Abstract
Malignancy has long been implicated with hypercoagulability, leading to an increased rate of both venous and arterial thromboembolic events (VTE and ATE). Immunotherapy has established itself as a cornerstone of modern cancer therapy by promoting antitumor immune responses, though there have been some suggestions that immune-related adverse events could include increased rates of VTE and ATE. In this review, we examine the available evidence regarding the use of immune checkpoint inhibitors (ICIs) and thrombosis. First, we describe the potential mechanisms by which ICIs might lead to thrombophilia given the overlap between the immune system, coagulation cascade, and platelet adhesion and activation. In addition, while there are some preclinical data evaluating immunotherapy-associated ATEs in animal models, there is a paucity of evidence exploring potential mechanism of VTEs in ICIs. Second, we review the incidence of ATE and VTE in patients receiving ICIs in the published literature. Finally, we discuss current limitations in understanding, areas of conflicting evidence, and approaches to further investigation.
Keywords: immune checkpoint inhibitors, immunotherapy, thrombosis
1 |. INTRODUCTION
Cancer portends an increased risk of thromboembolism, and as such, cancer patients suffer significant morbidity and mortality associated with venous and arterial thromboembolic events (VTE and ATE). Thromboembolism is the second leading cause of mortality among cancer patients.1 This propensity can be seen in incident rates as high as 110/1000 patient-years in pancreatic cancer.2 While tumor biology may promote thrombosis, the increased risk is multifactorial and certain treatments for various neoplasia can contribute to the development of thromboembolic disease. Platinum-based chemotherapy, vascular endothelial growth factor (VEGF)–targeted therapies, lenalidomide for the treatment of multiple myeloma, and newer agents such as cyclin-dependent kinase inhibitors have all been implicated in VTE/ATE.3–8
Immunotherapy has quickly evolved to be a key treatment modality for a variety of cancers. It offers effective treatment by disabling pathways that regulate immune activation. Immune checkpoint inhibitors (ICIs), specifically, block tumor-mediated immune inhibition, upregulating immune responses toward malignant cells.9 ICIs typically target one of three immune components: cytotoxic T-lymphocyte–associated protein 4 (CTLA-4), and programmed cell death protein 1 and its ligand (PD-1 and PD-L1, respectively). While they aim to promote an antitumor immune response, there is a notable potential for off-target inflammation and immune-related adverse events (irAEs) such as autoimmune colitis, pneumonitis, and immune-mediated endocrinopathies.10 There has been a suggestion that VTE and ATE should also be included in the list of irAEs, as outlined throughout some of the clinical studies included in this manuscript, although the potential for a causal relationship remains controversial. While controlled data are lacking, several published studies have attempted to evaluate this risk of VTE/ATE.
In this review, we examine the available evidence regarding the use of ICIs and thromboembolic disease in two parts. First, we discuss mechanisms by which ICIs impact development and propagation of immunothrombosis. This is supported by preclinical studies, which show clear disturbance in hemostatic parameters with blockade of PD-1 and PD-L1. We also present the available patient data on ICI therapy, rates of thrombosis, and areas for further investigation.
2 |. PRECLINICAL DATA
Certain forms of chemotherapy, especially platinum-based therapy, are commonly associated with thrombosis as seen in multiple studies.3,4 These drugs are toxic to the vascular endothelium, and release cytokines and procoagulant factors from tumor cells. Another set of agents known to be thrombogenic are those interfering with the vascular endothelial growth factor (VEGF) pathway, which impair endothelial cell function, and increase the risk of thromboembolic phenomenon.5,6 In contrast, there is a sparse mechanistic understanding of how ICIs could promote thrombosis. Here, we present several potential mechanisms, summarized in Figure 1.
FIGURE 1.
