Abstract
Phosphorene, also known as black phosphorus (BP), is a two-dimensional (2D) material that has gained significant attention in several areas of current research. Its unique properties such as outstanding surface activity, an adjustable bandgap width, favorable on/off current ratios, infrared-light responsiveness, good biocompatibility, and fast biodegradation differentiate this material from other two-dimensional materials. The application of BP in the biomedical field has been rapidly emerging over the past few years. This article aimed to provide a comprehensive review of the recent progress on the unique properties and extensive medical applications for BP in bone, nerve, skin, kidney, cancer, and biosensing related treatment. The details of applications of BP in these fields were summarized and discussed.
1. Introduction
Phosphorene, also known as black phosphorus (BP) when presented as multi-layers, is a 2D material with a unique combination of properties that include high surface activity, adjustable bandgap, favorable on/off current ratio, excellent carrier mobility, near-infrared (NIR) light responsiveness, biocompatibility, and nontoxic biodegradation products.1–26 In nature, phosphorus is present as one of three main allotropes, i.e., white phosphorus, red phosphorus, and BP. White phosphorus is the most reactive and unstable due to the large bonding strain that is present in its tetrahedral structure, while BP is the most stable and unreactive allotrope under ambient conditions.27,28 However, BP is the most difficult of the three allotypes to prepare, a fact that has limited the exploration of its potential in biomedical applications until relatively recently.27
Over the past decade, the application of BP in medicine has gained great attention owing to recently developed methods to exfoliate bulk BP powder or crystals into nanostructures, including BP nanosheets (or nanodots, nanoparticles),29–52 BP quantum dots,53–70 and BP nanoribbons.71 Similar to other 2D materials, stacked layers of BP are held together with relatively weak van der Waals forces.1 Exfoliation leads to the shearing of these layers, resulting in the separation of single- or multi-layer BP structures or nanosheets depending on the method and duration of treatment. Various methods for the fabrication of these nano-BP structures have been reported, including ultrasonic exfoliation, mechanical exfoliation, solvothermal treatment, shear force, laser irradiation, and microwave treatment.13,72–82
The application of BP for tissue regeneration and disease treatment, however, is still in its infancy.83,84 Here we summarized the current reports on applications of BP for tissue regeneration and disease therapy, including bone and nerve regeneration, wound healing, kidney injury, biosensing, and cancer treatment. In addition to these applications, we believe the unique properties of BP are widely applicable for a large variety of biomedical applications that aim to promote tissue repair or disease treatment. Therefore, we foresee a considerable growth in BP-related biomedical research in the near future.
2. Properties of phosphorene
The structural anisotropy of BP imbues the material with unique thermomechanical, electrical, and optical properties which have provided an opportunity for implementation of this material in electronic devices, batteries, photodetectors, optoelectronic devices, and as a metal-free semiconductor.5,10,17,18,85–99 The biocompatibility of biodegradable BP products has renewed interest for medical applications,100–102 where BP’s supplementary material, mechanical, and electrical characteristics have shown promise in the fields of bio-imaging, biosensors, cancer therapy, and regenerative medicine (Fig. 1).93,96,103
Fig. 1.
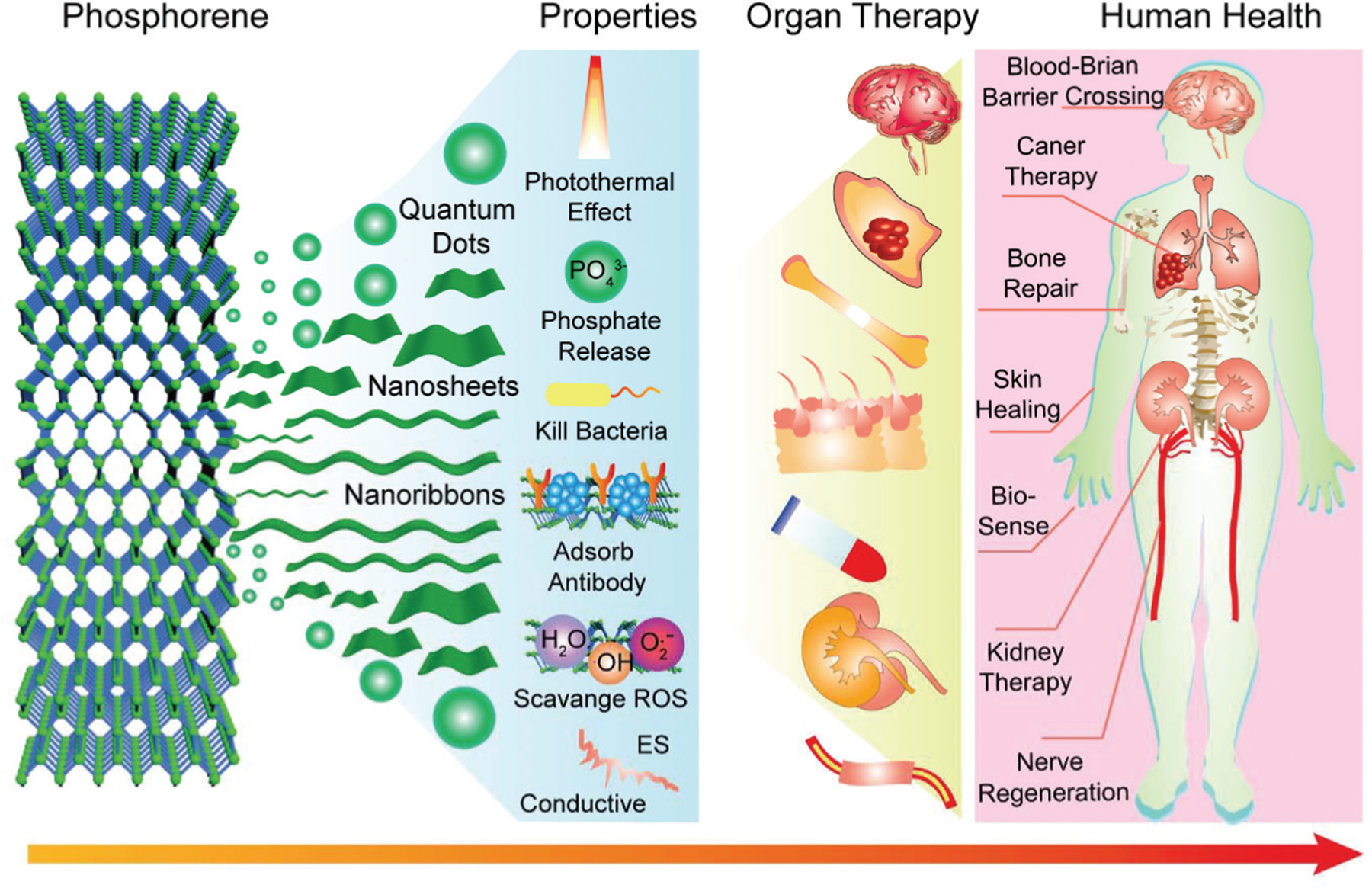
Extensive applications of BP based on unique properties for tissue engineering, regenerative medicine, cancer, and biosensing.
2.1. Phosphorus release
The water-assisted oxidation of BP leads to the release of phosphate ions and phosphonates, making the material biodegradable.104 Phosphate is one of the primary bio-components found in the human body, including the phospholipid bilayer of cell membranes, the sugar–phosphate backbones of deoxyribonucleic acid (DNA), as well as bone, skeletal muscle, blood, skin, and nerve tissue.75,105–108 Phosphate also has key functional roles during the development of bone, where phosphate ions regulate osteogenesis, osteointegration, and fracture regeneration.84,109–112 Due to the ubiquity of phosphate and its roles within tissue growth and maintenance, BP holds great potential for a wide range of biomedical applications where the localized delivery of phosphate is technically useful or beneficial to the regeneration of tissues.31,48,96,113–122
2.2. Biomineralization
The release of phosphate from BP nanosheets that results from environmental oxidation has profound implications for its use as an induction agent for biomineralization. Phosphate (PO43−) ions act as anionic ligands for positive calcium ions (Ca2+), resulting in the attraction, binding, and accumulation of free calcium ions from the physiological environment.84 This process subsequently leads to the formation of calcium phosphate (CaP) nanoparticles, which can support the formation of hydroxyapatite (HAP), an important structural component of bone and enamel.123 This property of BP paves the way for next-generation biomedical platforms that can guide the regeneration and remodeling of bone.84
2.3. Metal ion capture
The presence of phosphorus atoms in BP nanosheets enables it to have a strong binding ability to metal ions. BP nanosheets have been reported to capture several common transition-metal ions in the human body including Ca2+, Cu2+, Mg2+, Fe2+, Fe3+, and Zn2+ ions.124,125 High selectivities in the absorption of Cu2+ further enable BP nanosheets to function as antioxidants.124,125 These interesting and unique properties make BP nanosheets promising therapeutics for neurodegenerative disorders (ND) regeneration through the regulation of Cu2+ concentrations and the reduction of oxidative stress-associated symptoms.
2.4. Reactive oxygen species (ROS) capture
Reactive oxygen species (ROS) are a group of free radicals derived from oxygen, including hydrogen peroxide (H2O2) and hydroxyl radicals (OH). The accumulation of ROS in cells has been linked to DNA damage, the dysregulation of biological pathways, apoptosis, and necrosis.126 As a result, excess ROS have been associated with a range of diseases, including Alzheimer’s, sepsis, strokes, Parkinson’s, and acute kidney injury (AKI).127 BP holds promise in the development of therapies for ROS-associated diseases due to its high reduction potential. Early in vitro cell studies confirmed that BP nanosheets can alleviate oxidative-pressure-induced cell apoptosis by scavenging ROS.128 With this advantage, BP nanosheets hold immense potential for ROS related disease therapies.83,102,129–131
2.5. Photothermal effect
BP nanoparticles absorb a broad range of wavelengths, eliciting a photo-thermal response. When deployed in combination with near-infrared radiation (NIR), BP excitation leads to localized hyperthermia, with temperatures greater than 43 °C effectively ablating cancerous tumor cells.132,133 This application, along with other studies showing that NIR can stimulate tissue regeneration, prevent bacterial infection, and promote wound healing, make the photothermal properties of BP a promising tool for biomedical applications that aim to kill cancer and promote tissue regeneration.84,134
3. Synthesis of nanosheets, quantum dots, and nanoribbons
Bulk BP powder or crystals can be synthesized by different routes such as mechanical milling,135,136 high-pressure treatment,1,137,138 and chemical vapor transport.139–141 However, the top-down exfoliation of BP powder or crystals is the easiest and most commonly used technique for the fabrication of few-layered BP nanosheets for tissue engineering, regenerative medicine, cancer, and biosensing applications. The unique weak interlayer cohesion of black phosphorus layers enables easy cleavage of a single or a few layers from bulk BP under mechanical or ultrasonic force.142 Several strategies have been developed for the top-down exfoliation of bulk BP including mechanical milling, liquid-phase exfoliation, ultra-sonication, shear-assisted exfoliation, plasma-assisted fabrication, pulsed laser irradiation, and solvothermal treatment. Depending on the method and time of treatment, bulk BP can be exfoliated into nanosheets, quantum dots, or nano-ribbons, as summarized in Table 1.
Table 1.
Commonly used methods for BP nanosheets, quantum dots, and nanoribbons fabrication
| Method | Solvent/substrate | Parameters | Thickness (nm) | Reference | |
|---|---|---|---|---|---|
| Nanosheets | Sonication | NMP | 1.5–24 h, 200–820 W | 0.6 to 60 | 41, 45–47, 49, 111, 149, 150, 153, 156 and 162–167 |
| Sonication | Water | 0.5 to 8 h, ∼100 W | 2 to 20 | 44, 101, 128, 141, 171 and 172 | |
| Sonication | DMF, DMSO | ∼130 W, 12–15 h | 5.8 to 26 | 176 and 178 | |
| Sonication | CHP | 750 W, 5 h | 2 | 177 | |
| Sonication | Ethanol | 400 W, 43 h | 4 to 25 | 174 | |
| Sonication | IPA, NMP, EA | 2–3 h | 5 to 10 | 173 and 175 | |
| Sonication | Ionic liquids | 100 W, 24 h | 3 to 9 | 73 | |
| Sonication | Acetone, DMF, ethanol, hexane, IPA, NMP | 30 W, 1 h | 16 to 128 | 169 | |
| Sonication & milling | DMF, NMP, DIGLYM, AN | >6 h, 0.5 h, 400 W | 20 to 30 | 139 | |
| Shear | NMP | 15 500–22 000 rpm, 15 min | 0.9 to 3.5 | 182 | |
| Shear mixing | NMP | 5000 rpm, 4 h | 1–2 layers | 183 | |
| Mechanical | SiO2/Si | 0.7 to 47 | 1, 106 and 184–194 | ||
| Mechanical | Glass | 20–200 | 195 | ||
| Quantum dots |
Sonication | NMP | 3–16 h, 150–1200 W | 0.4–5 | 55, 56, 67 and 201–217 |
| Sonication | NVP | 5 h/20 W | 5 | 220 | |
| Sonication | IPA | 3 h/500 W | 5.2 | 219 | |
| Sonication | DMF | 20 h, 1 h/250 W | 2 to 10 | 218 | |
| Shear force | DMSO | 40 min | 0.58–1.45 | 224 | |
| Pulsed laser | Diethyl ether | 20 min | 7 | 225 | |
| Solvothermal | NMP | 6–12 h | 1 to 3 | 221–223 | |
| Microwave | NMP | 120 °C, 30 min | 3.59 | 59 | |
| Nanoribbons | Ionic scissoring | Li:NH3, NMP | Li:NH3 24 h; sonicate 50 W, 1 h; 2000 rpm, 20 min | Width: 4–50 nm; thickness: ∼1 L; length: 75 μm | 71 |
Abbreviations: AN: acetonitrile; CHP: N-cyclohexyl-2-pyrrolidone; DIGLYM: bis(2-methoxyethyl) ether; DMF: dimethylformamide; DMSO: dimethyl sulphoxide; EA: ethyl alcohol; IPA: isopropanol; NMP: N-methyl pyrrolidone; NVP: N-vinyl pyrrolidone.
3.1. Nanosheets fabrication
Various approaches have been developed for the synthesis of BP nanosheets, with the majority of tissue engineering, regenerative medicine, cancer, and biosensing applications using ultra-sonication.16,31–36,38–52,81,82,103,143–161 Ultra-sonication can be conducted with an ice bath or a tip sonicator, with either technique yielding good quality BP nanosheets. To date, N-methyl pyrrolidone (NMP) is the most widely used solvent for the liquid-phase-based ultra-sonication of BP nanosheets.41,45,47,49,149,150,156,162–167
3.1.1. NMP-based sonication.
Liquid-phase ultra-sonification of BP in combination with NMP has been shown to yield BP nanosheets ranging in size from 0.6 nm to 60 nm, depending on the length of time (from 15 to 24 hours) and power (from 200 to 820 W) used.41,45,47,49,149,150,156,162–167 NMP is well-suited for BP liquid exfoliation because of its high boiling point and excellent surface tension, leading to reproducible separation of BP layers.41,156,168–170 However, the high toxicity of NMP limits the direct application of BP nanosheets prepared through this method in tissue engineering, regenerative medicine, cancer, and biosensing applications.
Fortunately, performing a solvent exchange in the presence of water or other biocompatible solutions may be able to mitigate the cytotoxic effects of NMP. Guo et al. reported a modified method of liquid-exfoliation using a NaOH-saturated NMP solution for the exfoliation of BP nanosheets, which allowed for the control of nanosheet size, resulted in increased nanosheet stability in water, and produced a higher yield than NMP alone.163 The general synthesis procedure for basic NMP exfoliation is presented in Fig. 2a. Briefly, NaOH is added in excess to an NMP solution, generating a basic NMP solution. Then 30 mL of saturated NaOH NMP solution is added to 15 mg bulk BP powder and the mixture is bath sonicated at 40 kHz for 4 hours. The unexfoliated bulk BP is then removed by centrifugation (3000 rpm, 10 min) to obtain the completely exfoliated BP nanosheets.
Fig. 2.
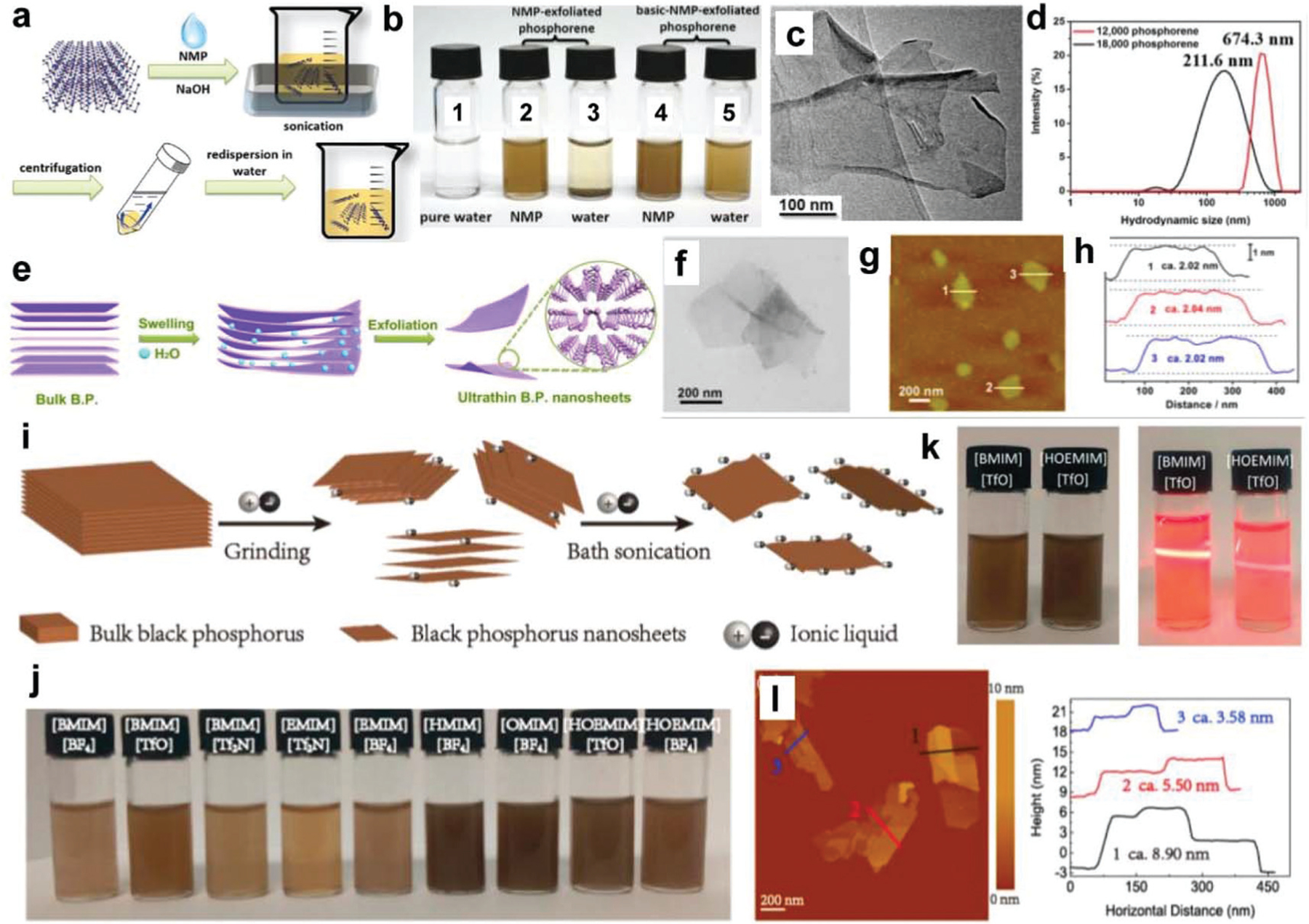
Fabrication of BP nanosheets. (a) Schematic demonstration of the exfoliation of BP nanosheets using NaOH–NMP solvent. (b) Photographs of BP nanosheets dispersed in NMP and water. The dispersion solutions are: (1) pure water, (2) NMP-exfoliated phosphorene in NMP, (3) NMP-exfoliated phosphorene in water, (4) basic NMP-exfoliated phosphorene in NMP, and (5) basic NMP-exfoliated phosphorene in water. (c) TEM and (d) hydrodynamic sizes of the obtained BP nanosheets. Reproduced with permission.163 Copyright 2015, John Wiley and Sons. (e) The exfoliation of BP nanosheets using water as the solvent. Morphology characterization of BP nanosheets by (f) TEM, (g) AFM, and (h) corresponding height analysis. Reproduced with permission.141 Copyright 2015, American Chemical Society. (i) Schematic of green exfoliation of bulk BP into BP nanosheets using ionic liquids as the solvent. (j) Photograph of BP nanosheets dispersed in different ILs after centrifugation at 4000 rpm. (k) Photograph of the BP nanosheets dispersed in [BMIM][TfO] and [HOEMIM][TfO] (left) and the Tyndall effect of diluted BP nanosheets dispersions (right). (l) AFM image of the obtained BP nanosheets and the height analysis from the tested results. Reproduced with permission.73 Copyright 2015, American Chemical Society.
BP nanosheet fabrication using basic NMP has become increasingly common,46,153 in part due to improved dispersion in both NMP and water (Fig. 2b) and reproducible sheet-like morphologies when imaged under transmission electron microscopy (TEM) (Fig. 2c). When employing this method, the thickness of nanosheets is related to the centrifugation speed used during separation. For example, centrifuging at 12 000 rpm for 20 min results in thicknesses of 5.3 ± 2.0 nm (5 to 12 layers) with a diameter of around 670 nm (Fig. 2d). When repeated at 18 000 rpm for 20 min, the thickness of BP nanosheets decreases, resulting in thicknesses of 2.8 ± 1.5 nm (1 to 7 layers) with a diameter of around 210 nm.
3.1.2. Water-based sonication.
In addition to NMP, water is also widely used for BP nanosheets fabrication.44,101,128,141,171 Being a biocompatible medium, this method can prevent the need for a solvent exchange step. BP nanosheets generated within the water can be used directly for functionalization after centrifugation. However, water-assisted oxidation poses a potential risk to BP during the sonication process. In most cases, therefore, the water–BP mixture needs to be bubbled with inert gas to replace oxygen and minimize the oxidation during sonication.
Wang et al. reported the use of distilled water as a solvent for the exfoliation of BP nanosheets, as demonstrated in Fig. 2e.141 The mixture was bubbled with argon gas to replace oxygen molecules and mitigate the issues with the oxidation. Exfoliated BP nanosheets were obtained after bath sonication and subsequent centrifugation. The morphologies of obtained BP nanosheets were observed by TEM and atomic force microscopy (AFM) imaging and are presented in Fig. 2f and g. The lateral size of the BP nanosheets was around 200–300 nm and the thickness of obtained BP nanosheets is around 2–3 nm (Fig. 2h).141 Lee et al. also employed distilled water for BP nanosheet fabrication.101 The BP–water mixture was sonicated for 30 min (20 kHz, 100 W) before collecting the supernatant and ultrasonicated further for 10 min. After repeating this sonication and purification step two or more times, BP nanosheets were obtained with a thickness determined to be around 8.7 nm and an average diameter around 10 nm.101
Tip sonication using water as a solvent has also been reported as a highly effective method for the fabrication of BP nanosheets.172 Before sonication, bulk BP was ground into powders, dispersed in deionized (DI) water, and tip sonicated for 30 to 300 min. After centrifugation, a high concentration of BP nanosheets was obtained that were found to retain an impurity-free structure, high crystallinity, and excellent stability in water.172
3.1.3. Other solvent-based sonication.
In addition to NMP and water, other solvents that are reported for the fabrication of BP nanosheets include dimethylformamide (DMF), dimethyl sulphoxide (DMSO), N-cyclohexyl-2-pyrrolidone (CHP), ethanol, isopropanol (IPA), ethyl alcohol (EA), bis(2-methoxyethyl) ether (DIGLYM), acetonitrile (AN), ionic liquids, acetone, chloroform, and hexane.73,139,169,173–178 The size of BP nanosheets obtained using these solvents ranges from a few nanometers to several hundred nanometers depending on the solvent type and sonication power used (Table 1). Ionic liquids (ILs) are a type of molten salt having high thermal stability and high viscosity.179,180 The viscosity of ILs can be 1–3 orders of magnitude higher than that of conventional organic solvents. In addition, ILs have larger surface tensions that can be used to easily break the van der Waals forces between BP layers to form nanosheets while also preventing restacking.73,169,181
Zhao et al. reported the exfoliation of BP nanosheets using ILs with grinding and weak bath sonication, as demonstrated in Fig. 2i.73 A total of nine types of IL were used, namely 1-butyl-3-methylimidazolium tetrafluoroborate ([BMIM] [BF4]), [BMIM] trifluoromethane sulfonate ([TfO]), [BMIM] bis((trifluoromethyl)sulfonyl)imide ([Tf2N]), 1-ethyl-3-methyl-imidazolium ([EMIM]) [Tf2N], [EMIM] [BF4], 1-hexyl-3-methyl-imidazolium ([HMIM]) [BF4], 1-octyl-3-methylimidazolium ([OMIM]) [BF4], 1-hydroxyethyl-3-methylimidazolium ([HOEMIM]) [TfO], and [HOEMIM] [BF4]. Photographs of BP nanosheets dispersed in these solvents after sonication are presented in Fig. 2j, with nanosheets dispersed in [BMIM][TfO] and [HOEMIM][TfO] demonstrating a significant Tyndall effect (Fig. 2k).73 AFM characterization also demonstrated separated BP nanosheets obtained by using ILs as solvent (Fig. 2l), the height of which was found to be around 3–9 nm (Fig. 2l).73
3.1.4. Shear-force based exfoliation.
Shear-force based exfoliation methods have been used to break van der Waals forces between BP layers and form nanosheets using appropriate solvents.182,183 Woomer et al. reported the exfoliation of large-scale bulk BP (10 g) using a shear mixer with NMP.183 This process required the mixing of bulk BP (6 g) with NMP solvent (100 mL), followed by bath sonication for 2 hours and shear mixing at 5000 rpm for another 4 hours. BP–NMP dispersions were further sonicated for 3 hours and shear mixed for an additional 1 h at 5000 rpm before exfoliated BP nanosheets were finally collected and centrifuged. Almost 25% of the BP nanosheets exfoliated by the shear mixing were found to be monolayered, with a lateral size distribution comparable to those generated by bath sonication.183 Xu et al. also reported shear exfoliation of BP nanosheets in an argon atmosphere.182 First, bulk BP was mixed with NMP in a conical tube equipped with a mixing head. The whole system was placed inside a glove box filled with argon gas and shear exfoliated to form a turbid dispersion. After centrifugation, BP nanosheet dispersions were obtained with brown to pale yellow color, depending on the centrifugation speed used.182
3.1.5. Mechanical exfoliation.
Mechanical exfoliation (ME) has been extensively used for BP nanosheet fabrication in large part due to its simplicity and high-quality product.1,106,184–194 The most commonly used substrate during exfoliation is a SiO2/Si mixture which facilitates a wide range of BP sheet thicknesses of less than 1 nm to more than 40 nm (Table 1). Glass has also been used as an exfoliation substrate for the production of larger BP nanosheets, ranging from 20 nm to more than 200 nm.195 Although ME is a simple and effective technique for BP nanosheet fabrication, it is also time-consuming and often struggles to produce uniform-sized nanosheets.196 As the unique optical, electronic, and thermal properties of BP can shift depending on the sizes and number of layers within nanosheets,195,197 the uniformity of these parameters has been shown to determine the performance of several BP-based electronics.195,197 In this respect, BP nanosheets produced through ME may affect the reproducibility and efficacy of their use in tissue engineering, regenerative medicine, cancer, and biosensing applications if these issues are not adequately addressed.
3.2. Quantum dots fabrication
Apart from nanosheets, ultra-small particles of BP known as BP quantum dots (BPQDs) have also attracted interest for a variety of applications, including the treatment of cancer,54–56,58,60,63–70,198–200 sensors,60,70 information storage,65 and catalysts.55,59 BPQDs are typically less than 10 nm in size and display similar photothermal and optical properties to BP nanosheets.201 To date, various techniques and methods have been reported for the fabrication of BPQDs including mechanical crushing, ultrasonic liquid-phase exfoliation, solvothermal treatment, blender shear-breaking, and pulsed laser irradiation, as summarized in Table 1.
3.2.1. Ultrasonic exfoliation.
Ultrasonic liquid-phase exfoliation is the most widely reported technique to exfoliate bulk BP into BPQDs.56,67,202 Zhang et al. were the first to report the synthesis of BPQDs from bulk BP powder using liquid-phase ultrasonic exfoliation with NMP as a solvent.201 A BP–NMP mixture was sonicated in an ice bath (200 W, 3 hours) followed by centrifugation at a high speed (7000 rpm) for 20 min to obtain a supernatant containing BPQDs. Sun et al. reported a two-step ultrasonic exfoliation method for the fabrication of BPQDs from BP powder with NMP as the solvent.203 Bulk BP was first exfoliated into BP nanosheets through probe-sonication before subsequent ice-bath sonication, as demonstrated in Fig. 3a. The integration of probe sonication with bath sonication increased the reproducibility of BPQDs as well as enabled the direct use of commercial bulk BP powders as precursors. TEM and AFM characterization of obtained BPQDs showed that they were ultra-small with both thickness and lateral size in a few nm ranges, as presented in Fig. 3b–f.
