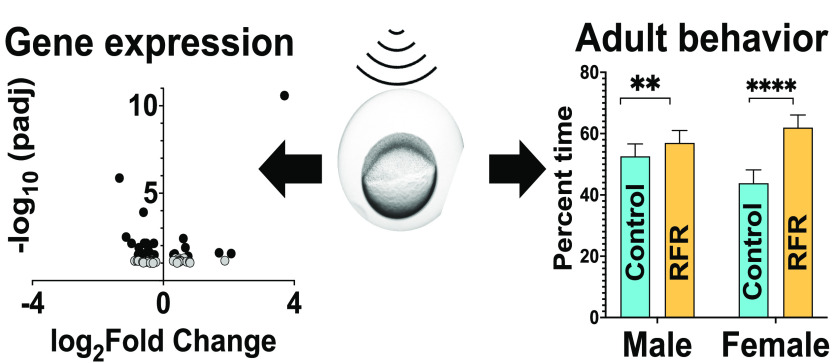
Abstract
The rapid deployment of the fifth-generation (5G) spectrum by the telecommunication industry is intended to promote better connectivity and data integration among various industries. However, concerns among the public about the safety and health effects of radiofrequency radiations (RFRs) emitted from the newer-generation cell phone frequencies remain, partly due to the lack of robust scientific data. Previously, we used developmental zebrafish to model the bioactivity of 3.5 GHz RFR, a frequency used by 5G-enabled cell phones, in a novel RFR exposure chamber. With RFR exposures from 6 h post-fertilization (hpf) to 48 hpf, we observed that, despite no teratogenic effects, embryos showed subtle hypoactivity in a startle response behavior assay, suggesting abnormal sensorimotor behavior. This study builds upon the previous one by investigating the transcriptomic basis of RFR-associated behavior effects and their persistence into adulthood. Using mRNA sequencing, we found a modest transcriptomic disruption at 48 hpf, with 28 differentially expressed genes. KEGG pathway analysis showed that biochemical pathways related to metabolism were significantly perturbed. Embryos were grown to adulthood, and then a battery of behavioral assays suggested subtle but significant abnormal responses in RFR-exposed fish across the different assays evaluated that suggest potential long-term behavioral effects. Overall, our study suggests the impacts of RFRs on the developing brain, behavior, and the metabolome should be further explored.
Introduction
The gradual rollout of fifth-generation (5G) frequencies has spurred increased scrutiny of their potential health effects over the past several years. Radiofrequency radiations (RFRs) emitted from these signals are non-ionizing, but skepticism about their safety remains, partly due to the lack of robust scientific data. This knowledge gap has contributed to valid public concerns about fully understanding 5G cell signal safety but has also been misappropriated to fuel baseless and dangerous conspiracy theories; e.g., a fabricated causal link between 5G and COVID-19 occurrence has led to violence against telecommunication engineers in Britain.1,2 Our hope is that robust studies of the health effects of 5G RFRs will provide scientific facts to dampen the unscientific noise.
Several studies, including a recent one conducted by the National Institute of Health National Toxicology Program, have addressed specific health effects of pre-5G RFR frequencies and have shown that exposure to these frequencies can lead to oxidative stress, neurological outcomes, and, in rare cases, carcinogenesis.3−6 5G frequencies penetrate less through the skin and, therefore, should be less capable of impacting biology than lower-frequency (pre-5G) RFR. Because higher-frequency RFRs are more readily blocked and hence absorbed by the skin, the obvious risk is from thermal effects. However, adverse thermal effects would require a large multi-watt per square meter dose of 5G RFR, far beyond that encountered with cell phone use or by living in the proximity of 5G infrastructure. Other than improbable thermal effects to the general public, the possibility that 5G RFR penetration, however weak, could cause resonance in some protein structures leading to altered cellular homeostasis remains. Thus, investigating any detectable, nonthermal biological effects from exposure to 5G or higher-frequency RFRs is important.