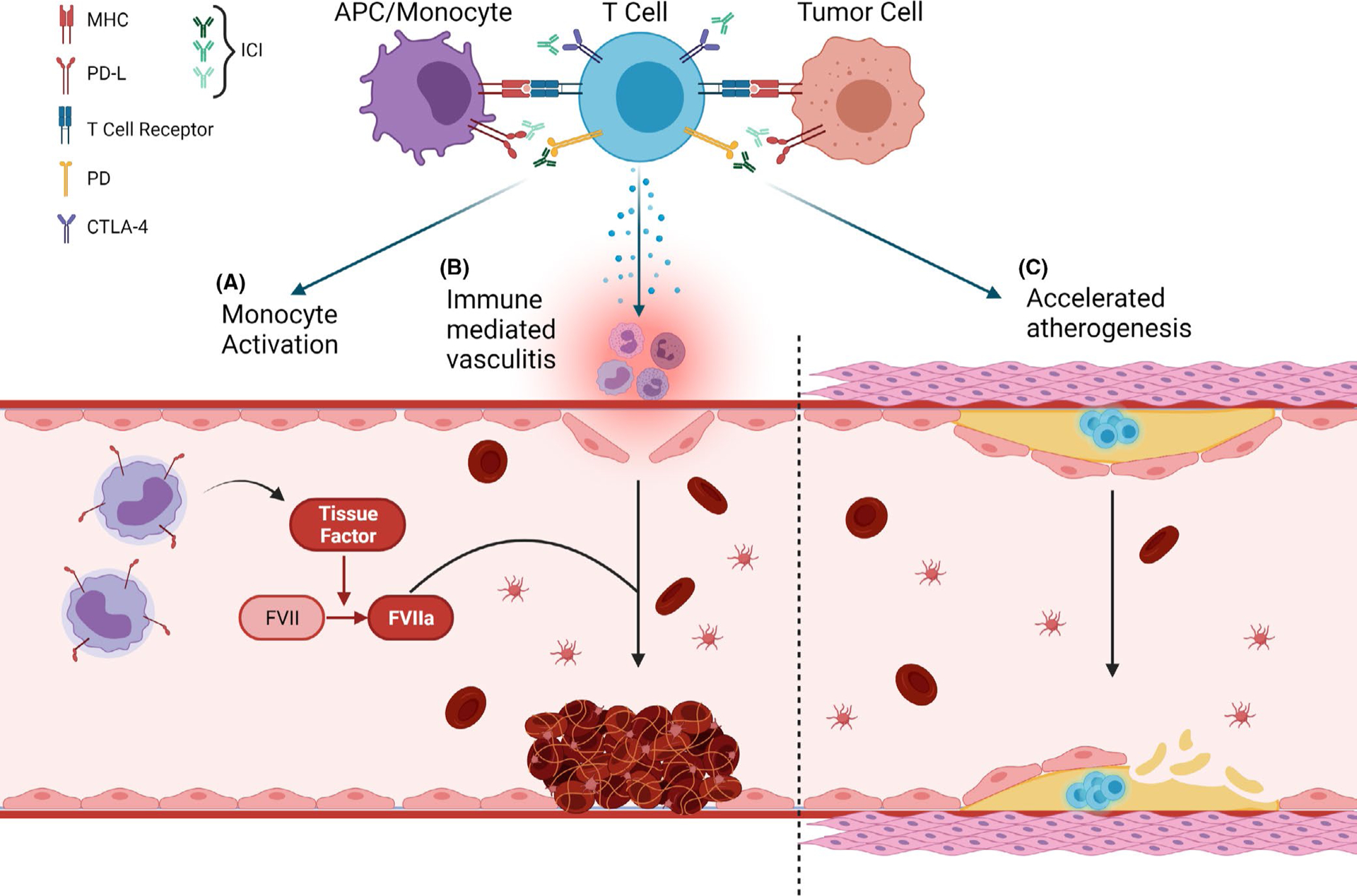
Possible mechanism by which immune checkpoint inhibitors may lead to thrombosis. T-cell activation after anti-PD-1/PD-L1 or anti-CTLA-4 leads to (A) monocyte activation, which leads to the release of tissue factor that initiates the coagulation cascade, (B) immune-mediated vasculitis, which causes endothelial damage and initiates vascular events to form a thrombus at the site of damage, and (C) deficiency in PD-1, which is known to aggravate hypercholesterolemia and increase macrophage infiltration of atherosclerotic plaques and enhance vascular inflammation and accelerate atherosclerosis
Immune checkpoint inhibitors bypass immune tolerance (checkpoints), and aid in activation of immune-related pathways that are initiated by ligand-receptor interaction between T cells and antigen-presenting cells or tumor cells. This leads to restoration of a previously inhibited antitumoral immune response and, as an adverse effect, also leads to inflammatory responses in normal organs. Off-target immune responses in the vascular endothelium can lead to clinically relevant vasculitis. Rare cases of vasculitis have been reported after initiation of ICIs, such as the development of giant cell arteritis after CTLA-4–directed therapy.11 Vasculitis is a well-known risk factor for thrombosis due to endothelial damage and activation of coagulation factors. The inflammatory state generated by other irAEs may cause thrombosis by similar mechanisms.12,13
Immune checkpoint inhibitors may also directly activate coagulation factors via the indirect release of tissue factor. ICIs block inhibitory ligands, thereby increasing the activation of T cells. These T cells in turn activate monocytes. Under physiologic circumstances, the activated monocytes will upregulate PD-L1 to counterbalance the effects of the T cells. Sato et al.14 demonstrated that not only do T cell–activated monocytes have greater PD-L1 expression but they also express greater levels of tissue factor. The blockade of PD-L1 can lead to constitutively activated monocytes and hypothetically elevated levels of tissue factor. Tissue factor, classically, is known to activate factor VII to initiate the extrinsic pathway of thrombogenesis. Tissue factor production by tumor cells is thought to be at least partially responsible for the thrombophilia of malignancy and may be key to the development of any potential ICI-associated thromboses.
