 10.1186/s12933-016-0350-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer, M. , Anker, S. D. , Butler, J. , Filippatos, G. , Pocock, S. J. , Carson, P. , Januzzi, J. , Verma, S. , Tsutsui, H. , Brueckmann, M. , Jamal, W. , Kimura, K. , Schnee, J. , Zeller, C. , Cotton, D. , Bocchi, E. , Böhm, M. , Choi, D.‐J. , Chopra, V. , … Zannad, F. (2020). Cardiovascular and renal outcomes with empagliflozin in heart failure. New England Journal of Medicine, 383(15), 1413–1424. 10.1056/NEJMoa2022190 [DOI] [PubMed] [Google Scholar]
- Palliyaguru, D. L. , Moats, J. M. , Di Germanio, C. , Bernier, M. , & de Cabo, R. (2019). Frailty index as a biomarker of lifespan and healthspan: Focus on pharmacological interventions. Mechanisms of Ageing and Development, 180, 42–48. 10.1016/j.mad.2019.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschoal, V. A. , Amano, M. T. , Belchior, T. , Magdalon, J. , Chimin, P. , Andrade, M. L. , Ortiz‐Silva, M. , Castro, É. , Yamashita, A. S. , Rosa Neto, J. C. , Câmara, N. O. , & Festuccia, W. T. (2017). MTORC1 inhibition with rapamycin exacerbates adipose tissue inflammation in obese mice and dissociates macrophage phenotype from function. Immunobiology, 222(2), 261–271. 10.1016/j.imbio.2016.09.014 [DOI] [PubMed] [Google Scholar]
- Perkovic, V. , Jardine, M. J. , Neal, B. , Bompoint, S. , Heerspink, H. J. L. , Charytan, D. M. , Edwards, R. , Agarwal, R. , Bakris, G. , Bull, S. , Cannon, C. P. , Capuano, G. , Chu, P.‐L. , de Zeeuw, D. , Greene, T. , Levin, A. , Pollock, C. , Wheeler, D. C. , Yavin, Y. , … Mahaffey, K. W. (2019). Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. New England Journal of Medicine, 380(24), 2295–2306. 10.1056/NEJMoa1811744 [DOI] [PubMed] [Google Scholar]
- Pietrocola, F. , Castoldi, F. , Markaki, M. , Lachkar, S. , Chen, G. , Enot, D. P. , Durand, S. , Bossut, N. , Tong, M. , Malik, S. A. , Loos, F. , Dupont, N. , Mariño, G. , Abdelkader, N. , Madeo, F. , Maiuri, M. C. , Kroemer, R. , Codogno, P. , Sadoshima, J. , … Kroemer, G. (2018). Aspirin recapitulates features of caloric restriction. Cell Reports, 22(9), 2395–2407. 10.1016/j.celrep.2018.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman, H. , Kumar, D. , Liu, T. , Okwundu, N. , Lum, D. , Florell, S. R. , Burd, C. E. , Boucher, K. M. , VanBrocklin, M. W. , & Grossman, D. (2021). Aspirin protects melanocytes and keratinocytes against UVB‐induced DNA damage in vivo. The Journal of Investigative Dermatology, 141(1), 132–141.e3. 10.1016/j.jid.2020.06.003 [DOI] [PubMed] [Google Scholar]
- Ravid, M. , Brosh, D. , Levi, Z. , Bar‐Dayan, Y. , Ravid, D. , & Rachmani, R. (1998). Use of enalapril to attenuate decline in renal function in normotensive, normoalbuminuric patients with type 2 diabetes mellitus. A randomized, controlled trial. Annals of Internal Medicine, 128(12 Pt 1), 982–988. 10.7326/0003-4819-128-12_part_1-199806150-00004 [DOI] [PubMed] [Google Scholar]
- Rianon, N. , Ambrose, C. G. , Pervin, H. , Garcia, M. , Mama, S. K. , Schwartz, A. V. , Lee, B. , & Harris, T. (2017). Long‐term use of angiotensin‐converting enzyme inhibitors protects against bone loss in African‐American elderly men. Archives of Osteoporosis, 12(1), 94. 10.1007/s11657-017-0387-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker, P. M. , Cook, N. R. , Lee, I.‐M. , Gordon, D. , Gaziano, J. M. , Manson, J. A. E. , Hennekens, C. H. , & Buring, J. E. (2005). A randomized trial of low‐dose aspirin in the primary prevention of cardiovascular disease in women. New England Journal of Medicine, 352(13), 1293–1304. 10.1056/NEJMoa050613 [DOI] [PubMed] [Google Scholar]
