Summary
Pathologic T cell-B cell interactions underlie many autoimmune diseases. The T cells that help B cells in autoimmune diseases vary in phenotype and include T cells that lack typical features of T follicular helper cells, such as expression of CXCR5 and BCL6. A population of PD-1hi CXCR5− T peripheral helper (Tph) cells has now been recognized in multiple autoantibody-associated diseases. Tph cells display a distinctive set of features, merging the ability to provide B cell help with the capacity to migrate to inflamed peripheral tissues. Here we review the scope of immune-related conditions in which Tph cells have been implicated and provide a perspective on their potential contributions to pathologic B cell activation in autoimmune diseases. We discuss Tph cells as a promising therapeutic strategy in autoimmunity and consider the utility of tracking Tph cells in blood as a biomarker to quantify aberrant T cell-B cell activation in patients with autoimmune diseases.
Keywords: T peripheral helper, T follicular helper, Autoimmune disease, IL-21, CXCL13
Introduction
Pathologic T cell activation is a core feature of multiple autoimmune diseases. A critical role for T cell recognition of autoantigens in autoimmune diseases is perhaps most clearly illustrated by the towering effects of MHC class II loci as risk alleles across multiple autoimmune diseases. The principal function of MHC class II is to present antigen to CD4+ T cells, strongly suggesting that presentation of relevant antigens to CD4+ T cells is a critical determinant in the development of autoimmune diseases such as rheumatoid arthritis, lupus, multiple sclerosis, and type 1 diabetes. Yet defining the identities, characteristics, and functions of pathologic T cell populations in autoimmune diseases remains a major challenge.
T cell interactions with B cells, and the subsequent production of disease-specific autoantibodies has provided one valuable foothold. Aberrant T cell-B cell interactions and production of class switched, somatically mutated autoantibodies are characteristic features of many autoimmune diseases. Generation of these antibodies requires antigen recognition by B cell receptors of autoreactive B cells and help from autoreactive T cells; thus, breaks in both B cell tolerance and T cell tolerance are necessary. Autoantibodies are often detectable years before clinical onset of disease,1,2 suggesting that the initial loss of T cell and B cell tolerance occurs quietly, in a phase of silent autoreactivity, before autoimmune pathology progresses to clinically evident tissue inflammation or injury.
Historically, the discovery of disease-specific autoantibodies in serum has preceded detection of disease-specific autoreactive T cells, largely due to technical reasons. Serum samples are easily and commonly banked and can be readily assayed for autoantibodies, which are typically present in the circulation, through a variety of assays. In contrast, identifying T cell antigens poses greater technical challenges, as it typically requires study of live T cells, which are harder to isolate and store. T cells recognize processed peptide antigens bound within an MHC molecule, and their reactivity is restricted to MHC alleles present in that individual, thus multiple components are required, often in an individualized fashion. Further, relevant T cell populations may be largely localized to target tissues or secondary lymphoid organs, with little representation in blood.
Recognizing these challenges, there are several potential approaches to identify potentially pathogenic T cell populations in autoimmune diseases.
1). Defining over-represented T cell populations in patient samples
A straightforward approach is to assume that T cell populations over-represented in blood or tissue of patients with an autoimmune disease, as compared to non-autoimmune disease comparator patients, may reflect the characteristics of the T cell response in that disease. This approach can be applied to both blood and tissue; however, studies of tissue are more likely to be informative and can demonstrate the prominent T cell phenotypes that accumulate within the inflamed autoimmune target site. Numerous studies using single cell RNA-seq and mass cytometry have begun to define ‘landscapes’ of lymphocytes in autoimmune target tissues.3–8 However, abundance does not necessarily imply pathogenicity, as bystander cells may accumulate in large numbers, and compensatory or regulatory cells may also accumulate in inflamed tissues. Applying this approach to blood samples is also reasonable, and may detect circulating T cell phenotypes that are also enriched within tissues;9 however, this amplifies the challenge of discriminating the disease-relevant T cells phenotypes from the many innocent circulating T cells that have no role in the disease. In rheumatoid arthritis, and likely other diseases as well, the T cell phenotypes expanded in blood represent a small portion of the total circulating T cell pool, with relatively subtle features of activation or function, compared to larger, robustly active effector populations present at the inflamed tissue site.9,10
2). Identifying activated T cells in patient samples
T cells acquire expression of a variety of surface receptors after activation. Expression of these receptors, including CD69, CD25, CD38, PD-1, HLA-DR, make it possible to identify cells that have potentially been recently activated in a patient with an autoimmune disease. Features such as expression of the transcription factor Nur77 maybe allow even more specific detection of recent TCR triggering.11 Analyses of T cells in tumors also suggest CD39 as a marker that distinguishes chronically antigen-activated cells from bystander cells.12 Thus, focusing on T cells with features of recent activation may allow one to enrich for cells that have recently detected relevant antigen through their TCR, even if the antigen is not known. This approach is particularly appealing when trying to tease out the small numbers of disease-relevant T cells in circulation from the much larger populations of circulating unrelated T cells. However, T cells in both blood and tissue express varied combinations of activation markers, such that it is not simple to define a uniform identifier of activated T cells. In addition, specific functional T cell subsets can persistently express these markers. For example, expression of CD25 is a defining feature of Tregs, while expression of PD-1 and ICOS are typical features of T follicular helper (Tfh) and T peripheral helper (Tph) cells. Thus, there is often uncertainty as to whether expression of these markers indicates a functional T cell subset (e.g. PD-1+ ICOS+ Tfh cells) or an activated effector cell with unspecified function. Decoding the functional implications of expression of different combinations of activation markers remains a work in progress.
3). Characterizing antigen-specific T cells in patient samples
If autoantigens are known, quantifying and characterizing antigen-specific T cells is clearly of interest. MHC-peptide tetramers loaded with disease-relevant peptides can be used to isolate T cells that recognize those peptides in the context of the appropriate MHC molecule. Alternatively, T cells can be stimulated with relevant antigens in vitro in the presence of appropriate antigen presenting cells, and the activated T cells isolated for interrogation. One major challenge with this approach is that autoimmune diseases such as RA and SLE may involve many different autoantigen targets; thus, assessing the overall scope of antigen-specific T cells is challenging.
As we discuss in the following sections, recent reports have provided examples of each of these approaches identifying PD-1hi CXCR5− CD4 T cells as a likely relevant contributor to chronic autoimmune responses. Here we begin by reviewing analyses of rheumatoid arthritis (RA) synovium that initially revealed a PD-1hi CXCR5− CD4 T peripheral helper (Tph) cell population, which displayed a distinctive combination of B cell-helper functions combined with a migratory program targeting inflamed tissues. We then review evidence for the presence of Tph cells across multiple autoimmune diseases, discuss aspects of Tph cells function and regulation, and consider implications of Tph cells as a therapeutic target and a predictive biomarker in autoimmunity.
Detection of a Tph cell population in RA joints
Multiple lines of evidence indicate that T cells are critical drivers of the autoimmune response in RA. In addition to the strong genetic association with MHC class II alleles,13 there is an abundance of T cells in inflamed synovium, and T cell targeted therapies such as abatacept (CTLA4-Ig) are effective in treating RA.14 To define the key features of T cells that infiltrate the synovium in RA, we employed mass cytometry to phenotype synovial T cells using a mass cytometry panel that included several markers of T cell activation. These analyses highlighted a prominent population of CD4+ T cells in RA synovial fluid and synovial tissue, comprising ~25% of synovial fluid CD4+ T cells, marked by very high expression of PD-1, with varied co-expression of ICOS and HLA-DR.10 Notably, these cells were elevated specifically in samples from patients with seropositive RA (positive for either rheumatoid factor or anti-CCP autoantibodies) and present at much lower levels in patients with seronegative arthritides such as seronegative RA and psoriatic arthritis.10 By flow cytometry, the PD-1hi population displayed a distinct contour that separated these cells from the many cells with intermediate PD-1 expression. The presence of this PD-1hi cell contour was easily apparent in the seropositive RA samples and largely missing from the seronegative samples; we could see this simply by lining up the flow cytometry plots.
Functional and transcriptomic analyses demonstrated that these PD-1hi CD4+ T cells in RA synovial samples bore striking resemblance to Tfh cells, the prototypical B cell-helper T cell population, despite the lack of expression of CXCR5, a defining feature of Tfh cells. PD-1hi CXCR5− cells from RA synovial fluid expressed high levels of IL-21, a cytokine critical for B cell differentiation into plasmablasts and plasma cells,15 as well as very high levels of the B cell chemoattractant CXCL13,16–20 consistent with prior observations.21,22 Transcriptomic analyses revealed a gene signature strongly overlapping with Tfh cells, including high expression of genes such as MAF, TIGIT, SLAMF5, CD200. In vitro co-culture experiments confirmed that PD-1hi CXCR5− cells from RA synovial fluid could stimulate B cell differentiation into plasmablasts in an IL-21 dependent manner.10
The most prominent difference between synovial PD-1hi CXCR5− -cells and Tfh cells was a substantially distinct migratory program. Synovial PD-1hi cells not only lacked CXCR5, but also expressed a set of chemokine receptors associated with migration to inflamed peripheral tissues, including CCR2 and CCR5. Consistent with altered migratory program, synovial PD-1hi cells lacked high expression of Bcl6, a transcription factor associated with CXCR5+ Tfh cells, and instead expressed higher levels of the counter-regulator Blimp1 (discussed in detail below). This PD-1hi CXCR5− population thus displayed a combination of B cell-helper functions coupled with a migratory program targeting peripheral tissues.