Lastly, inflammation has long been shown to be influential in the evolution of arterial plaques, and there may be a specific regulatory role for the PD-1/PD-L1 pathway. Deficiency of PD-1 in mice leads to aggravated hypercholesterolemia and larger atherosclerotic lesions compared with controls.15,16 The plaques demonstrated a significant increase in macrophage and T-cell infiltration, particularly with cytotoxic CD8+ T cells, providing a route for plaque destabilization/rupture and ATE.
3 |. CLINICAL DATA IN CANCER PATIENTS RECEIVING ICIS
3.1 |. Case reports
While there are many limitations to drawing conclusions from case reports, we identified five published case reports where immunotherapy was proposed to be the cause of a resultant thrombosis. Four case reports, all representing varying forms of cancer (two with lung adenocarcinoma, one with urothelial carcinoma, and one with lymph node adenocarcinoma), describe patients who underwent therapy with pembrolizumab monotherapy as either a first- or a second-line therapy. Two case reports describe the development of acute pulmonary thromboembolism (PE) and deep vein thrombosis (DVT) in both patients.17,18 The remaining case reports described the development of cutaneous necrosis and pembrolizumab-related disseminated intravascular coagulation.18,19 All patients had no prior history of thrombophilia and generally had different medical histories. A fifth patient with lung adenocarcinoma developed acute massive saddle thrombosis in the pulmonary artery after initiation of nivolumab monotherapy.20 Between all five patients, no instances of thrombosis were detected prior to the initiation of ICIs and all patients were placed on anticoagulation therapy, leading to successful recoveries. Any conclusions drawn from these case reports should be tempered by the fact that spontaneous thrombosis is well known to occur in patients with malignancy.
3.2 |. Retrospective cohort studies
Several published cohort studies have attempted to evaluate whether the observations described in the previous case reports represented unique presentations or reflected a true drug class effect (Table 1). The following sections outline the most relevant studies investigating thrombosis risk and ICIs, beginning with the studies that evaluated specific tumor types.
TABLE 1.