- Ritsinger, V. , Lagerqvist, B. , Lundman, P. , Hagström, E. , & Norhammar, A. (2020). Diabetes, metformin and glucose lowering therapies after myocardial infarction: Insights from the SWEDEHEART registry. Diabetes & Vascular Disease Research, 17(6), 1479164120973676. 10.1177/1479164120973676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos, C. M. , Zhang, B. , Palmer, A. K. , Ogrodnik, M. B. , Pirtskhalava, T. , Thalji, N. M. , Hagler, M. , Jurk, D. , Smith, L. A. , Casaclang‐Verzosa, G. , Zhu, Y. I. , Schafer, M. J. , Tchkonia, T. , Kirkland, J. L. , & Miller, J. D. (2016). Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell, 15(5), 973–977. 10.1111/acel.12458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu, Y.‐K. , Go, J. , Park, H.‐Y. , Choi, Y.‐K. , Seo, Y. J. , Choi, J. H. , Rhee, M. , Lee, T. G. , Lee, C.‐H. , & Kim, K.‐S. (2020). Metformin regulates astrocyte reactivity in Parkinson’s disease and normal aging. Neuropharmacology, 175, 108173. 10.1016/j.neuropharm.2020.108173 [DOI] [PubMed] [Google Scholar]
- Saccon, T. D. , Nagpal, R. , Yadav, H. , Cavalcante, M. B. , Nunes, A. D. D. C. , Schneider, A. , Gesing, A. , Hughes, B. , Yousefzadeh, M. , Tchkonia, T. , Kirkland, J. L. , Niedernhofer, L. J. , Robbins, P. D. , & Masternak, M. M. (2021). Senolytic combination of dasatinib and quercetin alleviates intestinal senescence and inflammation and modulates the gut microbiome in aged mice. The Journals of Gerontology: Series A, 76(11), 1895–1905. 10.1093/gerona/glab002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha, B. , Cypro, A. , Martin, G. M. , & Oshima, J. (2014). Rapamycin decreases DNA damage accumulation and enhances cell growth of WRN‐deficient human fibroblasts. Aging Cell, 13(3), 573–575. 10.1111/acel.12190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaras, K. , Makkar, S. , Crawford, J. D. , Kochan, N. A. , Wen, W. , Draper, B. , Trollor, J. N. , Brodaty, H. , & Sachdev, P. S. (2020). Metformin use is associated with slowed cognitive decline and reduced incident dementia in older adults with type 2 diabetes: The Sydney memory and ageing study. Diabetes Care, 43(11), 2691–2701. 10.2337/dc20-0892 [DOI] [PubMed] [Google Scholar]
- Santos, E. L. , de Picoli Souza, K. , da Silva, E. D. , Batista, E. C. , Martins, P. J. F. , D’Almeida, V. , & Pesquero, J. B. (2009). Long term treatment with ACE inhibitor enalapril decreases body weight gain and increases life span in rats. Biochemical Pharmacology, 78(8), 951–958. 10.1016/j.bcp.2009.06.018 [DOI] [PubMed] [Google Scholar]
- Savarese, G. , Costanzo, P. , Cleland, J. G. F. , Vassallo, E. , Ruggiero, D. , Rosano, G. , & Perrone‐Filardi, P. (2013). A meta‐analysis reporting effects of angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers in patients without heart failure. Journal of the American College of Cardiology, 61(2), 131–142. 10.1016/j.jacc.2012.10.011 [DOI] [PubMed] [Google Scholar]
