Abstract
There are sex-related differences in the epidemiology, presentation, diagnostic testing, and management of Ischemic heart disease (IHD) in women compared with men. The adjusted morbidity and mortality are persistently higher, particularly in younger women and blacks. Women have more angina but less obstructive CAD which affects delays in presentation and diagnosis and testing accuracy. The non-biological factors play a significant role in access to care, IHD management, and guideline adherence. Future research focus includes sex-specific outcomes, characterization of the biologic differences and implementation science around quality of clinical care.
Keywords: Ischemic Heart Disease, Coronary artery disease, sex differences, women
Ischemic heart disease (IHD) remains the leading cause of death in men and women. However, the manifestation of disease, treatment and health outcomes are influenced by both sex and gender factors. Sex differences are based on biological factors whereas gender differences are based on behavior, individual identity, and sociocultural roles. (1) Cardiovascular health and prevention strategies are impacted by both sex and gender factors. Women between the ages of 45 −74 have a higher mortality after a first myocardial infarction (MI) when compared to men. (2) Women with IHD have higher morbidity and experience chronic angina symptoms, more hospitalization and have overall worse health status. Also, there are female specific conditions that increase the risk of IHD such as early menopause, adverse pregnancy outcomes and cardiotoxic breast cancer treatments. Early detection and implementation of guideline directed treatment of IHD reduces morbidity and mortality and may be particularly important for women who may present with atypical symptoms. (3) The term IHD encompasses both obstructive and nonobstructive coronary artery disease. The later is more inclusive of disorders, such as microvascular and endothelial dysfunction, more commonly found in women and the broader definition may improve the undertreatment and ultimately morbidity and mortality in women, Therefore, it is imperative that clinicians understand the sex-specific IHD differences in women to help improve outcomes.
Epidemiology of sex/gender differences
Public awareness of IHD symptoms has increased and is greater in women than men (54.4% versus 45.6%). (4) However, awareness varies by sociodemographic factors. Among younger (<55 years of age) patients hospitalized with myocardial infarction, only 48.7% of females and 52.9% of males were previously told they were at risk for IHD. (5) Similarly, in a national sample, only 21% of women reported discussions about IHD risk with their doctors - rates were lowest among Hispanic women. (6) Approximately 50% of women are aware that heart disease is the number one cause of death in women. (7) Higher cardiovascular risk, younger age and low socioeconomic status are associated with reduced knowledge of IHD. (8)
The prevalence of CAD is higher for men than women (8.3% versus 6.2%) and increases with age. The average age for first MI in women is 72.0 years compared to 65.6 years for men. (2) Also, the incidence of MI or fatal IHD in women increases with age with a higher incidence in Black women than White women at all ages. (2) (Table 1) This pattern was found in another study with three large cohorts; however, these differences. (9)
Table 1.
Prevalence of CAD and angina in Women
| Prevalence of CAD | Prevalence of angina | |
|---|---|---|
| Non-Hispanic White women | 6.0% | 4.0% |
| Non-Hispanic Black women | 7.2% | 4.7% |
| Non-Hispanic Asian women | 3.2% | 2.2% |
| Hispanic women | 6.4% | 4.3% |
Data from Virani S, Alonso A, Aparicio A, et al. Heart Disease and Stroke Statistics—2021 Update. A Report From the American Heart Association. Circulation. 2021;143(8): e254-e743.
Yet, IHD related deaths are lower in women than men overall. As of 2018, the age-adjusted death rates per 100,000 for Non-Hispanic White women, Non-Hispanic Black women and Hispanic Black women were 64.9, 79.7 and 50.3, respectively. (2) Despite lower rates of IHD in women, when women have a IHD event, their mortality is higher. Using data pooled from several epidemiological cohorts, it is estimated that within 1 year of a first MI, 23% of women and 18% of men will die; within 5 years, 47% of women and 36% of men will die. (2)
Differences in mortality rates are greatest at the younger age groups. Since 2000 there has been minimal improvement in IHD mortality (−1% estimated annual percentage change) in young women (<55 years of age). (8) In hospital mortality with acute MI is higher in women and is influenced by age, race and ethnicity. (10) Among those less than 65 years of age, in-hospital mortality rates are highest for Hispanic women (3.7%) when compared to Black (3.1%) and White women (2.5%). (7)
Morbidity associated with IHD is higher in women. Women have more symptoms, more frequent hospitalizations, worse health status and more complications after procedures. (11) Some of the difference in women is due to higher burden of nonobstructive coronary disease. From the large, prospective ACC CathPCI Registry, women with stable angina and with ASC had lower risk-adjusted OR for obstructive CAD (0.34 and 0.47 respectively) and Black women had the lowest OR. (12) Similar finding were noted in the WISE trial, yet equal or greater degrees of ischemia and mortality. (13) The lack of data on treatment options for endothelial and microvascular dysfunction and spontaneous coronary artery dissection contributes to morbidity.