Fig. 3.
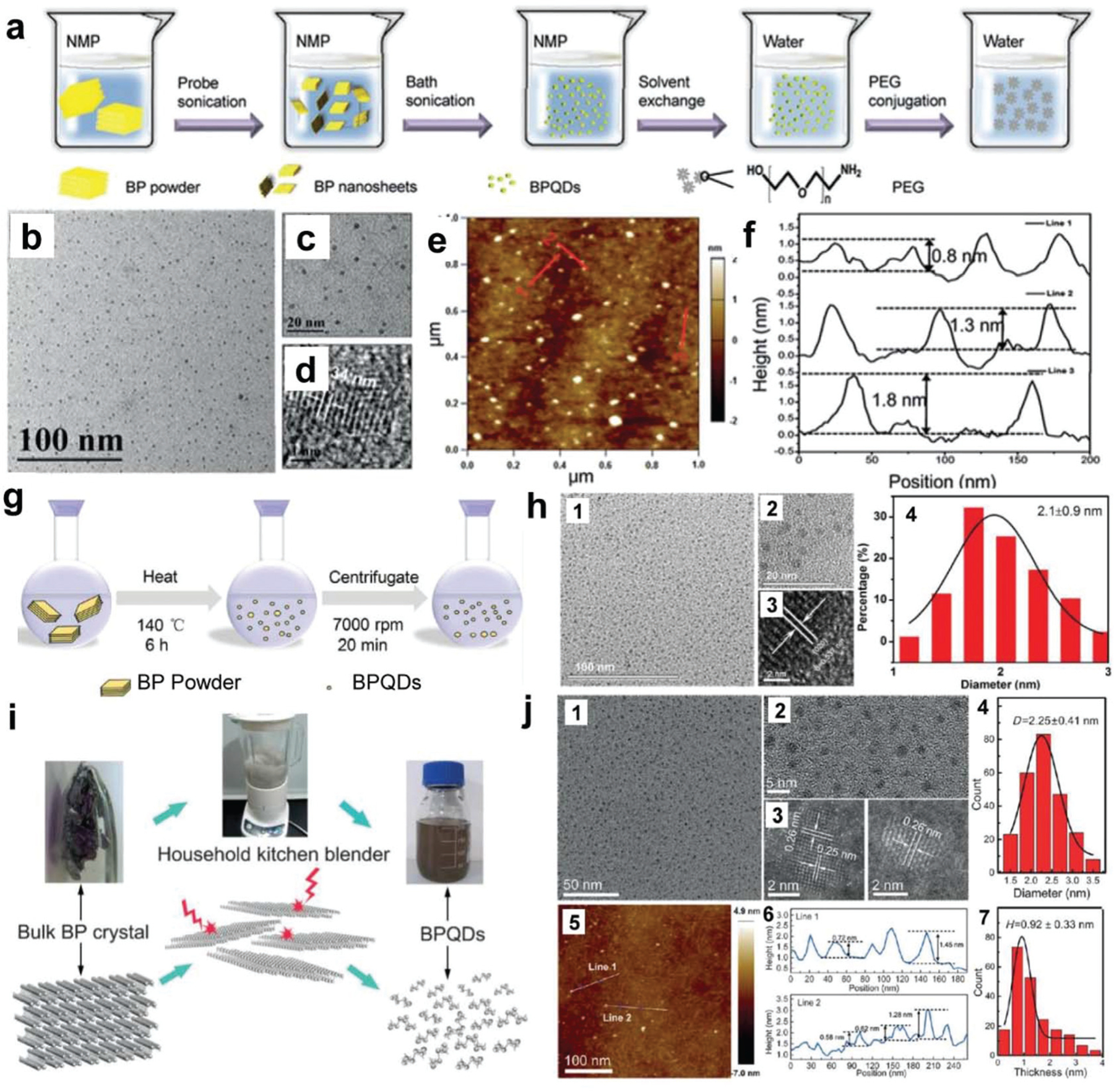
Synthesis and characterization of BPQDs. (a) Ultra-sonication-based synthesis of BPQDs using NMP as a solvent. (b) TEM image, (c) magnified TEM image, and (d) HRTEM image of the obtained BPQDs. (e) AFM image and (f) height analysis of the obtained BPQDs. Reproduced with permission.203 Copyright 2015, American Chemical Society. (g) Schematic demonstration of the synthesis of BPQDs by the solvothermal method using NMP as a solvent. (h) Characterizations of BPQDs: (1) TEM image, (2) magnified TEM image, (3) HRTEM image, and (4) statistical analysis of the lateral sizes of 100 BPQDs observed in TEM. Reproduced with permission.223 Copyright 2016, John Wiley and Sons. (i) Schematic demonstration of BPQDs fabrication using a household kitchen blender. (j) Characterizations of obtained BPQDs: (1) typical TEM bright-field image, (2) magnified TEM image of BPQDs, (3) STEM high-angle dark-field (HAADF) images, (4) statistical analysis of BPQDs sizes as measured from TEM images, (5) AFM image, (6) height profile, and (7) statistical analysis of BPQDs heights as measured by AFM. Reproduced with permission.224 Copyright 2016, John Wiley and Sons.
Several ensuing studies have explored various solvents and treatment conditions for the ultrasonic liquid-phase based fabrication of BPQDs. As summarized in Table 1, NMP is the most commonly studied solvent for ultrasonic liquid-phase-based BPQDS fabrication using bulk BP crystals or powders.55,204–217 Besides NMP, several other types of solvent were also explored for BPQDs fabrications, including dimethylformamide (DMF),218 isopropanol (IPA),219 and N-vinyl pyrrolidone (NVP).220
3.2.2. Thermal treatment fabrication.
In addition to ultrasonic exfoliation, exfoliation routes that employ thermal energy have also been used to fabricate BPQDs.221–223 Thermal treatment fabrication of BPQDs has been reported to reliably produce ultra-small size distributions within a range of 1 to 3 nm. Xu et al. reported a thermal-based method for the large-scale fabrication of BPQDs from BP powder precursors,223 as outlined in Fig. 3g. Briefly, bulk BP crystals were first ground into a fine powder followed by the addition of a NaOH-saturated NMP (basic NMP) solution to enhance quantum dot stability. The mixture was then vigorously stirred at 140 °C in a nitrogen protected atmosphere for 6 hours. With this method, ultra-small BPQDs were obtained through centrifugation with an average lateral size of 2.1 ± 0.9 nm (Fig. 3h). Using a similar method, Wang et al. reported the fabrication of BPQDs with an average lateral size of about 2.48 nm.222 With an extended 12 h treatment under these conditions, Gu et al. were able to achieve an even smaller average size of 1.76 nm and thicknesses around 1.2 nm.221
3.2.3. Shear, laser, and microwave treatment.
Facile and cost-effective methods that employ turbulent shear forces to produce BPQDs from bulk BP have been reported.224,225 Zhu et al. utilized a common household kitchen blender to provide shear force for BPQDs generation, as presented in Fig. 3i.224 When using this method, dimethyl sulphoxide (DMSO) is often used as a solvent to enhance the breakdown of van der Waals forces in BP layers. This method was found to produce a large quantity of BPQDs with an average size of 2.25 nm and thicknesses ranging from 0.58 to 1.45 nm (Fig. 3j). In addition to shear force, pulsed laser ablation is also a facile and clean method for the fabrication of BPQDs. Ge et al. reported the application of pulsed laser treatment of bulk BP using diethyl ether as a solvent for the fabrication of phosphorene QDs (PQDs).225 In brief, bulk BP was mixed with diethyl ether and irradiated with a Nd:YAG pulse laser that emitted light at 1064 nm for 20 min. PQDs generated through this method were approximately 7 nm, with the majority less than 10 nm in size.
Fabrication methods that employ microwaves have also been used to generate high-quality BPQDs using NMP as the solvent.59 For this fabrication method, the finely ground BP crystals were mixed with NMP at a concentration of 5 mg mL−1 and heated using a commercial microwave system with a maximum power of 600 W. The heating cycle involved initial heating to 50 °C for 10 min to first obtain BP nanosheets and further heating to 120 °C for 30 min to obtain BPQDs, which were collected through centrifugation at 6000 rpm for 30 min. This simple microwave approach generated a stable dispersion of BPQDs with an average lateral size of around 2.95 nm and thicknesses around 3.59 nm.59
3.3. Nanoribbons fabrication
Nanoribbons are unique structures that integrate the flexibility and unidirectional characters of one-dimensional (1D) materials with the high surface area to volume ratios of 2D nanomaterials. The edge effects and electron-confinement in nanoribbons can be used for the precise control of electronic band structure to generate new architectures and novel phenomena for a wide range of applications.226–241 Numerous theoretical calculations have predicted extraordinary properties for phosphorene nanoribbons (PNRs) due to the unique anisotropic structure of phosphorene.227–229,231–241
Watts et al. reported a method for the fabrication of high-quality, individual phosphorene nanoribbons (PNRs), i.e., BP nanoribbons (BPNR), through ionic scissoring of bulk black phosphorus crystals.71 A two-step process, lithium-ion intercalation of BP crystals was first performed and then followed by sonication to form a BPNR dispersion. As presented in Fig. 4a, the macroscopic black phosphorus crystals were outgassed at 100 °C under vacuum for 1 week and then were loaded with lithium metal (stoichiometric ratio of Li/P = 1/8) in a fused silica reaction tube. The tube was then placed in a propan-2-ol bath at −50 °C and condensed with high-purity ammonia gas to facilitate the dissolution of the alkali metal in the black phosphorus/lithium mixture (<1 mol% metal) for 24 hours.
Fig. 4.
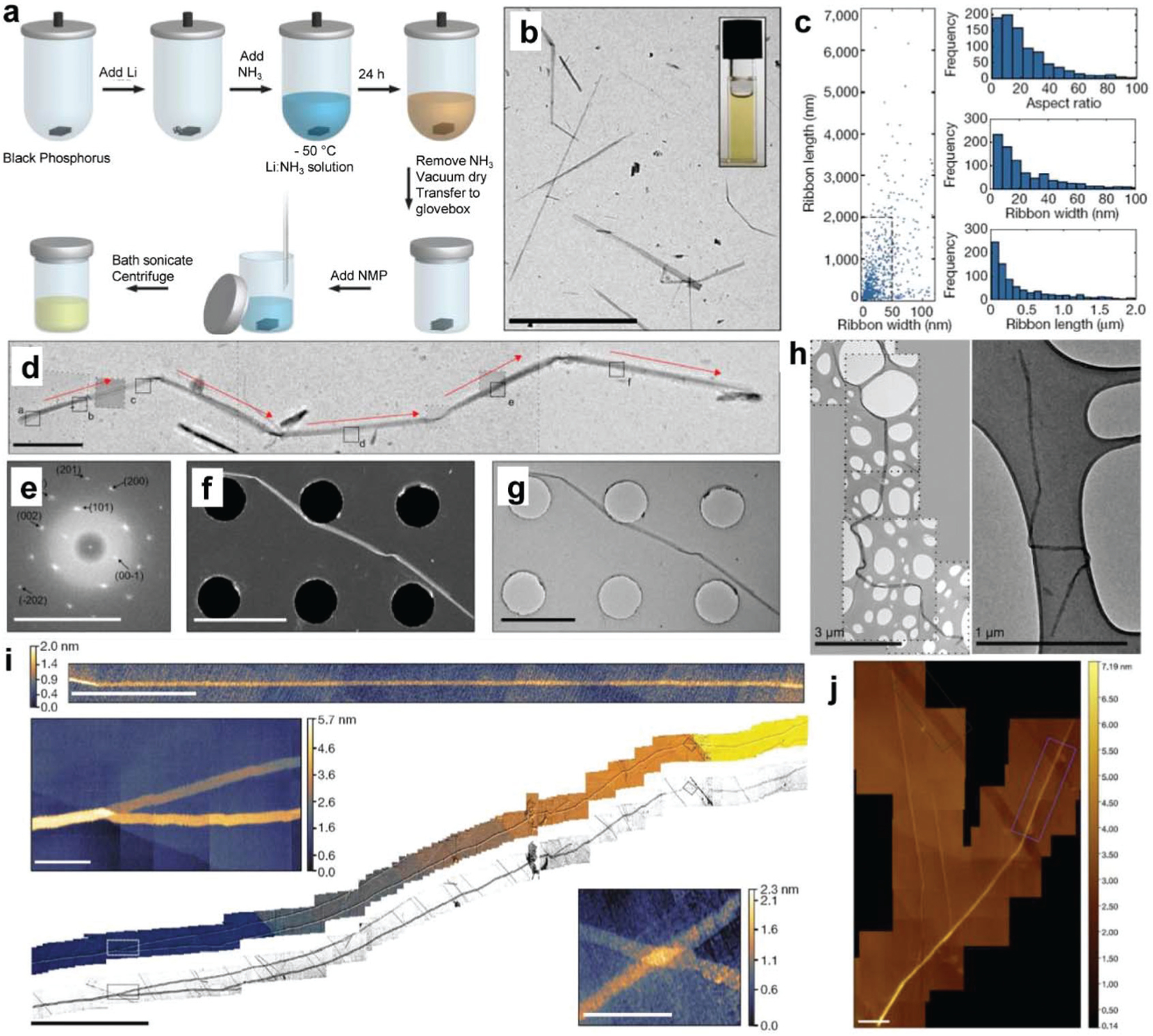
Synthesis and characterizations of BP nanoribbons (BPNR). (a) Schematic of fabrication procedures of BPNR. (b) TEM images of fabricated BPNRs. Scale bar, 10 µm. (c) Scatterplot of BPNR length and width for 940 BPNRs as measured by TEM and histograms showing aspect ratios, widths, and lengths for the fabricated BPNRs. (d) TEM images of a roughly 11 μm BPNR that has folded upon drying. Red arrows indicate the zigzag crystallographic direction. (e) Indexed FFT transform of the HRTEM images. Scale bar, 10 nm. (f) The STEM (scale bar, 2.5 µm) and (g) TEM (scale bar, 2 µm) images of the fabricated BPNR. (h) TEM images of BPNRs showing flexibility. (i) HS-AFM micrograph of a monolayer BPNR (scale bar, 500 nm) and large-area composite HS-AFM image of a BPNR longer than 75 µm (center image, scale bar, 10 µm) with magnified sections corresponding to the dashed boxes on the central image (top left and bottom right image, scale bars, 500 nm). (j) HS-AFM images (scale bars, 200 nm) of areas over which the stability study of BPNR was undertaken. Reproduced with permission.71 Copyright 2019, Nature Publishing Group.
After the removal of ammonia by cryo-pumping, the intercalated BP crystal was dried in a vacuum for 1 hour and transferred to a glovebox filled with high-purity argon gas. Under argon protection, 1 mL of anhydrous aprotic solvent was mixed with 1 mg of the intercalated Li–BP crystal in a glass vial and sealed. The sealed vial was then sonicated for 1 hour in an ultrasonic bath (Ultrawave QS3, 50 W) with water temperature below 40 °C. Large Li–BP crystal fragments were removed by low-g centrifugation for 20 min at 385g (2000 rpm) to obtain exfoliated BPNRs. Characterization of BPNRs fabricated through this top-down exfoliation method was found to generate stable liquid dispersion (Fig. 4b). The typical width of obtained BPNRs ranged from 4–50 nm, with the majority exfoliated into single-layer black phosphorus (Fig. 4c). The length was about 75 µm with aspect ratios up to 1000 (Fig. 4c). These nanoribbons were a single layer flat atomic crystal that aligned in the zigzag crystallographic orientation (Fig. 4d–g). In addition, nanoribbons were extremely flexible and were able to maintain uniform widths over their entire length (Fig. 4h–i).
Black phosphorus is well-acknowledged to be air-sensitive, oxidizing quickly under ambient conditions.191,242–245 The air-stability of BPNRs was measured using AFM over a six-day period from the same site on the ribbon as shown in Fig. 4j. Results showed no substantial changes in the height of the BPNRs over this period.191,243,245 However, a narrowing in the width of BPNRs was observed most likely due to the slow edge erosion of a few layers of black phosphorus.244 These unique properties of BPNRs are promising for an exotic state investigation and transformative studies as predicted theoretically for BPNRs.227,228,232,234,237,238,240,241 These BPNRs may be explored for broad applications in thermoelectric devices, integrated high-speed electronic circuits, and high-capacity fast-charging batteries.232,236,239,241,246–248
4. Biomedical application of phosphorene
The inclusion of BP has been proposed in a wide range of biomedical applications. Here we outline the recent contributions of BP to these applications, focusing specifically on studies that use BP to facilitate bone regeneration, nerve regeneration, wound healing, ROS-based diseases, cancer, and biosensor (Table 2).
Table 2.
Current progresses of 2D phosphorene in various biomedical applications
| Material | Application | Formulation | Cell and animal model | Outcomes | Ref. |
|---|---|---|---|---|---|
| BPNSs | Tumor killing and bone repair | BP/bioglass 3D printed scaffold | Osteosarcoma model Rat model of cranial defects |
NIR photothermal conversion kills osteosarcoma tumor Promote osteogenesis |
84 |
| BPNSs | Bone repair | BP/graphene-oxide 3D printed scaffold | In vitro pre-osteoblast cell study | GO facilitates cell adhesion Phosphate release from BP promotes cell proliferation Enhance osteogenesis |
263 |
| BPNSs | Tumor killing and bone repair | BP/PLGA/β-TCP/DOX 3D printed scaffold | Bone-tumor-bearing nude mice Rat cranial defects |
Strengthen mechanics Phosphate release Mitigate DOX toxicity Enhance osteogenesis |
262 |
| BPNSs | Bone repair | BP/PEA/GelMA hydrogel | Rabbit cranial defects | Sustained phosphorus supply Enhance gel mechanics |
274 |
| BPNSs | Bone repair | BP/PHEA/PDMA/PAM double network hydrogel | Rat cranial defects | BP induced CaP mineralization Enhance mechanics Favorable ECM microenvironment to mediate greater osteogenesis and bone regeneration |
273 |
| BPNSs | Bone repair | BPNSs/chitosan/PRP hydrogel | Rat with rheumatoid arthritis | NIR generates ROS to reduce inflammation Enhance osteogenesis and reduce arthritis friction |
343 |
| BPNSs | Bone repair | BPNSs/gelatin-methacryloyl (GelMA) hydrogel | Human mesenchymal stem cells (hMSC) Rat cranium defect model |
Photothermal antibacterial effect Strengthen crosslinking Promote in vitro osteogenesis without osteoinductive factors Significant cranial new bone formation |
42 |
| BPNSs | Bone repair | BPNSs/CNTpega/OPF hydrogel | In vitro pre-osteoblast cell study | Enhance adhesion, proliferation, and osteogenesis Elevated expression of osteogenic pathway genes under electric stimulation In situ gelation to fill femur defects, vertebral body cavities, and spinal fusion sites |
275 |
| BPQDs | Bone repair | BPQDs/aptamer-bioinspired matrix vesicles (Apt-bioinspired MVs) | Rat cranial defects | Targeted delivery and biomineralization Stimulating expression of heat shock proteins and alkaline phosphatase Outstanding bone regeneration |
285 |
| BPNSs | Bone repair | BP-SrCl2/PLGA microspheres | Rat femoral defects | Local release of Sr2+ at an optimal time NIR-responsive delivery Promote bone growth |
111 |
| BPNSs | Bone repair | BP/PCL/collagen nanofibers | In vitro cell experiment | Increase cell adhesion and proliferation Improve osteogenesis |
171 |
| BPNSs | Brain disorder | BP dispersed in saline | BALB/c mice injected with Evans blue | NIR facilitates blood–brain barrier (BBB) penetration Capture of Cu2+ and protect neuronal cells from Cu2+-induced neurotoxicity |
124 |
| BP nano plates | Nerve regeneration | BP/polycaprolactone nerve tubes | Sprague-Dawley rats with 20 mm of sciatic nerve defect | Induced angiogenesis and neurogenesis Stimulated calcium-dependent axon regrowth and remyelination |
121 |
| BPNSs | Skin wound healing | BP/silk fibroin sponges | Kunming mice with 5 mm2 wound | Silk fibroin prevents rapid oxidation of BPNS NIR-induced anti-bacterial effect promotes wound repair |
134 |
| BPNSs | Acute kidney injury therapy | BPNSs in PBS | Human embryonic kidney 293 cells Glycerol-induced acute kidney injury mice |
Alleviate oxidative-pressure-induced cellular apoptosis Consumed ROS in kidneys Cure acute kidney injury |
|
| BPNSs | Biosensing of cardiac biomarkers | BP/poly-L-lysine/anti-myoglobin (Mb) aptamer electrodes | In vitro detection in potassium ferricyanide/potassium ferrocyanide solution | Record-low detection limit and sensitivity toward Mb Dynamic response range for myoglobin |
302 |
| BPNSs | Biosensing of cancer biomarkers | BP/antibody/tilted fiber biosensor | In vitro detection with custom-made microchannel container | Ultrahigh sensitivity of 4 orders of magnitude lower than the cutoff value of small cell lung cancer Enhanced sensitivity of 100-fold higher than graphene oxide or AuNPs biosensors |
115 |
| BPNSs | Biosensing of immunoglobulin | BP/Al2O3/Au/antibody field-effect transistor biosensor | In vitro detection with Keithley 4200 semiconductor system | High sensitivity and selectivity Lower limit of detection ∼10 ng ml−1 |
344 |
| BPNSs | Biosensing of nucleic acids and proteins | BP/polydopamine/aptamer Probes | In vitro detection in serum diluted samples or in living cells | High selectively of thrombin High sensitivity of ssDNA Senses mRNAs (C-myc, and actin) in living cells |
345 |
| BPQDs | Cancer immunotherapy |
BPQD/erythrocyte membranes nanovesicle (BPQD-RMNV) | Basal-like 4T1 breast tumor Cells BALB/c mice with 4T1 breast tumor |
Long circulation time and tumor accumulation in vivo NIR induces tumor apoptosis and recruits DCs to capture tumor antigens Combined with aPD-1 to delay metastatic tumor growth |
310 |
| BP nano particles | Cancer immunotherapy | BP/poly-L-histidine/ILsi/paclitaxel/erythrocyte membrane-YSA | C57BL/6 mice with MC-38 tumor | EM cloaking suppressed undesirable rapid paclitaxel release Anchoring YSA enhanced tumor targetability and endosomal escape Induce sufficient antitumor immune responses (CD8+ T cells, IFN-γ, and TNF-α) |
316 |
| BP nano Flakes | Cancer immunotherapy | BP/TGF-β inhibitor/neutrophil membrane | 4T1 lung metastatic tumor-bearing mice | PDT-induced inflammation in tumor TGF-β inhibitor-induced potent immune activation and effectively inhibited lung metastasis | 346 |
| BPQDs | Cancer immunotherapy | BPQDs/polyethylene glycol/sensitive poly(propylene sulfide) (PPS) vesicles | 4T1 tumor-bearing BALB/c mice | Enhanced photo-absorption in the NIR region High loading efficiency of immunoadjuvant CpG oligodeoxynucleotides (CpG ODNs) |
311 |
| BPQDsCancer immunotherapy | Cancer immunotherapy | BPQDs/PLGA/mesenchymal stem cells (MSC) | U251 tumor-bearing balb/c nude mice | PLGA/BPQDs transported from MSC to U251 cells and kill by irradiation Treated the U251 glioma tumor with longer retention times Enhanced photothermal effectivity on U251 glioma tumor |
117 |
| BPNSs | Cancer therapy | BPNSs/NIR-II-responsive carbon dots (NIR-II-CDs) | 4T1 tumor-bearing mice | Carbon dots improve stability of BP by isolating from water and oxygen Strengthen light-harvesting and achieve high photothermal conversion Excellent PTT and achieve complete tumor eradication |
145 |
| BPNSs | Cancer therapy | BPNSs/gold nanobipyramids (GNBPs) | Mice bearing orthotopic A549 human lung tumors | Increased 1O2 production by plasmon-enhanced light absorption Higher photothermal conversion Tumor inhibition by dual-modality phototherapy |
320 |
| BPQDs | Cancer therapy | BPQDs/polydopamine (PDA) | Nude mice bearing A375 human melanoma tumors | PDA scavenges reactive oxygen and prevents oxidation and stabilizes BPQDs in water PDA with NIR absorption improve photothermal conversion of BPQDs |
56 |
| BPNSs | Cancer therapy | BPNSs/human serum albumin (HSA)/paclitaxel (PTX) | In vitro study using U87MG human glioblastoma cells | Great photothermal performance Excellent biodegradability, biocompatibility, effective drug loading NIR-induced hyperthermia to improve drug delivery and antitumor effect |
309 |
Abbreviations: aPD-1: programmed cell death protein 1 antibody; β-TCP: β-tricalcium phosphate; BPNRs: black phosphorous nanoribbons; BPNSs: black phosphorus nanosheets; BPQDs: black phosphorus quantum dots; CNTpega: carbon nanotube poly(ethylene glycol) acrylate; Col: collagen; DC: dendritic cells; DOX: doxorubicin; ILsi: interleukin-1α silencing small interfering RNA; NIR: near-infrared; OPF: oligo(poly(ethylene glycol) fumarate); PAM: polyacrylamide; PCL: poly(ε-caprolactone); PDMA: poly(N,N-dimethyl acrylamide); PDT: photodynamic therapy; PHEA: [poly(2-hydroxyethylacrylate); PLGA: poly(lactic-co-glycolic acid); PRP: platelet-rich plasma; PTT: photothermal therapy; ROS: reactive oxygen species; TGF-β: transformation growth factor-β; YSA: ephrin-A2 receptor-specific peptide.
4.1. BP for bone regeneration
BP has the potential for bone tissue engineering applications due to its unique structural properties, conductivity, biocompatibility, and ability to support biomineralization through the release of phosphate during biodegradation. BP nanosheets and quantum dots have been integrated into broad bone tissue engineering in various forms, e.g., 3D-printed scaffolds and delivery systems, as demonstrated in Fig. 5.
Fig. 5.
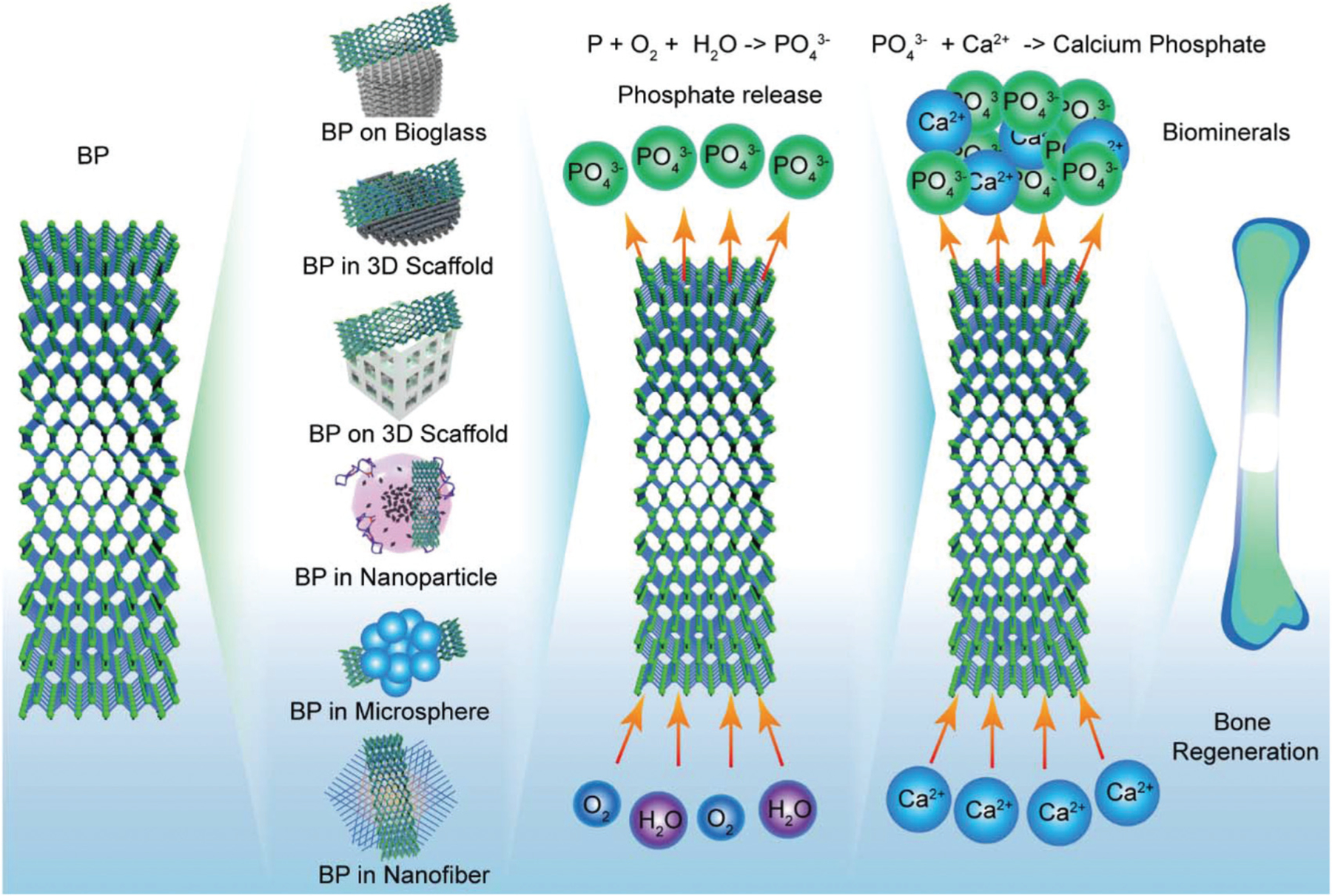
The application of BP in various forms for bone regeneration.
