Abstract
Cyclin dependent kinase 4 of 6 inhibitors (CDKi) are key therapeutics in the treatment of advanced breast cancer and have recently been approved in small cell lung cancer for the prevention of myelosuppression. Thrombotic events have emerged as a significant treatment related adverse event in up to 5% of patients in clinical trials and has been reported at higher rates, up to 10%, in real world analysis. The prothrombotic mechanisms of CDKis, however, remain unknown. Cancer specific risk assessment models exist to identify who may be at highest risk of thrombosis and who could potentially benefit from prophylactic anticoagulation. However, these models may not be accurate in patients taking CDKis and may not fully capture recently identified thrombotic risk factors such as tumor specific somatic mutations. In the following manuscript, we summarize the literature on thrombotic events with CDKis in clinical trials and real-world settings, review the existing thrombosis risk assessment models for ambulatory cancer patients, and discuss the literature on tumor mutations and role in cancer associated thrombosis.
Introduction
Thrombosis is a well-known complication of cancer and contributes to diminished patient outcomes.1–4 Thrombosis is also the leading cause of death in cancer patients after progression of malignancy. 1 Multiple factors contribute to thrombogenesis such as malignancy type and stage, specific medical treatments offered, procedures undertaken, patient medical history, and lifestyle factors. Certain cancer specific treatments, such as the immunomodulatory drugs (IMiDs) used in multiple myeloma, are well-known to be thrombogenic and require risk stratification that may necessitate incorporation of prophylactic anticoagulation or antiplatelet therapy to mitigate thrombotic risk. Additionally, tumor somatic mutations have recently been identified as independent risk factors for cancer associated thrombosis.5, 6 Risk assessment models for thrombosis specific to ambulatory cancer patients exist, however, the models do not broadly apply in all settings and lack incorporation of personalized data such as tumor mutational status, making identification of those at highest risk a challenge. Despite an increasing understanding of patient specific, tumor specific, and treatment specific risk factors for thrombosis which could potentially identify high risk patients who would benefit for thromboprophylaxis, thrombosis remains a major complication of cancer care.
In the last decade, multiple cyclin dependent kinase 4 of 6 inhibitors (CDKis) have advanced the care of patients with hormone receptor positive (HR+) human epidermal growth factor receptor 2 negative (HER2−) breast cancer, however thrombosis has arisen as a complication of their use. Overexpression of cyclin D1 and CDK4/6 is common in HR+/HER2− breast cancer.7 CDKis work by selectively inhibiting CDK 4 and CDK 6 which are needed to bind cyclin D1. Inhibiting the cyclin D1 and CDK4/6 complex prohibits phosphorylation of the tumor suppressor Rb protein, allowing it to remain suppressive, and act as a key blockade of aberrant proliferation (Fig 1).8 In metastatic HR+/HER2− breast cancer, the CDKis palbociclib, ribociclib and abemaciclib are frontline therapeutics. Recently, trilaciclib became the fourth CDKi granted federal drug administration (FDA) approval for use in small cell lung cancer to lower the risk of severe neutropenia. 9 At the time of this manuscript, over 120 active clinical trials with CDKis are un-derway and broadened use of CDKis into earlier stage breast cancer and other malignancies is anticipated.10 CDKis are overall well tolerated, however, venous thromboembolism events (VTE) complicated the treatment of up to 5% of patients in the trials and up to 10% of patients in real world settings.11, 12 The prothrombotic mechanisms by which CDKi use evokes thrombosis have yet to be defined.
Fig. 1.
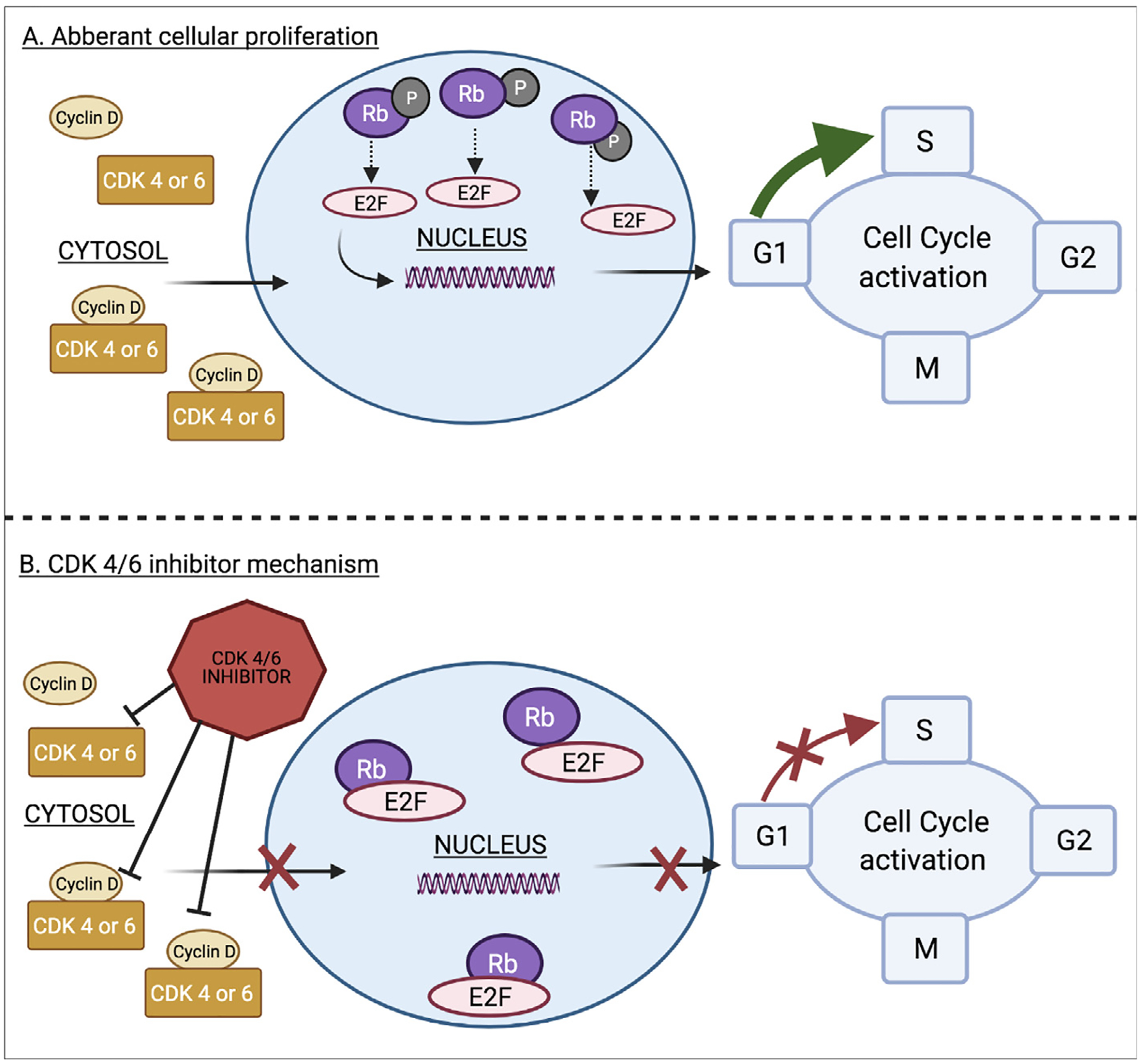
A. Cyclin D and CDK4/6 are overexpressed in HR + /HER2− breast cancer cells leading to phosphorylation and inactivation of the Rb tumor suppressor protein. Bound E2F transcription factor is released, and stimulates gene expression and cell cycle proliferation. B. CDK 4/6 inhibitors block CDK 4/6, preventing formation of the CDK4/6 and cyclin D activating complex. This allows the Rb tumor suppressor protein to remain hypophosphorylated and active, keeping E2F bound, inhibiting cellular proliferation
This article summarizes the existing trial and post-trial data of CDKi associated thrombotic events, reviews the existing thrombotic risk assessment models and prophylactic strategies in cancer, and touches on novel personalized thrombotic risk factors including tumor specific somatic mutations.