In sum, there are significant sex-differences in the incidence, prevalence and outcome of IHD with increased understanding of the broader spectrum of nonobstructive CAD and unique pathophysiology of atherosclerosis including more plaque erosion in women.
Presentation, Risk Assessment and Treatment
Sex differences exist in the presentation of IHD. Women less often report chest pain and diaphoresis and more often complain of back pain, jaw pain, epigastric pain, palpitations, lightheadedness; the additional, nonspecific symptoms can mislead both patients and providers. The National Registry of Myocardial Infarction demonstrated that 42% of men vs. 31% of women presented with chest pain in the setting of myocardial infarction. (14) In the VIRGO trial, the poorer 1-year outcomes among middle-aged women (<55) were largely attributed to less frequent reporting of typical chest pain. (15) This atypical presentation in women as well as social and behavioral barriers contributes to delays in seeking care, diagnosis and delivery of appropriate treatment strategies, resulting in worse outcomes.
Men and women share similar modifiable traditional cardiac risk factors for IHD, but diabetes and smoking are more potent in women. (16) Additionally, women are at increased risk due to female-specific risk-enhancing factor such as early menopause, inflammatory diseases (systemic lupus erythematosus, rheumatoid arthritis), pregnancy complications (preeclampsia, gestational diabetes) and cardiotoxic breast cancer treatment. (TABLE 2) These have been included in the 2018 AHA/ACC Cholesterol Guidelines and 2019 ACC/AHA primary prevention Guidelines to be consider in the discussion with patients for assessment and treatment. (17)
Table 2.
Female-specific risk enhancing factors.
| Female Risk Enhancers | |
|---|---|
| Pregnant Females | Pre-eclampsia |
| Gestational hypertension | |
| Gestational diabetes | |
| Pre-term delivery | |
| Delivery for small for gestational age infants | |
|
Younger Females
(< 40 years old) |
Premature ovarian failure (<40 years) |
| Polycystic ovarian syndrome (PCOS) | |
| Hormonal contraceptive use | |
| Menarche | |
| Transgender | |
|
Older Females
(> 40 years old) |
Menopause |
| Post-menopausal hormone therapy | |
There are sex-specific limitations to the traditional 10-year ASCVD risk calculator. It is less reliable for women because it does not identify high risk women under the age of 40, does not consider sex-specific risk factor potency or risk enhancers, and can over or underestimate risk due to late onset of CVD in women. (18)
Still, there remains an implementation gap with women having lower rates of assessment, education and treatment for primary and secondary prevention. Primary care physicians ranked heart disease after weight issues and breast health as their top clinical priority for women. (19) Also, women were 65% less likely to have assessment of their smoking status, body habitus, blood pressure and lipid profile (20) and less likely to be prescribed statin and antiplatelet therapy, compared with men. (21) With established IHD, women were less likely to achieve guideline-directed secondary prevention targets for lipids (OR, 0.5), glucose (OR, 0.78), physical activity (OR, 0.74), or body mass index (OR, 0.82). (22) The undertreatment is worse for younger women, young women (35–54 years) were 37% less likely to be prescribed antihypertensive, statin and antiplatelet medications than men in the same age group. (22) Also, women were 32% less likely be referred to cardiac rehab and 36% less likely to enroll in cardiac rehabilitation. (21)
Diagnostic Testing
Because women have higher burden of traditional risk factors and sex-specific risk enhancing factors and have more nonobstructive disease on angiography, accurate risk stratification and early identification is paramount. Coronary artery calcium has emerged as one of the more powerful predictors of subclinical atherosclerosis, and to effectively risk stratify women, particularly those with an intermediate Framingham risk score (FRS). (23) The MESA study revealed CAC was present in 32% of women who was previously classified as low CHD risk by FRS. Moreover, 4% had CAC ≥300 Agatston score reclassifying them high risk. (24)
Diagnostic imaging has an expanded role in the evaluation of symptomatic women to detect the full spectrum of IHD including nonobstructive CAD and ischemia from endothelial and microvascular dysfunction. (3) With Bayesian analysis of pretest probability of disease, each of the diagnostic modalities have comparable diagnostic accuracies as well as specific benefits and limitations that are of importance in women. (TABLE 3)
Table 3.