Select retrospective series of thrombosis in patients receiving immunotherapy
| Author | Number of patients | Type of malignancy | Type of immunotherapy | VTE incidence | ATE incidence |
|---|---|---|---|---|---|
| Moik et al.26 | 672 | Melanoma (30.4%), non-small-cell lung cancer (24.1%), renal cell carcinoma (11.0%), head and neck squamous cell carcinoma (10.4%), and urothelial cancer (4.9%) | Nivolumab (42.0%), pembrolizumab (40.0%), ipilimumab (6.7%), ipilimumab and nivolumab (6.0%), atezolizumab (30, 4.5%), and avelumab (0.9%) | 12.9% | 1.8% |
| Sussman et al.21 | 228 | Melanoma | Pembrolizumab (38.7%), ipilimumab plus nivolumab (29.4%), ipilimumab (20%), and nivolumab (12.3%) | 16.2% | 6.1% |
| Roopkumar et al.25 | 1686 | Lung (49.6%) and melanoma (13.2%) | Nivolumab, pembrolizumab, atezolizumab, ipilimumab, avelumab, durvalumab, nivolumab, and ipilimumab | 24% | N/A |
| Icht et al.23 | 345 (but only 176 received immunotherapy) | NSCLC | ICIs | 4.5% | N/A |
| Ando et al.29 | 122 | Lung cancer, kidney cancer, stomach cancer, urothelial carcinoma, or malignant melanoma | Nivolumab (N = 85) or pembrolizumab (N = 37) | 4.1% | 4.9% |
| Gutierrez-Sainz et al.28 | 229 | Lung cancer (48.0%), melanoma (23.6%), and renal cell carcinoma (11.8%) | Pembrolizumab (40.2%) and nivolumab plus ipilimumab (8.4%) | 7% | N/A |
| Guven et al.27 | 133 | Renal cell carcinoma (26.3%) and melanoma (24.1%) | ICI | 11.3% | N/A |
| Deschênes-Simard et al.22 | 593 | NSCLC | Anti-PD-1 (91.9%), with nivolumab (53.5%) | 9.9% | 1.3% |
| Ibrahimi et al.30 | 154 | Lung cancer (20.8%), melanoma (20.1%), and ovarian cancer (12.3%) | Nivolumab (47.4%), pembrolizumab (22.7%), other investigational agents (22.7%), and atezolizumab (7.2%) | 10% | 0% |
3.2.1 |. Melanoma
Melanoma was one of the first settings in which ICIs displayed a clinical benefit. However, the presence and magnitude of any thromboembolic risk imparted by ICIs in melanoma has yet to be established.21 A retrospective study of melanoma patients receiving ICIs at the Cleveland Clinic Taussig Cancer Institute aimed to evaluate these risks. The study comprised 228 melanoma patients receiving ICIs. Most patients had stage IV disease (n = 181, 81.1%), and just over 10% had brain metastases (n = 25) at the initiation of ICI. There were a total of 51 thromboembolic events (TE) in 47 patients (TE rate of 21%, of which 37 (73%) were VTE and 14 (28%) were ATE). The majority of TE were observed within 12 months of the initiation of ICI therapy. Of all VTEs, most were DVT (n = 17, 46%), PE (n = 9, 24%) or DVT with PE (n = 9, 24.3%). A smaller subset of events included visceral vein thromboses (n = 2, 5%) or DVT with visceral vein thrombosis (n = 1, 3%). Of those who developed VTE, 16 (43%) were receiving the combination of ipilimumab and nivolumab, whereas only 7 (19%) were on ipilimumab monotherapy and only 3 (8%) on nivolumab monotherapy. The authors concluded that ICI is associated with a high incidence of TE in patients with melanoma, especially in conjunction with combination therapy. This is concerning due to TE being associated with substantially worse survival rates.
3.2.2 |. Non–small-cell lung cancer
A retrospective cohort study involving three centers, the University of Montreal Health Centre (CHUM) in Montreal (Quebec, Canada), the Dijon Bourgogne University Hospital in Dijon (France), and the Quebec Heart and Lung Institute (IUCPQ) in Quebec City (Quebec, Canada), aimed to define the association of VTEs with mortality and disease progression in ICI-treated non–small-cell lung cancer (NSCLC) patients, as well as to identify potential risk factors for the development of VTEs within this population. The study included 593 patients from June 2013 to September 2020 receiving ICI in different lines.22 Considering the independent risks that chemotherapy presents, it is important to note that prior to immunotherapy, most patients had received chemotherapy (71%). Overall, a total of 59 (10%) patients developed venous thrombotic events; 36 (6%) had PE, 26 (4%) had DVT, and 2 (0.3%) had an extensive superficial venous thrombosis requiring anticoagulation.22 Additionally, one patient had a deep vein thrombosis related to a peripherally inserted central catheter and a total of 6 patients had venous thrombosis in more than one site. Patients receiving concomitant chemotherapy had a similar risk to those receiving ICIs alone (HR = 0.84, 95% CI 0.26–2.73), but the authors note that the overall incidence of 76.5 VTEs per 1000 person-years is similar to that observed in patients treated with chemotherapy alone (anywhere from 31 to 130 VTEs per 1000 person-years). Moreover, the study found no significant correlation between VTE occurrence and survival or clinical response to ICIs.