It is valuable to put these observations in the context of the Tfh cell paradigm. Tfh cells represent the prototype of B cell-helper T cells and have largely defined our expectations of what B cell-helper T cells look like. Tfh cells are characteristically express high levels of CXCR5, PD-1, and the transcription factor BCL6,23 such that these features have often been prioritized in searching for B cell-helper T cells in different contexts. However, observations from RA synovium, as well as several other studies of both patient samples and murine models have provided clear examples of B cell-helper T cells that do not fit a typical definition of Tfh cells.24–29 RA synovial samples contain a large population of PD-1hi CXCR5− CD4 T cells that can provide B cell help, yet show low expression of BCL6 and higher expression of BLIMP1 and CCR2. These cells clearly do not fit the mold of Tfh cells. We called these T ‘peripheral helper’ (Tph) cells to capture the idea that these cells could help B cells in inflamed peripheral tissues, a conceptual parallel to help from T ‘follicular helper’ (Tfh) cells in follicles of secondary lymphoid organs. Throughout this review, we use the term Tph cells to refer to PD-1hi CXCR5− CD4+ T cells that show features associated with B cell helper function, including ICOS, IL21, and/or CXCL13, with premise that these cells are relevant in T-B cell interactions in peripheral tissues. In the literature, cells with this phenotype have also been referred to under a broader term of ‘Tfh-like’ cells,30,31 ‘Tfhx13’ cells,25 and other variations on the theme; here we will use the term Tph cells for simplicity in a manner consistent with recent nomenclature proposal.31
Single cell RNA-seq analyses of T cells from RA synovium performed by the Accelerating Medicines Partnership RA/SLE Network, as well as independent studies, have confirmed the presence of a PD-1hi CXCL13+ Tph cell population and have helped further define the transcriptomic features of these cells. CXCL13 expression is particularly well captured by single cell RNA-seq analyses, likely because it is expressed at very high levels, and appears to mark tissue Tph and Tfh cells quite clearly in single cell RNA-seq datasets. Indeed, single cell RNA-seq analyses of RA synovium highlighted Tph cells as the principal source of CXCL13 in RA synovium, with CXCL13 expression that far outpaces any other synovial cell type.3,32 This suggests that infiltrating Tph cells are a predominant source of the major B cell chemoattractant in RA synovium. Comparisons of T cells from RA synovium and osteoarthritis synovium by single cell RNA-seq identified Tph cells as significantly expanded in RA synovial samples.4 Further, bulk RNA-seq analyses comparing total T cells from RA synovium to those from OA synovium identified CXCL13 as the most highly upregulated gene in RA T cells compared to OA T cells, with several other Tph-associated genes also highly upregulated.33 Thus, both single cell RNA-seq and bulk RNA-seq analyses have highlighted CXCL13+ Tph cells as a defining feature of the T cell infiltrate in RA synovium.
Notably, CXCL13 has emerged as a potential prognostic biomarker in RA. Elevated levels of serum CXCL13 in RA patients have been linked to worse prognosis and more severe joint destruction; RA patients with higher baseline serum CXCL13 had increased total Sharp score as well as progression at 11 years.34 Reports regarding the relationship of serum CXCL13 levels and clinical disease activity have been conflicting; however, baseline serum CXCL13 levels have been linked with better response to anti-TNF antibody therapy in patients with RA.35,36 The link between CXCL13 and anti-TNF antibody response presents questions regarding the mechanism of this relationship.
Circulating Tph cells in autoantibody-associated autoimmune diseases
While Tph cells are abundant in inflamed joints of RA patients, a cytometrically and transcriptomically similar population can also be found in the circulation of RA patients. Circulating Tph cells, gated as PD-1hi CXCR5− CD4+ T cells (often with co-expression of ICOS, HLA-DR, and TIGIT), are increased in the circulation of patients with seropositive RA, but not seronegative RA, compared to non-inflammatory control patients, consistent with the patterns in synovial fluid.10,37 Distinguishing Tph cells from other activated T cells, which may express intermediate levels of PD-1, in the circulation is not trivial. By cytometry, synovial fluid Tph cells can display a distinct contour of cells with very high PD-1 expression, comparable to that of germinal center Tfh cells, making it straightforward to gate these cells.10 In contrast, in blood samples, only a small portion of T cells achieve this level of PD-1 expression. We gate Tph cells in blood attempting to capture these cells with the highest levels of PD-1 expression.38 Applied to samples from RA and SLE patients, this gating strategy captures cells with the highest expression levels of IL-21 and CXCL13, consistent with a B cell-helper function.38,39 Tph cells sorted in this way from the circulation of both RA and SLE patients show qualitatively similar transcriptomic profiles to those from RA joints, including expression of MAF, IL21, CXCL13, TIGIT, and CD200, albeit with lower levels of IL21 and CXCL13 than from synovial Tph cells, likely because the proportion of strongly activated cells is much higher in samples obtained from inflamed autoimmune target sites. Functionally, Tph cells sorted from blood can stimulate B cell differentiation into plasmablasts in an IL-21-dependent manner.10,39 Together, these observations demonstrate that Tph cells can be identified and captured from the circulation. Still, the distinction between Tph cells and other activated T cells remains suboptimal and can likely be improved with additional discriminating surface markers, with ICOS and TIGIT as promising candidates.38,40
The recognition of Tph cells in the circulation has facilitated interrogation of Tph cells in a range of autoimmune diseases. Among these, expansion of Tph cells relative to non-inflammatory controls appears particularly pronounced in SLE. Dimensional reduction and clustering approaches applied to mass cytometry analyses of CD4 T cells from biopsy-demonstrated lupus nephritis patients identified the expansion of PD-1hi CXCR5− Tph cells as the most prominent cytometric abnormality among circulating CD4 T cells in SLE patients.39 By RNA-seq analysis, expression of IL21 and CXCL13 also tend to be higher in Tph cells sorted from SLE patients compared to controls.39
The expansion of Tph cells exceeded that of Tfh cells, and Tph cell frequency, but not Tfh cell frequency, correlated with clinical disease activity in lupus nephritis patients.39 Both Tfh and Tph cell frequencies were higher in SLE patients with a detectable anti-double stranded DNA antibodies as compared to anti-dsDNA antibody-negative patients. The elevated frequency of Tph cells in lupus nephritis patients is consistent with prior observations of an increased abundance of PD-1hi ICOShi CXCR5low T cells in SLE patients,41 and has now been observed in multiple, diverse SLE cohorts.42,43 Together, these studies highlight Tph cell expansion as a prominent, reproducible feature of the immune dysregulation in SLE. The expansion of Tph cells in the circulation of patients with established SLE appears more robust and more pronounced than the expansion of Tfh cells in the same cohorts, though this may vary with the time course of the disease (discussed further below).
Correlative studies of different lymphocyte populations in patient samples have highlighted an interesting relationship between Tph cells and CD11c+ CD21− B cells, often referred to as age-associated B cells (ABC) or DN2 cells.44,45 In both SLE and RA patients, the frequency of Tph cells, but not Tfh cells, correlated with the frequency of CD11c+ CD21− B cells.39 This suggests a potential interaction between Tph cells and ABCs, though the nature of this interaction is not clear. Both Tph cells and ABCs share a propensity to traffic to peripheral tissues; it is tempting to speculate that they may migrate to inflamed sites using similar homing programs and that they may interact within those tissues. Tph cells have been suggested to preferentially induce ABC differentiation,46 though this has not be consistently demonstrated.39ABCs have also been demonstrated to promote differentiation of Tfh cells,3 yet their comparative ability to induce Tph cells versus Tfh cells has not been fully defined.
In addition to RA and SLE, multiple other autoimmune diseases involve an increased frequency of PD-1hi CXCR5− Tph cells. Recent reports have identified an expansion of circulating T cells with a Tph phenotype in type 1 diabetes,40,47 primary biliary cholangitis,48 Sjogren’s syndrome,49 ulcerative colitis,50 IgA nephropathy,51 juvenile idiopathic arthritis,46 and autoimmune hepatitis.52 Notably these are all diseases with some extent of autoantibody production. In contrast, Tph cells do not appear to be expanded in either joints or circulation of patients with seronegative RA or seronegative spondyloarthropathies such as psoriatic arthritis or ankylosing spondylitis.10 Interestingly, the relative expansion of circulating Tph cells and Tfh cells appears to vary depending on the disease (Figure 1). For example, the increase in circulating PD-1hi CXCR5− Tph cells appears to be larger than the increase in circulating PD-1hi CXCR5+ Tfh cells in patients with established SLE. In contrast, in IgG4-related disease, circulating Tfh cells appear more expanded Tph cells.53–55 This is consistent with the observation that IgG4-related disease lesions contain large numbers of BCL6+ CXCR5+ Tfh cells and few Tph cells.53
Figure 1.