CDK 4/6 inhibitor associated thrombotic events in breast cancer clinical trials
The fundamental treatment strategy in metastatic hormone receptor positive breast cancer has been endocrine therapy since the advent of selective estrogen receptor modulators in the 1960s.13, 14 A half century later, the PALOMA-1 phase 2 clinical trial described the efficacy of palbociclib, the first CDK4/6 inhibitor to be approved for the disease.
PALOMA-1 demonstrated that when used in combination with the aromatase inhibitor, letrozole, palbociclib improved the median progression free survival (PFS) to over 20 months compared with ten months. Palbociclib was later shown to trend toward improved median overall survival (OS) by 6.9 months vs. using endocrine therapy alone in PALOMA-3 (P = 0.09).15–17, 21 This promising data has spawned multiple additional trials involving palbociclib, abemaciclib (MONARCH trials), and ribociclib (MONALEESA trials). The trials summarized in Table 1 have all shown promising data establishing CDKis as the new standard regimen for HR+/HER2− metastatic breast cancer.
Table 1.
Summary of clinical trials and real-world data on thrombotic risk
| Trial | Study | Drug and Treatment Arms | Median PFS (months) | Median OS (months) | # participants enrolled | Thrombotic events (affected/at risk) | % | Thrombotic events (affected/at risk) | % | Start of study | Date of most recent publication |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CDKi Arm | Control Arm | ||||||||||
| PALOMA-1/TR1O-18 | Phase 2 | Palbociclib + Letrozole vs Letrozole | 20.2 vs 10.2 | 37.5 vs 33.3 | 165 | 4/83 | 4.82% | 0/82 | 0.00% | 9/2008 | 5/2017 |
| PALOMA-2 | Phase 3 | Palbociclib + Letrozole vs placebo + letrozole | 24.8 vs 14.5 | Not Matured | 666 | 7/444 | 1.58% | 4/222 | 1.80% | 2/2013 | 2/2019 |
| PALOMA-3 | Phase 3 | Palbociclib + Fulvestrant vs placebo + fulvestrant | 9.2 vs 3.8 | 39.7 vs 29.7 | 521 | 5/345 | 1.45% | 0/172 | 0.00% | 9/2013 | 11/2018 |
| MONARCH-1 | Phase 2 | Abemaciclib | 6 | 17.7 | 132 | 2/132 | 1.52% | NA | NA | 6/2014 | 9/2017 |
| Next MONARCH | Phase 2 | Abemaciclib 150 + tamoxifen vs abemaciclib 150 vs abemaciclib 200 + loperamide | 9.07 vs 6.48 vs 7.43 | 24.2 vs 20.8 vs 17 | 234 | 14/234 | 5.98% | NA | NA | 9/2016 | 9/2020 |
| MONARCH-2 | Phase 3 | Abemaciclib + Fulvestrant vs placebo + fulvestrant | 16.4 vs 9.3 | 46.7 vs 37.3 | 669 | 21/441 | 4.76% | 2/223 | 0.90% | 7/2014 | 9/2019 |
| MONARCH-3 | Phase 3 | Abemaciclib + NSAI vs placebo + NSAI | 28.18 vs 14.76 | Not reported | 493 | 20/327 | 6.12% | 1/161 | 0.62% | 11/2014 | 1/2019 |
| MONARCH-Plus | Phase 3 | Abemaciclib + NSAI vs Placebo + NSAI; Abemaciclib + Fulvestrant vs Placebo + Fulvestrant | NA vs 14.73; 11.7 vs 5.59 | 56 vs 30/38.5 vs 7.5 | 463 | 8/304 | 2.63% | 0/152 | 0.00% | 12/2016 | 10/2020 |
| MONALEESA-2 | Phase 3 | Ribociclib + Letrozole vs Placebo + Letrozole | NA vs 14.7 | NR vs 33 | 668 | 2/334 | 0.60% | 1/330 | 0.30% | 12/2013 | 7/2018 |
| MONALEESA-3 | Phase 3 | Ribociclib + Fulvestrant vs Placebo + Fulvestrant | 20.8 vs 12.8 | NR vs 40 | 726 | 25/483 | 5.18% | 2/241 | 0.83% | 6/2015 | 2/2020 |
| MONALEESA-7 | Phase 3 | Ribociclib + NSAI/Tamoxifen + Goserelin vs Placebo + NSAI/Tamoxifen + Goserelin | 23.8 vs 13 | NR vs 40.7 | 672 | 10/335 | 2.99% | 3/337 | 0.89% | 11/2014 | 7/2019 |
| Trial | Study | Drug and Treatment Arms | Median PFS (months) | Median OS (months) | # participants enrolled | Thrombotic events (affected/at risk) | % | >1 Event | Arterial thrombotic Events | ||
| Gervaso et al. | Retro | Palbociclib (390, 91.9%) Ribociclib (4, 1%), abemaciclib (3, .7%), multiple (27, 6.4%) | 13.4 (overall) HR 1.25 in pt w/ VTE | 27.7 HR 1.6 in pt w/ VTE | 424 | 38 events (34 patients) | 4 | ||||
| West et al. | Retro | Palbociclib (236, 89%), Ribociclib (18, 7%), abemaciclib (37, 14%) | 7.3 vs 35.7 | 266 | 29 events (26 patients) | 3 | 10 |
CDKis are overall well tolerated, with the added benefit of being oral. However, evidence of an elevated risk of thrombosis relative to endocrine therapy alone subsequently emerged. In the phase 2 trial PALOMA-1, there was a 4.8% event rate of pulmonary embolism compared to 0% in the control arm.15, 16 The risk of VTE in subsequent studies PALOMA-2 and PALOMA-3 was closer to 1.5%, likely because these trials excluded patients who had a symptomatic PE within 6 months.18–21
In the MONALEESA trials evaluating ribociclib, publications make little mention of thrombotic events.22–24 Slamon et al. note that there was a death secondary to PE in MONALEESA-3, which the authors describe as unrelated to treatment. In that trial, there was a 5.2% rate of thrombotic events in the treatment arm compared with 0.8% rate in the placebo arm.23, 25 Another treatment related side effect of pneumonitis and interstitial lung disease (ILD) emerged, seen at rates of 1–3% and resulted in the FDA warning for these adverse events with palbociclib and ribociclib.26 Notably, the rates of thromboembolic events are comparable or greater than the rates of ILD or pneumonitis for both of these drugs (1%–5%).16, 17, 21, 25, 27, 28
The MONARCH series of trials investigated abemaciclib.29, 30 Investigators of the nextMonarch trial noted elevated incidence of VTE (9%) but suggested it may be due to tamoxifen included in a treatment arm. There was no placebo arm. The two other cohorts also included abemaciclib and found a 4%–5% incidence of VTE.30 Data from the remaining series of trials emerged to show thrombotic event rates ranging from 1.5% in the single-arm phase 2 trial of MONARCH-1 to 6.1% in the phase 3 trial of MONARCH-3 compared to 0.62% in the endocrine therapy only control arm.29, 31–37 In total, there were three fatal outcomes related to pulmonary embolism and resulted in the Food and Drug Administration (FDA) warning for VTE risk.34, 38
Thromboembolic risks in other cancer types