- Schafer, M. J. , White, T. A. , Iijima, K. , Haak, A. J. , Ligresti, G. , Atkinson, E. J. , Oberg, A. L. , Birch, J. , Salmonowicz, H. , Zhu, Y. I. , Mazula, D. L. , Brooks, R. W. , Fuhrmann‐Stroissnigg, H. , Pirtskhalava, T. , Prakash, Y. S. , Tchkonia, T. , Robbins, P. D. , Aubry, M. C. , Passos, J. F. , … LeBrasseur, N. K. (2017). Cellular senescence mediates fibrotic pulmonary disease. Nature Communications, 8(1), 14532. 10.1038/ncomms14532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinaman, J. M. , Rana, A. , Ja, W. W. , Clark, R. I. , & Walker, D. W. (2019). Rapamycin modulates tissue aging and lifespan independently of the gut microbiota in Drosophila . Scientific Reports, 9(1), 7824. 10.1038/s41598-019-44106-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, A. J. , Ellison, M. , & Sinclair, D. A. (2021). The economic value of targeting aging. Nature Aging, 1(7), 616–623. 10.1038/s43587-021-00080-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea, C. , & Witham, M. D. (2020). Association between the use of angiotensin‐blocking medications with hip fracture and death in older people. The Journal of Frailty & Aging, 9(2), 107–110. 10.14283/jfa.2019.38 [DOI] [PubMed] [Google Scholar]
- Shi, J. , Liu, B. , Wang, H. , Zhang, T. , & Yang, L. (2019). Association of metformin use with ovarian cancer incidence and prognosis: A systematic review and meta‐analysis. International Journal of Gynecological Cancer: Official Journal of the International Gynecological Cancer Society, 29(1), 140–146. 10.1136/ijgc-2018-000060 [DOI] [PubMed] [Google Scholar]
- Shin, Y. J. , Cho, D. Y. , Chung, T. Y. , Han, S. B. , Hyon, J. Y. , & Wee, W. R. (2011). Rapamycin reduces reactive oxygen species in cultured human corneal endothelial cells. Current Eye Research, 36(12), 1116–1122. 10.3109/02713683.2011.614372 [DOI] [PubMed] [Google Scholar]
- Sierra, F. , Caspi, A. , Fortinsky, R. H. , Haynes, L. , Lithgow, G. J. , Moffitt, T. E. , Olshansky, S. J. , Perry, D. , Verdin, E. , & Kuchel, G. A. (2021). Moving geroscience from the bench to clinical care and health policy. Journal of the American Geriatrics Society, 69(9), 2455–2463. 10.1111/jgs.17301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra‐Ramirez, A. , López‐Aceituno, J. L. , Costa‐Machado, L. F. , Plaza, A. , Barradas, M. , & Fernandez‐Marcos, P. J. (2020). Transient metabolic improvement in obese mice treated with navitoclax or dasatinib/quercetin. Aging (Albany NY), 12(12), 11337–11348. 10.18632/aging.103607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, A. K. , Singh, S. , Garg, G. , & Rizvi, S. I. (2016). Rapamycin alleviates oxidative stress‐induced damage in rat erythrocytes. Biochemistry and Cell Biology = Biochimie Et Biologie Cellulaire, 94(5), 471–479. 10.1139/bcb-2016-0048 [DOI] [PubMed] [Google Scholar]
- Sink, K. M. , Leng, X. , Williamson, J. , Kritchevsky, S. B. , Yaffe, K. , Kuller, L. , Yasar, S. , Atkinson, H. , Robbins, M. , Psaty, B. , & Goff, D. C. (2009). Angiotensin converting enzyme inhibitors and cognitive decline in older adults with hypertension: Results from the cardiovascular health study. Archives of Internal Medicine, 169(13), 1195–1202. 10.1001/archinternmed.2009.