Another retrospective study of patients at the Institute of Oncology at the Rabin Medical Center supported the previous study’s conclusions by gathering data on 345 NSCLC patients receiving single-agent ICI (n = 176) or chemotherapy (n = 169).23 The 6-month cumulative incidence of VTE within both cohorts was 7.1% in the chemotherapy patients and 4.5% in the ICI patients (HR for chemotherapy = 1.6, 95% CI 0.66–3.9). Moreover, among chemotherapy-treated patients, the high-risk Khorana score (KS) group had a trend toward a higher VTE incidence compared with patients with a low-risk KS (HR 3.04, 95% CI 0.82–11.22), and the ICI-treated patients’ high-risk KS group had a trend toward a lower VTE incidence compared with their low-risk group (HR 0.17, 95% CI 0.02–1.36).23 The study concluded that KS did not identify patients at higher risk for VTE who were treated with ICI, and recommended the development of an ICI-specific model.
For reference, many studies performed in other settings have estimated the overall incidence of VTEs as high as 14% in NSCLC. Treatment with chemotherapy is an independent risk factor for VTE and can increase the risk up to 6.5-fold compared with the general population. Moreover, platinum-based chemotherapy and cisplatin appear to be associated with an even higher VTE risk compared with other chemotherapeutic agents.24
3.2.3 |. Retrospective analysis involving multiple tumor types
Other studies pooled together patients with various malignancies who were receiving ICI therapy. A retrospective cohort study from the Cleveland Clinic Taussig Cancer Institute evaluated venous thrombosis risk in 1686 patients with various malignancies who received ICIs.25 Of the 404 (24%) patients that developed VTE, 172 had DVT, 134 had PE, 63 had both, and 24 had visceral vein thrombosis. There were no significant differences in the rates of VTE in patients treated with different ICIs. Additionally, the incidence of VTE did not change in patients receiving single versus combination immunotherapy. The cumulative VTE incidence rates for all immune therapies were 7.1% after 6 months, increasing to 10.9% at one year. The median survival in patients with VTE was significantly lower: 365 days for patients with VTE compared to 453 for individuals without VTE. Decreased survival in patients with VTE was also observed when the two most common malignancies in the study (lung cancer and melanoma) were evaluated leading the authors to view thrombosis as an important irAE.
A retrospective study of patients at the Medical University in Vienna reinforced the previous study’s findings. The study identified 672 patients treated with ICI.26 The most common types of cancer in the cohort were melanoma (30%) and NSCLC (24%). The patients were then screened for VTE and ATE and, over a mean follow-up time of 8.5 months, identified 47 (13%) VTE and 9 (2%) ATE. The most frequent types of VTE present were PE in 18 patients and DVT in 17 patients. The median life span after the occurrence of VTE was significantly reduced at 11.6 months compared to 25.5 months in those without VTE. Similar mortality risks were not found with ATE.
Two smaller European retrospective cohort studies, one at the Hacettepe University Cancer Center and one at the La Paz University Hospital, conducted similar analyses.27 The former, a cohort study involving 133 patients, observed 15 (11%) VTE with the majority being PE (n = 10, 67%). Over a mean follow-up time of 10.1 months, the median survival in patients was numerically shorter than in patients with VTEs (15.8 vs. 24.9 months, respectively, p = .303); however, it did not reach statistical significance.27 The latter study comprised a cohort of 229 patients, primarily with advanced malignancy. Sixteen patients (7%, 95% CI 4–10) developed VTE, of whom six patients had DVT, 7 patients had PE, and three patients had DVT and PE. VTE occurred in 10 of 110 patients with lung cancer (9%), five of 54 patients with melanoma (9%), and one of 27 patients with renal cell carcinoma (4%). Female sex and melanoma were independently associated with an increased risk of VTE. In this study, patients with VTE did have significantly shortened overall survival compared to patients without VTE (3.53 months vs. 18.76 months).28 Another retrospective study conducted at the Fujita Health University Hospital evaluated patients who were administered nivolumab or pembrolizumab.29 In their cohort of 122 patients, 10 (8%) developed VTE/ATE. Among these trials, there was no consistency in factors associated with thrombosis.
A retrospective study from the University of Oklahoma contested both the association of ICI with thrombosis and the mortality detriment associated with VTE.30 In their dataset of 154 patients on anti-PD-1 or PDL-1 ICI, 16 (10%) had VTE (9 DVT, 6 PE, and 1 both). The authors found that the occurrence of VTE was not significantly associated with worse progression-free survival. They also note that the incidence of VTE appears to be comparable to the previously reported incidence in patients on non-ICI therapy for their cancer. Notably, all these studies only looked at patients on ICI and did not offer any comparative cohort of patients not on an ICI.