The developmental zebrafish, i.e., from 0–120 h post-fertilization (hpf), is a human-health-relevant model that eliminates some of the challenges of using rodent models such as physical size, generation time, internal development, and high cost.7 We used the model to understand how RFR affects biology, using behavioral and transcriptomic end points as readouts. The developmental stages of zebrafish are sensitive to stressor impacts because the entire molecular repertoire is active and, therefore, molecular targets can be easily detected from whole animal samples. Neonatal exposure to 5G-level RFRs will likely be lower than previous sub-gigahertz (GHz) frequencies due to lower penetration as the frequency increases throughout the millimeter wavelength part of the spectrum, though this has not yet been adequately modeled in humans. We believe that the sensitivity of the zebrafish embryo makes it a useful model for detecting nonthermal RFR hazards, essentially a worst-case scenario of RFR hazard, and thus a benchmark for addressing public concerns. Identification of molecular targets is crucial to understanding any biological pathways that are affected, regardless of any apparent phenotypes, because underlying molecular changes can regulate long-term health effects. By leveraging the strengths of zebrafish for rapid and robust detection of phenotypic and molecular responses to RFR exposures, we designed an RFR proof-of-concept study to investigate potential phenotypic and molecular effects of RFR exposures.7 We constructed a temperature-controlled Faraday box as the RFR exposure chamber for zebrafish embryos (ref (8) and Materials and Methods) and transiently exposed embryos to a 3.5 GHz frequency at a 30–32 dBm signal power for ∼2 days, followed by developmental toxicity (devtox) assays for morphology and behavior. The frequency represents a midband frequency typically used in cell phone signals. The calculated specific absorption rate (SAR) was 8.27 W/kg, ∼10–100 times higher than what a human would be exposed to from a cell phone, but this enabled us to evaluate potential effects at a higher exposure limit. While there was no evidence of teratogenicity, embryos displayed a subtle but significantly heightened startle response at 120 hpf, suggesting some aspect of a sensorimotor deficit.8 In the study presented here, we characterize the transcriptomic basis of the sensorimotor deficits and determine whether the deficits in development persist into adulthood and manifest as social and anxiogenic behaviors. Our goal was to understand the long-term behavioral effects of short-term developmental RFR exposures.
Materials and Methods
Zebrafish Husbandry
Adult tropical 5D zebrafish were raised at Sinnhuber Aquatic Research Laboratory (SARL) at Oregon State University under standard laboratory conditions (28 °C with a 14 h light/10 h dark photocycle). Adult zebrafish were fed size appropriate Gemma Micro (Skretting Inc., Tooele, France) twice daily without any live food supplement.8 Adult care and reproductive techniques followed approved Institutional Animal Care and Use Committee protocol 5113 at Oregon State University.
RFR Chamber Setup and Exposure Regime
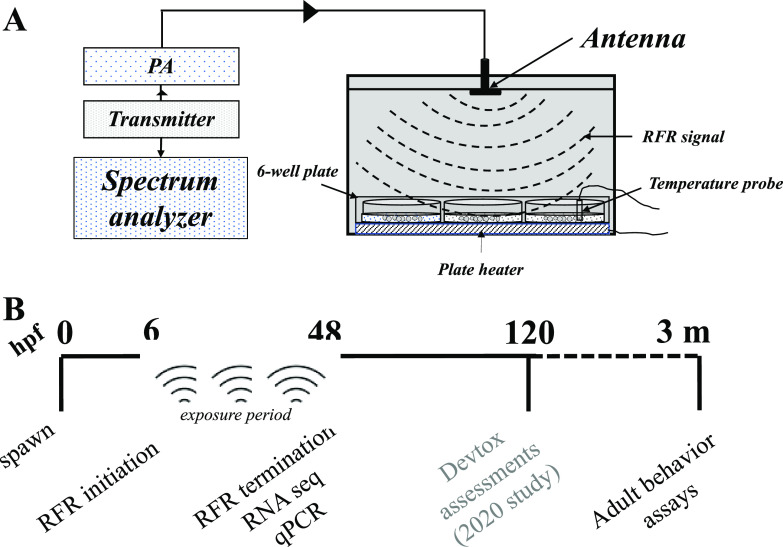
Our zebrafish RFR exposure chamber and exposure paradigm are illustrated in Figure 1, and details of the chamber design were extensively described in our previous study8 and are described in the Supporting Information. Briefly, Faraday boxes (exposure and sham), housing a six-well microtiter plate, were constructed of copper and had a 5G antenna fit to the underside of the box lid. A signal output power of 30–32 dBm at 3.5 GHz was delivered to the box. The signal was generated from a transmitter and boosted via a power amplifier (PA) upstream of the antenna. The signal power was measured at the output of the PA using a spectrum analyzer. The sham chamber received no signal. Each chamber contained a six-well flat-bottom plate (Falcon, Corning) with 30 embryos per well in 3 mL of embryo medium on a Cell MicroControls well plate heater (model HWPT-96) controlled remotely