Abstract
Background
Using magnetic resonance imaging (MRI), widths of ventral tissue bridges demonstrated significant predictive relationships with future pinprick sensory scores, and widths of dorsal tissue bridges demonstrated significant predictive relationships with future light touch sensory scores, following spinal cord injury (SCI). These studies involved smaller participant numbers, and external validation of their findings is warranted.
Objectives
The purpose of this study was to validate these previous findings using a larger independent data set.
Methods
Widths of ventral and dorsal tissue bridges were quantified using MRI in persons post cervical level SCI (average 3.7 weeks post injury), and pinprick and light touch sensory scores were acquired at discharge from inpatient rehabilitation (average 14.3 weeks post injury). Pearson product-moments were calculated and linear regression models were created from these data.
Results
Wider ventral tissue bridges were significantly correlated with pinprick scores (r = 0.31, p < 0.001, N = 136) and wider dorsal tissue bridges were significantly correlated with light touch scores (r = 0.31, p < 0.001, N = 136) at discharge from inpatient rehabilitation.
Conclusion
This retrospective study’s results provide external validation of previous findings, using a larger sample size. Following SCI, ventral tissue bridges hold significant predictive relationships with future pinprick sensory scores and dorsal tissue bridges hold significant predictive relationships with future light touch sensory scores.
Keywords: light touch, MRI, pinprick, sensory testing, spinal cord injury, tissue bridge
Introduction
Spinal cord injuries (SCIs) are catastrophic events for patients and their families1 and lead to heterogenous clinical presentations on a case-by-case basis.2 Magnetic resonance imaging (MRI) offers quantitative approaches to characterizing residual neural tissue sparing after SCI.3,4 These imaging approaches could help in subgrouping persons post SCI to reduce heterogeneity and provide tailored interventions to each group.2
One approach is the quantification of midsagittal tissue bridges, which are the measured widths of spared tissue ventral and dorsal to the spinal cord lesion, using a midsagittal T2-weighted image of the cord.5–9 Recently, ventral tissue bridges demonstrated significant predictive relationships with future pinprick sensory scores (n = 44),9 and dorsal tissue bridges demonstrated significant predictive relationships with future light touch sensory scores (n = 28).8 Midsagittal tissue bridges could be used to stratify patients into subgroups most at risk for specific sensory loss or development of neuropathic pain,9 and tissue bridge quantification appears generalizable to both thoracic and cervical level SCI.5–10 Emerging interventions such as spinal cord stimulation or virtual reality could be implemented early in this group to optimize sensory function.11,12
Although the results are promising, these ventral and dorsal tissue bridge studies involved relatively smaller participant numbers (n = 44 and n = 28),8,9 thus external validation of their findings is warranted. Accordingly, the purpose of this study was to validate these previous findings that ventral tissue bridges hold significant predictive relationships with future pinprick sensory scores and that dorsal tissue bridges hold significant predictive relationships with future light touch sensory scores.
Methods
This retrospective study was approved by local institutional review boards. Participant data were selected from the local SCI Model Systems Center. As part of a parent study (NIH R03 HD094577), only images from participants with cervical SCI were available for analyses. Inclusion criteria were status post spinal cord injury, clinical MRIs available for analyses (acquired no later than 12 weeks post injury), and available admission and discharge outcomes data. Exclusion criteria were concurrent traumatic brain injury beyond concussion and significant preexisting neurological history.
Magnetic resonance imaging and tissue bridge quantification
Postoperative routine clinical T2-weighted scans were used for MRI analyses, using a General Electric 1.5 T Signa Excite MR Scanner equipped with the 8-channel cervical-thoracic-lumbar (CTL) spine array coil. Sagittal T2-weighted images of the cervical spinal cord were acquired with a two-dimensional fast relaxation fast spin echo sequence (slice thickness = 3 mm, slice spacing = 4 mm, field-of-view = 240 × 240 mm2, matrix size = 256 × 256, in-plane resolution = 0.94 mm2, interpolated in-plane resolution = 0.47 × 0.47 mm2).