3.2.4 |. Meta-analysis
A meta-analysis encompassing over 20,000 patients receiving immunotherapy aimed to evaluate the incidence of VTEs and ATEs in patients undergoing immunotherapy alone or in combination with other treatments.31 Among 68 retrospective and prospective included studies, there were a total of 390 VTEs and 59 ATEs, with incidence rates of 2.7% (95% CI 1.8%–4%) and 1.1% (95% CI 0.5%–2.1%), respectively. VTE rates based on the type of ICI agent administered were as follows: atezolizumab, 4.2%; pembrolizumab, 3.3%; ipilimumab, 2.9%; durvalumab, 2.8%; avelumab, 1.2%; and nivolumab, 1%. Additionally, within the entire cohort, the rate of pulmonary embolism was 1.6% (95% CI 0.7%–3.2%) and deep venous thrombosis was 2.7% (95% CI 1.4%–5.4%). Fatalities linked to VTEs and ATEs were 0.02% and 0.1%, respectively. VTE rates in all ICI arms were as follows: ovarian cancer, 28.6%; pancreatic cancer, 23.5%; urothelial cancer, 6%; endometrial cancer, 5.7%; sarcoma, 4.8%; mesothelioma, 3.7%; glioblastoma, 3.6%; renal cell carcinoma, 2.8%; lung cancer, 2%; melanoma, 1.5%; breast cancer, 1.2%; and prostate cancer, 0.3%. The study found a similar incidence of VTE and ATE in trials where ICIs (alone or in combination without other antineoplastic agents) were the only treatment agent (2.5%, 95% CI 2.5%–4.1%) as in studies where ICIs were coupled with chemotherapy (2.8%, 95% CI 0.8%–9.9%).
4 |. DISCUSSION
While malignancy and conventional chemotherapies have been associated with an increased risk of thrombosis, there remains uncertainty as to whether immunotherapy carries an increased risk of VTE due to immune activation. The use of other therapies and malignancy itself are confounding variables that alter the risk of thrombosis. It remains unclear whether immune checkpoint inhibition leads to aberrant thrombus formation. Given concern for potentially increased risk of thrombosis with immunotherapy, we provide a bottom-up discussion of the preclinical understanding of immune thrombosis and a top-down discussion originating from observed clinical findings.
The mechanisms underlying immunothrombosis remain unclear, but are believed to involve multiple overlapping pathways including the adaptive and innate immune system, coagulation cascade, and platelet adhesion and activation.32 Hypotheses include activation of the inflammatory process that results from the surge in T cells and monocytes, possibly leading to increased tissue factor expression.17,32 While there are studies exploring the mechanism of ATE due to checkpoint inhibition, there are no dedicated studies exploring the mechanism of VTE in these patients.
The limited data exploring thrombogenicity of ICIs could be due to the wide variation in the prevalence of VTE and ATE, and conflicting results as shown in our review. The majority of studies are limited by factors such as retrospective design, sample size, lack of control group, and heterogeneity of the patient population. While prospective clinical trials document the incidence of thrombosis, it has been suggested that therapeutic-directed clinical trials under-report VTE.33
Rates of VTE reported in the various studies that inform this review vary by tumor type, with estimates ranging from 4.5% in NSCLC and 20% in melanoma, though these rates are not convincingly increased compared with patients receiving conventional chemotherapy.20,22 Validated risk scores for cancer patients receiving chemotherapy such as the Khorana score may not be as predictive for patients receiving immunotherapy.23 More research is needed to understand whether the combination of immune checkpoint inhibitors and conventional chemotherapies contributes to higher VTE risk. Further work is also needed to identify novel biomarkers that may predict thrombosis in patients receiving immunotherapy. This will help identify those patients that may benefit from thromboprophylaxis. As mechanisms of immunothrombosis are further elucidated, effective existing and novel pharmacologic treatments may be identified.
5 |. CONCLUSION
Our review found a reasonable incidence of VTE and ATE in patients receiving ICIs, although it remains unclear how these rates compare to baseline for many of these high-risk populations. There is a wide variation in the incidence as noted in our literature search. While prospective studies are by far the best way to estimate the incidence of such events, the accuracy of VTE and ATE rates reported in randomized controlled trials of cancer-directed therapeutics is questionable.34 Though there are preclinical studies exploring the mechanisms underlying immunotherapy-induced ATE, there is a paucity of mechanistic studies evaluating the same with VTE. There are hypothetical pathways for which ICIs may propagate thrombogenesis via the overlap between the adaptive and innate immune system with coagulation, but current real-world data showing such are less than definitive.