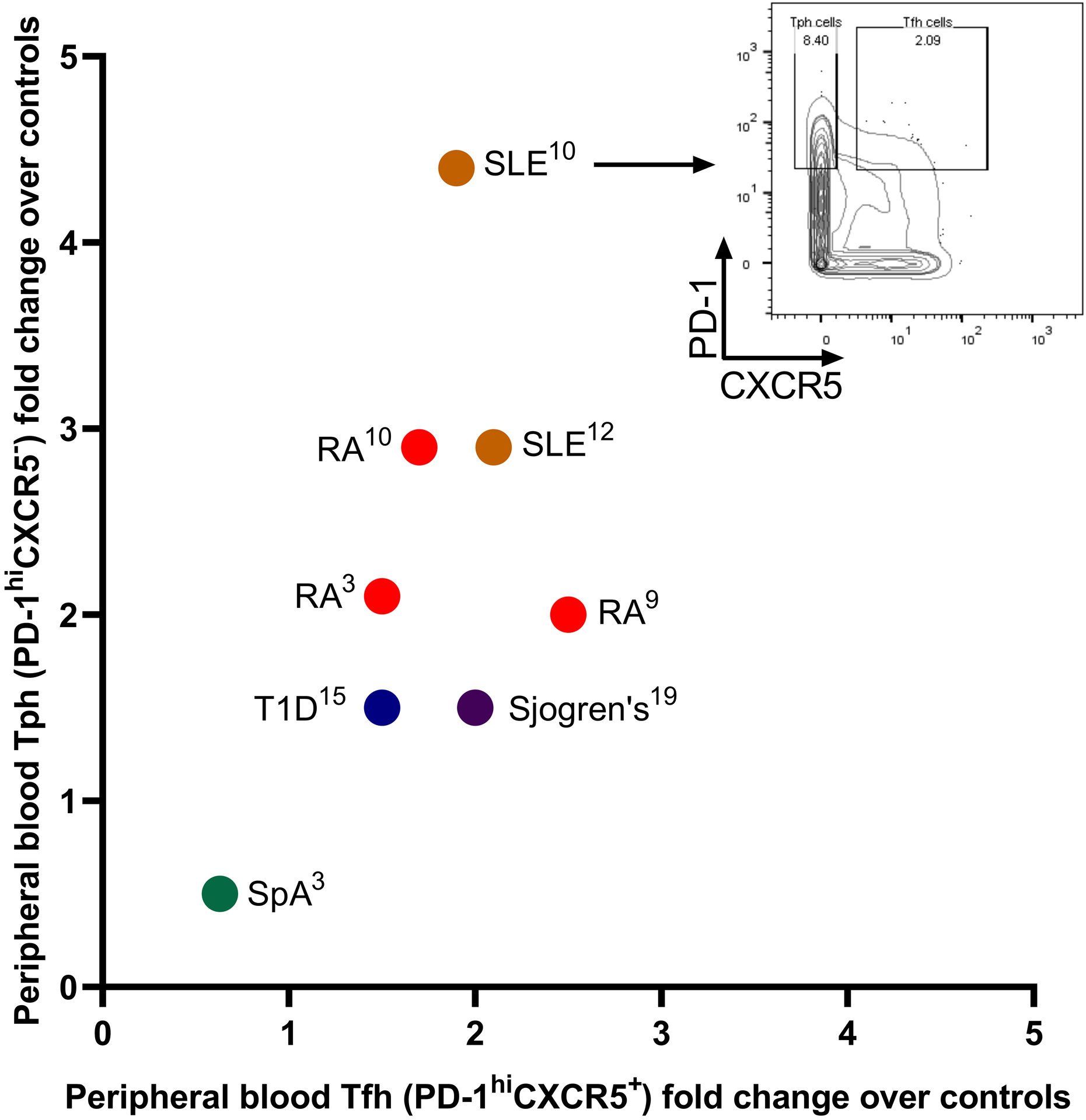
Expansion of Tph cells and Tfh cells in autoimmune diseases. Tph cells and Tfh cells were considered as PD-1hiCXCR5− and PD-1hiCXCR5+, respectively, and are represented in each indicated disease as fold over controls according to the cited publications. Publications were included if they analyzed both Tph and Tfh cells in both the disease patients and controls and gated them by cytometry as PD-1hiCXCR5− and PD-1hiCXCR5+. Representative mass cytometry plot demonstrates peripheral blood memory CD4+ T cells from Bocharnikov et al.
Expansion of Tph cells is not limited to autoimmunity and appears to also occur in other settings of chronic immune activation, including infection and cancer. Tph cell frequency is increased in blood samples from patients with chronic hepatitis B,33 and a similar appearing T cell population has been identified in patients with HIV.56–58 Surprisingly, a PD-1hi CXCR5− CD4 population resembling Tph cells could also be identified within lymph nodes of HIV patients;58 comparison of the TCR repertoires indicated substantial overlap between PD-1+ CXCR5+ Tfh cells and PD-1+ ICOS+ CXCR5− cells obtained from lymph nodes, indicating a shared ontogeny. ATAC-seq analyses demonstrated open chromatin at the CXCR5 locus in PD-1+ CXCR5− cells, suggesting an increased potential for these cells to express CXCR5.58 This suggests that it may be possible for CXCR5+ Tfh cells in lymph nodes to downregulate CXCR5 and acquire a CXCR5− Tph cell phenotype.
Analyses of tumor infiltrating lymphocytes have also at times yielded cells with a Tph phenotype; this was initially clearly demonstrated in T cells from breast cancer.25 A PD-1hi ICOS+ CXCR5− CD4 T cell population was found to be the dominant source of CXCL13 expression in human breast cancer samples, and expression of CXCL13 was positively associated with patient disease-free survival.25 Recent murine studies indicate a key role for peri-tumor T-B cell interactions and IL-21-producing Tfh cells in promoting a productive cytotoxic CD8 T cell response.59 It is possible that local aggregates of CXCL13+ Tph cells and B cells may act similarly in human tumors and potentially in target tissues in autoimmunity as well. CXCL13+ CD4+ and CD8+ T cells have now been observed in multiple studies of tumor infiltrating lymphocytes, with CXCL13 expressed along with multiple other genes associated with dysfunction, such that CXCL13 expression in tumor-infiltrating T cells has come to be regarded as a marker of dysfunctional cells.3,23,60,61 Tph cells (and Tfh cells) from RA and SLE patients also display some features associated with exhaustion, including high expression of multiple inhibitory receptors and low IL-2 production;10 understanding the similarities and differences between Tph/Tfh cells and dysfunctional cells is of major interest.
Expansion of Tph cells early in autoimmune disease course
Given the recognition of Tph cells across multiple autoimmune conditions, one important question that has emerged is whether Tph cell activation can occur early in the course of an autoimmune disease, or whether it occurs only later as a product of a chronic, dysfunctional response. Initial observations from different patient cohorts are starting to provide insights into this question. In IgA nephropathy, blood samples evaluated at the time of diagnostic kidney biopsy demonstrated >2-fold increase in PD-1hi CXCR5− ICOS+ Tph cells, indicating an increase in Tph cells in patients with newly diagnosed disease.51 In RA patients, increased Tph cell and Tfh cell frequencies can also be detected in patients with early disease, defined as disease duration <6 months and the absence of disease-modifying drug therapy or corticosteroids.37 In these patients, treatment with methotrexate reduced circulating Tph cell, but not Tfh cell, frequency.37 In patients with a new diagnosis of SLE, defined as disease duration less than 6 months and absence of immunosuppressive drugs except hydroxychloroquine and prednisone <10mg/day, both Tph and Tfh cells are elevated.39 Interestingly, Tfh cell frequency appeared to be relatively higher in a cohort of early SLE patients as compared to established SLE patients, while Tph cell frequencies were high in both cases.39 Consistent with this pattern observed in cross-sectional cohorts, longitudinal analyses of a cohort of early SLE patients demonstrated that Tfh cell frequency decreased over the first year of disease, while Tph cell frequency remained elevated.62 These patterns appeared independent of changes in disease activity or immunosuppressive drug use.
Establishing the time course of autoimmune disease initiation, and what constitutes ‘early’ disease, is challenging given that breaks in T cell and B cell tolerance and production of autoantibodies often precede development of clinically evident autoimmune disease by several years, including in RA,2 SLE,1 and T1D.63 Whether there are immune cell changes that can be detected during the transition from a clinically silent, autoantibody+ stage to a clinically evident inflammatory condition remains unclear, though initial insights from T1D suggest that this may be possible. Tph cells are elevated in T1D patients, particularly patients with multiple autoantibodies.40 In a small cohort of individuals with T1D-associated autoantibodies but without T1D, Tph cells were elevated specifically in the subset of individuals who progressed to T1D during the 3–5 years of follow-up, but not elevated in individuals who did not progress to T1D.40 Though preliminary, these observations support the idea that circulating Tph cells may reflect pathologic immune activation and risk of progression to disease. Currently studies are underway to evaluate this idea across multiple autoimmune diseases – for example, are Tph cells elevated in patients with an anti-CCP antibody but without clinically evident arthritis?
The presence of Tph cells early in clinical autoimmune disease suggests that these cells may play a role in immune responses more generally, rather than being solely the product of a chronic, dysfunctional response. A recent preprint indicates an expansion of Tph cells in patients with acute SARS-CoV-2 infection, in which Tph cells demonstrated the ability to stimulate plasmablast differentiation in vitro and correlated with the abundance of tissue-homing plasmablasts in patients with stable, but not severe, COVID-19.64 This suggests that Tph cells can be part of the early adaptive immune response to pathogens, perhaps encouraging differentiation of plasmablasts at extrafollicular sites of T-B cell interaction.65 Preliminary observations also suggest the potential for baseline Tph cell levels to predict response to COVID-19 vaccination in organ transplant recipients.66 The role of Tph cells in productive responses to pathogens or vaccines remains largely unexplored.
Searches for antigen-specific cells highlight Tph cells
The preceding discussion focused on studies that evaluated changes in the abundance of T cells with defined phenotypes without respect to antigen specificity. Evaluating the phenotypes and functions of antigen-specific T cells in human autoimmunity is challenging for several reasons; experimental approaches need to model peptide presentation in restricted MHC molecules, antigen-specific T cells are present at very low frequencies in circulation, and the relevant antigens are incompletely defined. For example, despite the well-recognized immune response to citrullinated proteins in RA, detecting citrullinated peptide-reactive T cells through in vitro stimulation or peptide-MHC tetramers is challenging and limited by the small number of antigen-specific T cells in blood samples67,68 and still relatively small numbers in synovial fluid samples (range ~1:500,000–1:30,000 synovial fluid CD4+ T cells reactive to citrullinated enolase peptide tetramer).69 It remains unclear to what extent Tph cells are represented among antigen-specific T cells in RA or in SLE, a disease in which it is perhaps even harder to define antigen-specific T cells.