4.1.1. 3D-printed scaffolds with BP for bone regeneration.
Three-dimensional (3D) printing is a revolutionary technique that can realize a wide array of sophisticated architectures, leading to its broad application in multiple fields including manufacturing, engineering, and biomedicine.249–260 For bone regeneration specifically, 3D printing has been used to fabricate biocompatible scaffolds or stents that can be implanted into bone fracture sites.253 To successfully support bone regeneration, scaffolds must provide immediate structural support while also mimicking the surface chemistry, material properties, and biological properties of bone.261 The unique surface chemistry, material properties, and biocompatibility of BP make it an attractive candidate for the surface functionalization of 3D-printed scaffolds that, on their own, fail to support robust osteoblast attachment, proliferation, differentiation, and biomineralization.
Yang et al. were among the first to develop BP nanosheet-coated 3D-printed scaffolds for the treatment of osteosarcoma, the mechanism of which is outlined in Fig. 6a.84 BP nanosheets (200–500 nm in lateral sizes) were coated onto bio-glass scaffolds through a simple surface modification method using solutions containing either 0, 50, 100, or 200 ppm BP (Fig. 6b). BP integration was confirmed with SEM-assisted elemental mapping of the surface of the coated BP–BG scaffolds (Fig. 6c). The BP–BG scaffolds were then implanted into osteosarcoma sites in tumor-bearing mice, followed by treatment of the affected area with near-infrared radiation (NIR). NIR treatment resulted in BP excitation, producing a localized photothermal effect that resulted in hyperthermia-induced tumor ablation (Fig. 6d). The subsequent biodegradation of BP nanosheets released a large quantity of PO43− ions into the surrounding body fluid, which enhanced local biomineralization through the formation of calcium phosphate (CaP) minerals, which was measured in vivo using the rat cranial defect model. As shown in Fig. 6e, 3D reconstruction of micro-CT images demonstrated that implanted BP-BG scaffolds supported greater bone regeneration than the controls, while H&E staining of harvested sections showed significant degradation of BP–BG scaffolds and the regeneration of new osseous tissue (Fig. 6f).
Fig. 6.
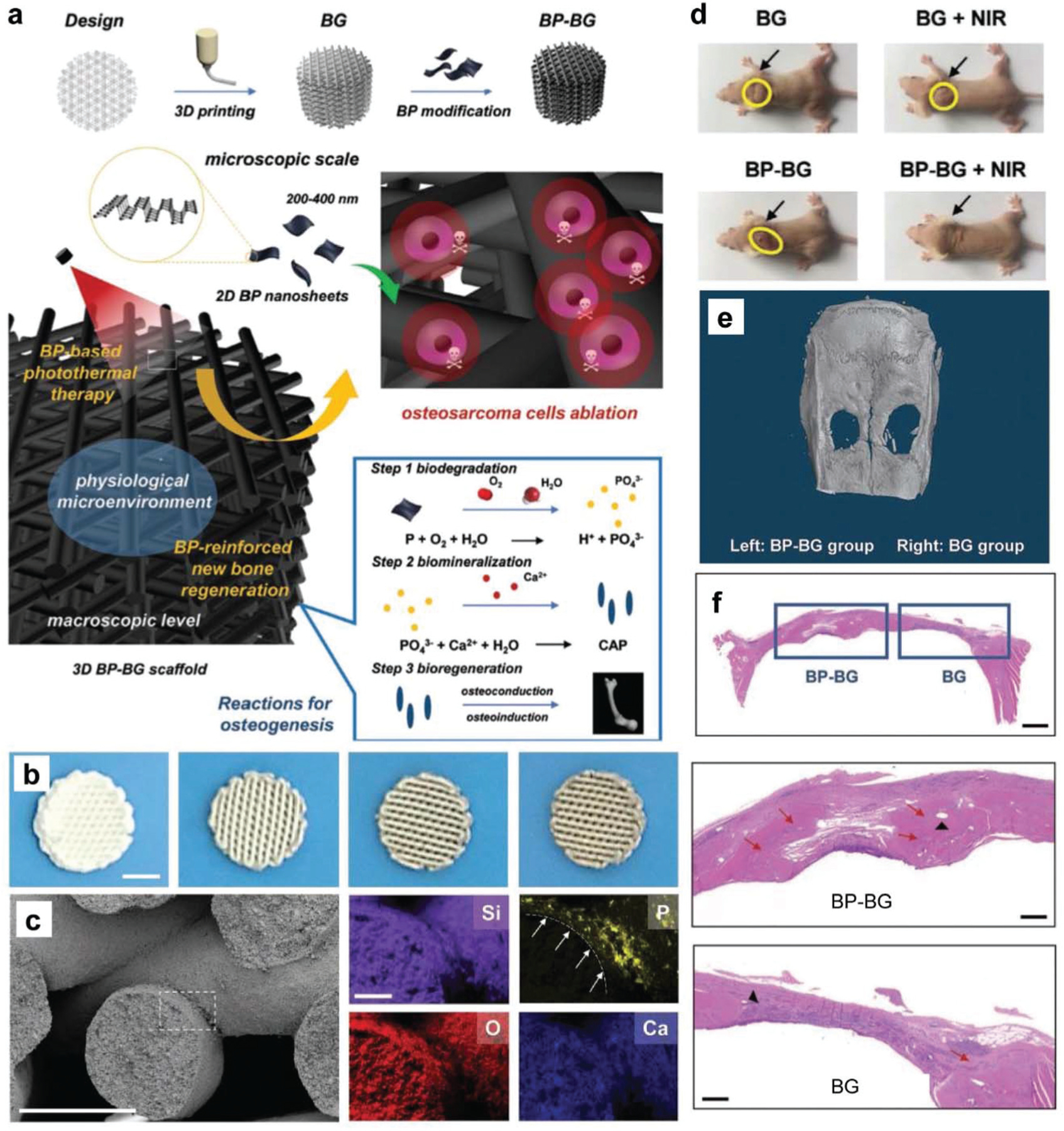
BP on 3D-printed inorganic bio-glass scaffolds. (a) Schematic demonstration of the BP-BG scaffold fabrication, therapeutic approach for osteosarcoma, and bone osteogenesis by BP-BG. (b) Photographs of BP-BG scaffolds with a 3D geometrical structure that were coated with different BP concentrations (from left to right, 0, 50, 100, 200 ppm; scale bar, 3 mm). (c) Morphologies of a BP-BG scaffold (scale bar, 300 µm) with the mappings of varied elements (Si, O, Ca, and P element; scale bar, 75 µm). (d) Osteosarcoma-bearing mice with varied treatments on day 14. (e) 3D micro-CT images showing new bone formation in cranial defect sites with the same original area around 5 mm in diameter. (f) H & E staining of craniums (scale bar, 1 mm) in week 6, with enlarged images showing the detail in two cranial defects implanted with BP-BG and BG scaffolds (scale bar, 400 µm). Red arrows show newborn osseous tissue that is mineralized. The black triangle indicates the residual scaffolds. Reproduced with permission.84 Copyright 2018, John Wiley and Sons.
In a similar application, Wang et al. developed a multifunctional 3D-printed PLGA scaffold using micro-extrusion that incorporated BP nanosheets, β-tricalcium phosphate (β-TCP), doxorubicin (DOX), and bone morphogenic protein-2-like osteogenic peptide (P24).262 BP nanosheets and DOX were included together to facilitate the photo-thermal and chemical ablation of tumors, while β-TCP and P24 were added to enhance bone regeneration, as outlined in Fig. 7a. Interestingly, the incorporation of BP nanosheets within scaffolds rather than just on the surface prolonged the photo-thermal effect of BP, which lasted up to 4 weeks after irradiation, increasing the release of DOX and P24 and decreasing the proliferation of MG63-osteosarcoma cells.
Fig. 7.
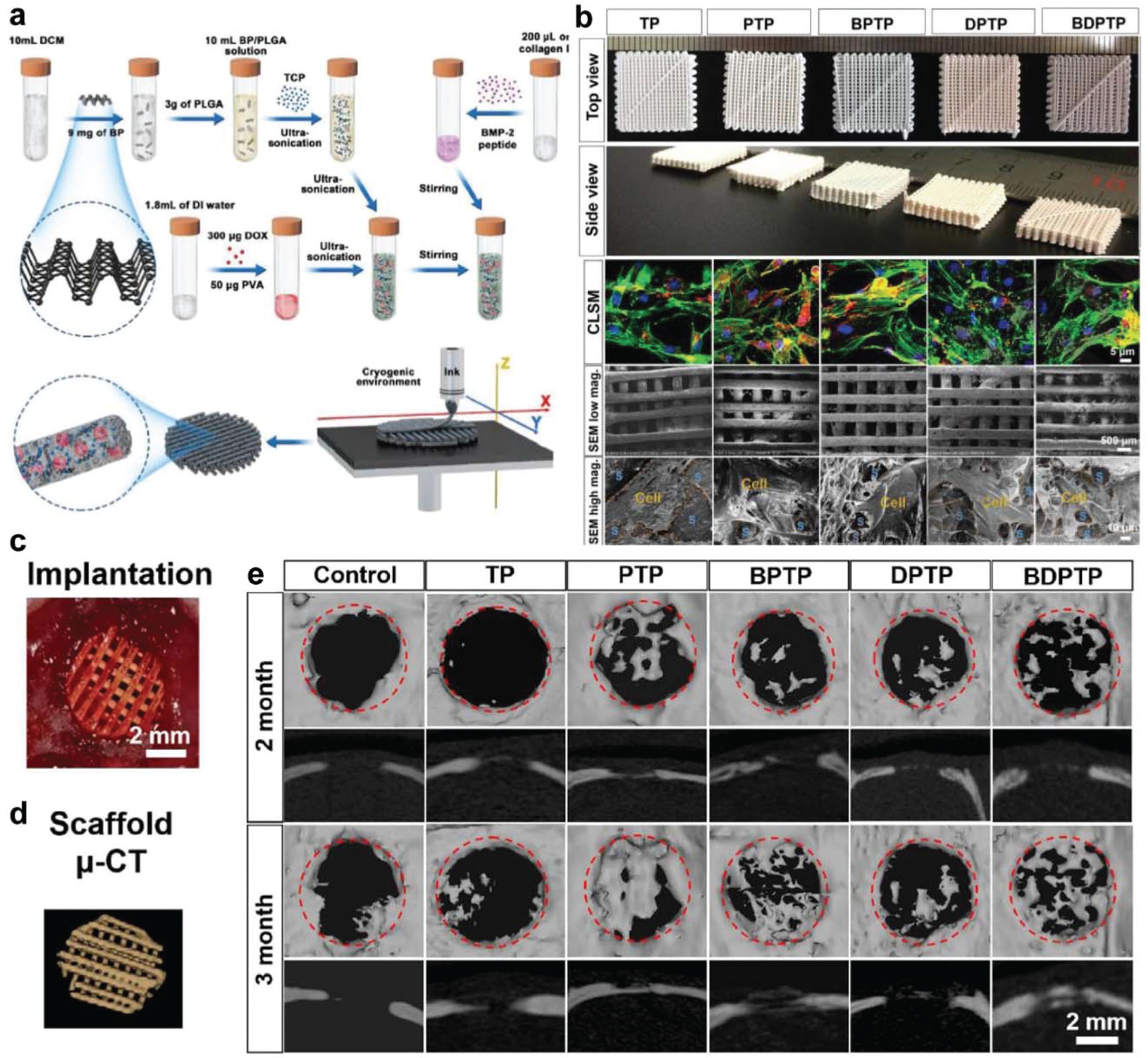
Cryogenic 3D printed BP scaffolds for bone regeneration. (a) Schematic demonstration of the preparation of multi-delivery inks and cryogenic 3D printing of multi-functional scaffolds. (b) Photographs of varied scaffolds and rBMSCs growing on these scaffolds (green: vinculin; red: F-actin). TP: TCP/PLGA; PTP: P24/TCP/PLGA; BPTP: BP/P24/TCP/PLGA; DPTP: DOX/P24/TCP/PLGA; BDPTP: DOX/P24/BP/TCP/PLGA. (c) In vivo implantation of BP incorporated scaffolds in cranial bone defects of rats and (d) micro-CT image of implanted scaffolds. (e) The micro-CT images of rat cranial defects that were implanted with varied scaffolds after 2 and 3 months. Reproduced with permission.262 Copyright 2020, IOP.
In vitro study showed excellent proliferation of rBMSCs on all scaffolds with spreading morphology, demonstrating that these porous scaffolds were favorable for cell spreading and proliferation (Fig. 7b). In vivo validation of BP/DOX scaffolds after implantation within tumors grown on nude mice showed smaller tumors after 16 days, with NIR treatment reducing tumor volume from 200 to 0 mm3 after 4 days. It is worth noting that the photo-thermal activity of BP was not sufficient to prevent tumor recurrence on its own, but was effective in combination with DOX. The osteogenic capacity of the scaffolds was also tested using the rat cranial bone defect model (Fig. 7c), evaluating bone growth with the aid of micro-CT after 2 and 3 months (Fig. 7d). As shown in Fig. 7e, scaffolds that included β-TCP and P24 showed significantly improved biomineralization within bone defects.
A third example was developed in our laboratory, wherein BP and graphene oxide (GO) nanosheets were used together to enhance the osteogenic capacity of the 3D-printed polypropylene fumarate (PPF) scaffold.263 PPF is a self-crosslinkable, biodegradable, and biocompatible polymer that is extensively applied as injectable formulations and 3D scaffolds for bone regeneration.264–269 PPF polymer scaffolds were first printed by 3D stereolithography using a PPF resin before undergoing surface ammonolyzation (Fig. 8a). GO nanosheets were used to increase the surface area available for cell adhesion while simultaneously maximizing the ability of cells to uptake the continuous release of phosphate ions from BP (Fig. 8b). Surface functionalization was achieved by wrapping BP nanosheets with negatively charged GO nanosheets (BP@GO) (Fig. 8c). The BP@GO composite was adsorbed together onto positively charged 3D scaffolds in solution (Fig. 8d). The morphology of the 3D printed scaffolds is relatively clear, as shown in Fig. 8e.
Fig. 8.
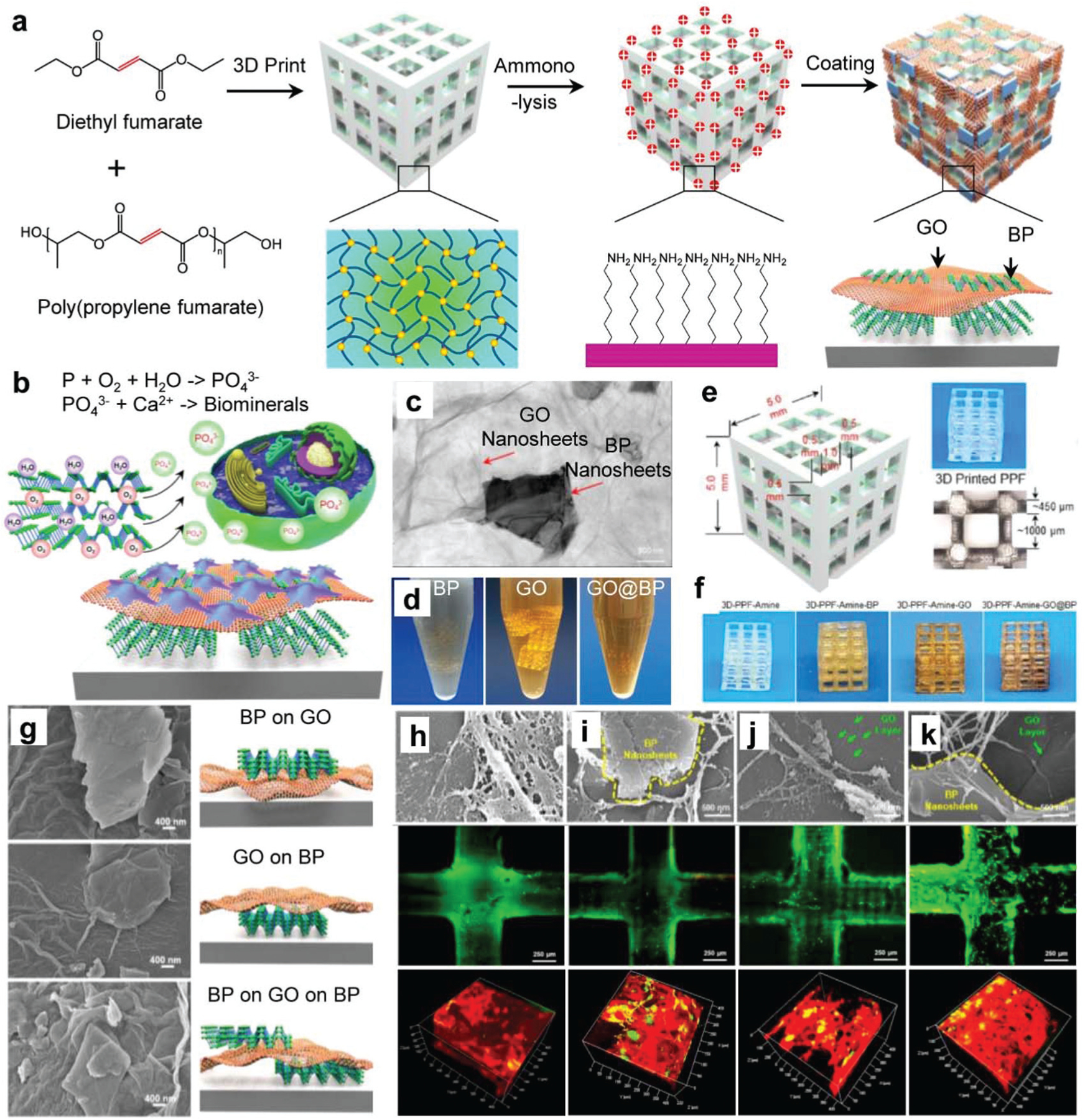
BP on 3D-printed polymer scaffolds for bone regeneration. (a) Schematic demonstration of the fabrication of 3D printed scaffolds functionalized with two-dimensional GO and BP nanosheets. (b) BP nanosheets release phosphate ions by oxidation, which enhances the proliferation of pre-osteoblasts and further helps to form biominerals for bone formation. (c) TEM images of BP nanosheets that wrapped together with GO nanosheets. (d) 3D scaffolds functionalization in GO, BP, and GO@BP solutions. (e) The printed 3D-PPF scaffolds and (f) scaffolds after functionalization. (g) SEM images of various morphologies of BP and GO nanosheets coated on the surfaces of the 3D scaffolds. The SEM of cells, live cell (green) and dead cell (red) staining, and immunofluorescence staining of vinculin (green) and F-actin (red) in MC3T3 pre-osteoblast cells that grow on the (h) pure 3D-PPF-amine scaffolds and scaffolds functionalized with (i) BP, (j) GO, and (k) GO@BP nanosheets. Reproduced with permission.263 Copyright 2019, American Chemical Society.
After functionalization in different solutions containing BP, GO, and GO@BP nanosheets, the scaffolds showed a slightly changed appearance (Fig. 8f). When observed under SEM, the BP@GO functionalization resulted in unique morphological surface features, with GO underlying BP, BP underlying GO, and double-layer structures (Fig. 8g). The in vitro live/dead test and SEM imaging using the MC3T3 pre-osteoblast cell line showed all the scaffolds had good biocompatibilities and cells grew on or surrounding the BP and GO nanosheets on different types of scaffold (Fig. 8h–k). Further immunofluorescent staining and confocal imaging observed enhanced cell adhesion, spreading, and proliferation on the BP@GO functionalized scaffolds (Fig. 8h–k). The unique features of BP@GO were also found to act synergistically to promote osteogenesis, increasing the mRNA expression of a number of osteogenic markers such as alkaline phosphatase (ALP), collagen, and osteocalcin (OCN).263
4.1.2. Hydrogels with BP for bone regeneration.
Hydrogels have been developed for several biomedical applications due to their biocompatibility, hydrophilicity, dynamic water absorption, and ability to encapsulate living cells.270 Comprising either synthetic or natural polymers, hydrogels can be more flexible in their application in 3D printed structures, with a number of groups developing injectable systems that crosslink in situ to facilitate integration with surrounding biological tissues.271 For bone regeneration, hydrogels embedded with growth factors or inorganic molecules have shown promise for the initiation of biomineralization while providing space for the development of bone matrix through accelerated biodegradation.272 To this end, the incorporation of BP into hydrogels to accelerate bone regeneration is an exciting new direction that stands to address the disadvantages inherent in tissue engineering.42
For example, Wang et al. incorporated BP nanosheets into functionalized nanoengineered (NE) hydrogels that employed a double crosslinked network design, as shown in Fig. 9a.273 The first network consisted of the biocompatible synthetic macromolecules poly(2-hydroxyethyl acrylate) (PHEA), poly(N, N-dimethyl acrylamide) (PDMA), or polyacrylamide (PAM), while the second was made from biocompatible natural polymers consisting of chitosan methacrylate (ChiMA), alginate methacrylate (AlgMA), or gelatin methacrylate (GelMA). After mechanical property screening, AlgMA or ChiMA hydrogels with PAM were selected for further study and infused with BP nanosheets to enhance their ability to induce calcium phosphate (CaP) matrix formation, as shown in Fig. 9b.
Fig. 9.
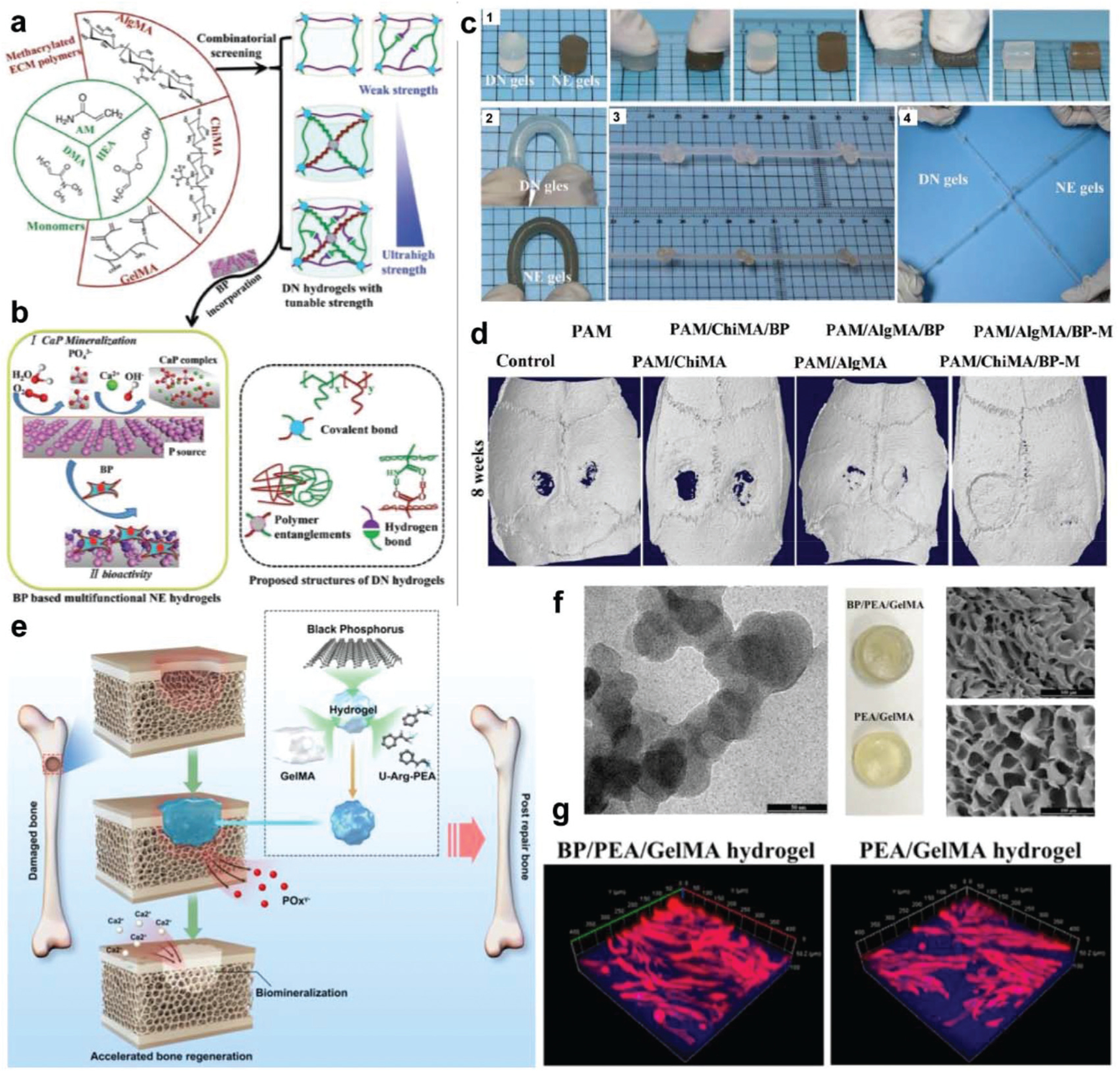
BP in hydrogel for bone tissue engineering. (a) Schematic demonstration of double network (DN) hydrogels with black phosphorus (BP) nanosheets for biomedical applications, e.g., bone regeneration. The first ductile network was prepared using PHEA, PDMA, or PAM synthetic macro-molecules. The second brittle network was prepared using ChiMA, AlgMA, or GelMA. (b) The BP nanosheets were incorporated to obtain hydrogels that can induce CaP matrix formation and enhance bone regenerations. (c) The obtained PAM/AlgMA gels and PAM/AlgMA/BP gels showed excellent mechanical toughness and could bear strong deformation by (1) compression, (2) bending, (3) knotting, and (4) crossover knotted stretching. (d) Micro-CT images of rat calvarial bone defects with varied gels at 8 weeks post-implantation. The original circular defects were around 4.5 mm in diameter. Reproduced with permission.273 Copyright 2019, John Wiley and Sons. (e) 3D hydrogel with BP nanosheets supplying phosphate ions that can capture calcium ions and thus promote bone defect biomineralization and enhance bone regeneration. (f) TEM image of BP nanosheets and photographs of PEA/GelMA and BP/PEA/GelMA hydrogels. (g) Immunofluorescence images of F-actin filaments (red) and cell nuclei (blue) for human dental pulp stem cells (hDPSCs) cultured on BP/PEA/GelMA hydrogels and PEA/GelMA hydrogels. Reproduced with permission.274 Copyright 2019, American Chemical Society.
These hydrogels were observed to sustain a high level of deformation under bending, knotting, and stretching without any ruptures, an effect that was not impacted by BP incorporation (Fig. 9c). Upon incubation in a calcium-containing solution, BP-containing hydrogels developed homogeneous calcium phosphate nanospheres (100–150 nm) distributed on their surface, which in turn promoted the attachment, proliferation, and osteogenic differentiation of both pre-osteoblasts and stem cells. Further in vivo investigation using the rat calvarial defect model showed that BP-containing hydrogels significantly improved bone healing compared to the parental DN hydrogels without BP nanosheets, resulting in higher bone volume to total volume ratios (BV/TV) and greater bone mineral density (BMD) values (Fig. 9d).