CDK4/6 inhibitors are being investigated in settings other than advanced breast cancer which may identify other thromboembolic risks. For example, in a study of abemaciclib in patients with stage IV squamous non-small cell lung cancer, 3/106 (2.8%) patients receiving abemaciclib experienced a thromboembolism vs. 0/52 in the docetaxel group.39 The JUNIPER trial compared abemaciclib to erlotinib in patients with previously treated KRAS mutated lung cancer. In 265 patients receiving abemaciclib, there were 5 thromboembolic events (1.89%) as opposed to 2 of 175 (1.1%) of those who received erlotinib.40, 41 Lastly, in a study of the CDK4/6 inhibitor abemaciclib alone or in combination with other agents for pancreatic ductal adenocarcinoma, there was a total thromboembolic event rate of 6/72 (8.3%) in the arms containing abemaciclib vs. 2 of 26 (7.7%) in the arm without it.42 As of this writing, there has also been approved a fourth CDK4/6 inhibitor, trilaciclib, for the purpose of reducing myelosuppression when used in combination with standard chemotherapy in the treatment of extensive-stage small cell lung cancer. This approval was based on data from three separate trials.43–45 The authors of one trial report that while hematologic adverse events were most common (as seen with the other drugs of the same class) there were fewer when compared with those receiving chemotherapy without trilaciclib. There were three fatal adverse events in the trilaciclib arm: two related to respiratory failure and one cerebrovascular accident.45 It is unclear if the cerebrovascular event is thromboembolic in nature, however unpublished data reported in the FDA package insert indicate that trilaciclib regimens carried a 7% risk of thrombosis compared to 2% risk in placebo groups.9, 46
Metanalysis data
The thromboembolic risk of the three CDKi in advanced HR +/HER2− breast cancer was noticed and led to a meta-analysis to better quantify the risk.47 The investigators examined eight studies (PALOMA-1, PALOMA-2, PALOMA-3, MONARCH-2, MONARCH-3, MONALEESA-2, MONALEESA-3 and MONALEESA-7), excluding trials without a control arm. Data were weighted to create a pooled relative risk for VTE. There were 2,793 total patients in the CDKi arms vs. 1,764 in the placebo arms. VTE events totaled 56 (2%) in the former group and 10 (0.5%) in the latter. The authors report a pooled relative risk for VTE of 2.62. That number increases to 3.18 over a median follow up of 36 months. Subgroup analysis shows the risk for VTE to be highest with abemaciclib, with a pooled relative risk of 6.8% (P = 0.009) compared to 2.3% for palbociclib and 2.2% for ribociclib although the latter 2 were not statistically significant. These numbers, however, underestimate the overall thrombotic risk based on new data released after the completion of this analysis. Notably, the number of VTE reported in this paper for MONARCH-2 is 9 and for MONALEESA-3 is 1 (0.2%). As the data matured, the investigators for those studies have revealed the actual number of VTEs to be 21 (4.8%) and 25 (5.2%), respectively.25, 37 It is expected that as time progresses there would be greater incidence of thromboembolic events. A calculation of incidence of thromboembolic events per year would be most useful to assess the risk of CDKis. There is currently not enough information provided in the data from the trials to make a temporal assessment, however, Table 1 includes the initial dates of trial commencement and the dates of the most recent publication regarding each trial.
Arterial thromboembolic events in clinical trials of CDK4/6 inhibitors
While the majority of thromboembolic events described in the trials represented VTE, it is worth noting that there were a few instances of arterial thromboemboli. In PALOMA-2 there was one thrombotic cerebral infarction and in MONARCH-1 there was one arterial thrombosis in the CDKi arm.48, 49 MONARCH-Plus reported 3 events of peripheral arterial occlusive disease in the abemaciclib arm.50 Most of the studies excluded patients who have a baseline elevated risk for arterial thromboembolic events, and thus arterial events reported in these trials may not reflect real world rates. The risk of both venous and arterial thromboembolic events may become more readily apparent in the post-trial setting.
Thrombotic events in real world studies of CDK4/6 inhibitors
Several published studies have evaluated the real-world risk of VTE in patients with breast cancer taking CDKi: two retrospective reviews and an analysis of FDA post marketing data published recently.11, 12, 51 Gervaso et al were the first to describe the rate of VTE in patients receiving CDKi in a general practice environment.11 Their cohort was comprised of 424 patients with a median age of 55 years at diagnosis of which 91% were on palbociclib. The authors found a VTE rate of 9% within 18.5 months with a median time to first VTE of 314 days. Of all VTEs observed, 47% were DVT, 18% were PE, 21% were visceral vein thrombosis and 10% of patients experienced 2 VTE events. Furthermore, the study showed VTE was associated with worse PFS and OS, with hazard ratios of 1.40 and 1.70, respectively. In this cohort, no statistically significant associations were found between VTE occurrence and patient or tumor related characteristics, though 34% of patients who developed VTE on CDKis had a history of prior VTE. None of the 57 patients on therapeutic anticoagulation for other indications developed VTE.
A second retrospective analysis revealed similar incidence of VTE in the real-world population, with 29 events in 26 out of 266 women (10%).12 This study was the first to quantify rate of arterial thromboembolism in a real-world cohort and found 34% of the total thrombotic events were arterial. BMI ≥35kg/m2, WBC <11K/μL, and hemoglobin <10g/dL were associated with elevated hazard ratios for VTE, but only hemoglobin less than 10g/dL showed a statistically significant association. Another important finding from this study was a lack of association between Khorana VTE risk scores and the development of VTE, highlighting the need for further investigation into risk factors for development of thrombotic events while on CKDi and overall need for a unique risk stratification model in this population.
Post marketing data from the FDA adverse reporting system evaluated 1,722 thromboembolic events and found elevated rates of VTE in palbociclib, ribociclib and abemaciclib.51 Rates of thromboembolic events were compared against all other drugs in the database, and also against other oncologic drugs. Abemaciclib had the highest reporting odds ratio for DVT and PE, followed by ribociclib and palbociclib. Interestingly, ribociclib also had an elevated reporting odds ratio for myocardial infarction compared to the other CDKi. Overall, the elevated thromboembolic rates with CDKi in combination with the real world and trial data illuminate the need for thrombosis risk stratification.