by an mTCII microtemperature controller to maintain a constant temperature of ∼28 °C, and a thermocouple temperature probe (Fluke, type K), remotely connected to a hand-held thermometer (Fluke model 51-2), was placed in the water column of one well of each plate to serve as a secondary monitor of temperature. Estimations showed that the specific absorption rate for the embryos with this setup was 8.27 W/kg. Embryos were collected in water from our recirculating system, sorted, and staged at 6 hpf (50% epiboly) according to ref (9), and static RFR exposures were conducted in embryo medium (EM; consisting of 15 mM NaCl, 0.5 mM KCl, 1 mM MgSO4, 0.15 mM KH2PO4, 0.05 mM Na2HPO4, and 0.7 mM NaHCO3) from 6 to 48 hpf. Following this, plates were removed from the Faraday cage and embryos were either (1) subsampled for RNA sequencing (this study), (2) transferred to 96-well plates with one embryo in 100 μL of EM in each well and incubated at 28 °C until 120 hpf for larval morphological and behavioral assessments (study published in ref (8)), or (3) grown in Petri dishes in EM until 120 hpf and transferred to 2.8 L tanks for further growth and adult behavioral assays (this study). The experiments were repeated over 3 days to ensure reproducibility.
Figure 1.
RFR exposure chamber and exposure regimen. (A) Faraday cage fitted with an antenna, containing a six-well plate with 50 embryos/well. The antenna receives the RF signal from a transmitter and was boosted by a power amplifier (PA). The frequency and power (dBm) were validated using a spectrum analyzer. Adapted from ref (8). (B) Time line of experiments for this study. Spawning and fertilization at 0 hpf; adult behavior at 3 m (months).
RNA Sequencing and Bioinformatics
For mRNA-seq, each well of a six-well plate (containing 50 embryos) per day represented a biological replicate (resulting in 18 biological replicate wells over three repeated experiment days). At 48 hpf, four biological replicates were collected over three separate days; each replicate represented 10 pooled embryos collected from a well. Total RNA extractions were performed as described previously.10 The RNA integrity (RIN) was assessed using an Agilent Bioanalyzer (Agilent, Santa Clara, CA), and RNA samples with RIN values of >8 were processed for library construction (see the Supporting Information for details) and sequencing on a BGISEQ-500 platform at the Beijing Genomic Institute (BGI) Americas (www.bgi.com) with a sequencing length of 100 bp paired-end. Following mRNA sequencing, low-quality reads were filtered, clean reads were annotated and mapped to the zebrafish GRz11 genome using Bowtie2, and differential gene expression was estimated using the DESeq2 platform. Sequencing quality, mapping percentages, and a list of differentially expressed genes (with padj < 0.05) are presented in Tables S1–S3. Differentially expressed genes with a padj of <0.05 were imported into gprofiler (https://biit.cs.ut.ee/gprofiler/gost) for Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis. Raw sequencing data have been deposited to NCBI under GEO accession number GSE197627.
qPCR for Selected Genes
To confirm the expression levels of selected genes from the sequencing data, we performed qPCR analyses for three genes with >1.5 log2-fold change values and padj values of <0.05: hnrnpdl, LOC103911943 (an unannotated gene), and mkrn2. We used NCBI’s Primer-BLAST to design forward and reverse primers specific to each target (primer sequences listed in Table S4) and synthesized primers from Integrated DNA Technologies (San Diego, CA). Primers were validated by determining the band size on a real time PCR, combined with melt curve analysis using qPCR. Details of primer selection and qPCR are presented in the Supporting Information. For each qPCR, we used eight biological replicates collected from three separate days of experimentation. Expression values were normalized to β-actin and analyzed using the 2–ΔΔCt method.11t tests were used to estimate the statistical significance of treatment; Grubbs’ test was used to eliminate outliers (both at the p < 0.05 level).