In another study by Huang et al., phosphorus-releasing hydrogels were developed by encapsulating BP nanosheets within (GelMA) and unsaturated arginine poly(ester amide) (PEA) derived photo-crosslinkable hydrogels for use in bone regeneration applications (Fig. 9e).274 The cationic nature of arginine-functionalized PEA further facilitated the electrostatic interaction of the hydrogel with the BP nanosheets, which led to the sustained release of phosphorus, enhanced hydrogel compressive strength, and the ability to capture calcium ions. TEM imaging of BP/PEA/GelMA hydrogels confirmed that BP nanosheets were successfully embedded within the gels, resulting in a slight color change (Fig. 9f). In vitro investigation showed that hydrogels containing BP supported greater attachment and proliferation of human dental pulp stem cells (hDPSCs), with the highest degree of cell spreading observed on the surface of BP/PEA/GelMA hydrogels (Fig. 9g). Similarly, in vivo characterization of the biomineralization of BP/PEA/GelMA hydrogels in the rabbit critical-size cranial defect model further elucidated the role of BP nanosheets in promoting in vivo bone healing, as was evident through immunohistological staining that showed the presence of BP nanosheets contributed to the vascularization on defect regions, resulting in mature bone formation at 12 weeks.274
In 2020, Liu et al. reported a novel injectable BP hydrogel embedded with carbon nanotubes (CNT) to achieve improved mechanical strength, electrical conductivity, and sustained phosphate ion release for bone tissue engineering.275 The hydrogel was fabricated by crosslinkable and biodegradable oligo(poly(ethylene glycol) fumarate) (OPF) polymer as the main matrix. CNTs are acknowledged to enhance hydrogel conductivity and promote cell proliferation.276–281 Therefore, crosslinkable CNT-PEG acrylate (CNTpega) was added to enhance gel mechanics and conductivity. BPNSs were also embedded to provide sustained phosphate ion release results from in situ oxidation of phosphorus element. In vitro studies showed that this gel is able to enhance preosteoblast cell adhesion, proliferation, and osteogenic differentiation. Under electric stimulation, the gel was also found to support osteogenic pathway gene expressions in preosteoblast cells. By utilizing X-ray imaging, the BP-CNTpega-gel was observed to have appropriate in situ gelation ability and have potential as injectable formulations for various bone applications such as femur defects fill, vertebral body cavities fill, and posterolateral spinal fusion.275
4.1.3. BP in delivery systems for bone regeneration.
As living tissue, bone is formed through a highly regulated developmental process that requires the coordinated temporal and spatial delivery of growth factors. Various delivery systems were developed to regulate cell growth and biomineralization during bone formation, as demonstrated in Fig. 10a.282–284 Wang et al. developed bioinspired PLGA matrix vesicles (MV), filled with ultrasmall BP quantum dots (BPQDs) and functionalized with osteoblast-specific aptamers to enable targeted bone tissue regeneration (Fig. 10b).285 Spherical MVs with diameters less than 100 nm in size were fabricated through emulsion solvent evaporation, followed by aptamer functionalization (Fig. 10b). When cultured with osteoblasts, BPQD released phosphate from MVs and enhanced ALP and RUNX2 osteogenic marker expression. After NIR irradiation, the mineralization in cells containing Apt-bioinspired MVs was enhanced greatly, demonstrating that the BPQDs in Apt-bioinspired-MVs have excellent capability in inducing osteoblast mineralization under NIR irradiation. Further in vivo investigation using the mouse bone defect model showed significant healing of defects after treatment with bioinspired MVs, an effect that was magnified after NIR treatment, as shown in Fig. 10c.285
Fig. 10.
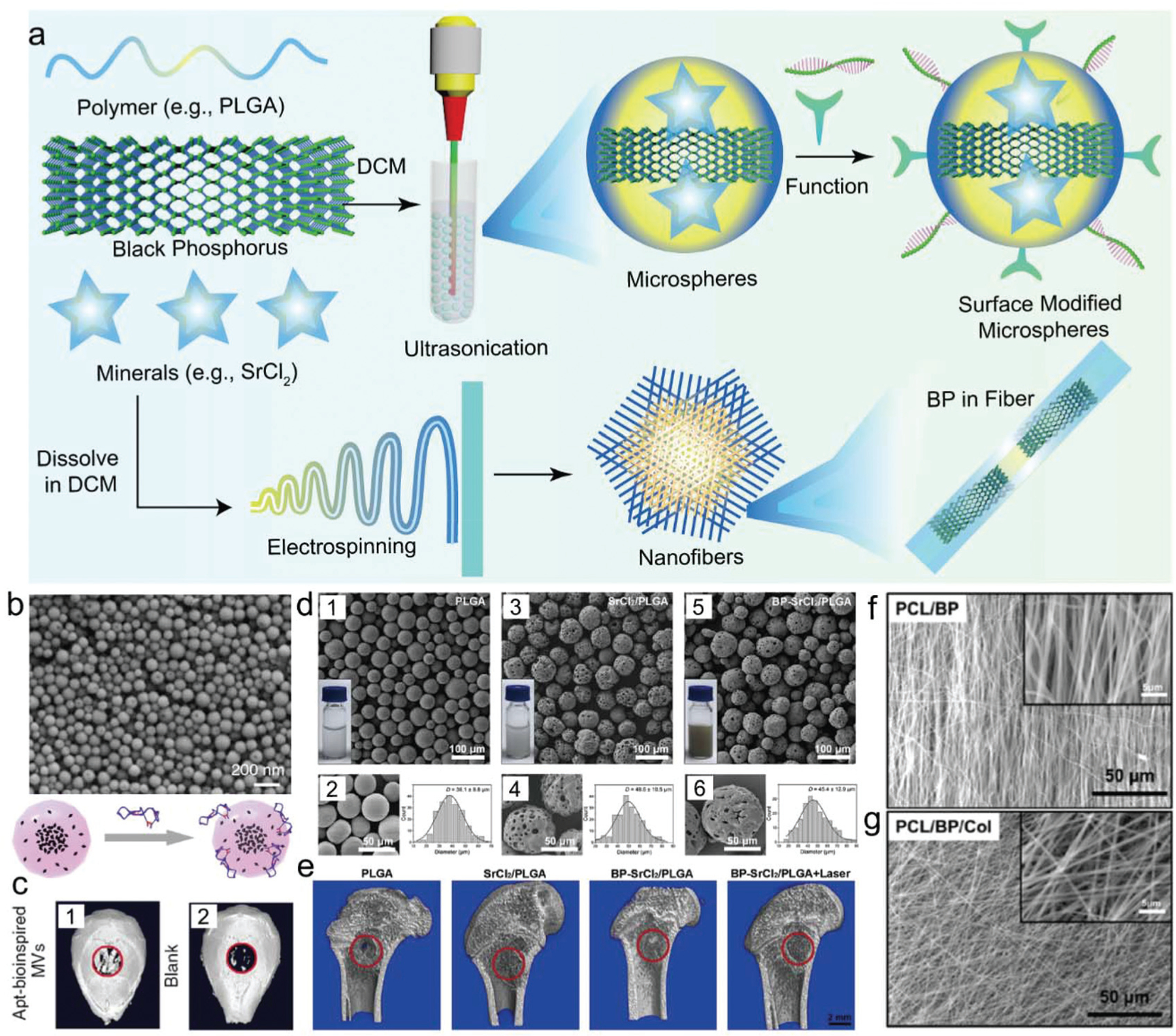
(a) BP in delivery nanovesicles, microparticles, or nanofibers for bone regeneration. (b) SEM of extracellular vesicles (EVs) containing BP quantum dots for bone regeneration and (c) micro-CT image of mouse bone defect after treatment with vesicles. Reproduced with permission.285 Copyright 2019, Nature Publishing Group. (d) SEM imaging of PLGA microspheres encapsulated with BP nanosheets and SrCl2 and statistical analysis of the average diameter of fabricated (1–2) PLGA microspheres, (3–4) SrCl2/PLGA microspheres, and (5–6) BP-SrCl2/PLGA microspheres. (e) The micro-CT analysis of in vivo bone regeneration in femoral bone defects after implantation for 8 weeks in rats. Reproduced with permission.111 Copyright 2018, Elsevier. SEM images of (f) PCL nanofibers and (g) PCL nanofibers incorporated with BP for bone regeneration. Reproduced with permission.171 Copyright 2019, Elsevier.
In another study, Wang et al. reported a near-infrared (NIR) light-triggered delivery platform by the encapsulation of BP nanosheets together with SrCl2 within poly(lactic-co-glycolic acid) (PLGA) microspheres.111 BP-SrCl2/PLGA microspheres were fabricated using a simple oil-in-water emulsion solvent method, resulting in uniformly spherical microspheres with size ranges of 10–100 microns, with the majority within the 30–50 micron range (Fig. 10d). SrCl2/PLGA and BP-SrCl2/PLGA microspheres had a porous surface structure when viewed under SEM, compared to the smooth surface of pure PLGA (Fig. 10d). BP-SrCl2/PLGA microspheres showed robust BPQD-mediated NIR photothermal activity, with irradiation also leading to the release of Sr2+ and phosphate ions. In vivo implantation of BP-SrCl2/PLGA microspheres within the rat femoral defect model showed excellent tissue biocompatibility, as well as outstanding bone regeneration after NIR irradiation treatment (Fig. 10e).111 This microsphere platform is an example of how BP and cations can be delivered together to direct biomineralization.
Electrospinning is a useful technique for the development of nanofibers with specific diameters, chemical properties, and unique architectures that support cell proliferation and differentiation.286–290 For bone tissue engineering, electrospinning has the potential to replicate the unique architecture, high surface-area-to-volume ratio, and porosity of the bone matrix.291 Lee et al. used electrospinning to fabricate bone tissue scaffolds from poly(ε-caprolactone) (PCL) and collagen nanofibers incorporated with BP to support bone tissue regeneration.171 Obtained nanofibers were structurally close to the natural extracellular matrix (ECM), with a nanoscale diameter and a very large surface area (Fig. 10f and g). In vitro evaluation of PCL/BP/Col nanofiber showed good support of MC3T3-E1 pre-osteoblast adhesion, proliferation, mineralization, and elevated expression of the ALP osteogenic marker.171
4.2. BP for brain disease treatment
Transition-metal ions such as Cu2+ can induce the production of cytotoxic ROS within biological tissues, resulting in the apoptosis of neuronal cells in neurodegenerative disorders (ND).292 The elimination of Cu2+ ions from BP may be developed for the treatment of ND and promote neuronal regeneration (Fig. 11a). Chen et al. reported that 2D BP nanosheets can effectively capture Cu2+ ions for ND treatment and also could enhance penetration of the blood–brain barrier (BBB) due to the photothermal effect after NIR-laser irradiation (Fig. 11b).124 TEM images showed non-obvious size changes for BP nanosheets before and after the capture of Cu2+ ions (Fig. 11c). The capture efficiency of various metal ions by BP was explored, and results showed the highest efficiency in capturing Cu2+ ions for BP nanosheets (Fig. 11d). The photothermal effects of BP nanosheets were apparent under NIR irradiation, with permeability tests demonstrating that the amount of BP nanosheets that crossed transwell mesh plates was six times more after NIR irradiation (Fig. 11e and f). The ability of BP nanosheets to cross the BBB was investigated using a mouse head model with Evans blue staining as an indicator.124
Fig. 11.
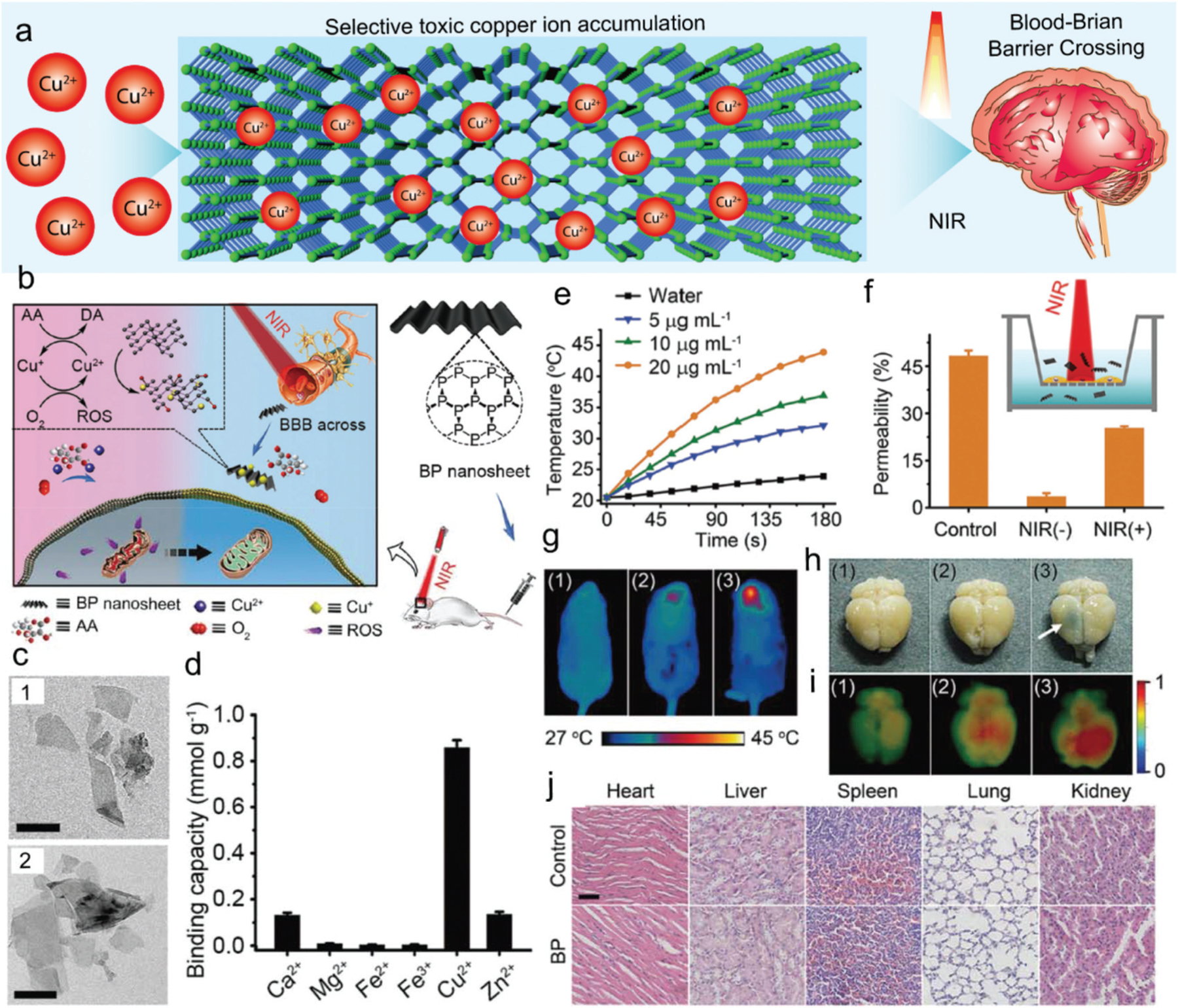
(a) Schematic demonstration of BP for neurodegenerative disorder. (b) BP nanosheets functioning as a BBB penetrable nanocaptor to capture copper ions and thus reduce the oxidative stress to enhance ND-related regeneration. (c) TEM images of BP nanosheets and BP nanosheets with captured Cu ions (scale bar, 200 nm). (d) The binding capacity of BP to various metal ions. (e) The photothermal effect of BP nanosheets as evidenced by temperature change in solutions containing varying concentrations of BP nanosheets with water as a control. (f) The photothermal effect of BP nanosheets facilitated the BBB permeability rate as tested in vitro. (g) In vivo photothermal effect of BP nanosheets in a mouse model with 808 nm laser irradiation. (1: BP nanosheets; 2: NIR treatment; 3: BP nanosheets + NIR treatment). (h) Photographs of mouse brains with Evans blue as BBB indicator after varied treatments (1: BP nanosheets; 2: NIR treatment; 3: BP nanosheets + NIR treatment). (i) Fluorescence photographs of mouse brains with varied treatments linked with polyethylene glycol (PEG) polymer chain and a fluorescence marker (1: Cy5-PEG-BP nanosheets; 2: Cy5-PEG + NIR treatment; 3: Cy5-PEG-BP nanosheets + NIR treatment). (j) Histological analysis of cytotoxicity of functionalized BP nanosheets to various organs. Reproduced with permission.124 Copyright 2017, John Wiley and Sons.
As shown in Fig. 11g, after laser irradiation, the head temperature in mice with BP nanosheet injection increased to 42.2 °C. After 24 h, BP-treated brains were harvested and were found to be stained blue whether or not NIR irradiation was employed, as presented in Fig. 11h. Further in vivo and ex vivo NIR fluorescence imaging showed that fluorescence intensities were significantly higher in mice treated with Cy5-PEG-BP nanosheets with NIR laser irradiation than other groups (Fig. 11i).124 Histological analysis showed no obvious cytotoxicity of the functionalized BP nanosheets on various organs after injection (Fig. 11j). These results indicate BP nanosheets are a great potential candidate material for efficient neuroprotection for ND therapy or neuronal regeneration.
4.3. BP for skin wound healing
Wound healing is a complex biological process that requires the coordinated temporal and spatial maintenance of hemostasis, inflammation, cell proliferation, and tissue remodeling.293,294 The replication of this process can pose a difficult surgical task, especially when repairing sensitive tissues that are prone to infection.295 In this context, nanomaterials that contain BP hold promise for tissue engineering, regenerative medicine, cancer, and biosensing applications that aim to speed wound healing and promote cell proliferation through biodegradation and the constant release of phosphorus.
BP nanosheets in combination with silk fibroin (SF) have been proposed as an exciting material in wound healing applications by Huang et al. in recent studies.134 As shown in Fig. 12a, BP nanosheets were successfully exfoliated in an SF solution (Fig. 12b), improving their stability through the prevention of rapid oxidation and degradation in the physiological environment (Fig. 12c). BP nanosheet–SF complexes were also able to be easily molded into different shapes, facilitating the preparation of flexible BP@SF films, BP@SF fibers (Fig. 12f and g), and BP@SF sponges with porous structures (Fig. 12h and i). To facilitate the wound healing process, BP@SF nanocomposites in the presence of NIR irradiation were observed to possess photo-thermal-induced antibacterial properties. Increased mortality of E. coli and B. subtilis was observed in the presence of BP nanosheets-SF with NIR irradiation compared to the individual treatments, as shown in Fig. 12j and k.
Fig. 12.
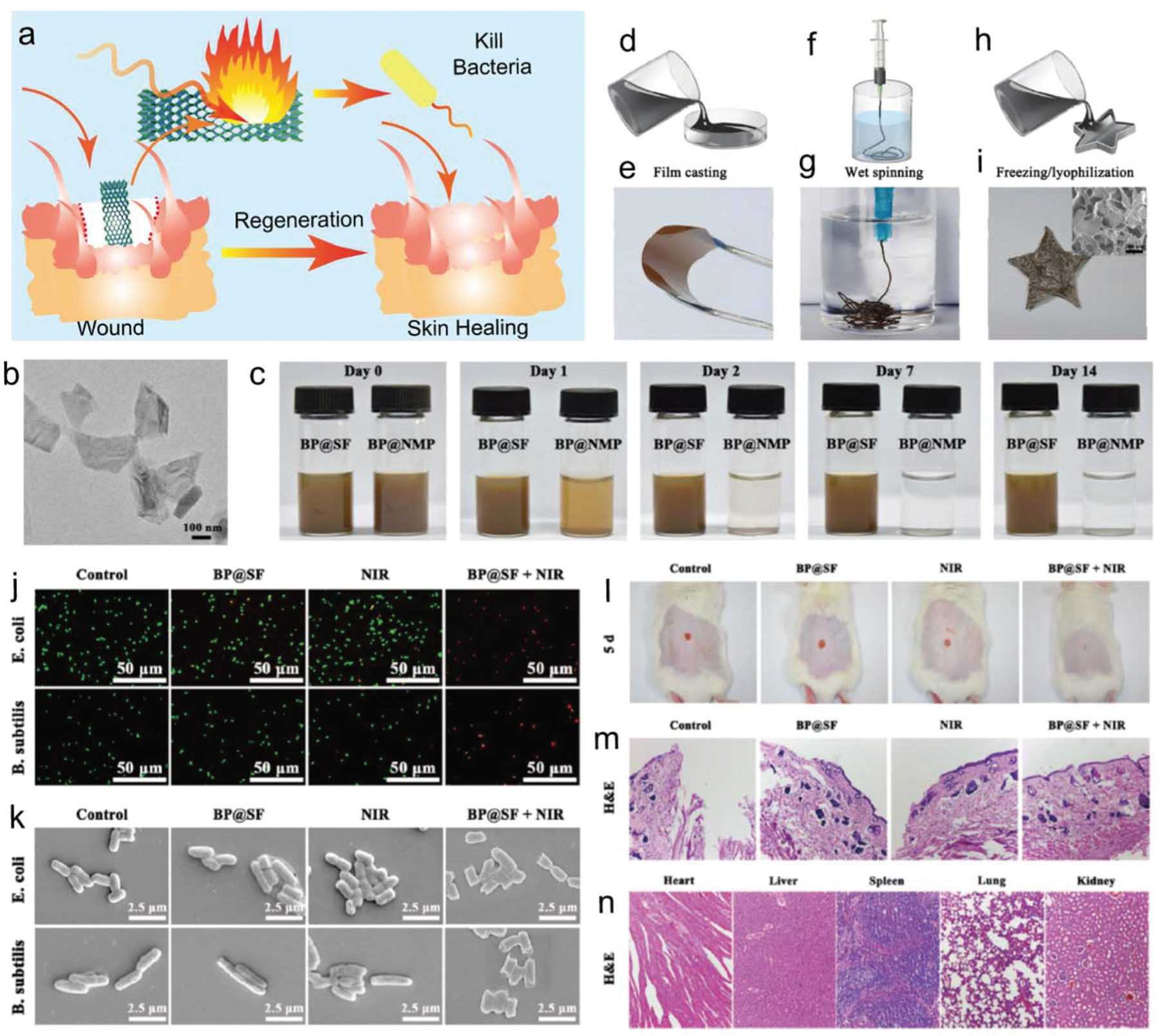
(a) Photothermal guided bacterial inhibition for wound healing and skin regeneration. (b) TEM images of exfoliated BP@SF nanosheets. (c) Stability evaluation with photographs of BP@SF and BP@NMP solutions exposed to air under ambient conditions. (d) Solution casting demonstration to prepare BP@SF films and (e) photographs of fabricated BP@SF films with excellent flexibility. (f) Wet-spinning technique to fabricate BP@SF fibers and (g) photographs of fabricated BP@SF fibers that could be twisted into knots. (h) Freezing drying process to fabricate BP@SF sponges and (i) photographs of fabricated BP@ SF sponges. Photothermal antibacterial effect of BP@SF with (j) confocal images of viability assay for E. coli and B. subtilis cells (live: green; dead: red) and (k) typical morphology of the bacteria after treatment with varied combinations. (l) Photographs showing regeneration of E. coli infected skin wounds in the mouse after treatment with varied combinations. (m) Histological staining of regenerated skin wound sites after being treated with varied combinations and (n) the major organs of mice treated with BP@SF material. Reproduced with permission.134 Copyright 2018, American Chemical Society.
When implanted into rats in combination with NIR irradiation, BP nanosheet–SF constructs were shown to promote significant wound repair with no signs of erythema and edema post 5 days of implantation (Fig. 12l). The histological analysis of tissue harvested from this group showed an intact epidermis compared to the fragmented epidermis observed with other treatments (Fig. 12m). In vivo biosafety of BP@SF was conducted by collecting and H&E staining of the major organs, including the heart, liver, spleen, lungs, and kidneys. As shown in Fig. 12n, no obvious histological abnormalities were observed in these organs, indicating good biocompatibility of BP@SF materials in vivo. These results indicate that the BP@SF-based material could effectively inhibit bacterial infection, and further facilitate wound regeneration without obvious damage to major organs in vivo.134
4.4. BP for biosensing and diagnosis
The conductivity and high surface area properties of BP nanosheets render them a promising biomaterial for biosensing, as shown in Fig. 13a. In 2019, Zhou et al. reported an optic fiber biosensor functionalized with BP nanosheets for ultrasensitive diagnosis of cancer biomarkers (Fig. 13b).115 The procedures for creating BP-coated fibers and further function with anti-human neuron-specific enolase (anti-NSE) biomarkers are illustrated in Fig. 13c. The surface morphological properties of the BP functionalized fibers were determined by AFM and showed BP nanosheets were successfully deposited on the fiber (Fig. 13d). The anti-NSE immobilized BP-TFG biosensor has ultra-high sensitivity of NSE biomarkers by showing a detection limit as low as 1.0 pg mL−1, much lower than the NSE cut off value in lung cancer cells (Fig. 13e). The sensitivity of this BP-TFG is reported to be 100 times higher than the previously reported AuNPs and graphene oxide sensors.296,297 The biosensor showed specific affinity binding of NSE over IgG, PSA, and PBS, indicating a strong selectivity for specific NSE detection (Fig. 13f). This BP nanosheet functionalized fiber-optic biosensor, therefore, is believed to provide a novel bio-nano-photonic platform for neuron-specific enolase (NSE) monitoring in biomedicine, food, and environmental applications.115
Fig. 13.
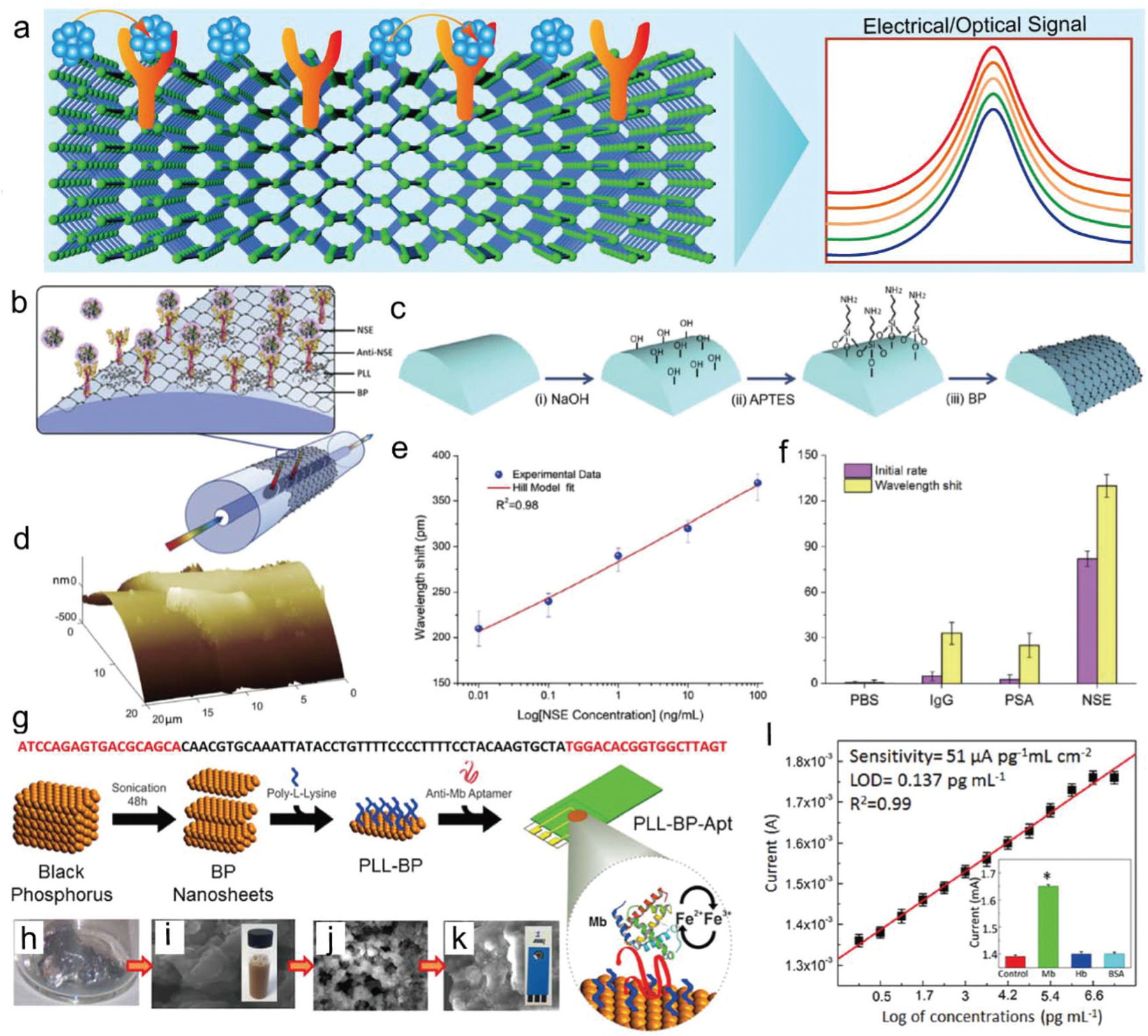
(a) BP-based antibody conjugation system for biosensing applications. (b) Ultrasensitive biosensing platform based on biofunctionalized BP nanosheet deposited tilted fiber grating (BP-TFG) for the detection of human neuron-specific enolase (NSE). (c) BP deposition procedures: (i) silica fiber surficial treatment using alkaline solution, (ii) surficial silanization using APTES, (iii) deposition of BP multiple times. (d) Three-dimensional AFM mapping of BP-coated cylindrical fibers. (e) Correlation of wavelength shift versus NSE concentrations with the Hill model fit curve (red line). (f) Ultrasensitive specificity for BP-TFG biosensor against PBS, IgG, PSA, and NSE. Reproduced with permission.115 Copyright 2019, Elsevier. (g) BP nanosheets on an electrode combined with the aptamer DNA sequence as a biosensor for Mb biomarker detection. Photographs of (h) bulk BP, (i) liquid-phase exfoliated BP nanosheets with SEM images. SEM image of BP nanosheets (j) surface-functionalized with PLL chains and (k) functionalized with both poly-L-lysine (PLL) and anti-Mb aptamer (PLL-BP-Apt). (l) Calibration signal plot for targeting Mb biomarkers with varied concentrations. Inset shows the specificity for detecting Mb biomarkers versus non-specific hemoglobin (Hb) protein and bovine serum albumin (BSA) protein. Reproduced with permission.302 Copyright 2016, American Chemical Society.