Ventral and dorsal midsagittal tissue bridges were measured on all participants by researchers blinded to the clinical outcome measures, using OsiriX (Pixmeo Sarl, Geneva, Switzerland). Tissue bridges were quantified as the width of spared tissue at the minimum distance from cerebrospinal fluid to the hyperintensity (see Figure 1).5–9,13 For these measures, a high level of intra- and interrater reliability has been demonstrated previously.5,10,14 For the current study, inter- and intrarater reliability testing was performed by the two primary raters on a subset of 20 participants’ images (details in Statistical Analysis below).
Figure 1.
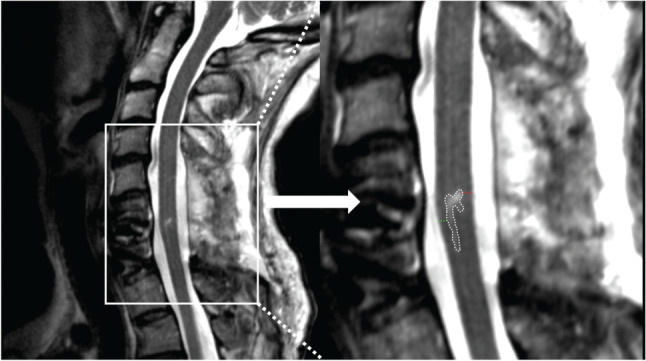
A representative participant’s T2-weighted midsagittal magnetic resonance image, seen on the left panel and zoomed in on the right panel. The lesion hyperintensity is delineated in white, the measured width of ventral tissue bridge in green, and dorsal tissue bridge in red.
Sensory testing
Pinprick and light touch sensory scores were acquired at discharge from inpatient rehabilitation using International Standards for the Neurological Classification of Spinal Cord Injury (ISNCSCI) testing. Each participant was assessed for normal (score of 2), altered (score of 1), or absent (score of 0) light touch and pinprick function in 56 standardized sensory points throughout the body for a possible total score of 112.15 This assessment also demonstrates favorable psychometric properties, although it may have lower reliability/repeatability in persons with incomplete SCIs compared to complete SCIs.15
Statistical analysis
Data analysis was performed using SPSS (version 27.0, Chicago, IL). Kolmogorov-Smirnov tests were used to test for normal distribution in the tissue bridge variables and sensory testing variables. Two-way mixed effects model, absolute agreement type, and average measures intraclass correlation coefficients (ICC) were computed for inter- and intrarater reliability testing. To examine the associations between ventral tissue bridges and pinprick score and dorsal tissue bridges and light touch score, the Pearson product-moment was used. Separate linear regression models for pinprick and light touch scores and were conducted, controlling for significant demographic variables and the nonpredictive tissue bridge widths. Pearson correlations were used to assess the relationships between ventral tissue bridges and pinprick score and dorsal tissue bridges and light touch score in subgroups of those with motor complete SCIs and motor incomplete SCIs.
Results
One hundred thirty-six participants were included in the retrospective analyses. The average age was 41.86 years (range, 15–81), with more males than females (82% males). For injury severity according to the American Spinal Injury Association Impairment Scale (AIS), 37 participants were classified as AIS A, 19 as AIS B, 33 as AIS C, and 47 as AIS D. The average ventral tissue bridge width was 0.55 mm (± 0.79), and the average dorsal tissue bridge width was 0.34 mm (± 0.47). The average number of weeks between imaging and discharge ISNCSCI sensory testing was 11.3 weeks (± 7.0 weeks). The average time from date of injury to date of discharge ISNCSCI sensory testing was 14.3 weeks (± 6.8 weeks).
Ventral/dorsal tissue bridge variables and light touch/pinprick variables all met assumptions for normal distribution. Tissue bridge measures demonstrated high reliability metrics (ICCinterrater, ICCintrarater1, and ICCintrarater2 = 0.99, p < .001) Independent of other variables, wider ventral tissue bridges at 3.7 weeks post injury (± 2.6 weeks) were significantly correlated with pinprick scores at 14.3 weeks post injury (± 6.8 weeks) (r = 0.31, p < .001; see Figure 2). For the same time points and independent of other variables, wider dorsal tissue bridges were significantly correlated with light touch scores (r = 0.31, p < .001; see Figure 2). Age was significantly correlated with both pinprick scores and light touch scores (r = 0.28, p = .001, and r = 0.26, p = .002, respectively). Sex assigned at birth was not significantly correlated with either outcome variable and was therefore not entered into either regression model.
Figure 2.