Adult Behavioral Assays
At 120 hpf, larvae were transferred to 2.8 L tanks for grow out with larvae from each day of experimentation housed in separate tanks. We did not observe any abnormal growth or survival of the fish during the grow-out phase. At ∼3 months of age, the fish were run through a battery of behavioral assays, including startle response, innate predator avoidance, and social cohesion. These assays were adapted from our previous publication,12 with some modifications. Briefly, individual fish were transferred to an array of eight tanks (105 mm × 105 mm × 130 mm) with an attached LCD monitor on the side. The assays included (1) novel tank (measurement of the distance traveled within the first 10 min after introduction into the tanks), (2) predator avoidance (flight response to the video of a predator on the screen), (3) schooling (social response to the video of schooling on the screen), and (4) startle response to a series of five taps (one tap every 20 s) on the tank bottoms. For schooling and predator response assays, the assay arena was divided into three zones: close (nearest to the video), middle, and far (farthest from the video). A fish, under normal conditions, will socialize with another fish in the video in the close zone but swim away to the far zone in response to a predator in the video. Therefore, the interpretation of data of the biological response was based on the percent of time spent in the close zone for the schooling assay and the far zone for the predator assay. For each assay, data were acquired for 2 min: 60 s prior to the start of the video (acclimation) followed by a 60 s video period. The duration of time spent in each zone was measured using Noldus Ethovision (version 11.5). Data analysis and statistical estimations were performed as previously described.12 For novel tank, a two-way analysis of variance (ANOVA) was executed, with treatment (control or RFR exposure) and sex of the fish as variables. For schooling and predator response, three-way ANOVAs for the percent of time spent in each zone were executed with treatment, status (acclimation or video periods), and sex of the fish as variables, followed by Tukey’s post hoc tests. Data representation and interpretation were performed on the “video” period of the assays. For startle response, a two-way repeated measures ANOVA was executed for the average distance traveled during the 20 s after each tap, with treatment and sex as the main variables and tap as a nested, repeated measures variable. In all cases, a p of <0.05 was considered statistically significant.
Results and Discussion
High-Power 3.5 GHz RFR-Induced Transcriptomic Deficits Were Associated with Metabolic Processes
Our overarching goal was to conduct a quality scientific investigation of the potential biological impacts of 5G RFR. Our immediate goal was to identify transcriptome-wide markers that accompanied subtle sensorimotor deficits observed following short-term, high-dose RFR exposures in our previous study8 and investigate whether such deficits persisted into later life stages. Our transcriptomic data revealed modest disruptions at 48 hpf to 3 days prior to the larval behavior phenotype (Figure 2A). Gene ontology assessments revealed that metabolic processes were strongly perturbed (Figure 2B). Among differentially expressed genes, mRNA levels of hnrnpdl (a pre-RNA processing and RNA metabolism gene) and mkrn2 (a protein modification gene) were increased. mRNA levels of an uncharacterized gene, LOC103911943, predicted to be a noncoding RNA, were also increased (Figure 2A,C–E). Some of these genes have been implicated in diseases; e.g., hnrnpdl plays an important role in muscle development, and its knockdown in zebrafish resulted in myopathy.13 However, we did not observe specific developmental, neurological, or signaling pathway perturbation by RFR exposures that could readily explain the sensorimotor effects observed in our previous study. Although some previous studies have shown transcriptome-level disruption in response to RFR exposure,14 the studies were limited to in vitro systems in which RFR penetration was likely much higher than that of any whole animal model. The lack of impacts on specific pathways in our study was not surprising, because GHz range RFR penetrates the skin less than sub-GHz RFR used in previous studies. Our developmental zebrafish data did suggest that relatively high-power 3.5 GHz RFR impacted basic nucleic acid synthesis and metabolic pathways that could perturb cellular homeostasis and development.