Cardiovascular diseases are major causes of adult deaths in the 30–70 year age group and the development of biosensors for the diagnosis of cardiac pathology is therefore of great interest to clinicians. Affinity-based assays utilize disease-related specific antibodies or nucleic acid (DNA or RNA) as targets for biosensing, which can reach ultra-strong sensitivity and specificity.298,299 Aptamers, a type of nucleic acid-based targeting ligand, were applied widely as an alternative to protein-based antibodies.300,301 Kumar et al. reported the development of aptamer-functionalized BP nanosheet electrodes for the electrochemical diagnosis of the redox-active cardiac biomarker myoglobin (Mb).302 The BP nanosheets were functionalized with poly-L-lysine (PLL) chains to generate a positively charged surface, which can be used for the binding of anti-Mb DNA aptamers onto electrodes (Fig. 13g). The SEM images of BP nanosheets that were functionalized with PLL chains or functionalized with both PLL and anti-Mb aptamer (PLL-BP-Apt) are presented in Fig. 13h–k. Biosensing tests showed that this aptamer sensor has extraordinary sensitivity with a record-low detection limit of ∼0.524 pg mL−1 and ultra-strong sensitivity of 36 μA pg−1 mL cm−2 toward the Mb biomarker (Fig. 13l). The response of the Mb biomarker in serum samples can be detected with a range from 1 pg mL−1 to 16 μg mL−1. Such BP nanosheet-based biosensors can provide a new strategy toward the multiplexed diagnosis of low level biomarkers for cardiovascular diseases.302
4.5. BP for kidney injury treatment
Acute kidney injury (AKI), known in the past as acute renal failure, is a syndrome that results in a rapid decrease in renal excretory function, often over hours or days.303,304 While the specific mechanism that leads to AKI remains controversial, disease onset has been linked to the presence of reactive oxygen species, localized within the kidneys.305 In this context, the ability of BP nanosheets to scavenge ROS has prompted researchers to explore their application in the treatment of AKI, as demonstrated in Fig. 14a.
Fig. 14.
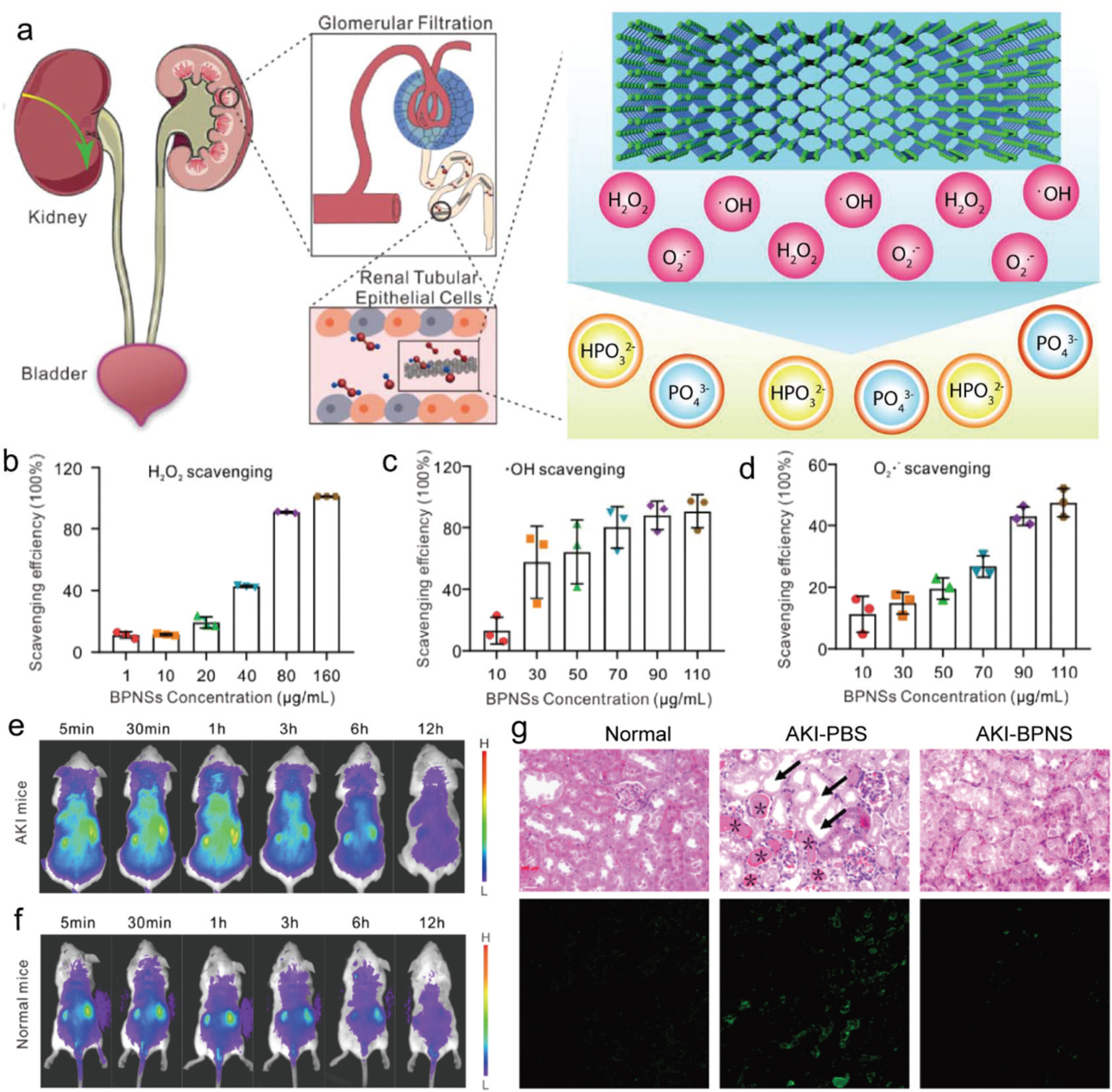
BP for kidney injury cure. (a) Schematic demonstration of BP nanosheet accumulation directed to the kidneys and alleviation of acute kidney injury (AKI) by reacting with reactive oxygen species (ROS) to safeguard renal function. The scavenging efficiency of BP for various ROS species including (b) hydrogen peroxide (H2O2), (c) hydroxyl radical (•OH), and (d) scavenging, and superoxide (•O2−). Track of BP nanosheet biodistribution after injection of cy5-BP nanosheets by in vivo imaging of cy5 intensity at different time points in (e) AKI mice and (f) healthy mice. (g) Hematoxylin and eosin staining of kidney tissues with different treatment (top row) and in situ TUNEL assay images with green fluorescence showing the cellular apoptosis (bottom row). Reproduced with permission.128 Copyright 2020, American Chemical Society.
Hou et al. reported the robust flake-like shape of BP nanosheets enabled their passive absorption into the kidneys where the desired scavenging treatment was achieved.128 BP nanosheets were tested to scavenge ROS such as H2O2, •OH, and O2•− in a fast and efficient manner, as demonstrated in Fig. 14b–d. This capability of BP nanosheets to scavenge the ROS was observed on H2O2 absorbed HEK293 cells. After the treatment with BP nanosheets, a significant reduction in H2O2 was observed in those cells. In vivo work in mice showed 80% accumulation of BP nanosheets in the kidneys instead of the heart, liver, spleen, and lungs, 12 h after injection (Fig. 14e and f). Using a glycerol-induced AKI model, it was shown that the BP-nanosheet-injected group had lower levels of creatinine and blood urea nitrogen, levels of which are elevated in blood during AKI, maintained a healthy weight, and demonstrated higher ROS scavenging. The therapeutic outcome of BP nanosheet treatment was shown to outperform the established drug-induced treatment of AKI, as evident from the H&E staining images of kidney sections (Fig. 14g).128 These results indicate that BP nanosheets could be a promising candidate for the therapeutic treatment of AKI and other renal diseases.
4.6. BP for nerve regeneration
Based on the fact that neuron cells are electroactive, the electrical conductivity of BP nanosheets may lead to their application for nerve regeneration, as shown in Fig. 15a. With this idea, Qian et al. used BP to develop concentric-layered polycaprolactone (PCL) conduits with BP material for sciatic nerve treatment.121 Varied BP concentrations of 0.25%, 0.5%, and 1% were incorporated to fabricate a series of scaffolds.121 In a typical fabrication procedure, the BP/PCL suspension was sprayed using multiple nozzles onto the surface of a rotating conduit-shaped mold. A flip microneedle arc panel was repeatedly buttoned onto the dried BP/PCL layer to punch pore structures, as demonstrated in Fig. 15b. BP nanoplates were observed to distribute within the scaffolds in various regions, with nanocomposite scaffolds displaying variable elastic moduli and electric conductivity depending on the concentration of BP.
Fig. 15.
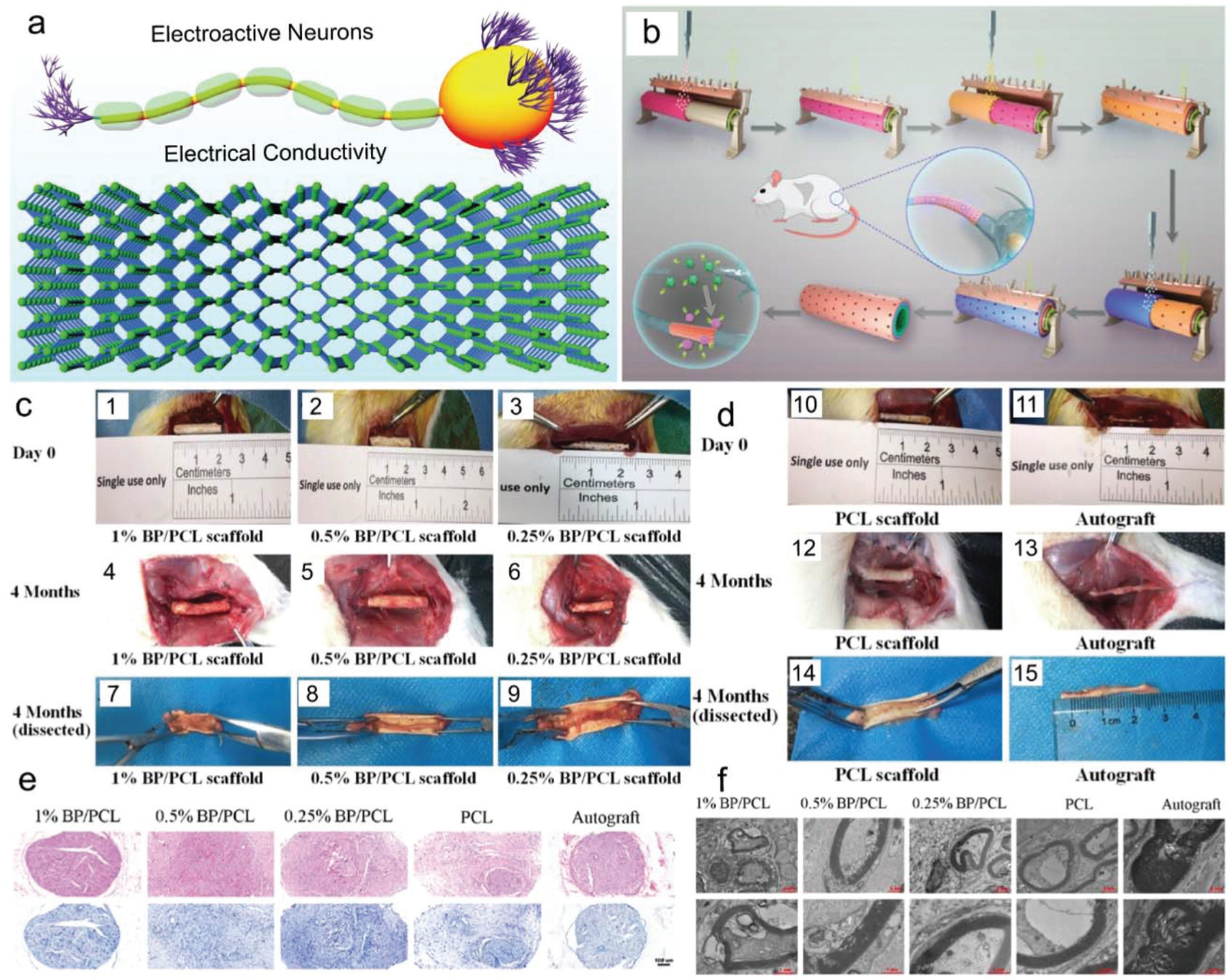
(a) Utilizing the conductivity of BP nanosheets scaffolds for sciatic nerve regeneration. (b) Schematic demonstration of layer-by-layer assembly of BP nanosheets and PCL to generate a composite nano-scaffold. (c) Photographs of BP-based nerve conduits in vivo at implantation (1–3), 4 months after surgery (4–6), and regenerated sciatic nerves (7–9). (d) PCL scaffold and autograft at implantation (10–11), 4 months after surgery (12–13), and regenerated sciatic nerves (14–15). (e) HE staining (top row) and TB staining (bottom row) of in vivo sciatic nerves regenerated in rats using varied BP-based scaffold and autograft groups (scale bar = 100 μm). (f) TEM imaging of regenerated myelin sheath and axons generated in vivo using varied BP-based scaffold and autograft groups. Reproduced with permission.121 Copyright 2019, American Chemical Society.
Scaffolds were both locally and systemically non-toxic when implanted within a sciatic nerve defect, resulting in normal levels of blood biochemical parameters including albumin, alkaline phosphatase, creatine kinase, and uric acid with no evidence of significant harm to the normal functions of the kidneys and liver. Furthermore, lower fibrosis and inflammatory reactions were observed for the 0.25% and 0.5% groups compared to the PCL control. The proangiogenic potential of BPs was also evident in these two groups through the upregulation of vascular endothelial growth factors (VEGFs). For neuronal regeneration, these two groups showed a higher number of axons, axonal area, and number of myelinated axons after 4 months of implantation in rats (Fig. 15c–f). In particular, the 0.5% groups promoted the expression of neuro-filament 200 and β-III-tubulin which are the important markers of peripheral nerve regeneration. The electrical conductivity imparted by BPs to these scaffolds facilitated the improvement in local neurite extension, remyelination, and microvessel formation.121
4.7. BP for cancer treatment
Photothermal therapy (PTT) is proven to be effective in triggering the in situ release of tumor neoantigen, which could be promising cancer immunotherapy. BP was encapsulated in various delivery systems, e.g., nanovesicles or nanoparticles, stem cells, or polymer self-assembly nanostructures, for treatment of a large variety of cancers utilizing the PTT effect of the BP sheets (Fig. 16a).56,306–308 In addition to the pure PTT effect, BP was also used together with the chemotherapy or immunotherapy reagents for cancer treatment.309 The photo-thermal effect of BP can be also utilized to trigger the release of immunotherapy reagents after NIR irradiation.
Fig. 16.
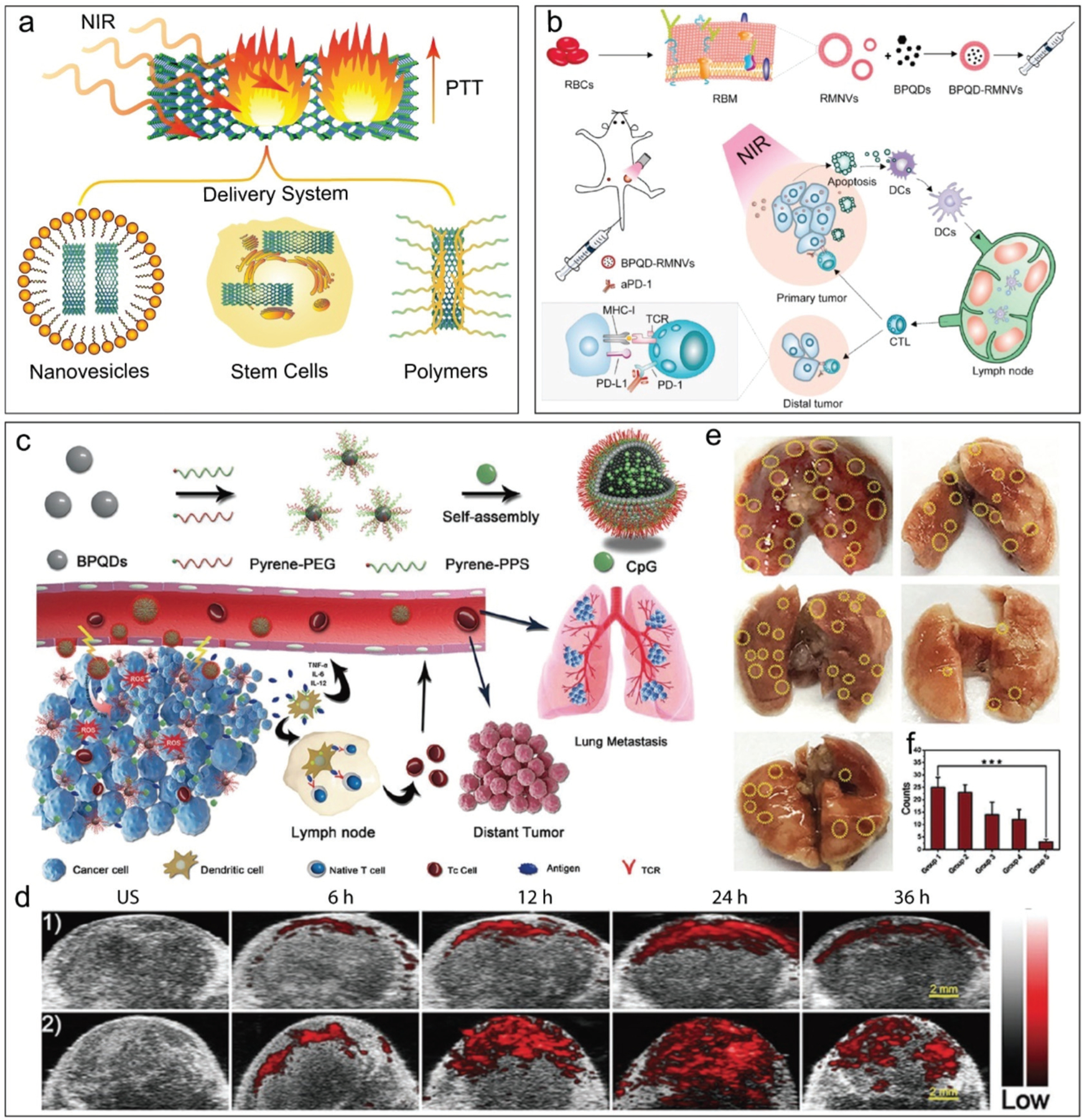
(a) Schematic demonstration of BP encapsulation in nanovesicles, stem cells, or self-assembled polymers for photothermal based cancer treatment. (b) The photothermal cancer immunotherapy using encapsulated BPQD-RMNVs with aPD-1 component. Reproduced with permission.310 Copyright 2019, Elsevier. (c) Schematic demonstration of CpG-loaded BPQD system (BPNVs-CpG) fabrication through self-assembly of BPQD conjugated with pyrene-PEG and ROS sensitive pyrene-PPS polymer, and the photodynamic immunotherapy for cancer. (d) In vivo PA imaging of tumors over time under NIR or no NIR irradiation in 4T1 tumor-bearing BALB/c mice injected with BPNVs-CpG. (e) Images for pulmonary nodules obtained from a metastasis animal model and (f) quantitative analysis of metastatic nodules observed in the mouse lungs with varied NIR treatments (group 1: PBS, group 2: PBS + NIR irradiation, group 3: CpG alone, group 4: BPNVs + NIR irradiation, and group 5: BPNVs-CpG + NIR irradiation). Reproduced with permission.311 Copyright 2019, John Wiley and Sons.
For example, basal-like breast cancer has a triple-negative phenotype together with a poor prognosis. However, the high mutation rate renders it a good fit for immunotherapy. In 2019, Liang et al. reported the use of BP quantum dot (BPQDs) and near-infrared (NIR) laser irradiation to trigger the immune system to eliminate metastatic cancer cells.310 NIR-responsive red blood cell membrane (RM) coated BP quantum dot nanovesicles (BPQD-RMNV) were fabricated as a carrier system (Fig. 16b). The PD-1 antibody (aPD-1) was administrated together with the nanovesicles system to stimulate the immune system and thus kill metastatic cancer cells. The basic scenario for this functioning process is that cancer cell apoptosis caused by NIR laser could lead to dendritic cell (DCs) recruitment followed by neoantigens capture. Thereafter, the tumor-specific CD8+ T cells will be activated to attack secondary tumors. The administration of functionalized PD-1 assists with stimulation of CD8+ T cells from exhaustion and further searching and eliminating cancer cells.
Li et al. applied BPQDs to devise the ROS-sensitive CpG oli-godeoxynucleotides-loaded nanovesicles and for the photodynamic immunotherapy against the tumor.311 The BPQD nanovesicles (BPNVs) were developed by the self-assembly of poly(ethylene glycol) and poly(propylene sulfide) (PPS) grafted BPQDs (Fig. 16c). The overall approach was to use the NIR irradiation of BPQDs to generate ROS, which further trigger the disintegration of ROS-sensitive PPS, thus leading to disassembly of BPNVs and release of encapsulated CpG to the tumor site. The CpG at the target tumor site can trigger the secretion of several cytokines that would lead to the enhancement of antitumor immune responses through the activation of cytotoxic T cells (Fig. 16c). In vivo injection studies in 4T1 tumor-bearing BALB/c mice resulted in a well-organized spatiotemporal distribution of CpG-loaded vesicles as indicated by the photoacoustic (PA) intensity in Fig. 16d. The PA signals from the tumor site were significantly stronger under NIR irradiation due to the dissociation of BPNVs with CpG at 24 h after injection. The distant tumor proliferation and lung metastases were also repressed by the BPNVs/CpG-NIR treatment. As shown in Fig. 16e and f, the lowest metastatic pulmonary nodule formation was found in the metastasis rat model with BPNVs/CpG-NIR treatment.311
In addition, the light-induced thermal effects of BP in amalgamation with an excellent tumor tropism of mesenchymal stem cells (MSC) was shown to be beneficial for the development of a targeted cancer therapy system.117 MSC was applied to encapsulate and deliver the BPQDs to the target cancer cells where the photo-induced thermal killing of cancer cells was desired (Fig. 17a). These composite nanoparticles were able to elicit higher photothermal efficacy compared to BPQDs only because PLGA prevented the undesired degradation of BPQDs at physiological conditions. After the intravenous injection of MSC loaded with BPQDs/PLGA nanoparticles, an apparent higher biodistribution of BPQDs/PLGA was observed at the target tumor site as indicated by the fluorescence intensities (Fig. 17b and c). This excellent distribution of BPQDs at the tumor site further enhanced the photothermal effects after irradiation with the NIR laser. This demonstrated a perfect synergism of functionalities between MSC and BPQDs to increase the targeted cancer therapeutic efficacy.117
Fig. 17.
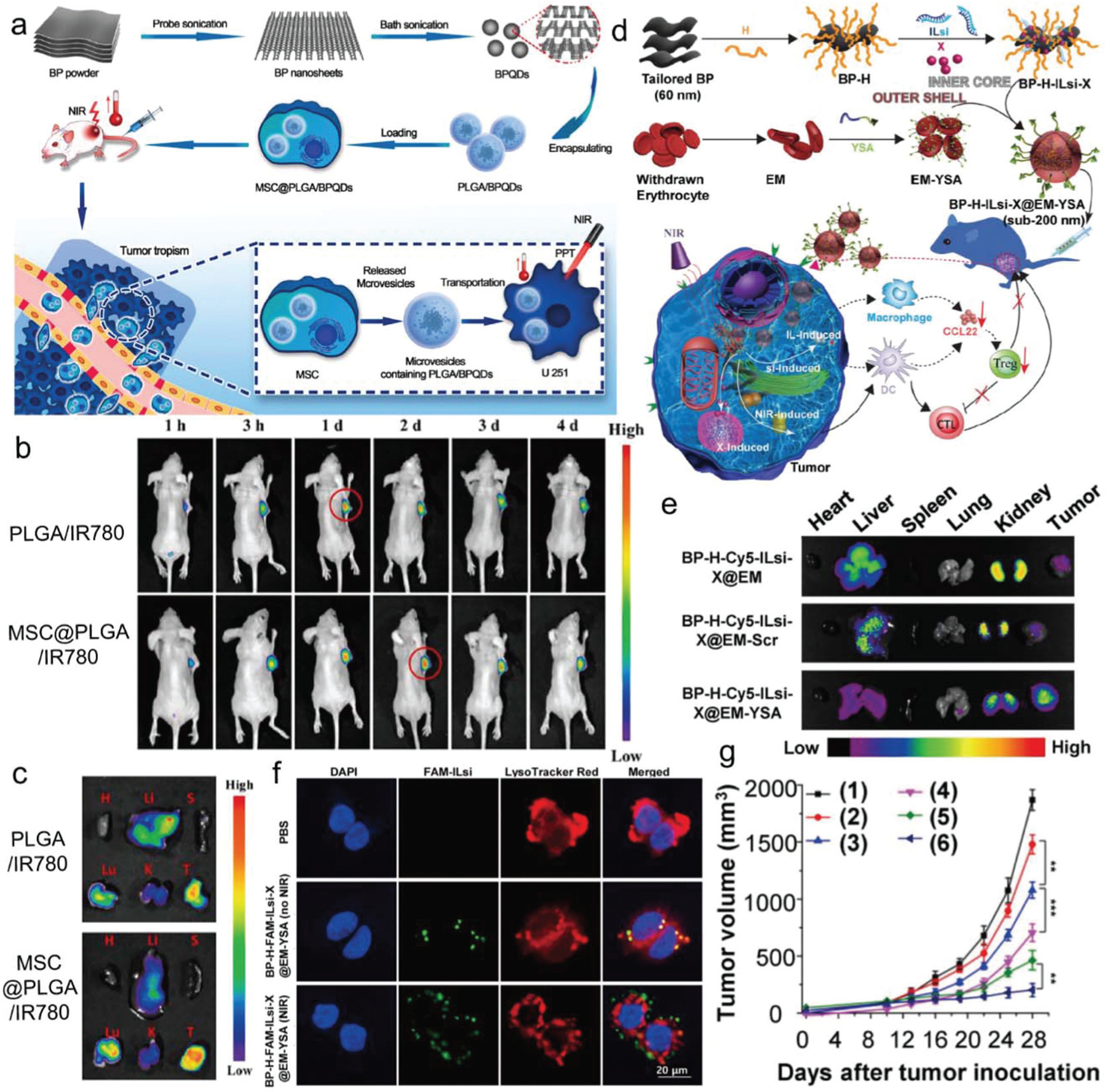
BP in stem cells and polymer assemblies for tumor treatment. (a) Enhanced photothermal therapy of tumors using mesenchymal stem cells (MSC) loaded with PLGA/BPQD under NIR irradiation. (b) U251 tumor-bearing nude mice fluorescence observation over time. (c) Fluorescence images of major organs and tumors in mice at 2 days post-intravenous-injection (H: heart; Li: liver; S: spleen; Lu; lung; K: kidney; T: tumor). Reproduced with permission.117 Copyright 2020, Elsevier. (d) Illustration of the preparation of a BP-H-ILsi-X@EM-YSA core@shell architecture for immunotherapy of tumors. (e) Ex vivo fluorescence imaging for tumors and other major organs in MC-38 tumor-bearing mice at 24 h after injection. (f) Confocal imaging of endosomal release of ILsi from MC-38 cells treated with BP-H-FAM-ILsi-X@EM-YSA under NIR or without NIR irradiation treatment (blue: DAPI; green: FAM; red: LysoTracker red). (g) Tumor volume in MC-38 tumor-bearing mice over time after injection of various components of (1) PBS, (2) ILsi-X, (3) BP-H-ILsi-X@EM, (4) BP-H-ILsi-X@EM-YSA, (5) BP-H-X@EM(NIR), (6) BP-H-ILsi-X@EM-YSA(NIR). Reproduced with permission.316 Copyright 2019, Ivyspring International Publisher.
Macrophages, lymphocytes, erythrocytes, or biomimetic artificial vesicles were extensively used as delivery nano-systems, taking advantage of biodegradability, biocompatibility, and non-immunogenicity.312,313 Erythrocyte membranes (EMs) with cargoes were applied for naturally enhancing immune responses, targeting and penetrating into tumors with a lowered amount of therapeutics and minimum side effects.314,315 Ou et al. developed an erythrocyte membrane nanosystem with surface-anchored tumor-specific ephrin-A2 receptor-specific peptide (YSA) for targeted cancer chemo-photo-immunotherapy (Fig. 17d).316 BP nanoflakes grafted with poly-L-histidine (H), interleukin-1α silencing small interfering RNA (ILsi), and paclitaxel (X) drug were the inner core of the nanosystem. After embedding within the YSA-anchored EM, a novel BP-H-ILsi-X@EM-YSA nanosystem delivery system was fabricated (Fig. 17d). Specific accumulation of the BP-H-Cy5-ILsi-X@EM-YSA nanosystem was observed in tumors (Fig. 17e). As presented in Fig. 17f, the green dots (FAM-ILsi) overlapped within the lysosome under a no-NIR-irradiation condition. However, after NIR treatment, the green dots (FAM-ILsi) were observed to have been released out of the lysosome. This indicates that NIR treatment could trigger an endosomal release of ILsi, i.e., the interleukin-1α silencing small interfering RNA. As demonstrated in Fig. 17g, with NIR irradiation, the BP-H-Cy5-ILsi-X@EM-YSA nanosystem showed stronger tumor sup-pression than that of other delivery systems.