Existing risk assessment models and prophylaxis strategies in ambulatory cancer patients that could be adapted for CDKi use
Multiple risk assessment models exist for use in outpatient cancer patients to predict those at highest risk of VTE events. These scores incorporate patient, treatment, and malignancy related risk factors and based on the score prophylactic anticoagulation recommendations may be suggested. Unfortunately, the scores do not predict VTE risk uniformly in all cancer subtypes and have focused on gastrointestinal pathologies known to be at higher risk of VTE. Thrombosis risk assessment models for breast cancer are limited and represent an emerging need given treatment related thrombosis risk as summarized above seen with CDKi therapies.
Clinical data on thromboprophylaxis in cancer
Safety and efficacy of low molecular weight heparin (LMWH) and direct oral anticoagulants (DOACs) for VTE prophylaxis in ambulatory patients with malignancy have been described. A recent Cochran Review including over 30 0 0 patients on primary prophylaxis for VTE in ambulatory cancer patients receiving chemotherapy notes that thromboprophylaxis with LMWH reduced the incidence of symptomatic VTE, although increased the risk of major bleeding events.52
To date, two major studies have evaluated the use of DOACs for VTE prophylaxis in ambulatory patients with malignancy who were receiving chemotherapy. The CASSINI trial randomized 841 patients to either 10mg daily rivaroxaban or placebo, and the AVERT trial randomized 573 patients to either 2.5mg twice daily apixaban or placebo. Compared to placebo, patients receiving rivaroxaban had a lower incidence of VTE: 2.5% vs. 6% VTE during chemotherapy treatment and 6.4% vs 8.8% at 180 days (P = 0.10). This was also seen with apixaban where compared to placebo, lower rates of VTE were seen: 4.2% vs. 10.2% (P < 0.001). A higher incidence of major bleeding events was seen in both, with rates doubled compared to placebo (2.0% vs 1.0% (HR 1.6, 95% CI 0.59–6.49) for rivaroxaban, and 3.5% vs 1.8% (P = 0.046) for apixaban.33, 53 Of note, both of these studies only included patients with Khorana risk scores greater than or equal to 2 and excluded certain subgroups of cancer patients who are at higher risk of bleeding such as those with brain metastasis and certain hematologic malignancies. Based in part on the results of these studies, the International Initiative on Thrombosis and Cancer working group has made a grade 1B recommendation which was endorsed by the International Society for Thrombosis and Haemostasis for primary prophylaxis with rivaroxaban or apixaban for ambulatory patients at intermediate to high risk of VTE who are not actively bleeding or at high risk of bleeding.54
Clinical VTE risk stratification models in cancer
In the outpatient oncology setting, there are multiple calculators to estimate short term VTE risk.55 These models were initially created to target cancers with the highest risk of VTE, and centered on colorectal, gastric, esophageal, pancreatic, and lung adenocarcinomas. Other models are focused on thrombosis risk specifically within multiple myeloma and are weighted for VTE risk contribution the immune modulatory drug treatments are known to bring. However, no risk assessment models exist that focus on thrombosis risk unique in breast cancer, where hormonal endocrine therapies and CDKi treatments may be combined in many patients.
The most well described and validated model is the Khorana score and is often what other scores are compared against. It is comprised of a six point system: 0–2 points assigned for cancer type (2 points for stomach and pancreas cancers, 1 point for lung, lymphoma, gynecologic malignancies, bladder, and testicular cancer and 0 points for all other malignancies including breast cancer), 1 point for a pre-chemotherapy platelet count greater than or equal to 350 × 109 /L, 1 point for a pre-chemotherapy hemoglobin less than 10 g/dL, 1 point for a pre-chemotherapy leukocyte count > 11 × 109 /L, and 1 point for a BMI greater than or equal to 35 kg/m.2 56–59 A Khorana risk score of 2 has a 2.0% risk of VTE at 6 months while a score of 3 carries a 6.7% risk.
Other risk stratification scores have evolved from the Khorana score and further focus on thrombosis risk with gastrointestinal malignancies. The Vienna CATS score adds soluble P-selectin level greater than 53.1 ng/L and D-dimer greater than or equal to 1.44 μg/L to the Khorana score model as additional risk factors for VTE risk.60 The PROTECHT score incorporates the chemotherapy regimen to be initiated, platinum-based therapy or gemcitabine.61 The CONKO score exchanges BMI for WHO performance status greater than or equal to 2, and the ONKOTEV score adds metastatic disease, personal history of VTE, and macroscopic vascular or lymphatic compression.57, 61–64 The Tic-ONCO score is unique in that it incorporates presence of germline genetic mutations into the score in addition to other patient specific risk factors.55 In this model, points are assigned for presence of a polymorphism in the coagulation pathway factor 5, 13 and SERPINA10, a member of the serpin family involved in regulation of the coagulation cascade.55
Other studies aimed to further stratify risk within cancers with lower or unique treatment related risks. The COMPASS-CAT score was developed specifically for breast, lung, colorectal and ovarian cancer and incorporates treatment related risks with points assigned for anthracycline or endocrine therapy, in addition to patient specific risk factors.58 SAVED and IMPEDE VTE scores were developed and validated in patients with multiple myeloma receiving IMiD treatments.65, 66 See Table 2 summarizing the VTE risk models.
Table 2.