Diagnostic Testing in Women
| Utilization | Limitations | Sensitivity & Specificity* | |
|---|---|---|---|
| Coronary artery calcium scoring | • Predictors of subclinical CHD particularly in women • Risk stratify women with an intermediate FRS |
Sensitivity 95% Specificity 66% |
|
| Exercise treadmill testing | • Initial diagnostic test for symptomatic women, capable of exercising | • Uninterpretable ECG • Exercise limitation |
Sensitivity 61% Specificity 70% |
| Dobutamine stress echocardiography | • Identification of obstructive CAD and estimation of prognosis in premenopausal woman • Detection of obstructive CAD and risk among symptomatic women with intermediate to high IHD risk |
• Image artifacts due to respiration and patient habitus/motion | Sensitivity 80% Specificity 84% |
| Exercise stress echocardiography | • Identification of obstructive CAD and estimation of prognosis for symptomatic women at intermediate-high IHD risk and with any of the following: (a) resting ST-segment abnormalities, (b) functional disability, or (c) indeterminate or intermediate-risk stress ECG • Diastolic function and pulmonary artery pressures may be reasonable in the echocardiographic evaluation of women presenting with dyspnea |
• Exercise limitation • Image artifacts due to respiration and patient habitus/motion |
Sensitivity 79% Specificity 83% |
| Single-photon emission tomography (SPECT) myocardial perfusion imaging (MPI) exercise | • Symptomatic women at intermediate-high IHD risk and with (a) resting ST-segment abnormalities, (b) functional disability, or (c) indeterminate or intermediate-risk stress ECG, stress MPI with SPECT or PET is recommended for identification of obstructive CAD and estimation of prognosis • Symptomatic women with an intermediate-high IHD risk who also have abnormal rest ST-segment changes or functional disability |
• Radiation exposure • Dense breast and obesity reduce specificity • Lower cardiac mass in women causing lower sensitivity for ischemia and inaccurate TID |
Sensitivity 82% Specificity 81% |
| Pharmacological stress myocardial perfusion imaging (MPI) | Sensitivity 93% Specificity 78% |
||
| Stress positron emission tomography (PET) | • Symptomatic women at intermediate-high IHD risk and with (a) resting ST-segment abnormalities, (b) functional disability, or (c) indeterminate or intermediate-risk stress ECG, stress MPI with SPECT or PET is recommended for identification of obstructive CAD and estimation of prognosis • Extent and severity of myocardial perfusion and wall-motion abnormalities, as well as LVEF assessment at rest and after stress. • Calculate absolute blood flow across the coronary vessels, which leads to the calculation of PET MFR, aid in the detection of microvascular CAD • Ability to quantify myocardial blood flow and coronary flow reserve to diagnose ischemia, even in the absence of obstructive CAD |
Sensitivity 90% Specificity 89% |
|
| Stress cardiac magnetic resonance (CMR) | • Premenopausal woman with functional disability • Symptomatic women at intermediate-high IHD risk and with (a) resting ST-segment abnormalities, (b) functional disability, or (c) indeterminate or intermediate risk, it may be reasonable to use stress CMR, especially vasodilator stress • Perfusion CMR, as the index procedure within the diagnostic evaluation • Assessment of subendocardial perfusion |
Sensitivity 89% Specificity 80% |
|
| Coronary CTA | • Detailed anatomical information and has a high diagnostic and prognostic accuracy for the detection of CHD • Risk stratification of women after an indeterminate stress test, also for women with persistent symptoms despite a negative stress test |
• Poor predictors of the hemodynamic significance of CAD | Sensitivity 96% Specificity 92% |
A sex- specific limitation in SPECT MPI is lower cardiac mass in women causing lower sensitivity for ischemia, and less accurate transient ischemic dilation evaluation. Specificity is also reduced due to dense breast, and/or obesity in women. (25) Stress positron emission tomography (PET) improves the diagnostic accuracy and prognostic value by evaluating the coronary microvasculature and macrovasculature with quantification of absolute myocardial blood flow and coronary flow reserve. Also, emerging areas in cardiac magnetic resonance (CMR) such as perfusion and metabolic imaging, blood oxygenation level-dependent imaging for microvascular disease, CMR spectroscopy, and exercise CMR show early promise for detection of IHD in women. (26)
Women with symptoms and/or ischemia on functional testing may undergo anatomic assessment by catheterization or CTA and have been shown to have a lower prevalence of obstructive CAD.
Complete diagnostic evaluation should include workup for vasospasm, microvascular dysfunction and coronary embolism. Spontaneous coronary artery dissection should also be suspected in young women presenting with ACS, particularly peripartum. (27) Importantly, the prognosis with symptomatic, nonobstructive disease is worse in women.
Sex specific Recommendations in Guidelines
Current ACC/AHA and ESC guidelines for the treatment of IHD offer recommendations which in general are not different between men and women but have added descriptive sections on sex- based differences. (TABLE 4) The 2013 ACCF/AHA ST elevation myocardial infarction (STEMI) guidelines highlight that 30% of patients presenting with STEMI are women and they remain undertreated despite universal recommendations for treatment. (28) Based on NCDR ACTION Registry data, women had longer door-to-fibrinolysis and door-to-balloon times and longer median first medical contact to device times compared to men (80 v. 75 min) (29) particularly in younger women. (30)
Table 4.