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, B. J. , Miller, R. A. , Ericsson, A. C. , Harrison, D. C. , Strong, R. , & Schmidt, T. M. (2019). Changes in the gut microbiome and fermentation products concurrent with enhanced longevity in acarbose‐treated mice. BMC Microbiology, 19(1), 130. 10.1186/s12866-019-1494-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, H.‐F. , Wang, S. , & Li, H.‐W. (2012). Effect of angiotensin receptor blockers in the prevention of type 2 diabetes and cardiovascular events: A meta‐analysis of randomized trials. Chinese Medical Journal, 125(10), 1804–1810. [PubMed] [Google Scholar]
- Spilman, P. , Podlutskaya, N. , Hart, M. J. , Debnath, J. , Gorostiza, O. , Bredesen, D. , & Galvan, V. (2010). Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid‐beta levels in a mouse model of Alzheimer’s disease. PLoS One, 5(4), e9979. 10.1371/journal.pone.0009979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens, R. J. , Ali, R. , Bankhead, C. R. , Bethel, M. A. , Cairns, B. J. , Camisasca, R. P. , Crowe, F. L. , Farmer, A. J. , Harrison, S. , Hirst, J. A. , Home, P. , Kahn, S. E. , McLellan, J. H. , Perera, R. , Plüddemann, A. , Ramachandran, A. , Roberts, N. W. , Rose, P. W. , Schweizer, A. , … Holman, R. R. (2012). Cancer outcomes and all‐cause mortality in adults allocated to metformin: Systematic review and collaborative meta‐analysis of randomised clinical trials. Diabetologia, 55(10), 2593–2603. 10.1007/s00125-012-2653-7 [DOI] [PubMed] [Google Scholar]
- Strong, R. , Miller, R. A. , Antebi, A. , Astle, C. M. , Bogue, M. , Denzel, M. S. , Fernandez, E. , Flurkey, K. , Hamilton, K. L. , Lamming, D. W. , Javors, M. A. , Magalhães, J. P. , Martinez, P. A. , McCord, J. M. , Miller, B. F. , Müller, M. , Nelson, J. F. , Ndukum, J. , Rainger, G. E. , … Harrison, D. E. (2016). Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an α‐glucosidase inhibitor or a Nrf2‐inducer. Aging Cell, 15(5), 872–884. 10.1111/acel.12496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong, R. , Miller, R. A. , Astle, C. M. , Floyd, R. A. , Flurkey, K. , Hensley, K. L. , Javors, M. A. , Leeuwenburgh, C. , Nelson, J. F. , Ongini, E. , Nadon, N. L. , Warner, H. R. , & Harrison, D. E. (2008). Nordihydroguaiaretic acid and aspirin increase lifespan of genetically heterogeneous male mice. Aging Cell, 7(5), 641–650. 10.1111/j.1474-9726.2008.00414.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong, R. , Miller, R. A. , Bogue, M. , Fernandez, E. , Javors, M. A. , Libert, S. , Marinez, P. A. , Murphy, M. P. , Musi, N. , Nelson, J. F. , Petrascheck, M. , Reifsnyder, P. , Richardson, A. , Salmon, A. B. , Macchiarini, F. , & Harrison, D. E. (2020). Rapamycin‐mediated mouse lifespan extension: Late‐life dosage regimes with sex‐specific effects. Aging Cell, 19(11), e13269. 10.1111/acel.13269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumukadas, D. , Witham, M. D. , Struthers, A. D. , & McMurdo, M. E. T. (2007). Effect of perindopril on physical function in elderly people with functional impairment: A randomized controlled trial. CMAJ: Canadian Medical Association Journal = Journal De L’association Medicale Canadienne, 177(8), 867–874. 10.1503/cmaj.061339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomopoulos, C. , Parati, G. , & Zanchetti, A. (2015). Effects of blood pressure lowering on outcome incidence in hypertension: 4. Effects of various classes of antihypertensive drugs–overview and meta‐analyses. Journal of Hypertension, 33(2), 195–211. 10.1097/HJH.0000000000000447 [DOI] [PubMed] [Google Scholar]