Summary of variables and relative weights in existing ambulatory cancer VTE risk assessment models
| VTE Variable | Khorana (6 pts) | Vienna CATS (8 pts) | Protecht (8 pts) | Conko (6 pts) | Onkotev (4 pts) | Tic-Onco | Compass-CAT (28 pt) | SAVED (8 pts) | IMPEDE (23 pts) |
|---|---|---|---|---|---|---|---|---|---|
| Cancer related risk factor: Type | |||||||||
| Pancreatic | 2 | 2 | 2 | 2 | 2 | + | − | − | − |
| Esophageal-Gastric | 2 | 2 | 2 | 2 | 2 | + | − | − | − |
| Gynecological | 1 | 1 | 1 | 1 | 2 | − | − | − | − |
| Lymphoma | 1 | 1 | 1 | 1 | 2 | − | − | − | − |
| Genitourinary/Bladder (not prostate) | 1 | 1 | 1 | 1 | 1 | − | − | − | − |
| Testicular | 1 | 1 | 1 | 1 | 1 | − | − | − | − |
| Lung | 1 | 1 | 1 | 1 | 1 | + | + | − | − |
| Colorectal | − | − | − | − | − | + | + | − | − |
| Ovarian/ Gynecologic | 1 | 1 | 1 | 1 | 1 | − | + | − | − |
| Breast | − | − | − | − | − | − | + | − | − |
| Head and Neck | − | − | − | − | − | − | − | − | − |
| High grade gliomas | 2 | − | − | − | − | ||||
| Multiple Myeloma | − | − | − | − | 1 | − | − | + | + |
| Cancer related risk factor: | |||||||||
| Advanced stage | − | − | − | − | 1 | + | 2 | − | − |
| Time since cancer diagnosis ≤ 6 months | − | − | − | − | − | − | 4 | − | − |
| Vascular/lymphatic macroscopic compression | − | − | − | − | 1 | − | − | − | − |
| Patient related risk factors | |||||||||
| Pre-chemotherapy WBC > 11 × 109/L | 1 | 1 | 1 | 1 | − | − | − | − | − |
| Pre-chemotherapy Hgb < 10g/dL or use of EPO agent | 1 | 1 | 1 | 1 | − | − | − | − | 1 |
| Pre-chemotherapy platelet ≥350 × 109/L | 1 | 1 | 1 | 1 | − | − | 2 | − | − |
| BMI ≥35kg/m2 | 1 | 1 | 1 | − | − | − | − | − | − |
| BMI ≥25kg/m2 | − | − | − | − | − | + | − | − | 1 |
| D dimer ≥1.44 μg/L | − | 1 | − | − | − | − | − | − | − |
| Soluble P-selectin ≥53.1ng/m2 | − | 1 | − | − | − | − | − | − | − |
| WHO performance status ≥2 | − | − | − | 1 | − | − | − | − | − |
| Asian race | − | − | − | − | − | − | − | −3 | −3 |
| Age ≥80 years | − | − | − | − | − | − | − | 1 | − |
| Factor V polymorphism | − | − | − | − | − | + | − | − | − |
| Factor XIII polymorphism | − | − | − | − | − | + | − | − | − |
| Serpina10 polymorphism | − | − | − | − | − | + | − | − | − |
| Surgery ≤90 days | − | − | − | − | − | − | − | 2 | − |
| Recent hospitalization ≤ 3 months | − | − | − | − | − | − | 5 | − | − |
| Pelvic, hip or femur fracture | 4 | ||||||||
| Presence of central venous catheter | − | − | − | − | − | − | 3 | − | 2 |
| History of 2 + (PAD, ischemic CVA, CAD, HTN, HLD, DM, obesity) | − | − | − | − | − | − | 5 | − | − |
| Personal History of VTE | − | − | − | − | 1 | − | 1 | 3 | 5 |
| Family History of VTE | − | − | − | − | + | − | − | − | |
| Khorana score ≥2 | − | − | − | − | 1 | − | − | − | − |
| Treatment related risk factors | |||||||||
| Gemcitabine chemotherapy | − | − | 1 | − | − | − | − | − | − |
| Platinum-based chemotherapy | − | − | 1 | − | − | − | − | − | − |
| Immunomodulatory drugs | − | − | − | − | − | − | − | − | 4 |
| Dexamethasone standard dose (120–160mg) | − | − | − | − | − | − | − | 1 | 2 |
| Dexamethasone high dose (> 160mg) | − | − | − | − | − | − | − | 2 | 4 |
| Anthracycline chemotherapy/ doxorubicin | − | − | − | − | − | − | − | − | 3 |
| Anti-Hormonal therapy if breast cancer | − | − | − | − | − | − | 6 | − | − |
| On existing therapeutic anticoagulation | − | − | − | − | − | − | − | − | −4 |
| On existing prophylactic LMWH or aspirin | − | − | − | − | − | − | − | − | −3 |
Abbreviations: WBC, white blood cell; Hgb, hemoglobin; BMI, body mass index; WHO, world health organization; PAD, peripheral arterial disease; CVA, cerebral vascular accident; HTN, hypertension; HLD, hyperlipidemia; DM, diabetes mellitus; LMWH, low molecular weight heparin
Khorana score: low risk: 0; intermediate risk 1–2; high risk ≥3
Vienna-CATS: 0–2 low-intermediate, ≥3 high risk
Protecht: 0–2 low-intermediate, ≥3 high risk
Conko: 0–2 low-intermediate, ≥3 high risk
Oncotev: high risk ≥2. Cancer type by weight according to KS risk model with inclusion of high-grade gliomas and multiple myeloma as noted.
Tic-Onco: + denotes included in risk model. High risk at specificity of ≥80% and low risk at sensitivity of ≥90%
Compass-CAT: low-intermediate risk: 0–6; high risk ≥7
Saved score: high risk ≥2
Impede score: low risk ≤ 3, high risk ≥8
An important consideration is that a cancer patient’s VTE risk is likely a dynamic, moving target as they transition from active treatment, between different agents with higher thrombotic complications (such as IMiDs, CDKi), remission, and relapse. Ideally, a simple risk assessment tool could be employed to guide the risk and benefit from thrombotic events and anticoagulation along the patient’s dynamic treatment journey. One clinical prediction algorithm has been described where only one clinical factor (tumor site) and one biomarker (D- dimer levels as a continuous variable) performed well in discriminating patients at low risk and high risk of VTE.67 Rendered as a hand-held user-friendly device, e.g. as simple to use as a modern glucometer in diabetes, could start addressing the above-mentioned challenges of targeting the right patient at the right time in their cancer journey for a tailored intervention.68
Predicting bleeding risk in patients with cancer
The clinician’s decision to anticoagulate or not, however, remains a difficult balance between the risk of bleeding and the risk of serious VTE. While cancer associated thrombosis is a major cause of cancer related morbidity and mortality, cancer patients receiving anticoagulation are at a 2-to-3-fold increased bleeding risk, especially in gastrointestinal and genitourinary cancers, compared to anticoagulated patients without cancer.69 The most commonly used score for bleeding risk is the HAS-BLED score which assigns one point each for hypertension, renal disease, liver disease, history of stroke, history of bleeding, aged > 65 years, use of NSAIDs or antiplatelet agents, and alcohol and/or drug abuse. Although initially validated in cohorts with atrial fibrillation, a recent study demonstrated good predictive validity in patients with VTE and also performed a robust analysis of bleeding risk in patients with cancer. Among all patients, cancer was the strongest predictor of major and overall bleeding and in patients with cancer, the HAS-BLED score was less predictive for bleeding events than in the non-cancer cohort.70 A separate score, VTE-BLEED, is a nine-point scale developed to predict the bleeding risk of patients with VTE on anticoagulation. In this model, six variables were assessed, of which active cancer was the strongest predictor of bleed and had the highest odds ratio of 4.18 (95% CI 2.50–7.02, P < 0.0001) compared to the other demographic and clinical variables.71 Unfortunately, this model did not specify bleeding risk by malignancy type and likely there is variation amongst active cancer subtypes, as gastrointestinal tumors are known to be at risk for bleeding.69
Unfortunately, our current ability to estimate risk of cancer associated arterial thromboembolic events is poor, and not well defined. It is unclear if cancer associated arterial events may be related to a prothrombotic state or an arrhythmia that may emerge associated with cancer or treatments.