Sex-specific Differences and Recommendations in Guidelines
| Clinical Practice Document | Noted Sex-specific Differences | Noted Sex-specific Recommendations | Knowledge Gaps |
|---|---|---|---|
| 2013 ACC/AHA STEMI Guideline | -30% are women -remain undertreated |
-none | -prehospital delay -bleeding risks |
| 2014 ACC/AHA NSTE-ACS | -pregnancy: revascularization if life-threatening complications | -early invasive strategy for high risk features | -antithrombotic dosing -MINOCA |
| 2012, 2014 update ACC/AHA Stable Ischemic heart disease | -none for PCI, medications, CABG | -Avoid estrogen replacement therapy in post-menopausal women | -nonobstructive disease diagnosis, treatment |
| 2011 ACC/AHA PCI | -higher in hospital mortality -higher procedural complications |
-none | -vascular access and bleeding risks |
| 2011 ACC/AHA CABG | -higher perioperative morbidity/mortality -similar long term outcomes |
-none -most data extrapolated from men |
-mitigating bleeding risks -improving complete revascularization |
| 2020 ESC ACS without STEMI | -none. noted to follow same treatment | -careful antithrombotic dosing periprocedural | -nonobstructive disease |
The 2014 AHA/ACC guidelines for non- ST elevation acute coronary syndrome (NSTE-ACS) offer a strong recommendation for an early invasive strategy in women with high-risk features. (31) For pregnant patients, revascularization is reasonable if an ischemia-guided strategy is ineffective in managing life-threatening complications.
The 2012 ACC/AHA guideline for stable ischemic heart disease and the 2014 Focused Update do not distinguish recommendations in terms of use of anti-ischemic medications, PCI to relieve symptoms, or revascularization with CABG to improve survival in left main or 3-vessel disease. (32) Randomized controlled trial data showed no sex differences in the benefits of goal directed medical therapy compared to goal directed medical therapy plus PCI (though few women were enrolled). (33) As no clinical trials have shown a reduction in cardiovascular risk or improved clinical outcomes from hormone therapy, the guidelines warn against estrogen therapy in postmenopausal women (Class III, LOE A No Benefit). (34) The 2011 ACCF/AHA/SCAI Guideline for percutaneous coronary intervention (PCI) highlight that woman have a 25–80% higher risk of in-hospital PCI mortality compared to men, due in part to higher procedural complications and greater number of risk factors. (35, 36) Drug-eluting stent is preferred without distinction by sex, given data on similar efficacy in clinical trial subgroup analyses. (37)
The 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery note that most recommendations for CABG in women are extrapolated from studies that predominantly studied men. (38) Studies of women receiving CABG demonstrate higher periprocedural morbidity and mortality, but these do not translate into worse longer-term outcomes. (39) Potential reasons for the higher periprocedural complications include older age, later or more severe presentation, smaller body surface area or coronary dimensions, and higher bleeding risk. With regards to the procedure itself, women are less likely to be completely revascularized and less likely to receive internal mammary arterial grafting, particularly bilateral. (40)
The Under-representation of Women in Cardiovascular Clinical Trials
Women have historically been underrepresented in cardiovascular clinical trials, which has resulted in uncertainty about the efficacy and safety of many therapies in women and has limited the potential to provide sex-specific guidelines and recommendations. Between 2010 and 2017, only 38.2% of all cardiovascular trial participants were female, with studies on acute coronary syndrome showing the most prominent under-representation of women (26.9% female participants). (41) In addition, sex-specific data of cardiovascular clinical trials are under-reported. Clinical trials need to prioritize inclusion of female subjects so that our recommendations for women are grounded in sex-specific data. (42) Statistically powered sex-specific subgroup analyses must be included in the study design, and the study population needs to be large enough to draw clinically relevant conclusions separately for women and men. (43) Ongoing mandates for inclusion of women from the NIH and other funding bodies are vital and must be followed. Additionally, given that female minorities present with unique biologic and social determinants of health, studies that evaluate the interplay between race and sex must be prioritized.
Strategies to increase the proportion of female participants in cardiovascular clinical trials include strict enforcement of existing mandates and policies, avoiding exclusion criteria that affect women more than men, or caps for men enrollment after a certain level of male participation is reached. It is also crucial to investigate and address barriers for women to participate in clinical trials and offer flexible hours or at-home follow-up. Including the primary care physician and family in the discussion about the risk and benefits of trial participation may further facilitate enrollment and retention of women in cardiovascular clinical trials.
Implementation Challenges and Solutions
The limited availability of robust data on women with CHD might also contribute to the ongoing challenge of implementing timely and appropriate guideline-driven treatment in women. Part of the solution also lies in improving gender representation among cardiovascular providers themselves. Despite evidence that patient-physician gender concordance and a more gender equitable physician workforce may result in improved cardiovascular outcomes, only 3% of PCIs are performed by women. (44) Dedicated efforts to reduce conscious and unconscious gender bias are required if we are to increase implementation of guideline-based therapy for all patients regardless of sex.