Geng et al. prepared the BP nanosheets from a liquid exfoliation method as shown in Fig. 18a.145 The diameter of the pristine BP is approximately 100–200 nm from the TEM images in Fig. 18b. Individual NIR-II-CDs were well decentralized on the BP nanosheet surface without aggregation (Fig. 18c). The enhanced NIR-II absorption of NIR-II-CD/BP hybrids is clearly illustrated in Fig. 18d. For NIR-II-CDs, deep defect states between the valence band (VB) and conduction band (CB) could be formed by graphitic N doping, leading to obvious absorption of NIR.317 For the NIR-II-CD/BP hybrids, according to the energy position and bandgap of few-layer BP and NIR-II-CDs, after defect states captured the excited electrons of NIR-II-CDs, the holes in the VB could transfer to the BP nanosheets, resulting in the abundant free carrier accumulation in the BP nanosheets. The enhanced NIR absorption might be ascribed to the abundant free carriers, which can lead to the localized surface plasmonic resonance (LSPR) effect.318,319 As depicted in Fig. 18e, the temperature of NIR-II-CD/BP (0.6 mg mL−1) increased by 25.7 °C under 1064 nm laser irradiation while that of NIR-II-CDs and BP only increased by 20.2 and 16.3 °C, respectively, indicating that the photothermal property of BP nanosheets was significantly enhanced after being passivated with NIR-II-CDs.
Fig. 18.
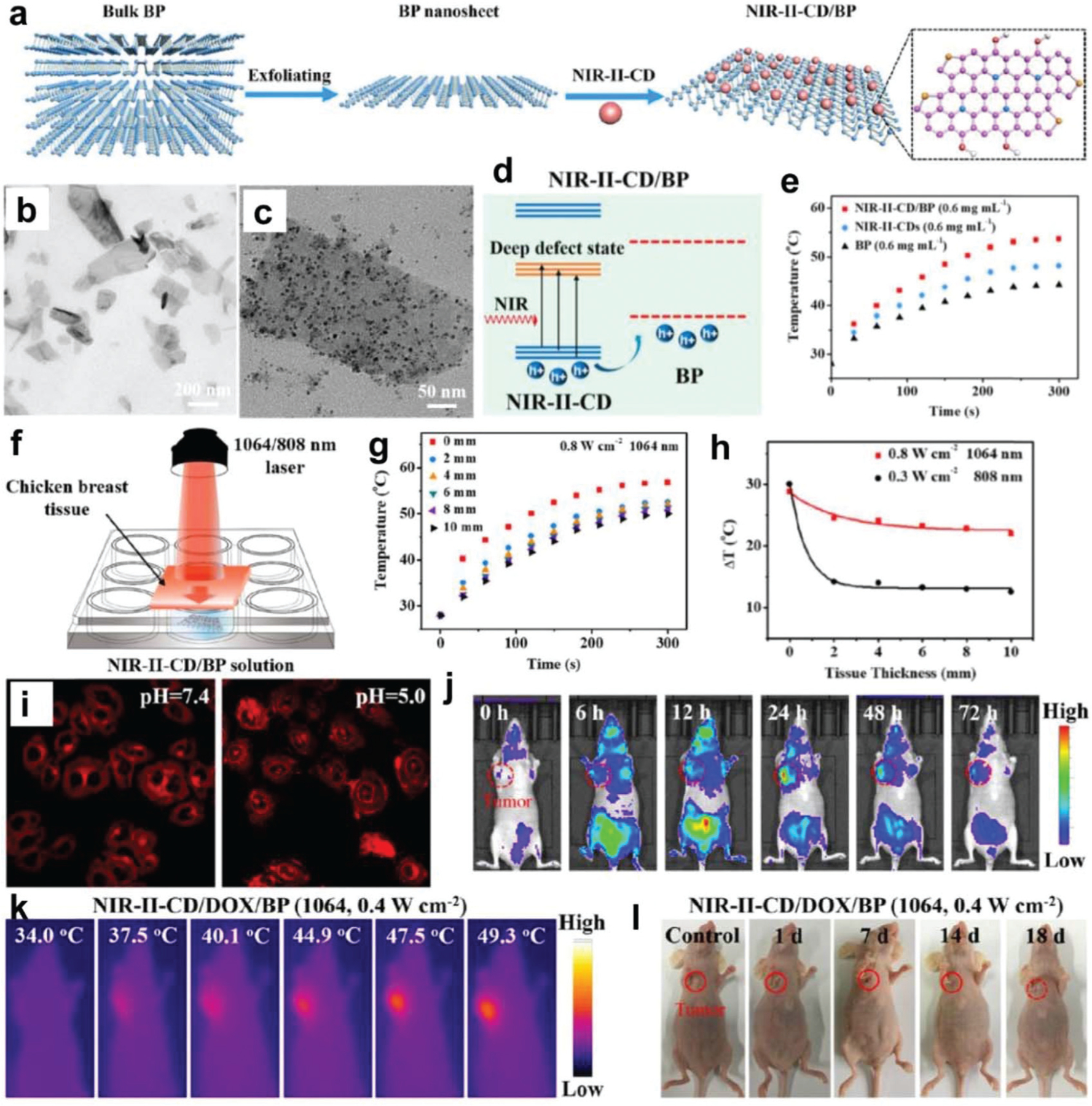
Carbon dot-passivated black phosphorus nanosheet hybrids for synergistic cancer therapy in the NIR-II window. (a) NIR-II-CD-loaded BP nanosheet preparation and morphology. TEM images of (b) pristine BP nanosheets and (c) NIR-II-CD/BP hybrids. (d) Schematic illustration of the NIR-IICD/BP hybrid energy position. (e) NIR-II-CDs, BP, and NIR-II-CD/BP temperature elevation upon 1064 nm laser irradiation at the same concentration. (f) In vitro deep-tissue PTT schematic illustration. (g) The temperature change of NIR-II-CD/BP solution irradiated by a 1064 nm laser. (h) Exponential decay of fitted temperature change of NIR-II-CD/BP hybrids upon 808 and 1064 nm laser irradiation. (i) Confocal pictures of HeLa cells cultured with NIR-II-CD/DOX/BP at pH 5.0 and 7.4. (j) NIR-II-CD/DOX/BP fluorescence image formation in vivo. (k) The PCT impact of NIR-II-CD/DOX/BP at 1064 nm. (l) Photographs of mice after receiving PCT treatment. Reproduced with permission.145 Copyright 2019, American Chemical Society.
The in vitro deep-tissue PTT effect of NIR-II-CD/BP hybrids in the presence of various additional tissue (2–10 mm) thicknesses were evaluated. The NIR-II-CD/BP hybrids covered by a chicken breast tissue were irradiated by an 808 or 1064 nm laser (Fig. 18f). Upon exposure to laser irradiation at 1064 nm, an elevated temperature of NIR-II-CD/BP hybrids could be attained (Fig. 18g). Particularly, at the tissue depth of 10 mm, the temperature of NIR-II-CD/BP hybrids under 1064 nm laser irradiation increased by 22.0 °C, compared to that of 12.5 °C under 808 nm laser irradiation (Fig. 18h). Cancer cells in deeper tumor tissues can be effectively ablated by such a temperature increment of 22.0 °C. The pH-triggered drug release was confirmed by cell imaging. The fluorescence was predominant in the cell cytoplasm in NIR-II-CD/DOX/BP-treated HeLa cells at pH 7.4 (Fig. 18i). Nevertheless, after the pH decreased to 5.0, rapid releasing of DOX can be indicated by the appearance of bright red fluorescence in the cell nucleus.
NIR-II-CD/DOX/BP under irradiation by NIR-II laser was further used to perform the in vivo PCT. The in vivo distribution and the passive targeting ability of NIR-II-CD/DOX/BP were examined from the in vivo fluorescence imaging. A high fluorescence was detected at 6 h post-injection and lasted more than 72 hours, proving that BP nanosheets can be accumulated in the tumor tissue (Fig. 18j). The tumor temperature of the NIR-II-CD/DOX/BP group increased from 34.0 to 49.3 °C within 5 min under 1064 nm laser irradiation, as presented by the infrared thermal imaging (Fig. 18k). Photographs of mice indicated the tumor was becoming smaller and smaller after receiving PCT treatment (Fig. 18l).
Wang et al. aimed to evaluate the efficacy of combined PDT and PTT using GNBPs anchored to the surface of BPNSs, as shown in Fig. 19a.320 Many shuttle-shaped GNBPs with an average length of 80 ± 4 nm covered the surface of the BPNSs (Fig. 19b and c). The GNBPs were uniformly distributed on the surface of the BPNSs. The successful formation of the nanocomposite was confirmed from the elemental mapping images (Fig. 19d). A better capability for PTT in tumor treatment was observed for the BPNS-GNBP nanocomposite upon NIR exposure. The semi-quantitative measurement of radiance showed that BPNS-GNBP achieved around an 8-fold reduction in tumor luminescence compared with PBS treatment groups (Fig. 19e and f). Infrared thermal imaging cameras recorded the infrared thermographic maps and the temperature change curves of the tumors. The tumor temperature of mice injected with the BPNS-GNBP nanocomposite rose by 20 °C rapidly within 200 s of NIR laser irradiation. The highest temperature of 58.8 °C was sufficient for tumor ablation. More than 20% of the Ag accumulated in the lung tumors (Fig. 19g). Images of lung masses harvested after 2 weeks clearly showed that mice treated with the BPNS-GNBP nanocomposite exhibited the poorest tumor invasion and lowest luminescence (Fig. 19h–j).
Fig. 19.
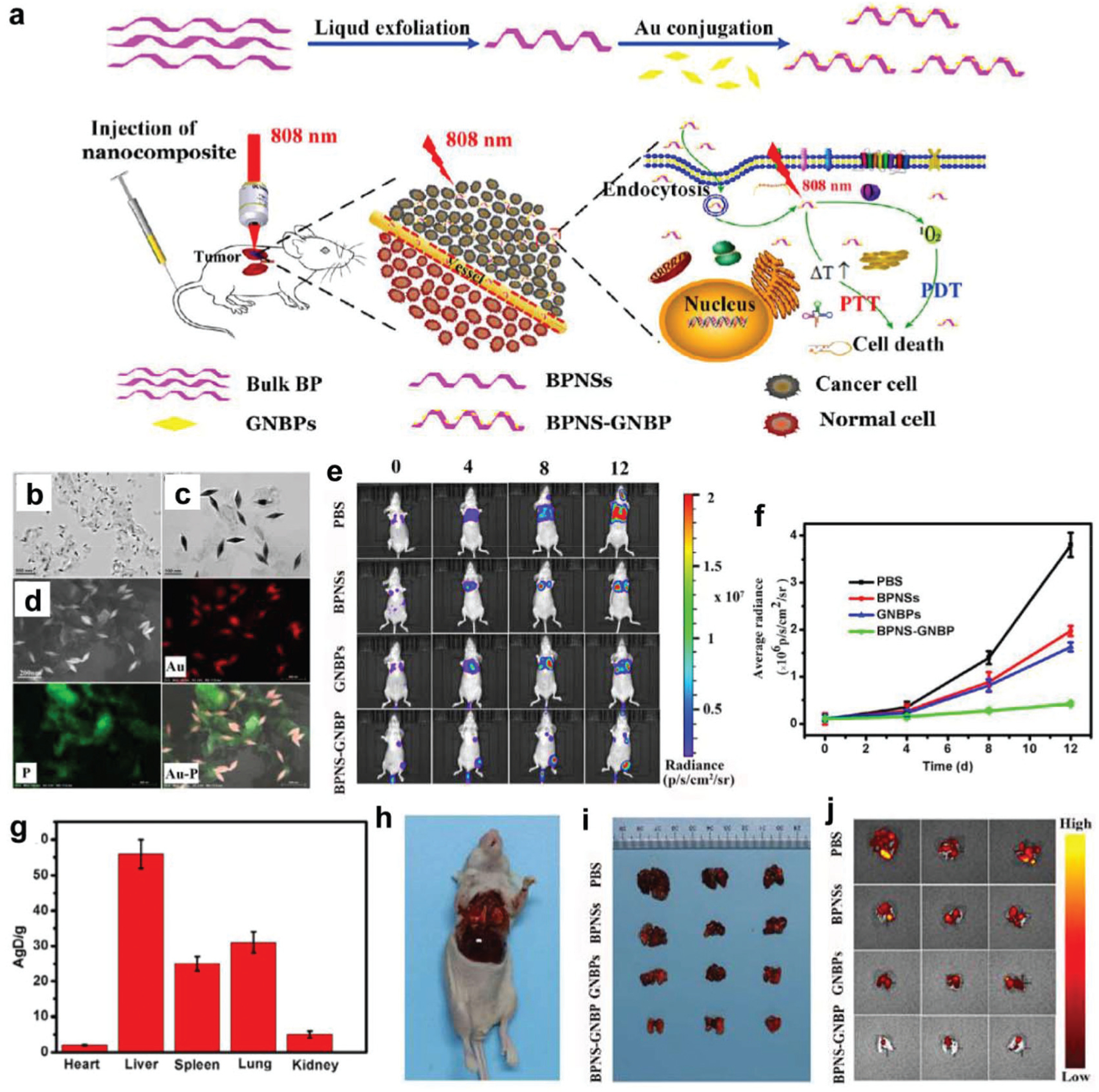
(a) Schematic illustration of the synthesis of the BPNS-GNBP nanocomposite and LSPR-enhanced PDT-PTT. (b) BPNS-GNBP nanocomposite TEM image. (c) BPNS-GNBP nanocomposite zoomed-in TEM image. (d) HAADF-SEM and elemental mapping (Au (red) and P (green)) images of the BPNS-GNBP nanocomposite. Tumor size change in mice determined by (e) luminescence imaging and (f) quantified luminescence levels after treatment with PBS, BPNSs, GNBPs, and the BPNS-GNBP nanocomposite. (g) Ag biodistributions in five main tissues at 12 h post-injection. Images of (h) mice after 2 weeks’ treatment, (i) lung masses removed from mice, and (j) corresponding luminescence levels determined by imaging. Reproduced with permission.320 Copyright 2020, Elsevier.
5. Conclusions and outlook
This review serves to highlight the structural and chemical properties of BP, with particular emphasis on biomedical applications. Composed of phosphorous atoms arranged in a puckered orthorhombic structure, BP is part of a larger class of ‘2D materials’ that exhibit in-plane anisotropy and form layers that are held together via van der Waals interactions.75,321,322 The unique electrochemical and structural properties of BP, e.g., good biocompatibility, mechanical strength, responsiveness to light, the capture of reactive oxygen species (ROS), and release of phosphorus ions during biodegradation, can be utilized to promote tissue regeneration and disease treatment.83,130,131 In this new context, we see BP exhibiting the needed potential to support significant advances in several key areas of biomedical research, including bone regeneration, wound closure, and the treatment of cancer.
The regeneration of bone loss due to osteoporosis, surgical removal, or trauma continues to be a major problem, imposing an estimated yearly economic burden of 3 billion USD within the United States alone.323 One of the major challenges when facilitating bone replacement is the design of novel materials that provide structural support while also supplying a chemical environment that promotes bone cell proliferation.324–336 Three-dimensional (3D) bone scaffolds that incorporate BP have the potential to address these issues. Under physiological conditions, BP-based materials undergo environmental oxidation, leading to the continuous release of phosphate ions. Studies suggest that phosphate can serve as an anionic ligand that aids in the aggregation of calcium ions, promoting osteoblast proliferation, differentiation, and in situ biomineralization.274,337 The biodegradation of BP makes it an attractive candidate for inclusion in future studies that aim to produce tissue scaffolds that support the natural process of bone remodeling and facilitate the integration of surrounding tissues.
Wound closure remains a difficult surgical task, especially in the case of sensitive internal tissues and organs that are prone to infection.295 In this context, the constant release of phosphorus from BP has been shown to speed wound healing, especially when the biodegradation of BP can be controlled through conjugation with stable polymers like silk fibroin.134 The photothermal properties of BP have also led to the development of novel wound closure systems that, when applied in conjunction with NIR irradiation, undergo gelation in situ and prevent bacterial infection.134,338 The application of BP in the development of wound closure systems is an exciting new direction within regenerative medicine that has the potential to address the inherent complications that arise when using surgical interventions in sensitive tissues that do not respond well to sutures, as is often the case in fatal surgery.339
The presence of reactive oxygen species (ROS) within tissues has been linked to the deceleration of wound repair and the inhibition of tissue regeneration.340 Excess ROS can result in an oxidative cytotoxic microenvironment that can lead to DNA damage, the dysregulation of important biological pathways, and the proliferation of cancer cells.341 The use of BP in biomedical applications that address ROS-induced diseases is a growing field due to BP’s ability to scavenge ROS and produce non-toxic by-products. In this regard, notable successes have been achieved in treating acute kidney injury (AKI), where BP nanosheets have been shown to prevent the formation of tubule abnormalities in the kidneys of mice.128 Conversely, BP can also be used to localize high doses of ROS to kill tumor cells during photodynamic therapy.342 These applications show that BP could be a promising component of future methods for the treatment of cancer and ROS-based disease, and modulate the inflammation and wound healing responses.
Acknowledgements
This work was supported by the National Institutes of Health grant R01 AR75037.
Biography

Xifeng Liu
Xifeng Liu received his B.S. (2008) from Hunan University and his Ph.D. (2014) in Materials Science & Engineer from the University of Tennessee, Knoxville. He is currently a research associate in the biomedical engineering and orthopedic surgery departments at Mayo Clinic College of Medicine. His research interests are mainly focused on the development of functionalized biomedical materials for tissue engineering.

Bipin Gaihre
Bipin Gaihre received his B.E. (2010) from Purbanchal University and Ph.D. (2019) from the University of Toledo, Ohio in Biomedical Engineering. He is currently a research fellow in the Department of Physiology and Biomedical Engineering at Mayo Clinic. His current research interests are focused on the development of functional biomaterials and 3-D bioprinting for tissue engineering applications.

Michael J. Yaszemski
Matthew N. George received a B. S. in biology from Gonzaga University in 2010 before going on to complete a Ph.D. in biology from the University of Washington in 2018. He is currently a Research Fellow at Mayo Clinic in the Department of Physiology and Biomedical Engineering researching tissue engineering strategies for the regeneration of bone and nerve. His interest is in the research and development of bio-inspired materials for use in biomedical applications.

Michael J. Yaszemski
Michael J. Yaszemski received both Bachelor’s (1977) and Master’s (1978) degrees in Chemical Engineering from Lehigh University, an M.D. from Georgetown University in 1983, and a Ph.D. in Chemical Engineering from Massachusetts Institute of Technology in 1995. He is the Krehbiel Family Endowed Professor of Orthopedic Surgery and Biomedical Engineering at the Mayo Clinic and director of its Tissue Engineering and Sarcoma Biology Laboratory. His research interests are in the synthesis and characterization of novel degradable polymers for use in bone regeneration, cartilage regeneration, nervous tissue regeneration, and controlled delivery of chemotherapeutic agents to musculoskeletal tumors.

Lichun Lu
Lichun Lu received her B.E. from Tsinghua University in 1994 and Ph.D. from Rice University, Houston, Texas in 1999, both in Chemical Engineering. She is currently a Professor of Biomedical Engineering and Orthopedics at Mayo Clinic and Director of the Biomaterials and Regenerative Medicine Laboratory. Her laboratory is focused on developing next-generation regenerative medicine therapies for clinical application, including finite element analysis models based on quantitative computed tomography scans for noninvasive spine fracture risk prediction, novel injectable and preformed composite scaffolds for large bone defect repair, and electrically conductive nerve guidance tubes for peripheral nerve regeneration and spinal cord injury.
Footnotes
Conflicts of interest
There are no conflicts to declare.
References
- 1.Li L, Yu Y, Ye GJ, Ge Q, Ou X, Wu H, Feng D, Chen H and Zhang Y, Nat. Nanotechnol, 2014, 9, 372–377.. [DOI] [PubMed] [Google Scholar]
- 2.Rodin A, Carvalho A and Neto AC, Phys. Rev. Lett, 2014, 112, 176801. [DOI] [PubMed] [Google Scholar]
- 3.Tran V, Soklaski R, Liang Y and Yang L, Phys. Rev. B: Condens. Matter Mater. Phys, 2014, 89, 235319. [Google Scholar]
- 4.Sakthivel T, Huang XY, Wu YC and Rtimi S, Chem. Eng. J, 2020, 379, 122297. [Google Scholar]
- 5.Xu YJ, Shi Z, Shi XY, Zhang K and Zhang H, Nanoscale, 2019, 11, 14491–14527. [DOI] [PubMed] [Google Scholar]
- 6.Zeng QS, Sun B, Du KZ, Zhao WY, Yu P, Zhu C, Xia J, Chen Y, Cao X, Yan QY, Shen ZX, Yu T, Long Y, Koh YK and Liu Z, 2D Mater, 2019, 6, 045009. [Google Scholar]
- 7.Ou PF, Song PF, Liu XY and Song J, Adv. Theory Simul, 2019, 2, 1800103. [Google Scholar]
- 8.Han FW, Zhao CX and Zhang YM, AIP Adv, 2019, 9, 055216. [Google Scholar]
- 9.Zhang ZQ, Liu HL, Liu Z, Zhang Z, Cheng GG, Wang XD and Ding JN, Appl. Surf. Sci, 2019, 475, 857–862. [Google Scholar]
- 10.Xing C, Zhang JH, Jing JY, Li JZ and Shi F, Chem. Eng. J, 2019, 370, 120–135. [Google Scholar]
- 11.Guo R, Zheng Y, Hu ZH, Zhang JL, Han C, Longhi E, Barlow S, Marder SR and Chen W, Chem. – Eur. J, 2020, 26(29), 6576–6582. [DOI] [PubMed] [Google Scholar]
- 12.Dai XY, Zhang LS, Wang ZC, Li J and Li H, Comput. Mater. Sci, 2019, 156, 292–300. [Google Scholar]
- 13.Jia CH, Zhao L, Cui MS, Yang F, Cheng G, Yang GH and Zeng ZP, J. Alloys Compd, 2019, 799, 99–107. [Google Scholar]
- 14.Penillard A, Beccacce L, Billot L, de Rossi A, Combrie S, Hole S and Geron E, J. Appl. Phys, 2019, 125(24), 243107. [Google Scholar]
- 15.Zhang LL, Vasenko AS, Zhao J and Prezhdo OV, J. Phys. Chem. Lett, 2019, 10, 1083–1091. [DOI] [PubMed] [Google Scholar]
- 16.Xu YF, Yu JY, Dong YH, You T and Hu XG, J. Tribolo.-Trans. ASME, 2019, 141, 072101. [Google Scholar]
- 17.Mu X, Wang J and Sun M, Mater. Today Phys, 2019, 8, 92–111. [Google Scholar]
- 18.Chen X, Ponraj JS, Fan DY and Zhang H, Nanoscale, 2020, 12, 3513–3534. [DOI] [PubMed] [Google Scholar]
- 19.Chen ZY and Qin R, Nanoscale, 2019, 11, 16377–16383. [DOI] [PubMed] [Google Scholar]
- 20.Liu YL, Zhou M, Zhang WC, Chen KQ, Mei AH, Zhang YY and Chen W, Nanoscale, 2019, 11, 5674–5683. [DOI] [PubMed] [Google Scholar]
- 21.Pradhan NR, Garcia C, Lucking MC, Pakhira S, Martinez J, Rosenmann D, Divan R, Sumant AV, Terrones H, Mendoza-Cortes JL, McGill SA, Zhigadlo ND and Balicas L, Nanoscale, 2019, 11, 18449–18463. [DOI] [PubMed] [Google Scholar]
- 22.Sun CC, Wang YX, Jiang YJ, Yang ZD, Zhang GL and Hu YY, New J. Chem, 2019, 43, 377–385. [Google Scholar]
- 23.Liu YY, Qi WH, Gong S, He J, Li Z and Li YJ, Phys. B, 2020, 579, 411903. [Google Scholar]
- 24.Wang JX, Wang Y, Liu GL, Wei L and Zhang GY, Phys. B, 2020, 578, 411755. [Google Scholar]
- 25.Chen QY, Liu MY, Cao C and He Y, Phys. E, 2020, 116, 113791. [Google Scholar]
- 26.Le PTT and Yarmohammadi M, Phys. E, 2019, 107, 11–17. [Google Scholar]
- 27.Khandelwal A, Mani K, Karigerasi MH and Lahiri I, Mater. Sci. Eng., B, 2017, 221, 17–34. [Google Scholar]
- 28.Pumera M, TrAC, Trends Anal. Chem, 2017, 93, 1–6. [Google Scholar]
- 29.Wang LJ, Hu YY, Qi F, Ding L, Wang JM, Zhang XY, Liu QW, Liu LZ, Sun HZ and Qu P, ACS Appl. Mater. Interfaces, 2020, 12, 8157–8167. [DOI] [PubMed] [Google Scholar]
- 30.Yang XY, Wang DY, Shi YH, Zou JH, Zhao QS, Zhang Q, Huang W, Shao JJ, Xie XJ and Dong XC, ACS Appl. Mater. Interfaces, 2019, 11, 43797–43797. [DOI] [PubMed] [Google Scholar]
- 31.Kumar A, ACS Appl. Nano Mater, 2019, 2, 2397–2404. [Google Scholar]
- 32.Li ZB, Wu L, Wang HY, Zhou WH, Liu HX, Cui HD, Li PH, Chu PK and Yu XF, ACS Appl. Nano Mater, 2019, 2, 1202–1209. [Google Scholar]
- 33.Li YJ, Pei W, He JT, Liu K, Qi WH, Gao XH, Zhou S, Xie HP, Yin K, Gao YL, He J, Zhao JJ, Hu JH, Chan TS, Li Z, Zhang GF and Liu M, ACS Catal, 2019, 9, 10870–10875. [Google Scholar]
- 34.Yuan YJ, Shen ZK, Song SX, Guan J, Bao L, Pei L, Su YB, Wu ST, Bai WF, Yu ZT, Ji ZG and Zou ZG, ACS Catal, 2019, 9, 7801–7807. [Google Scholar]
- 35.Zhang QZ, Huang SY, Deng JJ, Gangadharan DT, Yang F, Xu ZH, Giorgi G, Palummo M, Chaker M and Ma DL, Adv. Funct. Mater, 2019, 29(28), 1902486. [Google Scholar]
- 36.Ding HC, Tang ZR, Zhang L and Dong YP, Analyst, 2019, 144, 1326–1333. [DOI] [PubMed] [Google Scholar]
- 37.Hu JD, Chen DY, Mo Z, Li NJ, Xu QF, Li H, He JH, Xu H and Lu JM, Angew. Chem., Int. Ed, 2019, 58, 2073–2077. [DOI] [PubMed] [Google Scholar]
- 38.Liu YJ, Gao PF, Zhang TM, Zhu XJ, Zhang MM, Chen MQ, Du PW, Wang GW, Ji HX, Yang JL and Yang SF, Angew. Chem., Int. Ed, 2019, 58, 1479–1483. [DOI] [PubMed] [Google Scholar]
- 39.Yuan YH, Niu BY, Yu QH, Guo X, Guo ZH, Wen J, Liu T, Zhang HQ and Wang N, Angew. Chem, 2020, 59, 1220–1227. [DOI] [PubMed] [Google Scholar]
- 40.Zhang LL, Ding LX, Chen GF, Yang XF and Wang HH, Angew. Chem., Int. Ed, 2019, 58, 2612–2616. [DOI] [PubMed] [Google Scholar]
- 41.Li ST, Wang PF, Wang RD, Liu YF, Jing RS, Li Z, Meng ZL, Liu YY and Zhang Q, Appl. Surf. Sci, 2019, 497, 143682. [Google Scholar]
- 42.Miao YL, Shi XT, Li QT, Hao LJ, Liu L, Liu X, Chen YH and Wang YJ, Biomater. Sci, 2019, 7, 4046–4059. [DOI] [PubMed] [Google Scholar]
- 43.Xu YM, Wang X, Jin ML, Kempa K and Shui LL, ChemElectroChem, 2020, 7, 96–104. [Google Scholar]
- 44.Guo T, Lin Y, Jin GX, Weng RG, Song JB, Liu XZ, Huang GM, Hou L and Yang HH, Chem. Commun, 2019, 55, 850–853. [DOI] [PubMed] [Google Scholar]
- 45.Liu G, Tsai HI, Zeng XW, Qi JY, Luo MM, Wang XS, Mei L and Deng WB, Chem. Eng. J, 2019, 375, 121917. [Google Scholar]
- 46.Wu F, Zhang M, Chu XH, Zhang QC, Su YT, Sun BH, Lu TY, Zhou NL, Zhang J, Wang JX and Yi XY, Chem. Eng. J, 2019, 370, 387–399. [Google Scholar]
- 47.Yang XY, Wang DY, Zhu JW, Xue L, Ou CJ, Wang WJ, Lu M, Song XJ and Dong XC, Chem. Sci, 2019, 10, 3779–3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu WX, Zhang YN, Zhang YL and Dong A, Chem. – Eur. J, 2020, 26, 2478–2485.31756008 [Google Scholar]
- 49.Zhang F, Peng FF, Qin L, Yang DD, Li RR, Jiang SS, He HY and Zhang P, Colloids Surf., B, 2019, 180, 353–361. [DOI] [PubMed] [Google Scholar]
- 50.Li YJ, Liao CG, Tang KW, Zhang N, Qi WH, Xie HP, He J, Yin K, Gao YL and Wang CD, Electrochim. Acta, 2019, 297, 40–45. [Google Scholar]
- 51.Mei J, Zhang YW, Liao T, Peng XM, Ayoko GA and Sun ZQ, Energy Storage Mater, 2019, 19, 424–431. [Google Scholar]
- 52.Li P, Zeng L, Gao J, Yao LL, Zhao XC, Wu Q, Liu XL, Wang YY, Yang XX, Shi JB, Hu LG, Qu GB, Cai ZW and Jiang GB, Environ. Sci. Technol. Lett, 2020, 7, 35–41. [Google Scholar]
- 53.Xu ZY, Hu L, Yuan J, Zhang YY, Guo YJ, Jin ZY, Long FC, Long YJ, Liang HW, Ruan SC and Zeng YJ, Adv. Mater. Interfaces, 2020, 7, 1902075. [Google Scholar]