Thrombotic risk associated with specific tumor somatic mutations
Specific tumor somatic mutations have emerged as novel thrombotic risk factors, correlating with both venous and arterial thrombosis risk in certain malignancies.5, 6, 80, 72–79 In anaplastic lymphoma kinase (ALK) rearranged NSCLC, after other patient and treatment variables were controlled for, the presence of ALK rearrangements conferred a fourfold increased risk of VTE (hazard ratio 3.70, [95% confidence interval [CI]: 2.51–5.44, P < 0.001) and threefold increased risk for arterial thrombosis (HR 3.15 [95% CI: 1.18–8.37, P = 0.021).79 Several other studies have also described the NSCLC and increased VTE risk from genomic alterations in ALK rearrangement, ROS1 rearrangement, EGFR mutated as well as with elevated PDL1 expression.5, 72–76 KRAS mutated colon cancer likewise carries an increased VTE risk compared to wild type.5 Further, brain gliomas with wildtype isocitrate dehydrogenase 1 (IDH1) have increased rates of VTE after 6 months of observation; 18.2%, compared to 0% in patients with mutated IDH1 expression.77, 78
A study of 14,000 pooled solid tumor samples which included 14% breast cancer samples, found that somatic tumor mutations of STK11, KRAS, CTNNB1, KEAP1, CDKN2B and MET were associated with a statistically significant increased risk of VTE in solid tumor patients. Mutations in CDK4, CDKN2A, SMAD4, MHC, TP53, and SMARCA4 were also were also associated with elevated hazard ratios for thrombosis but did not meet statistical significance.6 Somatic tumor mutations in HR +/HER2− metastatic breast cancer often include genes involved in the aberrant CDK 4/6 and cyclin D1 pathway and can include CDK4, CDKN2A, CDKN2B in addition to others. It is unclear if these mutations play a role in thrombogenesis in the breast cancer setting but warrant further evaluation and may be an important risk factor to consider in considering a patient’s overall thrombosis risk when on CDKi treatment.
The mechanisms underlying how somatic mutations may alter thrombotic risk are just beginning to be understood. Mechanisms may relate to over-expression and secretion of various pro-coagulant factors by the tumor, including tissue factor (TF) and TF-bearing microparticles; activation of platelets and/or leukocytes by tumor secreted factors including pro-inflammatory cytokines; and/or secondary effects of tumor cells on surrounding tissue microenvironment and vasculature as a result of aberrant proangiogenic growth factor stimulation.81, 82 Further research to define the mechanism represents an area of needed research.
Conclusion
CDKis are important therapeutic agents for many patients with advanced breast cancer and widening application into more clinical settings is anticipated. However, these agents are associated with increased rates of thrombosis. In the breast cancer trials leading to the FDA approvals of palbociclib, abemaciclib and ribociclib, increased rates of thromboembolic events were reported compared to control arms. Trials of CDKi in other malignancy types also revealed elevated thromboembolic events compared to control, including in the most recently FDA approved CDKi, trilaciclib which is indicated for use with myelosuppression management in small cell lung cancer. Meta-analysis and real-world studies of CDKi similarly confirm high rates of thrombotic events associated with CDKi, with real-world data reflecting rates up to 10% including a significant portion of arterial events.
Thromboprophylaxis with LMWH and direct oral anticoagulants are an effective means of preventing VTE in high-risk ambulatory cancer patients but carry an increased risk of bleeding. Existing validated risk assessment models to identify those at highest risk of VTE, such as the Khorana score, center on gastrointestinal and lung adenocarcinomas, and appear to not be predictive of thromboembolic events in those on CDKi with breast cancer.12 Other validated risk assessment models to guide thromboprophylaxis decisions are disease specific, such as with multiple myeloma, but no breast cancer specific models have been established. No risk assessment model has incorporated tumor somatic mutations as part of the score and represents a growing area in predicting those at highest risk for cancer associated thromboembolic events.
Development of a risk assessment model specific to those with breast cancer indicated for CDKi would be useful to maximize the treatment potential of these key agents while minimizing the potentially dangerous thromboembolic side effect. CDKis are often given in combination with hormonal therapies, which may confer additive thrombotic risk. Future research exploring breast tumor somatic mutations and the role these may have with thromboembolic risk is needed. Creation of a personalized breast cancer thrombosis risk model that incorporates patient, treatment and tumor mutation data may provide the best means to predict who may benefit from thromboprophylaxis when taking these important treatments.
Research support
J.J.S. is supported by the National Institutes of Health, National Heart, Lung, and Blood Institute (HL151367).
Footnotes
Conflict-of-interest
J.J.S. is a consultant for Aronora INC. The remaining authors have no disclosures.
References
- 1.Khorana AA, Francis CW, Culakova E, et al. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J. Thromb. Haemost. 2007;5:632–634. [DOI] [PubMed] [Google Scholar]
- 2.Levitan N, Dowlati A, Remick SC, et al. Rates of initial and recurrent thromboembolic disease among… : medicine. Medicine (Baltimore). 1999;78:285–291. [DOI] [PubMed] [Google Scholar]
- 3.Sørensen HT, Mellemkjær L, Olsen JH, et al. Prognosis of Cancers Associated with Venous Thromboembolism. N. Engl. J. Med. 2000;343:1846–1850. [DOI] [PubMed] [Google Scholar]
- 4.Lee AYY, Levine MN. 2003. Venous thromboembolism and cancer: risks and outcomes [DOI] [PubMed]
- 5.Abufarhaneh M, Pandya RK, Alkhaja A, et al. association between genetic mutations and risk of venous thromboembolism in patients with solid tumor malignancies: a systematic review and meta-analysis. Blood. 2020;136(Supplement 1):33–34. [DOI] [PubMed] [Google Scholar]
- 6.Dunbar A, Bolton KL, Devlin SM, et al. Genomic profiling identifies somatic mutations predicting thromboembolic risk in patients with solid tumors. Blood. 2020;137:2103–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buckley M, Sweeney K, Hamilton J, et al. Expression and amplification of cyclin genes in human breast cancer - PubMed. Oncogene. 1993;8:2127–2133. [PubMed] [Google Scholar]
- 8.Knudsen ES, Witkiewicz AK. 2017. The strange case of cdk4/6 inhibitors: mechanisms, resistance, and combination strategies [DOI] [PMC free article] [PubMed]
- 9.G1 Therapeutics, Inc.. Cosela (trilaciclib)[package insert]. U.S. Food and Drug Administration; 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214200s000lbl.pdf. [Google Scholar]
- 10.ClinicalTrials.gov. https://clinicaltrials.gov/ct2/results?recrs=&cond=cdk+4%2F6&term=&cntry=&state=&city=&dist=
- 11.Gervaso L, Montero AJ, Jia X, et al. Venous thromboembolism in breast cancer patients receiving cyclin-dependent kinase inhibitors. J. Thromb. Haemost. 2020;18:162–168. [DOI] [PubMed] [Google Scholar]
- 12.West MT, Smith CE, Kaempf A, et al. CDK 4/6 inhibitors are associated with a high incidence of thrombotic events in women with breast cancer in real-world practice. Eur. J. Haematol. ejh.. 2021;106:13590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zarros A, Fornace AJ, Simoncini T, et al. Tamoxifen from failed contraceptive pill to best-selling breast cancer medicine: a case-study in pharmaceutical innovation. Front. Pharmacol. | www.frontiersin.org.. 2017;8:620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jordan C Historical perspective on hormonal therapy of advanced breast cancer. Clin. Ther. 2002;24(SUPPL. A):A3–16. [DOI] [PubMed] [Google Scholar]