We recommend:
Dedicated efforts to train providers may help to reduce delays to initial diagnosis. (45)
Patient- oriented efforts to increase awareness in women, such as the AHA Go Red for Women campaign, may also reduce prehospital delays.
Standardized approaches to care can elevate all quality, such as the ACC/AHA Door to Balloon Alliance helped to standardize timely PCI through partnership between hospitals, providers, and payers. (46)
Risk prediction tools that are tailored to women should be further developed and consider the differential risk of traditional risk factors and emerging risk factors, such as early menopause, pregnancy related factors, and autoimmune disorders.
Technical procedural approaches that may have advantages in women, such as radial access to reduce bleeding, should be further studied, and implemented.
Further research to better characterize diagnostic and treatment options for the spectrum of nonobstructive CAD is needed.
Ongoing mandates for inclusion of women and minorities as research subjects and investigators is needed.
Conclusions
In summary, there are well characterized differences in epidemiology, presentation, diagnostic testing, and management in women compared with men. Though women typically present at an older age and with more comorbidities, adjusted morbidity and mortality for all ages remains unacceptably high. The clinical presentation of IHD may be more varied in women leading to delays in recognition and in care by both patients and providers. Women have lower prevalence of obstructive CAD, but do not have a better prognosis. There are sex-specific differences in diagnostic testing modalities and those that can assess for nonobstructive disease such as endothelial and microvascular dysfunction are need refinement. The management of acute and chronic IHD as well as primary prevention, should be driven by our guidelines, the implementation of which still lags to a greater degree in women compared with men. Non-biological factors play a significant role in access to care, IHD screening, management, disease progression and outcome for women. We must focus research not only on the biology of the disease and quality of clinical care, but also on social science and public policy that effect the social determinants of health which drive over 80% of health outcomes.
Research must include more women of all age and racial groups; specific methodologies for recruiting women and minorities into clinical trials is needed. Targeted efforts to increase women cardiologists, researchers and principal investigators, mandates to report on sex-based differences in clinical trials, and open data access to patient level data for subsequent larger, meta -analysis can help drive progress. Lastly, ongoing education of the public and providers is needed to close these gaps in care and outcomes. (Central illustration)
Central Illustration: Sex-Related Differences in Ischemic Heart Disease.
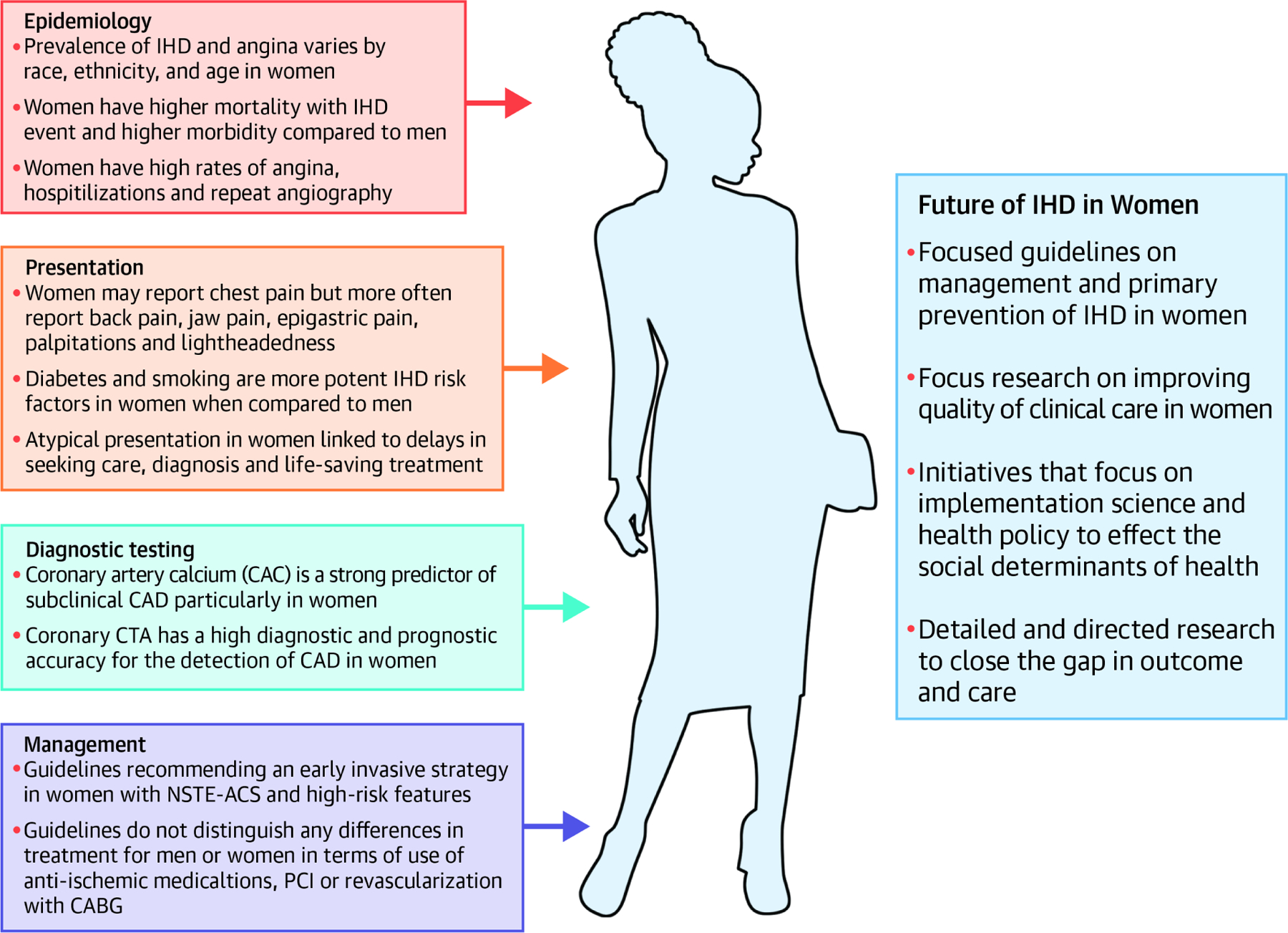
Visual diagram illustrating the epidemiology, presentation, diagnostic testing, and management of ischemic heart disease (IHD) in women as well as the means to improve the future care of IHD in women.
Highlights.
There are differences in the presentation, diagnosis, and management of ischemic heart disease in man and women.
Women more often have nonobstructive coronary disease than men but face higher morbidity and mortality.
Initiatives to address sex-based differences in clinical research could improve outcomes for women with ischemic heart disease.
Disclosures
Dr. Daugherty received support from National Institute of Health grants HL133343, DC0169065 and a grant from PCORI.