Figure 2.
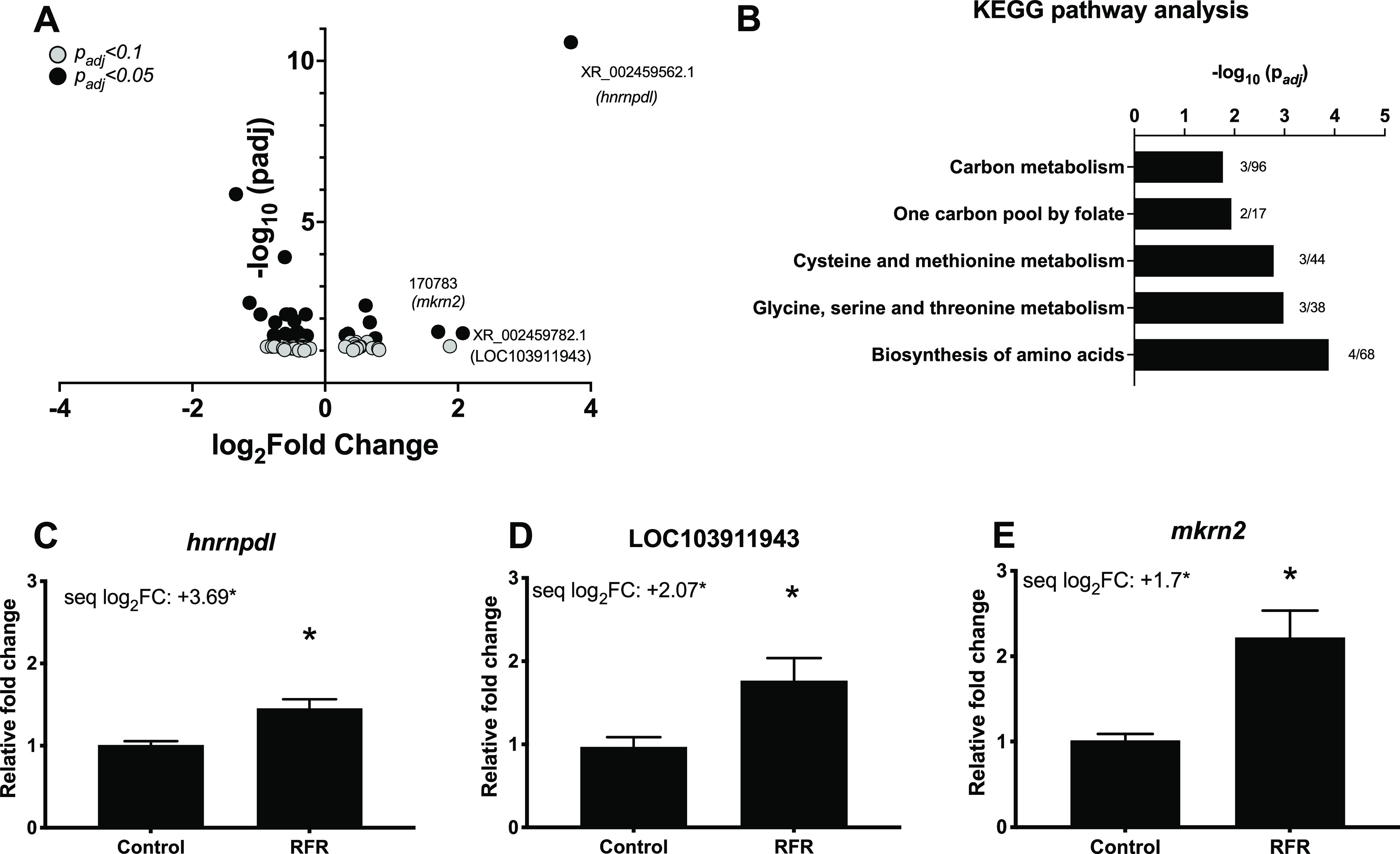
Transcriptomic effects of 3.5 GHz RFR exposures. Embryos were exposed to RFR from 6 to 48 hpf, followed by gene expression assessments. (A) Volcano plot showing differentially expressed genes with padj values of <0.1 and <0.05. (B) KEGG pathway analysis of differentially expressed mRNAs with padj values of <0.05. Numbers next to bars represent differentially expressed genes divided by the total number of genes in that pathway. (C–E) qPCR validation for the top three differentially expressed genes with padj values of <0.05. Data expressed as relative fold change ± the standard error of the mean. N = 6–8 biological replicates. An asterisk indicates a statistically significant difference using a t test (p < 0.05). “seq log2FC” indicates a log2-fold change from the mRNA sequencing data.