- Tocci, G. , Paneni, F. , Palano, F. , Sciarretta, S. , Ferrucci, A. , Kurtz, T. , Mancia, G. , & Volpe, M. (2011). Angiotensin‐converting enzyme inhibitors, angiotensin II receptor blockers and diabetes: A meta‐analysis of placebo‐controlled clinical trials. American Journal of Hypertension, 24(5), 582–590. 10.1038/ajh.2011.8 [DOI] [PubMed] [Google Scholar]
- Tomita, I. , Kume, S. , Sugahara, S. , Osawa, N. , Yamahara, K. , Yasuda‐Yamahara, M. , Takeda, N. , Chin‐Kanasaki, M. , Kaneko, T. , Mayoux, E. , Mark, M. , Yanagita, M. , Ogita, H. , Araki, S.‐I. , & Maegawa, H. (2020). SGLT2 inhibition mediates protection from diabetic kidney disease by promoting ketone body‐induced mTORC1 inhibition. Cell Metabolism, 32(3), 404–419.e6. 10.1016/j.cmet.2020.06.020 [DOI] [PubMed] [Google Scholar]
- Tong, J.‐J. , Chen, G.‐H. , Wang, F. , Li, X.‐W. , Cao, L. , Sui, X. U. , Tao, F. , Yan, W.‐W. , & Wei, Z.‐J. (2015). Chronic acarbose treatment alleviates age‐related behavioral and biochemical changes in SAMP8 mice. Behavioural Brain Research, 284, 138–152. 10.1016/j.bbr.2015.01.052 [DOI] [PubMed] [Google Scholar]
- Tseng, C.‐H. (2012). Diabetes, metformin use, and colon cancer: A population‐based cohort study in Taiwan. European Journal of Endocrinology, 167(3), 409–416. 10.1530/EJE-12-0369 [DOI] [PubMed] [Google Scholar]
- Tseng, C. (2018). Metformin and risk of hypertension in taiwanese patients with type 2 diabetes mellitus. Journal of the American Heart Association, 7(13), e008860. 10.1161/JAHA.118.008860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- UK Prospective Diabetes Study (UKPDS) Group (1998). Effect of intensive blood‐glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet (London, England), 352(9131), 854–865. [PubMed] [Google Scholar]
- van Vark, L. C. , Bertrand, M. , Akkerhuis, K. M. , Brugts, J. J. , Fox, K. , Mourad, J.‐J. , & Boersma, E. (2012). Angiotensin‐converting enzyme inhibitors reduce mortality in hypertension: A meta‐analysis of randomized clinical trials of renin‐angiotensin‐aldosterone system inhibitors involving 158,998 patients. European Heart Journal, 33(16), 2088–2097. 10.1093/eurheartj/ehs075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton, R. G. , Dungan, C. M. , Long, D. E. , Tuggle, S. C. , Kosmac, K. , Peck, B. D. , Bush, H. M. , Villasante Tezanos, A. G. , McGwin, G. , Windham, S. T. , Ovalle, F. , Bamman, M. M. , Kern, P. A. , & Peterson, C. A. (2019). Metformin blunts muscle hypertrophy in response to progressive resistance exercise training in older adults: A randomized, double‐blind, placebo‐controlled, multicenter trial: The MASTERS trial. Aging Cell, 18(6), e13039. 10.1111/acel.13039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H. , Wang, Z. , Huang, Y. U. , Zhou, Y. , Sheng, X. , Jiang, Q. , Wang, Y. , Luo, P. , Luo, M. , & Shi, C. (2020). Senolytics (DQ) mitigates radiation ulcers by removing senescent cells. Frontiers in Oncology, 9, 1576. 10.3389/fonc.2019.01576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, R. , Yu, Z. , Sunchu, B. , Shoaf, J. , Dang, I. , Zhao, S. , Caples, K. , Bradley, L. , Beaver, L. M. , Ho, E. , Löhr, C. V. , & Perez, V. I. (2017). Rapamycin inhibits the secretory phenotype of senescent cells by a Nrf2‐independent mechanism. Aging Cell, 16(3), 564–574. 