Dr. Mehran reports institutional research grants from Abbott, Abiomed, Alleviant Medical, AM-Pharma, Applied Therapeutics, Arena, AstraZeneca, Bayer, Biosensors, Biotronik, Boston Scientific, CardiaWave, CellAegis, CeloNova, CERC, Chiesi, Concept Medical, CSL Behring, DSI, Duke University, Humacyte, Insel Gruppe AG, Janssen, Medtronic, OrbusNeich, Philips, Vivasure, Zoll; personal fees from ACC, Boston Scientific, California Institute for Regenerative Medicine (CIRM), Cine-Med Research, Janssen, WebMD, SCAI; consulting fees paid to the institution from Medtronic, Idorsia Pharmaceuticals, Novartis, Philips; Equity <1% in Applied Therapeutics, Elixir Medical, STEL, CONTROLRAD (spouse); Scientific Advisory Board for AMA, Biosensors (spouse); Faculty CRF (no fee).
The remaining authors have no disclosures.
ABBREVIATIONS:
- IHD
Ischemic heart disease
- MI
Myocardial infarction
- CAD
Coronary Artery Disease
- SPECT
Single-photon emission tomography
- MPI
Myocardial perfusion imaging
- CMR
Cardiac magnetic resonance
- CTA
Computed tomography angiography
- NSTE-ACS
Non- ST elevation acute coronary syndrome
- CABG
Coronary artery bypass graft surgery
- PCI
Percutaneous coronary intervention
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Sex Regitz-Zagrosek V. and gender differences in health. Science & Society Series on Sex and Science. EMBO Rep 2012;13(7):596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Virani S, Alonso A, Aparicio A, et al. Heart Disease and Stroke Statistics—2021 Update. A Report From the American Heart Association. Circulation 2021;143(8): e254–e743. [DOI] [PubMed] [Google Scholar]
- 3.Mieres JH, Gulati M, Bairey Merz N, et al. Role of noninvasive testing in the clinical evaluation of women with suspected ischemic heart disease: a consensus statement from the American Heart Association. Circulation 2014; 130:350–79. [DOI] [PubMed] [Google Scholar]
- 4.Fang J, Luncheon C, Ayala C, Odom E, Loustalot F. Awareness of Heart Attack Symptoms and Response Among Adults - United States, 2008, 2014, and 2017. MMWR Morb Mortal Wkly Rep 2019;68(5):101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leifheit-Limson EC, D’Onofrio G, Daneshvar M, et al. Sex Differences in Cardiac Risk Factors, Perceived Risk, and Health Care Provider Discussion of Risk and Risk Modification Among Young Patients With Acute Myocardial Infarction: The VIRGO Study. J Am Coll Cardiol 2015;66(18):1949–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lundberg GP, Mehta LS, Sanghani RM, Patel HN. Heart Centers for Women. Historical Perspective on Formation and Future strategies to reduce cardiovascular disease. Circulation 2018;138(11):1155–1165. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez F, Foody JM, Wang Y, López L. Young Hispanic Women Experience Higher In-Hospital Mortality Following an Acute Myocardial Infarction. Journal of the American Heart Association 2015;4(9):e002089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilmot KA, O’Flaherty M, Capewell S, Ford ES, Vaccarino V. Coronary Heart Disease Mortality Declines in the United States From 1979 Through 2011: Evidence for Stagnation in Young Adults, Especially Women. Circulation 2015;132(11):997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colantonio LD, Gamboa CM, Richman JS, et al. Black-White Differences in Incident Fatal, Nonfatal, and Total Coronary Heart Disease. Circulation 2017;136(2):152–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langabeer JR 2nd, Champagne-Langabeer T, Fowler R, Henry T. Gender-based outcome difference for emergency department presentation ofnon-STEMI acute coronary syndrome. Am J Emerg Med 2019;37(2):179–182. [DOI] [PubMed] [Google Scholar]
- 11.Okunrintemi V, Valero-Elizondo J, Patrick B, et al. Gender Differences in Patient-Reported Outcomes Among Adults With Atherosclerotic Cardiovascular Disease. Journal of the American Heart Association 2018;7(24):e010498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaw LJ, Shaw RE, Merz CN et al. Impact of ethnicity and gender differences on angiographic coronary artery disease prevalence and in-hospital mortality in the American College of Cardiology-National Cardiovascular Data Registry. Circulation 2008; 117:1787–801. [DOI] [PubMed] [Google Scholar]