- 15.Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study. Lancet Oncol 2015;16:25–35. [DOI] [PubMed] [Google Scholar]
- 16.Finn RS, Crown JP, Ettl J, et al. Efficacy and safety of palbociclib in combination with letrozole as first-line treatment of ER-positive, HER2-negative, advanced breast cancer: Expanded analyses of subgroups from the randomized pivotal trial PALOMA-1/TRIO-18. Breast Cancer Res 2016;18:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finn RS, Crown J, Lang I, et al. Overall survival results from the randomized phase II study of palbociclib (P) in combination with letrozole (L) vs letrozole alone for frontline treatment of ER+/HER2− advanced breast cancer (PALO-MA-1; TRIO-18). J. Clin. Oncol. 2017;35(15_suppl):1001. [Google Scholar]
- 18.Finn RS, Martin M, Rugo HS, et al. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2016;375:1925–1936. [DOI] [PubMed] [Google Scholar]
- 19.Rugo HS, Finn RS, Diéras V, et al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res. Treat. 2019;174:719–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phas. Lancet Oncol 2016;17:425–439. [DOI] [PubMed] [Google Scholar]
- 21.Turner NC, Slamon DJ, Ro J, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N. Engl. J. Med. 2018;379:1926–1936. [DOI] [PubMed] [Google Scholar]
- 22.Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N. Engl. J. Med. 2016;375:1738–1748. [DOI] [PubMed] [Google Scholar]
- 23.Slamon DJ, Neven P, Chia S, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor–positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J. Clin. Oncol. 2018;36:2465–2472. [DOI] [PubMed] [Google Scholar]
- 24.Tripathy D, Im SA, Colleoni M, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol 2018;19:904–915. [DOI] [PubMed] [Google Scholar]
- 25.Slamon DJ, Neven P, Chia S, et al. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N. Engl. J. Med. 2020;382:514–524. [DOI] [PubMed] [Google Scholar]
- 26.Raschi E, Fusaroli M, Ardizzoni A, et al. Cyclin-dependent kinase 4/6 inhibitors and interstitial lung disease in the FDA adverse event reporting system: a pharmacovigilance assessment. Breast Cancer Res. Treat. 2020;186:219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hortobagyi GN, Stemmer SM, Burris HA, et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann. Oncol. 2018;29:1541–1547. [DOI] [PubMed] [Google Scholar]
- 28.Im S-A, Lu Y-S, Bardia A, et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N. Engl. J. Med. 2019;381:307–316. [DOI] [PubMed] [Google Scholar]
- 29.Dickler MN, Tolaney SM, Rugo HS, et al. MONARCH 1, a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, n patients with refractory HR + /HER2− metastatic breast cancer. Clin. Cancer Res. 2017;23:5218–5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamilton E, Cortes J, Ozyilkan O, et al. nextMONARCH: abemaciclib monotherapy or combined with tamoxifen for metastatic breast cancer. Clin. Breast Cancer. 2020;21:181–190. [DOI] [PubMed] [Google Scholar]
- 31.Cortés J, Rugo HS, Tolaney SM, et al. Analysis of overall survival by tumor response in MONARCH 1, a phase 2 study of abemaciclib, a CDK4 and CDK6 inhibitor, in women with HR+/HER2− metastatic breast cancer (MBC) after chemotherapy for advanced disease. Ann. Oncol. 2017;28:v80. [Google Scholar]
- 32.Sledge GW, Toi M, Neven P, et al. MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2− advanced breast cancer who had progressed while receiving endocrine therapy. J. Clin. Oncol. 2017;35:2875–2884. [DOI] [PubMed] [Google Scholar]
- 33.Carrier M, Abou-Nassar K, Mallick R, et al. Apixaban to Prevent Venous Thromboembolism in Patients with Cancer. N. Engl. J. Med. 2019;380:711–719. [DOI] [PubMed] [Google Scholar]
- 34.Goetz MP, Toi M, Campone M, et al. MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. J. Clin. Oncol. 2017;35:3638–3646. [DOI] [PubMed] [Google Scholar]
- 35.Johnston S, Martin M, Di Leo A, et al. MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. npj Breast Cancer. 2019;5:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang QY, Sun T, Yin YM, et al. MONARCH plus: abemaciclib plus endocrine therapy in women with HR+/HER2− advanced breast cancer: the multinational randomized phase III study. Ther. Adv. Med. Oncol. 2020;12:1758835920963925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goetz MP, Okera M, Wildiers H, et al. Safety and efficacy of abemaciclib plus endocrine therapy in older patients with hormone receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer: an age-specific subgroup analysis of MONARCH 2 and 3 trials. Breast Cancer Res. Treat. 2021;186:417–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eli Lilly and Company. Highlights of Prescribing Information (VERZENIO). 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/208855s000lbl.pdf.
- 39.A Study of Abemaciclib (LY2835219) in Participants With Stage IV Squamous Non-small Cell Lung Cancer - Study Re- sults - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/results/NCT02450539?term=abemaciclib&rslt=With&draw=2&rank=7.
- 40.Goldman JW, Mazieres J, Barlesi F, et al. A Randomized Phase III Study of Abemaciclib Versus Erlotinib in Patients with Stage IV Non-small Cell Lung Cancer With a Detectable KRAS Mutation Who Failed Prior Platinum-Based Therapy: JUNIPER. Front. Oncol. 2020;10:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.A Study of Abemaciclib (LY2835219) in Participants With Previously Treated KRAS Mutated Lung Cancer - Study Re- sults - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/results/NCT02152631?term=abemaciclib&rslt=With&draw=2&rank=14.
- 42.A Study of Abemaciclib (LY2835219) Alone or in Combination With Other Agents in Participants With Previously Treated Pancreatic Ductal Adenocarcinoma - Study Results - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/results/NCT02981342?term=abemaciclib&rslt=With&draw=2&rank=17.
- 43.Rocha Lima CMS, Roberts PJ, Priego VM, et al. Trilaciclib (G1T28): A cyclin dependent kinase 4/6 inhibitor, in combination with etoposide and carboplatin (EP) for extensive stage small cell lung cancer (ES-SCLC)—Phase 1b results. J. Clin. Oncol. 2017;35(15_suppl):8568. [Google Scholar]
- 44.Daniel D, Kuchava V, Bondarenko I, et al. Trilaciclib (T) decreases myelosuppression in extensive-stage small cell lung cancer (ES-SCLC) patients receiving first-line chemotherapy plus atezolizumab. Ann. Oncol. 2019;30:v713. [Google Scholar]
- 45.Hart LL, Ferrarotto R, Andric ZG, et al. Myelopreservation with trilaciclib in patients receiving topotecan for small cell lung cancer: results from a randomized, double-blind, placebo-controlled phase II study. Adv. Ther. 2021;38:350–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Safety | COSELATM (trilaciclib) for injection, for IV use. https://www.cosela.com/integrated-safety-analysis.
- 47.Thein KZ, Htut TW, Ball S, et al. Venous thromboembolism risk in patients with hormone receptor-positive HER2-negative metastatic breast cancer treated with combined CDK 4/6 inhibitors plus endocrine therapy versus endocrine therapy alone: a systematic review and meta-analysis of randomiz. Breast Cancer Res. Treat. 2020;183:479–487. [DOI] [PubMed] [Google Scholar]
- 48.A Study of Palbociclib (PD-0332991) + Letrozole vs. Letrozole For 1st Line Treatment Of Postmenopausal Women With ER + /HER2− Advanced Breast Cancer (PALOMA-2) - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT01740427?term=paloma&draw=3&rank=5.