10.1111/acel.12587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiemer, M. , Seth, A. , Chandra, P. , Neuzner, J. , Richardt, G. , Piek, J. J. , Desaga, M. , Macaya, C. , Bol, C. J. , Miquel‐Hebert, K. , De Roeck, K. , & Serruys, P. W. (2008). Systemic exposure of everolimus after stent implantation: A pharmacokinetic study. American Heart Journal, 156(4), 751.e1–7. 10.1016/j.ahj.2008.07.005 [DOI] [PubMed] [Google Scholar]
- Wilcock, G. K. , Gauthier, S. , Frisoni, G. B. , Jia, J. , Hardlund, J. H. , Moebius, H. J. , Bentham, P. , Kook, K. A. , Schelter, B. O. , Wischik, D. J. , Davis, C. S. , Staff, R. T. , Vuksanovic, V. , Ahearn, T. , Bracoud, L. , Shamsi, K. , Marek, K. , Seibyl, J. , Riedel, G. , … Wischik, C. M. (2018). Potential of low dose leuco‐methylthioninium bis(hydromethanesulphonate) (LMTM) monotherapy for treatment of mild Alzheimer’s disease: Cohort analysis as modified primary outcome in a phase III clinical trial. Journal of Alzheimer’s Disease: JAD, 61(1), 435–457. 10.3233/JAD-170560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wischik, C. M. , Staff, R. T. , Wischik, D. J. , Bentham, P. , Murray, A. D. , Storey, J. M. D. , Kook, K. A. , & Harrington, C. R. (2015). Tau aggregation inhibitor therapy: An exploratory phase 2 study in mild or moderate Alzheimer’s disease. Journal of Alzheimer’s Disease: JAD, 44(2), 705–720. 10.3233/JAD-142874 [DOI] [PubMed] [Google Scholar]
- Wiviott, S. D. , Raz, I. , Bonaca, M. P. , Mosenzon, O. , Kato, E. T. , Cahn, A. , Silverman, M. G. , Zelniker, T. A. , Kuder, J. F. , Murphy, S. A. , Bhatt, D. L. , Leiter, L. A. , McGuire, D. K. , Wilding, J. P. H. , Ruff, C. T. , Gause‐Nilsson, I. A. M. , Fredriksson, M. , Johansson, P. A. , Langkilde, A.‐M. , & Sabatine, M. S. (2019). Dapagliflozin and cardiovascular outcomes in type 2 diabetes. New England Journal of Medicine, 380(4), 347–357. 10.1056/NEJMoa1812389 [DOI] [PubMed] [Google Scholar]
- Woo, Y. , & Jung, Y.‐J. (2017). Angiotensin II receptor blockers induce autophagy in prostate cancer cells. Oncology Letters, 13(5), 3579–3585. 10.3892/ol.2017.5872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, K. , Liu, F. , Liu, J. , Xu, J. , Wu, Q. , & Li, X. (2020). The effect of metformin on lung cancer risk and survival in patients with type 2 diabetes mellitus: A meta‐analysis. Journal of Clinical Pharmacy and Therapeutics, 45(4), 783–792. 10.1111/jcpt.13167 [DOI] [PubMed] [Google Scholar]
- Xiong, Z.‐M. , Choi, J. Y. , Wang, K. , Zhang, H. , Tariq, Z. , Wu, D. I. , Ko, E. , LaDana, C. , Sesaki, H. , & Cao, K. (2016). Methylene blue alleviates nuclear and mitochondrial abnormalities in progeria. Aging Cell, 15(2), 279–290. 10.1111/acel.12434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, Z.‐M. , O’Donovan, M. , Sun, L. , Choi, J. Y. , Ren, M. , & Cao, K. (2017). Anti‐aging potentials of methylene blue for human skin longevity. Scientific Reports, 7(1), 2475. 