Significance of topic.
This review focuses on the available evidence regarding the use of immune checkpoint inhibitors (ICIs) and thrombosis. There have been some suggestions that immune-related adverse events could include increased rates of arterial and venous thrombosis. We review the current available literature and describe the potential mechanism of thromboembolism in patients receiving ICIs.
Footnotes
CONFLICT OF INTEREST
Dr Joseph J Shatzel is a consultant for Aronara Inc. Others have no disclosures.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1.Khorana AA. Venous thromboembolism and prognosis in cancer. Thromb Res. 2010;125(6):490–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahajan A, Brunson A, White R, Wun T. The epidemiology of cancer-associated venous thromboembolism: an update. Semin Thromb Hemost. 2019;45(4):321–325. [DOI] [PubMed] [Google Scholar]
- 3.Moore RA, Adel N, Riedel E, et al. High incidence of thromboembolic events in patients treated with cisplatin-based chemotherapy: a large retrospective analysis. J Clin Oncol off J Am Soc Clin Oncol. 2011;29(25):3466–3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seng S, Liu Z, Chiu SK, et al. Risk of venous thromboembolism in patients with cancer treated with cisplatin: a systematic review and meta-analysis. J Clin Oncol. 2012;30(35):4416–4426. [DOI] [PubMed] [Google Scholar]
- 5.Nalluri SR, Chu D, Keresztes R, Zhu X, Wu S. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis. JAMA. 2008;300(19):2277–2285. [DOI] [PubMed] [Google Scholar]
- 6.Choueiri TK, Schutz FA, Je Y, Rosenberg JE, Bellmunt J. Risk of arterial thromboembolic events with sunitinib and sorafenib: a systematic review and meta-analysis of clinical trials [Internet]. Database of Abstracts of Reviews of Effects (DARE): Quality-assessed Reviews [Internet]. Centre for Reviews and Dissemination (UK); 2010. [cited 2021 Aug 30]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK80124/ [Google Scholar]
- 7.Zonder JA, Barlogie B, Durie BGM, McCoy J, Crowley J, Hussein MA. Thrombotic complications in patients with newly diagnosed multiple myeloma treated with lenalidomide and dexamethasone: benefit of aspirin prophylaxis. Blood. 2006;108(1):403–404. [DOI] [PubMed] [Google Scholar]
- 8.West MT, Smith CE, Kaempf A, et al. CDK 4/6 inhibitors are associated with a high incidence of thrombotic events in women with breast cancer in real-world practice. Eur J Haematol. 2021;106(5):634–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol. 2021;24(16):223–249. [DOI] [PubMed] [Google Scholar]
- 10.Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol. 2016;2(10):1346–1353. [DOI] [PubMed] [Google Scholar]
- 11.Boland P, Heath J, Sandigursky S. Immune checkpoint inhibitors and vasculitis. Curr Opin Rheumatol. 2020;32(1):53–56. [DOI] [PubMed] [Google Scholar]
- 12.Foley JH, Conway EM. Cross talk pathways between coagulation and inflammation. Circ Res. 2016;118(9):1392–1408. [DOI] [PubMed] [Google Scholar]
- 13.Jackson SP, Darbousset R, Schoenwaelder SM. Thromboinflammation: challenges of therapeutically targeting coagulation and other host defense mechanisms. Blood. 2019;133(9):906–918. [DOI] [PubMed] [Google Scholar]
- 14.Sato R, Imamura K, Sakata S, et al. Disorder of coagulation-fibrinolysis system: an emerging toxicity of anti-PD-1/PD-L1 monoclonal antibodies. J Clin Med. 2019;8(6):762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bu D, Tarrio M, Maganto-Garcia E, et al. Impairment of the programmed cell death-1 pathway increases atherosclerotic lesion development and inflammation. Arterioscler Thromb Vasc Biol. 2011;31(5):1100–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cochain C, Chaudhari SM, Koch M, Wiendl H, Eckstein H-H, Zernecke A. Programmed cell death-1 deficiency exacerbates T cell activation and atherogenesis despite expansion of regulatory T cells in atherosclerosis-prone mice. PLoS One. 2014;9(4):e93280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunimasa K, Nishino K, Kimura M, et al. Pembrolizumab-induced acute thrombosis. Medicine (Baltimore). 2018;97(20):e10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsukamoto J, Monteiro M, Vale S, et al. Thromboembolic events related to treatment with checkpoint inhibitors: report of two cases. Case Rep Oncol. 2018;11(3):648–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alberti A, Mancin M, Cortinovis D, Bidoli P, Sala L. Disseminated intravascular coagulation in advanced lung adenocarcinoma during first-line pembrolizumab. Immunotherapy. 2020;12(9):629–633. [DOI] [PubMed] [Google Scholar]