Detailed studies in celiac disease provide one compelling example supporting the idea that Tph cells can be antigen-specific. A study using MHC class II tetramers loaded with gluten-derived peptides in high-dimensional mass cytometry panels demonstrated that gluten-specific T cells obtained from gut biopsies of gluten-challenged celiac disease patients show a quite uniform cytometric phenotype consistent with Tph cells.70 Gluten peptide-reactive T cells were almost uniformly PD-1hi ICOS+ CXCR5− cells with little expression of CD25. RNA-seq analyses of these cells demonstrated elevated expression of IL-21 and CXCL13, strongly suggesting that gluten-specific T cells in celiac disease are Tph cells.
An elegant study using peptide stimulations to identify antigen-reactive T cells in autoimmune hepatitis provided a second example of antigen-specific Tph cells. In this work, T cells from autoimmune hepatitis patients were stimulated in vitro with peptides derived from soluble liver antigen, an autoantibody target in autoimmune hepatitis, and the phenotype and repertoire of responsive T cells were characterized.52 Remarkably, the T cells that responded to soluble liver antigen peptides were highly enriched in a Tph cell phenotype, with expression of PD-1 but not CXCR5, and single cell RNA-seq analyses revealed a typical Tph signature, with expression of IL21, ICOS, MAF, TIGIT, and SLAMF6. Peripheral blood cell analyses confirmed an increased frequency of PD-1+ CXCR5− Tph cells in the circulation of patients with autoimmune hepatitis; these cells could stimulate B cell responses in vitro, consistent with a B cell-helper function. In addition, the PD-1+ CXCR5− T subset in these patients was highly enriched for T cells with TCRs that recognize soluble liver antigen peptides, strongly implicating Tph cells as antigen-specific CD4+ T cells in autoimmune hepatitis.
These two examples suggest the possibility that the Tph cell population may be highly enriched for antigen-specific T cells across autoimmune diseases. Perhaps chronic autoantigen detection leads cells to adopt a Tph cell phenotype, which shares some features with chronically activated or exhausted cells. It will be of major interest going forwards to understand the extent of oligoclonality and extent of autoreactivity represented within the Tph cell population in different autoimmune diseases.
Regulation and function of Tph cells
Despite the similarities between Tph and Tfh cells, there are key differences in their transcriptional regulation. Tph cells express low levels of Bcl6, the transcription factor that characteristically regulates Tfh cells.10 Tph cells instead express higher levels of Blimp-1, the reciprocal transcription factor for Bcl6, suggesting that Tph cells function well as B cell helpers without high Bcl6 function.10,71,72 Murine models provide some support for the idea that some B cell-helper T cells can function despite lack of Bcl6 expression. Although Bcl6-deficient mice fail to generate Tfh cells and germinal centers, they generate sufficient antibody responses to H1N1 and H5N1 to protect mice from acute influenza infection.73,74 Along these lines, a recent publication also addressed formation of antibodies against SARS-CoV-2 in Bcl6-deficient mice.73 Bcl6-deficient mice could generate specific antibody responses following immunization of several different methods especially by mRNA vaccine.73 This is consistent with the observations in a mouse lung inflammation model that accumulation of antigen specific T cells and B cells in inflamed lung tissue occurs without the presence of Bcl6- or CXCR5-expressing T cells.30 Mouse T cells lacking Bcl6 can still produce IL-21 both following in vitro differentiation and in vivo following immunization with the immunogenic antigen keyhole limpet haemocyanin.75 Consistent with these results, lentiviral overexpression of Bcl6 in human CD4+ T cells from tonsil does not result in increased expression of IL-21 or IL-4.72 Similarly, we find that human T cells lacking BCL6 due to CRISPR-mediated deletion still produce IL-21 at normal levels (unpublished observations).
Regulation of IL-21 production in Tph cells as well as in Tfh cells should then be attributed to transcriptional regulators other than Bcl6. Maf is a transcription factor that belongs to the AP-1 superfamily and is expressed in several subsets of CD4+ T cells.76 Both Tph and Tfh cells highly express Maf.72 While overexpression of Bcl6 does not result in changes in IL-21 expression, overexpression of Maf promotes IL-21 production from human CD4 T cells.77 Additionally, Maf binds to the promoter and enhancer regions of IL-21 following stimulation with IL-6 and TGFβ.77 Our lab has demonstrated that CRISPR-mediated deletion of Maf in primary human CD4+ T cells results in reduced production of IL-21.39 Moreover, deletion of Maf in Tph cells obtained from SLE patients results in a reduction in capacity to induce plasmablast formation in vitro.39
The significance of Maf for IL-21 production does not minimize the importance of Bcl6 for Tfh cells, but rather illustrates that IL-21-dependent B cell helper function can be separated from Bcl6 expression and a CXCR5+ Tfh identity. We hypothesize that IL-21-producing B cell-helper T cells can acquire different migratory programs depending on the transcriptional programs. For example, Bcl6 and Ascl2 expression promote and sustain CXCR5 and a follicular homing program, taking CXCR5+ Tfh cells to follicles of secondary lymphoid organs, where they promote a germinal center B cell response.78 In contrast, expression of CCR2 and CCR5 in synovial Tph cells may be controlled by alternate transcription factors that control a peripheral homing program.39 Tph cells themselves may utilize different migratory programs to home to different organs or areas of inflammation. A good example of this hypothesis is migration of CCR2+ Tph cells to the synovium of an RA patient. CCL2, a ligand for CCR2, is produced at high levels by synovial fibroblasts in RA synovium.79 Alternatively, it is possible that Tph cells that express CCR9 may migrate to the salivary gland in a patient with Sjogren’s disease or home to the gut to potentiate B cell activation in celiac disease.80,81
B cell-helper T cell populations also exist in other flavors; in between germinal center Tfh cells and synovial Tph cells on the spectrum of B cell-helper T cells are T extrafollicular helper (Tefh) cells (Figure 2). CXCR4+ Tefh cells reside near the red pulp of the spleen and drive IgG production from B cells in splenic extrafollicular sites.24 In addition, tissue resident T cells with variable expression of CXCR5 and CXCR6 accumulate in lungs following influenza infection; these cells functionally resemble Tfh cells and contribute to plasmablast differentiation in a Bcl6-dependent manner.28,29 These cells have been termed T resident helpers (Trh) and are transcriptomically distinct from Tfh cells and resident memory Th cells. Thus, B cell-helper T cells that make IL-21 can take on a range of migratory phenotypes, leading to accumulation at different anatomic sites. To visualize this in a patient, one might imagine a patient with RA who has Tfh cells in germinal centers in draining lymph nodes and in spleen, and CCR2+ T cells accumulating in the joints. It is possible that such a patient may also have Tefh cells in the spleen. Perhaps due to lung mucosal inflammation,82 or due to lung inflammation related to interstitial lung disease,83 such a patient might also have Trh cells or Tph cells in the lungs, supported by the recognition of T cells and lymphoid aggregates in lungs of RA patients and in murine models.84,85 Further, a patient with RA and concurrent Sjogren’s syndrome, a not uncommon pairing, could potentially have a CCR9+ Tph cell population in the salivary glands. Going further, B cell-helper T cells that provide help via mechanisms other than IL-21 production may also be widely distributed, for example CXCR3+ PD1hi CD4+ T cells that do not express Bcl6, CXCR5 or IL-21 yet help B cells via IL-10 and succinate.27
Figure 2.