10.1038/s41598-017-02419-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, M. , Pirtskhalava, T. , Farr, J. N. , Weigand, B. M. , Palmer, A. K. , Weivoda, M. M. , Inman, C. L. , Ogrodnik, M. B. , Hachfeld, C. M. , Fraser, D. G. , Onken, J. L. , Johnson, K. O. , Verzosa, G. C. , Langhi, L. G. P. , Weigl, M. , Giorgadze, N. , LeBrasseur, N. K. , Miller, J. D. , Jurk, D. , … Kirkland, J. L. (2018). Senolytics improve physical function and increase lifespan in old age. Nature Medicine, 24(8), 1246–1256. 10.1038/s41591-018-0092-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, W.‐W. , Chen, G.‐H. , Wang, F. , Tong, J.‐J. , & Tao, F. (2015). Long‐term acarbose administration alleviating the impairment of spatial learning and memory in the SAMP8 mice was associated with alleviated reduction of insulin system and acetylated H4K8. Brain Research, 1603, 22–31. 10.1016/j.brainres.2015.01.042 [DOI] [PubMed] [Google Scholar]
- Yilmaz, Ö. H. , Katajisto, P. , Lamming, D. W. , Gültekin, Y. , Bauer‐Rowe, K. E. , Sengupta, S. , Birsoy, K. , Dursun, A. , Yilmaz, V. O. , Selig, M. , Nielsen, G. P. , Mino‐Kenudson, M. , Zukerberg, L. R. , Bhan, A. K. , Deshpande, V. , & Sabatini, D. M. (2012). MTORC1 in the Paneth cell niche couples intestinal stem‐cell function to calorie intake. Nature, 486(7404), 490–495. 10.1038/nature11163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, X. , Jiang, D. , Wang, J. , Wang, R. , Chen, T. , Wang, K. , Durgahee, M. S. A. , Wei, X. , & Cao, S. (2019). Metformin prescription and aortic aneurysm: Systematic review and meta‐analysis. Heart (British Cardiac Society), 105(17), 1351–1357. 10.1136/heartjnl-2018-314639 [DOI] [PubMed] [Google Scholar]
- Yun, P. , Du, A. , Chen, X. , Liu, J. , & Xiao, H. (2016). Effect of acarbose on long‐term prognosis in acute coronary syndromes patients with newly diagnosed impaired glucose tolerance. Journal of Diabetes Research, 2016, 1602083. 10.1155/2016/1602083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakaria, A. , Hamdi, N. , & Abdel‐Kader, R. M. (2016). Methylene blue improves brain mitochondrial ABAD functions and decreases Aβ in a neuroinflammatory Alzheimer’s disease mouse model. Molecular Neurobiology, 53(2), 1220–1228. 10.1007/s12035-014-9088-8 [DOI] [PubMed] [Google Scholar]
- Zhang, C. , Chen, M. , Zhou, N. , & Qi, Y. (2020a). Metformin prevents H2O2‐induced senescence in human lens epithelial B3 cells. Medical Science Monitor Basic Research, 26, e923391–1‐e923391‐9. 10.12659/MSMBR.923391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, K. , Yang, W. , Dai, H. , & Deng, Z. (2020b). Cardiovascular risk following metformin treatment in patients with type 2 diabetes mellitus: Results from meta‐analysis. Diabetes Research and Clinical Practice, 160, 108001. 10.1016/j.diabres.2020.108001 [DOI] [PubMed] [Google Scholar]
- Zhang, P. , Kishimoto, Y. , Grammatikakis, I. , Gottimukkala, K. , Cutler, R. G. , Zhang, S. , Abdelmohsen, K. , Bohr, V. A. , Misra Sen, J. , Gorospe, M. , & Mattson, M. P. (2019a). Senolytic therapy alleviates Aβ‐associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer’s disease model. Nature Neuroscience, 22(5), 719–728. 10.1038/s41593-019-0372-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S. , Zhang, R. , Qiao, P. , Ma, X. , Lu, R. , Wang, F. , Li, C. , E, L. , & Liu, H. (2021). Metformin‐induced microRNA‐34a‐3p downregulation alleviates senescence in human dental pulp stem cells by targeting CAB39 through the AMPK/mTOR signaling pathway. Stem Cells International, 2021, e6616240. 10.1155/2021/6616240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. , Jiang, N. , Li, J. , Zhang, D. , & Lv, X. (2019b). Rapamycin alleviates proinflammatory cytokines and nociceptive behavior induced by chemotherapeutic paclitaxel. Neurological Research, 41(1), 52–59. 10.1080/01616412.2018.1531199 [DOI] [PubMed] [Google Scholar]