- 20.Abbas W, Dixit G, Rao RR, Gupta VG, Popli S. Immunotherapy-induced acute pulmonary thromboembolism: a case report. South Asian J Cancer. 2019;08(03):172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sussman TA, Li H, Hobbs B, Funchain P, McCrae KR, Khorana AA. Incidence of thromboembolism in patients with melanoma on immune checkpoint inhibitor therapy and its adverse association with survival. J Immunother Cancer. 2021;9(1):e001719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deschênes-Simard X, Richard C, Galland L, et al. Venous thrombotic events in patients treated with immune checkpoint inhibitors for non-small cell lung cancer: a retrospective multicentric cohort study. Thromb Res. 2021;1(205):29–39. [DOI] [PubMed] [Google Scholar]
- 23.Icht O, Darzi N, Shimony S, et al. Venous thromboembolism incidence and risk assessment in lung cancer patients treated with immune checkpoint inhibitors. J Thromb Haemost. 2021;19(5):1250–1258. [DOI] [PubMed] [Google Scholar]
- 24.Zahir MN, Shaikh Q, Shabbir-Moosajee M, Jabbar AA. Incidence of Venous Thromboembolism in cancer patients treated with Cisplatin based chemotherapy — a cohort study. BMC Cancer. 2017;17(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roopkumar J, Swaidani S, Kim AS, et al. Increased incidence of venous thromboembolism with cancer immunotherapy. Med N Y N. 2021;2(4):423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moik F, Chan W-SE, Wiedemann S, et al. Incidence, risk factors, and outcomes of venous and arterial thromboembolism in immune checkpoint inhibitor therapy. Blood. 2021;137(12):1669–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guven DC, Aksun MS, Sahin TK, et al. Poorer baseline performance status is associated with increased thromboembolism risk in metastatic cancer patients treated with immunotherapy. Support Care Cancer. 2021;29(9):5417–5423. [DOI] [PubMed] [Google Scholar]
- 28.Gutierrez-Sainz L, Martinez-Marin V, Viñal D, et al. Incidence of venous thromboembolic events in cancer patients receiving immunotherapy: a single-institution experience. Clin Transl Oncol. 2021;23(6):1245–1252. [DOI] [PubMed] [Google Scholar]
- 29.Ando Y, Hayashi T, Sugimoto R, et al. Risk factors for cancer-associated thrombosis in patients undergoing treatment with immune checkpoint inhibitors. Invest New Drugs. 2020;38(4):1200–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ibrahimi S, Machiorlatti M, Vesely SK, et al. Incidence of vascular thromboembolic events in patients receiving immunotherapy: a single institution experience. Blood. 2017;130(Suppl. 1):4864. [Google Scholar]
- 31.Solinas C, Saba L, Sganzerla P, Petrelli F. Venous and arterial thromboembolic events with immune checkpoint inhibitors: a systematic review. Thromb Res. 2020;1(196):444–453. [DOI] [PubMed] [Google Scholar]
- 32.Gaertner F, Massberg S. Blood coagulation in immunothrombosis—At the frontline of intravascular immunity. Semin Immunol. 2016;28(6):561–569. [DOI] [PubMed] [Google Scholar]
- 33.Stuijver DJF, Romualdi E, van Zaane B, et al. Under-reporting of venous and arterial thrombotic events in randomized clinical trials: a meta-analysis. Intern Emerg Med. 2015;10(2):219–246. [DOI] [PubMed] [Google Scholar]
- 34.Chiasakul T, Patell R, Maraveyas A, Carrier M, Zwicker JI. Discordant reporting of VTE in pancreatic cancer: a systematic review and meta-analysis of thromboprophylaxis versus chemotherapeutic trials. J Thromb Haemost JTH. 2021;19(2):489–501. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