- 13.Sharaf BL, Pepine CJ, Kerensky RA, et al. Detailed angiographic analysis of women with suspected ischemic chest pain (pilot phase data from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation [WISE] Study Angiographic Core Laboratory). Am J Cardiol 2001; 87:937–41 [DOI] [PubMed] [Google Scholar]
- 14.Canto JG, Rogers WJ, Goldberg RJ et al. Association of age and sex with myocardial infarction symptom presentation and in-hospital mortality. JAMA 2012; 307:813–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lichtman JH, Leifheit EC, Safdar B et al. Sex Differences in the Presentation and Perception of Symptoms Among Young Patients With Myocardial Infarction: Evidence from the VIRGO Study (Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients). Circulation 2018; 137:781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L; INTERHEART Study Investigators. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004. Sep 11-17;364(9438):937–52. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 17.Agarwala A, Liu J, Ballantyne CM, Virani SS. The Use of Risk Enhancing Factors to Personalize ASCVD Risk Assessment: Evidence and Recommendations from the 2018 AHA/ACC Multi-society Cholesterol Guidelines. Curr Cardiovasc Risk Rep 2019;13(7):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elder P, Sharma G, Gulati M, Michos E. Indetification of female-specific risk enhancers throughout the lifespan of women to improve cardiovascular disease prevention. American Journal of Preventive Cardiology 2020. Jun; 2(9):100028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bairey Merz CN, Andersen H, Sprague E et al. Knowledge, Attitudes, and Beliefs Regarding Cardiovascular Disease in Women: The Women’s Heart Alliance. J Am Coll Cardiol 2017; 70:123–132. [DOI] [PubMed] [Google Scholar]
- 20.Zhao M, Vaartjes I, Graham I et al. Sex differences in risk factor management of coronary heart disease across three regions. Heart 2017; 103:1587–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colella TJ, Gravely S, Marzolini S et al. Sex bias in referral of women to outpatient cardiac rehabilitation? A meta-analysis. Eur J Prev Cardiol 2015; 22:423–41. [DOI] [PubMed] [Google Scholar]
- 22.Hyun KK, Redfern J, Patel A et al. Gender inequalities in cardiovascular risk factor assessment and management in primary healthcare. Heart 2017; 103:492–498. [DOI] [PubMed] [Google Scholar]
- 23.Okwuosa TM, Greenland P, Ning H et al. Distribution of coronary artery calcium scores by Framingham 10-year risk strata in the MESA (Multi-Ethnic Study of Atherosclerosis) potential implications for coronary risk assessment. J Am Coll Cardiol 2011; 57:1838–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lakoski SG, Greenland P, Wong ND et al. Coronary artery calcium scores and risk for cardiovascular events in women classified as “low risk” based on Framingham risk score: the multi-ethnic study of atherosclerosis (MESA). Arch Intern Med 2007; 167:2437–42. [DOI] [PubMed] [Google Scholar]
- 25.Taqueti VR, Dorbala S, Wolinsky D et al. Myocardial perfusion imaging in women for the evaluation of stable ischemic heart disease-state-of-the-evidence and clinical recommendations. J Nucl Cardiol 2017; 24:1402–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baldassarre LA, Raman SV, Min JK et al. Noninvasive Imaging to Evaluate Women With Stable Ischemic Heart Disease. JACC Cardiovasc Imaging 2016; 9:421–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamis-Holland JE, Jneid H, Reynolds HR, et al. ; American Heart Association Interventional Cardiovascular Care Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; and Council on Quality of Care and Outcomes Research. Contemporary Diagnosis and Management of Patients With Myocardial Infarction in the Absence of Obstructive Coronary Artery Disease: A Scientific Statement From the American Heart Association. Circulation 2019. Apr 30;139(18): e891–e908. [DOI] [PubMed] [Google Scholar]
- 28.O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013. Jan 29;61(4): e78–140. [DOI] [PubMed] [Google Scholar]