High-Power 3.5 GHz RFR Resulted in Long-Term Impacts on Behavior
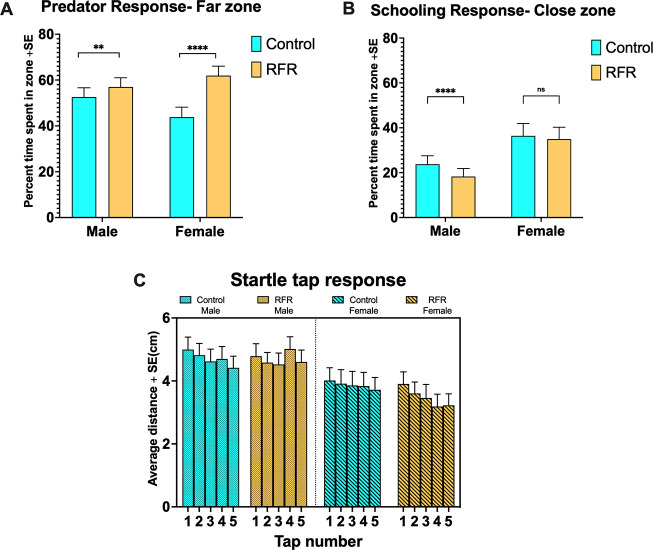
To assess whether short-term developmental exposures could have persistent consequences for behavior, we raised control (non-exposed) and RFR-exposed embryos to adulthood and evaluated their social and individual behaviors and predator avoidance and startle responses. Detailed statistics are listed in Tables S5–S8. It should be noted that, henceforth, “RFR-exposed fish” means adult fish that were developmentally exposed to RFR (prior to sexual differentiation), raised to adulthood in groups, and separated by their sex prior to the performance of behavioral assays. RFR-exposed fish did not exhibit any significant behavioral changes in a new surrounding [novel tank assay (Figure S2)]. For predator and schooling assays (Figure 3A,B), the basal female response was significantly lower and higher, respectively, than that of males (p < 0.00001 in each case) during the video period. For the predator assay (Figure 3A), RFR-exposed fish (both male and female) spent statistically significantly more time away from the predator video (far zone), with females displaying a significantly stronger predator avoidance response [∼41% increase over respective controls (p < 0.00001)] compared to that of males [∼8% increase over respective controls (p = 0.009)]. When the conspecific preference was measured in the schooling assay (Figure 3B), RFR-exposed males showed a weaker preference for the near zone than their respective controls (∼25% decrease; p < 0.00001), while the RFR-exposed females displayed no differential responses. In the startle response assay (Figure 3C), irrespective of treatment or tap, males moved ∼30% more following taps than did females (p < 0.00001). All treatment groups, except for male RFR-exposed fish, exhibited the expected habituation (less activity with successive taps) to the startle, and there was a treatment effect (p = 0.003) when taps were nested within treatment.
Figure 3.
Adult behavioral assay responses. Individual fish were introduced into a custom box with a side view of an LCD video monitor, and their acclimation to a novel environment (novel tank) was measured for the first 10 min, followed by (A) predator response (response to video of a predator) and (B) schooling (response to video of another zebrafish). For both, the first 60 s of the measurement period is the “acclimation phase” (not shown here), followed by 60 s of the “video” phase. **p < 0.01; ****p < 0.00001; ns, not significant. (C) Startle response (response to a total of five taps).