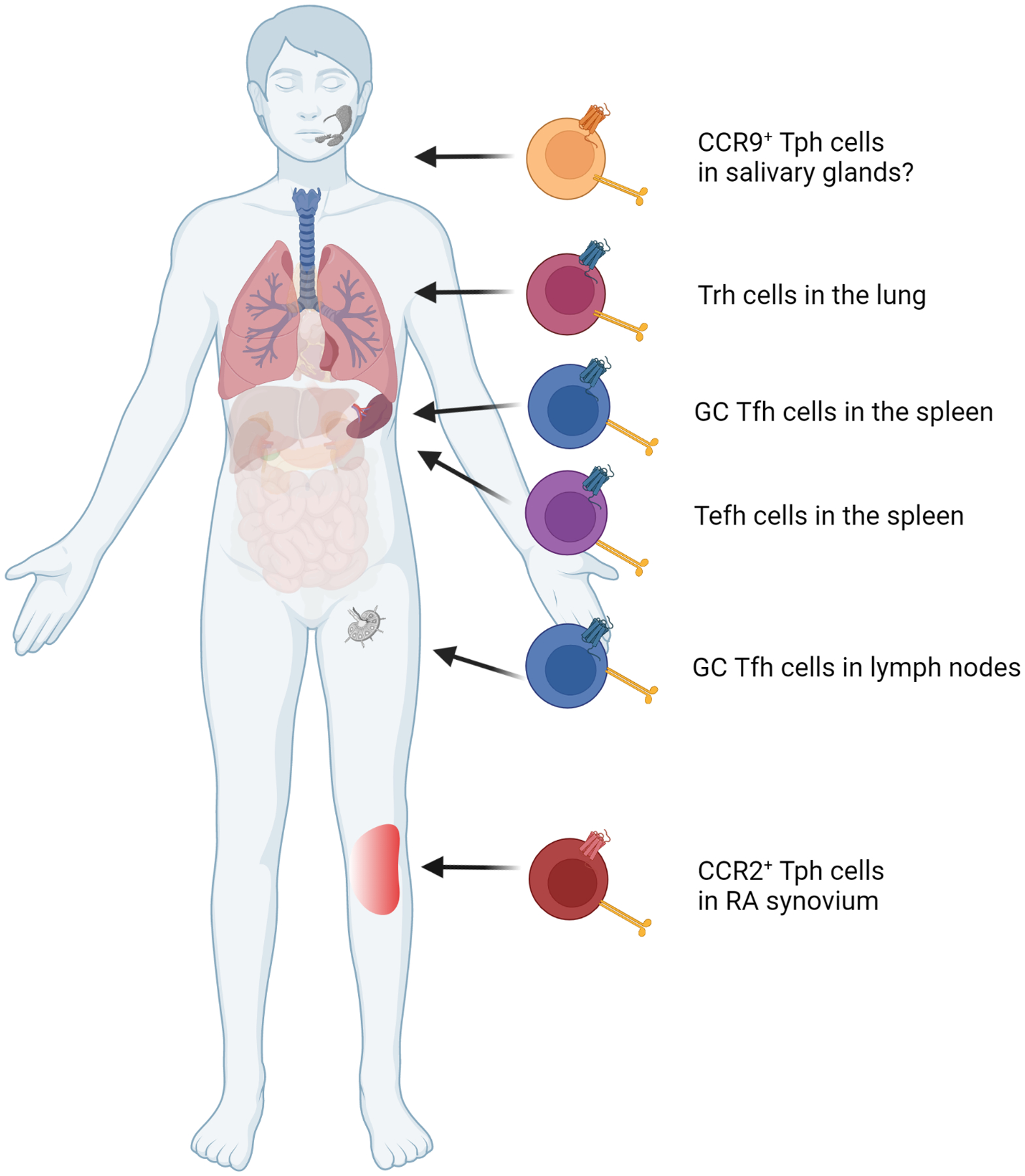
Potential populations of B cell-helper T cells in a patient with RA and Sjogren’s syndrome. Germinal center Tfh cells are CXCR5+ and present in the spleen and lymph nodes while CXCR4+ Tefh cells can be found in the spleen.24 CCR2+ Tph cells are found in high numbers in the an inflamed joint39 while CCR9+ Tph cells accumulate in salivary glands.81 Trh cells may reside in the lungs, possibly due to previous infection or interstitial lung disease.28,29,83
Though we have largely focused on T cell production of IL-21 to influence B cells, it is important to note that production of IL-21 by Tfh or Tph cells may also influence other leukocyte populations. IL-21 produced by Th17 cells or other CD4+ T cells promotes proliferation and differentiation of Th17 cells as demonstrated through in vitro mouse experiments and human psoriatic arthritis patients.86,87 Furthermore, IL-21 produced by Tfh cells directs effective granzyme B production by tumor-infiltrating CD8+ T cells leading to tumor clearance in mice.59,88 Similar to the effect on CD8+ T cells, IL-21 further augments cytotoxicity in NK cells, promoting reduction in tumor volume.88,89 Additionally, IL-21 increases CXCL8 production by macrophages resulting in increased neutrophil recruitment, but in healthy macrophages as well as RA synovial fluid macrophages has a suppressive effect resulting in production of less TNF-α, IL-6, IL-8, IL-1β, and IL-12 following LPS stimulation.90,91
In addition to IL-21, CXCL13 is produced at high levels by Tfh and Tph cells in both humans and primates, although not rodents.72,92 CXCL13 is a potent B cell chemoattract, sufficient to induce CXCR5+ B cell recruitment into a tissue.93 Indeed, mice that overexpress CXCL13 in pancreatic islet cells driven by an insulin promoter form lymphoid structures in the pancreas without any further stimulation.93 Given the prominent production of CXCL13 by tissue Tph cells, we hypothesize that Tph cell-derived CXCL13 attracts CXCR5+ B cells to a site of antigen reactivity or of ongoing inflammation.93,94 High production of CXCL13 likely also attracts CXCR5+ Tfh cells. We envision a model of Tph cell function in peripheral tissues in which Tph cells infiltrate into an inflamed tissue, become activated in response to antigen, and then produce CXCL13, leading to recruitment of B cells and subsequent formation of lymphoid aggregates. Tph cells in these sites also produce IL-21, which sustains the attracted B cells and induces their differentiation to plasmablasts. These sites of Tph-B cell interaction may multiply in size, eventually maturing to become ectopic lymphoid structures.
Interestingly, T cell production of CXCL13 is not regulated through the same pathways as IL-21. No role for Maf has been identified in regulation of CXCL13; rather, overexpression of Bcl6 results in increased expression of CXCL13.72 While IL-12 strongly induces IL-21 production from human T cells, it does not induce CXCL13, and in some cases inhibits CXCL13 expression.95 The importance of TGFβ in directing CD4+ T cells to produce CXCL13 has been described.21,96,97 Addition of TGFβ to memory or naïve CD4+ T cells in vitro induces CXCL13 production in a dose-dependent manner. Further study on the molecular regulation of CXCL13 production by Tph cells is needed. It would be valuable to interrogate the importance of Tph-derived CXCL13 in vivo in murine models, however, modeling Tph cells in mice presents a challenge since murine T cells do not produce CXCL13. Furthermore, it is not yet clear to what extent Tph cells are generated in mice and to what extent these cells will resemble Tph cells from chronic human autoimmune conditions.
Inhibition of T cell activation using CTLA4-Ig is now a well-established approach to treat RA, but in addition to lacking efficacy in some patients, it lacks T cell subset specificity, blocking T cell activation broadly.14,98 In light of their pathogenic function in inflammatory autoimmune diseases, Tph cells have emerged as a possible therapeutic target for SLE or RA, in particular due to their increased abundance in autoimmune disease patients as compared to controls. Multiple strategies to target Tfh cells have been considered in autoimmunity, for example targeting CXCR5 or BCL6; however, given that mice lacking Bcl6, and consequently lacking functional Tfh cells, still mount antibody responses, this approach risks missing potentially pathogenic CXCR5− Tph cell populations.73,74 One approach to target both Tph and Tfh cells could leverage the very high expression of PD-1 on these cells. A recent publication was successful at selectively reducing PD-1hi T cells by engineering CAR NK cells expressing a portion of PD-L1 to target PD-1hi T cells.99 These CAR NK cells were successful at selectively killing human tonsil Tfh cells in vitro, inhibited plasmablast formation in B-T coculture experiments, and reduced splenomegaly in an induced SLE mouse model.99 We hypothesize that targeting Tph cells specifically could be effective at reducing pathogenic B cell responses by reducing formation of lymphoid aggregates or ectopic lymphoid structures and pathogenic autoantibody production; still, strategies to selectively target Tph cells remain to be developed. Alternatively targeting B cell migration or plasmablast formation by inhibiting Tph cell-produced CXCL13 or IL-21 could potentially be beneficial, though the broader roles of IL-21 raise the possibility of increased immunosuppression with this approach.
Tph cell abundance as a disease biomarker
There are few, if any, cellular biomarkers in common use in the clinical care of patients with autoimmune diseases. We hypothesize that the prominent increase in circulating Tph cells in patients with autoimmune diseases may provide a valuable tool in the clinical assessment of adaptive immune activation in patients. The case for Tph cells as a potential cellular biomarker of disease activity is perhaps strongest in SLE, where increased abundance of Tph cells on average has been clearly and reproducibly demonstrated in SLE patients across multiple cohorts, disease manifestations, and disease phases.39,42,43,62 Although SLE patients demonstrate dramatic interpatient variability, flares of disease activity in SLE are often characterized by transient increases in circulating anti-double stranded DNA antibodies and an elevation in circulating ABCs,45,54 both of which may be a product of extrafollicular B cell activation.45,65 Determining the extent to which Tph cells fluctuate with disease activity is of pressing importance.
Though Tph cells are elevated on average in SLE patients, there is wide range, with some patients demonstrating consistently high Tph levels, and others with levels in the normal range, even among patients with biopsy-demonstrated proliferative lupus nephritis.39,62 Thus it is possible that Tph cell and ABC cell frequencies will identify a subset of patients with more persistently active T cell-B cell interactions, perhaps distinct from patients with more innate immune activation. Importantly, expansion of Tph cells is not unique to SLE, but rather appears to be a common feature of autoantibody-associated diseases, such that quantification of these cells in peripheral blood may serve as a generalizable marker of active T cell-B cell interactions across many conditions, conceptually similar to how C-reactive protein levels can be used to assess total body inflammation across many inflammatory conditions.
There are several tangible clinical utilizations of such a marker; we will highlight 2 such scenarios.
i). Considering the diagnosis of an autoantibody associated disease.
It is not uncommon in rheumatology clinics to consider the diagnosis of SLE in a young woman with a positive anti-nuclear antibody test and multiple non-specific symptoms, such as fatigue, joint pains, and rashes. Given the clear increase in Tph cell frequency in patients with established SLE, it is possible that Tph cell quantification may provide insight into the extent of T cell-B cell activation in such a patient and may influence clinical assessment of the likelihood that the patient has ongoing aberrant adaptive immune activation. Such information may prove valuable in predicting the likelihood of progression to full-blown SLE.
ii). Considering treatment options in a patient with an autoimmune disease.
There are now multiple treatment options for rheumatoid arthritis, which provide mechanistically distinct strategies to intervene on the disease, including blockade of TNF, blockade of IL-6 signaling, inhibition of T cell costimulation, and B cell depletion. In seropositive RA patients, circulating Tph cells are higher in patients with active disease, and fall with effective treatment.10,37 Recognition of a high frequency of Tph cells may suggest that a patient is more likely to respond to therapies that target T cell-B cell interactions, such as abatacept (CTLA4-Ig) or rituximab (anti-CD20 antibody).
Prospective studies are currently ongoing to evaluate these uses of Tph cells and related populations as useful predictive biomarkers in clinical decision making. Quantification of CD38+ ICOS+ Tfh cells provides a thematically similar approach.100 Further correlative studies may provide additional insights as to whether these and related B cell-helper T cell populations should be considered together as a total activated B cell-helper compartment detected in blood, or whether there are unique features of the adaptive immune response captured by individual subpopulations (e.g. Tph cells vs Tfh cells). The specific association of Tph cells with ABCs in the circulation suggests that some specific characteristics of the T cell-B cell interactions, for example more GC/follicular vs peripheral, may be decoded from the relative proportions of Tfh and Tph cell populations.