- 29.Roswell RO, Kunkes J, Chen AY, et al. Impact of sex and contact-to-device time on clinical outcomes in acute ST-segment elevation myocardial infarction-findings from the National Cardiovascular Data Registry. J Am Heart Assoc 2017;6: e004521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D’Onofrio G, Safdar B, Lichtman JH, et al. Sex differences in reperfusion in young patients with ST-segment-elevation myocardial infarction: results from the VIRGO study. Circulation 2015; 131:1324–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non–ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014. Dec 23;64(24): e139–228. [DOI] [PubMed] [Google Scholar]
- 32.Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: executive summary. J Am Coll Cardiol 2012. Dec 18;60(24):2564–603. [DOI] [PubMed] [Google Scholar]
- 33.Boden WE, O’Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007; 356:1503–16. [DOI] [PubMed] [Google Scholar]
- 34.Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA 1998; 280:605–13. [DOI] [PubMed] [Google Scholar]
- 35.Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol 2011. Dec 6;58(24): e44–122. [DOI] [PubMed] [Google Scholar]
- 36.Lichtman JH, Wang Y, Jones SB, et al. Age and sex differences in in-hospital complication rates and mortality after percutaneous coronary intervention procedures: evidence from the NCDR. Am Heart J 2014; 167:376–83. [DOI] [PubMed] [Google Scholar]
- 37.Solinas E, Nikolsky E, Lansky AJ, et al. Gender-specific outcomes after sirolimus-eluting stent implantation. J Am Coll Cardiol 2007; 50:2111–6. [DOI] [PubMed] [Google Scholar]
- 38.Hillis LD, Smith PK, Anderson JL, et al. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in collaboration with the American Association for Thoracic Surgery, Society of Cardiovascular Anesthesiologists, and Society of Thoracic Surgeons. J Am Coll Cardiol 2011. Dec 6;58(24): e123–210. [DOI] [PubMed] [Google Scholar]
- 39.Kim C, Redberg RF, Pavlic T, et al. A systematic review of gender differences in mortality after coronary artery bypass graft surgery and percutaneous coronary interventions. Clin Cardiol 2007; 30:491–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jabagi H, Tran DT, Hessian R, Glineur D, Rubens FD. Impact of gender on arterial revascularization strategies for coronary artery bypass grafting. Ann Thorac Surg 2018; 105:62–68. [DOI] [PubMed] [Google Scholar]
- 41.Jin X, Chandramouli C, Allocco B, Gong E, Lam CSP, Yan LL. Women’s Participation in Cardiovascular Clinical Trials From 2010 to 2017. Circulation 2020. Feb 18;141(7):540–548. doi: 10.1161/CIRCULATIONAHA.119.043594. Epub 2020 Feb 17. [DOI] [PubMed] [Google Scholar]
- 42.Institute of Medicine (US) Committee on Women’s Health Research. Women’s Health Research: Progress, Pitfalls, and Promise Washington, DC: National Academies Press; 2010. [PubMed] [Google Scholar]
- 43.Harris DJ, Douglas PS. Enrollment of women in cardiovascular clinical trials funded by the National Heart, Lung, and Blood Institute. N Engl J Med 2000. Aug 17;343(7):475–80. doi: 10.1056/NEJM200008173430706. [DOI] [PubMed] [Google Scholar]
- 44.Wang TY, Grines C, Ortega R, et al. Women in interventional cardiology: Update in percutaneous coronary intervention practice patterns and outcomes of female operators from the National Cardiovascular Data Registry. Catheter Cardiovasc Interv 2016. Mar;87(4):663–8. [DOI] [PubMed] [Google Scholar]
- 45.Lutfiyya MN, Cumba MT, McCullough JE, Barlow EL, Lipsky MS. Disparities in adult African American women’s knowledge of heart attack and stroke symptomatology: an analysis of 2003–2005 Behavioral Risk Factor Surveillance Survey data. Journal of women’s health (2002) 2008;17(5):805–813. [DOI] [PubMed] [Google Scholar]
- 46.Krumholz HM, Bradley EH, Nallamothu BK, et al. A campaign to improve the timeliness of primary percutaneous coronary intervention: door-to-balloon: an alliance for quality. JACC Cardiovasc Interv 2008; 1:97–104. [DOI] [PubMed] [Google Scholar]