- 54.Liu HJ, Su YY, Deng DY, Song HJ and Lv Y, Anal. Chem, 2019, 91, 9174–9180. [DOI] [PubMed] [Google Scholar]
- 55.Liu YT, Li D, Yu JY and Ding B, Angew. Chem., Int. Ed, 2019, 58, 16439–16444. [DOI] [PubMed] [Google Scholar]
- 56.Li ZJ, Xu H, Shao JD, Jiang C, Zhang F, Lin J, Zhang H, Li JQ and Huang P, Appl. Mater. Today, 2019, 15, 297–304. [Google Scholar]
- 57.Shi R, Liu FL, Wang Z, Weng YX and Chen Y, Chem. Commun, 2019, 55, 12531–12534. [DOI] [PubMed] [Google Scholar]
- 58.Cheng H, Xie J, Cao GS, Lu YH, Zheng D, Jin Y, Wang KY and Zhao XB, Energy Storage Mater, 2019, 23, 684–692. [Google Scholar]
- 59.Batmunkh M, Myekhlai M, Bati ASR, Sahlos S, Slattery D, Benedetti T, Goncales VR, Gibson CT, Gooding JJ, Tilley RD and Shapter JG, J. Mater. Chem. A, 2019, 7, 12974–12978. [Google Scholar]
- 60.Guo WL, Zhu LX, Song HZ, Wang J and Yan SC, J. Nanosci. Nanotechnol, 2019, 19, 5762–5768. [DOI] [PubMed] [Google Scholar]
- 61.Yuan HW, Zhao YY, Wang YD, Duan JL, He BL and Tang QW, J. Power Sources, 2019, 410, 53–58. [Google Scholar]
- 62.Zhang D, Lin ZG, Lan SY, Sun HY, Zeng YY and Liu XL, Mater. Chem. Front, 2019, 3, 2775–2775. [Google Scholar]
- 63.Yue QL, Hu YY, Tao LX, Zhang BQ, Liu C, Wang YP, Chen CY, Zhao JS and Li CZ, Microchim. Acta, 2019, 186(9), 1–9. [DOI] [PubMed] [Google Scholar]
- 64.Pan XY, Shang CQ, Chen ZH, Jin ML, Zhang YG, Zhang Z, Wang X and Zhou GF, Nanomaterials, 2019, 9(9), 1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cao YM, Zhang B, Tian XY, Gu MC and Chen Y, Nanoscale, 2019, 11, 3527–3533. [DOI] [PubMed] [Google Scholar]
- 66.Shi XT, Tong ZR, Zhang WH, Qin J, Pan HG and Sun C, Opt. Eng, 2019, 58(10), 106103. [Google Scholar]
- 67.Luo MM, Cheng W, Zeng XW, Mei L, Liu G and Deng WB, Pharmaceutics, 2019, 11(5), 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fang DD, Zhao DD, Zhang SP, Huang YT, Dai H and Lin YY, Sens. Actuators, B, 2020, 305, 127544. [Google Scholar]
- 69.Yang WT, Chen JH, Lian XM, Li J, Yao FF, Wu G, Qiu WM, Jin CH, Heremans P and Chen HZ, Solar RRL, 2019, 3(8), 1900132. [Google Scholar]
- 70.Liu H, Zhang Y, Dong YP and Chu XF, Talanta, 2019, 199, 507–512. [DOI] [PubMed] [Google Scholar]
- 71.Watts MC, Picco L, Russell-Pavier FS, Cullen PL, Miller TS, Bartus SP, Payton OD, Skipper NT, Tileli V and Howard CA, Nature, 2019, 568, 216–220. [DOI] [PubMed] [Google Scholar]
- 72.Late DJ, Microporous Mesoporous Mater, 2016, 225, 494–503. [Google Scholar]
- 73.Zhao W, Xue Z, Wang J, Jiang J, Zhao X and Mu T, ACS Appl. Mater. Interfaces, 2015, 7, 27608–27612. [DOI] [PubMed] [Google Scholar]
- 74.Xu J-Y, Gao L-F, Hu C-X, Zhu Z-Y, Zhao M, Wang Q and Zhang H-L, Chem. Commun, 2016, 52, 8107–8110. [DOI] [PubMed] [Google Scholar]
- 75.Dhanabalan SC, Ponraj JS, Guo Z, Li S, Bao Q and Zhang H, Adv. Sci, 2017, 4, 1600305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kumar A, Telesio F, Forti S, Al-Temimy A, Coletti C, Serrano-Ruiz M, Caporali M, Peruzzini M, Beltram F and Heun S, 2D Mater, 2019, 6, 015005. [Google Scholar]
- 77.Luo F, Wang DY, Zhang JD, Li XN, Liu DM, Li H, Lu M, Xie XJ, Huang L and Huang W, ACS Appl. Nano Mater, 2019, 2, 3793–3801. [Google Scholar]
- 78.Zhu MS, Fujitsuka M, Zeng LX, Liu MH and Majima T, Appl. Catal., B, 2019, 256, 117864. [Google Scholar]
- 79.Castillo AED, Reyes-Vazquez CD, Rojas-Martinez LE, Thorat SB, Serri M, Martinez-Hernandez AL, Velasco-Santos C, Pellegrini V and Bonaccorso F, FlatChem, 2019, 18, 100131. [Google Scholar]
- 80.Baboukani AR, Khakpour I, Drozd V, Allagui A and Wang CL, J. Mater. Chem. A, 2019, 7, 25548–25556. [Google Scholar]
- 81.Chang YK, Nie AM, Yuan SJ, Wang BC, Mu CP, Xiang JY, Yang BC, Li L, Wen FS and Liu ZY, Nanotechnology, 2019, 30, 035701. [DOI] [PubMed] [Google Scholar]
- 82.Su SP, Xu BY, Ding JH and Yu HB, New J. Chem, 2019, 43, 19365–19371. [Google Scholar]
- 83.Luo M, Fan T, Zhou Y, Zhang H and Mei L, Adv. Funct. Mater, 2019, 29, 1808306. [Google Scholar]
- 84.Yang B, Yin J, Chen Y, Pan S, Yao H, Gao Y and Shi J, Adv. Mater, 2018, 30, 1705611. [DOI] [PubMed] [Google Scholar]
- 85.Qiao J, Kong X, Hu Z-X, Yang F and Ji W, Nat. Commun, 2014, 5, 4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xia F, Wang H and Jia Y, Nat. Commun, 2014, 5, 4458. [DOI] [PubMed] [Google Scholar]
- 87.Ling X, Wang H, Huang S, Xia F and Dresselhaus MS, Proc. Natl. Acad. Sci. U. S. A, 2015, 201416581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang X, Jones AM, Seyler KL, Tran V, Jia Y, Zhao H, Wang H, Yang L, Xu X and Xia F, Nat. Nanotechnol, 2015, 10, 517. [DOI] [PubMed] [Google Scholar]
- 89.Yi Y, Yu X-F, Zhou W, Wang J and Chu PK, Mater. Sci. Eng., R, 2017, 120, 1–33. [Google Scholar]
- 90.Fang T, Liu TR, Jiang ZN, Yang R, Servati P and Xia GR, ACS Appl. Nano Mater, 2019, 2, 3138–3145. [Google Scholar]
- 91.Huang L, Dong BW, Guo X, Chang YH, Chen N, Huang X, Liao WG, Zhu CX, Wang H, Lee C and Ang KW, ACS Nano, 2019, 13, 913–921. [DOI] [PubMed] [Google Scholar]
- 92.Guo Z, Ding WD, Liu XW, Sun ZJ and Wei L, Appl. Mater. Today, 2019, 14, 51–58. [Google Scholar]
- 93.Wang Z, Liu ZM, Su CK, Yang BW, Fei XX, Li Y, Hou YQ, Zhao HN, Guo YX, Zhuang ZF, Zhong HQ and Guo ZY, Curr. Med. Chem, 2019, 26, 1788–1805. [DOI] [PubMed] [Google Scholar]
- 94.He LD, Lian PC, Zhu YZ, Lu QJ, Wang C and Mei Y, J. Nanosci. Nanotechnol, 2019, 19, 5361–5374. [DOI] [PubMed] [Google Scholar]
- 95.Kim J and Park B, Mater. Lett, 2019, 254, 367–370. [Google Scholar]
- 96.Anju S, Ashtami J and Mohanan PV, Mater. Sci. Eng., C, 2019, 97, 978–993. [DOI] [PubMed] [Google Scholar]
- 97.Lin SH, Li YY, Qian JS and Lau SP, Mater. Today Energy, 2019, 12, 1–25. [Google Scholar]
- 98.Li BS, Lai C, Zeng GM, Huang DL, Qin L, Zhang MM, Cheng M, Liu XG, Yi H, Zhou CY, Huang FL, Liu SY and Fu YK, Small, 2019, 15(8), 1804565. [DOI] [PubMed] [Google Scholar]
- 99.Yi Y, Sun ZB, Li J, Chu PK and Yu XF, Small Methods, 2019, 3, 1900165. [Google Scholar]
- 100.Latiff NM, Teo WZ, Sofer Z, Fisher AC and Pumera M, Chem. – Eur. J, 2015, 21, 13991–13995. [DOI] [PubMed] [Google Scholar]
- 101.Lee HU, Park SY, Lee SC, Choi S, Seo S, Kim H, Won J, Choi K, Kang KS, Park HG, Kim H-S, An HR, Jeong K-H, Lee Y-C and Lee J, Small, 2016, 12, 214–219. [DOI] [PubMed] [Google Scholar]
- 102.Choi JR, Yong KW, Choi JY, Nilghaz A, Lin Y, Xu J and Lu X, Theranostics, 2018, 8, 1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Qin L, Jiang SS, He HY, Ling GX and Zhang P, J. Controlled Release, 2020, 318, 50–66. [DOI] [PubMed] [Google Scholar]
- 104.Childers DL, Corman J, Edwards M and Elser JJ, Bioscience, 2011, 61, 117–124. [Google Scholar]
- 105.Shao J, Xie H, Huang H, Li Z, Sun Z, Xu Y, Xiao Q, Yu X-F, Zhao Y, Zhang H, Wang H and Chu PK, Nat. Commun, 2016, 7, 12967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wood JD, Wells SA, Jariwala D, Chen K-S, Cho E, Sangwan VK, Liu X, Lauhon LJ, Marks TJ and Hersam MC, Nano Lett, 2014, 14, 6964–6970. [DOI] [PubMed] [Google Scholar]
- 107.Munro S, Cell, 2003, 115, 377–388. [DOI] [PubMed] [Google Scholar]
- 108.Huo S, Li H, Boersma AJ and Herrmann A, Adv. Sci, 2019, 6, 1900043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.George MN, Liu X, Miller AL, Xu H and Lu L, J. Biomed. Mater. Res., Part A, 2020, 108, 515–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Olthof MG, Kempen DH, Liu X, Dadsetan M, Tryfonidou MA, Yaszemski MJ, Dhert WJ and Lu L, J. Tissue Eng. Regener. Med, 2018, 12, 1339–1351. [DOI] [PubMed] [Google Scholar]
- 111.Wang X, Shao J, El Raouf MA, Xie H, Huang H, Wang H, Chu PK, Yu X-F, Yang Y and AbdEl-Aal AM, Biomaterials, 2018, 179, 164–174. [DOI] [PubMed] [Google Scholar]
- 112.Olthof MG, Tryfonidou MA, Liu X, Pouran B, Meij BP, Dhert WJ, Yaszemski MJ, Lu L, Alblas J and Kempen DH, Tissue Eng., Part A, 2018, 24, 819–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xu YF, Ren F, Liu HH, Zhang H, Han YB, Liu Z, Wang WL, Sun Q, Zhao CJ and Li Z, ACS Appl. Mater. Interfaces, 2019, 11, 21399–21407. [DOI] [PubMed] [Google Scholar]
- 114.Zhou WH, Pan T, Cui HD, Zhao Z, Chu PK and Yu XF, Angew. Chem., Int. Ed, 2019, 58, 769–774. [DOI] [PubMed] [Google Scholar]
- 115.Zhou L, Liu C, Sun ZB, Mao HJ, Zhang L, Yu XF, Zhao JL and Chen XF, Biosens. Bioelectron, 2019, 137, 140–147. [DOI] [PubMed] [Google Scholar]
- 116.Zhen ZG, Tang YH, Tao JW and Yun Z, ChemElectroChem, 2019, 6, 1129–1133. [Google Scholar]
- 117.Luo MM, Zhou Y, Gao NS, Cheng W, Wang XS, Cao JX, Zeng XW, Liu G and Mei L, Chem. Eng. J, 2020, 385, 123942. [Google Scholar]
- 118.Pal S, Verma A, Saini JP and Prajapati YK, IET Optoelectron, 2019, 13, 196–201. [Google Scholar]
- 119.Ji XY, Kang Y, Fan TJ, Xiong QQ, Zhang SP, Tao W and Zhang H, J. Mater. Chem. A, 2020, 8, 323–333. [Google Scholar]
- 120.Jia GY, Huang ZX, Zhang YL, Hao ZQ and Tian YL, J. Mater. Chem. C, 2019, 7, 3843–3851. [Google Scholar]
- 121.Qian Y, Yuan WE, Cheng Y, Yang YQ, Qu XH and Fan CY, Nano Lett, 2019, 19, 8990–9001. [DOI] [PubMed] [Google Scholar]
- 122.Qiu M, Singh A, Wang D, Qu JL, Swihart M, Zhang H and Prasad PN, Nano Today, 2019, 25, 135–155. [Google Scholar]
- 123.Cai Y and Tang R, J. Mater. Chem, 2008, 18, 3775–3787. [Google Scholar]
- 124.Chen W, Ouyang J, Yi X, Xu Y, Niu C, Zhang W, Wang L, Sheng J, Deng L, Liu YN and Guo S, Adv. Mater, 2018, 30, 1703458. [DOI] [PubMed] [Google Scholar]
- 125.Hu K, Xie L, Zhang Y, Hanyu M, Yang Z, Nagatsu K, Suzuki H, Ouyang J, Ji X and Wei J, Nat. Commun, 2020, 11, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sureshbabu A, Ryter SW and Choi ME, Redox Biol, 2015, 4, 208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Halliwell B, Am. J. Med, 1991, 91, S14–S22. [Google Scholar]
- 128.Hou J, Wang H, Ge Z, Zuo T, Chen Q, Liu X, Mou S, Fan C, Xie Y and Wang L, Nano Lett, 2020, 20, 1447–1454. [DOI] [PubMed] [Google Scholar]
- 129.Ge X, Xia Z and Guo S, Adv. Funct. Mater, 2019, 29, 1900318. [Google Scholar]
- 130.Qu G, Xia T, Zhou W, Zhang X, Zhang H, Hu L, Shi J, Yu X-F and Jiang G, Chem. Rev, 2020, 2288–2346. [DOI] [PubMed] [Google Scholar]
- 131.Thurakkal S and Zhang XY, Adv. Sci, 2020, 7, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tao W, Zhu X, Yu X, Zeng X, Xiao Q, Zhang X, Ji X, Wang X, Shi J and Zhang H, Adv. Mater, 2017, 29, 1603276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chen W, Ouyang J, Liu H, Chen M, Zeng K, Sheng J, Liu Z, Han Y, Wang L, Li J, Deng L, Liu Y-N and Guo S, Adv. Mater, 2017, 29(5), 1603864. [DOI] [PubMed] [Google Scholar]
- 134.Huang X-W, Wei J-J, Zhang M-Y, Zhang X-L, Yin X-F, Lu C-H, Song J-B, Bai S-M and Yang H-H, ACS Appl. Mater. Interfaces, 2018, 10, 35495–35502. [DOI] [PubMed] [Google Scholar]
- 135.Nagao M, Hayashi A and Tatsumisago M, J. Power Sources, 2011, 196, 6902–6905. [Google Scholar]
- 136.Sun J, Zheng G, Lee H-W, Liu N, Wang H, Yao H, Yang W and Cui Y, Nano Lett, 2014, 14, 4573–4580. [DOI] [PubMed] [Google Scholar]
- 137.Sun L-Q, Li M-J, Sun K, Yu S-H, Wang R-S and Xie H-M, J. Phys. Chem. C, 2012, 116, 14772–14779. [Google Scholar]
- 138.Li D, Jussila H, Karvonen L, Ye G, Lipsanen H, Chen X and Sun Z, Sci. Rep, 2015, 5, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sofer Z, Bousa D, Luxa J, Mazanek V and Pumera M, Chem. Commun, 2016, 52, 1563–1566. [DOI] [PubMed] [Google Scholar]
- 140.Wang L, Sofer Z and Pumera M, ChemElectroChem, 2015, 2, 324–327. [Google Scholar]
- 141.Wang H, Yang X, Shao W, Chen S, Xie J, Zhang X, Wang J and Xie Y, J. Am. Chem. Soc, 2015, 137, 11376–11382. [DOI] [PubMed] [Google Scholar]
- 142.Sreshtt V, Padua AAH and Blankschtein D, ACS Nano, 2015, 9, 8255–8268. [DOI] [PubMed] [Google Scholar]
- 143.Ding XW, Hong C, Zhang GL, Liu JQ, Ouyang H, Wang MY, Dong LN, Zhang W, Xin HB and Wang XL, Nanoscale Horiz, 2019, 4, 1277–1285. [Google Scholar]
- 144.Gao NS, Xing CY, Wang HF, Feng LW, Zeng XW, Mei L and Peng ZC, Front. Pharmacol, 2019, 10, 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Geng BJ, Shen WW, Li P, Fang FL, Qin H, Li XK, Pan DY and Shen LX, ACS Appl. Mater. Interfaces, 2019, 11, 44949–44960. [DOI] [PubMed] [Google Scholar]
- 146.Hu ZZ, Li YX, Hussain E, Huang XN, Zhang YY, Niu N, Shahzad SA and Yu C, Talanta, 2019, 197, 270–276. [DOI] [PubMed] [Google Scholar]
- 147.Nakhanivej P, Yu X, Park SK, Kim S, Hong JY, Kim HJ, Lee W, Hwang JY, Yang JE, Wolverton C, Kong J, Chhowalla M and Park HS, Nat. Mater, 2019, 18(2), 156–162. [DOI] [PubMed] [Google Scholar]
- 148.Ouyang H, Zheng ZL, Chen YT, Liu Y, Hong C, Zhu YL, Deng JJ, Ding XW, Zhou WM and Wang XL, J. Mater. Chem. B, 2019, 7, 6099–6108. [DOI] [PubMed] [Google Scholar]
- 149.Qin L, Ling GX, Peng FF, Zhang F, Jiang SS, He HY, Yang DD and Zhang P, J. Colloid Interface Sci, 2019, 556, 232–238. [DOI] [PubMed] [Google Scholar]
- 150.Wang KX, Dong NN, Liu ZW, Shi MK, Zhang B, Wang J and Chen Y, Polym. Chem, 2019, 10, 6003–6009. [Google Scholar]
- 151.Wang X, Xiang YR, Zhou BQ, Zhang YM, Wu JT, Hu R, Liu LW, Song J and Qu JL, J. Colloid Interface Sci, 2019, 534, 1–11. [DOI] [PubMed] [Google Scholar]
- 152.Xu JQ, Qiao XJ, Wang Y, Sheng QL, Yue TL, Zheng JB and Zhou M, Microchim. Acta, 2019, 186, 238. [DOI] [PubMed] [Google Scholar]
- 153.Xue Y, Min SX and Wang F, Int. J. Hydrogen Energy, 2019, 44, 21873–21881. [Google Scholar]
- 154.Cai W, Hu YX, Pan Y, Zhou X, Chu FK, Han LF, Mu XW, Zhuang ZY, Wang X and Xing WY, J. Colloid Interface Sci, 2020, 561, 32–45. [DOI] [PubMed] [Google Scholar]
- 155.Maity A, Sui XY, Pu HH, Bottum KJ, Jin B, Chang JB, Zhou GH, Lu GH and Chen JH, Nanoscale, 2020, 12, 1500–1512. [DOI] [PubMed] [Google Scholar]
- 156.Mo JB, Xu Y, Wang XX, Wei W and Zhao J, Nanoscale, 2020, 12, 1742–1748. [DOI] [PubMed] [Google Scholar]
- 157.Sun YR, Li XP, Fan SH, Li C, Shang ZF, Gu MM, Liang SF and Tian X, J. Nanosci. Nanotechnol, 2020, 20, 659–667. [DOI] [PubMed] [Google Scholar]
- 158.Xu YM, Wang X, Jin ML, Zhou GF and Shui LL, J. Mater. Sci, 2020, 55, 5499–5509. [Google Scholar]
- 159.Zhang HM, Han QQ, Yin XL and Wang YQ, Spectrochim. Acta, Part A, 2020, 227, 117662. [DOI] [PubMed] [Google Scholar]
- 160.Zhang Q, Zhang JH, Zhang L, Cao MT, Yang FL and Dai WL, Appl. Surf. Sci, 2020, 504, 144366. [Google Scholar]
- 161.Zou B, Qiu SL, Ren XY, Zhou YF, Zhou F, Xu ZM, Zhao ZX, Song L, Hu Y and Gong XL, J. Hazard. Mater, 2020, 383, 121039. [DOI] [PubMed] [Google Scholar]
- 162.Brent JR, Savjani N, Lewis EA, Haigh SJ, Lewis DJ and O’Brien P, Chem. Commun, 2014, 50, 13338–13341. [DOI] [PubMed] [Google Scholar]
- 163.Guo Z, Zhang H, Lu S, Wang Z, Tang S, Shao J, Sun Z, Xie H, Wang H, Yu X-F and Chu PK, Adv. Funct. Mater, 2015, 25, 6996–7002. [Google Scholar]
- 164.Ma J, Lu S, Guo Z, Xu X, Zhang H, Tang D and Fan D, Opt. Express, 2015, 23, 22643–22648. [DOI] [PubMed] [Google Scholar]
- 165.Luo Z-C, Liu M, Guo Z-N, Jiang X-F, Luo A-P, Zhao C-J, Yu X-F, Xu W-C and Zhang H, Opt. Express, 2015, 23, 20030–20039. [DOI] [PubMed] [Google Scholar]
- 166.Zheng X, Chen R, Shi G, Zhang J, Xu Z, Cheng X. a. and Jiang T, Opt. Lett, 2015, 40, 3480–3483. [DOI] [PubMed] [Google Scholar]
- 167.Sun J, Lee H-W, Pasta M, Yuan H, Zheng G, Sun Y, Li Y and Cui Y, Nat. Nanotechnol, 2015, 10, 980–U184. [DOI] [PubMed] [Google Scholar]
- 168.O’Neill A, Khan U, Nirmalraj PN, Boland J and Coleman JN, J. Phys. Chem. C, 2011, 115, 5422–5428. [Google Scholar]
- 169.Kang J, Wood JD, Wells SA, Lee J-H, Liu X, Chen K-S and Hersam MC, ACS Nano, 2015, 9, 3596–3604. [DOI] [PubMed] [Google Scholar]
- 170.Coleman JN, Lotya M, O’Neill A, Bergin SD, King PJ, Khan U, Young K, Gaucher A, De S, Smith RJ, Shvets IV, Arora SK, Stanton G, Kim H-Y, Lee K, Kim GT, Duesberg GS, Hallam T, Boland JJ, Wang JJ, Donegan JF, Grunlan JC, Moriarty G, Shmeliov A, Nicholls RJ, Perkins JM, Grieveson EM, Theuwissen K, McComb DW, Nellist PD and Nicolosi V, Science, 2011, 331, 568–571. [DOI] [PubMed] [Google Scholar]
- 171.Lee YB, Song SJ, Shin YC, Jung YJ, Kim B, Kang MS, Kwon IK, Hyon SH, Lee HU, Jung SH, Lim D and Han DW, J. Ind. Eng. Chem, 2019, 80, 802–810. [Google Scholar]
- 172.Chen L, Zhou G, Liu Z, Ma X, Chen J, Zhang Z, Ma X, Li F, Cheng H-M and Ren W, Adv. Mater, 2016, 28, 510–517. [DOI] [PubMed] [Google Scholar]
- 173.Lu SB, Miao LL, Guo ZN, Qi X, Zhao CJ, Zhang H, Wen SC, Tang DY and Fan DY, Opt. Express, 2015, 23, 11183–11194. [DOI] [PubMed] [Google Scholar]
- 174.Mu H, Lin S, Wang Z, Xiao S, Li P, Chen Y, Zhang H, Bao H, Lau SP, Pan C, Fan D and Bao Q, Adv. Opt. Mater, 2015, 3, 1447–1453. [Google Scholar]
- 175.Zhang B, Lou F, Zhao R, He J, Li J, Su X, Ning J and Yang K, Opt. Lett, 2015, 40, 3691–3694. [DOI] [PubMed] [Google Scholar]
- 176.Yasaei P, Kumar B, Foroozan T, Wang C, Asadi M, Tuschel D, Indacochea JE, Klie RF and Salehi-Khojin A, Adv. Mater, 2015, 27, 1887–1892. [DOI] [PubMed] [Google Scholar]
- 177.Hanlon D, Backes C, Doherty E, Cucinotta CS, Berner NC, Boland C, Lee K, Harvey A, Lynch P, Gholamvand Z, Zhang S, Wang K, Moynihan G, Pokle A, Ramasse QM, McEvoy N, Blau WJ, Wang J, Abellan G, Hauke F, Hirsch A, Sanvito S, O’Regan DD, Duesberg GS, Nicolosi V and Coleman JN, Nat. Commun, 2015, 6, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yasaei P, Behranginia A, Foroozan T, Asadi M, Kim K, Khalili-Araghi F and Salehi-Khojin A, ACS Nano, 2015, 9, 9898–9905. [DOI] [PubMed] [Google Scholar]
- 179.Cao Y and Mu T, Ind. Eng. Chem. Res, 2014, 53, 8651–8664. [Google Scholar]
- 180.Hapiot P and Lagrost C, Chem. Rev, 2008, 108, 2238–2264. [DOI] [PubMed] [Google Scholar]
- 181.Tariq M, Freire MG, Saramago B, Coutinho JAP, Lopes JNC and Rebelo LPN, Chem. Soc. Rev, 2012, 41, 829–868. [DOI] [PubMed] [Google Scholar]
- 182.Xu F, Ge B, Chen J, Nathan A, Xin LL, Ma H, Min H, Zhu, Xia W, Li Z, Li S, Yu K, Wu L, Cui Y, Sun L and Zhu Y, 2D Mater, 2016, 3, 025005. [Google Scholar]
- 183.Woomer AH, Farnsworth TW, Hu J, Wells RA, Donley CL and Warren SC, ACS Nano, 2015, 9, 8869–8884. [DOI] [PubMed] [Google Scholar]
- 184.Koenig SP, Doganov RA, Schmidt H, Castro Neto AH and Oezyilmaz B, Appl. Phys. Lett, 2014, 104, 103106. [Google Scholar]
- 185.Tayari V, Hemsworth N, Fakih I, Favron A, Gaufres E, Gervais G, Martel R and Szkopek T, Nat. Commun, 2015, 6, 7702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhang S, Yang J, Xu R, Wang F, Li W, Ghufran M, Zhang Y-W, Yu Z, Zhang G, Qin Q and Lu Y, ACS Nano, 2014, 8, 9590–9596. [DOI] [PubMed] [Google Scholar]
- 187.Castellanos-Gomez A, Vicarelli L, Prada E, Island JO, Narasimha-Acharya KL, Blanter SI, Groenendijk DJ, Buscema M, Steele GA, Alvarez JV, Zandbergen HW, Palacios JJ and van der Zant HSJ, 2D Mater, 2014, 1, 025001. [Google Scholar]
- 188.Ge S, Li C, Zhang Z, Zhang C, Zhang Y, Qiu J, Wang Q, Liu J, Jia S, Feng J and Sun D, Nano Lett, 2015, 15, 4650–4656. [DOI] [PubMed] [Google Scholar]
- 189.Cui S, Pu H, Wells SA, Wen Z, Mao S, Chang J, Hersam MC and Chen J, Nat. Commun, 2015, 6, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Anugrah Y, Robbins MC, Crowell PA and Koester SJ, Appl. Phys. Lett, 2015, 106, 103108. [Google Scholar]
- 191.Favron A, Gaufres E, Fossard F, Phaneuf-L’Heureux A-L, Tang NYW, Levesque PL, Loiseau A, Leonelli A, Francoeur S and Martel R, Nat. Mater, 2015, 14, 826–832. [DOI] [PubMed] [Google Scholar]
- 192.Saito Y and Iwasa Y, ACS Nano, 2015, 9, 3192–3198. [DOI] [PubMed] [Google Scholar]
- 193.Island JO, Steele GA, van der Zant HSJ and Castellanos-Gomez A, 2D Mater, 2015, 2, 011002. [Google Scholar]
- 194.Yang J, Xu R, Pei J, Myint YW, Wang F, Wang Z, Zhang S, Yu Z and Lu Y, Light: Sci. Appl, 2015, 4(7), e312. [Google Scholar]
- 195.Wang Z, Jia H, Zheng X, Yang R, Wang Z, Ye GJ, Chen XH, Shan J and Feng PXL, Nanoscale, 2015, 7, 877–884. [DOI] [PubMed] [Google Scholar]
- 196.Huo C, Yan Z, Song X and Zeng H, Sci. Bull, 2015, 60, 1994–2008. [Google Scholar]
- 197.Buscema M, Groenendijk DJ, Blanter SI, Steele GA, van der Zant HSJ and Castellanos-Gomez A, Nano Lett, 2014, 14, 3347–3352. [DOI] [PubMed] [Google Scholar]