Conclusions:
Diverse investigational approaches to study samples from patients with autoimmune diseases have highlighted development of PD-1hi CXCR5− T peripheral helper cells as a common feature of human autoimmunity. Tph cells and other CXCR5− Tfh-like populations amplify B cell activation and autoantibody production within inflamed target tissues and appear likely to interact with other arms of the immune response as well. The developmental relationship between Tph cells and Tfh cells remains to be fully defined, yet understanding both the common and distinct regulatory pathways in Tph cells and Tfh cells may provide new tools selectively disrupt these T cell populations. Ongoing studies appear well poised to interrogate the translational utility of Tph cells as a biomarker of aberrant T-B cell activation in autoimmune disease patients.
Acknowledgements
This was work was supported by NIH National Institute of Arthritis and Musculoskeletal and Skin Diseases grants K08-AR072791, P30-AR070253, and R01-AR078769, as well as grants from the Lupus Research Alliance, Burroughs Welcome Fund Career Award for Medical Scientists, and Doris Duke Charitable Foundation Clinical Scientist Development Award to DAR.
Footnotes
Disclosures
Dr. Rao reports personal fees from Pfizer, Janssen, Merck, Scipher Medicine, GlaxoSmithKline, and Bristol-Myers Squibb and grant support from Janssen, Merck, and Bristol-Myers Squibb. Dr. Rao is a coinventor on a patent submitted on T peripheral helper cells.
References
- 1.Arbuckle MR, McClain MT, Rubertone MV, et al. Development of Autoantibodies before the Clinical Onset of Systemic Lupus Erythematosus. New England Journal of Medicine. 2003;349(16):1526–1533. doi: 10.1056/NEJMoa021933 [DOI] [PubMed] [Google Scholar]
- 2.Nielen MM, van Schaardenburg D, Reesink HW, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. Feb 2004;50(2):380–6. doi: 10.1002/art.20018 [DOI] [PubMed] [Google Scholar]
- 3.Zhang F, Wei K, Slowikowski K, et al. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat Immunol. Jul 2019;20(7):928–942. doi: 10.1038/s41590-019-0378-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arazi A, Rao DA, Berthier CC, et al. The immune cell landscape in kidneys of patients with lupus nephritis. Nat Immunol. Jul 2019;20(7):902–914. doi: 10.1038/s41590-019-0398-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaeger N, Gamini R, Cella M, et al. Single-cell analyses of Crohn’s disease tissues reveal intestinal intraepithelial T cells heterogeneity and altered subset distributions. Nat Commun. Mar 26 2021;12(1):1921. doi: 10.1038/s41467-021-22164-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Penkava F, Velasco-Herrera MDC, Young MD, et al. Single-cell sequencing reveals clonal expansions of pro-inflammatory synovial CD8 T cells expressing tissue-homing receptors in psoriatic arthritis. Nat Commun. Sep 21 2020;11(1):4767. doi: 10.1038/s41467-020-18513-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaydosik AM, Tabib T, Domsic R, Khanna D, Lafyatis R, Fuschiotti P. Single-cell transcriptome analysis identifies skin-specific T-cell responses in systemic sclerosis. Ann Rheum Dis. Nov 2021;80(11):1453–1460. doi: 10.1136/annrheumdis-2021-220209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao Y, Dunlap G, Elahee M, Rao DA. Patterns of T-Cell Phenotypes in Rheumatic Diseases From Single-Cell Studies of Tissue. ACR Open Rheumatology. 2021;3(9):601–613. doi: 10.1002/acr2.11296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fonseka CY, Rao DA, Teslovich NC, et al. Mixed-effects association of single cells identifies an expanded effector CD4(+) T cell subset in rheumatoid arthritis. Sci Transl Med. Oct 17 2018;10(463)doi: 10.1126/scitranslmed.aaq0305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rao DA, Gurish MF, Marshall JL, et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature. Feb 01 2017;542(7639):110–114. doi: 10.1038/nature20810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashouri JF, Weiss A. Endogenous Nur77 Is a Specific Indicator of Antigen Receptor Signaling in Human T and B Cells. J Immunol. Jan 15 2017;198(2):657–668. doi: 10.4049/jimmunol.1601301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simoni Y, Becht E, Fehlings M, et al. Bystander CD8+ T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature. 2018/05/01 2018;557(7706):575–579. doi: 10.1038/s41586-018-0130-2 [DOI] [PubMed] [Google Scholar]
- 13.Diogo D, Okada Y, Plenge RM. Genome-wide association studies to advance our understanding of critical cell types and pathways in rheumatoid arthritis: recent findings and challenges. Curr Opin Rheumatol. Jan 2014;26(1):85–92. doi: 10.1097/BOR.0000000000000012 [DOI] [PubMed] [Google Scholar]
- 14.Kremer JM, Westhovens R, Leon M, et al. Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA4Ig. N Engl J Med. Nov 13 2003;349(20):1907–15. doi: 10.1056/NEJMoa035075 [DOI] [PubMed] [Google Scholar]
- 15.Moens L, Tangye SG. Cytokine-Mediated Regulation of Plasma Cell Generation: IL-21 Takes Center Stage. Front Immunol. 2014;5:65. doi: 10.3389/fimmu.2014.00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luther SA, Lopez T, Bai W, Hanahan D, Cyster JG. BLC expression in pancreatic islets causes B cell recruitment and lymphotoxin-dependent lymphoid neogenesis. Immunity. May 2000;12(5):471–81. [DOI] [PubMed] [Google Scholar]
- 17.Forster R, Mattis AE, Kremmer E, Wolf E, Brem G, Lipp M. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell. Dec 13 1996;87(6):1037–47. [DOI] [PubMed] [Google Scholar]
- 18.Gunn MD, Ngo VN, Ansel KM, Ekland EH, Cyster JG, Williams LT. A B-cell-homing chemokine made in lymphoid follicles activates Burkitt’s lymphoma receptor-1. Nature. Feb 19 1998;391(6669):799–803. doi: 10.1038/35876 [DOI] [PubMed] [Google Scholar]
- 19.Legler DF, Loetscher M, Roos RS, Clark-Lewis I, Baggiolini M, Moser B. B cell-attracting chemokine 1, a human CXC chemokine expressed in lymphoid tissues, selectively attracts B lymphocytes via BLR1/CXCR5. The Journal of experimental medicine. Feb 16 1998;187(4):655–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ansel KM, Ngo VN, Hyman PL, et al. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. Jul 20 2000;406(6793):309–14. doi: 10.1038/35018581 [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi S, Murata K, Shibuya H, et al. A distinct human CD4+ T cell subset that secretes CXCL13 in rheumatoid synovium. Arthritis Rheum. Dec 2013;65(12):3063–72. doi: 10.1002/art.38173 [DOI] [PubMed] [Google Scholar]
- 22.Manzo A, Vitolo B, Humby F, et al. Mature antigen-experienced T helper cells synthesize and secrete the B cell chemoattractant CXCL13 in the inflammatory environment of the rheumatoid joint. Arthritis Rheum. Nov 2008;58(11):3377–87. doi: 10.1002/art.23966 [DOI] [PubMed] [Google Scholar]
- 23.Crotty S T Follicular Helper Cell Biology: A Decade of Discovery and Diseases. Immunity. May 21 2019;50(5):1132–1148. doi: 10.1016/j.immuni.2019.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Odegard JM, Marks BR, DiPlacido LD, et al. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. Journal of Experimental Medicine. 2008;205(12):2873–2886. doi: 10.1084/jem.20080840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gu-Trantien C, Migliori E, Buisseret L, et al. CXCL13-producing TFH cells link immune suppression and adaptive memory in human breast cancer. JCI Insight. Jun 2 2017;2(11)doi: 10.1172/jci.insight.91487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vu Van D, Beier KC, Pietzke LJ, et al. Local T/B cooperation in inflamed tissues is supported by T follicular helper-like cells. Nat Commun. 2016;7:10875. doi: 10.1038/ncomms10875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caielli S, Veiga DT, Balasubramanian P, et al. A CD4(+) T cell population expanded in lupus blood provides B cell help through interleukin-10 and succinate. Nat Med. Jan 2019;25(1):75–81. doi: 10.1038/s41591-018-0254-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Son YM, Cheon IS, Wu Y, et al. Tissue-resident CD4+ T helper cells assist the development of protective respiratory B and CD8+ T cell memory responses. Science Immunology. 2021;6(55):eabb6852. doi:doi: 10.1126/sciimmunol.abb6852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swarnalekha N, Schreiner D, Litzler LC, et al. T resident helper cells promote humoral responses in the lung. Science Immunology. 2021;6(55):eabb6808. doi:doi: 10.1126/sciimmunol.abb6808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vu Van D, Beier KC, Pietzke L-J, et al. Local T/B cooperation in inflamed tissues is supported by T follicular helper-like cells. Nature Communications. 2016/02/26 2016;7(1):10875. doi: 10.1038/ncomms10875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eisenbarth SC, Baumjohann D, Craft J, et al. CD4(+) T cells that help B cells - a proposal for uniform nomenclature. Trends Immunol. Aug 2021;42(8):658–669. doi: 10.1016/j.it.2021.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stephenson W, Donlin LT, Butler A, et al. Single-cell RNA-seq of rheumatoid arthritis synovial tissue using low-cost microfluidic instrumentation. Nat Commun. Feb 23 2018;9(1):791. doi: 10.1038/s41467-017-02659-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang L, Li H, Ren H, Hu P. Circulating PD-1(hi)CXCR5(+)CD4(+) T cells are associated with a decrease in hepatitis B surface antigen levels in patients with chronic hepatitis B who are receiving peginterferon-alpha therapy. Mol Immunol. Nov 2018;103:270–278. doi: 10.1016/j.molimm.2018.10.011 [DOI] [PubMed] [Google Scholar]