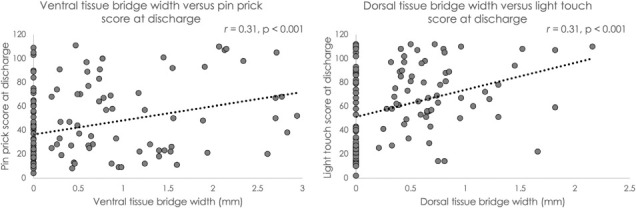
The left panel shows the relationship of ventral tissue bridge widths versus pinprick scores acquired at discharge from inpatient rehabilitation (r = 0.31, p < .001). The right panel shows the relationship of dorsal tissue bridge widths versus light touch scores acquired at discharge from inpatient rehabilitation (r = 0.31, p < .001).
For the first regression model, after controlling for age and dorsal tissue bridges, ventral tissue bridges significantly predicted total pinprick sensory score at discharge (β = 6.91, p = .048; 95% CI, 0.58, 13.76). For the second model, after controlling for age and ventral tissue bridges, dorsal tissue bridges significantly predicted light touch sensation at the time of discharge (β = 16.88, p = .007; 95% CI, 4.64, 29.12).
Significant relationships remained in the subgroups of those with motor complete SCIs (AIS A and B) and motor incomplete SCIs (AIS C and D). For the motor complete SCI group, ventral tissue bridges were significantly related to discharge pinprick scores (n = 56, R = 0.39, p = .003) and dorsal tissue bridges were significantly related to discharge light touch scores (n = 56, R = 0.39, p = .002). For the motor incomplete SCI group, ventral tissue bridges were significantly related to discharge pinprick scores (n = 80, R = 0.26, p = .023) and dorsal tissue bridges were significantly related to discharge light touch scores (n = 80, R = 0.26, p = .022).
Discussion
With a larger sample size (N = 136), our results provide external validation of previous findings.8,9 Namely, independent of other variables, ventral tissue bridges hold significant predictive relationships with future pinprick sensory scores.9 Likewise, dorsal tissue bridges hold significant predictive relationships with future light touch sensory scores, independent of other variables.8 The timing of MRI in this present study (average 3.7 weeks post injury) was in line with the two previous studies (average 4 to 5 weeks).8,9
Interestingly, age was a significant covariate and was used in both models, as it was positively correlated with both sensory scores. Typically, older age tends to be related to worse clinical outcomes.16 Although we are unsure of the exact reasons for our finding, it is possible that patients with SCI from this SCI Model Systems Center who are younger tend to have more severe, traumatic injuries compared to those who are older (unpublished data). The mechanisms of injury were unavailable for this project, and this is an acknowledged limitation.
Our correlation and first linear regression model demonstrated a slightly lower explanation of the variance in future pinprick score (R2 < 0.20) compared to the previous study (previously reported R2 = 0.385). However, our correlation and second linear regression model demonstrated explanations of variance in future light touch score (R2 = 0.10 and 0.13) that were comparable to the other previous study (previously reported R2 = 0.16).8 Collectively, these data suggest that true, albeit weak, statistically significant predictive relationships exist between midsagittal tissue bridges and their associated future ISNCSCI sensory scores. These straightforward MRI measures are quick and easy to perform, and this information could be used, along with clinical examination, to inform diagnostic workups and prognosis of specific sensory recovery.6,8,9 Tissue bridges may also be used to stratify subgroups of patients with SCI for prospective outcomes and interventional clinical trials.6,8,9
Conclusion
This retrospective study’s results provide external validation of previous findings, using a larger sample size (N = 136). Following cervical spinal cord injury, at ~4 weeks post injury using a T2-weighted midsagittal image, ventral tissue bridges hold significant predictive relationships with future pinprick sensory scores and dorsal tissue bridges hold significant predictive relationships with future light touch sensory scores. These simple, straightforward MRI-measured surrogates of spared neural tissue may be used, along with clinical examination, for prognosis of specific sensory recovery and patient stratification for prospective clinical trials.
Footnotes
Financial Support
This work was supported by a National Institute of Child Health and Development, National Center for Medical Rehabilitation Research, National Institutes of Health award R03HD094577 and grant K12 HD055931.
Conflicts of Interest
The authors report no conflicts of interest.