- 198.Fu XY, Chen ZD, Zhang YL, Han DD, Ma JN, Wang W, Zhang ZR, Xia H and Sun HB, Nanoscale, 2019, 11, 9133–9140. [DOI] [PubMed] [Google Scholar]
- 199.Kong ZZ, Chen XZ, Ong WJ, Zhao XJ and Li N, Appl. Surf. Sci, 2019, 463, 1148–1153. [Google Scholar]
- 200.Wang K, Chen YX, Zheng JL, Ge YQ, Ji JH, Song YF and Zhang H, Nanotechnology, 2019, 30, 415202. [DOI] [PubMed] [Google Scholar]
- 201.Zhang X, Xie H, Liu Z, Tan C, Luo Z, Li H, Lin J, Sun L, Chen W, Xu Z, Xie L, Huang W and Zhang H, Angew. Chem., Int. Ed, 2015, 54, 3653–3657. [DOI] [PubMed] [Google Scholar]
- 202.Lan SY, Lin ZG, Zhang D, Zeng YY and Liu XL, ACS Appl. Mater. Interfaces, 2019, 11, 9804–9813. [DOI] [PubMed] [Google Scholar]
- 203.Sun Z, Xie H, Tang S, Yu X-F, Guo Z, Shao J, Zhang H, Huang H, Wang H and Chu PK, Angew. Chem., Int. Ed, 2015, 54, 11526–11530. [DOI] [PubMed] [Google Scholar]
- 204.Pan L, Zhu X-D, Sung K-N, Liu Y-T, Xie X-M and Ye X-Y, Nano Energy, 2016, 30, 347–354. [Google Scholar]
- 205.Gao L-F, Xu J-Y, Zhu Z-Y, Hu C-X, Zhang L, Wang Q and Zhang H-L, Nanoscale, 2016, 8, 15132–15136. [DOI] [PubMed] [Google Scholar]
- 206.Li Y, Liu Z, Hou Y, Yang G, Fei X, Zhao H, Guo Y, Su C, Wang Z, Zhong H, Zhuang Z and Guo Z, ACS Appl. Mater. Interfaces, 2017, 9, 25098–25106. [DOI] [PubMed] [Google Scholar]
- 207.Han S-T, Hu L, Wang X, Zhou Y, Zeng Y-J, Ruan S, Pan C and Peng Z, Adv. Sci, 2017, 4, 160043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Yin F, Hu K, Chen S, Wang D, Zhang J, Xie M, Yang D, Qiu M, Zhang H and Li Z.-g., J. Mater. Chem. B, 2017, 5, 5433–5440. [DOI] [PubMed] [Google Scholar]
- 209.Liu S, Lin S, You P, Surya C, Lau SP and Yan F, Angew. Chem., Int. Ed, 2017, 56, 13717–13721. [DOI] [PubMed] [Google Scholar]
- 210.Mu X, Wang J-Y, Bai X, Xu F, Liu H, Yang J, Jing Y, Liu L, Xue X, Dai H, Liu Q, Sun Y-M, Liu C and Zhang X-D, ACS Appl. Mater. Interfaces, 2017, 9, 20399–20409. [DOI] [PubMed] [Google Scholar]
- 211.Chen R, Zheng X and Jiang T, Opt. Express, 2017, 25, 7507–7519. [DOI] [PubMed] [Google Scholar]
- 212.Wang YW, Liu S, Zeng BW, Huang H, Xiao J, Li JB, Long MQ, Xiao S, Yu XF, Gao YL and He J, Nanoscale, 2017, 9, 4683–4690. [DOI] [PubMed] [Google Scholar]
- 213.Sun Z, Zhao Y, Li Z, Cui H, Zhou Y, Li W, Tao W, Zhang H, Wang H, Chu PK and Yu X-F, Small, 2017, 13(11), 1602896. [DOI] [PubMed] [Google Scholar]
- 214.Jiang X-F, Zeng Z, Li S, Guo Z, Zhang H, Huang F and Xu Q-H, Materials, 2017, 10, 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Du J, Zhang M, Guo Z, Chen J, Zhu X, Hu G, Peng P, Zheng Z and Zhang H, Sci. Rep, 2017, 7, 42357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Gu W, Yan Y, Pei X, Zhang C, Ding C and Xian Y, Sens. Actuators, B, 2017, 250, 601–607. [Google Scholar]
- 217.Chen L, Zhang C, Li L, Wu H, Wang X, Yan S, Shi Y and Xiao M, J. Phys. Chem. C, 2017, 121, 12972–12978. [Google Scholar]
- 218.Meng M, Gan Z, Zhang J, Liu K, Wang L, Li S, Yao Y, Zhu Y and Li J, Phys. Status Solidi B, 2017, 254, 1700011. [Google Scholar]
- 219.Chen W, Li K, Wang Y, Feng X, Liao Z, Su Q, Lin X and He Z, J. Phys. Chem. Lett, 2017, 8, 591–598. [DOI] [PubMed] [Google Scholar]
- 220.Wang W, Niu X, Qian H, Guan L, Zhao M, Ding X, Zhang S, Wang Y and Sha J, Nanotechnology, 2016, 27, 505204. [DOI] [PubMed] [Google Scholar]
- 221.Gu W, Pei X, Cheng Y, Zhang C, Zhang J, Yan Y, Ding C and Xian Y, ACS Sens, 2017, 2, 576–582. [DOI] [PubMed] [Google Scholar]
- 222.Wang Z, Xu Y, Dhanabalan SC, Sophia J, Zhao C, Xu C, Xiang Y, Li J and Zhang H, IEEE Photonics J, 2016, 8, 1–10. [Google Scholar]
- 223.Xu Y, Wang Z, Guo Z, Huang H, Xiao Q, Zhang H and Yu X-F, Adv. Opt. Mater, 2016, 4, 1223–1229. [Google Scholar]
- 224.Zhu C, Xu F, Zhang L, Li M, Chen J, Xu S, Huang G, Chen W and Sun L, Chem. – Eur. J, 2016, 22, 7357–7362. [DOI] [PubMed] [Google Scholar]
- 225.Ge S, Zhang L, Wang P and Fang Y, Sci. Rep, 2016, 6, 27307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Cai J, Ruffieux P, Jaafar R, Bieri M, Braun T, Blankenburg S, Muoth M, Seitsonen AP, Saleh M, Feng X, Muellen K and Fasel R, Nature, 2010, 466, 470–473. [DOI] [PubMed] [Google Scholar]
- 227.Guo H, Lu N, Dai J, Wu X and Zeng XC, J. Phys. Chem. C, 2014, 118, 14051–14059. [Google Scholar]
- 228.Carvalho A, Rodin A and Neto AC, EPL, 2014, 108, 47005. [Google Scholar]
- 229.Lee S, Yang F, Suh J, Yang S, Lee Y, Li G, Choe HS, Suslu A, Chen Y, Ko C, Park J, Liu K, Li J, Hippalgaonkar K, Urban JJ, Tongay S and Wu J, Nat. Commun, 2015, 6, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Yagmurcukardes M, Peeters FM, Senger RT and Sahin H, Appl. Phys. Rev, 2016, 3, 041302. [Google Scholar]
- 231.Yao Q, Huang C, Yuan Y, Liu Y, Liu S, Deng K and Kan E, J. Phys. Chem. C, 2015, 119, 6923–6928. [Google Scholar]
- 232.Nourbakhsh Z and Asgari R, Phys. Rev. B, 2016, 94, 035437. [Google Scholar]
- 233.Wu Q, Shen L, Yang M, Cai Y, Huang Z and Feng YP, Phys. Rev. B: Condens. Matter Mater. Phys, 2015, 92, 035436. [Google Scholar]
- 234.Yang Y-R, Zhang Z-Q, Gu L and Fu H-H, RSC Adv, 2016, 6, 44019–44023. [Google Scholar]
- 235.Poljak M and Suligoj T, Nano Res, 2016, 9, 1723–1734. [Google Scholar]
- 236.Das PM, Danda G, Cupo A, Parkin WM, Liang L, Kharche N, Ling X, Huang S, Dresselhaus MS, Meunier V and Drndic M, ACS Nano, 2016, 10, 5687–5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Sisakht ET, Fazileh F, Zare M, Zarenia M and Peeters F, Phys. Rev. B, 2016, 94, 085417. [Google Scholar]
- 238.Wu X, Zhang X, Wang X and Zeng Z, AIP Adv, 2016, 6, 045318. [Google Scholar]
- 239.Hu W, Lin L, Zhang R, Yang C and Yang J, J. Am. Chem. Soc, 2017, 139, 15429–15436. [DOI] [PubMed] [Google Scholar]
- 240.Yang G, Xu S, Zhang W, Ma T and Wu C, Phys. Rev. B, 2016, 94, 075106. [Google Scholar]
- 241.Sorkin V, Cai Y, Ong Z, Zhang G and Zhang Y-W, Crit. Rev. Solid State Mater. Sci, 2017, 42, 1–82. [Google Scholar]
- 242.Zhou Q, Chen Q, Tong Y and Wang J, Angew. Chem., Int. Ed, 2016, 55, 11437–11441. [DOI] [PubMed] [Google Scholar]
- 243.Abellan G, Wild S, Lloret V, Scheuschner N, Gillen R, Mundloch U, Maultzsch J, Varela M, Hauke F and Hirsch A, J. Am. Chem. Soc, 2017, 139, 10432–10440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Zhang T, Wan Y, Xie H, Mu Y, Du P, Wang D, Wu X, Ji H and Wan L, J. Am. Chem. Soc, 2018, 140, 7561–7567. [DOI] [PubMed] [Google Scholar]
- 245.Abate Y, Akinwande D, Gamage S, Wang H, Snure M, Poudel N and Cronin SB, Adv. Mater, 2018, 30(29), 1704749. [DOI] [PubMed] [Google Scholar]
- 246.Liu Y, Feng XB, Qin YG and Wang QJ, Appl. Sci, 2019, 9, 2014. [Google Scholar]
- 247.Forte JDS, de Sousa DJP and Pereira JM, Phys. E, 2019, 114, 113578. [Google Scholar]
- 248.Li ZF, Ruan BX, Zhu JQ, Guo J, Dai XY and Xiang YJ, Results Phys, 2019, 15, 102677. [Google Scholar]
- 249.Mironov V, Boland T, Trusk T, Forgacs G and Markwald RR, Trends Biotechnol, 2003, 21, 157–161. [DOI] [PubMed] [Google Scholar]
- 250.Murphy SV and Atala A, Nat. Biotechnol, 2014, 32, 773. [DOI] [PubMed] [Google Scholar]
- 251.Siqueira G, Kokkinis D, Libanori R, Hausmann MK, Gladman AS, Neels A, Tingaut P, Zimmermann T, Lewis JA and Studart AR, Adv. Funct. Mater, 2017, 27, 1604619. [Google Scholar]
- 252.Sultan S, Abdelhamid HN, Zou X and Mathew AP, Adv. Funct. Mater, 2019, 29, 1805372. [Google Scholar]
- 253.Bose S, Vahabzadeh S and Bandyopadhyay A, Mater. Today, 2013, 16, 496–504. [Google Scholar]
- 254.Pham M-S, Liu C, Todd I and Lertthanasarn J, Nature, 2019, 565, 305–311. [DOI] [PubMed] [Google Scholar]
- 255.Cui H, Hensleigh R, Yao D, Maurya D, Kumar P, Kang MG, Priya S and Zheng XR, Nat. Mater, 2019, 18, 234–241. [DOI] [PubMed] [Google Scholar]
- 256.Koffler J, Zhu W, Qu X, Platoshyn O, Dulin JN, Brock J, Graham L, Lu P, Sakamoto J and Marsala M, Nat. Med, 2019, 25, 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Kelly BE, Bhattacharya I, Heidari H, Shusteff M, Spadaccini CM and Taylor HK, Science, 2019, 363, 1075–1079. [DOI] [PubMed] [Google Scholar]
- 258.De Beer MP, Van Der Laan HL, Cole MA, Whelan RJ, Burns MA and Scott TF, Sci. Adv, 2019, 5, eaau8723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Wang J, Lu T, Yang M, Sun D, Xia Y and Wang T, Sci. Adv, 2019, 5, eaau8769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Zhu C, Qi Z, Beck VA, Luneau M, Lattimer J, Chen W, Worsley MA, Ye J, Duoss EB and Spadaccini CM, Sci. Adv, 2018, 4, eaas9459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Bose S, Roy M and Bandyopadhyay A, Trends Biotechnol, 2012, 30, 546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Wang C, Ye X, Zhao Y, Bai L, He Z, Tong Q, Xie X, Zhu H, Cai D, Zhou Y, Lu B, Wei Y, Mei L, Xie D and Wang M, Biofabrication, 2020, 12, 035004. [DOI] [PubMed] [Google Scholar]
- 263.Liu X, Miller AL, Park S, George M, Waletzki BE, Xu H, Terzic A and Lu L, ACS Appl. Mater. Interfaces, 2019, 11, 23558–23572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Dilla RA, Motta CM, Snyder SR, Wilson JA, Wesdemiotis C and Becker ML, ACS Macro Lett, 2018, 7, 1254–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Liu X, Miller AL, Xu H, Waletzki BE and Lu L, Biomacromolecules, 2019, 20, 3352–3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Liu X, George MN, Park S, Miller AL II, Gaihre B, Li L, Waletzki BE, Terzic A, Yaszemski MJ and Lu L, Acta Biomater, 2020, 111, 129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Kasper FK, Tanahashi K, Fisher JP and Mikos AG, Nat. Protoc, 2009, 4, 518–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Trachtenberg JE, Placone JK, Smith BT, Piard CM, Santoro M, Scott DW, Fisher JP and Mikos AG, ACS Biomater. Sci. Eng, 2016, 2, 1771–1780. [DOI] [PubMed] [Google Scholar]
- 269.Liu X, Paulsen A, Giambini H, Guo J, Miller AL, Lin P-C, Yaszemski MJ and Lu L, Tissue Eng., Part A, 2017, 23, 223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Guvendiren M, Lu HD and Burdick JA, Soft Matter, 2012, 8, 260–272. [Google Scholar]
- 271.Li Y, Rodrigues J and Tomas H, Chem. Soc. Rev, 2012, 41, 2193–2221. [DOI] [PubMed] [Google Scholar]
- 272.Gkioni K, Leeuwenburgh SC, Douglas TE, Mikos AG and Jansen JA, Tissue Eng., Part B, 2010, 16, 577–585. [DOI] [PubMed] [Google Scholar]
- 273.Wang Z, Zhao J, Tang W, Hu L, Chen X, Su Y, Zou C, Wang J, Lu WW, Zhen W, Zhang R, Yang D and Peng S, Small, 2019, 15, e1901560. [DOI] [PubMed] [Google Scholar]
- 274.Huang K, Wu J and Gu Z, ACS Appl. Mater. Interfaces, 2019, 11, 2908–2916. [DOI] [PubMed] [Google Scholar]
- 275.Liu X, George MN, Li L, Gamble D, Miller AL II, Gaihre B, Waletzki BE and Lu L, ACS Biomater. Sci. Eng, 2020, 6, 4653–4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Ahadian S, Yamada S, Ramon-Azcon J, Estili M, Liang XB, Nakajima K, Shiku H, Khademhosseini A and Matsue T, Acta Biomater, 2016, 31, 134–143. [DOI] [PubMed] [Google Scholar]
- 277.Liu X, Miller AL II, Park S, Waletzki BE, Terzic A, Yaszemski MJ and Lu L, J. Mater. Chem. B, 2016, 4, 6930–6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Martinelli V, Bosi S, Peña B, Baj G, Long CS, Sbaizero O, Giacca M, Prato M and Mestroni L, ACS Appl. Bio Mater, 2018, 1, 1530–1537. [DOI] [PubMed] [Google Scholar]
- 279.Pok S, Vitale F, Eichmann SL, Benavides OM, Pasquali M and Jacot JG, ACS Nano, 2014, 8, 9822–9832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Liu X, Miller AL, Park S, Waletzki BE, Zhou Z, Terzic A and Lu L, ACS Appl. Mater. Interfaces, 2017, 9, 14677–14690. [DOI] [PubMed] [Google Scholar]
- 281.Liu X, Kim JC, Miller AL, Waletzki BE and Lu L, New J. Chem, 2018, 42, 17671–17681. [Google Scholar]
- 282.Paliwal R and Palakurthi S, J. Controlled Release, 2014, 189, 108–122. [DOI] [PubMed] [Google Scholar]
- 283.Chen F-M, Zhang M and Wu Z-F, Biomaterials, 2010, 31, 6279–6308. [DOI] [PubMed] [Google Scholar]
- 284.Anderson HC, Curr. Rheumatol. Rep, 2003, 5, 222–226. [DOI] [PubMed] [Google Scholar]
- 285.Wang Y, Hu X, Zhang L, Zhu C, Wang J, Li Y, Wang Y, Wang C, Zhang Y and Yuan Q, Nat. Commun, 2019, 10, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Xie J, Li X and Xia Y, Macromol. Rapid Commun, 2008, 29, 1775–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Zheng JK, Hua G, Yu JY, Lin F, Wade MB, Reneker DH and Becker ML, ACS Macro Lett, 2015, 4, 207–213. [DOI] [PubMed] [Google Scholar]
- 288.Lin F, Yu JY, Tang W, Zheng JK, Xie SB and Becker ML, Macromolecules, 2013, 46, 9515–9525. [Google Scholar]
- 289.Pham QP, Sharma U and Mikos AG, Tissue Eng, 2006, 12, 1197–1211. [DOI] [PubMed] [Google Scholar]
- 290.Saraf A, Lozier G, Haesslein A, Kasper FK, Raphael RM, Baggett LS and Mikos AG, Tissue Eng., Part C, 2009, 15, 333–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Tanaka Y, Nakayamada S and Okada Y, Curr. Drug Targets: Inflammation Allergy, 2005, 4, 325–328. [DOI] [PubMed] [Google Scholar]
- 292.Knight JA, Ann. Clin. Lab. Sci, 1997, 27, 11–25. [PubMed] [Google Scholar]
- 293.Koh TJ and DiPietro LA, Expert Rev. Mol. Med, 2011, 13, e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Greaves NS, Ashcroft KJ, Baguneid M and Bayat A, J. Dermatol. Sci, 2013, 72, 206–217. [DOI] [PubMed] [Google Scholar]
- 295.Tajirian AL and Goldberg DJ, J. Cosmet. Laser Ther, 2010, 12, 296–302. [DOI] [PubMed] [Google Scholar]
- 296.Zhou W-H, Zhu C-L, Lu C-H, Guo X, Chen F, Yang H-H and Wang X, Chem. Commun, 2009, 6845–6847. [DOI] [PubMed] [Google Scholar]
- 297.Qu F, Li T and Yang M, Biosens. Bioelectron, 2011, 26, 3927–3931. [DOI] [PubMed] [Google Scholar]
- 298.Villalonga A, Pérez-Calabuig AM and Villalonga R, Anal. Bioanal. Chem, 2020, 1–18. [DOI] [PubMed] [Google Scholar]
- 299.Xiao M, Lai W, Man T, Chang B, Li L, Chandrasekaran AR and Pei H, Chem. Rev, 2019, 119, 11631–11717. [DOI] [PubMed] [Google Scholar]
- 300.Wang L, Liu X, Zhang Q, Zhang C, Liu Y, Tu K and Tu J, Biotechnol. Lett, 2012, 34, 869–874. [DOI] [PubMed] [Google Scholar]
- 301.Sabherwal P, Mutreja R and Suri CR, TrAC, Trends Anal. Chem, 2016, 82, 12–21. [Google Scholar]
- 302.Kumar V, Brent JR, Shorie M, Kaur H, Chadha G, Thomas AG, Lewis EA, Rooney AP, Nguyen L and Zhong XL, ACS Appl. Mater. Interfaces, 2016, 8, 22860–22868. [DOI] [PubMed] [Google Scholar]
- 303.Ricci Z, Cruz DN and Ronco C, Nat. Rev. Nephrol, 2011, 7, 201. [DOI] [PubMed] [Google Scholar]
- 304.Prowle JR, Echeverri JE, Ligabo EV, Ronco C and Bellomo R, Nat. Rev. Nephrol, 2010, 6, 107. [DOI] [PubMed] [Google Scholar]
- 305.Aksu U, Demirci C and Ince C, in Controversies in Acute Kidney Injury, Karger Publishers, 2011, vol. 174, pp. 119–128. [DOI] [PubMed] [Google Scholar]
- 306.Huang K, Zhang Y, Lin J and Huang P, Biomater. Sci, 2019, 7, 472–479. [DOI] [PubMed] [Google Scholar]
- 307.Yang G, Liu Z, Li Y, Hou Y, Fei X, Su C, Wang S, Zhuang Z and Guo Z, Biomater. Sci, 2017, 5, 2048–2055. [DOI] [PubMed] [Google Scholar]
- 308.Zhao Y, Tong L, Li Z, Yang N, Fu H, Wu L, Cui H, Zhou W, Wang J and Wang H, Chem. Mater, 2017, 29, 7131–7139. [Google Scholar]
- 309.Wang S, Weng J, Fu X, Lin J, Fan W, Lu N, Qu J, Chen S, Wang T and Huang P, Nanotheranostics, 2017, 1, 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Liang X, Ye XY, Wang C, Xing CY, Miao QW, Xie ZJ, Chen XL, Zhang XD, Zhang H and Mei L, J. Controlled Release, 2019, 296, 150–161. [DOI] [PubMed] [Google Scholar]
- 311.Li Z, Hu YH, Fu QR, Liu Y, Wang J, Song JB and Yang HH, Adv. Funct. Mater, 2020, 30, 1905758. [Google Scholar]
- 312.Burns JM, Vankayala R, Mac JT and Anvari B, ACS Appl. Mater. Interfaces, 2018, 10, 27621–27630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Hu CMJ, Fang RH and Zhang L, Adv. Healthcare Mater, 2012, 1, 537–547. [DOI] [PubMed] [Google Scholar]
- 314.Liu W, Ruan M, Wang Y, Song R, Ji X, Xu J, Dai J and Xue W, Small, 2018, 14, 1801754. [Google Scholar]
- 315.Su J, Sun H, Meng Q, Yin Q, Zhang P, Zhang Z, Yu H and Li Y, Adv. Funct. Mater, 2016, 26, 7495–7506. [Google Scholar]
- 316.Ou W, Byeon JH, Soe ZC, Kim BK, Thapa RK, Gupta B, Poudel BK, Ku SK, Yong CS and Kim JO, Theranostics, 2019, 9, 6780–6796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Schiros T, Nordlund D, Pálová L, Prezzi D, Zhao L, Kim KS, Wurstbauer U, Gutiérrez C, Delongchamp D and Jaye C, Nano Lett, 2012, 12, 4025–4031. [DOI] [PubMed] [Google Scholar]
- 318.Ding X, Liow CH, Zhang M, Huang R, Li C, Shen H, Liu M, Zou Y, Gao N and Zhang Z, J. Am. Chem. Soc, 2014, 136, 15684–15693. [DOI] [PubMed] [Google Scholar]
- 319.Cao Y, Li S, Chen C, Wang D, Wu T, Dong H and Zhang X, Biomaterials, 2018, 158, 23–33. [DOI] [PubMed] [Google Scholar]
- 320.Wang J, Zhang H, Xiao X, Liang D, Liang X, Mi L, Wang J and Liu J, Acta Biomater, 2020, 107, 260–271. [DOI] [PubMed] [Google Scholar]
- 321.Eswaraiah V, Zeng Q, Long Y and Liu Z, Small, 2016, 12, 3480–3502. [DOI] [PubMed] [Google Scholar]
- 322.Kurapati R, Kostarelos K, Prato M and Bianco A, Adv. Mater, 2016, 28, 6052–6074. [DOI] [PubMed] [Google Scholar]
- 323.Desai BM, Am. J. Orthop, 2007, 36, 8–11. [PubMed] [Google Scholar]
- 324.Wang W and Yeung KW, Bioact. Mater, 2017, 2, 224–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Winkler T, Sass F, Duda G and Schmidt-Bleek K, Bone Joint Res, 2018, 7, 232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Kakar S and Shin AY, Chir. Main, 2010, 29(Suppl 1), S104–S111. [DOI] [PubMed] [Google Scholar]
- 327.Nair MB, Kretlow JD, Mikos AG and Kasper FK, Curr. Opin. Biotechnol, 2011, 22, 721–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Oftadeh R, Perez-Viloria M, Villa-Camacho JC, Vaziri A and Nazarian A, J. Biomech. Eng, 2015, 137, 010802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Lewis G, J. Biomed. Mater. Res., Part B, 2017, 105, 1260–1284. [DOI] [PubMed] [Google Scholar]
- 330.Saleh KJ, El Othmani MM, Tzeng TH, Mihalko WM, Chambers MC and Grupp TM, J. Orthop. Res, 2016, 34, 737–744. [DOI] [PubMed] [Google Scholar]
- 331.Fernandez de Grado G, Keller L, Idoux-Gillet Y, Wagner Q, Musset A-M, Benkirane-Jessel N, Bornert F and Offner D, J. Tissue Eng, 2018, 9, 2041731418776819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Shridhar P, Chen Y, Khalil R, Plakseychuk A, Cho S, Tillman B, Kumta P and Chun Y, Materials, 2016, 9, 821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Kondiah PJ, Choonara YE, Kondiah PP, Marimuthu T, Kumar P, du Toit LC and Pillay V, Molecules, 2016, 21, 1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Magnan B, Bondi M, Maluta T, Samaila E, Schirru L and Dall’Oca C, Musculoskelet. Surg, 2013, 97, 93–100. [DOI] [PubMed] [Google Scholar]
- 335.Kretlow JD and Mikos AG, Tissue Eng, 2007, 13, 927–938. [DOI] [PubMed] [Google Scholar]
- 336.Arora M, Chan EK, Gupta S and Diwan AD, World J. Orthop, 2013, 4, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Mancardi G, Tamargo CEH, Di Tommaso D and De Leeuw NH, J. Mater. Chem. B, 2017, 5, 7274–7284. [DOI] [PubMed] [Google Scholar]
- 338.Shao J, Ruan C, Xie H, Li Z, Wang H, Chu PK and Yu XF, Adv. Sci, 2018, 5, 1700848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Turner CG and Fauza DO, Clin.Perinatol, 2009, 36, 473–488. [DOI] [PubMed] [Google Scholar]
- 340.Bryan N, Ahswin H, Smart N, Bayon Y, Wohlert S and Hunt JA, Eur. Cells Mater, 2012, 24, e65. [DOI] [PubMed] [Google Scholar]
- 341.Liou G-Y and Storz P, Free Radical Res, 2010, 44, 479–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Yang D, Yang G, Yang P, Lv R, Gai S, Li C, He F and Lin J, Adv. Funct. Mater, 2017, 27, 1700371. [Google Scholar]
- 343.Pan W, Dai C, Li Y, Yin Y, Gong L, Machuki J. O. a., Yang Y, Qiu S, Guo K and Gao F, Biomaterials, 2020, 239, 119851. [DOI] [PubMed] [Google Scholar]
- 344.Chen Y, Ren R, Pu H, Chang J, Mao S and Chen J, Biosens. Bioelectron, 2017, 89, 505–510. [DOI] [PubMed] [Google Scholar]
- 345.Jiang H, Xia Q, Liu D and Ling K, Anal. Chim. Acta, 2020, 1121, 1–10. [DOI] [PubMed] [Google Scholar]
- 346.Su YJ, Wang TT, Su YN, Li M, Zhou JP, Zhang W and Wang W, Mater. Horiz, 2020, 7, 574–585. [Google Scholar]