- 34.Greisen SR, Mikkelsen C, Hetland ML, et al. CXCL13 predicts long-term radiographic status in early rheumatoid arthritis. Rheumatology. 2021;doi: 10.1093/rheumatology/keab763 [DOI] [PubMed] [Google Scholar]
- 35.Han BK, Kuzin I, Gaughan JP, Olsen NJ, Bottaro A. Baseline CXCL10 and CXCL13 levels are predictive biomarkers for tumor necrosis factor inhibitor therapy in patients with moderate to severe rheumatoid arthritis: a pilot, prospective study. Arthritis Research & Therapy. 2016/04/22 2016;18(1):93. doi: 10.1186/s13075-016-0995-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greisen SR, Schelde KK, Rasmussen TK, et al. CXCL13 predicts disease activity in early rheumatoid arthritis and could be an indicator of the therapeutic ‘window of opportunity’. Arthritis Res Ther. Sep 24 2014;16(5):434. doi: 10.1186/s13075-014-0434-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fortea-Gordo P, Nuno L, Villalba A, et al. Two populations of circulating PD-1hiCD4 T cells with distinct B cell helping capacity are elevated in early rheumatoid arthritis. Rheumatology (Oxford). May 5 2019;doi: 10.1093/rheumatology/kez169 [DOI] [PubMed] [Google Scholar]
- 38.Wacleche VS, Wang R, Rao DA. Identification of T Peripheral Helper (Tph) Cells. In: Graca L, ed. T-Follicular Helper Cells: Methods and Protocols. Springer US; 2022:59–76. [DOI] [PubMed] [Google Scholar]
- 39.Bocharnikov AV, Keegan J, Wacleche VS, et al. PD-1hi CXCR5- T peripheral helper cells promote B cells responses in lupus via MAF and IL-21. JCI Insight. Sep 19 2019;doi: 10.1172/jci.insight.130062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ekman I, Ihantola EL, Viisanen T, et al. Circulating CXCR5(−)PD-1(hi) peripheral T helper cells are associated with progression to type 1 diabetes. Diabetologia. Jul 3 2019;doi: 10.1007/s00125-019-4936-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi JY, Ho JH, Pasoto SG, et al. Circulating follicular helper-like T cells in systemic lupus erythematosus: association with disease activity. Arthritis Rheumatol. Apr 2015;67(4):988–99. doi: 10.1002/art.39020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Makiyama A, Chiba A, Noto D, et al. Expanded circulating peripheral helper T cells in systemic lupus erythematosus: association with disease activity and B cell differentiation. Rheumatology (Oxford). Oct 1 2019;58(10):1861–1869. doi: 10.1093/rheumatology/kez077 [DOI] [PubMed] [Google Scholar]
- 43.Lin J, Yu Y, Ma J, Ren C, Chen W. PD-1+CXCR5-CD4+T cells are correlated with the severity of systemic lupus erythematosus. Rheumatology (Oxford). Dec 1 2019;58(12):2188–2192. doi: 10.1093/rheumatology/kez228 [DOI] [PubMed] [Google Scholar]
- 44.Rubtsov AV, Rubtsova K, Fischer A, et al. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c(+) B-cell population is important for the development of autoimmunity. Blood. Aug 4 2011;118(5):1305–15. doi: 10.1182/blood-2011-01-331462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jenks SA, Cashman KS, Zumaquero E, et al. Distinct Effector B Cells Induced by Unregulated Toll-like Receptor 7 Contribute to Pathogenic Responses in Systemic Lupus Erythematosus. Immunity. Oct 16 2018;49(4):725–739 e6. doi: 10.1016/j.immuni.2018.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fischer J, Dirks J, Klaussner J, et al. Effect of Clonally Expanded PD-1highCXCR5–CD4+ Peripheral T Helper Cells on B Cell Differentiation in the Joints of Patients With Antinuclear Antibody–Positive Juvenile Idiopathic Arthritis. Arthritis & Rheumatology. n/a(n/a)doi: 10.1002/art.41913 [DOI] [PubMed] [Google Scholar]
- 47.Edner NM, Heuts F, Thomas N, et al. Follicular helper T cell profiles predict response to costimulation blockade in type 1 diabetes. Nature Immunology. 2020/10/01 2020;21(10):1244–1255. doi: 10.1038/s41590-020-0744-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yong L, Chunyan W, Yan Y, et al. Expanded circulating peripheral helper T cells in primary biliary cholangitis: Tph cells in PBC. Mol Immunol. Mar 2021;131:44–50. doi: 10.1016/j.molimm.2020.09.007 [DOI] [PubMed] [Google Scholar]
- 49.Pontarini E, Murray-Brown WJ, Croia C, et al. Unique expansion of IL-21+ Tfh and Tph cells under control of ICOS identifies Sjögren’s syndrome with ectopic germinal centres and MALT lymphoma. Annals of the Rheumatic Diseases. 2020;79(12):1588–1599. doi: 10.1136/annrheumdis-2020-217646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Long Y, Xia C, Sun Y, et al. Increased circulating PD-1(hi)CXCR5- peripheral helper T cells are associated with disease severity of active ulcerative colitis patients. Immunol Lett. May 2021;233:2–10. doi: 10.1016/j.imlet.2021.03.001 [DOI] [PubMed] [Google Scholar]
- 51.Wang X, Li T, Si R, Chen J, Qu Z, Jiang Y. Increased frequency of PD-1(hi)CXCR5(−) T cells and B cells in patients with newly diagnosed IgA nephropathy. Sci Rep. Jan 16 2020;10(1):492. doi: 10.1038/s41598-019-57324-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Renand A, Cervera-Marzal I, Gil L, et al. Integrative molecular profiling of autoreactive CD4 T cells in autoimmune hepatitis. bioRxiv. 2020: 10.1101/2020.01.06.895938. [DOI] [PubMed] [Google Scholar]
- 53.Kamekura R, Takano K, Yamamoto M, et al. Cutting Edge: A Critical Role of Lesional T Follicular Helper Cells in the Pathogenesis of IgG4-Related Disease. J Immunol. Oct 15 2017;199(8):2624–2629. doi: 10.4049/jimmunol.1601507 [DOI] [PubMed] [Google Scholar]
- 54.Wang S, Wang J, Kumar V, et al. IL-21 drives expansion and plasma cell differentiation of autoreactive CD11c(hi)T-bet(+) B cells in SLE. Nat Commun. May 1 2018;9(1):1758. doi: 10.1038/s41467-018-03750-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kamekura R, Yamamoto M, Takano K, et al. Circulating PD-1+CXCR5−CD4+ T cells underlying the immunological mechanisms of IgG4-related disease. Rheumatology Advances in Practice. 2018;2(2)doi: 10.1093/rap/rky043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buranapraditkun S, Pissani F, Teigler JE, et al. Preservation of Peripheral T Follicular Helper Cell Function in HIV Controllers. J Virol. Jul 15 2017;91(14)doi: 10.1128/jvi.00497-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morou A, Brunet-Ratnasingham E, Dube M, et al. Altered differentiation is central to HIV-specific CD4(+) T cell dysfunction in progressive disease. Nature immunology. Aug 2019;20(8):1059–1070. doi: 10.1038/s41590-019-0418-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Del Alcazar D, Wang Y, He C, et al. Mapping the Lineage Relationship between CXCR5(+) and CXCR5(−) CD4(+) T Cells in HIV-Infected Human Lymph Nodes. Cell Rep. Sep 17 2019;28(12):3047–3060 e7. doi: 10.1016/j.celrep.2019.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cui C, Wang J, Fagerberg E, et al. Neoantigen-driven B cell and CD4 T follicular helper cell collaboration promotes anti-tumor CD8 T cell responses. Cell. 2021/12/09/ 2021;184(25):6101–6118.e13. doi: 10.1016/j.cell.2021.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thommen DS, Koelzer VH, Herzig P, et al. A transcriptionally and functionally distinct PD-1+ CD8+ T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nature Medicine. 2018/07/01 2018;24(7):994–1004. doi: 10.1038/s41591-018-0057-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Workel HH, Lubbers JM, Arnold R, et al. A Transcriptionally Distinct CXCL13+CD103+CD8+ T-cell Population Is Associated with B-cell Recruitment and Neoantigen Load in Human Cancer. Cancer Immunology Research. 2019;7(5):784–796. doi: 10.1158/2326-6066.Cir-18-0517 [DOI] [PubMed] [Google Scholar]
- 62.Sasaki T, Bracero S, Keegan J, et al. Longitudinal immune cell profiling in early systemic lupus erythematosus. bioRxiv. 2021:2021.11.08.467791. doi: 10.1101/2021.11.08.467791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sosenko JM, Skyler JS, Herold KC, Palmer JP, TrialNet tTD, Groups DPTTS. The Metabolic Progression to Type 1 Diabetes as Indicated by Serial Oral Glucose Tolerance Testing in the Diabetes Prevention Trial–Type 1. Diabetes. 2012;61(6):1331–1337. doi: 10.2337/db11-1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Asashima H, Mohanty S, Comi M, et al. PD-1highCXCR5−CD4+ Peripheral Helper T (Tph) cells Promote Tissue-Homing Plasmablasts in COVID-19. medRxiv. 2021:2021.03.13.21253527. doi: 10.1101/2021.03.13.21253527 [DOI] [Google Scholar]