REFERENCES
- 1.SCI Facts and Figures. J Spinal Cord Med . 2017;40(6):872–873. doi: 10.1080/10790268.2017.1379938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krishna V, Andrews H, Varma A, Mintzer J, Kindy MS, Guest J. Spinal cord injury: How can we improve the classification and quantification of its severity and prognosis? J Neurotrauma . 2014;31(3):215–227. doi: 10.1089/neu.2013.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rejc E, Smith AC, Weber KA et al. Spinal cord imaging markers and recovery of volitional leg movement with spinal cord epidural stimulation in individuals with clinically motor complete spinal cord injury. Front Syst Neurosci . 2020;14:559313. doi: 10.3389/fnsys.2020.559313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith AC, Albin SR, O’Dell DR et al. Axial MRI biomarkers of spinal cord damage to predict future walking and motor function: A retrospective study [published online ahead of print October 6, 2020] Spinal Cord . doi: 10.1038/s41393-020-00561-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huber E, Lachappelle P, Sutter R, Curt A, Freund P. Are midsagittal tissue bridges predictive of outcome after cervical spinal cord injury? Ann Neurol . 2017;81(5):740–748. doi: 10.1002/ana.24932. [DOI] [PubMed] [Google Scholar]
- 6.Pfyffer D, Vallotton K, Curt A, Freund P. Predictive value of midsagittal tissue bridges on functional recovery after spinal cord injury [published online ahead of print November 16, 2020] Neurorehabil Neural Repair . doi: 10.1177/1545968320971787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Dell DR, Weber KA, Berliner JC et al. Midsagittal tissue bridges are associated with walking ability in incomplete spinal cord injury: A magnetic resonance imaging case series[published online ahead of print October 22, 2018] J Spinal Cord Med . doi: 10.1080/10790268.2018.1527079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vallotton K, Huber E, Sutter R, Curt A, Hupp M, Freund P. Width and neurophysiologic properties of tissue bridges predict recovery after cervical injury. Neurology . 2019;92(24):e2793–e2802. doi: 10.1212/WNL.0000000000007642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pfyffer D, Vallotton K, Curt A, Freund P. Tissue bridges predict neuropathic pain emergence after spinal cord injury. J Neurol Neurosurg Psych . 2020;91(10):1111–1117. doi: 10.1136/jnnp-2020-323150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfyffer D, Huber E, Sutter R, Curt A, Freund P. Tissue bridges predict recovery after traumatic and ischemic thoracic spinal cord injury. Neurology . 2019;93(16):e1550–e1560. doi: 10.1212/WNL.0000000000008318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inanici F, Samejima S, Gad P, Edgerton VR, Hofstetter CP, Moritz CT. Transcutaneous electrical spinal stimulation promotes long-term recovery of upper extremity function in chronic tetraplegia. IEEE Trans Neural Syst Rehabil Eng . 2018;26(6):1272–1278. doi: 10.1109/TNSRE.2018.2834339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shokur S, Gallo S, Moioli RC et al. Assimilation of virtual legs and perception of floor texture by complete paraplegic patients receiving artificial tactile feedback. Sci Reports . 2016;6:32293. doi: 10.1038/srep32293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berliner JC, O’Dell DR, Albin SR et al. The influence of conventional T2 MRI indices in predicting who will walk outside one year after spinal cord injury [published online ahead of print April 2, 2021] J Spinal Cord Med . doi: 10.1080/10790268.2021.1907676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cummins DP, Connor JR, Heller KA et al. Establishing the inter-rater reliability of spinal cord damage manual measurement using magnetic resonance imaging. Spinal Cord Series Cases . 2019;5:20. doi: 10.1038/s41394-019-0164-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marino RJ, Jones L, Kirshblum S, Tal J, Dasgupta A. Reliability and repeatability of the motor and sensory examination of the international standards for neurological classification of spinal cord injury. J Spinal Cord Med . 2008;31(2):166–170. doi: 10.1080/10790268.2008.11760707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hicks KE, Zhao Y, Fallah N et al. A simplified clinical prediction rule for prognosticating independent walking after spinal cord injury: A prospective study from a Canadian multicenter spinal cord injury registry. Spine J . 2017;17(10):1383–1392. doi: 10.1016/j.spinee.2017.05.031. [DOI] [PubMed] [Google Scholar]