Our data here point to impacts on adult social and anxiety-related behavior from RFR exposures. A striking feature of our findings was that behavioral interference in adults, albeit subtle, may have resulted from short-term developmental RFR exposure 3 months prior. The impacts on zebrafish anxiety-related behavior are consistent with previous studies in rodent models that showed ongoing GHz or sub-GHz RFRs could alter behavior and sleep patterns.6,15 While there was no direct correspondence between an abnormal larval behavior and an abnormal adult behavior, it is likely that the adult effects were manifestations of RFR impacts on development. Studies with embryonic neural stem cells have shown that RFR exposures can inhibit neural outgrowth that can potentially impact nervous system function in the long term.5 While this is a possibility for our exposures, further investigation of the effects of high-power, low-Ghz frequencies on the brain and behavior may be warranted to better understand the health effects of such radiations. Although our data also provide evidence of sex-specific responses to RFR exposures across individual assays, there was no consensus for a specific sex driving collective behavioral deficits. The previous body of work has also shown equivocal evidence of impacts of ionizing and non-ionizing radiation independently on males and females.3,16 It is highly possible that there may be sex specificity in responses to RFR exposures at different biological levels of organization, and more studies are needed to address these effects.
Implications
High-dose, low-GHz RFR-induced effects on zebrafish development were subtle, but sensorimotor effects were persistent. The underlying transcriptomic changes pointed to components of nucleic acid synthesis and metabolism as the RFR targets. Perturbations of such important biochemical processes during development could impair neurodevelopment. This study is unique in taking a systems biology approach to studying RFR effects using a human-health-relevant model, along multiple levels of biological organization. While our exposures were short-term and single-frequency exposures, the next steps should test the impacts of longer-term exposures with rapid frequency modulation to better represent 5G cell phone use and residence near 5G infrastructure.
Acknowledgments
The project was funded through internal pilot project funds to SARL as well as a pilot grant awarded to H.L., S.D., and M.T.S. by the Pacific Northwest Center for Translational Environmental Health Research, a National Institute of Environmental Health Sciences (NIEHS)-funded P30 center (Grant P30 ES030287). The authors thank Carrie Barton and Jane La Du of Sinnhuber Aquatic Research Laboratory (SARL) for help with zebrafish husbandry and primer design. The authors also thank Tingwei Zhang and Guangxin Wang (Liu lab members, School of Electrical Engineering and Computer Science, Oregon State University) for their assistance in the setup of RFR chambers.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.estlett.2c00037.
Detailed exposure setup for RFRs, details of library preparation and sequencing, details of qPCR, supporting figures for adult behavior assays, RNA sequencing metrics (clean reads and quality scores), full list of differentially expressed genes, details of qPCR primers, and detailed statistics for behavioral assays (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Bruns A.; Harrington S.; Hurcombe E. ‘Corona 5G or both’: the dynamics of COVID-19/5G conspiracy theories on Facebook. Media International Australia 2020, 177 (1), 12–29. 10.1177/1329878X20946113. [DOI] [Google Scholar]
- Jolley D.; Paterson J. L. Pylons ablaze: Examining the role of 5G COVID-19 conspiracy beliefs and support for violence. British journal of social psychology 2020, 59 (3), 628–640. 10.1111/bjso.12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyde M.; Cesta M.; Blystone C.; Elmore S.; Foster P.; Hooth M.; Kissling G.; Malarkey D.; Sills R.; Stout M. Report of partial findings from the national toxicology program carcinogenesis studies of cell phone radiofrequency radiation in Hsd: Sprague Dawley® SD rats (whole body exposures). bioRxiv 2018, 10.1101/055699. [DOI] [Google Scholar]