- 65.Elsner RA, Shlomchik MJ. Germinal Center and Extrafollicular B Cell Responses in Vaccination, Immunity, and Autoimmunity. Immunity. Dec 15 2020;53(6):1136–1150. doi: 10.1016/j.immuni.2020.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lemieux JE, Siddle KJ, Shaw BM, et al. Phylogenetic analysis of SARS-CoV-2 in the Boston area highlights the role of recurrent importation and superspreading events. medRxiv. 2020:2020.08.23.20178236. doi: 10.1101/2020.08.23.20178236 [DOI] [Google Scholar]
- 67.Rims C, Uchtenhagen H, Kaplan MJ, et al. Citrullinated Aggrecan Epitopes as Targets of Autoreactive CD4+ T Cells in Patients With Rheumatoid Arthritis. Arthritis Rheumatol. Apr 2019;71(4):518–528. doi: 10.1002/art.40768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scally SW, Petersen J, Law SC, et al. A molecular basis for the association of the HLA-DRB1 locus, citrullination, and rheumatoid arthritis. J Exp Med. Nov 18 2013;210(12):2569–82. doi: 10.1084/jem.20131241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pieper J, Dubnovitsky A, Gerstner C, et al. Memory T cells specific to citrullinated α-enolase are enriched in the rheumatic joint. J Autoimmun. Aug 2018;92:47–56. doi: 10.1016/j.jaut.2018.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Christophersen A, Lund EG, Snir O, et al. Distinct phenotype of CD4(+) T cells driving celiac disease identified in multiple autoimmune conditions. Nat Med. Mar 25 2019;doi: 10.1038/s41591-019-0403-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Johnston RJ, Poholek AC, DiToro D, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. Aug 21 2009;325(5943):1006–10. doi: 10.1126/science.1175870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kroenke MA, Eto D, Locci M, et al. Bcl6 and Maf cooperate to instruct human follicular helper CD4 T cell differentiation. Journal of immunology. Apr 15 2012;188(8):3734–44. doi: 10.4049/jimmunol.1103246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen JS, Chow RD, Song E, et al. High-affinity, neutralizing antibodies to SARS-CoV-2 can be made without T follicular helper cells. Science Immunology. 0(0):eabl5652. doi:doi: 10.1126/sciimmunol.abl5652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miyauchi K, Sugimoto-Ishige A, Harada Y, et al. Protective neutralizing influenza antibody response in the absence of T follicular helper cells. Nature immunology. Dec 2016;17(12):1447–1458. doi: 10.1038/ni.3563 [DOI] [PubMed] [Google Scholar]
- 75.Nurieva RI, Chung Y, Martinez GJ, et al. Bcl6 mediates the development of T follicular helper cells. Science. Aug 21 2009;325(5943):1001–5. doi: 10.1126/science.1176676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Imbratta C, Hussein H, Andris F, Verdeil G. c-MAF, a Swiss Army Knife for Tolerance in Lymphocytes. Review. Frontiers in Immunology. 2020-February-14 2020;11(206)doi: 10.3389/fimmu.2020.00206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hiramatsu Y, Suto A, Kashiwakuma D, et al. c-Maf activates the promoter and enhancer of the IL-21 gene, and TGF-beta inhibits c-Maf-induced IL-21 production in CD4+ T cells. Journal of leukocyte biology. Apr 2010;87(4):703–12. doi: 10.1189/jlb.0909639 [DOI] [PubMed] [Google Scholar]
- 78.Liu X, Chen X, Zhong B, et al. Transcription factor achaete-scute homologue 2 initiates follicular T-helper-cell development. Nature. Mar 27 2014;507(7493):513–8. doi: 10.1038/nature12910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moadab F, Khorramdelazad H, Abbasifard M. Role of CCL2/CCR2 axis in the immunopathogenesis of rheumatoid arthritis: Latest evidence and therapeutic approaches. Life Sciences. 2021/03/15/ 2021;269:119034. doi: 10.1016/j.lfs.2021.119034 [DOI] [PubMed] [Google Scholar]
- 80.Cosorich I, McGuire HM, Warren J, Danta M, King C. CCR9 Expressing T Helper and T Follicular Helper Cells Exhibit Site-Specific Identities During Inflammatory Disease. Frontiers in immunology. 2019;9:2899–2899. doi: 10.3389/fimmu.2018.02899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Blokland SLM, Hillen MR, Kruize AA, et al. Increased CCL25 and T Helper Cells Expressing CCR9 in the Salivary Glands of Patients With Primary Sjogren’s Syndrome: Potential New Axis in Lymphoid Neogenesis. Arthritis & rheumatology. Oct 2017;69(10):2038–2051. doi: 10.1002/art.40182 [DOI] [PubMed] [Google Scholar]
- 82.Catrina AI, Svensson CI, Malmström V, Schett G, Klareskog L. Mechanisms leading from systemic autoimmunity to joint-specific disease in rheumatoid arthritis. Nat Rev Rheumatol. Feb 2017;13(2):79–86. doi: 10.1038/nrrheum.2016.200 [DOI] [PubMed] [Google Scholar]
- 83.McDermott GC, Doyle TJ, Sparks JA. Interstitial lung disease throughout the rheumatoid arthritis disease course. Curr Opin Rheumatol. May 1 2021;33(3):284–291. doi: 10.1097/bor.0000000000000787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Reynisdottir G, Olsen H, Joshua V, et al. Signs of immune activation and local inflammation are present in the bronchial tissue of patients with untreated early rheumatoid arthritis. Ann Rheum Dis. Nov 3 2015;doi: 10.1136/annrheumdis-2015-208216 [DOI] [PubMed] [Google Scholar]
- 85.Shilling RA, Williams JW, Perera J, et al. Autoreactive T and B cells induce the development of bronchus-associated lymphoid tissue in the lung. Am J Respir Cell Mol Biol. Apr 2013;48(4):406–14. doi: 10.1165/rcmb.2012-0065OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shi Y, Chen Z, Zhao Z, et al. IL-21 Induces an Imbalance of Th17/Treg Cells in Moderate-to-Severe Plaque Psoriasis Patients. Original Research. Frontiers in Immunology. 2019-August-07 2019;10(1865)doi: 10.3389/fimmu.2019.01865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wei L, Laurence A, Elias KM, O’Shea JJ. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J Biol Chem. 2007;282(48):34605–34610. doi: 10.1074/jbc.M705100200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu Rev Immunol. 2008;26:57–79. doi: 10.1146/annurev.immunol.26.021607.090316 [DOI] [PubMed] [Google Scholar]
- 89.Ugai S-i, Shimozato O, Yu L, et al. Transduction of the IL-21 and IL-23 genes in human pancreatic carcinoma cells produces natural killer cell-dependent and -independent antitumor effects. Cancer Gene Therapy. 2003/10/01 2003;10(10):771–778. doi: 10.1038/sj.cgt.7700630 [DOI] [PubMed] [Google Scholar]
- 90.Pelletier M, Bouchard A, Girard D. In Vivo and In Vitro Roles of IL-21 in Inflammation. The Journal of Immunology. 2004;173(12):7521–7530. doi: 10.4049/jimmunol.173.12.7521 [DOI] [PubMed] [Google Scholar]
- 91.Jian L, Li C, Wang X, Sun L, Ma Z, Zhao J. IL-21 impairs pro-inflammatory activity of M1-like macrophages exerting anti-inflammatory effects on rheumatoid arthritis. Autoimmunity. 2021:1–11. doi: 10.1080/08916934.2021.2007374 [DOI] [PubMed] [Google Scholar]
- 92.Chevalier N, Jarrossay D, Ho E, et al. CXCR5 expressing human central memory CD4 T cells and their relevance for humoral immune responses. J Immunol. May 15 2011;186(10):5556–68. doi: 10.4049/jimmunol.1002828 [DOI] [PubMed] [Google Scholar]
- 93.Luther SA, Lopez T, Bai W, Hanahan D, Cyster JG. BLC Expression in Pancreatic Islets Causes B Cell Recruitment and Lymphotoxin-Dependent Lymphoid Neogenesis. Immunity. 2000;12(5):471–481. doi: 10.1016/S1074-7613(00)80199-5 [DOI] [PubMed] [Google Scholar]
- 94.Luther SA, Ansel KM, Cyster JG. Overlapping roles of CXCL13, interleukin 7 receptor alpha, and CCR7 ligands in lymph node development. The Journal of experimental medicine. 2003;197(9):1191–1198. doi: 10.1084/jem.20021294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yoshitomi H, Kobayashi S, Miyagawa-Hayashino A, et al. Human Sox4 facilitates the development of CXCL13-producing helper T cells in inflammatory environments. Nature Communications. 2018/09/19 2018;9(1):3762. doi: 10.1038/s41467-018-06187-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kobayashi S, Watanabe T, Suzuki R, et al. TGF-beta induces the differentiation of human CXCL13-producing CD4(+) T cells. Eur J Immunol. Feb 2016;46(2):360–71. doi: 10.1002/eji.201546043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rao DA. T Cells That Help B Cells in Chronically Inflamed Tissues. Front Immunol. 2018;9:1924. doi: 10.3389/fimmu.2018.01924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Westhovens R, Verschueren P. The efficacy and safety of abatacept in rheumatoid arthritis. Ther Adv Musculoskelet Dis. 2010;2(2):89–94. doi: 10.1177/1759720X09360429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Reighard SD, Cranert SA, Rangel KM, et al. Therapeutic Targeting of Follicular T Cells with Chimeric Antigen Receptor-Expressing Natural Killer Cells. Cell Rep Med. Apr 21 2020;1(1)doi: 10.1016/j.xcrm.2020.100003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Herati RS, Silva LV, Vella LA, et al. Vaccine-induced ICOS(+)CD38(+) circulating Tfh are sensitive biosensors of age-related changes in inflammatory pathways. Cell Rep Med. May 18 2021;2(5):100262. doi: 10.1016/j.xcrm.2021.100262 [DOI] [PMC free article] [PubMed] [Google Scholar]