- 49.A Study of Abemaciclib (LY2835219) In Participants With Previously Treated Breast Cancer That Has Spread - Study Results - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/results/NCT02102490?term=monarch&draw=3&rank=6.
- 50.A Study of Abemaciclib (LY2835219) in Participants With Breast Cancer - Study Results - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/results/NCT02763566?term=monarch&draw=3&rank=12.
- 51.Raschi E, Fusaroli M, Ardizzoni A, et al. Thromboembolic events with cyclin-dependent kinase 4/6 inhibitors in the fda adverse event reporting system. Cancers (Basel). 2021;13:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rutjes AWS, Porreca E, Candeloro M, et al. 2020. Primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy [DOI] [PMC free article] [PubMed]
- 53.Khorana AA, Soff GA, Kakkar AK, et al. Rivaroxaban for thromboprophylaxis in high-risk ambulatory patients with cancer. N. Engl. J. Med. 2019;380:720–728. [DOI] [PubMed] [Google Scholar]
- 54.Farge D, Frere C, Connors JM, et al. 2019. 2019 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer [DOI] [PubMed]
- 55.Martín AJM, Ortega I, Font C, et al. 2018. Multivariable clinical-genetic risk model for predicting venous thromboembolic events in patients with cancer [DOI] [PMC free article] [PubMed]
- 56.Khorana AA, Francis CW. Risk prediction of cancer-associated thrombosis: Appraising the first decade and developing the future. Thromb. Res. 2018;164:S70–S76. [DOI] [PubMed] [Google Scholar]
- 57.Cella CA, Di Minno G, Carlomagno C, et al. Preventing venous thromboembolism in ambulatory cancer patients: The ONKOTEV Study. Oncologist. 2017;22:601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gerotziafas GT, Taher A, Abdel-Razeq H, et al. A predictive score for thrombosis associated with breast, colorectal, lung, or ovarian cancer: the prospective COMPASS–cancer-associated thrombosis study. Oncologist. 2017;22:1222–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khorana A, Kuderer NM, Culakova E, et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902–4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Es N, Di Nisio M, Cesarman G, et al. Comparison of risk prediction scores for venous thromboembolism in cancer patients: A prospective cohort study. Haematologica. 2017;102:1494–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Verso M, Agnelli G, Barni S, et al. 2012. A modified Khorana risk assessment score for venous thromboembolism in cancer patients receiving chemotherapy: The Protecht score [DOI] [PubMed]
- 62.Leiva O, Connors JM, Al-Samkari H. 2020. Impact of tumor genomic mutations on thrombotic risk in cancer patients [DOI] [PMC free article] [PubMed]
- 63.Ay C, Dunkler D, Marosi C, et al. Prediction of venous thromboembolism in cancer patients. Blood. 2010;116:5377–5382. [DOI] [PubMed] [Google Scholar]
- 64.Pelzer U, Sinn M, Stieler J, et al. Primäre medikamentöse Thromboembolieprophylaxe bei ambulanten Patienten mit fortgeschrittenem Pankreaskarzinom unter Chemotherapie? Dtsch. Medizinische Wochenschrift. 2013;138:2084–2088. [DOI] [PubMed] [Google Scholar]
- 65.Li A, Wu Q, Luo S, et al. Derivation and validation of a risk assessment model for immunomodulatory drug-associated thrombosis among patients with multiple myeloma. JNCCN J. Natl. Compr. Cancer Netw.. 2019;17:840–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Khorana AA, DeSancho MT, Liebman H, et al. Prediction and prevention of cancer-associated thromboembolism. Oncologist. 2021;26:e2–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Douce DR, Holmes CE, Cushman M, et al. Risk factors for cancer-associated venous thromboembolism: The venous thromboembolism prevention in the ambulatory cancer clinic (VTE-PACC) study. J. Thromb. Haemost. 2019;17:2152–2159. [DOI] [PubMed] [Google Scholar]
- 68.Maraveyas A Latest advances in preventing thromboembolic disease in the ambulatory oncology patient. Thromb. Res. 2020;191:S91–S98. [DOI] [PubMed] [Google Scholar]
- 69.Al-Samkari H, Connors JM. 2019. Managing the competing risks of thrombosis, bleeding, and anticoagulation in patients with malignancy [DOI] [PMC free article] [PubMed]
- 70.Brown JD, Goodin AJ, Lip GYH, et al. Risk stratification for bleeding complications in patients with venous thromboembolism: Application of the HAS-BLED bleeding score during the first 6 months of anticoagulant treatment. J. Am. Heart Assoc. 2018;7:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Klok FA, Hösel V, Clemens A, et al. Prediction of bleeding events in patients with venous thromboembolism on stable anticoagulation treatment. Eur Respir J 2016;48:1369–1376. [DOI] [PubMed] [Google Scholar]
- 72.Xiong W, Du H, Ding W, et al. The association between pulmonary embolism and the cancer-related genomic alterations in patients with NSCLC. Respir. Res. 2020;21:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Roopkumar J, Poudel SK, Gervaso L, et al. Risk of thromboembolism in patients with ALK- and EGFR-mutant lung cancer: A cohort study. J. Thromb. Haemost. 2021;19:822–829. [DOI] [PubMed] [Google Scholar]
- 74.Wang J, Hu B, Li T, et al. The EGFR-rearranged adenocarcinoma is associated with a high rate of venous thromboembolism. Ann. Transl. Med. 2019;7:724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chiari R, Ricciuti B, Landi L, et al. ROS1-rearranged non–small-cell lung cancer is associated with a high rate of venous thromboembolism: analysis from a phase II, prospective, multicenter, two-arms trial (METROS). Clin. Lung Cancer.. 2020;21:15–20. [DOI] [PubMed] [Google Scholar]
- 76.Ng TL, Smith DE, Mushtaq R, et al. ROS1 Gene rearrangements are associated with an elevated risk of peridiagnosis thromboembolic events. J. Thorac. Oncol. 2019;14:596–605. [DOI] [PubMed] [Google Scholar]
- 77.Unruh D, Schwarze SR, Khoury L, et al. Mutant IDH1 and thrombosis in gliomas. Acta Neuropathol. 2016;132:917–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mir Seyed Nazari P, Riedl J, Preusser M, et al. Combination of isocitrate dehydrogenase 1 (IDH1) mutation and podoplanin expression in brain tumors identifies patients at high or low risk of venous thromboembolism. J. Thromb. Haemost. 2018;16:1121–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Al-Samkari H, Leiva O, Dagogo-Jack I, et al. Impact of ALK rearrangement on venous and arterial thrombotic risk in NSCLC. J. Thorac. Oncol. 2020;15:1497–1506. [DOI] [PubMed] [Google Scholar]
- 80.Leiva O, Connors JM, Al-Samkari H. Impact of tumor genomic mutations on thrombotic risk in cancer patients. Cancers. 2020;12:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Furie B, Furie B. Mechanism of thrombus formation. N. Engl. J. Med. 2008;359:938–949. [DOI] [PubMed] [Google Scholar]
- 82.Najem MY, Couturaud F, Lemarié CA. 2020. Cytokine and chemokine regulation of venous thromboembolism [DOI] [PubMed]