- Ertilav K.; Uslusoy F.; Ataizi S.; Nazıroǧlu M. Long term exposure to cell phone frequencies (900 and 1800 MHz) induces apoptosis, mitochondrial oxidative stress and TRPV1 channel activation in the hippocampus and dorsal root ganglion of rats. Metabolic brain disease 2018, 33 (3), 753–763. 10.1007/s11011-017-0180-4. [DOI] [PubMed] [Google Scholar]
- Chen C.; Ma Q.; Liu C.; Deng P.; Zhu G.; Zhang L.; He M.; Lu Y.; Duan W.; Pei L.; Li M.; Yu Z.; Zhou Z. Exposure to 1800 MHz radiofrequency radiation impairs neurite outgrowth of embryonic neural stem cells. Sci. Rep. 2014, 4 (1), 1–10. 10.1038/srep05103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z.-q.; Zhang Y.; Wan Y.-M.; Zhou Q.; Liu C.; Wu H.-X.; Mu Y.-Z.; He Y.-F.; Rauniyar R.; Wu X.-N. Testing of behavioral and cognitive development in rats after prenatal exposure to 1800 and 2400 MHz radiofrequency fields. Journal of radiation research 2020, 61 (2), 197–206. 10.1093/jrr/rrz097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia G. R.; Noyes P. D.; Tanguay R. L. Advancements in zebrafish applications for 21st century toxicology. Pharmacology & therapeutics 2016, 161, 11–21. 10.1016/j.pharmthera.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta S.; Wang G.; Simonich M. T.; Zhang T.; Truong L.; Liu H.; Tanguay R. L. Impacts of high dose 3.5 GHz cellphone radiofrequency on zebrafish embryonic development. PloS one 2020, 15 (7), e0235869 10.1371/journal.pone.0235869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel C. B.; Ballard W. W.; Kimmel S. R.; Ullmann B.; Schilling T. F. Stages of embryonic development of the zebrafish. Developmental dynamics 1995, 203 (3), 253–310. 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Dasgupta S.; Dunham C. L.; Truong L.; Simonich M. T.; Sullivan C. M.; Tanguay R. L. Phenotypically Anchored mRNA and miRNA Expression Profiling in Zebrafish Reveals Flame Retardant Chemical Toxicity Networks. Front. Cell Dev. Biol. 2021, 9, 654. 10.3389/fcell.2021.663032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J.; Schmittgen T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2- ΔΔCT method. Methods 2001, 25 (4), 402–408. 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- García-Jaramillo M.; Beaver L. M.; Truong L.; Axton E. R.; Keller R. M.; Prater M. C.; Magnusson K. R.; Tanguay R. L.; Stevens J. F.; Hord N. G. Nitrate and nitrite exposure leads to mild anxiogenic-like behavior and alters brain metabolomic profile in zebrafish. PloS one 2020, 15 (12), e0240070 10.1371/journal.pone.0240070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira N. M.; Naslavsky M. S.; Licinio L.; Kok F.; Schlesinger D.; Vainzof M.; Sanchez N.; Kitajima J. P.; Gal L.; Cavaçana N.; et al. A defect in the RNA-processing protein HNRPDL causes limb-girdle muscular dystrophy 1G (LGMD1G). Hum. Mol. Genet. 2014, 23 (15), 4103–4110. 10.1093/hmg/ddu127. [DOI] [PubMed] [Google Scholar]
- Zhao R.; Zhang S.; Xu Z.; Ju L.; Lu D.; Yao G. Studying gene expression profile of rat neuron exposed to 1800 MHz radiofrequency electromagnetic fields with cDNA microassay. Toxicology 2007, 235 (3), 167–175. 10.1016/j.tox.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Liu L.; Deng H.; Tang X.; Lu Y.; Zhou J.; Wang X.; Zhao Y.; Huang B.; Shi Y. Specific electromagnetic radiation in the wireless signal range increases wakefulness in mice. Proc. Natl. Acad. Sci. U. S. A. 2021, 118 (31), e2105838118. 10.1073/pnas.2105838118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendran N.; Luzhna L.; Kovalchuk O. Sex difference of radiation response in occupational and accidental exposure. Front. Genet. 2019, 10, 260. 10.3389/fgene.2019.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.