- Zhao, X. , Liu, L. , Jiang, Y. , Silva, M. , Zhen, X. , & Zheng, W. (2020). Protective effect of metformin against hydrogen peroxide‐induced oxidative damage in human retinal pigment epithelial (RPE) cells by enhancing autophagy through activation of AMPK pathway. Oxidative Medicine and Cellular Longevity, 2020, 2524174. 10.1155/2020/2524174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, D. , Chen, L. , & Mou, X. (2021). Acarbose ameliorates spontaneous type‐2 diabetes in db/db mice by inhibiting PDX‐1 methylation. Molecular Medicine Reports, 23(1), 72. 10.3892/mmr.2020.11710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, H. , Wang, S. , Zhu, P. , Hu, S. , Chen, Y. , & Ren, J. (2018). Empagliflozin rescues diabetic myocardial microvascular injury via AMPK‐mediated inhibition of mitochondrial fission. Redox Biology, 15, 335–346. 10.1016/j.redox.2017.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, J.‐B. , Tang, X. , Han, M. , Yang, J. , & Simó, R. (2020). Impact of antidiabetic agents on dementia risk: A Bayesian network meta‐analysis. Metabolism: Clinical and Experimental, 109, 154265. 10.1016/j.metabol.2020.154265 [DOI] [PubMed] [Google Scholar]
- Zhou, Y.‐Y. , Zhu, G.‐Q. , Liu, T. , Zheng, J.‐N. , Cheng, Z. , Zou, T.‐T. , Braddock, M. , Fu, S.‐W. , & Zheng, M.‐H. (2016). Systematic review with network meta‐analysis: Antidiabetic medication and risk of hepatocellular carcinoma. Scientific Reports, 6, 33743. 10.1038/srep33743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, X. , Shen, J. , Feng, S. , Huang, C. , Liu, Z. , Sun, Y. E. , & Liu, H. (2020). Metformin improves cognition of aged mice by promoting cerebral angiogenesis and neurogenesis. Aging, 12(18), 17845–17862. 10.18632/aging.103693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, Y. I. , Tchkonia, T. , Pirtskhalava, T. , Gower, A. C. , Ding, H. , Giorgadze, N. , Palmer, A. K. , Ikeno, Y. , Hubbard, G. B. , Lenburg, M. , O'Hara, S. P. , LaRusso, N. F. , Miller, J. D. , Roos, C. M. , Verzosa, G. C. , LeBrasseur, N. K. , Wren, J. D. , Farr, J. N. , Khosla, S. , … Kirkland, J. L. (2015). The Achilles’ heel of senescent cells: From transcriptome to senolytic drugs. Aging Cell, 14(4), 644–658. 10.1111/acel.12344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziehm, M. , Kaur, S. , Ivanov, D. K. , Ballester, P. J. , Marcus, D. , Partridge, L. , & Thornton, J. M. (2017). Drug repurposing for aging research using model organisms. Aging Cell, 16(5), 1006–1015. 10.1111/acel.12626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinman, B. , Wanner, C. , Lachin, J. M. , Fitchett, D. , Bluhmki, E. , Hantel, S. , Mattheus, M. , Devins, T. , Johansen, O. E. , Woerle, H. J. , Broedl, U. C. , & Inzucchi, S. E. (2015). Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. New England Journal of Medicine, 373(22), 2117–2128. 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]


